Abstract
Individuals with a history of epilepsy are at higher risk for bone fractures compared to the general population. Although clinical studies support an association between low bone mineral density (BMD) and anti-seizure medications, little is known on whether a history of seizures is linked to altered bone health. Therefore, in this study we tested the hypothesis that bone mass, morphology, and bone mineralization are altered by seizures in genetically epileptic animals and in animals subjected to an episode of status epilepticus. In this study, we used NS-Pten conditional knockout mice (a well-studied genetic model of epilepsy). We used microCT analysis to measure BMD, morphology, and mineralization in NS-Pten+/+ (wildtype) and NS-Pten−/− (knockout) mice at 4 and 8 weeks, as well as adult Kv4.2+/+ and Kv4.2−/− mice. We measured BMD, bone morphology, and mineralization in adult NS-Pten+/+ mice that received status epilepticus through kainic acid (20 mg/kg intraperitoneal). Further, we measured locomotion for NS-Pten+/+ and NS-Pten−/− mice at 4 and 6 weeks. We found that NS-Pten−/− mice exhibited low BMD in the tibial metaphysis and midshaft compared to non-epileptic mice. Morphologically, NS-Pten−/− mice exhibited decreased trabecular volume fraction, and endocortical expansion in both the metaphyeal and diaphyseal compartments. In the midshaft, NS-Pten−/− mice exhibited reduced tissue mineral density, indicating impaired mineralization in addition to morphological deficits. NS-Pten−/− mice exhibited hyperactivity in open field testing, suggesting low bone mass in NS-Pten−/− mice was not attributable to hypoactivity. Differences in BMD were not observed following kainate-induced seizures or in the Kv4.2−/− model of seizure susceptibility. Our findings suggest that deletion of Pten in the brain results in impaired bone mass and mineralization, which may contribute to weaker bones and thereby a higher fracture risk.
Keywords: PI3K, mTOR, osteoporosis, fracture, BMD, seizure, neurodevelopmental disorder, epilepsy
1. Introduction
Seizures can induce long-term structural and biochemical changes and the occurrence of at least one spontaneous seizure can dramatically augment the risk for future seizures (Ben-Ari et al., 2008). While the importance of immediate treatment is clear, the choice of treatment strategy can have a significant impact on long-term health. In children, a long-term consequence that is important to understand is bone health (Sheth, 2004). Approximately 95% of the total bone development occurs by 18 years of age with a peak of bone mineral density (BMD) that occurs by 25 and declines after 40 (Kemper, 2000). Therefore, any process that reduces bone mass or alters mineralization during the early developmental period or early adulthood will likely result in an increased susceptibility to bone fractures in the long-term (Melton et al., 1988).
The overall bone fracture rate of individuals with epilepsy is 2 to 6 times higher than the general population (Harden, 2003; Koppel et al., 2005), and thereby represents an important clinical problem. The bone fracture rate in epilepsy is not solely due to seizure-related falls. For instance, in one study, investigators followed 750 patients with epilepsy over a 7 year period (Sheth et al., 2006). While 61% of the fractures were due to seizure-related falls, the remainder (39%) were pathological (i.e., they occurred without adequate trauma and thus were associated with preexistent bone weakening). The authors predicted that the occurrence of pathological fractures should be highest in those over 50 years of age. However, they found that the incidence of pathological fractures was 56% in individuals younger than 50 and 15% in those younger than 20 years of age. The high incidence of bone fractures in epilepsy may be associated with changes in bone structural integrity. Clinical studies support that anti-seizure medications reduce BMD and thereby bone strength. For instance, carbamazepine treatment correlates with an 8% reduction in BMD in the lumbar spine (Sheth et al., 1995). Twenty-three percent of individuals receiving valproate monotherapy have osteoporosis (i.e., BMD 2.5 standard deviation or more the average for a young adult) and 37% have osteopenia (i.e., 1-2.5 standard deviations below) (Petty et al., 2016b). While these studies suggest the possibility that anti-seizure medications alter bone mineral density, not all studies have reported consistent effects. Children treated with carbamazepine (> 12 months) displayed similar BMD compared to healthy children (Akin et al., 1998). One theory is that treatment with cytochrome P450 enzyme-inducing antiepileptic drugs (EIAEDs) can contribute to a higher fracture risk compared to non-EIAEDs potentially by increasing the metabolism of vitamin D in the liver (Pack et al., 2005). The reduction of vitamin D is widely thought to result in a decrease in BMD. However, one recent review found inconclusive evidence that EIAEDs produce a higher risk to fractures than non-EIAEDS (Fraser et al., 2015). Taken together these studies support the idea that anti-seizure medications influence bone metabolism. However, the impact of a history of seizures on accrual of bone mass and mineralization is unknown. Therefore, in this study we tested the hypothesis that bone mass, morphology, and bone mineralization may be altered by seizures in untreated epileptic animals and in animals subjected to an episode of status epilepticus.
For the experiments in this study, we used mouse models of genetic epilepsy and acquired epilepsy. The genetic model of epilepsy is a neuronal-subset specific phosphatase and tensin homologue (PTEN) knockout mice. The mice possess a conditional deletion for the PI(3,4,5)P3 lipid phosphatase PTEN (Maehama and Dixon, 1998), which results in enlarged cortical neurons, hyperactivation of the mTOR pathway, and spontaneous recurrent seizures starting by 6-7 weeks of age which progress in duration and intensity over the lifespans of the mice (Kwon et al., 2001; Ljungberg et al., 2009; Nguyen et al., 2015; Sunnen et al., 2011). We performed an in-depth characterization of bone phenotype in NS-Pten−/− mice and NS-Pten+/+ mice at 4 and 8 weeks of age. To study the effects of a single episode of status epilepticus on bone mineral density, in parallel we used mouse models of seizures (kainate-induced) and increased seizure susceptibility (Kv4.2 knockout mice). Kv4.2 subunits contribute to the transient, A-type K+ current (A-current) in CA1 pyramidal cell dendrites. Mice with deletion of Kv4.2 have a decrease in the latency to seizures and status epilepticus following kainate (Barnwell et al., 2009). When presented with bicuculline, brain slices from Kv4.2 knockout mice show an increase in epileptiform bursting in the CA1 region of the hippocampus. However, they do not develop spontaneous seizures.
