Abstract
A quarter of the global population meets diagnostic criteria for metabolic syndrome (MetS). MetS prevalence stratifies by socioeconomic status (SES), such that low SES is associated with higher MetS risk starting in childhood. Despite this trend, some low-SES children maintain good metabolic health across the lifespan, but the factors responsible for their resilience are not well understood. This study examined the role of threat vigilance as either a moderator or mediator of the effects of low early-life SES on adult metabolic risk. 325 Canadians aged 15-55 participated (M = 36.4 years, SD = 10.7; 55.4% female). We coded parental occupational status between the ages of 0 and 5 to index early-life SES. We used the International Diabetes Federation case definition for MetS based on waist circumference, blood pressure, triglyceride levels, HDL cholesterol, and glycosylated hemoglobin measures. Threat vigilance was assessed using the Weapons Identification Procedure, a visual discrimination paradigm that captures implicit perceptions of threat. Analyses supported the moderator hypothesis: low early-life SES was associated with metabolic syndrome diagnosis exclusively among those with high levels of threat vigilance. This suggests that low early life-SES environments that heighten vigilance to threat might be particularly detrimental for metabolic health. Conversely, low threat vigilance may buffer against the metabolic risks associated with socioeconomic disadvantage.
Keywords: metabolic syndrome, socioeconomic status, threat vigilance
Metabolic syndrome (MetS) is a clustering of cardiometabolic risk factors including abdominal obesity, elevated blood pressure, dyslipidemia, and insulin resistance (Alberti, Zimmet, & Shaw, 2006; Cornier et al., 2008). Approximately a quarter of the world’s population meets diagnostic criteria for MetS (Cornier et al., 2008; Grundy, 2016; Y.-W. Park et al., 2003), and its prevalence is projected to continue to rise (Grundy, 2008; Hossain, Kawar, & El Nahas, 2007). Furthermore, MetS is often comorbid with several conditions that can be disabling in their own right, including psychopathology (e.g., depression), sleep apnea, and reproductive disorders (Grundy, 2008). MetS is also associated with greater mortality risk, with those diagnosed with MetS having a two-fold increased risk of cardiovascular events or death (Gami et al., 2007) and a five-fold increased risk of incident type 2 diabetes mellitus (Cornier et al., 2008), which increases odds of mortality 1.8-fold (The Emerging Risk Factors Collaboration, 2011).
Socioeconomic disparities in MetS prevalence have been observed worldwide, in both developed countries, such as the United States (Y.-W. Park et al., 2003), United Kingdom (Brunner et al., 1997) and Finland (Silventoinen, Pankow, Jousilahti, Hu, & Tuomilehto, 2005), and developing nations, such as Korea (M. J. Park, Yun, Lee, Cho, & Park, 2007) and Tunisia (Gannar et al., 2015). There is also increasing recognition that these disparities emerge early in life (Faienza, Wang, Fruhbeck, Garruti, & Portincasa, 2016; Pervanidou & Chrousos, 2012), and that early disadvantage forecasts adult patterns of disease (Choi, Lee, Chun, & Lee, 2014; Gustafsson & Hammarström, 2012; Gustafsson, Persson, & Om, 2011; Lehman, Taylor, Kiefe, & Seeman, 2005; Non et al., 2014; Tamayo, Christian, & Rathmann, 2010). Importantly, most studies show that early-life socioeconomic status (SES) predicts MetS independently of adult SES (Chichlowska et al., 2009; Langenberg, Kuh, Wadsworth, Brunner, & Hardy, 2006; Lawlor, Ebrahim, & Smith, 2002; Lehman et al., 2005; Non et al., 2014; Parker et al., 2003; Schooling et al., 2008, but see also Lucove, Kaufman, & James, 2007 for a contrary result). In an earlier analysis of the dataset used in the present report, we found a stronger role for early-life SES in explaining adult metabolic risk compared to current SES (Hostinar, Ross, Chen, & Miller, 2017). MetS prevalence was assessed in four groups of adults who, by design, were stratified by early-life and current SES. Across three distinct operational definitions of MetS, we found consistent evidence that those experiencing low early-life SES exhibited greater metabolic risk in adulthood relative to high early-SES individuals, whereas the main effects of current SES were non-significant across all models tested. This finding motivated our current follow-up exploration of possible moderating and mediating pathways explaining the association between low early-life SES and metabolic risk.
Although prior research suggests an association between low early-life SES and increased risk for MetS, not all individuals exposed to low early-life SES go on to develop MetS. For example, analyses from the national Midlife in the United States (MIDUS) study found an association between early-life disadvantage and MetS prevalence in adulthood (Miller, Lachman, et al., 2011). But the later metabolic risks were only apparent in about 50% of those exposed to low childhood SES (Miller, Lachman, et al., 2011). Findings like these suggest that vulnerability and protective factors exist which moderate the metabolic risks of disadvantaged youth (Chen & Miller, 2013). In particular, parental nurturance and positive caregiving may be one such protective factor. For instance, early-life maternal warmth buffers against the effects of low SES on allostatic load in adolescence (Evans, Kim, Ting, Tesher, & Shannis, 2007), pro-inflammatory signaling in adulthood (Chen, Miller, Kobor, & Cole, 2011), and metabolic risk in middle and old age (Miller, Lachman, et al., 2011). In adolescents, perceived emotional support from parents, peers, and mentors buffers against cardiometabolic risk associated with neighborhood poverty (Brody, Lei, Chen, & Miller, 2014, but see also Doom, Gunnar, & Clark, 2016 for contrary evidence). These observations raise questions about the psychobiological characteristics that early caregiving instantiates.
Research on early-life attachment patterns has shown that receiving sensitive, responsive, and consistent caregiving teaches children that they live in a safe, predictable environment where their needs will be met (Ainsworth, Bell, & Stayton, 1974). This leads to secure attachment patterns, which are linked to a wide range of positive developmental outcomes later on, including better emotion-regulation skills, higher self-esteem, and competent social relationships across multiple contexts (Carlson, Sroufe, & Egeland, 2004; Sroufe, Egeland, Carlson, & Collins, 2005). Children who are securely attached to their parents also show dampened hypothalamic-pituitary-adrenocortical (HPA) stress responses when their parents are present (for a review, see Hostinar, Sullivan, & Gunnar, 2014). Furthermore, animal models support a causal role of high-quality early caregiving in programming fear and stress-response systems later in life via epigenetic mechanisms (Meaney & Szyf, 2005). Conversely, children experiencing insensitive or abusive care are more likely to show patterns of cognitive processing and social-emotional development that suggests they are hypervigilant to threat (Cicchetti & Valentino, 2007; Dodge & Pettit, 2003; Pollak, 2008).
In turn, frequent activations of threat-response systems like the HPA axis, autonomic nervous system (ANS), and the immune system are thought to contribute to metabolic dysfunction through multiple mechanisms (Abraham, Brunner, & Eriksson, 2007; Brunner et al., 2002; Dallman, 2010; Hotamisligil, 2006; Kyrou & Tsigos, 2009). For example, elevated levels of glucocorticoids promote increased motivation for food and storage of fat in abdominal regions rather than subcutaneously (Abraham et al., 2007; Dallman, 2010); dysregulation of the ANS can contribute to high blood pressure and insulin resistance (Abraham et al., 2007; Kyrou & Tsigos, 2009); and low-grade inflammation and obesity can potentiate each other over time (Hotamisligil, 2006; Kyrou & Tsigos, 2009). There is also increasing recognition of bidirectional transactions between the brain and peripheral physiology (i.e. HPA axis, ANS, immune system) such that neural and peripheral threat-response systems can amplify each other’s activity over time (Gianaros & Manuck, 2010; Nusslock & Miller, 2016). These findings suggest that factors that shape threat vigilance might play an important role in explaining individual differences among those growing up in low-SES environments, leaving some vulnerable and others protected against metabolic risk.
