Abstract
Purpose:
Tissue engineered heart valves (TEHV) are being investigated to address the limitations of currently available valve prostheses. In order to advance a wide variety of TEHV approaches, the goal of this study was to develop a cardiac valve bioreactor system capable of conditioning living valves with a range of hydrodynamic conditions as well as capable of assessing hydrodynamic performance to ISO 5840 standards. Methods: A bioreactor system was designed based on the Windkessel approach. Novel features including a purpose-built valve chamber and pressure feedback control were incorporated to maintain asepsis while achieving a range of hydrodynamic conditions. The system was validated by testing hydrodynamic conditions with a bioprosthesis and by operating with cell culture medium for 4 weeks and living cells for 2 weeks.
Results:
The bioreactor system was able to produce a range of pressure and flow conditions from static to resting adult left ventricular outflow tract to pathological including hypertension. The system operated aseptically for 4 weeks and cell viability was maintained for 2 weeks. The system was also able to record the pressure and flow data needed to calculate effective orifice area and regurgitant fraction.
Conclusions:
We have developed a single bioreactor system that allows for step-wise conditioning protocols to be developed for each unique TEHV design as well as allows for hydrodynamic performance assessment.
Keywords: tissue engineered heart valve, biomechanical stimulation, biochemical stimulation, three-dimensional tissue culture, ISO 5840
Introduction
Valvular heart disease has a prevalence of approximately 2.5% and results in 106,000 valve procedures performed annually in the US.4 Prosthetic valve replacements are an effective treatment option; however, mechanical prostheses require compliance with lifelong anticoagulation therapy and bioprostheses have limited durability. In addition, no currently available valve prosthesis can grow in a pediatric patient and multiple revision surgeries are usually required as a result.
Tissue engineered heart valves (TEHV) are being developed to address the limitations of current mechanical and bioprosthetic valves.30 TEHVs are able to repair and remodel to provide the possibility of indefinite durability. They may also be non-thrombogenic if coated with appropriate cells.16 Finally, they have the potential to grow, which is especially important in pediatric populations.
Various strategies have been adopted for creating a TEHV. Often, cells are seeded onto a scaffold and conditioned in a bioreactor prior to implantation.2, 10 Ideally, the cells would be autologous and obtained from blood, bone marrow, adipose tissue, skin, or myocardium either directly or via stem or progenitor cell populations.16 Scaffolds being investigated include decellularized allogenic and xenogenic valves, biological polymers, and synthetic polymers.15
Cell-seeded scaffolds may be conditioned in a bioreactor to develop and strengthen the tissue valve prior to implantation.2, 10 Appropriate biomechanical and biochemical stimulation may induce the cells to proliferate, migrate, differentiate, and remodel the scaffold into a suitable extracellular matrix (ECM). Over time, a mature tissue with appropriate composition, biological properties, mechanical properties, and function may develop. A bioreactor serves the additional purpose of providing a three-dimensional culture environment by generating convection to maintain the temperature, pO2, pCO2, and pH throughout the medium and within the TEHV. Finally, a bioreactor can be used to assess the functionality of a TEHV as it develops by providing pressure and flow measurements indicative of valve opening and closing characteristics.
Bioreactors have proven beneficial for conditioning of TEHVs,11, 27, 31 but current designs are typically limited to a narrow range of hydrodynamic conditions due to the challenge of producing fine control over pressures in time and space while generating large flow rates and maintaining asepsis. Some designs overcome this challenge by focusing on a subset of physiological hydrodynamic conditions such as diastolic strain,21 cyclic positive and negative pressure,6 or flow-flex-stretch.25 Other designs achieve either subphysiological18, 29, 34 or pulmonary17 hydrodynamic conditions, but designs capable of achieving aortic conditions are much less common.12 While progress is being made,33 it is largely unknown which bioreactor conditions are optimal and it is likely that different TEHV designs will require different conditioning protocols. It is also likely that a TEHV will need to be exposed to gradually increasing biomechanical stimulation as it matures, similar to the conditions under which a heart valve develops naturally.
Accordingly, the primary goal of this work was to develop a bioreactor system capable of accurately achieving an unprecedented range of hydrodynamic conditions in order to determine the best conditioning protocol for each unique TEHV design. Our valve bioreactor system is capable of exposing a TEHV to conditions ranging from static to resting adult left ventricular outflow tract (i.e. 5 L/min of flow, 120/80 mmHg of pressure on the aortic side, and 120/12 mmHg of pressure on the ventricular side) to pathological conditions (i.e. hypertension). The secondary goal of this work was to develop a bioreactor system capable of serving as a pulse duplicator for assessing hydrodynamic performance of TEHVs based on ISO 5840 standards.14 Current pulse duplicators are designed for non-living valve prostheses5 and a system capable of maintaining cell viability and asepsis is needed for translating TEHVs into clinical practice.
To accomplish these goals, we developed a bioreactor system with several novel features. First, a variable resistor is used on the inflow side of the valve to provide control over ventricular pressure during diastole. Second, a relief valve is used on the outflow side of the valve to provide control over pressure during systole. Third, a proportional-integral (PI) feedback loop is used to maintain desired pressures, particularly during low flow conditions. Fourth, a unique valve chamber was designed to allow for rapid, aseptic valve installation without compromising fluid flow characteristics. Finally, this is the first system to allow for both TEHV conditioning and hydrodynamic performance assessment.
Materials and Methods
Bioreactor design
Our specific design objectives were to create a system that was: (1) self-contained and independent of an incubator, (2) straightforward to sterilize and assemble, (3) capable of a range of operating modes and parameter settings, and (4) able to monitor and record critical parameters. The parameters we desired to control included stroke volume, stroke rate, systole:diastole ratio, ventricular side pressure, aortic side pressure, pO2, pCO2, pH, and temperature. The parameters we desired to measure included volumetric flow rate, ventricular side pressure, aortic side pressure, pO2, pCO2, pH, and temperature.