2. Methods
2.1. Subjects
GFAP-Cre; PtenloxP:loxP mice have been previously described (Backman et al., 2001; Ljungberg et al., 2009). We bred NS-PtenloxP/+ heterozygote parents to produce NS-Pten+/+ wild-type (WT), NS-PtenloxP/+ heterozygous (HT), and NS-Pten loxP/loxP knockout (KO) over 10 generations on a FVB-based mixed background strain. The Kv4.2 knockout mice used in this study were generated in a 129S6/SvEv background (Chen et al., 2006). The knockout mice were backcrossed with WT 129S6/SvEv to produce (F10 or greater) littermates that were used in these studies. The Kv4.2 knockout and wildtype animals were from the offspring of heterozygote parents. Mice were generated and housed at Baylor University at 22°C ambient temperature with 14-h light and 10-h dark (20:00 to 06:00) diurnal cycle. All mice were given ad libidum access to food and water. All procedures were conducted in compliance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the animal protocol was approved by Baylor University Animal Care and Use Committee. For experiments all mice used in experiments were 8 week old males, unless noted otherwise.
2.2. microCT Analysis
For microCT analysis, mice were euthanized by rapid decapitation and then stored at −20 °C until later analyzed. Mice were scanned at the proximal and mid diaphysis with a vivaCT40 microCT scanner (Scanco Medical, Switzerland) at 10.5μm voxel resolution using the following settings: (55 kVp, 145 μA, 2048 samples, 1000 proj/180°, 200 ms integration time). Analysis of trabecular parameters in the tibial metaphysis (BV: bone volume, TV: total volume, BV/TV: bone volume/total volume, Tb.N: trabecular number, and Tb.Th: trabecular thickness) and cortical parameters in the tibial midshaft (Ct.Th: cortical thickness, endocortical volume, and TMD: tissue mineral density) were performed as previously described (Poliachik et al., 2010).
2.3. Open Field
Locomotor activity was evaluated by use of an open field activity test as previously described.(Lugo et al., 2014) Mice were first allowed to acclimate to the room for 30 min before testing. Mice were then placed in a clear acrylic arena (40 cm × 40 cm × 30 cm) and their activity levels were observed over 30 min. Open field activity was collected by a computer-operated optical animal activity system (Fusion by Omnitech Electronics Inc., Columbus, USA). Total activity levels, stereotypy (self-grooming), and rearing behaviors were measured. The testing chamber was cleaned with 30% isopropanol between subjects.
2.4. Kainate-Induced Seizures
Seizures were induced in NS-Pten+/+ mice with kainic acid (20 mg/kg) via intraperitoneal (IP) injection on postnatal day 60. The presence of behavioral status epilepticus was determined using previously described methods (Barnwell et al., 2009). At approximately 1 hour following either saline or kainic acid injections all mice received a 20 mg/kg (IP) injection of pentobarbital to terminate seizure activity. A second 10 mg/kg dose was administered to those mice that continued to show behavioral seizure activity 1 hour after the initial pentobarbital injections. All seizures were induced in the mice while they were held in isolation. Seizure activity was monitored through behavioral observations for at least 2 hours following injections. They were then regularly checked on for several more hours, until all mice showed recovery. Following recovery, mice were returned to their home cages with their littermates. Mice that displayed continuous aggression and fighting behaviors, to the point of injury, were separated and singly housed for the duration of testing.
2.5. Statistical Analysis
All data were analyzed by using SPSS 21.0 for PC (SPSS, Chicago, IL) or by Graphpad Prism 6 for PC or Mac (San Diego, CA). For all comparisons, the level of significance was set at p < 0.05. We used independent samples, two-tailed t-tests assuming equal variances when comparing two groups.
3. Results
3.1. NS-Pten−/− Mice exhibit low bone mineral density
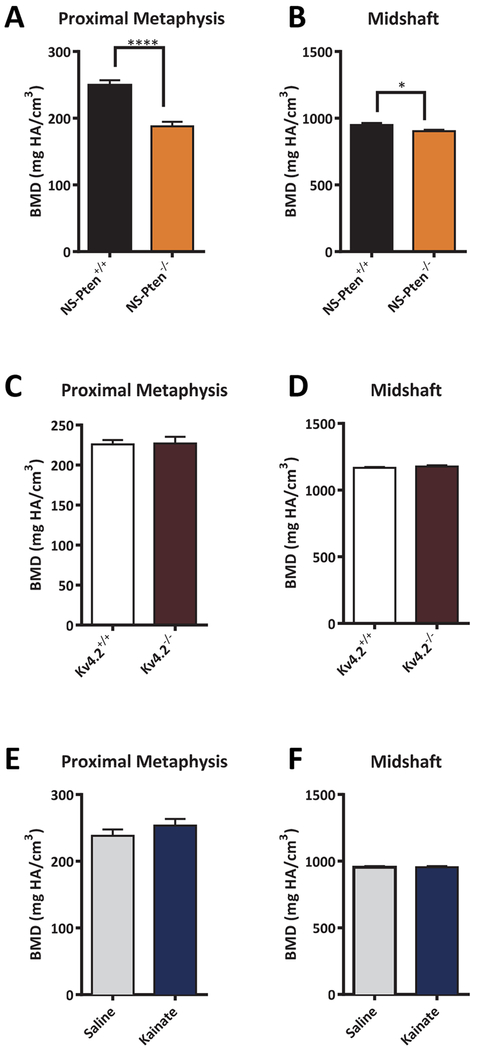
NS-Pten−/− mice are morphologically similar to NS-Pten+/+ controls and do not present obvious signs of gross skeletal abnormalities. There were no difference in weight between the NS-Pten+/+ (26.7g± 1.3) and NS-Pten−/− (27.3g± 1.0) mice. However, in depth analyses of bone structure volume and BMD revealed several group differences. MicroCT analysis of the proximal tibial metaphysis revealed that BMD in the trabecular compartment was decreased by 24.7% t(l, 24) = 6.02 p < 0.0001 (Fig. 1A). We computed a BMD Z-score of −2.1 in NS-Pten−/− mice, a value associated with osteoporosis in children (Baim et al., 2008; Gordon et al., 2014). To determine whether BMD deficits extended to cortical bone, we examined BMD in the tibial midshaft. Analysis revealed that midshaft BMD in NS-Pten−/− mice was also significantly reduced t(1,24) = 2.581 p < 0.05 (Fig. 1B), though at a decreased level (4.9%) compared to that in the metaphysis.
