Graphical abstract
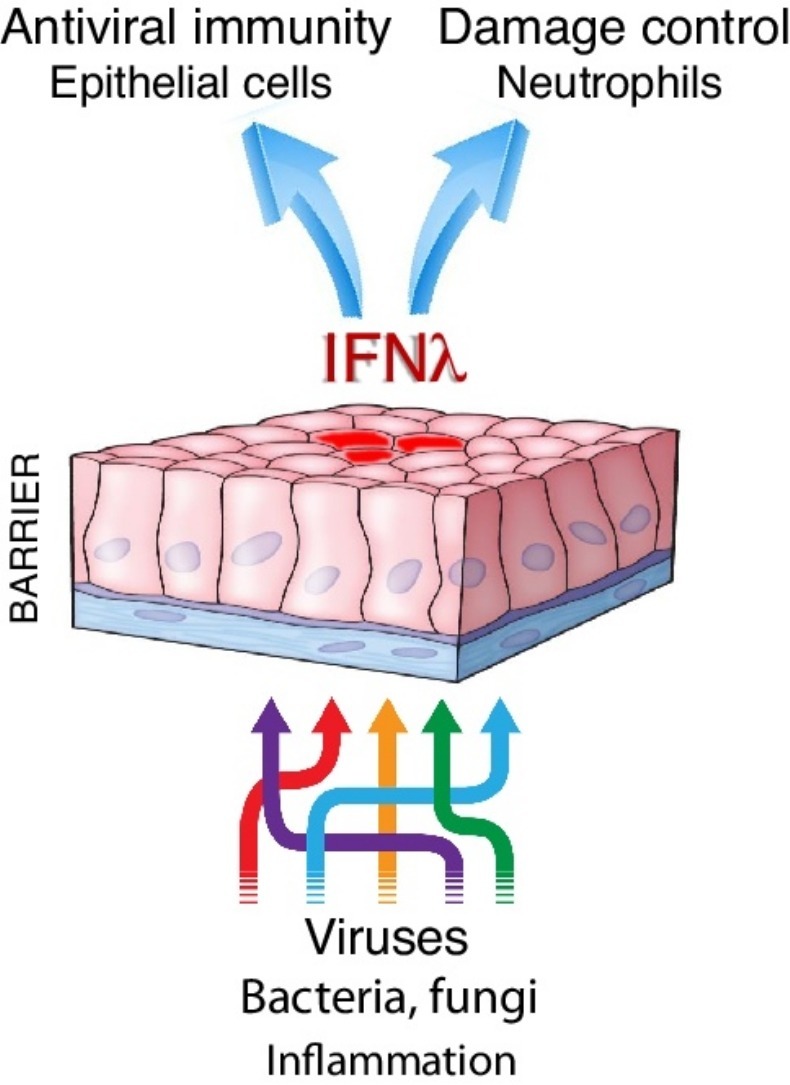
Abstract
Lambda interferons (IFNλs, type III IFNs or interleukins-28/29) were described fifteen years ago as novel cytokines sharing structural and functional homology with IL-10 and type I IFNs, respectively. IFNλs engage a unique receptor complex comprising IFNLR1 and IL10R2, nevertheless they share signaling cascade and many functions with type I IFNs, questioning their possible non-redundant roles and overall biological importance. Here, we review the latest evidence establishing the primacy of IFNλs in front line protection at anatomical barriers, mediating antiviral immunity before type I IFNs. We also discuss their emerging role in regulating inflammation and limiting host damage, a major difference to type I IFNs. IFNλs come thus to light as dual function cytokines mediating antiviral immunity and damage control.
Current Opinion in Immunology 2019, 56:67–75
This review comes from a themed issue on Innate immunity
Edited by Nicolas Manel and James Di Santo
For a complete overview see the Issue and the Editorial
Available online 3rd November 2018
https://doi.org/10.1016/j.coi.2018.10.007
0952-7915/© 2018 Elsevier Ltd. All rights reserved.
Introduction
For a long time, type I interferons (IFNs) have been considered as the primary antiviral defense system, acting in an autocrine and paracrine manner to induce resistance to infection and enhance innate and adaptive immune responses needed for viral clearance [1]. Moreover, they have attracted major interest in oncology and multiple sclerosis as biological response modifiers able to improve therapy [1]. However, although type I IFNs have been approved for diverse indications including genital warts, viral hepatitis, hairy cell leukemia and chronic myelogenous leukemia, their use in the clinic is limited due to the frequent and severe adverse effects (including flu-like disease and depression) they exhibit.
With the completion of the Human Genome Project, it became apparent that another cytokine family, termed lambda IFNs (IFNλs), type III IFNs or IL-28 and IL-29, exists and shares structural homology with the interleukin (IL)-10 family and functional homology with type I IFNs [2,3]. Similarly to type I IFNs, IFNλs are triggered by infection and induce multiple antiviral responses mediating viral clearance. They also exert pleiotropic effects on the immune system, many of which highly reminiscent to these of type I IFNs. This raised the question whether IFNλs and type I IFNs are redundant, and why our organism needs two IFN-based antiviral defense systems to confront infection. Here, we review the latest evidence highlighting the primacy of IFNλs in antimicrobial, and in particular antiviral, immunity. We survey their unique and common biology with type I IFNs, their co-operation with type I IFNs in the fine-tuning of antimicrobial immunity and their emerging role in damage control. We also discuss their potential as novel therapeutics that exhibit the beneficial effects, but lack the pro-inflammatory activities causing side effects, of type I IFNs.
