Abstract
We have described an Igtransgenic, autoreactive B cell clonotype that undergoes a novel tolerance pathway. Early in development this clonotype expresses average BCR levels, but these levels are progressively down-regulated as development proceeds efficiently to the mature, follicular compartment. This clonotype does not display conventional features of anergy and can be induced to undergo apoptosis and receptor editing in in vitro bone marrow cultures, but these pathways are not taken in vivo. These data suggested that autoantigen-driven down-regulation of BCR levels and, hence, avidity for autoantigen allows this clonotype to bypass conventional tolerance mechanisms. To test this idea, we enforced elevated levels of expression of BCR in this clonotype by making the transgenic Igh locus homozygous. This resulted in retarded clonotype development and L chain receptor editing in vivo. These data support a pivotal role for adaptive, autoantigen-induced adjustment of BCR expression levels in the regulation of primary B cell development and tolerance.
Three mechanisms of B lymphocyte tolerance induction have been extensively described: clonal deletion, receptor editing, and anergy (1–5). These result in either physical or functional elimination of autoreactivity from the mature B cell population. This is consistent with the forbidden clone corollary to the clonal selection hypothesis (6). However, removal of all auto-reactive B cells from the functional pool would severely limit the size of the anti-foreign Ag repertoire, as BCRs cannot be mono-specific (7, 8). In fact, many mature B cells display “multi” or “polyreactivity” (9–11), including autospecificities (11, 12). Also, a subset of autoreactive B cells that enter the periphery may not encounter sufficient quantities of their cognate autoantigen to induce tolerance pathways. Such B cells are said to be “ignorant” of or “indifferent” to self-Ags (13, 14). Nonetheless, some self-Ags clearly promote the positive selection of autoreactive B cells into the mature pool (15–18) and these B cells can serve useful functions (19). Collectively, these observations suggest that developing B cells expressing only certain types of autospecificity are subjected to physical or functional removal from the mature B cell compartment. The parameters that distinguish B cell-autoantigen interactions resulting in clonal deletion, receptor editing, and an-ergy vs complete and unimpeded developmental progression remain poorly understood.
Using a line of gene-targeted mice in which an Ab H chain variable (VH) region gene is inserted into the endogenous H chain locus, we recently discovered a novel fate available to developing autoreactive B cells that we term “learned ignorance” (20, 21). The VH knockin locus in these mice, termed HKIR, in combination with a single, unmutated, κ L chain gene (Vκ10A-Jκ1), encodes Abs termed “canonical” that have avidity for DNA-based autoantigens in in vitro assays, as well as affinity for the hapten p-azo-phenylarsonate (Ars)5 (20, 22). B cells expressing canonical HKIR BCRs develop to a mature follicular (FO) phenotype, are localized in follicles, and are not short-lived (21). Moreover, these B cells do not display features of anergy in vitro (23) and can respond to Ars immunizing by entering germinal centers in vivo (24). However, these B cells express levels of surface (s)IgM and sIgD that are 10-fold lower than the average levels in the FO B cell compartment (20, 21), suggesting that canonical HKIR B cells are not ignorant of self-Ag(s). Indeed, using an in vitro bone marrow (BM) culture system, we showed that modulation of canonical BCR levels on immature HKIR B cells is regulated via an adaptive feedback loop in which BCR engagement by a DNA-based autoantigen(s) is linked to transcriptional control of BCR-encoding loci (25). Although HKIR B cells in these cultures could be induced to undergo clonal deletion and receptor editing by anti-BCR Abs, engagement of the canonical HKIR BCR by cognate autoantigen(s) present in the cultures results only in BCR down-regulation (25).
Taken together, these results predicted that autoantigen engagement of the BCR on canonical HKIR B cells during their early developmental stages in vivo induced BCR down-regulation, resulting in a level of cellular avidity for this autoantigen(s) insufficient to subsequently trigger conventional tolerance pathways. Since we deemed testing this hypothesis via varying concentrations of endogenous autoantigens in vivo very difficult, we chose to attempt to alter levels of HKIR BCR expression instead. To this end, we bred the HKIR VH knockin locus to homozygosity. Strikingly, canonical B cell development is retarded in HKIR homozygous mice and they have a distinct peripheral compartment of B cells that have undergone L chain receptor editing. Thus, inhibition of the learned ignorance pathway leads to activation of alternative central tolerance mechanisms. These results strongly support the hypothesis that adaptive down-regulation of BCR levels by autoantigen(s) of the type recognized by canonical B cells must result in reduction in the avidity of the B cell-autoantigen interaction below a certain threshold if retarded developmental progression and receptor editing are to be avoided.
Materials and Methods
Mice
The HKIR VH knockin mice, Vκ10-Jκ1-transgenic mice, and double-transgenic HKIR/Vκ10 mice were previously described (20, 25). Mice expressing homozygous and heterozygous knockin H chain loci were genotyped by PCR for both the endogenous JH3 to JH4 region (absent in the HKIR line) and the knockin VDJ gene region. C57BL/6 (CD45.2+) and C57BL/6.SJL (CD45.1+) mice were purchased from The Jackson Laboratory. Mice were housed under specific pathogen-free conditions and given autoclaved food and water. All mice were 8–12 wk of age at the time of initiation of the experiments. The use of mice in these studies was conducted in compliance with institute guidelines and all protocols using animals were approved by the Institutional Animal Care and Use Committee.
Flow cytometry and cell sorting
Single-cell suspensions were prepared from lymphoid organs of 8- to 12-wk-old mice. Cells were stained with different combinations of the following Abs: anti-IgM (Jackson ImmunoResearch Laboratories), anti-IgD (11–26; Southern Biotechnology Associates), anti-κ (187.1; Southern Biotechnology Associates), anti-λ and anti-κ (goat anti-mouse PE and FITC; Southern Biotechnology Associates), anti-CD1d-PE (1B1), anti-CD3 (145–2C11), anti-CD21/35 (7G6), anti-CD22.2 (Cy34.1), anti-CD23 (B3B4), anti-CD45R (RA3–6B2; eBioscience), anti-CD45.2 (clone 104), anti-C1qRp (AA4.1; eBioscience), or anti-idiotypic mAb E4 (prepared in-house). In some experiments monovalent Fab of anti-IgM (Jackson ImmunoResearch Laboratories,) were used for flow cytometric analysis of surface IgM levels. All Abs were obtained from BD Pharmingen unless otherwise indicated. Streptavidin-CyChrome (BD Pharmingen) was used to detect biotinylated Abs. Peanut agglutinin-FITC was from Vector Laboratories. Cells were assayed on an EPICS Elite flow cytometer (Coulter) and data were analyzed using FlowJo software (Tree Star). In some experiments, B cell subpopulations were stained and purified using a MoFlo high-performance cell sorter (DakoCytomation).
