Abstract
This is a report on how 1H NMR-based metabonomics was employed to discriminate osteopenia from osteoporosis in postmenopausal women, identifying the main metabolites associated to the separation between the groups. The Assays were performed using seventy-eight samples, being twenty-eight healthy volunteers, twenty-six osteopenia patients and twenty-four osteoporosis patients. PCA, LDA, PLS-DA and OPLS-DA formalisms were used. PCA discriminated the samples from healthy volunteers from diseased patient samples. Osteopenia-osteoporosis discrimination was only obtained using Analysis Discriminants formalisms, as LDA, PLS-DA and OPLS-DA. The metabonomics model using LDA formalism presented 88.0% accuracy, 88.5% specificity and 88.0% sensitivity. Cross-Validation, however, presented some problems as the accuracy of modeling decreased. LOOCV resulted in 78.0% accuracy. The OPLS-DA based model was better: R2Y and Q2 values equal to 0.871 (p<0.001) and 0.415 (p<0.001). LDA and OPLS-DA indicated the important spectral regions for discrimination, making possible to assign the metabolites involved in the skeletal system homeostasis, as follows: VLDL, LDL, leucine, isoleucine, allantoin, taurine and unsaturated lipids. These results indicate that 1H NMR-based metabonomics can be used as a diagnosis tool to discriminate osteoporosis from osteopenia using a single serum sample.
Introduction
Osteoporosis is a multifactorial systemic skeletal disease that causes damage to the microarchitecture of bone tissue, increasing the risk of fractures [1]. Women in the postmenopausal period are the most affected by this problem because of the hormonal deficiency that occurs during this period. Reduction of estrogen levels promotes the homeostatic imbalance of the bone remodeling process, causing an increase in bone resorption, deterioration of the microarachitecture, and a decrease in bone mass. About 40% of women older than 50 years of age are diagnosed with postmenopausal osteoporosis, making it necessary to pay special attention to this patient group [2]. Estrogen hormone therapy has been considered the most effective for the prevention and treatment of postmenopausal osteoporosis. However, investigations showed that estrogen could lead to higher occurrences of endometrial cancer, stroke, cardiovascular diseases and breast carcinoma [3]
According to WHO criteria, the osteoporosis diagnosis is performed from bone mineral density (BMD) determination, using a T-score. Patients with bone mineral density values (in T-score) higher than –1.0 are classified as healthy, while patients with T-score minor than –2.5 are diagnosed with osteoporosis. Patients who have T-scores between –2.5 and –1.0 are not classified as having osteoporosis, but also present risk of fractures higher than the medium of the population. These patients are diagnosed with osteopenia [4].
The pharmacological approach is generally recommended for patients with osteoporosis and osteopenia, as indicated by the fracture risk assessment (FRAX), which takes into account other factors (BMI, used medications, previous fracture, etc.) in addition to BMD. However, the decision of pharmacological treatment carries with it the risk of adverse effects and side effects. Thus, early diagnosis of bone loss allows an intervention only with changes in patient lifestyle [5,6].
Some serum biomarkers of bone formation (or resorption) are used to this diagnosis. As the resorption process is faster than bone formation, levels of bone resorption biomarkers change more rapidly than levels of bone formation markers [7–9]. In addition, others metabolites are studied with correlation in bone metabolism. Tanko et al.[10], Orozco et al.[11] and Poiana et al.[12] have reported that postmenopausal women who presented abnormal lipid profile had lower lumbar and femoral BMD and, therefore, higher risk of fractures. They suggest an association between hyperlipidemia and osteopenia. Moreover, amine-terminal collagen type I (NTX-I) telopeptides, which are markers of bone resorption, present positive correlation with Total Cholesterol and LDL serum levels [13]. Lv et al.[14] identified changes in arachidonic acid, leucine, isoleucine, lactate, taurine and cholesterol serum levels and showed that these changes correlated with loss of bone mass.