IFNλ members, induction mechanisms and expression patterns
There are four IFNλ members in humans, IFNλ1/IL-29, IFNλ2/IL-28A, IFNλ3/IL-28B, IFNλ4, and two (IFNλ2/IL-28A, IFNλ3/IL-28B) in mice [2, 3, 4]. Much like type I IFNs, IFNλs are only transiently expressed following stimulation by viruses and microbial products. These include all major respiratory (influenza and parainfluenza viruses, rhinoviruses, respiratory syncytial viruses, coronaviruses etc), gastrointestinal (rotaviruses, reoviruses, noroviruses) and hepatotropic (hepatitis B and C) viruses [2,3,5,6], intracellular and extracellular bacteria (Listeria monocytogenes, Streptococcus pneumonia, Haemophilus influenzae, Staphylococcus aureus, Salmonella enterica, Shigella sonnei and Mycobacterium tuberculosis) [7,8,9•], and numerous microbial components and synthetic ligands (imidazoquinolines, polyinosinic-polycytidylic acid, flagellin, peptidoglycan and CpG oligodeoxynucleotides) [10,11].
Central to IFNλ production are pattern-recognition receptors (PRRs) such as toll-like receptor (TLR)3, TLR5, TLR7/8 and TLR9, RIG-I and MDA-5 which trigger a downstream signaling cascade leading to the activation of nuclear factor κB (NF-κB) and interferon regulatory factors (IRFs). Accordingly, IFNλ genes have binding sites for NF-κB, IRF1, IRF3 and IRF7 in their promoter regions similarly to type I IFNs [2,3,12]. Although this would suggest co-regulation, this is often not the case; IFNλs exhibit a restricted pattern of expression compared to type I IFNs which are almost ubiquitously expressed. IFNλs are most abundant at barrier surfaces including the respiratory and gastrointestinal tracts, and can be robustly produced by epithelia and epithelial-origin cells including hepatocytes and some immune cells [10,11,13,14]. This goes beyond the ability of all cells to respond to PRR engagement, suggesting the existence of additional cell-specific epigenetic, transcriptional and post-transcriptional regulation of IFNλ production. For example, RIG-I-like receptor signaling through mitochondrial antiviral signaling protein (MAVS) in peroxisomes [15], sensing through the cytosolic DNA sensor Ku70 [16], induction of the transcriptional co-activator Med23 [17] or presence of transcriptional repressors such as ZEB1 and BLIMP-1 [18,19] have all been associated with IFNλ production. Whether such mechanisms can broadly account for IFNλ expression patterns remains to be established.
Downstream signaling cascades, target cells and functional consequences
All IFNλ members engage a unique heterodimeric receptor complex, the IFNLR, that comprises IFNLR1 (IFNLRA, IL-28RA), and IL10R2 (IL-10RB). IFNLR1 confers ligand specificity and enables receptor assembly, while IL10R2 is shared with IL-10 family members and is required for signaling. Binding of IFNλ to IFNLR1 and IL10R2 occurs at a 1:1:1 stoichiometry compared to the 2:1:1 ratio between IL-10, IL10R1 and IL10R2 [20•]. IFNLR stimulation leads to the activation of the tyrosine kinases JAK1 and TYK2, and the transcription factors STAT1 and STAT2 which bind to IRF9, forming the IFN-stimulated gene factor 3 (ISGF3) complex [2,3]. ISGF3 then enters the nucleus and induces the transcription of hundreds of genes, termed IFN-stimulated genes (ISGs), that generally inhibit viral replication and spread, and exhibit broad antimicrobial functions against viral, bacterial and parasitic infections [21]. Notably, this pathway is also shared with type I IFNs, highlighting the very similar antiviral activity these two systems exhibit [22, 23, 24]. Thus, it has been difficult to segregate IFNλ from type I IFN functional or transcriptional responses, with IFNλ-induced genes typically representing a subset of all genes elicited by type I IFNs but exhibiting a delayed peak and longer duration [23, 24, 25, 26].
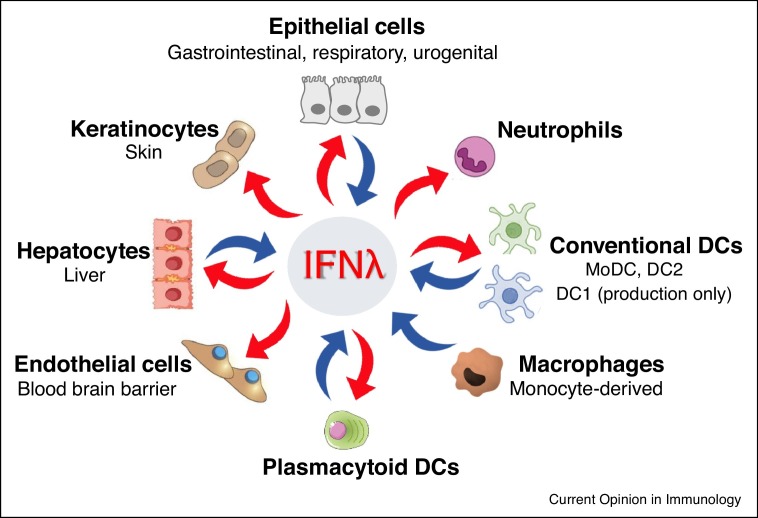
The most striking difference between the two IFN systems is their receptor distribution. The type I IFN receptor is expressed in almost all cell types. On the contrary, the IFNLR1/IL10RB complex is expressed primarily in cells of epithelial origin and few immune cells, mainly neutrophils and subsets of dendritic cells (DCs), conferring selective IFNλ responsiveness to them. This has been demonstrated for both mouse [27•,28••,29••] and human neutrophils [29••] and plasmacytoid DC (pDCs) [11,30], as well as human monocyte-derived DC [31,32], and mouse bone marrow-derived or lung-sorted conventional DC (cDC) of the DC2 phenotype [33] (Figure 1 ). The expression pattern of the receptors largely follows that of their ligands with human and mouse epithelial cells [2,3,13,28••,34], pDCs [10,11,30,35,36], DC1 [37] and DC2 [11,28••,36,38] cells, as well as human monocyte-derived DC [31,32,38,39] and macrophages [34,38,40] expressing high levels of IFNλs upon activation. This points to more specialized roles for IFNλs in localized immune responses at epithelial barrier surfaces conferred by epithelial and immune cells. However, subtler differences also exist. For example, JAK2 phosphorylation is induced only by IFNλs but not type I IFNs [15], while other less well-described pathways may also be important for antiviral functions [41••], indicating the existence of distinct signalling events that remain to be characterized.