Figure 1.
NS-Pten knockout (−/−) mice exhibit low BMD. BMD in the metaphyseal and midshaft compartments is decreased in NS-Pten−/− mice (A-B), but not in Kv4.2−/− mice (C-D) or following a single bout of kainate-induced seizures (E-F). The bars represent the mean and the error bars represent the standard error of the mean. * = p < 0.05; ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. NS-Pten+/+ n= 14; NS-Pten−/− n=12; Kv4.2+/+ n = 12; Kv4.2−/− n = 10; NS-Pten+/+ vehicle n = 12; NS-Pten+/+ kainate n = 9.
We sought to contrast the prevalence of this phenotype in a genetic model of epilepsy (NS-Pten knockout mice), a mouse model of acquired epilepsy in which a single prolonged seizure is associated with the development of epilepsy at a later time (kainate-induced seizures), and a mouse model characterized by a high seizure susceptibility (Kv4.2 knockout mice) (Barnwell et al., 2009; Lugo et al., 2012; Lugo et al., 2014). Neither Kv4.2−/− mice (proximal tibia: t(1,20) = 0.11, p = 0.91; midshaft: t(1,20) = 0.90, p = 0.38) (Fig. 1C–1D) or kainate-exposed mice (proximal tibia: t(1,20) = 0.11, p = 0.91; midshaft: t(1,20) = 0.90, p = 0.38) (Fig. 1E–1F) exhibited significant differences in BMD compared to their respective control groups. Thus, the low BMD phenotype exhibited by NS-Pten−/− mice was not recapitulated with a single bout of kainate-induced seizures or in mice with high susceptibility for seizures arising from Kv4.2 channel deletion. There were no differences in weight between the Kv4.2+/+ (29.45g ± 1.6) and Kv4.2−/− (28.5g ± 1.5) or between Pten saline (31.6g ±1.7) and Pten kainate groups (33.1g ±1.3).
3.2. Low BMD in NS-Pten−/− mice is attributable to deficits in bone mass and mineralization
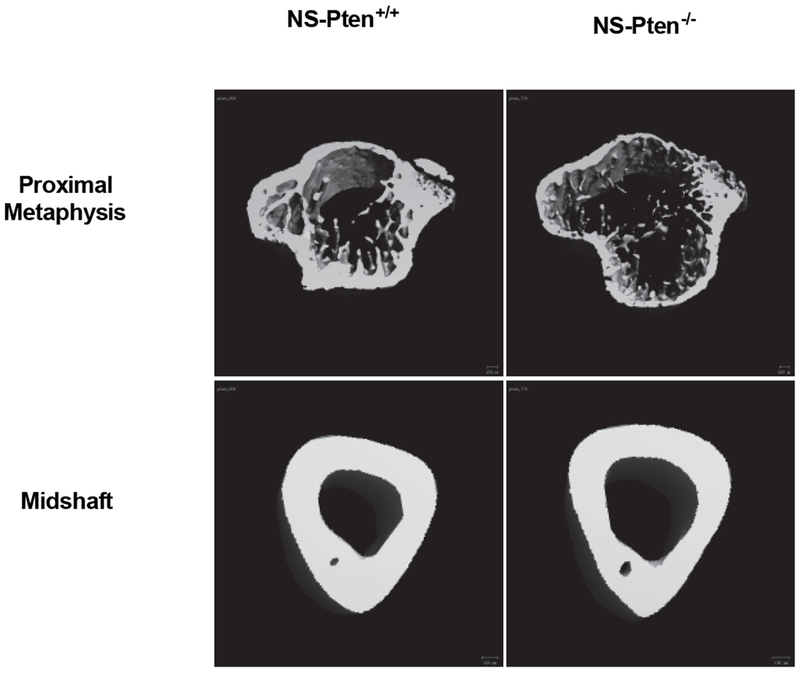
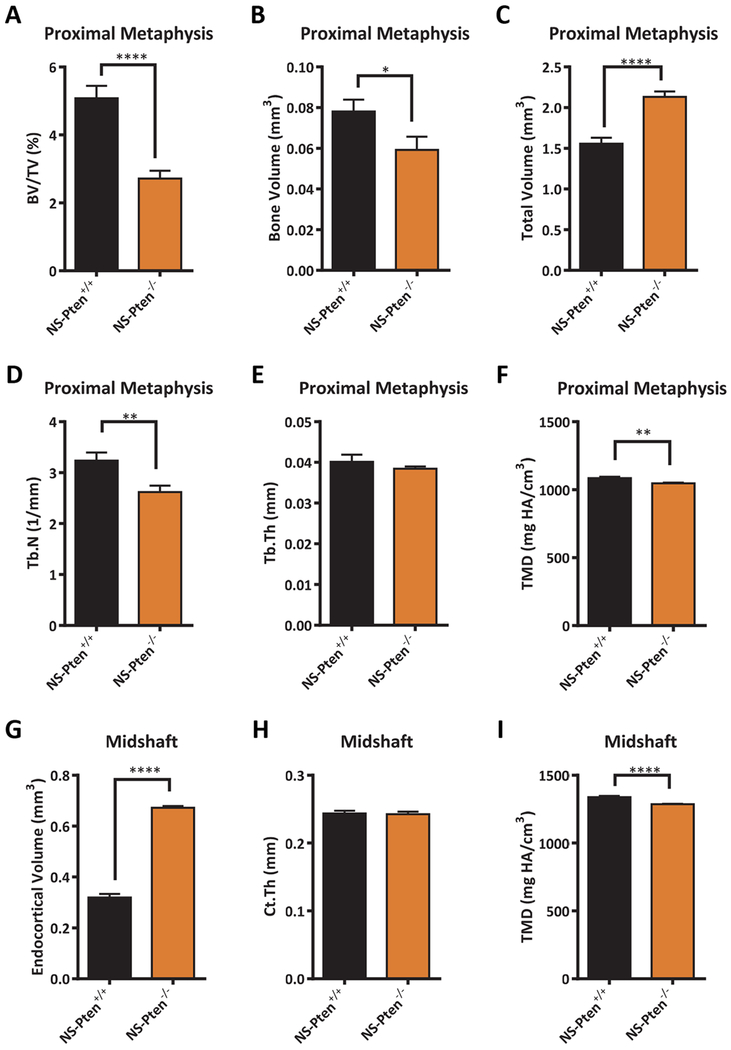
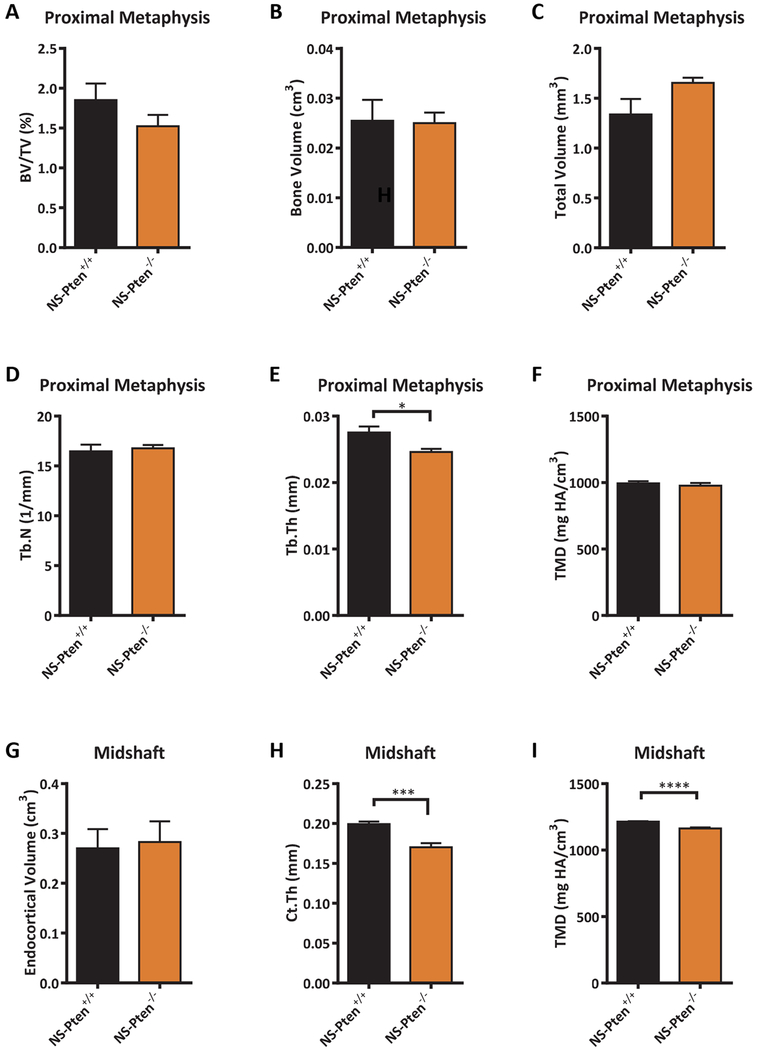