BM cultures
The S17 stromal cell line and IL-7 were used to generate BM cultures as previously described (25). Medium was supplemented with 16 ng/ml recombinant mouse IL-7 (R&D Systems). To block autoantigen binding to the BCR, monomeric p-azophenylarsonate (Ars)-L-tyrosine was added to the cultures at 0.1 mM for at least 12 h as described before (25). The hapten p-aminobenzoic acid-L-tyrosine, for which canonical Abs have no measurable affinity, was used as a control.
Immunohistochemistry and immunofluorescence
Spleens from 8- to 12-wk-old naive mice were frozen and cryosections were prepared and processed as previously described (26). Sections were stained with combinations of various mAbs (described above), as well as MOMA-1-FITC (SeroTech), analyzed on a fluorescence microscope, and digital images were captured with a confocal laser-scanning microscope (Zeiss 510 Meta).
Hybridomas and V region nucleotide sequencing
FACS purified B220+λ+ splenocytes from both HKIR+/+/Vκ10 and HKIR+/+ mice were stimulated with LPS (20 μg/ml) and IL-4 (50 ng/ml) for 3 days. Hybridomas were constructed using the SP2/0 fusion partner as previously described (27). Hybridomas were screened for λ+, κ+, and E4+ Ab production by ELISA. Hybridomas were subcloned by limiting dilution and supernatants from IgM-secreting hybridomas were collected and used for ELISA and anti-nuclear Ab (ANA) staining. V region RT-PCR and nucleotide sequencing of PCR products were conducted as described previously (20).
Autoreconstitution
Adult mice were exposed to a sublethal dose of whole-body gamma irradiation (550 rad) as described before (26) and were allowed to rest for 4 wk. Cells were then obtained from lymphoid organs, labeled, and analyzed by flow cytometry as described above.
Adoptive transfer and immunization
After FACS purification of λ+ and λ− splenic B cells from 8- to 12-wk-old mice, 0.5 × 106 cells of each population were injected into the tail vein of syngeneic B6.CD45.1 recipients that were immunized (i.p.) 12 h later with 100 µg of Ars-keyhole limpet hemocyanin (KLH) in alum. Germinal center responses were analyzed on day 6 as described before (24, 28).
ELISA
Total and dsDNA-binding IgM was measured by ELISA on 96-well plates (Immulon-4; Dynatech Laboratories) as previously described (22). Bound Abs were also elaborated using biotin-conjugated goat anti-mouse λ Ab or alkaline phosphatase-conjugated goat anti-mouse κ Ab (Southern Biotech-nology Associates).
ANA staining
Slides coated with human epithelioid Hep-2 cells (Antibodies) were used to determine the ANA reactivity of hybridoma supernatants according to the manufacturer’s instructions and as previously described (20). Fluorescein-labeled (FITC) affinity pure donkey anti-mouse IgM Ab, μ-chain specific (Jackson ImmunoResearch Laboratories), was used to detect the bound Abs.
Results
Increased expression of the canonical BCR in HKIR homozygous mice
We intercrossed the HKIR line to generate mice expressing homozygous H chain knockin loci. For many experiments, these mice were then crossed to a line of conventional Vκ10A-Jκ1-transgenic mice, resulting in mice homozygous or hemizygous for the HKIR knockin Igh locus and hemizygous for the canonical Vκ10A-Jκ1-transgenic L chain locus. The former mice are termed HKIR+/+/Vκ10 and the latter mice HKIR+/−/Vκ10.
To compare levels of canonical BCR expression on developing HKIR+/+/Vκ10 B cells under conditions when this BCR was engaging autoantigen(s) and when it was not, we used monomeric Arstyrosine to block canonical BCR-autoantigen interactions in BM cultures as described before (25). After 5 days, sIgM levels on immature B cells arising in HKIR+/−/Vκ10 BM cultures containing Arstyrosine were on average 1.7-fold higher than those on B cells in analogous HKIR+/−/Vκ10 cultures (Fig. 1A and legend). In addition, sIgM levels on both types of B cells in these cultures were 10-fold elevated as compared with the levels observed in cultures containing p-aminobenzoic acid-tyrosine, a control hapten for which canonical Abs have no measurable affinity (Fig. 1A). These levels approached those on nontransgenic control B cells. Also, both types of B cells down-regulated BCRs to comparable levels when autoantigen binding was not blocked (Fig. 1A).
FIGURE 1.
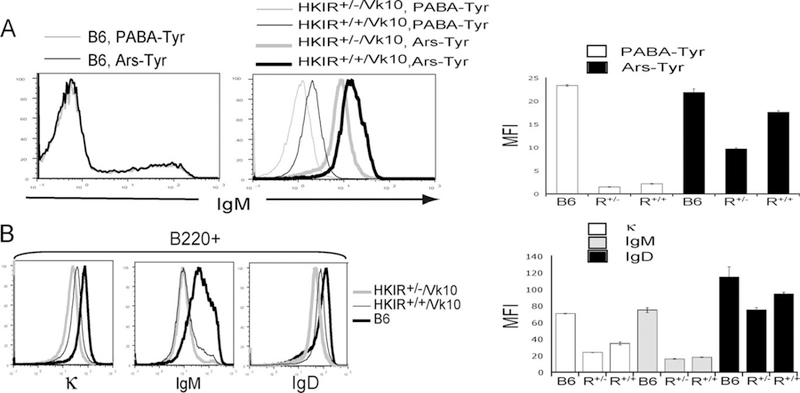
Surface BCR levels on B cells in HKIR+/+/Vκ10 mice and in BM cultures. A, Immature B cells from day 5 cultures of HKIR+/+/Vκ10 BM (R+/+), HKIR+/−/Vκ10 BM (R+/−), or C57BL/6 (B6) BM containing either Ars-Tyr or p-aminobenzoic acid (PABA)-Tyr (0.1 mM) were analyzed for sIgM levels via flow cytometry. The right panel shows the mean fluorescence intensity (MFI) of sIgM levels on sIgM cells illustrated in the left panels. These data were obtained using a fluorochrome-labeled intact allotype-nonspecific anti-IgM reagent and indicated that HKIR+/+/Vκ10 B cells in BM cultures containing Ars-Tyr express 1.5–1.7-fold more sBCRs than HKIR+/−/Vκ10 B cells in analogous cultures. When the analysis was done with a Fab anti-IgM reagent, this value was 1.8-fold (data not shown). B, Splenic B220 cells from the indicated mice were analyzed for surface levels of BCR via flow cytometry using allotype-nonspecific anti-κ, anti-IgM, and anti-IgD Abs. Overlays of staining intensity on splenic B cells from these mice are shown. The right panel shows the mean fluorescence intensities of the staining patterns illustrated in the left panel. Figures are representative of data obtained from at least four mice of each genotype in multiple experiments.