Figure 1.
Sources and targets of IFNλs among immune and non-immune cell populations. Blue arrows depict cells expressing and red arrows cells responding to IFNλs. MoDC, DC1 and DC2 cells produce IFNλs but only moDC and DC2 cells also respond to them. DCs: Dendritic cells; MoDC: monocyte-derived DCs; DC1: Dendritic cell subset 1; DC 2: Dendritic cell subset 2.
IFNλs in antiviral immunity
From the very first publications describing the discovery of IFNλs, it became evident that IFNλs exhibit potent antiviral activity [2,3]. Treatment of infected cells in culture or experimental animals with recombinant IFNλs has been effective against a very diverse range of viruses [42, 43, 44]. However, endogenous non-redundant roles of IFNλs that are not compensated by type I IFNs have been difficult to establish. Important progress has recently come from the study of gastrointestinal infections where it was shown that cell responsiveness to IFNλs is largely compartmentalized with IFNλs acting on intestinal epithelial cells (expressing high levels of IFNLR1) and having minimal effects on lamina propria cells (expressing low levels), while type I IFNs exhibiting the opposite effects [45,46••]. Accordingly, it was demonstrated that rotavirus — which infects epithelial cells — is solely controlled by IFNλs while reovirus -which replicates in both epithelial and non-epithelial cells and can spread systemically- requires the co-operative action of IFNλs with type I IFNs [45,46••,47•]. Similarly, norovirus that grows at the gastrointestinal epithelium but spreads systemically is confronted by both IFN systems [48••]. Interestingly, IFNλs can effectively clear norovirus in antibiotics-treated mice, an effect prohibited by gut commensals [49•], adding another level of complexity to the equation.
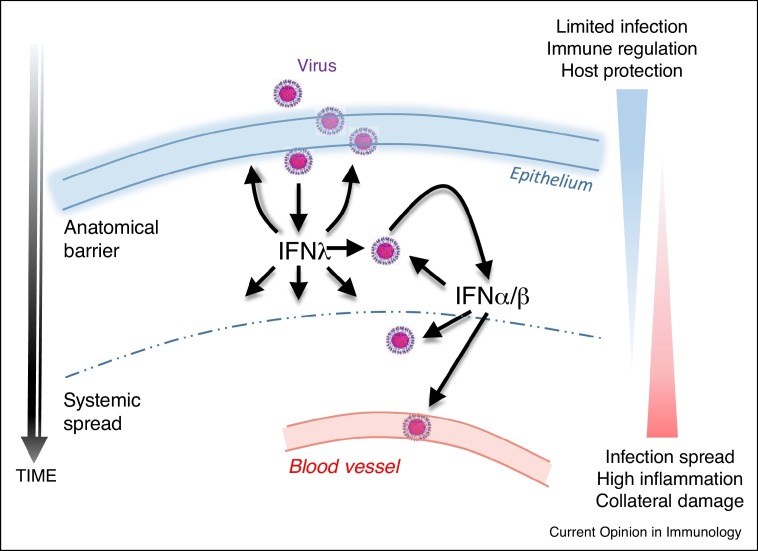
In the respiratory tract, such compartmentalization is less obvious. Respiratory epithelial cells express receptors for and respond to both types of IFNs, as do certain immune cell populations residing or infiltrating the lung. Moreover, although nose, bronchial and alveolar epithelial cells express high levels of IFNλs following infection [34,50], smooth muscle cells, fibroblasts, and DCs can also do so [10,11,51,52]. This suggested a degree of redundancy between the two IFN systems, supported by early work showing increased susceptibility to respiratory viral infections in mice deficient in both IFNLR1 and IFNAR1, but not in mice deficient in only IFNLR1 [23,53,54]. However, more recent studies have uncovered major and unique roles of IFNλs in antiviral immunity in the lung. They have revealed that IFNλs are induced first, at lower viral burden than type I IFNs, and act to limit initial infection spread [28••]. This is possibly due to the propensity of airway epithelial cells to selectively produce type III IFNs [23,55]. In line with that, it has been reported that IFNλs are essential for preventing virus transmission from the upper airways to the lungs, a process that takes place early during infection [56••]. Interestingly, IFNλs lack the strong pro-inflammatory effects of type I IFNs and are rather anti-inflammatory and tissue protective. As a result, IFNLR1−/− mice exhibit increased viral load, inflammation and host damage following infection [28••], whereas recombinant IFNλ2 treatment potently suppresses these outcomes [57]. This is also the case when mice are treated with IFNλ2/3 neutralizing antibodies [58]. These data support a model where IFNλs confer initial antiviral protection without provoking unnecessary inflammation, while type I IFNs come into play as a second line of defense to enhance antiviral responses at the expense of collateral damage (Figure 2 ).