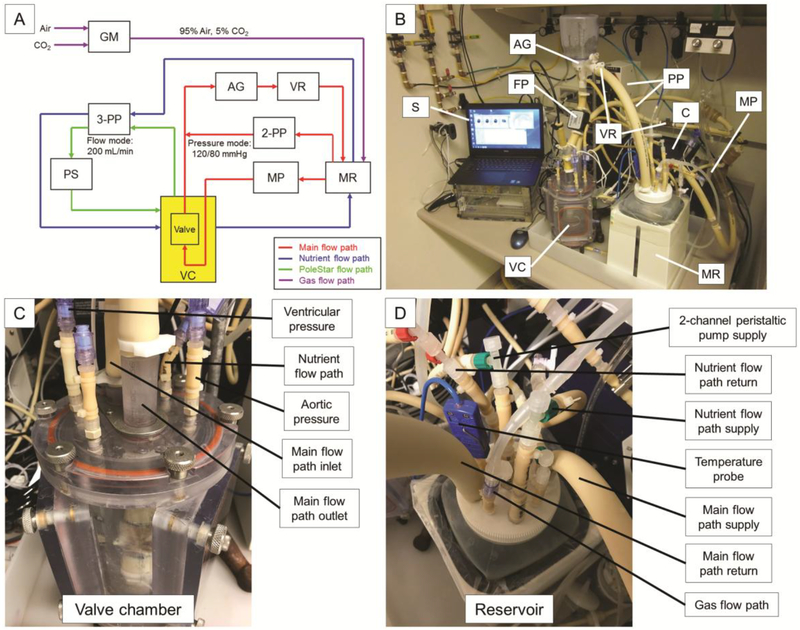
The bioreactor system is comprised of the following basic components (Fig. 1): main pulsatile pump (MP), peristaltic pumps (PP), heated medium reservoir (MR), afterload generator (AG) consisting of a compliance chamber and a relief valve, variable resistors (VR), valve chamber (VC), flow probe (FP), software (S), and controller (C). These components coordinate to control and monitor critical parameters.
Figure 1. Bioreactor system schematics.
(A) Schematic and (B-D) photographs of the bioreactor system showing critical components. The main flow path (red) utilizes the main pulsatile pump to generate flow through the valve lumen and additional components to achieve desired pressures above and below the valve throughout the cardiac cycle. The nutrient flow path (blue) circulates medium between the heated and oxygenated reservoir and the valve chamber. The PoleStar flow path (green) samples medium from the valve chamber to monitor pO2, pCO2, and pH. The gas flow path (purple) introduces a mixture of 95% air and 5% CO2 to the medium reservoir. Abbreviations: AG - afterload generator, C - controller, FP - flow probe, GM - gas mixer, MP - main pulsatile pump, MR - medium reservoir, 2-PP - 2-channel peristaltic pump, 3-PP - 3-channel peristaltic pump, PS - PoleStar optical process monitor, S -software, VC - valve chamber, VR - variable resistor.
The system includes three culture medium flow paths (main, nutrient, and PoleStar) and one gas flow path (Fig. 1A). The main flow path is: medium reservoir (MR) to main pulsatile pump (MP) to check-valve and variable resistor (VR) in parallel to valve lumen to afterload generator (AG) to variable resistor (VR) and back to reservoir. The main flow path also utilizes the 2-channel peristaltic pump (2-PP) in a pressure feedback control mode to pump fluid in either direction between the medium reservoir and the aortic side of the valve for the purpose of achieving and maintaining set pressures during systole and diastole. The nutrient flow path is: medium reservoir (MR) to 3-channel peristaltic pump (3-PP) to valve chamber (VC) and back to reservoir. The PoleStar flow path is: valve chamber (VC) to 3-channel peristaltic pump (3-PP) to PoleStar optical process monitor (PS) and back to valve chamber. The gas flow path delivers air and CO2 to a two gas mixer (GM), which creates a mixture of 95% air and 5% CO2. This mixture is then delivered to the culture medium within the medium reservoir (MR) via a submerged diffusion stone.
Flows through tissue valve lumen
Precise flow control is critical for providing the appropriate bending stress and fluid shear stress to the valve cusps during the systolic phase of the cardiac cycle when the cusps open and then close. The main pulsatile pump (Harvard Apparatus, Holliston, MA) generates unsteady flow through the valve lumen to simulate physiological blood flow. The stroke volume, stroke rate, and systole:diastole ratio can be independently controlled to achieve a desired flow profile. The stroke volume is set by a mechanical crank on the pump and the stroke rate and systole:diastole ratio are set in the software. Adult resting flow of 5 L/min can be generated with the following parameters: 70 mL stroke volume, 70 bpm stroke rate, and 35% systole (based on ISO 5840 standards). The volumetric flow rate can be scaled down by decreasing the stroke volume and/or stroke rate.
The volumetric flow rate is recorded using an ultrasonic flow probe (Transonic Systems Inc, Ithaca, NY) placed on the outflow side of the tissue valve. This can be used to verify the average volumetric flow rate as well as record the instantaneous flow rate over time to assess the valve for hydrodynamic performance (e.g. effective orifice area and regurgitant fraction) based on ISO 5840 standards.
Pressures on aortic and ventricular sides of the tissue valve
Precise pressure control is critical for providing the appropriate tensile stress to the valve cusps during the diastolic phase of the cardiac cycle when the cusps are closed. In a healthy adult, the aortic side of the valve typically experiences a peak pressure of 120 mmHg during systole and a minimum pressure of 80 mmHg during diastole. The ventricular side of the valve typically experiences a peak pressure of 120 mmHg during systole and a minimum pressure of 12 mmHg during diastole. Achieving these time varying pressure waveforms both above and below the valve requires the coordinated efforts of several system components. It may also be desirable to simulate pathological conditions including hypertension for testing purposes, but the current ISO 5840 standards do not require this for hydrodynamic performance or durability assessment.