BMD is an integrative measure of bone mass and mineralization. To distinguish the degree to which the low BMD in NS-Pten−/− mice was attributable to each characteristic, we performed in-depth phenotypic analysis of the metaphyseal and diaphyseal compartments. MicroCT reconstructions revealed expansion of the endocortical compartment (i.e., the volume encased by the inner cortical surface) in both the metaphysis and diaphysis of NS-Pten−/− mice, as well as a reduced number of trabeculae in the metaphysis (Fig. 2). Quantitative analysis of the metaphysis revealed that NS-Pten−/− mice exhibited a significant decrease in trabecular bone volume fraction t(1,24) = 5.32 p < 0.0001 (Fig. 3A) which was manifested by both a decrease in the volume of trabecular bone t(1,24) = 2.16 p < 0.05 (Fig. 3B), as well as an increase in the total volume of the trabecular compartment t(1,24) = 5.84 p < 0.0001 (Fig. 3C). Trabecular number was decreased in NS-Pten−/− mice t(1,24) = 2.93 p < 0.01 (Fig. 3D), but not trabecular thickness t(1,24) = 0.85 p = 0.40 (Fig. 3E). NS-Pten−/− mice also exhibited a significant reduction in tissue mineral density (TMD) (which measures tissue mineralization independent of bone mass) t(1,24) = 3.481 p < 0.01 (Fig. 3F). In the midshaft, endocortical volume was significantly increased in NS-Pten−/− mice t(1,23) = 22.11 p < 0.0001 (Fig. 3G), however changes in cortical thickness were not significant between NS-Pten−/− mice and NS-Pten+/+ t(1,23) = 0.2038 p =0.84 (Fig. 3H). Tibial midshaft TMD was also significantly lower for NS-Pten−/− mice t(1,23) = 4.588 p < 0.001 (Fig. 3I). Taken together, our studies suggest that skeletal deficits in NS-Pten−/− mice were characterized by both compartmental similarities and differences, with the metaphysis characterized by decreased trabecular bone mass and mineralization, the midshaft characterized by decreased mineralization but normal bone thickness, and endocortical expansion in both locations.
Figure 2.
MicroCT reconstructions of metaphysis and midshaft in NS-Pten−/− and NS-Pten+/+ mice. Note the low trabecular bone mass in the metaphysis of NS-Pten−/− mice, as well as the expanded endocortical volume in both locations.
Figure 3.