Figure 2.
Model of IFNλ action. IFNλs confer initial antiviral protection at anatomical barriers without provoking unnecessary inflammation. Type I IFNs come up as a second line of defense, following escape from IFNλ control, to enhance antiviral and immunoinflammatory responses, at the expense though of collateral damage.
This model of IFNλ action is consistent with the behavior of IFNλs in the gastrointestinal tract and may therefore explain their broader role in antiviral defense. Indeed, in the liver, IFNλs are also important in providing protective immunity against HBC and HCV infections as demonstrated by the many IFNλ gene polymorphisms linked to improved spontaneous or treatment-induced viral clearance, and the effectiveness of IFNλ therapy [59]. Interestingly, this is attributed to the ability of human hepatocytes to preferentially generate and respond to IFNλs similarly to respiratory epithelial cells [60]. Moreover, in the skin, IFNλs are predominantly expressed over type I IFNs and are associated with reduced incidence to infections [61].
IFNλs in antibacterial and antifungal immunity
In addition to viruses, bacterial and fungal infections can also trigger IFNλ production. L. monocytogenes, S. enterica and S. sonnei as well as several bacterial ligands induce IFNλs [7,9•], mainly in a MyD88-dependent manner [9•]. This is functionally important. in vitro, IFNλs enhanced epithelial barrier integrity, preventing bacterial dissemination [9•]. In vivo, in models of S. aureus or Pseudomonas aeruginosa infection, IFNLR1−/− mice exhibited lower bacterial loads and less pathology, although inflammatory cell infiltration was not affected [62]. Also, intranasal infection of IFNLR1−/− mice with S. aureus led to significantly increased bacterial clearance and, at the same time, decreased proinflammatory cytokines including IL-1β in the airways [63]. Interestingly, in this study IL-1β production appeared to be regulated by proteases released by neutrophils rather than NLRP3 and capsase-1 activation. Moreover, in a model of invasive aspergillosis with Aspergillus fumigatus, IFNLR1−/− mice developed aggravated disease, with higher fungal loads in the lungs and more severe fungal invasion [64••]. This was largely due to IFNλ-mediated STAT1 activation in neutrophils, that was shown to protect the host. Further studies are therefore needed to shed light into the wider role of IFNλs in antimicrobial defenses beyond viral infections.
IFNλs in immune modulation and damage control
In sharp contrast to type I IFNs, IFNλs and their receptor are not ubiquitously expressed in the immune system. Rather, IFNλs are produced by pDCs [10,11,30,35,36], cDCs (DC1 [37] and DC2 [11,28••,36,38]), and monocyte-derived DC [31,32,38,39] and macrophages [34,38,40], as earlier discussed, while IFNLR1/IL10RB is functional in neutrophils [27•,28••,29••], pDCs [11,30], monocyte-derived DCs [31,32] and DC2 cells [33] but not other leukocytes. IFNλs therefore signal on a restricted set of leukocytes, exerting selective immune modulatory functions which are only now starting to become understood.
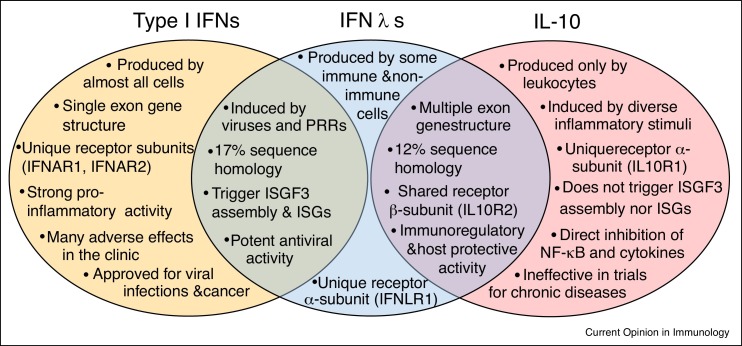
A primary emerging activity of IFNλs is the regulation of innate immunity. Contrary to type I IFNs, IFNλs suppress innate pro-inflammatory responses and limit the host damaging effects associated with inflammation. This is largely due to their selective action on neutrophils, preventing their pro-inflammatory activation and functions. Thus, in the context of influenza viral infection, IFNλs restrain the production of TNF, IL-1β and other tissue destructive mediators from neutrophils, while allowing effective antiviral responses to develop [28••]. Although it is unclear whether this is a direct effect, it nevertheless shows that IFNλs are critically involved in keeping inflammation under control and limiting collateral damage. In the context of chronic intestinal inflammation, IFNλs act directly on neutrophils to inhibit the generation of ROS and their degranulation through a process mediated by JAK2 [29••]. IFNλs can also induce additional beneficial effects by accelerating mucosal healing [65•]. Exogenous administration of recombinant IFNλs in experimental animal models with influenza virus infection or colitis has further confirmed their regulatory and anti-inflammatory activity, and has highlighted their therapeutic potential [29••,57]. Moreover, exogenous administration of recombinant IFNλs has shown therapeutic potential in other diseases where IFNλs may not be produced naturally such as collagen-induced arthritis [27•] and arterial thrombosis [66••]. In arthritis, the efficacy of IFNλ treatment was attributed to the inhibitory effects of IFNλs in neutrophil migration [27•]. In arterial thrombosis, IFNλ-mediated protection was due to the suppressive effects of IFNλs on neutrophil-extracellular traps (NETs) formation [66••]. This anti-inflammatory or immune-modulatory behavior of IFNλs is reminiscent of the activity of IL-10 with which IFNλs share sequence homology and the IL10RB subunit for signaling (Figure 3 ).