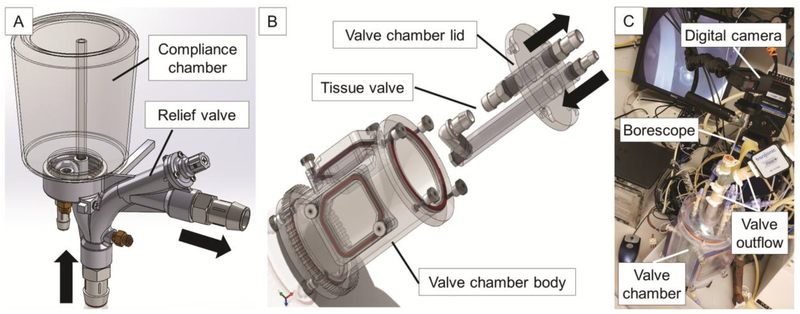
The custom-built afterload generator on the outflow side of the tissue valve consists of a compliance chamber and a relief valve (Fig. 2A). The relief valve is set to open at a prescribed pressure, which helps to determine the peak systolic pressure. The compliance chamber serves to mimic the compliance of the aorta and other large elastic arteries. The air in the compliance chamber can be pressurized to help maintain diastolic pressure on the aortic side of the valve. A variable resistor on the outflow side of the afterload generator is used to restrict the forward flow of medium and allows for energy to be stored during systole and released during diastole. This resistor mimics the resistance of the arterioles. The resistance can be set empirically such that the main pulsatile pump generates a peak pressure of 120 mmHg during systole. The compliance and resistance have the combined effect of smoothing the pressure waveforms and providing backpressure on the aortic side of the valve during diastole to help close the valve cusps and maintain pressure as in the classic Windkessel model of the cardiovascular system.
Figure 2. Schematics of afterload generator and valve chamber assembly.
(A) The afterload generator resides on the outflow side of the tissue valve and contains both a compliance chamber and a relief valve. The compliance chamber mimics the elasticity of the aorta and large elastic arteries while the relief valve helps to control the peak systolic pressure. (B) The valve chamber body is filled with cell culture medium. The tissue valve is installed between the barbed connectors of the lid assembly to allow for quick installation and submersion. Inflow and outflow directions are indicated by black arrows. (C) Photograph of borescope camera set-up showing placement above the outflow side of the tissue valve.
The 2-channel peristaltic pump (Cole-Parmer, MasterFlex, Vernon Hills, IL) is controlled by a PI feedback loop to maintain set pressures on the aortic side of the valve throughout the cardiac cycle and on the ventricular side of the valve during systole. Separate pressure targets can be set for systole and diastole and typical settings would be 120 mmHg and 80 mmHg, respectively, for a healthy adult. The software measures the pressure on the aortic side of the valve and runs the pump in the forward or reverse direction at the rate needed to achieve the target pressures. Running in the forward direction pushes fluid from the reservoir to the aortic side of the valve to increase pressure while running in the reverse direction pulls fluid from the aortic side of the valve to the reservoir to decrease pressure.
The outflow check-valve of the main pulsatile pump is placed in parallel with a variable resistor to allow for a controllable degree of backflow during diastole. The resistance can be set empirically to allow the pressure on the ventricular side of the valve to reach a desired minimum value during diastole, which is typically 12 mmHg for a healthy adult.
Thus, the main pulsatile pump, main pulsatile pump outflow check-valve and variable resistor in parallel, relief valve, compliance chamber, feedback controlled 2-channel peristaltic pump, and outflow variable resistor coordinate to achieve physiological pressure waveforms throughout the cardiac cycle on both the aortic and ventricular sides of the valve. Systolic pressure on both the aortic and ventricular sides of the valve is generated by the main pulsatile pump, fine-tuned by the feedback controlled 2-channel peristaltic pump, smoothed by the compliance chamber, and set to a maximum value by the relief valve and variable resistor. Aortic side diastolic pressure is maintained by the feedback controlled 2-channel peristaltic pump, compliance chamber, and variable resistor. Ventricular side diastolic pressure is controlled by the small amount of backflow allowed to bypass the main pulsatile pump’s outflow check-valve by the variable resistor in parallel.
Pressure transducers (PendoTECH, Princeton, NJ) measure the pressure on the aortic and ventricular sides of the valve and these values are recorded by the software. Ports in the lid of the valve chamber are connected to the flow path immediately upstream and downstream of the valve to provide easy access for direct pressure measurement. The mean pressure gradient during systole is used to calculate the effective orifice area according to ISO 5840 standards.
Nutrient delivery and monitoring
It is critical for the bioreactor system to provide a suitable three-dimensional tissue culture environment. Cells throughout the TEHV require precisely controlled nutrients, temperature, pO2, pCO2, and pH. The valve is bathed within culture medium in the valve chamber to allow for diffusion of these critical components and removal of metabolic waste.
The medium reservoir (Harvard Apparatus, Holliston, MA) has a 4 L capacity and is capable of aseptic medium exchanges to bring fresh nutrients and remove metabolic waste. The reservoir is heated to a set point programmed into the software to maintain physiological temperature of the culture medium. In practice, maintaining the reservoir 1–2 °C above the desired temperature will compensate for heat loss between the reservoir and the valve chamber. The software measures the reservoir temperature and powers the heating element as needed. The main pulsatile pump brings heated medium from the reservoir to the lumen of the valve via the main flow path. The 3-channel peristaltic pump generates continuous circulation between the reservoir and the valve chamber to provide additional convection of nutrients and thermal energy to the medium surrounding the valve. The reservoir also serves as a bubble trap for the system to remove air bubbles from the circulating medium which could have a detrimental effect on the valve. Air bubbles can be safely purged by priming the tubing with a low flow immediately following set-up.
The 3-channel peristaltic pump (Cole-Parmer, MasterFlex, Vernon Hills, IL) also carries culture medium from the valve chamber to the PoleStar optical process monitor (PoleStar Technologies, Needham Heights, MA) for monitoring of pO2, pCO2, and pH. The pO2 and pCO2 target values of 20.0% and 5.0%, respectively, are used to verify that the culture medium is properly equilibrated with air and CO2. The two gas mixer (Warner Instruments, Hamden, CT) and diffusion stone help equilibrate the culture medium with 95% air and 5% CO2 without causing excessive foaming of the medium. The reservoir is maintained at atmospheric pressure by using a sterile filter to allow for gas escape. In addition, physiological pH of 7.4 indicates the medium is aseptic as well as properly equilibrated with CO2. The PoleStar optical process monitor also measures temperature, which provides continuous verification of the valve chamber temperature.
Valve chamber
The tissue valve resides within the valve chamber (Harvard Apparatus, Holliston, MA), which has ports to access both the intraluminal and extraluminal spaces (Fig. 2B). These ports can be used to aseptically collect medium samples for microscopically analyzing the presence of contamination. The valve chamber bathes the valve in culture medium and seals to maintain asepsis. The main flow path routes through the intraluminal space of the tissue valve and is kept isolated from the nutrient and PoleStar flow paths that route to the extraluminal space within the valve chamber. This ensures the hydrodynamics of the main flow path are not affected by the other flow paths.