Analysis of bone mass, morphology, and mineralization in 8 wk NS-Pten−/− mice. Results shown for (A) bone volume/total volume, (B) bone volume, (C) total volume, (D) trabecular number, (E) trabecular thickness, (F) tissue mineral density (metaphysis), (G) endocortical volume, (H) cortical thickness, and (I) tissue mineral density (midshaft). The bars represent the mean and the error bars represent the standard error of the mean. * = p < 0.05; ** = p < 0.01, **** = p < 0.0001. NS-Pten+/+ n= 14; NS-Pten−/− n=12.
3.3. Low BMD in NS-Pten−/− Mice is not explained by decreases in activity
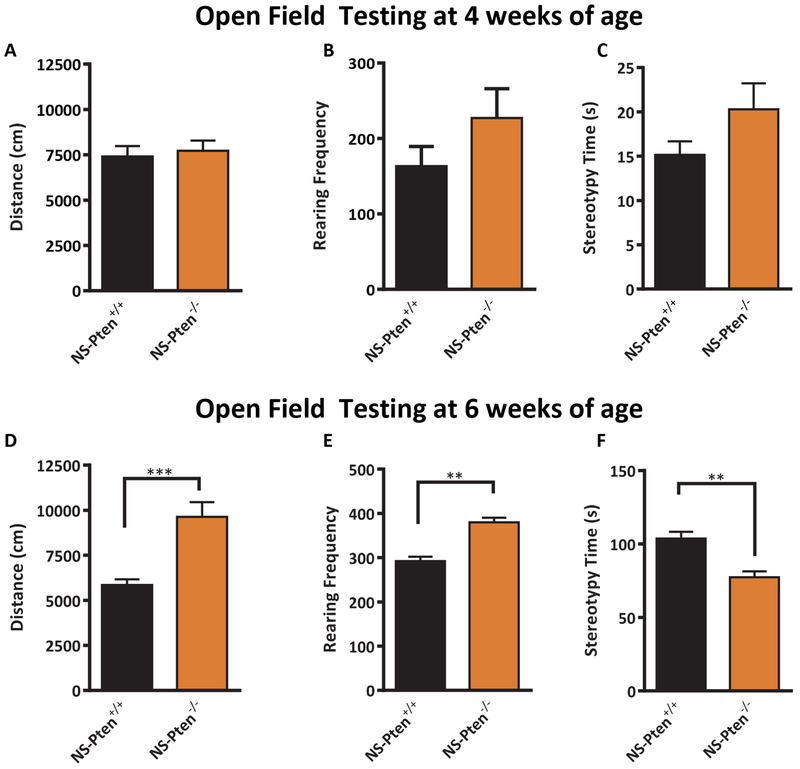
A major factor impacting skeletal health is exercise (Forwood and Burr, 1993), suggesting that low BMD in individuals with epilepsy may arise from decreased activity levels. (Wong and Wirrell, 2006). We ran two cohorts of mice in the open field activity test to assess whether the low BMD in NS-Pten−/− mice may be attributable in part to decreased locomotor activity. At four weeks of age, we found no significant differences in activity levels (Fig. 4A–C). The NS-Pten−/− had no difference in total distance compared to the NS-Pten+/+ mice t(1,30) = 0.39, p = 0.69 (Fig. 4A). Further, there was no difference in rearing activity t(1,30) = 1.39 p = 0.17 (Fig. 4B) or in stereotypy time t(1,30) = 1.6, p = 0.11 (Fig. 4C). The similar activity levels in 4 week old animals was in contrast to significant differences observed in NS-Pten−/− and NS-Pten+/+ mice at 6 weeks of age. We ran a separate cohort of mice in the open field test at this age. Six week old NS-Pten−/− mice had an increase in total distance t(1,29) = 5.2, p < 0.001 (Fig. 4D). There was also a significant increase in rearing activity t(1,29) = 3.5, p < 0.01 (Fig. 4E) and a significant decrease in stereotypy time t(1,29) = 3.5, p < 0.01 (Fig. 4F). The results from the open field test indicate that that NS-Pten −/− mice exhibit acute, age-dependent onset of hyperactivity. Further, they suggest that hypoactivity may not be a contributing factor to the low BMD phenotype observed in these animals.
Figure 4.
Behaviorial analysis reveals normal activity at 4 wk in NS-Pten knockout mice and hyperactivity in 6wk NS-Pten mice−/− mice. Results for open-field activity at 4 weeks of age for NS-Pten wildtype and knockout mice for total activity (A), rearing frequency (B), and stereotypy time (C). Results for open-field activity at 6 weeks of age for NS-Pten wildtype and knockout mice for total activity (D), rearing frequency (E), and stereotypy time (F). The bars represent the mean and the error bars represent the standard error of the mean. ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001. 4 weeks of age NS-Pten+/+ n= 17; NS-Pten−/− n=15; 4 weeks of age NS-Pten+/+ n= 21; NS-Pten−/− n=10.
3.4. NS-Pten−/− mice exhibit age-dependent onset of skeletal abnormalities
NS-Pten−/− mice exhibit spontaneous seizures by 6-7 weeks of age (Ljungberg et al., 2009), a phenotype that is preceded by the onset of hyperactivity between 4-6 weeks of age. Based on this progressive onset of behavioral abnormalities, we examined whether the skeletal deficits in 8 week old NS-Pten−/− mice were also preceded by early skeletal abnormalities, or manifested entirely following the onset of spontaneous seizures. We performed an in-depth phenotypic analysis of the proximal metaphysis and midshaft in young (4 week old) NS-Pten−/− mice. Unlike the 8 week old animals, young NS-Pten−/− mice exhibited largely normal trabecular bone morphology and mineralization. Analysis of the proximal metaphysis revealed no significant difference for trabecular bone volume fraction t(1,14) = 1.28 p = 0.22 (Fig. 5A), bone volume t(1,14) = 0.09 p = 0.93 (Fig. 5B), total volume t(1,14) = 1.93 p = 0.07 (Fig. 5C), trabecular number t(1,14) = 0.40 p = 0.70 (Fig. 5D), or trabecular TMD t(1,14) = 0.68 p=0.51 (Fig. 5F), but did identify a significant decrease in trabecular thickness t(1,14) = 2.89 p <0.05 (Fig. 5E). In contrast, the bone abnormalities were readily apparent in the cortical midshaft by 4 weeks of age. Analysis revealed a significant decrease in NS-Pten−/− mice for cortical thickness t(1,14) = 4.60 p < 0.001 (Fig. 5H) and TMD t(1,14) = 5.94 p < 0.0001 (Fig. 5I), but not endocortical volume t(1,14) = 1.16 p=0.83 (Fig. 5G). There were no difference in weight between the NS-Pten+/+ (20.7± .7) and NS-Pten−/− (20.8g± 0.4) mice. Taken together, our studies suggest that NS-Pten−/− mice exhibit mineralization deficits in cortical bone that are evident as early as 4 weeks of age, as well as deficits in trabecular and cortical thickness at 4 weeks that are not present at 8 weeks.