Figure 3.
Common and unique structural and functional characteristics of IFNλs with type I IFNs and IL-10. Antiviral activity is shared with type I IFNs whereas immune regulatory and host protective actions are shared with IL-10.
Another major activity of IFNλs is the regulation of adaptive immunity. IFNλs signal on DC2 cells [31, 32, 33] and pDCs [11,30], of both human and mouse origin, and modulate their function. in vitro, IFNλs enhance the propensity of human monocyte-derived DCs to generate Foxp3+ regulatory T cells [31,32]. in vivo, IFNλs suppress the ability of mouse DC2 cells to induce T helper 2 (Th2) and Th17 differentiation, and enhance Th1 cell development [33]. This is consistent with cell culture studies using mouse DC2 and T cells [33], or human peripheral blood mononuclear cells (PBMC) [67]. Interestingly, a SNP in the Ifnl3 locus (rs8099917) is linked to higher IFNλ3 production and Th1 skewing following PBMC stimulation with influenza virus [68]. A shift in IFNγ production from NK cells is also observed but this might be indirect as neither NK cells [11,13] nor T cells [11,33] seem to respond to IFNλs. The Th1 skewing effect of IFNλs may be related to their capacity to increase the expression of the Th1 polarizing cytokine IL-12 in a context-dependent manner [33,69]. Noteworthy, cytotoxic T cell responses may also be affected by IFNλs as increased CD8+ T cell responses have been reported in IFNLR1−/− mice following acute LCMV infection [70].
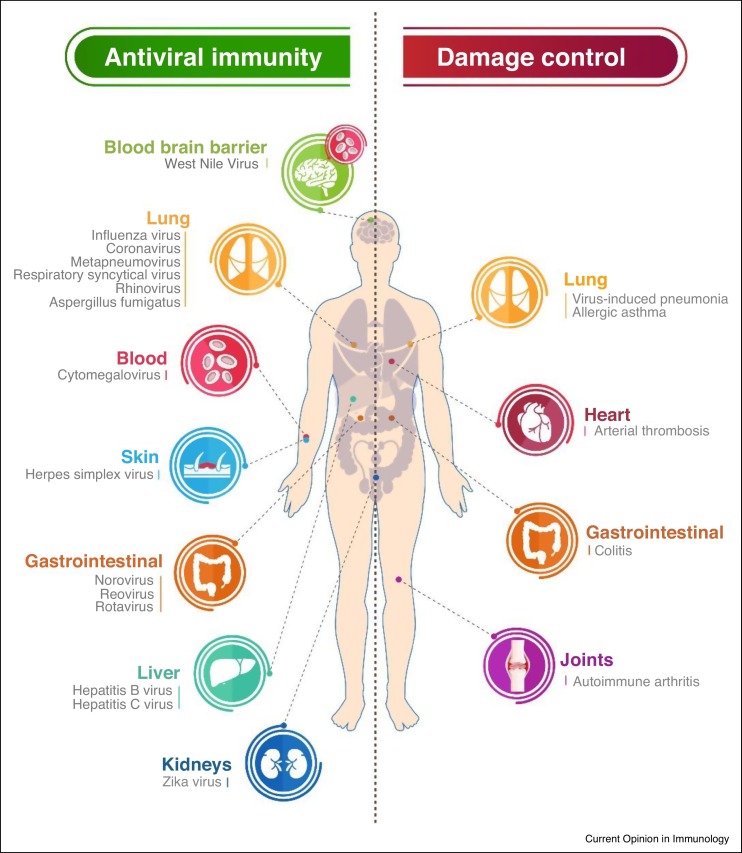
Interestingly, in T cell-driven diseases in experimental animals IFNλs are therapeutically effective. In allergic asthma, IFNλs potently suppress the activation of Th2 and Th17 responses, and the development of immunopathology [33]. In autoimmune arthritis, they also inhibit the induction of Th17 and γδ T cell responses and they ameliorate disease [27•]. IFNλs therefore appear to be broadly protective, in both acute and chronic inflammatory diseases, mediating immune modulatory actions aiming at restoring immunological balance and limiting direct tissue damage caused by the byproducts of host defense (Figure 4 ).
Figure 4.
IFNλs as dual function cytokines mediating antiviral activity and damage control. The schematic shows the unique and non-redundant roles of IFNλs in antiviral defense, and their immune regulatory actions mediating disease protection as emerging over the last years.
Conclusions
Over the last few years IFNλs have come out of the shadow of type I IFNs. They have emerged as a front line defense system mediating antiviral immunity at anatomical barriers such as the gastrointestinal and respiratory tracts. They have also emerged as novel immune regulatory cytokines with a special duty in damage control that act to maintain immunological balance and limit immunopathology. This extends beyond infections as IFNλs limit inflammation and prevent host damage in diverse other diseases including colitis, autoimmune arthritis and allergic asthma.