Metabonomics applies multivariate statistical formalisms to spectral data aiming to correlate them to biochemical status. In metabonomics strategy, the working hypothesis is that when life system is exposed to an external agent, by homeostasis, there are changes in the concentration of endogenous metabolites which can be associated to patients’ biochemical status. Therefore, it is possible develop metabonomics models aiming to diagnose or assess a clinical therapy [15,16]. There are various reports that make use of metabonomics for clinical diagnosis. Duarte and Gil [17] showed the use of metabonomics in the study of human biofluids to identify various types of cancer, using 1H NMR spectra. Qiao et al.[18] studied schizophrenia in patients treated with olanzapine through the metabolic analysis of blood plasma. Gouveia et al.[19] developed metabonomics models to investigate periportal liver fibrosis caused by mansonic schistosomiasis among patients diagnosed with viral hepatitis. Batista et al. [20] discriminated intermediate from advanced liver fibrosis in patients using 1H NMR-based metabonomics. Godoy et al.[21] used LDA metabonomics model to made hepatitis C virus diagnosis from urine 1H NMR spectra.
Statistical formalisms employed in metabonomics assays are divided in two categories: unsupervised, which doesn’t use class information, and supervised methods. Principal Components Analysis (PCA) is the unsupervised method more used to investigate natural grouping and outlies. The samples and original variables are projected in new coordinate system defined by Principal Components (PC), producing case and loading plots, respectively. Each PC explains part of variance contained in dataset. Among unsupervised methods, Discriminants Analysis formalisms are the more employed, with highlight to PLS-DA (Partial Least Discriminants Analysis), OPLS-DA (Orthogonal Partial Least Discriminants Analysis) and LDA (Linear Discriminants Analysis). The discriminants analysis formalisms are linear combinations of original variables that relate to class matrix (matrix Y). In PLS-DA are build Latent Variables, similar to PCA, but considering the variance contained into matrix Y; in OPLS-DA, the systematic variance contained in matrix X (dataset) is divided in two groups, where first component explains the higher variance contained into matrix Y associated to difference between the classes, while the second group, called orthogonal component, explains intraclass variance; LDA is a linear combination of the some original variables. Therefore, LDA needs to use a variable selection tool to build the discriminant function which will divide to space in two regions, where the samples of each class are projected [15,16,22].
Our study was to use metabonomics strategy to discriminate osteopenia from osteoporosis in postmenopausal women, using 1H NMR spectra of serum.
Materials and method
Patients and ethical procedure
The study was developed using samples of postmenopausal women arising from Cabo de Santo Agostinho city (Pernambuco/Brazil). These patients were recruited by spontaneous demand when they were to Rheumatology Ambulatory, where were submitted to anamnesis and the bone mineral density (T-scores) was determined using the Hologic Bone Densitometer Discovery Ci. For each patient, T-scores were measured in three regions–lumbar spine (L1-L4), femoral neck and femur total, being considered the site with minor T-score. Bone mineral density assays were performed until 90 days before 1H NMR analysis. The body mass index (BMI) of each patient and serum level of total cholesterol and alkaline phosphatase were determined. In the study were excluded patients with others associated chronic disease as well as those who were making use of drugs that affect BMD. After anamnesis, were recruited 78 volunteers who were distributed in three groups: (1) Healthy, containing twenty-eight women; (2) Osteopenia, being twenty-six patients; and (3) Osteoporosis, containing twenty-four patients. This study received approval from the Ethics Committee of the Universidade Federal de Pernambuco Health Sciences Center (Approval number 1.114.754/July 2015) and all volunteers signed the Free and Informed Consent Term.
Statistical analysis
The clinical parameters of participants (mean age, bone density, body mass index, total cholesterol and alkaline phosphatase) were submitted to statistical analysis through ANOVA and Tukey test with significance level of 5% (p > 0.05), using the GraphPad Prism version 7.0 (GraphPad Software Inc., USA).