Though we have emphasized threat vigilance as an individual difference factor (i.e., a moderator), it could also be conceptualized as a mediator of the SES – MetS association. In this scenario, children raised in low-SES contexts develop enhanced vigilance to threat, which in turn negatively affects components of MetS over time. There is some empirical support for this mediational scenario. Low SES has been linked to heightened threat responses at multiple levels of analysis, including psychological processes, peripheral physiology, and neural activity patterns. Psychologically, low-SES children, adolescents and young adults show a higher propensity to provide threatening interpretations of ambiguous situations (Chen, Langer, Raphaelson, & Matthews, 2004; Chen & Matthews, 2001, 2003). Physiologically, they also tend to exhibit greater cardiovascular reactivity to acute stressors or challenges (Gump, Matthews, & Räikkönen, 1999; Jackson, Treiber, Turner, Davis, & Strong, 1999; Treiber, Harshfield, Davis, Kapuku, & Moore, 1990) and greater cortisol output (Evans & English, 2002; Lupien, King, Meaney, & McEwen, 2000). At the neural level, SES indices have also been associated with structural or functional alterations in neural regions involved in threat processing and regulation, including the amygdala, hippocampus, and the prefrontal cortex (Brito & Noble, 2014; Hackman, Farah, & Meaney, 2010; Hackman & Farah, 2009; McEwen & Gianaros, 2010). In particular, low childhood SES has been associated with greater amygdala reactivity to threatening cues (fearful or angry facial expressions) (Gianaros et al., 2008; Javanbakht et al., 2015; Muscatell et al., 2012). Low-SES individuals also exhibit lower activation of prefrontal areas during emotion-regulatory tasks (Kim et al., 2013), suggesting that top-down cognitive mechanisms may not control negative affect as effectively. A few studies have linked threat responses to health-relevant outcomes and explicitly found evidence for a mediation model, whereby threat responsivity mediates the association between low SES and components of MetS, such as blood pressure (Chen et al., 2004; Chen & Matthews, 2001; Gump et al., 1999).
The Present Study
Most theories of how low early-life SES promotes poor health across the lifespan propose a role for threat responses and their neurobiological correlates (e.g., Evans & Kim, 2010; McEwen & Gianaros, 2010; Miller, Chen, et al., 2011; Taylor et al., 2004). However, it is unresolved whether threat vigilance acts as a moderator or mediator of the association between low early-life SES and MetS risk. The current study tested both moderator and mediator models of threat vigilance to further understand associations between early-life SES and adult cardio-metabolic risk in a sample of 325 healthy adults.
Methods
Participants
The study recruited 360 participants from Vancouver, BC, Canada through postings in local media and public transit. Recruitment and testing was conducted between February 2009 and May 2012. Only 9.7% of the sample (N = 35) had missing data for any of the variables of interest in the present analysis, thus results are presented for N = 325. These 325 participants were between the ages of 15 and 55 (M = 36.4 years, SD = 10.7; 55.4% female; 73.2% Caucasian, 14.8% Asian, and 12% other) and recruited to fit into one of four groups defined by early-life (low vs. high) and adulthood (low vs. high) SES (see Measures section and participant characteristics in Table 1). To minimize confounding by health status, participants had to be (a) free of infectious disease in the two weeks before testing, as evidenced by self-report and a normal complete blood count, and (b) without a history of serious and chronic medical illnesses including, but not limited to, cancer, diabetes mellitus, heart disease, stroke, autoimmune disease, HIV/AIDS, hepatitis, chronic obstructive pulmonary disorder, asthma, schizophrenia, bipolar disorder, and dementia. Participants who presented with acute infections were rescheduled after the signs had resolved. Candidates were also screened out if they were not fluent in English, if they were pregnant or had been pregnant in the prior year.
Table 1.
Characteristics of the sample (N = 325). Mean (SD) is presented unless otherwise noted.
| Low early-life SES (n = 167) | High early-life SES (n = 158) | p | |
|---|---|---|---|
| Age | 37.4 (10.2) | 35.4 (11.1) | .08 |
| Sex, Female, n(%) | 91 (54.5%) | 89 (56.3%) | .74 |
| Descent, European, n(%) | 108 (64.7%) | 130 (82.3%) | <.001 |
| Parental education (household max.), years | 12.1 (2.7) | 15.8 (2.4) | <.001 |
| Current education (household max.), years | 15.2 (2.8) | 15.8 (2.5) | .03 |
| Waist circumference (cm) | 87.5 (14.96) | 83.6 (12.94) | .01 |
| Systolic blood pressure | 107.9 (12.2) | 106.7 (10.98) | .32 |
| Diastolic blood pressure | 69.8 (10.5) | 68.8 (8.7) | .34 |
| Triglycerides | 1.12 (.69) | .99 (.56) | .07 |
| HDL cholesterol | 1.4 (.36) | 1.48 (.39) | .15 |
| Glycosylated hemoglobin | 5.4 (.39) | 5.3 (.32) | .27 |
| Heavy smoker, >=10 cig./day, n(%) | 16 (9.6%) | 12 (7.6%) | .47 |
| Heavy Alcohol user, >=10 drinks/week, n(%) | 16 (9.6%) | 28 (17.7%) | .10 |
| Physical activity, hours/week | 2.6 (2.8) | 3.0 (2.9) | .20 |
Of the 2880 who responded to study advertisements, 417 met all of the eligibility criteria described above, and volunteered to participate after learning about the study details. A total of 360 subsequently attended their scheduled laboratory session and 325 (90.3%) had complete measures for the research questions included in this report. The project was approved by the University of British Columbia’s Research Ethics Board and all participants gave written informed consent.
Procedure
All participants completed laboratory sessions between 8:00 a.m. and 10:00 a.m. following an overnight fasting period. After study details were explained, participants gave written consent, and had blood drawn by antecubital venipuncture to measure MetS components (see below). Participants also completed a battery of self-report measures and behavioral tasks, and had their height, weight, and waist circumference measured (see details in Measures).
Measures
Socioeconomic status.
Participants were recruited based on their early-life and current SES, as defined by occupational status ratings derived from the United Kingdom’s National Statistics Socio-economic Classification (NS-SEC). This system is used widely in epidemiology and has been updated regularly since 1911, allowing for comparability to previous research. It is also well-suited to the Canadian social structure. Occupational status was also chosen because it is often a more visible aspect of SES than educational attainment, and people can more reliably recall their parents’ occupations than income or education.
The NS-SEC system is highly standardized, coding occupational status using industry, occupation title, occupation description, self-employment status, supervisor status, and number of employees in the company/organization. It also provides specific procedures in case one piece of information among these is unavailable. Occupations were graded on an 8-point scale, which was reduced into three superordinate categories of low, middle, and high SES. Volunteers from either low or high SES categories for both childhood and current SES were enrolled. Low SES included routine and manual occupations, those who never worked and the long-term unemployed. This included positions such as cleaners, laborers, and transportation operatives. High SES included higher managerial and professional occupations, such as architects, engineers, and medical practitioners. For early-life SES, we coded parental occupational status during their first 5 years of life, using the higher of mother’s vs. father’s ratings. To classify current SES, we coded their occupational status over the past 5 years, as well as that of their romantic partner (the higher of the two ratings was used). A small minority of the subjects in our study were ages 15-23 (10.5%) and were full-time students, financially supported by their families. For these cases, we used parental occupation to categorize current SES, unless the subject was financially independent. All recruiters received extensive training prior to using this system. Coding consistency was regularly checked, and discrepancies resolved by consensus.
Metabolic outcomes.
Blood pressure was assessed with an automated oscillometric device (BpTRU, VSM Medtech; Burnaby, BC) while participants were seated in a comfortable chair. An initial reading was taken to acclimate participants to the procedure, but these data were not used in analysis. Three subsequent readings were taken at two-minute intervals, and their average was used for analysis. Cronbach’s alpha’s for systolic blood pressure (SBP) and diastolic blood pressure (DBP) measures were .93 and .95, respectively.
Waist circumference (cm) was read at the midpoint between the upper iliac crest and lower costal margin at the midaxillary line. Measures were taken at least twice, or until a consistent measure was obtained.
