Abstract
We report a catalytic asymmetric Nazarov cyclization of simple, acylic, alkyl-substituted divinyl ketones using our recently disclosed strong and confined imidodiphosphorimidate Brønsted acids. The corresponding monocyclic cyclopentenones are formed in good yields and excellent regio-, diastereo-, and enantioselectivities. Further, the chemical utility of the obtained enantiopure cyclopentenones is demonstrated.
Enantiopure cyclopentenones are frequently used as key building blocks toward, and are themselves present within, a variety of bioactive and/or complex natural products.1 Chemists have consequently devoted considerable effort to the development of enantioselective approaches to these important compounds. Commonly used techniques today include chemical or enzymatic resolutions,2,3 asymmetric functionalizations of existing cyclopentenone units,1c or derivatizations of chiral-pool reagents.4 While effective, each of these strategies is conceptually inferior to synthetic methods that introduce chirality during the construction of the cyclic unit from simple starting materials, such as asymmetric Pauson–Khand reactions or Nazarov cyclizations.5,6 Unfortunately, the relatively underdeveloped methodology of the latter techniques has limited their application. In fact, despite being considered one of the most direct and atom-economical transformations for the synthesis of cyclopentenones, the asymmetric Nazarov cyclization is arguably one of the least employed methods toward chiral cyclopentenones.7 The limited application of this strategy is likely an effect of systematic substrate specificity for given variants and, therefore, a lack of generality.
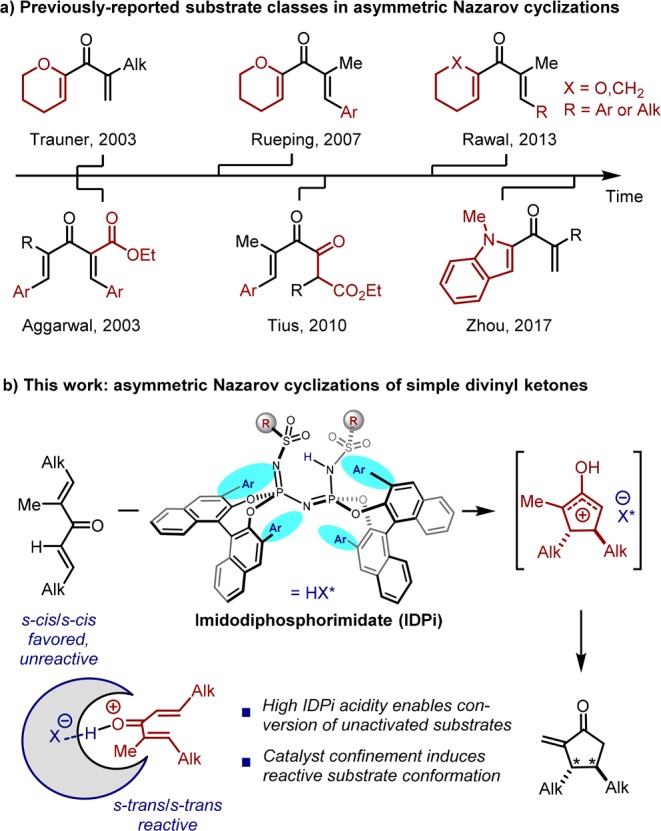
Since the first catalytic asymmetric Nazarov cyclization emerged from the Trauner group in 2003,7c this and subsequent methods have largely depended on designed substrates to overcome the relatively low reactivity of divinyl ketones and/or to circumvent challenges in regio- and stereoselectivity (Figure 1a). More specifically, these substrates are usually activated by adjacent heteroatoms to stabilize the oxyallyl cation, neighboring electron-withdrawing groups, and/or β-aryl substituents to polarize the divinyl ketone.6b Notably, in 2013, Rawal and co-workers disclosed two Nazarov cyclizations of electronically unactivated divinyl ketones; however, in each of these substrates, one of the olefins was within a cyclohexane unit, compromising the overall generality of the method.7j As such, we recognized that simple alkyl-substituted, acyclic divinyl ketones still remain an extremely challenging class of substrates for asymmetric Nazarov cyclizations and thereby undermine its synthetic application.
Figure 1.
(a) Previously reported systems for asymmetric Nazarov cyclizations. (b) Highly acidic and confined acid enables catalytic asymmetric Nazarov cyclization of simple divinyl ketones.