The valve chamber was designed to allow for aseptic installation of the valve within a laminar flow hood. The valve is clamped into the main flow path between two barbed adapters. The valve can be either directly clamped if there is sufficient tissue conduit above and below the cusps or a synthetic polymer conduit can be sutured above and below the valve to allow for clamping. Alternatively, the valve can be fitted within a plastic tube, with or without anchoring sutures, and the tube can be clamped to the barbed adapters. This method is especially effective with stented valve designs.
The barbed adapters securing the valve are purposefully assembled to the lid so that the valve can be quickly submerged into the medium-filled valve chamber by simply assembling the lid into position. The quick assembly minimizes the risk of contamination and minimizes the time the valve is not submerged in culture medium. However, this design requires the main flow path to undergo two 90° turns before reaching the valve. This part of the flow path had to be carefully designed to ensure the tissue valve would be exposed to symmetrical and non-rotating flow. Computational fluid dynamics (CFD) was used to compare candidate designs prior to fabrication.
The valve chamber also includes interchangeable windows. The windows can be removed in a laminar flow hood for quick access to the valve. Windows made of polycarbonate allow for unobstructed viewing of the valve. Windows with a silicon membrane allow for ultrasonic imaging of the valve cusps. In addition, a borescope camera (Medit Inc, Winnipeg, Canada) can be optionally inserted into the outflow tract of the valve chamber to allow for direct visualization of the valve cusps from the aortic side (Fig. 2C). Asepsis can be maintained by wrapping a sterilized, clear plastic bag around the boroscope and installing within a laminar flow hood.
Sterilization and assembly
All components are made from materials that can be steam sterilized (polycarbonate, polysulfone, polypropylene, rubber gaskets, and Masterflex PharMed® tubing). These materials can maintain their dimensions over repeated sterilization cycles in order to provide water-tight seals and minimize the risk of contamination. Following sterilization, the components are allowed to cool and then aseptically assembled in a laminar flow hood. The components assemble quickly, without the need for tools, and with minimal handling. Due to the unique design of the valve chamber, it can be filled with cell culture medium and the tissue valve can then be quickly installed and submerged with minimal handling inside of the laminar flow hood. The straightforward assembly of the components and quick installation of the tissue valve are intended to minimize the risk of contamination.
Once the bioreactor system is assembled and the valve is installed, the system can be relocated to any clean laboratory space for operation since the entire system is sealed from the environment other than a sterile air filter on the medium reservoir. Additional safeguard against contamination, for example operation within a laminar flow hood, cell culture incubator, or good manufacturing practice (GMP) cleanroom, should be considered for animal and clinical studies. The main pulsatile pump head is assembled to the motor shaft, the appropriate tubes are installed into the peristaltic pump heads, pressure transducers are connected, and the temperature transducer is connected. The software can then be used to operate the pumps and run the system.
Operation
The software (Harvard Apparatus, Holliston, MA) is used to set a variety of input parameters, control certain system elements, and record data. The user can set the reservoir temperature and the software will turn on the heating element at 0.1 °C below the set temperature and turn off the heating element at 0.1 °C above the set temperature. The software is also used to set the stroke rate and systole:diastole ratio of the main pulsatile pump, which are used to calculate the forward and reverse stroke times according to the following equations:
| (1) |
| (2) |
Where tsys is forward stroke time, tdia is reverse stroke time, bpm is stroke rate in beats per minute, and % systole is the percentage of each cycle that is forward stroke. The software is also used to set the flow rate of the peristaltic pumps, which can be constant, oscillatory, or based on pressure feedback control. In oscillatory mode, the maximum pressure, minimum pressure, forward flow rate, and reverse flow rate are set by the user in the software. In feedback control mode, the software uses the PID and Fuzzy Logic Toolkit (National Instruments Corporation, Austin, TX) to solve the following equation for controlling the pump speed:
| (3) |
Where u(t) is the pump speed control variable, e(t) is the error value, Kp is the proportional coefficient, and Ki is the integral coefficient. Target diastolic pressure target systolic pressure, maximum forward flow rate, maximum reverse flow rate, Kp, and Ki are set by the user in the software.
Parameters measured and recorded by the software include volumetric flow rate, systole/diastole state of the pulsatile pump, four pressure channels (e.g. aortic pressure, ventricular pressures, and valve chamber pressure), and four temperature channels (e.g. reservoir and valve chamber temperatures).
Finally, the software is capable of sending hourly status e-mails as well as text message alerts if system parameters exceed user-defined limits. The status e-mails show recent pressure, flow, and temperature waveforms as well as instantaneous values. The text message alerts can be enabled to trigger when system parameters including pressure, flow, and temperature exceed upper or lower limits.
Bioreactor validation and performance studies
A Freestyle™ aortic bioprosthesis (Medtronic, Minneapolis, MN) was installed into the system to validate the design. Pressure and flow were recorded under a variety of conditions from sub-physiological to physiological and an error analysis was performed. Sensitivity analyses were performed by slowly varying the stroke rate, inflow variable resistor, and outflow variable resistor. Effective orifice area and regurgitant fraction were calculated from measured parameters based on ISO 5840 standards.
CFD (ANSYS, Ansys Inc., Canonsburg, PA) was used to design a flow path that would establish symmetrical and non-rotating flow through the valve. The CFD analysis studied 5 L/min and 15 L/min of flow and used a κ-epsilon model for turbulence.
The bioreactor was sterilized by autoclave and assembled. The medium consisted of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and 1x antibiotic-antimycotic. DMEM is a Newtonian fluid with a dynamic viscosity of 0.84 cP at 37 °C. Half medium exchanges were performed twice per week to supply fresh nutrients, remove metabolic waste, and supply renewed antibiotic-antimycotic. The software was set to send hourly status e-mails as well as to send alert text messages if processes parameters including temperature, flow, and pressure exceeded certain levels (e.g. reservoir temperature below 37 °C or above 39 °C, instantaneous flow above 18 L/min, valve chamber pressure above 20 mmHg, and aortic pressure below 60 mmHg or above 140 mmHg).
NIH 3T3 fibroblasts (ATCC, Manassas, VA.) were cultured on tissue culture treated plastic or in Matrigel (Corning, Corning, NY) for 2 weeks in the bioreactor valve chamber. The cells were stained with a live/dead viability/cytotoxicity kit (Invitrogen, Carlsbad, CA) and visualized with fluorescence microscopy.