Fasting blood samples were obtained for the assessment of glycosylated hemoglobin, triglycerides and HDL cholesterol. A trained phlebotomist collected overnight fasting blood samples via antecubital venipuncture into Serum Separator and EDTA-treated tubes (Becton-Dickinson, Oakville, ON, Canada). Serum separator tubes were left standing for 60 minutes prior to being centrifuged at 1000g for 10 minutes, as per manufacturer’s instructions. Serum was harvested, and stored at −30 C. EDTA-treated whole blood samples were stored at 4C. Samples were assayed in batch at St. Paul’s Hospital Clinical Trials Laboratory, Vancouver, Canada. Glycosylated hemoglobin was measured in whole blood from EDTA-treated tubes via a Bio Rad D-10 ion exchange chromatography method (Bio-Rad Laboratories Inc., Hercules, CA). Assays yield the percentage of hemoglobin that is glycosylated out of total hemoglobin, with greater values indicating higher blood glucose exposure over the past 3 months. The lower range of detection was 1.2%, with an interassay coefficient of variation of 1.2%. Triglycerides were determined enzymatically on a Hitachi 747 (KyowaMedex, Japan) after hydrolysis to glycerol. This method has an interassay coefficient of variation of 1.1%. Fasting HDL was assayed in serum. Standard enzymatic techniques using cholesterol esterase and cholesterol oxidase were used, after low-density, intermediate density, and very-low density lipoproteins had been precipitated through centrifugation. Assays were done on a Hitachi 911 instrument (Kyowa Medex, Japan). The interassay coefficient of variation was 5.1% and the lower limit of detection (LLD) was .189 mmol/L.
We used the worldwide definition of metabolic syndrome provided by the International Diabetes Federation (IDF, Alberti, Zimmet, & Shaw, 2006; Cornier et al., 2008), which recommends racial/ethnic and sex-specific cutoffs to evaluate the presence of MetS components and overall syndrome diagnosis. The five MetS components (central adiposity, raised blood pressure, triglyceride and glucose levels, and low HDL levels) were assessed during laboratory visits. As recommended by IDF, waist circumference cutoffs for central adiposity were ≥ 94 cm for men and ≥ 80 cm for women of Europid and African descent, and ≥ 90 cm for men and ≥ 80 cm for women of Asian (including Asian-Indian) descent, for Ethnic South and Central Americans, and Aboriginal Canadians. The 18 participants of mixed racial backgrounds fit the same diagnostic classification irrespective of which racial origin was used for the cutoff. Following IDF criteria, the cutoff for raised blood pressure was defined as systolic readings ≥ 130 or diastolic readings ≥ 85 mm Hg. Cutoffs for blood-derived biomarkers were as follows: triglyceride levels ≥ 1.7 mmol/L, HDL levels < 1.03 mmol/L in males and < 1.29 mmol/L in females. The fifth criterion specified by IDF is raised plasma glucose (FPG ≥ 100) or a diagnosis of diabetes. We deliberately excluded individuals with diabetes from participation, and FPG was not measured. In its place, we use glycosylated hemoglobin, which research shows is an accurate surrogate when a cutoff of ≥ 5.7% is used (Ong et al., 2010).
For analyses, we considered three sets of outcome variables: a binary variable indicating whether the participant met the IDF case definition for MetS; a count of the MetS components for which the participant met IDF cutoffs; and a continuous measure of metabolic function obtained by extracting factor scores reflecting the common variance of the five continuous metabolic measures. Maximum likelihood factor analysis (SPSS Statistics 22) indicated that the five component measures loaded highly on a single factor (eigenvalue: 2.24; explained 44.9 % of variance). Factor loadings for waist circumference, blood pressure (an average of z-scored systolic and diastolic blood pressure), triglyceride levels, HDL cholesterol (reversed) and HbA1c levels were .74, .59, .62, .41 and .41, respectively. Using these different outcome measures also allows comparability with prior literature which sometimes uses the sum of MetS components (e.g., Davis et al., 2014) and in other instances uses factor analysis to capture multi-system dysregulation (e.g., Wiley, Gruenewald, Karlamangla, & Seeman, 2016).
Threat vigilance.
To assess threat vigilance, participants completed a modified version of the Weapons Identification Procedure (Payne, 2001, 2006). The original paradigm was designed to tap into automatic processing of racial cues by quantifying the tendency to misidentify neutral objects (e.g., hand tools) as threatening (e.g., guns) after being primed with images of faces belonging to different racial groups. False-positives occur when participants misidentify a tool as a gun (Payne, 2001). Results showed that the race of the primes influences the perceptual identification of weapons, or in other words, there was a racial bias in the misidentification of weapons (Payne, 2001).
Because our interest in this study was in threat vigilance more broadly rather than racial bias, we replaced the original primes with other images from the International Affective Picture System (IAPS). Table 2 describes the 12 negative and 12 neutral primes we incorporated from the IAPS. In studies by the IAPS developers (Lang, Bradley, & Cuthbert, 2008), all of the negative images we used had a mean valence rating below 3.5 on a scale from 1 to 9, and all neutral ones were in the 5.50 – 6.50 range. The negative and neutral primes were matched for content (see Table 2). Participants completed 208 trials, with 52 trials presented for each of the four possible combinations of prime (neutral or negative) and target image (gun or tool). In line with standard quality-control practices (Payne, 2001), we excluded trials with reaction times less than 100 ms or more than 1000 ms from analysis. False-positives (i.e., identifying guns when tools are shown) that occur after a neutral image provide a measure of general threat vigilance tendencies or the tendency to perceive threat even when not primed by anything potentially threatening (from here on labeled NL false-positive rate). False-positives that occur after a negative image reflect a response after activating threat systems (by the negative image in the prime, from here on labeled NEG false-positive rate).
Table 2.
Negative and neutral primes from the International Affective Picture System used in the Weapons Identification Procedure.
| Negatives IAPS# | Negatives Description | Neutrals IAPS# | Neutrals Description |
|---|---|---|---|
| 1050 | Snake | 1333 | Parrots |
| 1220 | Spider | 1670 | Cow |
| 1525 | Attack dog | 1942 | Turtles |
| 2799 | Funeral | 2850 | Tourist |
| 6370 | Attacker | 2493 | Man |
| 9050 | Plane crash | 8340 | Airplane |
| 9470 | Ruins of building | 7491 | Building |
| 9471 | Burnt out building | 7500 | Building |
| 9495 | Fire | 7493 | Man at building |
| 9611 | Plane crash | 7620 | Jet |
| 9600 | Ship sinking | 5390 | Boat |
| 9910 | Car wreck | 7096 | Car |
The NEG false-positive rate ranged from 0% to 100%, with M = 17.01%, SD = 15.8%. The NL false-positive rate was comparable but slightly lower, with the same range of 0% to 100%, and M = 15.9%, SD = 15.1%. In a pilot study, test-retest reliability for this measure over a 1-month period was .79. This measure was previously validated with self-report measures and shows correlations with other performance-based measures of implicit bias and cognitive control (Chan, Chen, Hibbert, Wong, & Miller, 2011; Payne, 2001, 2006).
Covariates.
Subjects provided information about basic demographics (age, sex, and race/ethnicity) and health behaviors (smoking, alcohol use, and exercise). We included these factors as covariates to rule out their possible role as confounds for the association between early-life SES and MetS risk. Given the racial/ethnic distribution of the sample (72.9% European descent, 13.8% Asian descent, 5% or less in any other ethnic group), the variable was recoded (1= European descent, 0 = non-European) to maximize statistical power for race/ethnicity effects. Using a previously validated instrument (Miller, Cohen, Rabin, Skoner, & Doyle, 1999), we collected information on daily smoking and alcohol use. Participants reported the number of cigarettes they smoked each day and the number of alcoholic drinks they consumed per week. A drink was considered a bottle or can of beer, a glass of wine, or a shot of hard liquor (Cohen, Tyrrell, Russell, Jarvis, & Smith, 1993). Regular physical activity was measured with the well-validated Paffenbarger Activity Scale (Paffenbarger, Blair, Lee, & Hyde, 1993), which estimates weekly hours of brisk physical activity. Demographics and health behavior variables (smoking, alcohol use and physical activity) were used as covariates in all analyses including metabolic outcomes. To isolate the independent contributions of early-life SES, all models also adjusted for current SES.
Data Analysis
Data preparation.