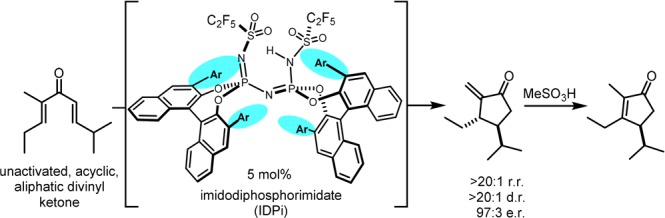
Recently, our group disclosed a novel class of chiral, highly acidic, and confined Brønsted acids, i.e., imidodiphosphorimidates (IDPis), and demonstrated their success in a variety of asymmetric transformations.8 We envisioned that these highly reactive catalysts might be uniquely suited for the Nazarov cyclization of unbiased divinyl ketones, as the confined chiral microenvironment not only induces asymmetry but furthermore may enhance reactivity by increasing the population of the reactive s-trans/s-trans conformer of the divinyl ketone (Figure 1b). Here, we report the fruition of these concepts with a unique catalytic asymmetric Nazarov cyclization of simple, acylic, and alkyl-substituted divinyl ketones.
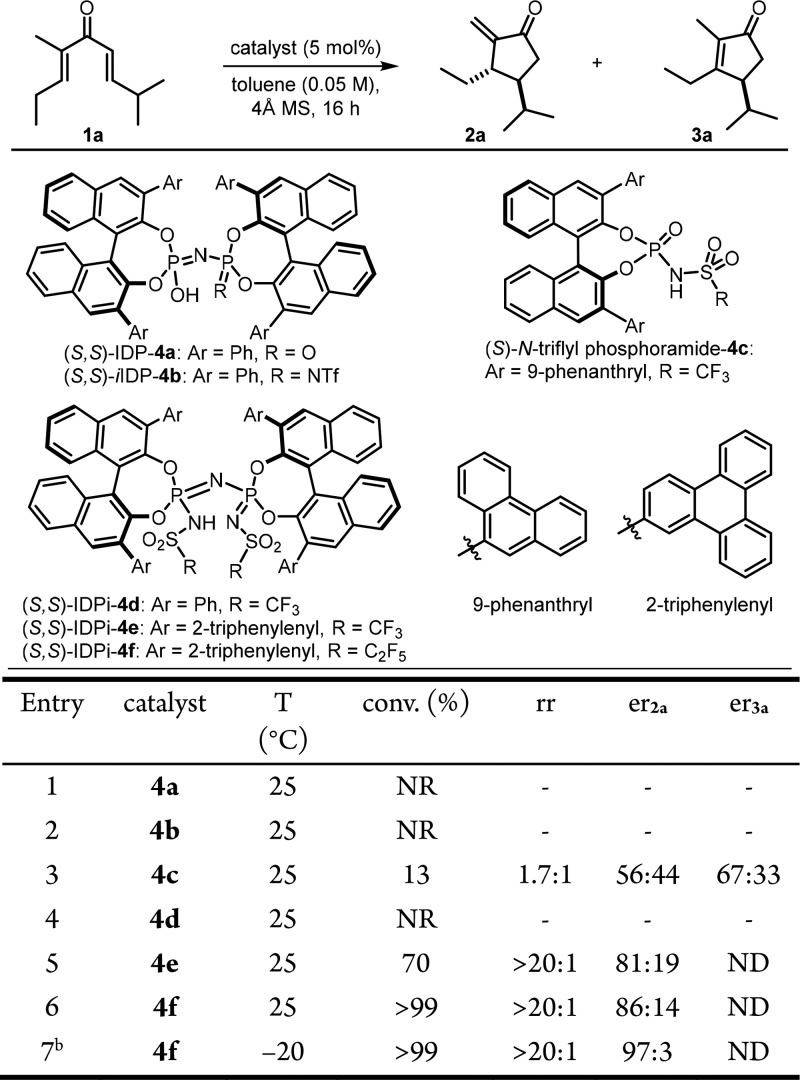
We initiated our studies by evaluating acyclic divinyl ketone 1a as a model substrate using a variety of chiral Brønsted acid catalysts in toluene at 25 °C (Scheme 1). As we anticipated, relatively weakly acidic and confined Brønsted acids, such as imidodiphosphoric acid (IDP) 4a and iminoimidodiphosphate (iIDP) 4b, did not provide any of the desired products (Table 1, entries 1 and 2). Interestingly, N-triflyl phosphoramide 4c, which Rueping and co-workers have already shown to be an efficient Brønsted acid of Nazarov cyclizations, resulted in poor conversion and regioselectivity (2a/3a = 1.7:1) and an enantiomeric ratio of 56:44 for 2a and 67:33 for 3a (entry 3).