Results
Bioreactor design validation
The system controls reservoir temperature, main pulsatile pump parameters (stroke rate, systole:diastole ratio, and stroke volume), and peristaltic pump flow rate as expected. To achieve the desired pressure response, the user can empirically set a variety of parameters. First, if using pressure feedback control mode, the user can empirically set Kp and Ki to achieve responsiveness with minimal overshoot and oscillation. Second, the user can adjust the relief valve and variable resistor on the outflow side of the tissue valve to achieve the desired maximum systolic pressure. Third, the user can adjust the variable resistor on the inflow side of the valve to achieve the desired minimum diastolic pressure on the ventricular side of the valve. Finally, the user can adjust the air pressure in the compliance chamber to achieve the desired minimum diastolic pressure on the aortic side of the valve. The system will typically converge within 10–20 cycles of any parameter change, allowing for rapid optimization.
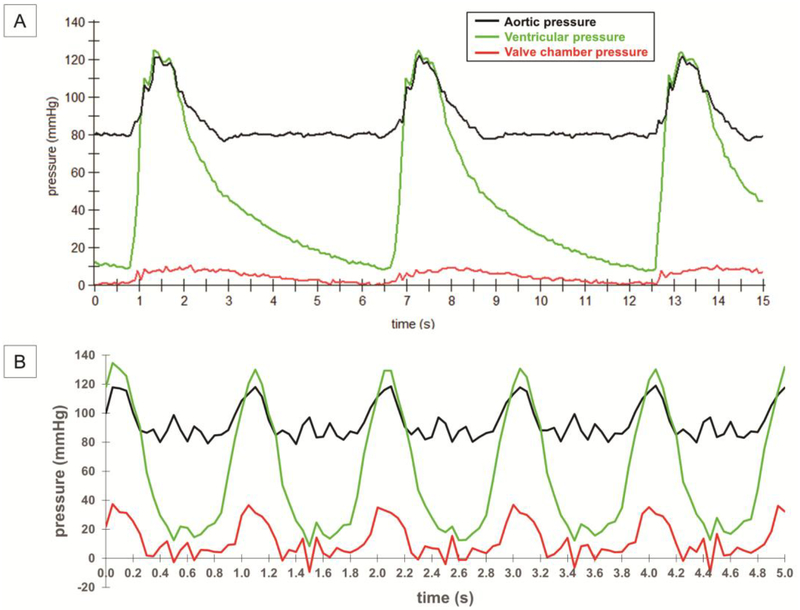
Pressure recordings demonstrate the system’s ability to generate a range of pressure conditions (Fig. 3). Pressures on the aortic side of the tissue valve vary between 120 mmHg and 80 mmHg over the course of a cardiac cycle. Pressures on the ventricular side of the tissue valve vary between 120 mmHg and 12 mmHg over the course of a cardiac cycle. The ventricular pressure is slightly higher than the aortic pressure during systole, indicating the cusps are open and the flow through the valve is encountering little resistance. The large separation between the ventricular pressure and aortic pressure during diastole indicates the cusps are closed and competent. The pressure waveforms are used to calculate effective orifice area, which is a key index of hydrodynamic performance under the ISO 5840 standards.
Figure 3. Pressure recordings from a tissue valve in the bioreactor.
Recordings of ventricular (green) and aortic (black) pressure over time at (A) 10 bpm and (B) 60 bpm. These waveforms are necessary for calculating effective orifice area according to ISO 5840 standards. Also shown is the pressure within the valve chamber (red), which is useful to monitor for ensuring the valve is securely installed and the flow paths are unobstructed.
An error analysis revealed the 2-channel peristaltic pump in pressure feedback control mode is highly effective at achieving target pressures at low stroke rates (Supplementary Table 1). At 10 bpm, all measured pressures are maintained within 1.7% of the target values. The 2-channel peristaltic pump in pressure feedback control mode is less effective at achieving target pressures at high stroke rates. At 60 bpm, the minimum aortic pressure is maintained within 7.5% of the target value at the start of diastole and improves to within 3.3% of the target value at the end of diastole.
A sensitivity analysis revealed increasing the stroke rate from 20 bpm to 70 bpm did not affect the target maximum aortic pressure (120 mmHg) or target minimum ventricular pressure (0 mmHg) (Supplementary Fig. 1A). The ventricular maximum pressure increased slightly over this range due to the increasing flow rate. The aortic minimum pressure dropped slightly over this range, but was near physiological levels (80 mmHg) at physiological stroke rates. The 2-channel peristaltic pump can be used to achieve the target aortic minimum pressure during diastole, especially at low stroke rates, but this feature was disabled for the purposes of the sensitivity analysis. The sensitivity analysis also revealed the inflow resistance can be adjusted to achieve minimum ventricular pressures ranging from −12 to 50 mmHg (Supplementary Fig. 1B) and the outflow resistance can be adjusted to achieve maximum aortic pressures ranging from 55 to 140 mmHg (Supplementary Fig. 1C).
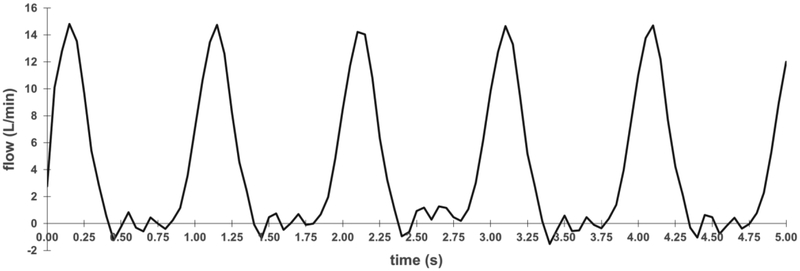
Flow recordings demonstrate the system’s ability to generate a range of flow conditions. At physiological conditions, the instantaneous flow rate varies between a maximum of 15 L/min and a minimum of −1 L/min over the course of a cardiac cycle (Fig. 4). The average volumetric flow rate is also confirmed to be 5.0 L/min. The rapid rise in flow rate during systole indicates little resistance provided by the tissue valve and the minimal degree of retrograde flow during diastole indicates the valve is closed and competent. The flow waveform is used to calculate effective orifice area and regurgitant fraction, both of which are key indices of hydrodynamic performance under the ISO 5840 standards.
Figure 4. Flow recording from a tissue valve in the bioreactor.