Central to the unique biology of IFNλs is their selective action on neutrophils, preventing their pro-inflammatory activation, inhibiting ROS production, degranulation and NET formation, and down regulating their migratory capacity. This is in sharp contrast to the pro-inflammatory effects of type I IFNs, which instead activate neutrophils and other leukocytes, and highlights the IL-10-like properties of IFNλs that remain to be further investigated. This may also explain the improved safety profile IFNλs exhibit in the clinic.
The dual antimicrobial and immune regulatory function of IFNλs makes them particularly attractive for the treatment of infectious diseases or chronic disorders such as asthma and colitis where infections can exacerbate their severity, as IFNλs can selectively up regulate antiviral responses while limiting host damaging inflammation and symptoms. It also advocates for the broader evaluation of IFNλs in the fine-tuning of immunity in diseases which involve neutrophilic inflammation and alterations of the Th1/Th2/Th7 balance. Further studies towards these directions are therefore eagerly awaited.
Conflict of interest statement
E. Andreakos has received consultancy fees from Vorso Corp and in Thought Research, and grants from Novosom AG and Janssen. I. Zanoni and I. Galani declare no relevant conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
EA is supported by the Hellenic Ministry of Education, Research and Religious Affairs (MIDAS, RESOLVE-ASTHMA) and the European Commission (PREDICTA, CURE, IMMUNAID, TAXINOMISIS). IEG is supported by the Hellenic Foundation for Research and Innovation (RELIEVE). IZ is supported by the National Institutes of Health (R01AI121066, R01DK115217), Harvard Digestive Diseases Center P30 DK034854 grant, CCFA Senior Research Award No. 412708, the Eleanor and Miles Shore 50th Anniversary Fellowship Program, and the Cariplo Foundation.
References
- 1.Borden E.C., Sen G.C., Uze G., Silverman R.H., Ransohoff R.M., Foster G.R., et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard P., Kindsvogel W., Xu W., Henderson K., Schlutsmeyer S., Whitmore T.E., et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 4.Prokunina-Olsson L., Muchmore B., Tang W., Pfeiffer R.M., Park H., Dickensheets H., et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robek M.D., Boyd B.S., Chisari F.V. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ank N., West H., Bartholdy C., Eriksson K., Thomsen A.R., Paludan S.R. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebreton A., Lakisic G., Job V., Fritsch L., Tham T.N., Camejo A., et al. A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response. Science. 2011;331:1319–1321. doi: 10.1126/science.1200120. [DOI] [PubMed] [Google Scholar]
- 8.Bierne H., Travier L., Mahlakoiv T., Tailleux L., Subtil A., Lebreton A., et al. Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta. PLoS One. 2012;7:e39080. doi: 10.1371/journal.pone.0039080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Odendall C., Voak A.A., Kagan J.C. Type III IFNs are commonly induced by bacteria-sensing TLRs and reinforce epithelial barriers during infection. J Immunol. 2017;199:3270–3279. doi: 10.4049/jimmunol.1700250. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meticulous study showing that IFNλs are induced by bacteria and diverse bacterial ligands.
- 10.Coccia E.M., Severa M., Giacomini E., Monneron D., Remoli M.E., Julkunen I., et al. Viral infection and toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 11.Ank N., Iversen M.B., Bartholdy C., Staeheli P., Hartmann R., Jensen U.B., et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 12.Osterlund P.I., Pietila T.E., Veckman V., Kotenko S.V., Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- 13.Sommereyns C., Paul S., Staeheli P., Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolou E., Kapsogeorgou E.K., Konsta O.D., Giotakis I., Saridaki M.I., Andreakos E., et al. Expression of type III interferons (IFNlambdas) and their receptor in Sjogren’s syndrome. Clin Exp Immunol. 2016;186:304–312. doi: 10.1111/cei.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odendall C., Dixit E., Stavru F., Bierne H., Franz K.M., Durbin A.F., et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat Immunol. 2014;15:717–726. doi: 10.1038/ni.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Brann T.W., Zhou M., Yang J., Oguariri R.M., Lidie K.B., et al. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J Immunol. 2011;186:4541–4545. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths S.J., Koegl M., Boutell C., Zenner H.L., Crump C.M., Pica F., et al. A systematic analysis of host factors reveals a Med23-interferon-lambda regulatory axis against herpes simplex virus type 1 replication. PLoS Pathog. 2013;9:e1003514. doi: 10.1371/journal.ppat.1003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel R., Eskdale J., Gallagher G. Regulation of IFN-lambda1 promoter activity (IFN-lambda1/IL-29) in human airway epithelial cells. J Immunol. 2011;187:5636–5644. doi: 10.4049/jimmunol.1003988. [DOI] [PubMed] [Google Scholar]
- 19.Elias S., Robertson E.J., Bikoff E.K., Mould A.W. Blimp-1/PRDM1 is a critical regulator of Type III Interferon responses in mammary epithelial cells. Sci Rep. 2018;8:237. doi: 10.1038/s41598-017-18652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Mendoza J.L., Schneider W.M., Hoffmann H.H., Vercauteren K., Jude K.M., Xiong A., et al. The IFN-lambda-IFN-lambdaR1-IL-10Rbeta complex reveals structural features underlying Type III IFN functional plasticity. Immunity. 2017;46:379–392. doi: 10.1016/j.immuni.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work unravelled the crystal structure of the IFNλR receptor and explained how the IL-10Rβ chain acts as a cross-reactive but also cytokine-specific receptor. This provides broader insight into cytokine recognition by the IL-10R family.