Scheme 1. Reaction Development.
Reactions were performed with substrate 1a (0.02 mmol), catalyst (5 mol %), 4 Å MS (10 mg) in toluene (0.4 mL); conversions (conv) and regioisomeric ratios (rr of 2a:3a) were obtained by 1H NMR analysis with Ph3CH as an internal standard; enantiomeric ratios (er) were measured by GC, unless otherwise indicated; all diastereomeric ratios (dr) of product 2a were >20:1.
Reaction was run for 3.5 days. NR = no reaction; ND = not determined.
Table 1. Scope of the Reactiona,b.
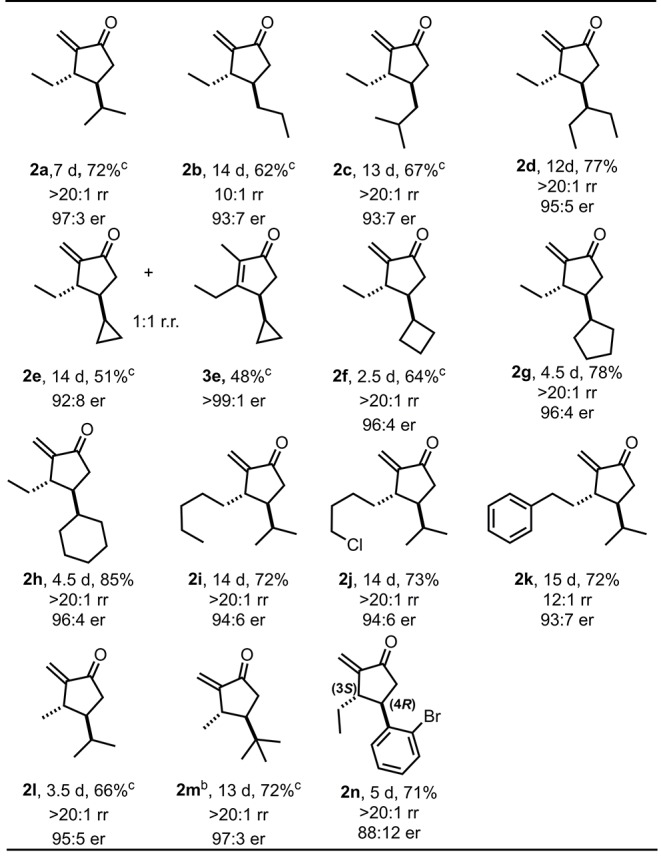
Reactions were carried out with 0.2 mmol of substrates 1, catalyst 4f (5 mol %), and 100 mg molecular sieves in 4 mL of toluene (0.05 M) at −20 °C for the specified reaction time. Regioisomeric ratios (rr of 2/3) and diastereomeric ratios (dr) were detected by 1H NMR of the crude reaction mixture. All diastereomeric ratios (dr) were >20:1. The enantiomeric ratios (er) were determined by GC or HPLC analysis.
7 mol % catalyst was used.
Yields of the volatile products were determined by 1H NMR analysis of the mixtures after column chromatography to remove toluene.
Remarkably, even highly acidic IDPi catalyst 4d (where Ar = Ph) proved to be inactive under the reaction conditions. However, based on our hypothesis that the confinement of the IDPi scaffold would be critical for the increased population of the necessary s-trans/s-trans conformer, we tested IDPi catalysts with sterically larger π-substituents in the 3,3′ positions. Indeed, upon testing IDPi catalysts 4e and 4f (where Ar = 2-triphenylenyl), 2a was formed in good yields with excellent diastereo- and regioselectivity (both >20:1) and moderate enantioselectivity (entries 5 and 6). IDPi catalyst 4f was found to be the best catalyst for this transformation in terms of enantioselectivity and was therefore selected for further optimizations. Gratifyingly, when the reaction was performed at −20 °C, full conversion of substrate 1a to enone 2a was observed with excellent regio- (>20:1), diastereo- (>20:1), and enantioselectivity (97:3).