Metabonomics assay
1H NMR spectra were performed using a VNMRS400 spectrometer operating at 400 MHz. Samples were prepared using 400 μL of serum and 200 μL of D2O. Acquisition used the following parameters: T2-filter associated to presaturation of water signal (Presat-CPMG) pulse sequence, as follows: spectral window equal to 6.4 kHz, acquisition time equal to 2.56 s, 128 transients, spin echo delay equal to 400 μs, 88 cycles, giving a total echo time equal to 70.4 ms and saturation delay equal to 2.0 s. [23] The signal attributed to methyl group of lactate (δ 1.33 ppm) was used as chemical shift reference. Spectra were binned in the region between δ 8.00 and 0.00 ppm, 0.04 ppm/bin, excluding the region between δ 5.12 and 4.48 ppm. Spectral data were collected in a matrix with 78 cases (lines) and 184 variables (column). Data set were preprocessed using normalization by sum (in line) and employed PCA, PLS-DA and OPLS-DA formalisms, using MetaboAnalyst online platform. Metabonomics models were validated using Leaving-One-Out Cross Validation (LOOCV) and permutation test, using 2000 permutations. LDA Model was performed using Statistical 10.0 software. The selection of variables to build LDA model was performed using Wilk’s Lambda.
Results
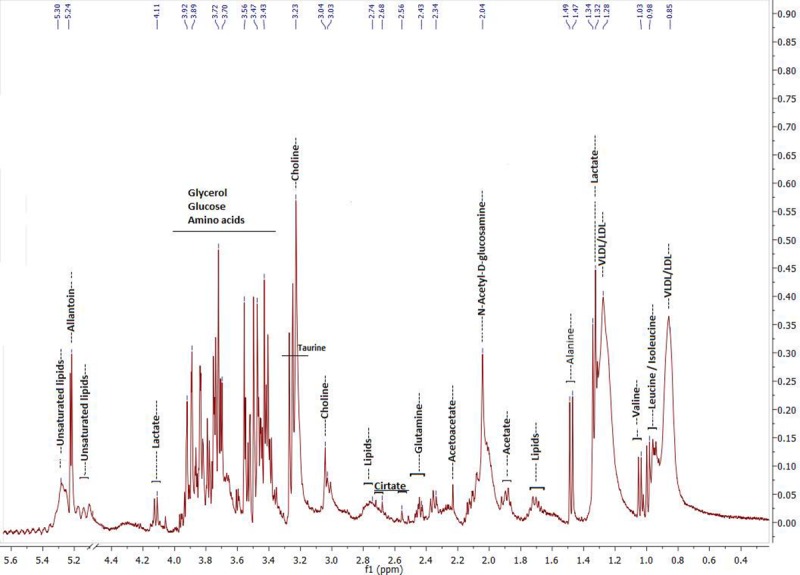
Table 1 shows clinical data of participants of each group, while Fig 1 presents a typical 1H NMR spectrum of serum obtained in this study and main assignments, identifying associated metabolites.
Table 1. Clinical data of studied volunteers.
| Studied Groups | ||||||
|---|---|---|---|---|---|---|
| Healthy volunteers (1) | Osteopenia patients (2) | Osteoporosis patients (3) | ANOVA p-valueb |
Tukey test p-valuec |
||
| Bone Density (T-scores)a | -0.11 (±0.7) | -1.64 (±0.4) | -3.11 (±0.5) | <0.0001 | <0.0001d | |
| BMI (kg.m-2) | 25.35 (±3.4) | 27.20 (±5.2) | 25.58 (±4.8) | 0.2165 | - | |
| Cholesterol Total (mg.L-1) | 216.28 (±28.2) | 226.4 (±37.3) | 217.7 (±40.8) | 0.503 | - | |
| Alkaline Phosphatase (mg.L-1) | 78.0 (±36.9) | 75.4 (±40.7) | 102.5 (±58.1) | 0.3351 | - | |
| Age (years old) | 60.38 (±6.2) | 61.88 (±7.9) | 60.80 (±6.0) | 0.5292 | - | |
| Ethnnicity | Afrodescendant | 20 [71%] | 17 [65%] | 16 [67%] | 0.9916 | - |
| Caucasian | 7 [25%] | 8 [31%] | 6 [25%] | |||
| Not declared | 1 [4%] | 1 [4%] | 2 [8%] | |||
| N | 28 | 26 | 24 | - | ||
aT-scores were measured in three regions–lumbar spine (L1-L4), femoral neck and femur total It was considered the site with minor value. BMD collected until 90 days before 1H NMR analysis.
b Fisher’s chi-square test.
c Only when p-value of ANOVA < 0.05
d It was observe the same p-value when compared all studied groups: (1) vs (2); (1) vs (3); and (2) vs (3).