- 21.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z., Hamming O.J., Ank N., Paludan S.R., Nielsen A.L., Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotta S., Davidson S., Mahlakoiv T., Desmet C.J., Buckwalter M.R., Albert M.L., et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 2013;9:e1003773. doi: 10.1371/journal.ppat.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle S.E., Schreckhise H., Khuu-Duong K., Henderson K., Rosler R., Storey H., et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 25.Marcello T., Grakoui A., Barba-Spaeth G., Machlin E.S., Kotenko S.V., MacDonald M.R., et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 26.Selvakumar T.A., Bhushal S., Kalinke U., Wirth D., Hauser H., Koster M., et al. Identification of a predominantly interferon-lambda-induced transcriptional profile in murine intestinal epithelial cells. Front Immunol. 2017;8:1302. doi: 10.3389/fimmu.2017.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Blazek K., Eames H.L., Weiss M., Byrne A.J., Perocheau D., Pease J.E., et al. IFN-lambda resolves inflammation via suppression of neutrophil infiltration and IL-1beta production. J Exp Med. 2015;212:845–853. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study showing that IFNλs act on neutrophils to suppress their migration to sites of inflammation.
- 28••.Galani I.E., Triantafyllia V., Eleminiadou E.E., Koltsida O., Stavropoulos A., Manioudaki M., et al. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity. 2017;46:875–890 e6. doi: 10.1016/j.immuni.2017.04.025. [DOI] [PubMed] [Google Scholar]; This study revealed the non-redundant role of IFNλs in front-line antiviral protection in the respiratory tract. It demonstrated that IFNλs mediate initial antiviral defenses, before type I IFNs, without provoking unnecessary inflammation and causing damage. It also uncovered the importance of both IFN systems in the fine-tuning of neutrophil function and antiviral immunity.
- 29••.Broggi A., Tan Y., Granucci F., Zanoni I. IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function. Nat Immunol. 2017;18(10):1084–1093. doi: 10.1038/ni.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that IFNλs possess direct anti-inflammatory activity, acting in neutrophils in a post-transcriptional and non-translational IFNλs manner to inhibit ROS generation and cell degranulation.
- 30.Yin Z., Dai J., Deng J., Sheikh F., Natalia M., Shih T., et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mennechet F.J., Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 32.Dolganiuc A., Kodys K., Marshall C., Saha B., Zhang S., Bala S., et al. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PLoS One. 2012;7:e44915. doi: 10.1371/journal.pone.0044915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koltsida O., Hausding M., Stavropoulos A., Koch S., Tzelepis G., Ubel C., et al. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3:348–361. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W., et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 35.Megjugorac N.J., Gallagher G.E., Gallagher G. IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra. Blood. 2010;115:4185–4190. doi: 10.1182/blood-2009-09-246157. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S., Kodys K., Li K., Szabo G. Human type 2 myeloid dendritic cells produce interferon-lambda and amplify interferon-alpha in response to hepatitis C virus infection. Gastroenterology. 2013;144:414–425. doi: 10.1053/j.gastro.2012.10.034. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauterbach H., Bathke B., Gilles S., Traidl-Hoffmann C., Luber C.A., Fejer G., et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillyer P., Mane V.P., Schramm L.M., Puig M., Verthelyi D., Chen A., et al. Expression profiles of human interferon-alpha and interferon-lambda subtypes are ligand- and cell-dependent. Immunol Cell Biol. 2012;90:774–783. doi: 10.1038/icb.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterlund P., Veckman V., Siren J., Klucher K.M., Hiscott J., Matikainen S., et al. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spann K.M., Tran K.C., Chi B., Rabin R.L., Collins P.L. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Lazear H.M., Daniels B.P., Pinto A.K., Huang A.C., Vick S.C., Doyle S.E., et al. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7:284ra. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first description of the antiviral role of IFNλs in the blood brain barrier.
- 42.Wack A., Terczynska-Dyla E., Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol. 2015;16:802–809. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazear H.M., Nice T.J., Diamond M.S. Interferon-lambda: immune functions at barrier surfaces and beyond. Immunity. 2015;43:15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreakos E., Salagianni M., Galani I.E., Koltsida O. Interferon-lambdas: front-line guardians of immunity and homeostasis in the respiratory tract. Front Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pott J., Mahlakoiv T., Mordstein M., Duerr C.U., Michiels T., Stockinger S., et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Mahlakoiv T., Hernandez P., Gronke K., Diefenbach A., Staeheli P. Leukocyte-derived IFN-alpha/beta and epithelial IFN-lambda constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog. 2015;11:e1004782. doi: 10.1371/journal.ppat.1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that in the gastrointestinal tract the type I and type III IFN systems are compartmentalized, with IFNλs acting at the epithelial barrier and type I IFNs at the lamina propria to confer protection against viral infections.
- 47•.Hernandez P.P., Mahlakoiv T., Yang I., Schwierzeck V., Nguyen N., Guendel F., et al. Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol. 2015;16:698–707. doi: 10.1038/ni.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that IFNλs acts synergistically with IL-22 to induce optimal antiviral responses and microbial clearance in the gastrointestinal tract.
- 48••.Nice T.J., Baldridge M.T., McCune B.T., Norman J.M., Lazear H.M., Artyomov M., et al. Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2015;347:269–273. doi: 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the ability of IFNλs to cure norovirus infections even in the absence of adaptive immunity.