With the optimized conditions in hand, we next explored the scope of this reaction.9 Substituents at R2 with linear (1b), branched (1c, 1d), and cyclic (1f–h) aliphatic groups were well tolerated, providing the corresponding enones in good yields with excellent regio- and enantioselectivities. Interestingly, cyclopropyl-substituted substrate 1e resulted in two regioisomers, 2e and 3e (rr = 1:1), under the reaction conditions. We suspect that the poor regioselectivity is a result of a relative increase in the thermodynamic stability of the endocyclic isomer 3e by virtue of the unique π-character of the cyclopropyl unit. The successful application of substrate 1j, containing an alkyl chloride, potentially allows for subsequent cyclization or functionalization. In the case of substrate 1k, a Friedel–Crafts-type interrupted Nazarov cyclization was not observed.10 We next turned our attention toward divinyl ketones 1l and 1m with a methyl substituent at R1. The desired enone 2l was obtained as a single regioisomer (rr > 20:1) and with an excellent enantiomeric ratio of 95:5. As for the more bulky substituted divinyl ketone 1m (R2 = t-Bu group), a slightly higher catalyst loading (7 mol %) was required to give cyclopentanone 2m in good yield (72%) and excellent enantioselectivity (97:3). Notably, o-bromophenyl divinyl ketone 1n, as a representative of an aryl-substituted substrate, was converted with a reasonable er of 88:12. The absolute configuration of the produced ketone 2n was determined to be 3S,4R following derivatization (see the SI). The relative configuration of all other products was assigned by analogy.
Encouraged by the success of our reaction design, we were eager to investigate the mechanism of this catalytic, asymmetric Nazarov cyclization. We envisioned two plausible scenarios, the first in which the free catalyst is the resting state and the second involving a covalent intermediate formed in a reaction between the oxyallyl cation and the anion of catalyst 4f, similar to that which was found in the imidodiphosphoric acid (IDP) catalyzed carbonyl–ene cyclization previously reported by our group.8i,11 In order to distinguish these two possible mechanisms, a kinetic study was performed using 1H NMR analysis. As shown in Figure 2a, the linear correlation between reaction rate and concentration of starting material suggests the reaction to be first order in substrate under the steady state approximation. We therefore propose that the free catalyst is the resting state in the catalytic cycle and coordinates to the substrate to form the complex A (Figure 2b). Subsequently, a conrotatory 4π-electrocyclization occurs to generate the oxyallyl ion pair B, followed by a kinetically controlled deprotonation (path a), presumably by the moderately basic O atoms of the sulfonyl group, which regenerates the catalyst and releases the product.
Figure 2.
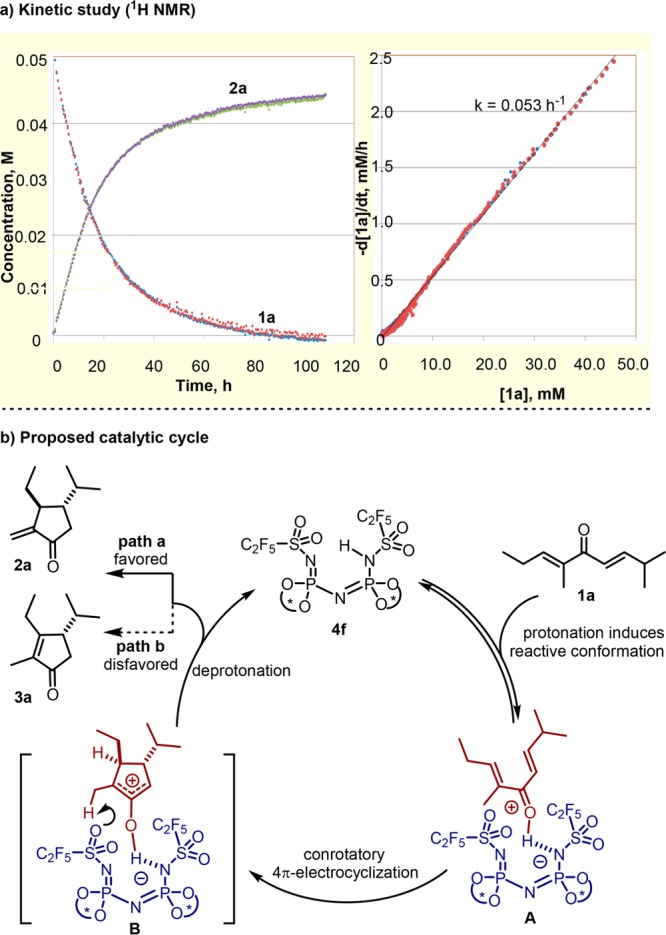
(a) Reaction profile for the reaction of substrate 1a with catalyst 4f in the presence of 4 Å molecular sieves at −20 °C in toluene-d8 and CH2Br2 as external standard. (b) Proposed mechanism.