Figure 5.
Analysis of bone mass, morphology, and mineralization in 4 wk NS-Pten−/− mice. Results shown for (A) bone volume/total volume, (B) bone volume, (C) total volume, (D) trabecular number, (E) trabecular thickness, (F) tissue mineral density (metaphysis), (G) endocortical volume, (H) cortical thickness, and (I) tissue mineral density (midshaft). The bars represent the mean and the error bars represent the standard error of the mean. * = p < 0.05; ** = p < 0.01, **** = p < 0.0001. NS-Pten+/+ n= 8; NS-Pten−/− n=8.
4. Discussion
The current study is the first to show evidence that neuron-specific deletion of PTEN in mice results in skeletal deficits reminiscent of the low BMD phenotype broadly observed in patients with epilepsy. Bone remodeling occurs through the coupled activity of bone-forming osteoblasts, and bone-resorbing osteoclasts. In the endocortical compartment, expansion occurs via osteoclast activity on the endocortical surface, coupled with osteoblast activity on the periosteal surface. If these activities are balanced, expansion occurs in the absence of changes in cortical thickness. In this context, 8 week old NS-Pten−/− mice exhibited enhanced endocortical volume but normal cortical thickness, as well as decreased trabecular volume fraction. Interestingly, the expanded endocortical volume and low trabecular bone mass observed in NS-Pten−/− mice are phenotypic hallmarks of human osteoporosis (Ofek et al., 2006). The osteoporosis phenotype suggest that NS-Pten−/− mice exhibit accelerated remodeling in the cortical compartment, as well as imbalanced remodeling in the trabecular compartment. Recently, it has been shown that the central nervous system is a potent regulator of bone remodeling, and can act through a variety of pathways including hypothalamic receptors for leptin and Neuropeptide Y, modulation of sympathetic and parasympathetic tone, and cannabinoid signaling (Ofek et al., 2006). In regard to the latter, it is interesting to note that mice deficient for the peripheral cannabinoid receptor CB2 are a morphological phenocopy of 8 week NS-Pten−/− mice (i.e., low trabecular volume fraction and endocortical expansion with normal cortical thickness).
The NS-Pten conditional knockout mouse used in our experiment is a well-characterized model of cortical dysplasia (Kwon et al., 2001; Ljungberg et al., 2009; Nguyen et al., 2015; Sunnen et al., 2011). In these mice, Cre is expressed under the control of a modified glial fibrillary acidic protein and the Cre recombinase is expressed in the mouse central nervous system (Kwon et al., 2001). The deletion of PTEN occurs in the granule cells in the dentate gyrus, which results in a dysplastic dentate gyrus due to enlargement of granule cells (Backman et al., 2001). The low BMD phenotype observed in NS-Pten−/− mice occurs in contrast to the high BMD phenotype observed following osteoblast specific Pten deletion (Liu et al., 2007). Specifically, osteocalcin-Cre; PtenloxP:loxP mice exhibit progressive increases in BMD and cortical and trabecular bone mass, suggesting that Pten may function as an anabolic checkpoint in osteoblasts. Importantly, the opposing bone phenotypes following GFAP- and osteocalcin-mediated recombination suggests that skeletal deficits in NS-Pten−/− mice may not be attributable to osteoblastic deletion of Pten (e.g., from low levels of GFAP expression). Additionally, conditional deletion of Pten in chondrocytes, which produces cartilage that later develops into bone, results in larger vertebrae and intervertebral discs compared to control mice (Hsieh et al., 2009). Our findings suggest the idea that neuronal Pten plays a critical role in regulating bone metabolism and thereby brings forth the question of whether Pten may mediate the association between epilepsy and bone health in humans. In animal models of epilepsy, aberrant activation of the mTOR pathway has been observed in Tuberous Sclerosis models along with chronic and acute seizure models of epilepsy (Buckmaster et al., 2009; Zeng et al., 2009; Zeng et al.; Zhang and Wong, 2012). Individuals with Tuberous Sclerosis have been found to have intracranial calcification and islands of sclerotic bone within the calvarium (Lagos et al., 1968).A recent found sclerotic bone lesions by abdominal MRI in children with tuberous sclerosis complex (Boronat et al., 2016). Future studies could use conditional deletion of TSC1 or TSC2 in the brain to determine whether neural specific deletion of either gene results in bone loss.
NS-Pten−/− mice also exhibit impaired bone mineralization in addition to morphological abnormalities. These mineralization defects mirror those observed in Wistar Albino Glaxo/Rijswijk rats, a rat model of human absence epilepsy (Garip et al., 2013). Bone hypomineralization (osteomalacia) and hypocalcemia are both known consequences of vitamin D deficiency. Further, vitamin D deficiency is highly prevalent in individuals with epilepsy (Hollo et al., 2012). In its active form, (1,25(OH)2D3) vitamin D maintains serum calcium by increasing intestinal absorption. However, if this is insufficient to maintain normal levels, (1,25(OH)2D3) mobilizes calcium in bone by increasing resorption and suppressing mineralization. In our studies, NS-Pten mice exhibit severe cortical bone mineralization deficits (TMD Z-score: −4.6) as early as 4 weeks of age, prior to the onset of detectable behavioral abnormalities. In this context, an intriguing question is whether the impaired mineral homeostasis in the skeletons of NS-Pten−/− mice may contribute to epilepsy pathophysiology. For instance, in humans, hypocalcaemia is strongly associated with seizures in children and adults (Han et al., 2015). Further, hypocalcemia-induced seizures have been observed in association with bone anti-resorptive therapies (bisphosphanates) (Maclsaac et al., 2002; Tsourdi et al., 2011), presumably due to disturbances in calcium and phosphate metabolism. The question is raised whether NS-Pten−/− mice have an altered enteric plexus or may have differences in the way they absorb vitamin D. Later studies could include measurements of vitamin d and calcium levels in the blood and measuring individual food intake.