Analysis of the peripheral B cell compartment of HKIR+/+/Vκ10 mice revealed that the majority of canonical B cells, as detected by the anti-clonotypic mAb E4, were indistinguishable in their FO locale in the spleens and lymph nodes as compared with HKIR+/−/Vκ10 mice (data not shown). However, although sIgM levels on splenic B cells were only slightly higher, sIgD and total sBCR (sIgκ) levels were ~1.5-fold higher in HKIR+/+/Vκ10 mice as compared with HKIR+/−/Vκ10 mice (Fig. 1B). These data indicate that most peripheral B cells in HKIR+/+/Vκ10 mice have not down-regulated surface BCR to levels characteristic of HKIR+/−/Vκ10 mice. Because we do not know whether either type of B cell expresses normal levels of sIgD at some point during primary development, an alternative explanation for these observations is that developing HKIR+/−/Vκ10 and HKIR+/+/Vκ10 B cells fail to up-regulate IgD expression, but levels of sIgD are higher on HKIR+/+/Vκ10 B cells since they express two copies of the transgenic Igh locus.
Retarded B cell development and λ+ B cells in autoreconstituting HKIR+/+/Vκ10 mice
To determine whether the elevated levels of autoreactive BCR expression in HKIR+/+/Vκ10 mice influenced the kinetics of B cell developmental progression, we used an autoreconstitution strategy. Four weeks after sublethal irradiation, the percentages of B220+, sIgM+sIgD+, and B220+ AA4.1+ splenic lymphocytes were reduced ~2-fold in HKIR+/+/Vκ10 as compared with HKIR+/−/Vκ10 mice. Also, lower percentages of CD23+ splenic B cells were observed in autoreconstituting HKIR+/−/Vκ10, as compared with HKIR+/−/Vκ10 mice (Fig. 2A). The absolute num-bers of both immature (AA4.1+) B cells and mature B cells in the spleens of autoreconstituting HKIR+/+/Vκ10 mice were substantially lower than in HKIR+/−/Vκ10 mice (Fig. 2B). All of these observations are consistent with slowed B cell developmental progression in HKIR+/+/Vκ10 mice. Surprisingly, λ+ B cells were also detected in autoreconstituting HKIR+/+/Vκ10 mice (Fig. 2C), an indication that L chain receptor editing was taking place (4). These λ+ cells could not have been derived from fetal precursors, indicating that the processes that produce them take place during BM B cell development.
FIGURE 2.
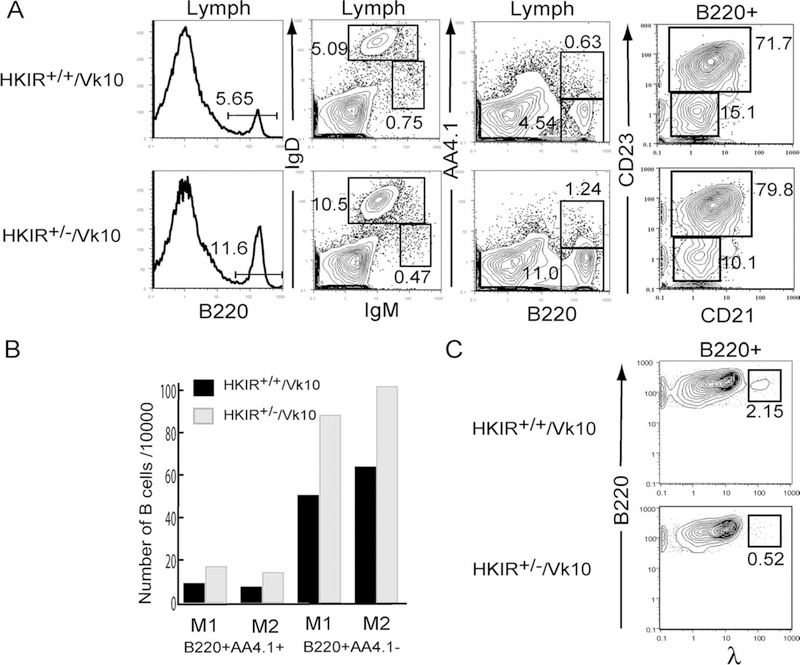
Retarded B cell autoreconstitution in HKIR+/+/Vκ10 mice. Mice of the indicated genotypes were given 550 rad whole-body irradiation, allowed to autoreconstitute for 4 wk, and then spleen cells were isolated, stained with mAbs specific for the indicated markers, and analyzed by flow cytometry. A, Percentages of B cells in these mice in the indicated gates are shown. B, The absolute number of mature and transitional B cells in each spleen sample were calculated from the data shown in A. C, Percentages of B220+λ+ B cells in the spleens of the indicated types of mice are shown. Data in A and C are representative of those obtained from two mice of each genotype. All of these data are illustrated in B. The data in A were used to generate the values illustrated in B.
Reduction of T1 and T2 transitional B cells in HKIR+/+/Vκ10 mice
To determine whether the efficiency of B cell development was also abnormal in unmanipulated HKIR+/+/Vκ10 mice, we next examined the BM and splenic transitional B cell compartments in these mice via flow cytometry. Comparison of the various BM B cell developmental subsets in HKIR+/+/Vκ10 and HKIR+/−/Vκ10 mice did not reveal any obvious qualitative or quantitative differences (data not shown). However, analyses of splenic B cells showed that the overall percentage of AA4.1+ transitional B cells was lower in HKIR+/+/Vκ10 mice as compared with HKIR+/−/Vκ10 mice (Fig. 3, left panels). This was specifically due to a reduction in the representation of sIgMhigh T1/T2 stage B cells (Fig. 3, middle and right panels). As such, the data presented in Figs. 2 and 3 suggest that the early stages of developmental progression in the periphery are slowed in the HKIR+/+/Vκ10 B cells. Whether this is accompanied by clonal deletion and can account for the elevated percentage of T3 transitional B cells in the spleens of these mice will require further investigation.
FIGURE 3.
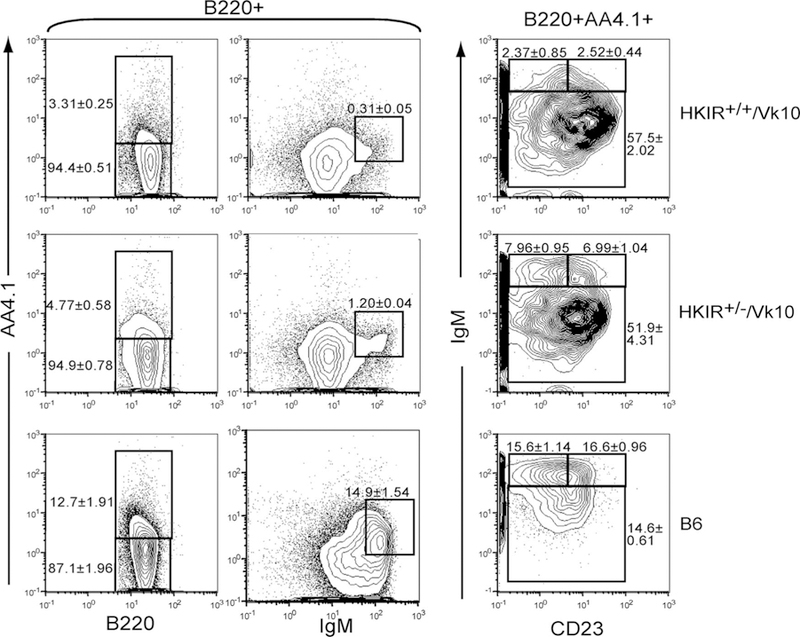
Reduction of T1 and T2 transitional B cells in HKIR+/+/Vκ10 mice. Splenic B cells from mice of the indicated genotypes were stained with Abs specific for the indicated markers and analyzed by four- color flow cytometry. The percentage of cells in each fraction are indicated next to the gates. The data are representative of at least three mice per experiment and multiple experiments.