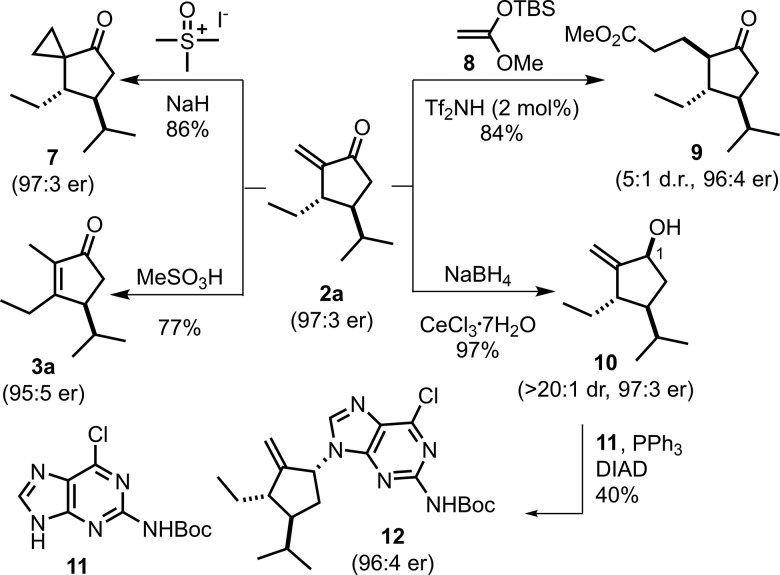
We also explored the synthetic utility of our enone products (Scheme 2). Indeed, unsaturated ketone 2a reacted as a Michael acceptor in a cyclopropanation and in a Mukaiyama–Michael addition. The resulting products, ketone 7 and cyclopentanone 9, were obtained without deterioration of enantioselectivity. The α-methylene unit of 2a could be isomerized to the fully substituted, thermodynamically more stable cyclopentenone 3a with an excess amount of methanesulfonic acid, again retaining the excellent enantioselectivity. Moreover, a Luche reduction of 2a furnished allylic alcohol 10 in excellent diastereoselectivity (dr > 20:1), which could then be utilized in a Mitsunobu reaction to install a purine-derivative and afford compound 12 with excellent C1 enantiopurity.12
Scheme 2. Functionalization of Nazarov Cyclization Product 2a.
In conclusion, we have developed a powerful catalytic, asymmetric Nazarov cyclization of simple, acyclic, aliphatic-substituted divinyl ketones using a strong and confined Brønsted acid. We propose that the confinement of the IDPi scaffold induces the reactive s-trans/s-trans conformation of the divinyl ketone substrate, thereby promoting the cyclization to give a variety of versatile enones in good yields and excellent enantio-, regio-, and diastereoselectivities. Our approach could be useful in other conformation-dependent transformations, and the developed Nazarov reaction may aid in the asymmetric synthesis of several biologically active natural products.
Acknowledgments
Generous support from the Max Planck Society, the Deutsche Forschungsgemeinschaft (Leibniz Award to B.L), and the European Research Council (Advanced Grant “C–H Acids for Organic Synthesis, CHAOS”) is gratefully acknowledged. Excellent service provided by our technicians and our NMR, GC, and HPLC departments is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b13899.
Additional detailed synthetic protocols, analytical data for all compounds, and computational strategy (PDF)
The authors declare the following competing financial interest(s): A patent, WO2017037141 (A1), has been filed by the MPI für Kohlenforschung covering the IDPi catalyst class and their applications in asymmetric synthesis.