Fig 1. Typical 1H NMR spectrum of serum (400 MHz, D2O, Presat-CPMG pulse sequence) used in the study.
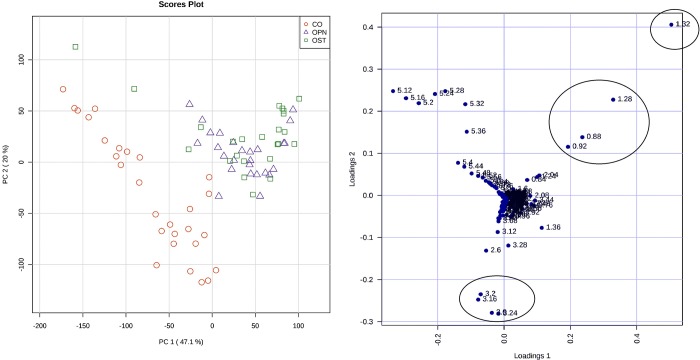
The study used serum samples from 78 volunteers. Exploratory analysis was performed using PCA formalism and results are presented in Fig 2. PC1 and PC2 explain 71% of variance contained in the dataset. Separation between healthy volunteers and patients (osteopenia or osteoporosis) samples can be observed. There is no natural discrimination, however, between the osteopenia and osteoporosis groups. The PCA loading plot indicates that regions δ 0.88–1.32 ppm and δ 3.12–3.28 ppm are important to discriminate diseased from healthy volunteers. The control group presented higher intensity to signal at δ 3.12–3.28 ppm, while diseased volunteers presented a more intense signal at δ 0.88–1.32 ppm.
Fig 2. PCA results using all dataset.
On the left, score plot (PC1xPC2, 71% of variance)—control (red circles), osteoporosis (green squares) and osteopenia (blue triangles); The right, loading plot indicates the most important variables for discrimination.
When PCA formalism was applied to the osteopenia/osteoporosis data, neither separation was observed. Then, supervised methods were employed, such as LDA (Linear Discriminant Analysis) and OPLS-DA (Orthogonal Partial Least Square–Discriminant Analysis), aiming to discriminate between the osteopenia and osteoporosis samples. A LDA model was built using six variables, as follows: δ 0.92, 1.28, 3.24, 3.68, 5.44 and 5.96 ppm. Table 2 shows the classification matrix using the LDA model.
Table 2. Samples classification based on scores obtained from LDA Model using fifty 1H NMR spectra– 26 osteopenia and 24 osteoporosis.
| Clinical Diagnosis | |||
|---|---|---|---|
| LDA Model | Osteopenia | Osteoporosis | |
| Osteopenia | 23 (21)* | 3 (5)* | |
| Osteoporosis | 3 (6)* | 21 (18)* | |
F(6,43) = 8.60 p<0.001
*Classification after LOOCV.
The LDA model presented sensitivity, specificity and accuracy values equal to 88.0%, 88.5% and 88.0%, respectively. The F-test with 6 (variables used) and 43 (50-6-1) degrees of freedom was equal to 8.60 (p<0.001). After LOOCV, LDA model presented 78.0% accuracy, 75% specificity and 80.8% sensitivity.
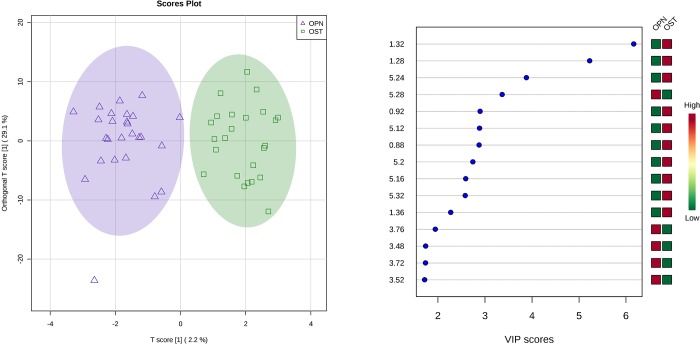
Fig 3 presents results obtained applying OPLS-DA formalism to the osteopenia-osteoporosis dataset (fifty samples). OPLS-DA model presented R2Y and Q2 values equal to 0.871 (p<0.001) and 0.415 (p<0.001), respectively, after validation by permutation test.