We did not observe differences in any of the bone measures of Kv4.2 KO mice compared to WT mice. We examined these mice because they are more susceptible to seizures and because we have previously reported that NS-Pten−/− mice have a significant reduction of Kv4.2 in the hippocampus (Lugo et al., 2014). We wanted to determine whether deletion of Kv4.2 was sufficient to result in bone changes. We also examined whether inducing seizures in the NS-Pten+/+ mice at 8 weeks of age would also be sufficient to induce changes in bone phenotype. We did not find changes in bone phenotype. It may be that we need to induce seizures at an earlier time in development or may need to induce multiple instances of seizures. Later studies could include intrahippocampal kainate model, which has been repeatedly been demonstrated to result in spontaneous seizures in mice (Ehninger and Silva; Folstein and Rosen-Sheidley, 2001).
Historically, loss of BMD in individuals with epilepsy is assumed to be attributed to antiseizure medications. The anti-seizure medications that are most commonly associated with an altered bone phenotype are drugs that induce the hepatic cytochrome P450 enzyme system (Pack et al., 2003). EIADS are widely believed to lower vitamin D levels and decrease BMD, but the mechanism of action for this change is unknown. A recent systematic review of the influence of EIADS did not provide unequivocal conclusions that the class of drugs results in a decrease in vitamin D levels and BMD (Fraser et al., 2015). They reviewed experiments that used EIADS (including carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, or topiramate) compared to non-EIADS for more than one year. Half of the studies found decreased BMD and the other half did not find any difference. They also found that 75% of conference abstracts they reviewed reported no differences in BMD or fracture incidence. Our studies add to growing evidence of an association between epilepsy and impaired skeletal health that may be incurred independently of anti-seizure medications and/or decreases in activity. One important caveat in our study is that we cannot rule out the effects of AEDs on bone growth. The impact of seizures and AEDs on bone growth are likely to be multifactorial and there is a high level of complexity of the impacts of AEDs and seizures on bone growth (Khanna et al., 2009). There is a recent study that found that the AEDs carbamazepine and phenytoin inhibit native sodium currents in mouse osteoblasts (Petty et al., 2016a). In order to investigate the mechanism of action of seizures on osteoblasts and osteoclasts future studies could use similar in vitro assays.
5. Conclusions
The identification of bone changes in the NS-Pten deletion mouse model suggests that a bone pathology may exist in individuals with epilepsy that share similar genetic conditions, independently of the anti-epileptic drug treatments. Future research will be needed to determine whether other types of epilepsies show changes in bone health. Our findings may lead to both finding ways to optimize therapeutic strategies for co-treatment of bone co-morbidities, as well as to identify pathophysiological links between impaired activity in the central nervous system and the skeleton. In regard to the former, such models are essential as even though there have been many case-controlled studies that have examined the effects of anti-seizure medications on BMD and other measures of bone morphology. It would be unethical to conduct a double-blind placebo study where one group receives an anti-seizure medication and one group receives a placebo to determine the effect of the anti-seizure medication on bone growth. To our knowledge, no studies have found definitive evidence of which type of anti-seizure medications affect bone growth, the length of time needed for the effect to occur, age-dependent effects, and the mechanism of action of medications on bone metabolism. In regard to the latter, our studies identified an early bone hypomineralization phenotype in NS-Pten−/− mice that precedes any detectable behavioral abnormalities. Thus, our findings open new opportunities to investigate early events by which the pathophysiology of epilepsy and osteomalacia may intersect, including the possibility for disrupted bone mineral homeostasis to contribute to seizure onset.
Acknowledgements
This research was funded by the Epilepsy Foundation, NIH NS088776 to JNL, and by a Baylor University Research Committee grant. RYK would like to acknowledge support from NIH AR066061, and the University of Washington Department of Orthopedics and Sports Medicine. We would also like to acknowledge the Baylor University Molecular Biosciences Core for the use of equipment for this study. We would like to thank Dr. Amy Brewster for her careful review and suggested modifications to the manuscript.