Elevated frequency of λ+ B cells in the spleens of HKIR+/+/Vκ10 and HKIR+/+ mice
Due to enforced expression of the transgenic Vκ10 gene, HKIR+/−/Vκ10 mice contain very few λ L chain-expressing B cells (Ref. 25 and see below). In contrast, Fig. 2A shows that there is a distinct subpopulation of λ+ B cells in the spleens of autoreconstituting HKIR+/+/Vκ10 mice. To determine whether this subpopulation was present in unmanipulated HKIR+/+/Vκ10 mice, we performed flow cytometric analysis on spleen cells from these mice as well as HKIR+/−/Vκ10, HKIR+/−, and HKIR+/− mice. Fig. 4 shows that HKIR+/+/Vκ10 mice indeed contain a distinct subpopulation of λ+ cells, comprising 1% of splenocytes that is essentially absent in HKIR+/−/Vκ10 mice. Interestingly, HKIR+/+ mice also contain levels of λ+ cells similar to HKIR+/+/Vκ10 mice. HKIR+/− mice also contain a subpopulation of λ+ cells that is less prevalent than that observed in HKIR+/+ mice. However, further studies showed that these largely express an endogenous H chain rather than the HKIR H chain (data not shown).
FIGURE 4.
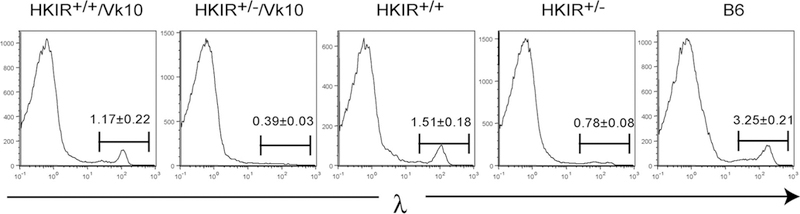
HKIR+/+/Vκ10 and HKIR+/+ mice contain elevated levels of λ-expressing B cells. Spleen cells from mice of the indicated genotypes were stained with anti-λ Abs and analyzed by flow cytometry. Gates are set on the λ+ subpopulations and the percentages of these cells of total splenocytes are indicated. The few λ+ B cells present in HKIR+/− mice mainly express the nontransgenic Igh (IgMb) allele (data not shown). The data were derived from at least three mice of each genotype.
L chain editing and isotypic inclusion in l B cells in HKIR+/+ mice
As mentioned above, the appearance of λ+ B cells in mice expressing an Igκ transgene is an indication of L chain receptor editing (4). Indeed, previous studies showed that κ+, λ+ dual L chain-expressing B cells present in the marginal zone (MZ) in another line of IgH antinuclear Ag transgenic mice usually are the product of receptor editing and L chain isotypic inclusion (29). Therefore, we costained splenic B cells in the various transgenic lines with Abs to κ and λ L chains. Whereas good L chain isotypic exclusion was seen in HKIR+/−/Vκ10 and HKIR+/− B cells (data not shown), nearly all λ+ B cells in HKIR+/+/Vκ10 mice stained at low intensity with anti-κ as well (Fig. 5, upper left panel). The isotypically included κ-chain on these cells is very likely the canonical Vκ10-Jκ1, since λ+ HKIR+/+/Vκ10 B cells also showed low levels of clono-type-specific E4 staining (Fig. 5, lower left panel).
FIGURE 5.
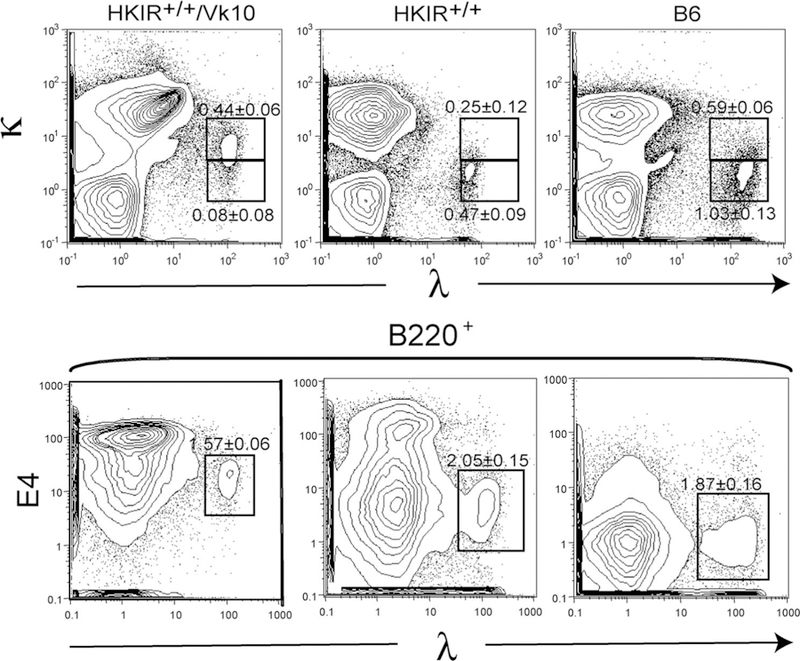
L chain isotypic inclusion in λ+ B cells in HKIR+/+/Vκ10 mice. Spleen cells from mice of the indicated genotypes were stained with anti-κ and anti-λ Abs (upper panels) and anti-B220, anti-λ and E4 Abs (lower panels), and analyzed by flow cytometry. Major subpopulations of λ+ B cells are gated. All data are representative of multiple experiments using at least three mice per genotype.
In contrast, the λ+ B cells in HKIR+/+ mice do not stain with anti-κ and are E4− (Fig. 5, middle panels). Interestingly, the λ+ subset in HKIR+/+/Vκ10 mice give rise to E4 staining intensities higher than “bulk” E4+ cells when costained with anti-λ-PE and E4-R670 (data not shown), but lower than bulk E4+ B cells when costained with anti-λ-FITC and E4-R670 (Fig. 5). This difference is likely due to fluorescence energy transfer between PE and R670 when these two fluorochromes are in close juxtaposition, as would be the case when anti-λ-PE and E4-R670 were staining the same BCR. Taken together with the data presented in Figs. 2 and 4, these results suggest that a subset of developing B cells in both HKIR+/+/Vκ10 and HKIR+/+ mice undergo L chain receptor editing, resulting in expression of high levels of λ-chain containing BCRs on their mature progeny.