Fig 3. OPLS-DA results using only osteoporosis (green squares) and osteopenia (blue triangles) samples from postmenopausal women.
Score plot (at left) and VIP score plot (at right).
The VIP scores plot (Fig 3, right) indicates some spectral regions which are responsible for discrimination, as follows: δ 3.48–3.76 ppm and δ 5.28 ppm, where the signal intensities are higher in the osteopenia group than in the osteoporosis group; δ 0.88–0.92 ppm; 1.28–1.36 ppm; and 5.12–5.32 ppm, where the signals are more intense in osteoporosis group.
Discussion
International Osteoporosis Foundation [24] data indicate that osteoporosis was diagnosed in more de 200 million women and is associated with 9 million fractures annually in the world. Generally, osteoporosis is associated to women and to aging, but can be diagnosed in the young and also in men. About 33% of women over 45 years old have a positive diagnosis for osteoporosis. When women over 80 are observed, the disease reaches about 73% of this population. This indicates that is important to develop diagnostic tools that are able to discriminate osteoporosis from osteopenia patients in this group (postmenopausal women). The present study investigated only postmenopausal women, aiming to discriminate osteopenia from osteoporosis, using 1H NMR-based metabonomics. There were three groups–Healthy (28 volunteers), osteopenia (26 patients) and osteoporosis (24 patients).
Table 1 indicates that there is only significant difference in bone mineral density. This is natural, since bone mineral density is the common criteria to diagnose a patient. The groups were similar in all the other parameters studied. The PCA loading plot (Fig 2) suggests that the lipid profile of patients was slightly different from healthy volunteers, since that variables δ 0.88–1.32 ppm are important to discriminate diseased from healthy samples. The Control group presented greater signal intensity at δ 3.12–3.28 ppm, while osteopenia/osteoporosis patients presented increased integration values at δ 0.88–1.32 ppm. Signals at δ 3.12–3.28 ppm were attributed to choline and taurine, while signals at δ 0.88–1.32 ppm were attributed to methylene and methyl groups of VLDL and LDL. These results are in according with reports that associated decrease in bone mineral density to increased LDL serum level [14,25]. PCA loading plot shows that the control group samples signals were more intense at δ 3.16–3.24 ppm, indicating that healthy volunteers had choline and taurine serum levels higher than the osteopenia/osteoporosis patients. This was also reported by Long et al.[26] and Lv et al.[20], who observed a decrease in choline concentration in patients diagnosed with osteoporosis. Taurine is one of the most abundant non-essential amino acids found in bones. This result corroborates with studies correlating taurine as an osteoclast formation inhibitor and osteoblast inductor [2,27,28]. Besides that, choline and tyrosine amino acids were identified as important for the discrimination between healthy volunteers and osteopenia-osteoporosis patients, with higher concentrations observed in the control group. This observation agrees with reports in the literature [14,26], [29,30] which indicate that choline and tyrosine concentrations decreased in patient with osteoporosis. Tyrosine is one of the amino acids present in thyroid hormones (T3 e T4) with an intimate relationship with the osseous metabolism and stimulates the expression of genes in the osteoblasts for the production of collagen [29,30].