Variables were examined for outliers and their approximation of the normal distribution before analyses. Values that exceeded four standard deviations from the mean were Winsorized and replaced with the value at the 99.9th percentile (waist circumference: n = 3; triglyceride levels: n = 4; HbA1c: n = 1; diastolic blood pressure: n = 1; Weapons Identification Procedure NEG false-positive rate: n = 5; NL false-positive rate: n = 6). Most variables approximated the normal distribution. In contrast, variables indexing the count of MetS components, daily cigarette smoking, alcohol use, weekly physical activity, and Weapons Identification Procedure false-positive rates had a pronounced right skew. Cigarette use, alcohol consumption and count of MetS components variables were too skewed to be corrected with mathematical transformations, and were therefore converted into new scales by recoding the values at the tail-end of the distributions. For smoking, the new variable was coded as 0 = non-smoker, 1 = less than 10 daily cigarettes, and 2 = 10 or more cigarettes per day. For alcohol use, it was 0 = zero drinks per week, 1 = less than 10 drinks per week, and 2 = 10 or more drinks per week. For MetS component counts, meeting criteria for 3 or more symptoms was coded as a 3. For the Paffenbarger index of physical activity, a square-root transformation of the total number of hours of brisk physical activity per week was sufficient to reduce skewness and kurtosis and normalize the distribution, thus the square-root of the variable was used in analyses. For the two false-positive variables from the Weapons Identification Procedure, a logarithmic transformation was applied (log 10) to normalize their distributions.
Missing data.
Only 9.7% (N = 35) of participants were missing data on any of the variables of interest here. Thus, data imputation was not necessary given that estimates are not likely to be biased when the rate of missingness is less than 10% (Bennett, 2001).
Statistical analyses
Our first aim was to test the potential role of threat vigilance as a moderator of the association between early-life SES and metabolic risk. To test this aim, we conducted moderation analyses using regression models implemented with the SPSS PROCESS macro (Hayes, 2013). NEG false-positive rate and NL false-positive rate were the two indices of threat vigilance that were tested in separate models as the moderator, early-life SES as the predictor, and the three metabolic risk indices were the outcomes in separate analyses. Logistic regression was used for the analysis predicting the binary MetS diagnosis, and multiple linear regression for the count of MetS components and for MetS factor scores. To probe any significant statistical interactions indicative of moderation, we tested the significance of simple slopes at high and low values of the moderator (we chose the widely-used values of +1SD and −1SD of the moderator). We also computed regions of significance using the Johnson-Neyman technique (Johnson & Neyman, 1936) implemented using the SPSS process macro (Hayes, 2013), which is a technique that provides the full range of values of the moderator for which the independent and dependent variable are significantly associated, rather than reporting single values through simple slopes analysis.
Our second aim was to estimate the viability of indirect pathways between early-life SES and each of the three MetS indices via threat vigilance. This was assessed through regression-based mediation analysis implemented with the SPSS PROCESS macro (Hayes, 2013). This macro conducts a series of regression models and estimates an indirect effect based on the coefficients from each regression model. Bootstrap bias-corrected confidence intervals based on 10000 samples were used for making inferences about indirect effects in mediation analyses (Hayes, 2013). This is due to the fact that the estimates for the indirect effects are seldom normally-distributed, thus normal theory significance testing is not recommended (Hayes, 2013). If the confidence interval (CI) for the estimate contained zero, we concluded that we could not rule out a null effect. However, if the CI did not include zero, then we interpreted the effect as significant. All the analyses adjusted for the set of covariates described above (sociodemographic characteristics and health behaviors).
Results
Bivariate correlations and descriptive statistics for the main study variables are shown in Table 3. All of the metabolic risk indicators were associated with low early-life SES, as previously reported (Hostinar et al., 2017). NEG false-positive rate was not associated with early-life SES, r(323) = −.01, p = .84, and neither was NL false-positive rate, r(323) = .03, p = .66. Finally, false-positive rates in both conditions were significantly and positively associated with MetS diagnosis (for negative condition: r(323) = .18, p =.001; for neutral condition: r(323) = .13, p = .02). Associations of false-positive rates with the count of MetS components and MetS factor scores were also positive but did not reach statistical significance (p’s >.10, Table 3).
Table 3.
Bivariate associations among primary variables and descriptive statistics. NEG = after negative prime; NL = after neutral prime; MetS = metabolic syndrome.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Low early-life SES | - | −.01 | .03 | .14* | .14* | .14* |
| 2. NEG false-positive rate (% trials) | - | .78** | .18** | .09 | .06 | |
| 3. NL false-positive rate (% trials) | - | .13* | .07 | .04 | ||
| 4. MetS diagnosis | - | .67** | .59** | |||
| 5. MetS components count | - | .78** | ||||
| 6. MetS factor score | - | |||||
| M | .49 | 1.1 | 1.06 | .10 | 1.00 | −.01 |
| SD | .50 | .40 | .42 | .30 | .99 | .88 |
p < .05;
p < .01.
Testing the Moderation Model
For our first aim, we tested the moderating role of threat vigilance for the association between early-life SES and MetS risk. We report results for NEG false-positive rate first, followed by analyses with NL false-positive rate. When using logistic regression to predict the binary MetS diagnosis, there was a significant main effect of threat vigilance indexed by NEG false-positive rate, B = 1.57, SE = .60, OR = 4.82 (CI: 1.48–15.63), p = .009, and a significant interaction of early-life SES and threat vigilance, B = 1.54, SE = .61, OR = 4.68 (CI: 1.42–15.39), p = .01 (see Table 4). This was independent of covariates, among which only smoking and age had significant effects (p = .046 for both), predicting higher metabolic risk. Simple slopes analysis indicated that low early-life SES was associated with higher odds of MetS diagnosis at high (+1SD) levels of threat vigilance, B = .94, SE = .31, OR = 2.56 (CI: 1.40–4.69), p = .002, but not low (−1SD) levels of threat vigilance, B = −.30, SE = .38, OR = 0.74 (CI: 0.35–1.55), p = .43 (Figure 1). When examining the region of significance for the association, low early-life SES and MetS diagnosis were only significantly associated for those whose threat vigilance was in the top 44% of scores. An identical interaction emerged in analyses using NL false-positive rate, B = 1.2, SE = .53, OR = 3.29 (CI: 1.17–9.26), p = .02. As above, early-life SES was only associated with greater odds of MetS for those with the highest 48.6% of NL false-positive rates.
Table 4.
Logistic regression results (N = 325) predicting MetS diagnosis (binary outcome). Threat vigilance is indexed by false-positive rates across negative prime trials in this analysis.
| B | S.E. | Wald | p | Exp(B) | 95% CI Lower | 95% CI Upper | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| Constant | −2.52 | .23 | 119.17 | .000* | .08 | ||
| EL SES | .28 | .23 | 1.44 | .23 | 1.32 | .84 | 2.08 |
| Threat vigilance | 1.35 | .58 | 5.45 | .02* | 3.87 | 1.24 | 12.05 |
| EL SES × Threat vigilance | 1.55 | .58 | 7.08 | .008* | 4.70 | 1.50 | 14.69 |
| Model 2 | |||||||
| Constant | −4.51 | 1.05 | 18.52 | .000* | .01 | ||
| EL SES | .32 | .24 | 1.75 | .19 | 1.38 | .86 | 2.22 |
| Threat vigilance | 1.57 | .60 | 6.85 | .009* | 4.82 | 1.48 | 15.63 |
| EL SES × Threat vigilance | 1.54 | .61 | 6.44 | .01* | 4.68 | 1.42 | 15.39 |
| Age | .04 | .02 | 3.97 | .046* | 1.04 | 1.00 | 1.09 |
| Sex (male) | .19 | .42 | .21 | .65 | 1.21 | .53 | 2.78 |
| Race/ethnicity (European) | .81 | .56 | 2.07 | .15 | 2.25 | .75 | 6.78 |
| Alcohol use | −.18 | .33 | .29 | .59 | .84 | .44 | 1.59 |
| Smoking | .58 | .29 | 3.98 | .046* | 1.78 | 1.01 | 3.14 |
| Physical activity | −.05 | .03 | 2.80 | .09 | .95 | .90 | 1.01 |
| Current SES | .00 | .23 | .00 | .99 | 1.00 | .64 | 1.55 |
p < .05;
EL SES = early-life SES.
Figure 1.