λ-expressing B cells in HKIR+/+/Vκ10 mice have an unusual cell surface phenotype and reside largely in the MZ
Canonical B cells in HKIR+/− and HKIR+/−/Vκ10 mice predominantly develop to FO phenotype and locale (20, 21, 25). To test whether the λ-expressing B cells in HKIR+/+ mice developed to FO phenotype, we first costained splenic cells with Abs to λ L chains as well as IgM and IgD. As shown in Fig. 6A, left panel, λ+ B cells in HKIR+/+/Vκ10 mice are largely IgMhighIgDhigh. In contrast, the λ+ B cells in HKIR+/+ mice are mainly IgMlowIgDhigh (Fig. 6A, middle panel), as expected of FO B cells.
FIGURE 6.
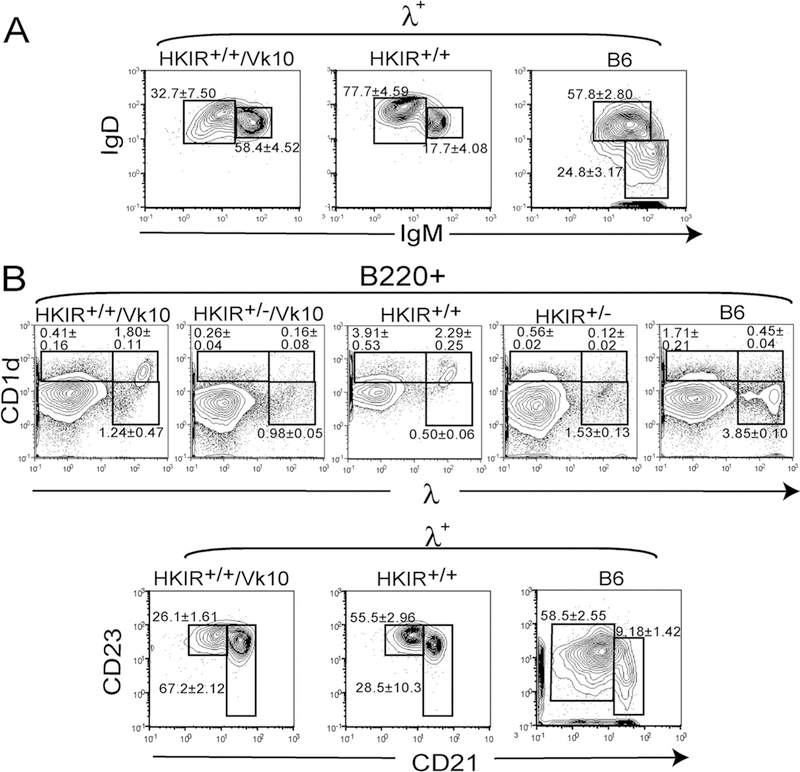
The λ-κ double-positive B cells in HKIR+/+ mice have MZ-like phenotypes. A, Spleen cells from mice of the indicated genotypes were stained with an anti-λ Ab as well as Abs specific for IgM and IgD and analyzed by flow cytometry. Panels show data obtained for cells gated on λ staining. Gates are set around areas expected to contain FO (left) or MZ (right) B cells, although the gates set for HKIR+/+/Vκ10 and HKIR+/+ cells do not exactly correspond to those in the B6 panel due to the unusual BCR expression levels of the former B cells. B, Upper panels, spleen cells from mice of the indicated genotypes were stained with Abs specific for B220, CD1d, and λ and analyzed by flow cytometry. Gates are set around areas expected to contain largely MZ (upper gates) or FO (lower gate) B cells. B, Lower panels, splenic B cells from the indicated mice were stained with an anti-λ Ab as well as Abs specific for CD21 and CD23 and analyzed by flow cytometry. Panels show data obtained for cells gated on λ staining. Gates are set around areas expected to contain FO (left) or MZ (right) B cells, although the MZ-like cells in HKIR+/+/Vκ10 and HKIR+/+ mice express higher levels of CD23 than those in B6 mice. All data are representative of those obtained from at least two experiments using three or more mice per experiment.
Given these results, we further analyzed these λ-expressing sub-populations with markers routinely used to distinguish FO from MZ B cells. Fig. 6B shows that the λ+ subpopulation in HKIR+/+/Vκ10 mice is CD21high and CD1dhigh as expected of MZ B cells, but expresses higher levels of CD23 than most MZ B cells in B6 mice. The λ+ subpopulation in HKIR+/+ mice revealed a phenotype intermediate between FO and MZ B cells in B6 mice. It is CD1dhigh and bimodal with respect to CD21 and CD23 expression, indicating it is composed of both FO and MZ-like B cells.
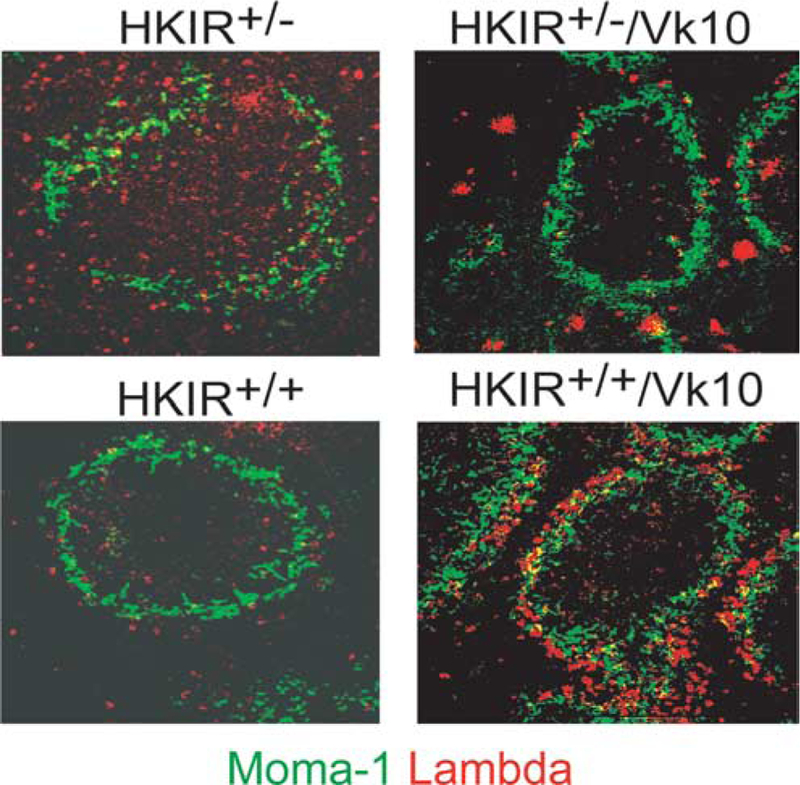
To expand on these flow cytometric studies, immunohistology was performed on the spleens of HKIR+/+/Vκ10, HKIR+/−/Vκ10, HKIR+/+ and HKIR+/− mice. Fig. 7 shows that the few λ+ cells observed in white pulp and MZ regions in HKIR+/− and HKIR+/−/Vκ10 spleens are located in both of these areas (the inner perimeter of the MZ is defined by MOMA-1 (green) staining). A substantially elevated frequency of λ+ cells is seen in HKIR+/+ spleens, but as in the other mice these are found in both white pulp and MZ regions. HKIR+/+/Vκ10 spleens also contain substantially elevated frequencies of λ+ B cells, but these are predominately located outside of or overlapping the MOMA-1 met-allophile “ring” in the MZ.