References
- Akin R, Okutan V, Sarici U, Altunbas A, Gokcay E, 1998. Evaluation of bone mineral density in children receiving antiepileptic drugs. Pediatr Neurol 19, 129–131. [DOI] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW, 2001. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet 29, 396–403. [DOI] [PubMed] [Google Scholar]
- Baim S, Leonard MB, Bianchi ML, Hans DB, Kalkwarf HJ, Langman CB, Rauch F, 2008. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Pediatric Position Development Conference. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry 11, 6–21. [DOI] [PubMed] [Google Scholar]
- Barnwell LF, Lugo JN, Lee WL, Willis SE, Gertz SJ, Hrachovy RA, Anderson AE, 2009. Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia 50, 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Crepel V, Represa A, 2008. Seizures beget seizures in temporal lobe epilepsies: the boomerang effects of newly formed aberrant kainatergic synapses. Epilepsy Curr 8, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat S, Barber I, Pargaonkar V, Chang J, Thiele EA, 2016. Sclerotic bone lesions at abdominal magnetic resonance imaging in children with tuberous sclerosis complex. Pediatric Radiology 46, 689–694. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X, 2009. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 29, 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D, 2006. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci 26, 12143–12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ, Increased Levels of Anxiety-related Behaviors in a Tsc2 Dominant Negative Transgenic Mouse Model of Tuberous Sclerosis. Behav Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B, 2001. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet 2, 943–955. [DOI] [PubMed] [Google Scholar]
- Forwood MR, Burr DB, 1993. Physical activity and bone mass: exercises in futility? Bone and mineral 21, 89–112. [DOI] [PubMed] [Google Scholar]
- Fraser LA, Burneo JG, Fraser JA, 2015. Enzyme-inducing antiepileptic drugs and fractures in people with epilepsy: A systematic review. Epilepsy Res 116, 59–66. [DOI] [PubMed] [Google Scholar]
- Garip S, Sahin D, Severcan F, 2013. Epileptic seizure-induced structural and functional changes in rat femur and tibia bone tissues: a Fourier transform infrared imaging study. Journal of biomedical optics 18, 111409. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Leonard MB, Zemel BS, International Society for Clinical, D., 2014. 2013 Pediatric Position Development Conference: executive summary and reflections. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry 17, 219–224. [DOI] [PubMed] [Google Scholar]
- Han P, Trinidad BJ, Shi J, 2015. Hypocalcemia-induced seizure: demystifying the calcium paradox. ASN neuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, 2003. Menopause and bone density issues for women with epilepsy. Neurology 61, S16–22. [DOI] [PubMed] [Google Scholar]
- Hollo A, Clemens Z, Kamondi A, Lakatos P, Szucs A, 2012. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav 24, 131–133. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Chen NT, Lo SH, 2009. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Mol Carcinog 48, 545–552. [DOI] [PubMed] [Google Scholar]
- Kemper HCG, 2000. Skeletal development during childhood and adolescence and the effects of physical activity (vol 12, pg 198, 2000). Pediatr Exerc Sci 12, 444–444. [Google Scholar]
- Khanna S, Pillai KK, Vohora D, 2009. Insights into liaison between antiepileptic drugs and bone. Drug Discovery Today 14, 428–435. [DOI] [PubMed] [Google Scholar]
- Koppel BS, Harden CL, Nikolov BG, Labar DR, 2005. An analysis of lifetime fractures in women with epilepsy. Acta Neurol Scand 111, 225–228. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ, 2001. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet 29, 404–411. [DOI] [PubMed] [Google Scholar]
- Lagos JC, Holman CB, Gomez MR, 1968. Tuberous sclerosis: neuroroentgenologic observations. Am J Roentgenol Radium Ther Nucl Med 104, 171–176. [DOI] [PubMed] [Google Scholar]
- Liu X, Bruxvoort KJ, Zylstra CR, Liu J, Cichowski R, Faugere MC, Bouxsein ML, Wan C, Williams BO, Clemens TL, 2007. Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc Natl Acad Sci U S A 104, 2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G, 2009. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech 2, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Brewster AL, Spencer CM, Anderson AE, 2012. Kv4.2 knockout mice have hippocampal-dependent learning and memory deficits. Learn Mem 19, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Smith GD, Arbuckle EP, White J, Holley AJ, Floruta CM, Ahmed N, Gomez MC, Okonkwo O, 2014. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front Mol Neurosci 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclsaac RJ, Seeman E, Jerums G, 2002. Seizures after alendronate. Journal of the Royal Society of Medicine 95, 615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE, 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273, 13375–13378. [DOI] [PubMed] [Google Scholar]
- Melton LJ 3rd, Kan SH, Wahner HW, Riggs BL, 1988. Lifetime fracture risk: an approach to hip fracture risk assessment based on bone mineral density and age. Journal of clinical epidemiology 41, 985–994. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Brewster AL, Clark ME, Regnier-Golanov A, Sunnen CN, Patil VV, D’Arcangelo G, Anderson AE, 2015. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia 56, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I, 2006. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A 103, 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AM, Morrell MJ, Marcus R, Holloway L, Flaster E, Done S, Randall A, Seale C, Shane E, 2005. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol 57, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack AM, Olarte LS, Morrell MJ, Flaster E, Resor SR, Shane E, 2003. Bone mineral density in an outpatient population receiving enzyme-inducing antiepileptic drugs. Epilepsy Behav 4, 169–174. [DOI] [PubMed] [Google Scholar]
- Petty SJ, Milligan CJ, Todaro M, Richards KL, Kularathna PK, Pagel CN, French CR, Hill-Yardin EL, O’Brien TJ, Wark JD, Mackie EJ, Petrou S, 2016a. The antiepileptic medications carbamazepine and phenytoin inhibit native sodium currents in murine osteoblasts. Epilepsia 57, 1398–1405. [DOI] [PubMed] [Google Scholar]
- Petty SJ, Wilding H, Wark JD, 2016b. Osteoporosis Associated with Epilepsy and the Use of Anti-Epileptics-a Review. Current osteoporosis reports 14, 54–65. [DOI] [PubMed] [Google Scholar]
- Poliachik SL, Bain SD, Threet D, Huber P, Gross TS, 2010. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 46, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth RD, 2004. Bone health in pediatric epilepsy. Epilepsy & Behavior 5, S30–S35. [DOI] [PubMed] [Google Scholar]
- Sheth RD, Gidal BE, Hermann BP, 2006. Pathological fractures in epilepsy. Epilepsy Behav 9, 601–605. [DOI] [PubMed] [Google Scholar]
- Sheth RD, Wesolowski CA, Jacob JC, Penney S, Hobbs GR, Riggs JE, Bodensteiner JB, 1995. Effect of carbamazepine and valproate on bone mineral density. J Pediatr 127, 256–262. [DOI] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE, 2011. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia 52, 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourdi E, Rachner TD, Gruber M, Hamann C, Ziemssen T, Hofbauer LC, 2011. Seizures associated with zoledronic acid for osteoporosis. The Journal of clinical endocrinology and metabolism 96, 1955–1959. [DOI] [PubMed] [Google Scholar]
- Wong J, Wirrell E, 2006. Physical activity in children/teens with epilepsy compared with that in their siblings without epilepsy. Epilepsia 47, 631–639. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M, 2009. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29, 6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M, Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of Tuberous Sclerosis Complex. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wong M, 2012. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia 53, 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]