Exploratory analysis, however, did not discriminate osteoporosis from osteopenia. Supervised formalisms were employed aiming at this discrimination. A LDA Model was built using six variables which were associated with leucine, isoleucine, lactate, taurine and unsaturated compounds. This metabonomics model is significant statistically, since the F-test with 6 and 43 degrees of freedom was equal to 8.60 (p<0.001). However, there was a grey area in the model, where the classification was doubtful. This was evidenced when LOOCV (Leave-One-Out Cross Validation) was carried out, resulting in a 78.0% accuracy. Alternatively, OPLS-DA was used and the metabonomics model built was able to discriminate between the groups, indicating that there was a significant difference between them. The validation using 2000 permutation resulted in R2Y and Q2 values equal to 0.871 (p<0.001) and 0.415 (p<0.001), respectively. Fig 3 shows four spectral regions important for discrimination: δ 0.88–0.92 ppm; δ 1.28–1.36 ppm; δ 3.48–3.76 ppm; and δ 5.12–5.32 ppm.
According to the OPLS-DA VIP score (Fig 3), there was an increase in the allantoin serum level in the osteopenia group while the osteoporosis groups presented higher serum levels of cholesterol, lactate and unsaturated lipids. These findings already have been reported by Chen et al.[31], who observed an increase in allantoin serum level after osteoporosis prophylaxis. Maritz et al. [25] and Lv et al.[20] reported an association between cholesterol serum level and osteoporosis diagnosis. Xue et al.[3] and Dixon and Sims[32] associated lactate serum level with osteoblasts inhibition and osteoclasts formation. While Xue et al.[3], Lv et al.[20] and Parhami et al.[33] all reported that the products of lipids oxidation are associated to osteoblast differentiation inhibition. Table 3 summarizes the metabolites that are associated to observed discriminations in this study, as well as indicates data from the literature that show the relation among these metabolites and the diagnosis of osteoporosis.
Table 3. Identification of metabolites in the metabonomics model responsible for discrimination among groups.
| Compound | nuclei and (δ/ppm) | ↑ group | References |
|---|---|---|---|
| Cholesterol (VLDL/LDL) | CH3 (0.88 ppm) CH2 (1.28 ppm) |
OST | [20], [25] |
| Leucine and isoleucine | γ-CH3 (0.92 ppm) | OST | [34] |
| Lactate | CH3 (1.32 ppm) | OST | [3], [32] |
| Tyrosine | β-CH2 (3.16 ppm) | Control | [30], [29] |
| Choline | CH3N (3.20 ppm) | Control | [26] [20] |
| Taurine | CH2N (3.24 ppm) | Control | [2,27,28] |
| Allantoin | CH (5.28 ppm) | OPN | [31]. |
| Unsaturated lipids | = CH (5.15–5.28 ppm) | OST | [33],.[3], [20]. |
OPN—Osteopenia; OST—Osteoporosis; ↑ higher concentration
Therefore, all formalisms employed indicated that it is possible to discriminate osteopenia from osteoporosis using serum 1H NMR spectra of patients. The best metabonomics model was built using OPLS-DA formalism which also revealed the metabolites associated to discrimination. These findings are important for clinical practice, since that is nothing change in the routine of patients is employed; the assay is minimally invasive; it is not necessary the presence of patient neither doctor during the analysis, contributing for decrease the length of stay of patients in the hospital environment and decreasing the probability of infections, for example. However, the main gain associated to introduction of 1H NMR-based metabonomics in the clinical practice will be to obtain patients’ systemic information in the first exams requested by doctors. Besides of metabonomics models to differential diagnosis of osteopenia-osteoporosis, others various metabonomics models can be built for disease different helping in the early diagnosis.
Conclusion
In this paper, three multivariate statistical tools were employed in serum 1H NMR spectra data aiming at an osteopenia-osteoporosis differential diagnosis. Principal Component Analysis discriminated healthy volunteers from osteopenia-osteoporosis patients, but didn’t discriminate osteopenia from osteoporosis. This differentiation was obtained only when supervised methods were used–Linear Discriminant Analysis and Orthogonal Partial Least Square-Discriminant Analysis. The best result was obtained using OPLS-DA which presented R2Y and Q2 values equal to 0.871 (p<0.001) and 0.415 (p<0.001), respectively. Moreover, the metabonomics strategy used identified the metabolites associated with the discrimination observed. This permits us to understand the disease evolution mechanism and make a rapid and early differential diagnosis of osteopenia and osteoporosis in postmenopausal women.