FIGURE 7.
Most λ+ splenic B cells in HKIR+/+/Vκ10 mice reside in the MZ. Spleen sections from mice of the indicated genotypes were stained with MOMA-1-FITC and anti-λ-PE and digital images were captured by immunofluorescence microscopy. MOMA-1 staining defines the border of the B cell follicle and the MZ. Original magnification, × 200. The intensely staining λ+ cells in HKIR+/−/Vκ10 spleens was reproducibly observed and appears to be plasma cells located in the red pulp. We currently have no explanation for the presence of these cells in this mouse line. The data are representative of those obtained from at least three mice per genotype.
HKIR+/+, λ+, and λ− B cells respond to Ars immunization in vivo
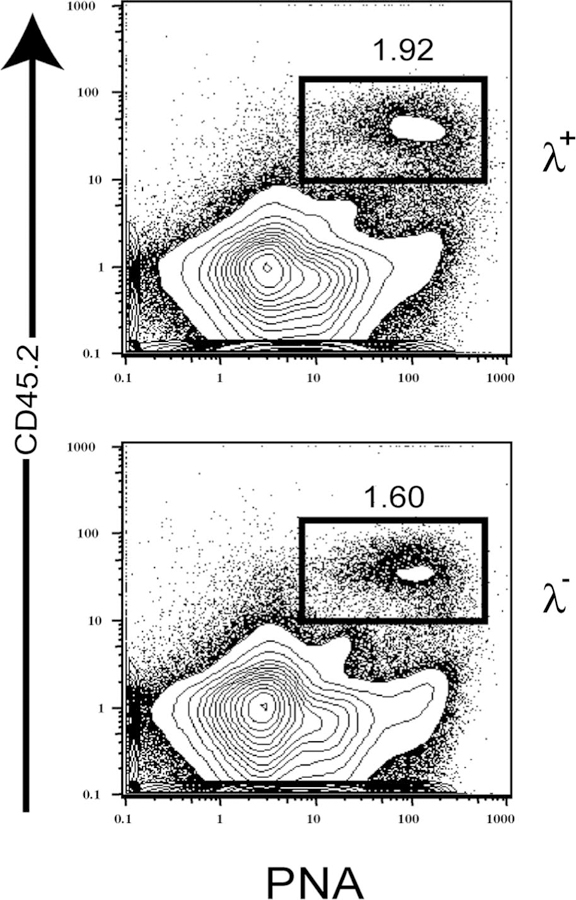
The data above show that λ+ B cells in HKIR+/+/Vκ10 mice express canonical H and Vκ10 L chains as well as a λ L chain, and they have a MZ-like phenotype and locale. To test whether these cells had been rendered anergic during their development, we isolated λ B cells and λ B cells by FACS from HKIR+/+/Vκ10 mice and transferred them into syngeneic C57BL/6.CD45.1 recipients that were immunized 12 h later with Ars-KLH. Similar numbers of CD45.2 donor B cells entered the peanut agglutinin-positive germinal center compartment in both cases (Fig. 8). These data demonstrate that HKIR+/+/Vκ10/λ+ B cells are responsive to immunization in vivo and, thus, cannot be considered anergic by conventional standards.
FIGURE 8.
λ+ B cells from HKIR+/+/Vκ10 mice respond to Ars-KLH immunization in vivo. FACS-purified λ+ and λ− splenic B cells from HKIR+/+/Vκ10 mice were transferred to recipient C57BL/6.CD45.1 mice that were immunized (i.p.) 12 h later with 100 μg of Ars-KLH in alum. Mice were sacrificed on day 6 after transfer and immunization, and splenocytes were stained with Abs specific for the indicated markers and analyzed by three-color flow cytometry. Gated percentages represent peanut agglutinin-positive (PNA+), CD45.2+ GC B cells. The data are representative of two experiments.
λ L chain-containing HKIR-encoded Abs display reduced antinuclear activity
The receptor editing hypothesis proposes that secondary L (and perhaps H) chain V(D)J rearrangements induced by autoantigen-BCR interactions take place until a new BCR is expressed that is less autoreactive (2). To evaluate whether this prediction was fulfilled by λ B cells in HKIR+/+/Vκ10 and HKIR+/+ mice, hybridomas were produced from the splenic B cells of these mice after polyclonal activation in vitro. As expected from the flow cytometric studies illustrated in Fig. 5, the majority of λ+ hybridomas derived from HKIR+/+/Vκ10 B cells expressed mAbs containing both κ and λ L chains and were E4+, indicating that the expressed κ L chain was derived from the Vκ10-Jκ1 transgene. In contrast, all of the λ hybridomas from HKIR+/+ mice expressed only λ L chains and were E4−. HKIR+/−/Vκ10 hybridomas had not lost expression of the canonical VH gene, since all were positive by ELISA for expression of several VH-dependent idiotypes in addition to E4, and two of these assayed were positive for expression of HKIR RNA as assessed by RT-PCR and nucleotide sequencing (data not shown). Although the mAbs produced by the HKIR+/−, λ+ hybridomas did not express detectable levels of VH-dependent idiotypes, two of these were shown to express an intact HKIR locus via RT-PCR and nucleotide sequencing (data not shown).
Because the autoantigen(s) with which canonical BCRs interact in vivo has not been biochemically defined, the resulting hybridomas were tested for general levels of autoreactivity using whole cell staining (ANA) assays. Fig. 9 shows that although all of the mAbs produced by representative hybridomas stained elements in the cytoplasm, the nuclear staining intensity of λ+ mAbs from HKIR+/+/Vκ10 mice was less than the levels obtained with mAbs obtained from HKIR+/−/Vκ10 mice, while such staining with λ+ mAbs obtained from HKIR+/− mice appeared to be even lower.
FIGURE 9.