- 49•.Baldridge M.T., Nice T.J., McCune B.T., Yokoyama C.C., Kambal A., Wheadon M., et al. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science. 2015;347:266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report showed that commensal microbes compromise the ability of IFNλs to deal with norovirus infections in the gastrointestinal tract.
- 50.Wang J., Oberley-Deegan R., Wang S., Nikrad M., Funk C.J., Hartshorn K.L., et al. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang K., Puel A., Zhang S., Eidenschenk C., Ku C.L., Casrouge A., et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–4678. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calven J., Yudina Y., Uller L. Rhinovirus and dsRNA induce RIG-I-like receptors and expression of interferon beta and lambda1 in human bronchial smooth muscle cells. PLoS One. 2013;8:e62718. doi: 10.1371/journal.pone.0062718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mordstein M., Kochs G., Dumoutier L., Renauld J.C., Paludan S.R., Klucher K., et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mordstein M., Neugebauer E., Ditt V., Jessen B., Rieger T., Falcone V., et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jewell N.A., Cline T., Mertz S.E., Smirnov S.V., Flano E., Schindler C., et al. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol. 2010;84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Klinkhammer J., Schnepf D., Ye L., Schwaderlapp M., Gad H.H., Hartmann R., et al. IFN-lambda prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. Elife. 2018;7 doi: 10.7554/eLife.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important report demonstrating that IFNλs are essential for preventing virus spread from the upper airways to the lungs and thus limiting transmission.
- 57.Davidson S., McCabe T.M., Crotta S., Gad H.H., Hessel E.M., Beinke S., et al. IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment. EMBO Mol Med. 2016;8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S., Kim M.J., Kim C.H., Kang J.W., Shin H.K., Kim D.Y., et al. The superiority of IFN-lambda as a therapeutic candidate to control acute influenza viral lung infection. Am J Respir Cell Mol Biol. 2017;56:202–212. doi: 10.1165/rcmb.2016-0174OC. [DOI] [PubMed] [Google Scholar]
- 59.Donnelly R.P., Dickensheets H., O’Brien T.R. Interferon-lambda and therapy for chronic hepatitis C virus infection. Trends Immunol. 2011;32:443–450. doi: 10.1016/j.it.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park H., Serti E., Eke O., Muchmore B., Prokunina-Olsson L., Capone S., et al. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection. Hepatology. 2012;56:2060–2070. doi: 10.1002/hep.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolk K., Witte K., Witte E., Raftery M., Kokolakis G., Philipp S., et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci Transl Med. 2013;5:204ra. doi: 10.1126/scitranslmed.3006245. [DOI] [PubMed] [Google Scholar]
- 62.Cohen T.S., Prince A.S. Bacterial pathogens activate a common inflammatory pathway through IFNlambda regulation of PDCD4. PLoS Pathog. 2013;9:e1003682. doi: 10.1371/journal.ppat.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pires S., Parker D. IL-1beta activation in response to Staphylococcus aureus lung infection requires inflammasome-dependent and independent mechanisms. Eur J Immunol. 2018 doi: 10.1002/eji.201847556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Espinosa V., Dutta O., McElrath C., Du P., Chang Y.J., Cicciarelli B., et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5357. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that, beyond viral infections, IFNλs are critical regulators of antifungal immunity.
- 65•.Chiriac M.T., Buchen B., Wandersee A., Hundorfean G., Gunther C., Bourjau Y., et al. Activation of epithelial signal transducer and activator of transcription 1 by interleukin 28 controls mucosal healing in mice with colitis and is increased in mucosa of patients with inflammatory bowel disease. Gastroenterology. 2017;153:123–138 e8. doi: 10.1053/j.gastro.2017.03.015. [DOI] [PubMed] [Google Scholar]; This indicates that IFNλs, though STAT1 activation, control proliferation of intestinal epithelial cells and accelerate mucosal healing in inflammatory bowel disease.
- 66••.Chrysanthopoulou A., Kambas K., Stakos D., Mitroulis I., Mitsios A., Vidali V., et al. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J Pathol. 2017;243(1):111–122. doi: 10.1002/path.4935. [DOI] [PubMed] [Google Scholar]; Important study showing that IFNλs suppress NET formation and arterial thrombosis through a post-transcriptional mechanism involving inhibition of autophagy.
- 67.Jordan W.J., Eskdale J., Boniotto M., Rodia M., Kellner D., Gallagher G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29) Genes Immun. 2007;8:13–20. doi: 10.1038/sj.gene.6364348. [DOI] [PubMed] [Google Scholar]
- 68.Egli A., Santer D.M., O’Shea D., Barakat K., Syedbasha M., Vollmer M., et al. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog. 2014;10:e1004556. doi: 10.1371/journal.ppat.1004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu B.S., Janssen H.L., Boonstra A. IL-29 and IFNalpha differ in their ability to modulate IL-12 production by TLR-activated human macrophages and exhibit differential regulation of the IFNgamma receptor expression. Blood. 2011;117:2385–2395. doi: 10.1182/blood-2010-07-298976. [DOI] [PubMed] [Google Scholar]
- 70.Misumi I., Whitmire J.K. IFN-lambda exerts opposing effects on T cell responses depending on the chronicity of the virus infection. J Immunol. 2014;192:3596–3606. doi: 10.4049/jimmunol.1301705. [DOI] [PMC free article] [PubMed] [Google Scholar]