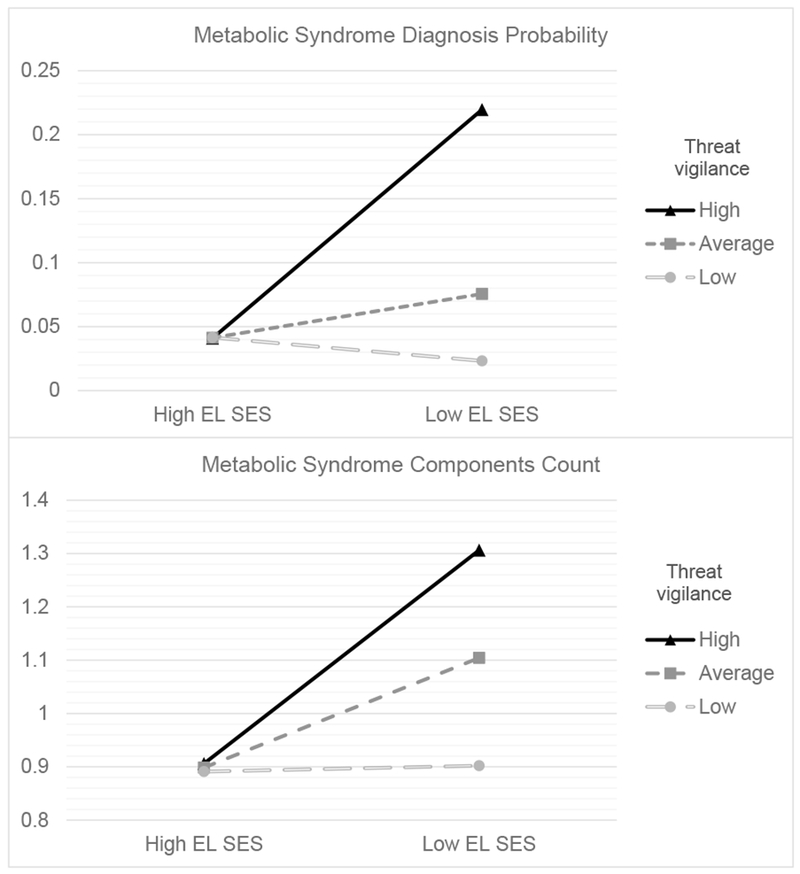
Metabolic risk was higher for those at high (+1SD) versus average or low (−1SD) levels of threat vigilance if they were exposed to low early-life SES (EL SES). The two panels display different metabolic outcomes: A) MetS diagnosis probability; and B) number of MetS components.
We observed similar results when using linear regression models predicting the count of MetS components (see Table 5). When considering threat vigilance indexed by NEG false-positive rate, there was a significant interaction of early-life SES and threat vigilance (b = .30, SE = .14, p = .03), in addition to a significant main effect of early-life SES (b = .14, SE = .05, p = .01) and non-significant effect of threat vigilance (b = .19, SE = .14, p = .17). The interaction became marginally significant when adjusting for our panel of covariates (b = .24, SE = .13, p = .07). Among the covariates, only age (p = .001), alcohol use (p = .051) and smoking (p = .048) had effects below p = .10 (all of them predicted a higher MetS component count, Table 5). Follow-up simple slopes analysis revealed that low early-life SES was significantly associated with a higher count of MetS components for those with high (+1SD) levels of threat vigilance (b = .20, SE = .08, p = .01), but not for those with low (−1SD) levels of threat vigilance (b = .01, SE = .08, p = .94, Figure 1). Low early-life SES and MetS components were only positively associated for those having the highest 52.3% of threat vigilance scores according to the region of significance obtained for this effect. Similar results emerged with threat vigilance indexed by NL false-positive rate (interaction of early-life SES and threat vigilance, b = .22, SE = .13, p = .08). But probing revealed the same basic pattern, with early-life SES significantly associated with elevated metabolic risk only among those with the highest 55.1% of threat vigilance scores.
Table 5.
Multiple regression results predicting MetS components count (N = 325).
| b | SE | β | t | p | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Constant | 1.00 | .05 | 18.55 | <.001* | |
| EL SES | .14 | .05 | .14 | 2.57 | .01* |
| Threat vigilance | .19 | .14 | .08 | 1.39 | .17 |
| EL SES × Threat vigilance | .30 | .14 | .12 | 2.17 | .03* |
| Model 2 | |||||
| Constant | .43 | .23 | 1.84 | .07Δ | |
| EL SES | .10 | .05 | .10 | 1.90 | .06Δ |
| Threat vigilance | .27 | .13 | .11 | 1.98 | .048* |
| EL SES × Threat vigilance | .24 | .13 | .10 | 1.82 | .07Δ |
| Age | .02 | .01 | .20 | 3.49 | .001* |
| Sex (male) | .10 | .11 | .05 | .92 | .36 |
| Race/ethnicity (European) | .03 | .13 | .01 | .25 | .80 |
| Alcohol use | −.15 | .08 | −.11 | −1.96 | .05Δ |
| Smoking | .19 | .09 | .11 | 1.98 | .048* |
| Physical activity | −.01 | .01 | −.07 | −1.31 | .19 |
| Current SES | .03 | .06 | .03 | .56 | .57 |
p < .05.
p < .10.
We next repeated the analysis with a continuous MetS score derived from factor analysis as the outcome. Unlike the two operational definitions above, it uses the full range of scores on each component, taking a more dimensional view of the phenomenon. With this definition, the interaction of early-life SES and threat vigilance (first indexed by NEG false-positive rate) was not significant in unadjusted analyses (b = .19, SE = .12, p = .12) or after adjustment for our panel of covariates (b = .12, SE = .11, p = .27, see Table 6 for full models). In the adjusted analysis, both early-life SES and threat vigilance had marginal effects (b = .08, SE = .05, p = .08, and b = .22, SE = .11, p = .05, respectively). In analyses including threat vigilance indexed by NL false-positive rate, there was a significant interaction of early-life SES and threat vigilance in unadjusted analyses (b = .23, SE = .12, p = .045), which became non-significant after covariate adjustment (b = .15, SE = .11, p = .15), thus simple slopes were not probed further.
Table 6.
Multiple regression results predicting MetS factor scores (N = 325).
| b | SE | β | t | p | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Constant | −.01 | .05 | −.29 | .77 | |
| EL SES | .12 | .05 | .14 | 2.48 | .01* |
| Threat vigilance | .10 | .12 | .05 | .87 | .39 |
| EL SES × Threat vigilance | .19 | .12 | .09 | 1.54 | .12 |
| Model 2 | |||||
| Constant | −.92 | .19 | −4.73 | <.001* | |
| EL SES | .08 | .04 | .09 | 1.78 | .08Δ |
| Threat vigilance | .22 | .11 | .10 | 1.96 | .05Δ |
| EL SES × Threat vigilance | .12 | .11 | .06 | 1.11 | .27 |
| Age | .02 | .00 | .28 | 5.16 | <.001* |
| Sex (male) | .53 | .09 | .30 | 6.01 | <.001* |
| Race/ethnicity (European) | .00 | .10 | .00 | .02 | .99 |
| Alcohol use | −.06 | .06 | −.05 | −.96 | .34 |
| Smoking | .09 | .08 | .06 | 1.15 | .25 |
| Physical activity | −.01 | .01 | −.11 | −2.08 | .04* |
| Current SES | −.01 | .05 | −.01 | −.20 | .84 |
p < .05.
p < .10.
Mediation Models
For our second aim, we estimated the viability of mediational paths involving threat vigilance using the PROCESS macros. None of the models supported a mediational scenario, primarily due to the non-significant associations between early-life SES and threat vigilance (b = −.001, SE = .02, p = .96). For each of the three measures of MetS risk, no significant mediation by threat vigilance was observed. For threat vigilance following negative primes, all indirect effects included zero in their confidence interval (MetS diagnosis: b = −.002, SE = .05, CI = [−.11, .10]; count of MetS components: b = −.0004, SE = .007, CI = [−.02, .01]; MetS factor scores: b = −.0003, SE = .006, CI = [−.01, .01]). The same result emerged for indirect effects via threat vigilance following neutral primes (MetS diagnosis: b = .02, SE = .04, CI = [−.05, .12]; MetS components count: b = .004, SE = .007, CI = [−.01, .02]; MetS factor scores: b = .003, SE = .005, CI = [−.005, .02]).