Supplementary Material
References
- a Hanna I. Synthesis of substituted furans using 1,4-dioxene. Tetrahedron Lett. 1999, 40, 2521–2524. 10.1016/S0040-4039(99)00283-X. [DOI] [Google Scholar]; b Gibson S. E.; Lewis S. E.; Mainolfi N. Transition metal-mediated routes to cyclopentenones. J. Organomet. Chem. 2004, 689, 3873–3890. 10.1016/j.jorganchem.2004.04.045. [DOI] [Google Scholar]; c Simeonov S. P.; Nunes J. P. M.; Guerra K.; Kurteva V. B.; Afonso C. A. M. Synthesis of Chiral Cyclopentenones. Chem. Rev. 2016, 116, 5744–5893. 10.1021/cr500504w. [DOI] [PubMed] [Google Scholar]
- Johnson C. R.; Penning T. D. Triply convergent synthesis of (−)-prostaglandin E2 methyl ester. J. Am. Chem. Soc. 1988, 110, 4726–4735. 10.1021/ja00222a034. [DOI] [Google Scholar]
- Pinot E.; Guy A.; Guyon A.-L.; Rossi J.-C.; Durand T. Enzymatic kinetic resolution of a functionalized 4-hydroxy-cyclopentenone: synthesis of the key intermediates in the total synthesis of isoprostanes. Tetrahedron: Asymmetry 2005, 16, 1893–1895. 10.1016/j.tetasy.2005.03.028. [DOI] [Google Scholar]
- Brill Z. G.; Condakes M. L.; Ting C. P.; Maimone T. J. Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products. Chem. Rev. 2017, 117, 11753–11795. 10.1021/acs.chemrev.6b00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Blanco-Urgoiti J.; Añorbe L.; Pérez-Serrano L.; Domínguez G.; Pérez-Castells J. The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules. Chem. Soc. Rev. 2004, 33, 32–42. 10.1039/B300976A. [DOI] [PubMed] [Google Scholar]; b Roche S. P.; Aitken D. J. Chemistry of 4-Hydroxy-2-cyclopentenone Derivatives. Eur. J. Org. Chem. 2010, 2010, 5339–5358. 10.1002/ejoc.201000704. [DOI] [Google Scholar]
- a Nazarov I. N.; Zaretskaya I. I. Izv. Akad. Nauk. SSSR, Ser. Khim. 1941, 211–224. [Google Scholar]; b Frontier A. J.; Collison C. The Nazarov cyclization in organic synthesis. Recent advances. Tetrahedron 2005, 61, 7577–7606. 10.1016/j.tet.2005.05.019. [DOI] [Google Scholar]; c Pellissier H. Recent developments in the Nazarov process. Tetrahedron 2005, 61, 6479–6517. 10.1016/j.tet.2005.04.014. [DOI] [Google Scholar]; d Tius M. A. Some New Nazarov Chemistry. Eur. J. Org. Chem. 2005, 2005, 2193–2206. 10.1002/ejoc.200500005. [DOI] [Google Scholar]; e Shimada N.; Stewart C.; Tius M. A. Asymmetric Nazarov cyclizations. Tetrahedron 2011, 67, 5851–5870. 10.1016/j.tet.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; f West F. G.; Scadeng O.; Wu Y. K.; Fradette R. J.; Joy S.. The Nazarov Cyclization. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Ed.; Elsevier: Amsterdam, 2014; pp 827–866. [Google Scholar]
- a Aggarwal V. K.; Belfield A. J. Catalytic Asymmetric Nazarov Reactions Promoted by Chiral Lewis Acid Complexes. Org. Lett. 2003, 5, 5075–5078. 10.1021/ol036133h. [DOI] [PubMed] [Google Scholar]; b He W.; Sun X.; Frontier A. J. Polarizing the Nazarov Cyclization: Efficient Catalysis under Mild Conditions. J. Am. Chem. Soc. 2003, 125, 14278–14279. 10.1021/ja037910b. [DOI] [PubMed] [Google Scholar]; c Liang G.; Gradl S. N.; Trauner D. Efficient Nazarov Cyclizations of 2-Alkoxy-1,4-pentadien-3-ones. Org. Lett. 2003, 5, 4931–4934. 