Recording of instantaneous volumetric flow over time at 60 bpm. These waveforms are necessary for calculating regurgitant fraction and effective orifice area according to ISO 5840 standards.
Two 90° turns in the main flow path just upstream of the tissue valve were necessary to ensure quick and aseptic installation of the valve. CFD was used to design an improved flow path consisting of a circular obstacle that separates and recombines the flow in a manner that cancels lateral flow momentum and directs the flow up through the tissue valve (Fig. 5). Two partitions across perpendicular diameters placed within the main flow path just below the tissue valve serve to eliminate flow rotation.
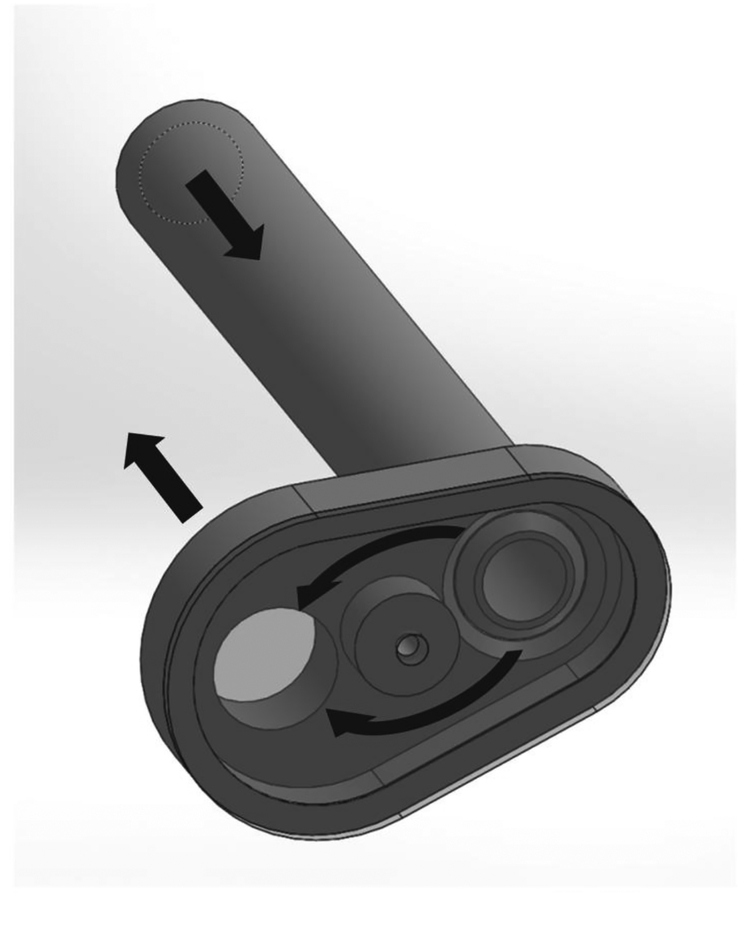
Figure 5. Computer rendering of flow correction component.
The main flow path executes two 90° turns before reaching the valve. This component guides the flow around a circular obstacle to minimize flow disturbances at the valve. Flow directions are indicated by black arrows.
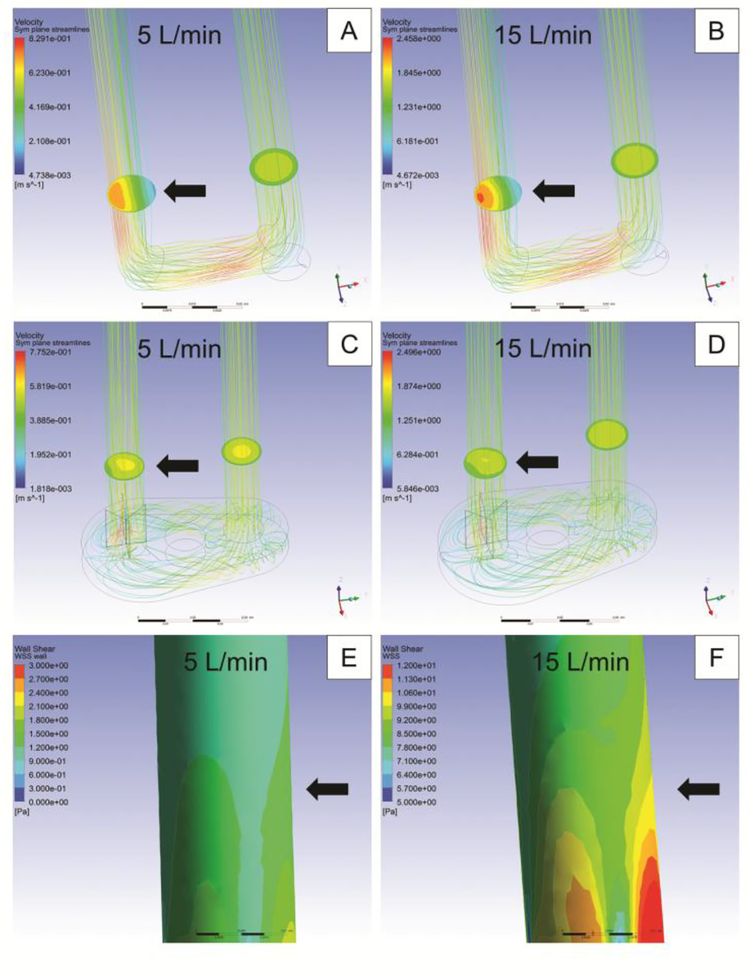
The results of the CFD analysis demonstrate an improvement in the flow profile through the tissue valve lumen as a result of utilizing this flow correction component (Fig. 6A–D). The flow is symmetrical and non-rotational through the valve, which ensures each of the valve cusps experiences the desired hydrodynamic conditions. Visualization of the valve confirmed uniform opening and closing of the cusps following implementation of the corrected flow path. The results of the CFD analysis also demonstrate that the wall shear stress in the vicinity of the tissue valve is 15 dyn/cm2 at 5 L/min of flow and 90 dyn/cm2 at 15 L/min of flow (Fig. 6EߝF).
Figure 6. Computational fluid dynamics analysis of flow path through valve.