Discussion
The global prevalence of metabolic syndrome is rising (Grundy, 2008) and disproportionately affects those from low SES backgrounds (Cornier et al., 2008; Saland, 2007). Extant evidence suggests that these metabolic health disparities begin in childhood (Faienza et al., 2016; Lehman et al., 2005; Pervanidou & Chrousos, 2012; Tamayo et al., 2010), and persist into adulthood (Choi et al., 2014; Gustafsson & Hammarström, 2012; Gustafsson et al., 2011; Lehman et al., 2005; Non et al., 2014; Tamayo et al., 2010). But not all individuals from a low early-life SES background go on to develop MetS, and much less is known about the vulnerability and protective factors that might moderate or mediate these risks. The present study sought to examine whether threat vigilance might serve as one such moderator or mediator for the association between low early-life SES (birth to 5 years old) and adult metabolic risk.
Our analyses supported a moderator role of threat vigilance, rather than a mediator role. Specifically, we found a significant interaction of early-life SES and threat vigilance in explaining risk of meeting MetS diagnostic criteria (binary outcome). The statistical interaction was such that low early-life SES was only associated with elevated metabolic risk in those with the highest 40 to 50% of threat vigilance scores. In contrast, those with low levels of threat vigilance had lower metabolic risk, comparable to that of high early-life SES individuals. This distribution is consistent with findings that roughly half of middle-aged and older adults who report experiencing low childhood SES do not exhibit MetS, whereas roughly half do (Miller, Lachman, et al., 2011). A similar pattern emerged for MetS components count, but the interaction became non-significant after adjustment for our panel of covariates. Nevertheless, a priori-planned simple slopes analysis revealed a similar pattern as the previous analyses (Figure 1). Results were in the same direction but became non-significant in the analysis involving MetS factor scores.
One interpretation of the statistical interaction we observed is that a proportion of those growing up with disadvantage possess protective factors that buffer their psychological and physiological responses to threat (for reviews of studies documenting such social buffering effects for various biological outcomes in low-SES individuals, see Chen & Miller, 2013; Hostinar et al., 2014). Attenuated threat responses might in turn prevent deleterious metabolic outcomes by reducing adrenocortical, autonomic and inflammatory mediators, which can unfavorably alter various metabolic processes (Abraham et al., 2007; Brunner et al., 2002; Dallman, 2010; Hotamisligil, 2006; Kyrou & Tsigos, 2009). A direct measure of early-life parenting quality was not available in this study to test this possibility. However, this hypothesis is supported by animal models (Meaney & Szyf, 2005; Meaney, 2010) and accumulating evidence in humans (Cicchetti & Blender, 2006; Fisher et al., 2016; Gunnar & Quevedo, 2007) that high-quality early-life maternal care is associated with less fearful and less stress-reactive phenotypes. Future studies will have to simultaneously assess maternal warmth and threat vigilance in addition to metabolic health and childhood SES to examine these possibilities.
We found almost identical results when using the false-positive rate after negative primes or false-positive rate after neutral primes as measures of threat vigilance. Because these two measures were highly correlated (r = .77, p <.001; Table 3), it is difficult to interpret these patterns with any certainty. They could signify the importance of threat vigilance in both emotionally neutral and activated situations. Alternatively, this could be a statistical artifact of our measure, which may not have fully captured differences in threat vigilance across contexts. From a theoretical standpoint, it will be important for future studies to conduct this task in a neuroimaging environment to explicitly test how neural activity after negative versus neutral primes moderates the effect of low SES. Negative IAPS images activate different neural networks compared to neutral IAPS images –e.g., they elicit greater activity in the amygdala and hippocampus (Aldhafeeri, Mackenzie, Kay, Alghamdi, & Sluming, 2014). More research is needed to understand whether these differences in neural activity matter for explaining the effect of low SES on health.
We did not find evidence supporting a mediational model, primarily due to non-significant associations between early-life SES and threat vigilance. This result is inconsistent with a number of studies documenting greater threat responses in low-SES youth psychologically (Chen et al., 2004; Chen & Matthews, 2001), physiologically (Gump et al., 1999; Jackson et al., 1999; Treiber et al., 1990), and at the neural level (Gianaros et al., 2008; Javanbakht et al., 2015; Muscatell et al., 2012). This discrepancy is unlikely due to limited variation or range restriction in behavioral threat responses in the current study. False-positive rates spanned the entire range, from 0% to 100%, and significant associations between threat vigilance measures and MetS diagnosis suggest that range restriction was unlikely. The different results might arise from national differences in exposure to threatening circumstances for those experiencing low SES. Most prior studies linking low childhood SES to heightened threat responses have been conducted in the United States. The present study, however, was conducted in Canada, where rates of gun-related deaths are estimated to be 7 times lower than in the United States (Grinshteyn & Hemenway, 2016). As such, it is possible that low early-life SES individuals in Canada are less prone to developing hypervigilance to handguns, relative to those in the US, given the comparative rarity of gun violence in Canada.
Although moderation and mediation models were tested independently, these models are not mutually exclusive conceptually or statistically. It is possible that associations between early-life SES and adult MetS risk are both moderated and mediated by threat vigilance, with different studies finding support for one or the other depending on various study characteristics. For example, results might be shaped by the type of socioeconomic adversities that are more frequent in the sample (e.g., material deprivation versus threats to safety and wellbeing), the developmental stages when SES, threat responses and health outcomes are assessed; and the timing and duration of poverty exposure. In particular, although the majority of individuals experience continuity of SES through life, the present study intentionally oversampled participants who experienced social mobility (half the participants experienced mobility from early life to adulthood). Even though this approach has the advantage of supplying greater statistical power to formally test the role of social mobility (for these analyses, see Hostinar et al., 2017), it may also underestimate the effects of early-life socioeconomic disadvantage in this sample compared to other studies and the population at large, where the majority of individuals experience continuity of SES throughout their life. Upwardly mobile individuals may have had access to family and neighborhood protective factors that facilitated their academic success and ascension on the social ladder, and at the same time buffered their threat responses. Consistent with this interpretation, we found that indeed in this sample upwardly-mobile individuals (with low early-life and high current SES) exhibited lower threat vigilance than those experiencing low SES continuously from early-life to adulthood (p = .007 for false-positive rate after negative primes and p = .04 after neutral primes). However, more research is needed to fully test the buffering processes considered at play. Future research should consider the role of social mobility, developmental stage, and timing and duration of poverty exposure to clarify these patterns.
The present study has a number of strengths, including the observational, computerized assessment of threat vigilance and the relatively large sample compared to other research assessing both biomarkers and behavior. The study is also not without limitations. First, the cross-sectional design precludes any definitive inferences about causality or the temporal ordering of phenomena. Multi-wave, prospective research will be needed to substantiate these findings and shed light on how they unfold developmentally. Nevertheless, reverse causality is an unlikely explanation for some of the associations we observed –e.g., it is difficult to imagine how levels of threat vigilance or metabolic risk in adulthood could affect parental occupational status many years prior. Second, the self-reported and retrospective assessment of early-life SES may be a source of measurement error due to memory problems or other sources of response bias. This limitation was mitigated by selecting occupational status as a proxy measure of SES (versus household income or parental educational level), since occupation is a more stable, visible, and easier to recall measure of SES than other indices. A third limitation is the concurrent assessment of our mediators and outcomes, which may underestimate mediation effects. Future studies should assess threat vigilance trajectories throughout development and prospectively examine their associations with subsequent health outcomes. Fourth, the marginally significant result in analyses predicting MetS component counts and non-significant result when predicting MetS factor scores suggest that our panel of covariates might be overly conservative. This is because several health behaviors (cigarette smoking, alcohol use, physical activity) are plausible mediators between low SES and metabolic risk, thus these adjusted analyses may be underestimating the total effects we report. For this reason, we also presented unadjusted results for each analysis. Finally, another limitation was the use of only one measure of threat vigilance in the present study. Even though we validated this measure in prior work (e.g., there were significant associations between threat vigilance assessed in the same manner and angry or fearful responses subsequent to viewing childhood family photographs, Chan et al., 2011), future studies could benefit from assessing threat vigilance at multiple levels of analysis within the same study (e.g., self-report, behavioral, psychophysiological, and neural measures) in order to replicate and extend our results.