Acknowledgments
Authors thank Analytical Central Laboratory of Universidade Federal de Pernambuco by 1H NMR spectra acquisition.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior to T. A. Pontes and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (BR) (APQ-0765-1.06/14) to R. O. Silva. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. , editors. Endotext; South Dartmouth (MA): MDText.com, Inc.; 2000. Available: http://www.ncbi.nlm.nih.gov/books/NBK279134/ [Google Scholar]
- 2.Xue L, Wang Y, Liu L, Zhao L, Han T, Zhang Q, et al. A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats. Int J Mol Sci. 2011;12: 7635–7651. 10.3390/ijms12117635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentice R. Postmenopausal Hormone Therapy and the Risks of Coronary Heart Disease, Breast Cancer, and Stroke. Semin Reprod Med. 2014;32: 419–425. 10.1055/s-0034-1384624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 2009;9: 1137–1141. 10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Oden A, Johansson H, Borgström F, Ström O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44: 734–743. 10.1016/j.bone.2009.01.373 [DOI] [PubMed] [Google Scholar]
- 6.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142: 155–170. 10.1016/j.jsbmb.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22: 391–420. 10.1007/s00198-010-1501-1 [DOI] [PubMed] [Google Scholar]
- 8.Vasikaran SD, Chubb SP, Ebeling PR, Jenkins N, Jones GR, Kotowicz MA, et al. Harmonised Australian Reference Intervals for Serum PINP and CTX in Adults. Clin Biochem Rev. 2014;35: 237–242. [PMC free article] [PubMed] [Google Scholar]
- 9.Chubb SAP, Byrnes E, Manning L, Beilby JP, Ebeling PR, Vasikaran SD, et al. Reference intervals for bone turnover markers and their association with incident hip fractures in older men: the Health in Men study. J Clin Endocrinol Metab. 2015;100: 90–99. 10.1210/jc.2014-2646 [DOI] [PubMed] [Google Scholar]
- 10.Tankó LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32: 8–14. [DOI] [PubMed] [Google Scholar]
- 11.Orozco P. Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur J Epidemiol. 2004;19: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 12.Poiana C, Radoi V, Carsote M, Bilezikian JP. New Clues that May Link Osteoporosis to the Circulating Lipid Profile. Bone Res. 2013;1: 260–266. 10.4248/BR201303004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You L, Sheng Z, Tang C, Chen L, Pan L, Chen J. High cholesterol diet increases osteoporosis risk via inhibiting bone formation in rats. Acta Pharmacol Sin. 2011;32: 1498–1504. 10.1038/aps.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv H, Jiang F, Guan D, Lu C, Guo B, Chan C, et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int J Mol Sci. 2016;17 10.3390/ijms17122018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao H, Lu Q, Liu X, Cong H, Zhao L, Wang H, et al. Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 2009;100: 782–785. 10.1111/j.1349-7006.2009.01086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindon JC, Holmes E, Nicholson JK. So what’s the deal with metabonomics? Anal Chem. 2003;75: 384A–391A. [DOI] [PubMed] [Google Scholar]
- 17.Duarte IF, Gil AM. Metabolic signatures of cancer unveiled by NMR spectroscopy of human biofluids. Prog Nucl Magn Reson Spectrosc. 2012;62: 51–74. 10.1016/j.pnmrs.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 18.Qiao Y, Zhang L, He S, Wen H, Yu Y-M, Cao C-H, et al. Plasma metabonomics study of first-Episode schizophrenia treated with olanzapine in female patients. Neurosci Lett. 2016;617: 270–276. 10.1016/j.neulet.