Computational fluid dynamics studies were used to confirm the effectiveness of the flow correction device. Before flow correction, the flow is greater on the outer curve of the main flow path through the valve at (A) 5 L/min and (B) 15 L/min. After flow correction, the flow is symmetrical and non-rotational through the valve at (C) 5 L/min and (D) 15 L/min. The wall shear stress is also shown for (E) 5 L/min and (F) 15 L/min. The black arrows indicate the approximate location of the valve in the fluid flow.
Bioreactor performance
Video recordings demonstrate the proper opening and closing of tissue valve cusps (Fig. 7A–B and Video 1). These large scale deformations are critical to stimulate the cells within the TEHV to proliferate, migrate, differentiate, and remodel ECM components. Especially noteworthy is the tensile strain that is evident during diastole after the cusps have coapted.
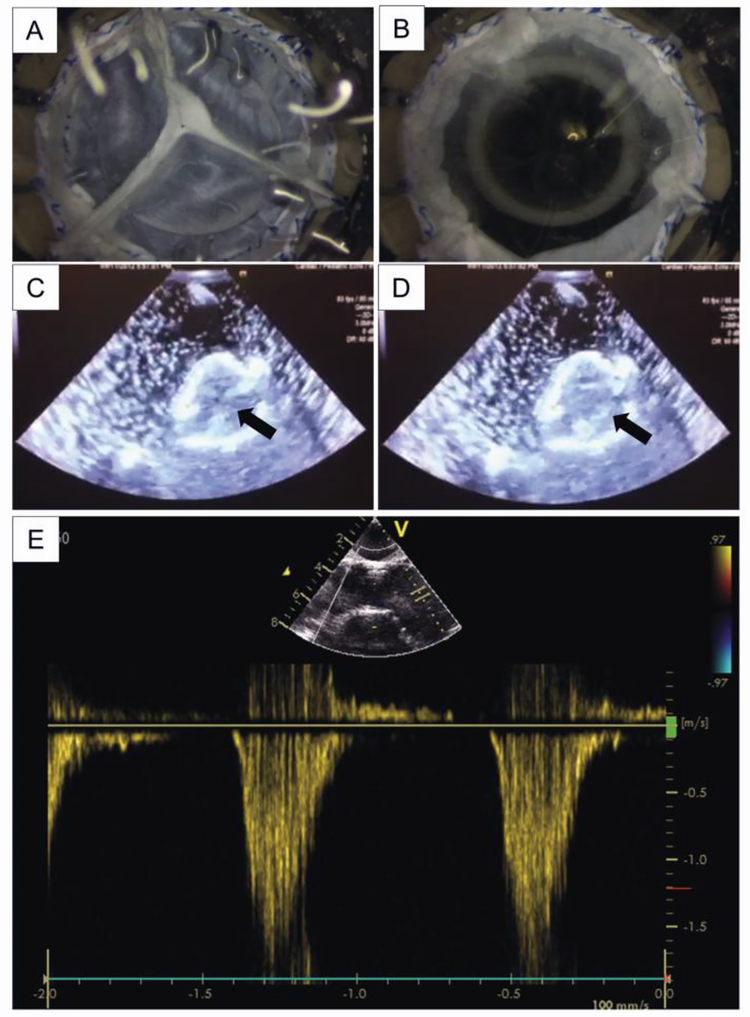
Figure 7. Imaging of a tissue valve in bioreactor.
Photographs of the tissue valve shown (A) closed during diastole and (B) open during systole. Visualization of the valve is helpful for monitoring and troubleshooting, but is not required for testing to ISO 5840 specifications. Echocardiographic images of the valve shown (C) closed during diastole and (D) open during systole. The arrows in (C) and (D) are pointing to the same cusp to indicate its movement. (E) Doppler recordings of flow through the valve orifice are useful for real time assessment of regurgitation and stenosis.
Noninvasive imaging with ultrasonography is an important feature for assessing valve function without altering flow characteristics or introducing the risk of contamination. Ultrasound of a valve was accomplished through the silicone membrane window in the valve chamber with sufficient resolution to visualize the movement of the cusps (Fig. 7C–D). Doppler ultrasonography can also be used to assess functional characteristics of the valve including effective orifice area, mean pressure gradient, and regurgitant fraction (Fig. 7E).
The bioreactor system was successfully run for 4 weeks without interruption of any parameters including flow and temperature. The status e-mails and alert text messages worked as intended. Importantly, no indications of contamination were observed during the 4 week study.
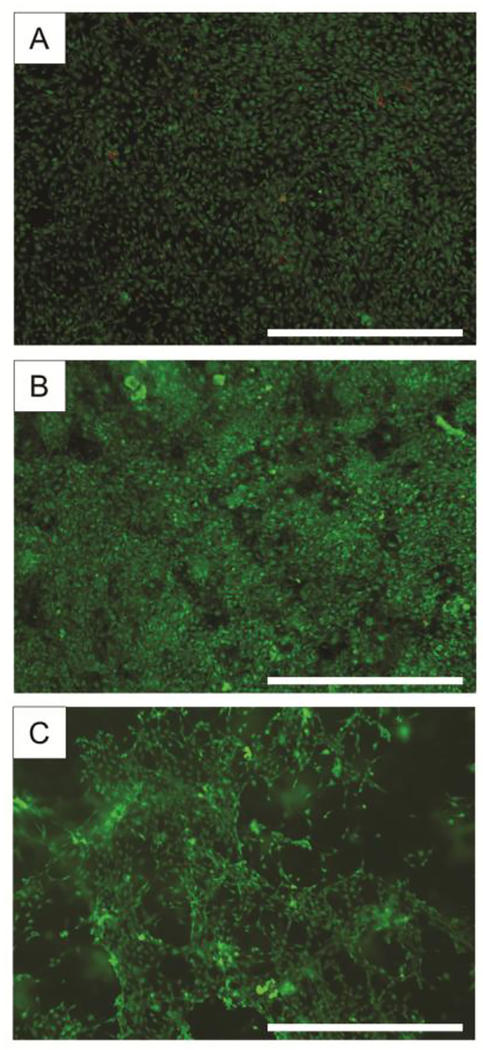
The cells cultured within the bioreactor on tissue culture plastic for 1 week and 2 weeks were confluent and virtually all viable while the cells cultured in Matrigel for 2 weeks were spread and virtually all viable as well (Fig. 8). This indicates the bioreactor is able to achieve proper convection of nutrients and gasses to support cell growth on two-dimensional surfaces as well as within a three-dimensional biomaterial scaffold.