Despite these limitations, this study inspires several avenues of research designed to reveal when and how low early-life SES is linked to greater metabolic risk, as moderated by threat vigilance. Which characteristics make individuals more likely to respond to cues of low social status with heightened threat responses? What types of neighborhoods and life experiences foster high versus low threat vigilance and risky versus resilient metabolic health pathways for low-SES individuals? Do social buffers such as early-life maternal warmth exert beneficial effects on metabolic health indirectly, by reducing threat vigilance, or are there more direct pathways? Answering these questions will bring us closer to understanding equifinality and multifinality (Cicchetti & Rogosch, 1996) in metabolic and cardiovascular health outcomes for those experiencing early-life disadvantage. It will also guide interventions to help minimize health disparities by suggesting targets that are both modifiable and influential agents in the pathways from early-life socioeconomic disadvantage and adult metabolic risk.
Another implication of these findings for interventions is that minimizing children’s exposure to threatening circumstances and environments might reduce health disparities, in addition to being a socially desirable goal. Preventing child maltreatment, reducing the incidence of gun violence within homes and neighborhoods, and promoting a sense of physical and emotional safety in contexts where children spend a substantial portion of their time (e.g., schools) are some of the avenues through which the health gap between low-SES and high-SES children might be reduced. Additionally, promoting supportive family relationships through clinical and educational interventions may be one way to buffer children’s threat responses and prevent the development of persistently high vigilance to threat. If the associations observed in the current study are causal in nature, then minimizing threat vigilance for low-SES children might promote favorable outcomes for metabolic health and these benefits might persist across the lifespan.
Acknowledgements:
We thank the participants for their contribution to this project. This research was supported by NIH Grants R01 HD058502 and F32 HD078048.
References
- Abraham NG, Brunner EJ, & Eriksson JW (2007). Metabolic syndrome: Psychosocial, neuroendocrine, and classical risk factors in type 2 diabetes. Annals of the New York Academy of Sciences, 275, 256–275. 10.1196/annals.1391.015 [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS, Bell SM, & Stayton DJ (1974). Infant–mother attachment and social development: Socialisation as a product of reciprocal responsiveness to signals In Richards MPM (Ed.), The integration of a child into a social world (pp. 99–137). New York, NY: Cambridge University Press. [Google Scholar]
- Alberti KGMM, Zimmet P, & Shaw J (2006). Metabolic syndrome -A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Medicine, 23, 469–480. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- Aldhafeeri F, Mackenzie I, Kay T, Alghamdi J, & Sluming V (2014). Regional brain responses to pleasant and unpleasant IAPS pictures: Different networks. Neuroscience Letters, 512(2), 94–98. 10.1016/j.neulet.2012.01.064 [DOI] [PubMed] [Google Scholar]
- Bennett DA (2001). How can I deal with missing data in my study? Australian and New Zealand Journal of Public Health, 25(5), 464–469. 10.1111/j.1467-842X.2001.tb00294.x [DOI] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 1–12. 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Lei M, Chen E, & Miller GE (2014). Neighborhood poverty and allostatic load in African American youth. Pediatrics, 134, e1362–e1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, … Marmot MG (2002). Adrenocortical, autonomic, and inflammatory causes of the Metabolic Syndrome. Circulation, 106, 2659–2665. 10.1161/01.CIR.0000038364.26310.BD [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Marmot M, Nanchahal K, Shipley M, Stansfeld S, Juneja M, & Alberti K (1997). Social inequality in coronary risk: Central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia, 40(11), 1341–1349. 10.1007/s001250050830 [DOI] [PubMed] [Google Scholar]
- Carlson EA, Sroufe LA, & Egeland B (2004). The construction of experience: a longitudinal study of representation and behavior. Child Development, 75(1), 66–83. 10.1111/j.1467-8624.2004.00654.x [DOI] [PubMed] [Google Scholar]
- Chan M, Chen E, Hibbert AS, Wong JHK, & Miller GE (2011). Implicit measures of early-life family conditions: Relationships to psychosocial characteristics and cardiovascular disease risk in adulthood. Health Psychology, 30(5), 570–578. 10.1037/a0024210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, & Matthews KA (2004). Socioeconomic status and health in adolescents: The role of stress interpretations. Child Development, 75(4), 1039–1052. 10.1111/j.1467-8624.2004.00724.x [DOI] [PubMed] [Google Scholar]
- Chen E, & Matthews KA (2001). Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine, 23(2), 101–111. 10.1207/S15324796ABM2302_4 [DOI] [PubMed] [Google Scholar]
- Chen E, & Matthews KA (2003). Development of the cognitive appraisal and understanding of social events (CAUSE) videos. Health Psychology, 22(1), 106–110. 10.1037/0278-6133.22.1.106 [DOI] [PubMed] [Google Scholar]
- Chen E, & Miller GE (2013). Socioeconomic status and health: Mediating and moderating factors. Annual Review of Clinical Psychology, 9, 723–49. 10.1146/annurev-clinpsy-050212-185634 [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, & Cole SW (2011). Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry, 16(7), 729–37. 10.1038/mp.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, & Heiss G (2009). Life course socioeconomic conditions and Metabolic Syndrome in adults: The Atherosclerosis Risk in Communities (ARIC) Study. Annals of Epidemiology, 19(12), 875–883. 10.1016/j.annepidem.2009.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Lee D, Chun E, & Lee J (2014). The relationship between Metabolic Syndrome and childhood maternal education level, job status. Findings from the Korean National Health and Nutrition Examination, 2007-2009. Korean Journal of Family Medicine, 35(4), 207–215. 10.4082/kjfm.2014.35.4.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Blender JA (2006). A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences, 1094, 248–58. 10.1196/annals.1376.029 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Cicchetti D, & Valentino K (2007). Toward the application of a multiple-levels-of-analysis perspective to research in development and psychopathology In Masten AS (Ed.), Multilevel Dynamics in Developmental Psychopathology. Pathways to the Future (pp. 243–284). Mahwah, NJ: Lawrence Erlbaum Associates; 10.4324/9780203936429 [DOI] [Google Scholar]
- Cohen S, Tyrrell D, Russell M, Jarvis M, & Smith A (1993). Smoking, alcohol consumption, and susceptibility to the common cold. American Journal of Public Health, 83(9), 1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, … Eckel RH (2008). The Metabolic Syndrome. Endocrine Reviews, 29(7), 777–822. 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF (2010). Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism, 21(3), 159–65. 10.1016/j.tem.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Usher N, Dearing E, Barkai A, Crowell-Doom C, Neupert S, … Crowell J (2014). Attachment and the Metabolic Syndrome in midlife: The role of interview-based discourse patterns. Psychosomatic Medicine, 76(8), 611–21. 10.1097/PSY.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, & Pettit GS (2003). A biopsychosocial model of the development of chronic conduct problems in adolescence. Psychological Bulletin, 39(2), 349–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR, & Clark CJ (2016). Maternal relationship during adolescence predicts cardiovascular disease risk in adulthood. Health Psychology, 35(4), 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development, 73(4), 1238–48. 10.1111/1467-8624.00469 [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences, 1186, 174–89. 10.1111/j.1749-6632.2009.05336.x [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, & Shannis D (2007). Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology, 43(2), 341–51. 10.1037/0012-1649.43.2.341 [DOI] [PubMed] [Google Scholar]
- Faienza MF, Wang DQH, Fruhbeck G, Garruti G, & Portincasa P (2016). The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Internal and Emergency Medicine, 11(2), 175–82. 10.1007/s11739-015-1382-6 [DOI] [PubMed] [Google Scholar]
- Fisher PA, Beauchamp KG, Roos LE, Noll LK, Flannery J, & Delker BC (2016). The neurobiology of intervention and prevention in early adversity. Annual Review of Clinical Psychology, 12, 331–357. 10.1146/annurev-clinpsy-032814-112855 [DOI] [PubMed] [Google Scholar]
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami L. a., Somers VK, & Montori VM (2007). Metabolic Syndrome and risk of incident cardiovascular events and death. Journal of the American College of Cardiology, 49(4), 403–414. 10.1016/j.jacc.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Gannar F, De León AC Díaz BB, Del M, Rodríguez C, Rodríguez IM, … Attia N. (2015). Social class and metabolic syndrome in populations from Tunisia and Spain. Diabetology & Metabolic Syndrome, 7(88), 1–7. 10.1186/s13098-015-0084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, & Cohen S (2008). Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience, 3, 91–96. 10.1093/scan/nsn003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, & Manuck SB (2010). Neurobiological pathways linking socioeconomic position and health. Psychosomatic Medicine, 72(5), 450–461. 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinshteyn E, & Hemenway D (2016). Violent death rates: The US compared with other high-income OECD Countries, 2010. The American Journal of Medicine, 129(3), 266–273. 10.1016/j.amjmed.2015.10.025 [DOI] [PubMed] [Google Scholar]