10.1021/ol036019z. [DOI] [PubMed] [Google Scholar]; d Liang G.; Trauner D. Enantioselective Nazarov Reactions through Catalytic Asymmetric Proton Transfer. J. Am. Chem. Soc. 2004, 126, 9544–9545. 10.1021/ja0476664. [DOI] [PubMed] [Google Scholar]; e Rueping M.; Ieawsuwan W.; Antonchick A. P.; Nachtsheim B. J. Chiral Brønsted Acids in the Catalytic Asymmetric Nazarov Cyclization–The First Enantioselective Organocatalytic Electrocyclic Reaction. Angew. Chem., Int. Ed. 2007, 46, 2097–2100. 10.1002/anie.200604809. [DOI] [PubMed] [Google Scholar]; f Walz I.; Togni A. Ni(II)-catalyzed enantioselective Nazarov cyclizations. Chem. Commun. 2008, 4315–4317. 10.1039/b806870d. [DOI] [PubMed] [Google Scholar]; g Basak A. K.; Shimada N.; Bow W. F.; Vicic D. A.; Tius M. A. An Organocatalytic Asymmetric Nazarov Cyclization. J. Am. Chem. Soc. 2010, 132, 8266–8267. 10.1021/ja103028r. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Bow W. F.; Basak A. K.; Jolit A.; Vicic D. A.; Tius M. A. Enamine-Iminium Ion Nazarov Cyclization of α-Ketoenones. Org. Lett. 2010, 12, 440–443. 10.1021/ol9025765. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Raja S.; Ieawsuwan W.; Korotkov V.; Rueping M. Asymmetric Brønsted Acid-Catalyzed Nazarov Cyclization of Acyclic α-Alkoxy Dienones. Chem. - Asian J. 2012, 7, 2361–2366. 10.1002/asia.201200391. [DOI] [PubMed] [Google Scholar]; j Hutson G. E.; Turkmen Y. E.; Rawal V. H. Salen promoted enantioselective Nazarov cyclizations of activated and unactivated dienones. J. Am. Chem. Soc. 2013, 135, 4988–4991. 10.1021/ja401908m. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Jolit A.; Walleser P. M.; Yap G. P. A.; Tius M. A. Catalytic Enantioselective Nazarov Cyclization: Construction of Vicinal All-Carbon-Atom Quaternary Stereocenters. Angew. Chem., Int. Ed. 2014, 53, 6180–6183. 10.1002/anie.201403587. [DOI] [PubMed] [Google Scholar]; l Kitamura K.; Shimada N.; Stewart C.; Atesin A. C.; Ateşin T. A.; Tius M. A. Enantioselective Palladium(0)-Catalyzed Nazarov-Type Cyclization. Angew. Chem., Int. Ed. 2015, 54, 6288–6291. 10.1002/anie.201500881. [DOI] [PubMed] [Google Scholar]; m Wang G.-P.; Chen M.-Q.; Zhu S.-F.; Zhou Q.-L. Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids. Chem. Sci. 2017, 8, 7197–7202. 10.1039/C7SC03183A. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Jin J.; Zhao Y.; Gouranourimi A.; Ariafard A.; Chan P. W. H. Chiral Brønsted Acid Catalyzed Enantioselective Dehydrative Nazarov-Type Electrocyclization of Aryl and 2-Thienyl Vinyl Alcohols. J. Am. Chem. Soc. 2018, 140, 5834–5841. 10.1021/jacs.8b02339. [DOI] [PubMed] [Google Scholar]; o Mietke T.; Cruchter T.; Larionov V. A.; Faber T.; Harms K.; Meggers E. Asymmetric Nazarov Cyclizations Catalyzed by Chiral-at-Metal Complexes. Adv. Synth. Catal. 2018, 360, 2093–2100. 10.1002/adsc.201701546. [DOI] [Google Scholar]; p Süsse L.; Vogler M.; Mewald M.; Kemper B.; Irran E.; Oestreich M. Enantioselective Nazarov Cyclizations Catalyzed by an Axial Chiral C6F5-Substituted Boron Lewis Acid. Angew. Chem., Int. Ed. 2018, 57, 11441–11444. 10.1002/anie.201806011. [DOI] [PubMed] [Google Scholar]