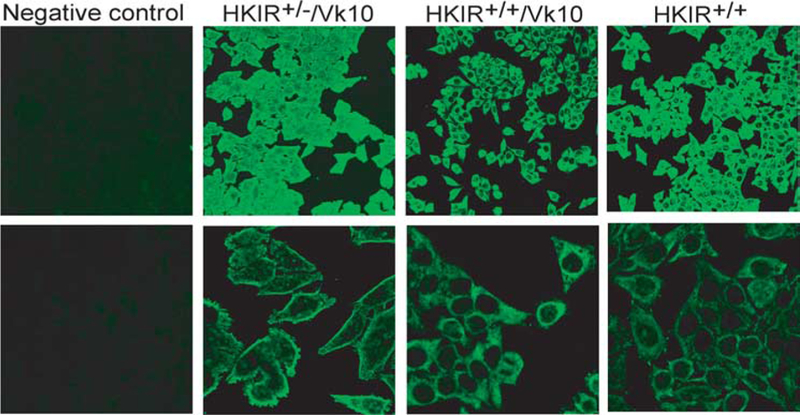
λ L chain-containing mAbs from HKIR+/+/Vκ10 and HKIR+/+ mice have little nuclear staining activity. Slides coated with fixed Hep-2 cells were stained with mAbs produced by IgM, λ-expressing hybridomas generated from the indicated mice and counterstained with a FITC-labeled anti-IgM Ab. Digital images were captured on a fluorescence microscope. Dilutions of culture supernatants containing equivalent amounts of IgM (determined by ELISA) from each hybridoma were used. Upper panels, × 200 original magnification; lower panels, × 630 original magnification. The staining patterns are representative of all IgM-λ-expressing hybridomas isolated from the indicated strains of mice.
Discussion
The data we present here show that when the learned ignorance pathway is inhibited in developing canonical clonotypes in vivo, B cell development is retarded and receptor editing is induced. These data illustrate the pivotal influence of adaptive regulation of BCR expression levels on the pathway(s) of development and tolerance taken by an immature B cell. They also add further weight to the conclusion that BCR-autoantigen interactions of the same specificity can lead to induction of different central tolerance mechanisms in vivo, depending on the avidity of these interactions (1).
Interestingly, BCR-transgenic B cells expressing another version of the canonical BCR, in which the H chain is encoded by a conventional transgenic locus and lacks the position 55 R mutation and the L chain contains numerous amino acid substitutions as compared with the Vκ10 gene (termed Ars/A1), appear not to undergo adaptive BCR down-regulation and become anergic (30). Although differences in the autoantigen specificities of Ars/A1 and HKIR/Vκ10 BCRs may contribute to the distinct fates of B cells expressing these two types of Ag receptors, we speculate that the inability of conventional transgenic H chain loci to undergo adaptive, autoantigen-driven down-regulation of expression of IgM and IgD in the early stages of B cell development may also be a factor in this regard.
Enforced expression of two copies of the canonical HKIR H chain locus resulted in increased expression levels of the canonical BCR on immature HKIR+/+/Vκ10 B cells developing in vitro under conditions where autoantigen engagement was blocked, as compared with HKIR+/−/Vκ10 B cells. This was also the case for peripheral HKIR+/+/Vκ10 B cells in vivo and a subpopulation of these B cells underwent receptor editing, leading to expression of high levels of HKIR BCRs that contained both κ and λ L chains. Such BCRs displayed reduced autoreactivity for nuclear Ags as compared with canonical HKIR/Vκ10 BCRs.
Previous analyses of another line of site-directed IgH-transgenic mice expressing antichromatin BCRs revealed the presence of κ-λ L chain isotypically included B cells in the MZ compartment (29). Because these B cells expressed an autoreactive IgH-λ BCR, it was argued that tolerance was maintained in this population due to a combination of a reduced level of autoreactivity due to dual L chain expression, as well as “sequestration” of these B cells in the MZ. Our data suggest that the κ-λ isotypically included population of MZ-like B cells present in HKIR+/+/Vκ10 mice may have followed a similar pathway of tolerance induction, although the relevance of B cell localization to the MZ for maintenance of tolerance in both of these cases is unclear at present. The data we illustrate in Fig. 8 show that the λ B cells in HKIR+/+/Vκ10 mice are able to respond to Ag in vivo and thus might be capable of responding to autoantigen if located in a supportive microenvironment such as the follicle.
In HKIR+/+ mice, receptor editing also appears to be taking place. However, the λ+ B cells found in HKIR+/+ mice do not coexpress a κ L chain. In addition, they are observed in both FO and MZ regions of the spleen and have a “mixed” FO and MZ phenotype. The phenotypic differences of these cells as compared with those found in HKIR+/+/Vκ10 mice may be due to the difficulty in editing or otherwise altering expression levels of the transgenic Vκ10 locus. These data argue that preferential selection into the MZ compartment of κ-λ isotypically included B cells in HKIR+/+/Vκ10 mice is due to their elevated levels of BCR expression, higher avidity for autoantigen, or both.
Clearly, the bulk of the developing B cells in both HKIR+/+/Vκ10 and HKIR+/+ mice do not undergo λ editing, as only 3–5% of peripheral B cells in these mice express a l L chain. Whether a major subpopulation of developing B cells in these mice follows pathways of receptor editing leading to expression of noncanonical κ L chains was not examined. If this is taking place, however, it apparently does not alleviate the retarded developmental progression displayed by HKIR+/+/Vκ10 B cells. This suggests that most κ L chains are not “good editors” of the autoreactivity characteristic of canonical HKIR BCRs. This may explain why the T1 and T2 stages of splenic B cell development are underrepresented in HKIR+/+/Vκ10 mice, and the T3 subset, which may be largely composed of anergic B cells (31), is overrepresented. This could also account for the fact that the frequency of λ+ B cells is similar in HKIR+/+/Vκ10 and HKIR+/+ mice. Further studies will be required to more directly address these issues. Finally, in agreement with a previous report in which B cell development was assessed in mice expressing two copies of the 3H9/56R antichromatin BCR (32), H chain editing does not seem to play a predominant role in the development of either HKIR+/+/Vκ10 or HKIR+/+ B cells, since hybridomas derived from such B cells express mAbs with canonical VH-dependent idiotopes, RNA derived from the intact HKIR locus, or both.
Although the data we present here demonstrate that B cells expressing a canonical HKIR BCR are competent to undergo L chain receptor editing in vivo, as we previously demonstrated was the case in vitro (25), the question of why developing canonical HKIR+/− B cells undergo learned ignorance as apposed to receptor editing remains incompletely resolved. Current data indicate that three parameters predominantly influence the tolerance pathway taken by a developing autoreactive B cell: the stage and locale of development when autoantigen is first encountered, the avidity of the BCR-autoantigen interaction, and the nature of the autoantigen itself. With regard to the latter factor, we considered that since the cognate autoantigen for canonical HKIR B cells in vivo is most likely nucleic acid-based, that TLR 3, 7, or 9 signaling might be increasing the efficiency of the learned ignorance process. However, we observed no differences in B cell development and BCR down-regulation in TLR9 or MyD88-deficient and sufficient versions of HKIR+/− and HKIR+/−/Vκ10 mice (Ref. 33 and F. Coffey and T. Manser, unpublished results).
With respect to the nature of the first two parameters, our in vitro studies suggest that the cognate autoantigen(s) for the canonical HKIR BCR is ubiquitous and likely present in the BM and can be derived from developing B cells themselves (25). This is consistent with past studies showing that intracellular autoantigens are externalized by apoptotic or postapoptotic necrotic cells (34, 35). However, when normal apoptotic cell scavenging and phagocytic pathways are operative, such Ags are probably present at only low concentrations and predominantly in soluble form in the milieu of developing B cells. This could favor the induction of learned ignorance over receptor editing early in B cell development, as our data show that the latter process requires higher avidity BCR-autoantigen interactions than the former.