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 19.Gouveia LR, Santos JC, Silva RD, Batista AD, Domingues ALC, Lopes EP de A, et al. Diagnosis of coinfection by schistosomiasis and viral hepatitis B or C using 1H NMR-based metabonomics. Hsieh MH, editor. PLOS ONE. 2017;12: e0182196 10.1371/journal.pone.0182196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batista AD, Barros CJP, Costa TBBC, de Godoy MMG, Silva RD, Santos JC, et al. Proton nuclear magnetic resonance-based metabonomic models for non-invasive diagnosis of liver fibrosis in chronic hepatitis C: Optimizing the classification of intermediate fibrosis. World J Hepatol. 2018;10: 105–115. 10.4254/wjh.v10.i1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godoy MMG, Lopes EPA, Silva RO, Hallwass F, Koury LCA, Moura IM, et al. Hepatitis C virus infection diagnosis using metabonomics: Hepatitis C diagnosis using metabonomics. J Viral Hepat. 2010;17: 854–858. 10.1111/j.1365-2893.2009.01252.x [DOI] [PubMed] [Google Scholar]
- 22.Schicho R, Shaykhutdinov R, Ngo J, Nazyrova A, Schneider C, Panaccione R, et al. Quantitative Metabolomic Profiling of Serum, Plasma, and Urine by 1 H NMR Spectroscopy Discriminates between Patients with Inflammatory Bowel Disease and Healthy Individuals. J Proteome Res. 2012;11: 3344–3357. 10.1021/pr300139q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckonert O, Keun HC, Ebbels TMD, Bundy J, Holmes E, Lindon JC, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2: 2692–2703. 10.1038/nprot.2007.376 [DOI] [PubMed] [Google Scholar]
- 24.IOF. Osteoporosis & Musculoskeletal Disorders [Internet]. 2018. Available: https://www.iofbonehealth.org/osteoporosis
- 25.Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol. 2001;21: 1636–1641. [DOI] [PubMed] [Google Scholar]
- 26.Long W-F, Li L, Chen H-Q, Tang Y, He X-L, Jing R-Z. [1H-NMR-based metabonomics analysis of plasma from osteoporotic rats induced by ovariectomy]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40: 843–847. [PubMed] [Google Scholar]
- 27.Hanaa HA, Amal HH. Potential Role of Arginine, Glutamine and Taurine in Ameliorating Osteoporotic Biomarkers in Ovariectomized Rats. Rep Opin. 2009;1 10.7537/marsroj010609.05 [DOI] [Google Scholar]
- 28.Liu Y-R, Huang R-Q, Xiao B-K, Yang J-Y, Dong J-X. 1H NMR metabolic profiling analysis offers evaluation of Nilestriol treatment in ovariectomised rats. Mol Cell Endocrinol. 2014;387: 19–34. 10.1016/j.mce.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 29.Varga F, Rumpler M, Luegmayr E, Fratzl-Zelman N, Glantschnig H, Klaushofer K. Triiodothyronine, a regulator of osteoblastic differentiation: depression of histone H4, attenuation of c-fos/c-jun, and induction of osteocalcin expression. Calcif Tissue Int. 1997;61: 404–411. [DOI] [PubMed] [Google Scholar]
- 30.da Silva RA, de Camargo Andrade AF, da Silva Feltran G, Fernandes CJ da C, de Assis RIF, Ferreira MR, et al. The role of triiodothyronine hormone and mechanically-stressed endothelial cell paracrine signalling synergism in gene reprogramming during hBMSC-stimulated osteogenic phenotype in vitro. Mol Cell Endocrinol. 2018;478: 151–167. 10.1016/j.mce.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 31.Chen S-Y, Yu H-T, Kao J-P, Yang C-C, Chiang S-S, Mishchuk DO, et al. An NMR metabolomic study on the effect of alendronate in ovariectomized mice. PloS One. 2014;9: e106559 10.1371/journal.pone.0106559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon SJ, Sims SM. P2 purinergic receptors on osteoblasts and osteoclasts: Potential targets for drug development. Drug Dev Res. 2000;49: 187–200. [DOI] [Google Scholar]
- 33.Parhami F, Garfinkel A, Demer LL. Role of Lipids in Osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20: 2346–2348. 10.1161/01.ATV.20.11.2346 [DOI] [PubMed] [Google Scholar]
- 34.Ma B, Liu J, Zhang Q, Ying H, A J, Sun J, et al. Metabolomic Profiles Delineate Signature Metabolic Shifts during Estrogen Deficiency-Induced Bone Loss in Rat by GC-TOF/MS. Chowen JA, editor. PLoS ONE. 2013;8: e54965 10.1371/journal.pone.0054965 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.