- a Kaib P. S.; Schreyer L.; Lee S.; Properzi R.; List B. Extremely Active Organocatalysts Enable a Highly Enantioselective Addition of Allyltrimethylsilane to Aldehydes. Angew. Chem., Int. Ed. 2016, 55, 13200–13203. 10.1002/anie.201607828. [DOI] [PubMed] [Google Scholar]; b Xie Y.; Cheng G.-J.; Lee S.; Kaib P. S. J.; Thiel W.; List B. Catalytic Asymmetric Vinylogous Prins Cyclization: A Highly Diastereo- and Enantioselective Entry to Tetrahydrofurans. J. Am. Chem. Soc. 2016, 138, 14538–14541. 10.1021/jacs.6b09129. [DOI] [PubMed] [Google Scholar]; c Lee S.; Kaib P. S. J.; List B. Asymmetric Catalysis via Cyclic, Aliphatic Oxocarbenium Ions. J. Am. Chem. Soc. 2017, 139, 2156–2159. 10.1021/jacs.6b11993. [DOI] [PubMed] [Google Scholar]; d Liu L.; Kim H.; Xie Y.; Fares C.; Kaib P. S. J.; Goddard R.; List B. Catalytic Asymmetric [4 + 2]-Cycloaddition of Dienes with Aldehydes. J. Am. Chem. Soc. 2017, 139, 13656–13659. 10.1021/jacs.7b08357. [DOI] [PubMed] [Google Scholar]; e Bae H. Y.; Höfler D.; Kaib P. S. J.; Kasaplar P.; De C. K.; Döhring A.; Lee S.; Kaupmees K.; Leito I.; List B. Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon–carbon bond forming reaction. Nat. Chem. 2018, 10, 888–894. 10.1038/s41557-018-0065-0. [DOI] [PubMed] [Google Scholar]; f Gatzenmeier T.; Kaib P. S. J.; Lingnau J. B.; Goddard R.; List B. The Catalytic Asymmetric Mukaiyama-Michael Reaction of Silyl Ketene Acetals with α,β-Unsaturated Methyl Esters. Angew. Chem., Int. Ed. 2018, 57, 2464–2468. 10.1002/anie.201712088. [DOI] [PubMed] [Google Scholar]; g Gatzenmeier T.; Turberg M.; Yepes D.; Xie Y.; Neese F.; Bistoni G.; List B. Scalable and Highly Diastereo- and Enantioselective Catalytic Diels–Alder Reaction of α,β-Unsaturated Methyl Esters. J. Am. Chem. Soc. 2018, 140, 12671–12676. 10.1021/jacs.8b07092. [DOI] [PubMed] [Google Scholar]; h Schreyer L.; Kaib P. S. J.; Wakchaure V. N.; Obradors C.; Properzi R.; Lee S.; List B. Confined acids catalyze asymmetric single aldolizations of acetaldehyde enolates. Science 2018, 362, 216–219. 10.1126/science.aau0817. [DOI] [PubMed] [Google Scholar]; i Tsuji N.; Kennemur J. L.; Buyck T.; Lee S.; Prévost S.; Kaib P. S. J.; Bykov D.; Farès C.; List B. Activation of olefins via asymmetric Brønsted acid catalysis. Science 2018, 359, 1501–1505. 10.1126/science.aaq0445. [DOI] [PubMed] [Google Scholar]; j Lee S.; Kaib P. S.; List B. N-Triflylphosphorimidoyl Trichloride: A Versatile Reagent for the Synthesis of Strong Chiral Brønsted Acids. Synlett 2017, 28, 1478–1480. 10.1055/s-0036-1588782. [DOI] [Google Scholar]
- A description of known limitations in the scope of the method is provided in the SI.
- Bender J. A.; Arif A. M.; West F. G. Nazarov-Initiated Diastereoselective Cascade Polycyclization of Aryltrienones. J. Am. Chem. Soc. 1999, 121, 7443–7444. 10.1021/ja991215f. [DOI] [Google Scholar]
- Liu L.; Leutzsch M.; Zheng Y.; Alachraf M. W.; Thiel W.; List B. Confined Acid-Catalyzed Asymmetric Carbonyl-Ene Cyclization. J. Am. Chem. Soc. 2015, 137, 13268–13271. 10.1021/jacs.5b09484. [DOI] [PubMed] [Google Scholar]
- Xu H.; Wang F.; Xue W.; Zheng Y.; Wang Q.; Qiu F. G.; Jin Y. Total Synthesis of Entecavir: A Robust Route for Pilot Production. Org. Process Res. Dev. 2018, 22, 377–384. 10.1021/acs.oprd.8b00007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.