Acknowledgments
We thank Dr. Larry Wysocki for p-aminobenzoic acid-tryosine and rabbit anti-CRI antiserum, the Kimmel Cancer Center Flow Cytometry and Bio-imaging Facilities, and all members of the Manser laboratory for their indirect contributions to this work.
The studies were supported by National Institutes of Health Grant AI038965 (to T.M.).
5. Abbreviations used in this paper:
- Ars
p-azophenylarsonate
- FO
follicular
- s
surface
- BM
bone marrow
- ANA
antinuclear Ab
- KLH
keyhole limpet hemocyanin
- MZ
marginal zone
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Goodnow CC 1992. Transgenic mice and analysis of B-cell tolerance. Annu. Rev. Immunol 10: 489–518. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee D 2000. Receptor editing in B cells. Adv. Immunol 74: 89–126. [DOI] [PubMed] [Google Scholar]
- 3.Gay D, Saunders T, Camper S, and Weigert M. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med 177: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiegs SL, Russell DM, and Nemazee D. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med 177: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nossal GJ 1993. Tolerance and ways to break it. Ann. NY Acad. Sci 690: 34–41. [DOI] [PubMed] [Google Scholar]
- 6.Burnet FM 1957. A modification of Jerne’s theory of antibody production using the concept of clonal selection. Aust. J. Sci 20: 67–69. [DOI] [PubMed] [Google Scholar]
- 7.Braden BC, and Poljak RJ. 1995. Structural features of the reactions between antibodies and protein antigens. FASEB J 9: 9–16. [DOI] [PubMed] [Google Scholar]
- 8.Foote J 2003. Immunology: isomeric antibodies. Science 299: 1327–1328. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau PG, Mallett CP, and Smith-Gill SJ. 1989. A substantial proportion of the adult BALB/c available B cell repertoire consists of multireactive B cells. Mol. Immunol 26: 993–1006. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix-Desmazes S, Kaveri SV, Mouton L, Avouba A, Malnchere E, Coutinho A, and Kazatchkine MD. 1998. Self-reactive antibodies (natural au-toantibodies) in healthy individuals. J. Immunol. Methods 216: 117–130. [DOI] [PubMed] [Google Scholar]
- 11.Limpanasithikul W, Ray S, and Diamond B. 1995. Cross-reactive antibodies have both protective and pathogenic potential. J. Immunol 155: 967–973. [PubMed] [Google Scholar]
- 12.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, and Wardemann H. 2007. Autoreactivity in human IgG memory B cells. Immunity 26: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodnow CC 1996. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA 93: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aplin BD, Keech CL, de Kauwe AL, Gordon TP, Cavill D, and McCluskey J. 2003. Tolerance through indifference: autoreactive B cells to the nuclear antigen La show no evidence of tolerance in a transgenic model. J. Immunol 171: 5890–5900. [DOI] [PubMed] [Google Scholar]
- 15.Cancro MP, and Kearney JF. 2004. B cell positive selection: road map to the primary repertoire? J. Immunol 173: 15–19. [DOI] [PubMed] [Google Scholar]
- 16.Pillai S, Cariappa A, and Moran ST. 2004. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol. Rev 197: 206–218. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, and Clarke SH. 2003. Evidence for a ligand-mediated positive selection signal in differentiation to a mature B cell. J. Immunol 171: 6381–6390. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, and Hardy RR. 1999. Positive selection of natural autoreactive B cells. Science 285: 113–116. [DOI] [PubMed] [Google Scholar]
- 19.Martin F, and Kearney JF. 2000. B-cell subsets and the mature preimmune repertoire: marginal zone and B1 B cells as part of a “natural immune memory.” Immunol. Rev 175: 70–79. [PubMed] [Google Scholar]
- 20.Notidis E, Heltemes L, and Manser T. 2002. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B cell development. Immunity 17: 317–327. [DOI] [PubMed] [Google Scholar]
- 21.Heltemes-Harris L, Liu X, and Manser T. 2004. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J. Immunol 172: 823–833. [DOI] [PubMed] [Google Scholar]
- 22.Hande S, and Manser T. 1998. Single amino acid substitutions in Vh CDR2 are sufficient to generate or enhance the specificity of two forms of an anti-arsonate antibody variable region for DNA. Mol. Immunol 34: 1281–1290. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, and Manser T. 2005. Antinuclear antigen B cells that down-regulate surface B cell receptor during development to mature, follicular phenotype do not display features of anergy in vitro. J. Immunol 174: 4505–4515. [DOI] [PubMed] [Google Scholar]
- 24.Alabyev B, Rahman ZS, and Manser T. 2007. Quantitatively reduced participation of anti-nuclear antigen B cells that down-regulate B cell receptor during primary development in the germinal center/memory B cell response to foreign antigen. J. Immunol 178: 5623–5634. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Wysocki LJ, and Manser T. 2007. Autoantigen-B cell antigen receptor interactions that regulate expression of B cell antigen receptor Loci. J. Immunol 178: 5035–5047. [DOI] [PubMed] [Google Scholar]
- 26.Heltemes-Harris L, Liu X, and Manser T. 2005. An antibody VH gene that promotes marginal zone B cell development and heavy chain allelic inclusion. Int. Immunol 17: 1447–1461. [DOI] [PubMed] [Google Scholar]
- 27.Manser T 1989. Evolution of antibody structure during the immune response: the differentiative potential of a single B lymphocyte. J. Exp. Med 170: 1211–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hande S, Notidis E, and Manser T. 1998. Bcl-2 obstructs negative selection of autoreactive, hypermutated antibody V regions during memory B cell development. Immunity 8: 189–198. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li H, and Weigert M. 2002. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med 195: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, and Wysocki LJ. 2001. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity 14: 33–43. [DOI] [PubMed] [Google Scholar]
- 31.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, and Cambier JC. 2006. Identification of anergic B cells within a wild-type repertoire. Immunity 25: 953–962. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Li L, and Mohan C. 2008. The role of rearrangement at the second Ig heavy chain locus in maintaining B cell tolerance to DNA. J. Immunol 180: 7721–7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffey F, Liu X, and Manser T. 2007. Primary development and participation in a foreign antigen-driven immune response of a chromatin-reactive B cell clonotype are not influenced by TLR9 or other MyD88-dependent TLRs. J. Immunol 179: 6663–6672. [DOI] [PubMed] [Google Scholar]
- 34.Cocca BA, Cline AM, and Radic MZ. 2002. Blebs and apoptotic bodies are B cell autoantigens. J. Immunol 169: 159–166. [DOI] [PubMed] [Google Scholar]
- 35.Navratil JS, Liu CC, and Ahearn JM. 2006. Apoptosis and autoimmunity. Immunol. Res 36: 3–12. [DOI] [PubMed] [Google Scholar]