- Grundy SM (2008). Metabolic syndrome pandemic. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(4), 629–636. 10.1161/ATVBAHA.107.151092 [DOI] [PubMed] [Google Scholar]
- Grundy SM (2016). Metabolic syndrome update. Trends in Cardiovascular Medicine, 26(4), 364–373. 10.1016/j.tcm.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, & Räikkönen K (1999). Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychology, 18(2), 140–150. 10.1037/0278-6133.18.2.140 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–73. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, & Hammarström A (2012). Socioeconomic disadvantage in adolescent women and metabolic syndrome in mid-adulthood: An examination of pathways of embodiment in the Northern Swedish Cohort. Social Science & Medicine, 74(10), 1630–1638. 10.1016/j.socscimed.2012.01.044 [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Persson M, & Om AH (2011). Life course origins of the Metabolic Syndrome in middle-aged women and men: The role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Annals of Epidemiology, 21, 103–110. 10.1016/j.annepidem.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews. Neuroscience, 11(9), 651–9. 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Hossain P, Kawar B, & El Nahas M (2007). Obesity and diabetes in the developing world — A growing challenge. New England Journal of Medicine, 356, 213–215. 10.1056/NEJMp068177 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, & Miller GE (2017). Early-life socioeconomic disadvantage and metabolic health disparities. Psychosomatic Medicine, 79(5), 514–523. doi: 10.1097/PSY.0000000000000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1), 256–82. 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature, 444(7121), 860–7. [DOI] [PubMed] [Google Scholar]
- Jackson RW, Treiber FA, Turner JR, Davis H, & Strong WB (1999). Effects of race, sex, and socioeconomic status upon cardiovascular stress responsivity and recovery in youth. International Journal of Psychophysiology, 31, 111–119. 10.1016/S0167-8760(98)00044-0 [DOI] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, & Liberzon I (2015). Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Frontiers in Behavioral Neuroscience, 9, 1–8. 10.3389/fnbeh.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, & Neyman J (1936). Tests of certain linear hypotheses and their applications to some educational problems. Statistical Research Memoirs, 1, 57–93. [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, & Swain JE (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, & Tsigos C (2009). Stress hormones: physiological stress and regulation of metabolism. Current Opinion in Pharmacology, 9, 787–793. 10.1016/j.coph.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. Gainesville, FL.: University of Florida. [Google Scholar]
- Langenberg C, Kuh D, Wadsworth MEJ, Brunner E, & Hardy R (2006). Social circumstances and education: life course origins of social inequalities in metabolic risk in a prospective national birth cohort. American Journal of Public Health, 96(12), 2216–2221. 10.2105/AJPH.2004.049429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, & Smith GD (2002). Socioeconomic position in childhood and adulthood and insulin resistance: Cross sectional survey using data from British women’s heart and health study. BMJ, 325, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman B, Taylor S, Kiefe C, & Seeman T (2005). Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine, 67, 846–854. 10.1097/01.psy.0000188443.48405.eb [DOI] [PubMed] [Google Scholar]
- Lucove JC, Kaufman JS, & James SA (2007). Association between adult and childhood socioeconomic status and prevalence of the Metabolic Syndrome in African Americans : The Pitt County Study. American Journal of Public Health, 97(2), 234–236. 10.2105/AJPH.2006.087429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry, 48(10), 976–980. 10.1016/S0006-3223(00)00965-3 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ (2010). Epigenetics and the biological definition of gene × environment interactions. Child Development, 81(1), 41–79. 10.1111/j.1467-8624.2009.01381.x [DOI] [PubMed] [Google Scholar]
- Meaney MJ, & Szyf M (2005). Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience, 7(2), 103–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Rabin BS, Skoner DP, & Doyle WJ (1999). Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain, Behavior, and Immunity, 123, 109–123. [DOI] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, & Seeman TE (2011). Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science, 22(12), 1591–9. 10.1177/0956797611419170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, … Eisenberger NI (2012). Social status modulates neural activity in the mentalizing network. NeuroImage, 60(3), 1771–1777. 10.1016/j.neuroimage.2012.01.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, … Kubzansky LD (2014). Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. American Journal of Epidemiology, 180(3), 263–271. 10.1093/aje/kwu127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KL, Tso AW, Lam KS, Cherny SS, Sham PC, & Cheung BM (2010). Using glycosylated hemoglobin to define the metabolic syndrome in United States adults. Diabetes Care, 33, 1856–1858. 10.2337/dc10-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Blair SN, Lee I-M, & Hyde RT (1993). Measurement of physical activity to assess health effects in free-living populations. Medicine and Science in Sports and Exercise, 25(1), 60–70. [DOI] [PubMed] [Google Scholar]
- Park MJ, Yun KE, Lee GU, Cho HJ, & Park HS (2007). A cross-sectional study of socioeconomic status and the Metabolic Syndrome in Korean adults. Annals of Epidemiology, 17(4), 320–326. 10.1016/j.annepidem.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, & Heymsfield SB (2003). The Metabolic Syndrome. Archives of Internal Medicine, 163, 1988–1994. 10.1001/archinte.163.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Lamont DW, Unwin N, Pearce MS, Bennett SMA, Dickinson HO, … Craft AW (2003). A lifecourse study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49 – 51 years. Diabetic Medicine, 20, 406–415. 10.1046/j.1464-5491.2003.00949.x [DOI] [PubMed] [Google Scholar]
- Payne BK (2001). Prejudice and perception: The role of automatic and controlled processes in misperceiving a weapon. Journal of Personality and Social Psychology, 81(2), 181–192. 10.1037/0022-3514.81.2.181 [DOI] [PubMed] [Google Scholar]
- Payne BK (2006). Weapon bias: Split-second decisions and unintended stereotyping. Current Directions in Psychological Science, 15(6), 287–291. 10.1111/j.1467-8721.2006.00454.x [DOI] [Google Scholar]
- Pervanidou P, & Chrousos GP (2012). Metabolic consequences of stress during childhood and adolescence. Metabolism, 61(5), 611–619. 10.1016/j.metabol.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Pollak SD (2008). Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science, 17(6), 370–375. 10.1111/j.1467-8721.2008.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland JM (2007). Update on the metabolic syndrome in children. Current Opinion in Pediatrics, 19(2), 183–191. 10.1097/MOP.0b013e3280208519 [DOI] [PubMed] [Google Scholar]
- Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, & Leung GM (2008). Life-course origins of social inequalities in metabolic risk in the population of a developing country. American Journal of Epidemiology, 167(4), 419–428. 10.1093/aje/kwm329 [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Pankow J, Jousilahti P, Hu G, & Tuomilehto J (2005). Educational inequalities in the metabolic syndrome and coronary heart disease among middle-aged men and women. International Journal of Epidemiology, 34(2), 327–334. 10.1093/ije/dyi007 [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson EA, & Collins WA (2005). The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. New York, NY: Guilford Press. [Google Scholar]
- Tamayo T, Christian H, & Rathmann W (2010). Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health, 10, 525 10.1186/1471-2458-10-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, & Seeman TE (2004). Early environment, emotions, responses to stress, and health. Journal of Personality, 72(6), 1365–1394. 10.1111/j.1467-6494.2004.00300.x [DOI] [PubMed] [Google Scholar]
- The Emerging Risk Factors Collaboration. (2011). Diabetes mellitus, fasting glucose, and risk of cause-specific death. New England Journal of Medicine, 364(9), 829–841. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber F, Harshfield G, Davis H, Kapuku G, & Moore D (1990). Stress responsivity and body fatness: Links between socioeconomic status and cardiovascular risk factors in youth. Annals of the New York Academy of Sciences, 6295, 435–438. 10.1111/j.1749-6632.1999.tb08163.x [DOI] [PubMed] [Google Scholar]
- Wiley JF, Gruenewald TL, Karlamangla AS, & Seeman TE (2016). Modeling multisystem physiological dysregulation. Psychosomatic Medicine, 78(3), 1–12. 10.1097/PSY.0000000000000288 [DOI] [PMC free article] [PubMed] [Google Scholar]