Figure 8. Fluorescence micrographs of cells culturing in bioreactor.
Fibroblasts were cultured on tissue culture treated plastic for (A) 1 week and (B) 2 weeks as well as in (C) Matrigel for 2 weeks all within the bioreactor valve chamber. Viable cells are stained green and non-viable cells are stained red. Scale bars = 1 mm.
The bioreactor was used to assess the hydrodynamic performance of a bioprosthesis and the results were compared to those obtained with a ViVitro Pulse Duplicator system (ViVitro Labs, Victoria, BC, Canada) (Table 1, Supplementary Fig. 2). The transvalvular pressure gradient measurements were within 4 mmHg, which resulted in the effective orifice area calculations being within 4%. The regurgitant fraction measurement in the ViVitro system was over 6% higher, which was attributed to paravalvular leakage due to suboptimal mounting in the ViVitro system.
Table 1.
Hydrodynamic performance assessment comparison.
| Transvalvular Pressure (mmHg) | Effective Orifice Area (cm2) | Regurgitant Fraction (%) | |
|---|---|---|---|
| Bioreactor | 7.4±0.5 | 0.78±0.05 | 0.1±0.3 |
| ViVitro Pulse Duplicator | 4.0±0.2 | 0.81±0.04 | 6.3±1.2 |
Data are shown as mean ± standard deviation.
Discussion
We have developed a bioreactor system capable of generating a range of flow and pressure conditions to stimulate the mechanical and biological maturation of a TEHV. A range of conditions is necessary to allow for optimization of a single- or multi-step protocol for each unique type of TEHV that can efficiently and reliably result in functional and durable valves that are suitable for implantation. Our system was designed with particular emphasis on accurately simulating the physiological conditions of the healthy adult left ventricular outflow tract. Additional emphasis was placed on self-containment, reliable sterilization, and monitoring of critical parameters to assess progress of TEHV functionality over time. The goal was to create a flexible system with considerations for clinical implementation including reliability, efficiency, and release criteria prior to implantation. In addition, our system provides the essential capability of testing TEHV prostheses to ISO 5840 standards, which is not possible with current valve testing systems designed for non-living valve prostheses.
Cardiac valve bioreactor systems were pioneered by Hoerstrup, et al. in 200013 and since that time many groups have developed systems with unique capabilities. The incorporation of resistance and capacitance elements allowed for independent control over pressure conditions,7 which led to a very sophisticated system capable of achieving physiological flow and pressure conditions with feedback control.12 Additional benefits were realized by designs with silicon tubing for gas exchange,32 cell seeding ports,19 Qadapters to accommodate multiple valve types,23, 29 a fluid column for providing afterload,9 a heated reservoir,20 and simultaneous conditioning of multiple valves.8 Other innovations allowed for active monitoring of the TEHV construct during conditioning including a digital camera,24 optical monitoring,34 and endoscopic visualization.18 Our design incorporates many of these concepts to achieve a flexible and highly capable system.
Properly designed bioreactors have been shown to develop the microarchitecture and biomechanical properties of TEHVs. For example, bioreactors have been shown to stimulate cell migration and proliferation.1, 3, 9 These cells, in turn, mediate the production and alignment of ECM.1, 3, 9,26 Importantly, these changes in cellular activity and ECM properties have translated into critical improvements in mechanical properties including stiffness and strength.21, 22, 28 The role of a bioreactor is, therefore, to provide the biomechanical and biochemical stimulation needed to direct cellular activities including proliferation, differentiation, migration, and extracellular matrix production and alignment. While acellular scaffolds or cell-seeded scaffolds cultured under static conditions can produce mechanically competent and functional valves, the value of bioreactor conditioning lies in developing not only the mechanical properties but also the biological properties of a valve including the capacity for remodeling, repair, and growth. These biological properties enable the improvements in durability, growth, and blood compatibility that TEHVs promise to deliver to the clinic.
The advantages of bioreactor conditioning must be weighed against the disadvantages. For example, extra time and cost are associated with bioreactor conditioning of a TEHV. Contamination risk is also significant which means good aseptic technique is critical and antibiotics should be added to the culture medium when possible. Aseptic cell culture conditions are a reasonable standard for a bioreactor and the duration of a conditioning protocol should be no longer than the minimum time required to generate a successful implant. We incorporated several design features to minimize the risk of contamination during the assembly and operation of our bioreactor system, including a novel valve chamber design.
Efforts to study optimal conditioning protocols are currently underway and will likely depend heavily upon the cells and materials that comprise the TEHV. Gradually increasing the flow and pressure conditions over time is likely necessary to strengthen the construct without causing structural damage. Once physiological conditions are reached and tolerated, the construct may still benefit from further conditioning in order to improve biocompatibility and performance under a range of super-physiological and pathological conditions. Our flexible bioreactor design is intended to accommodate the exploration of multi-step conditioning protocols in order to optimize outcomes. Ultimately, the conditioning process finishes within the host and the TEHV subsequently remodels, repairs, and grows as needed.
ISO 5840 standards were written for qualification of non-living cardiac valve prostheses. As TEHVs advance towards clinical application, dedicated standards for rigorous testing of functionality and durability will be essential. Our bioreactor has the unique capability of accommodating the living nature of the valve while enabling assessment of valve functionality and durability to ISO 5840 standards. While accelerated wear testing may not be feasible with a TEHV, our bioreactor can allow for extended durability assessment of a living valve in an environment that allows for the valve to remodel and repair at physiological frequencies.
A single system capable of bioreactor conditioning as well as hydrodynamic performance assessment has two distinct advantage compared with separate systems. First, the valve does not need to be removed from the bioreactor and loaded into the pulse duplicator in order to assess functionality. This serves to reduce the amount of handling and mitigate the risk of contamination. Second, the valve can be readily assessed at regular intervals for functionality to the ISO 5840 standards and other release criteria in order to avoid the costs and risks of unnecessary conditioning time. This approach is particularly important for clinical translation because TEHV strategies relying upon autologous cell sources will likely experience great variability in the minimum necessary conditioning time for each individual patient.
While our current bioreactor design allows for a range of conditioning parameters and extensive monitoring and testing, the added flexibility and capabilities come at the expense of added complexity. This requires a trained operator to clean, sterilize, assemble, and operate the bioreactor successfully. Additionally, there are many tubes and connections that can deteriorate after repeated sterilization cycles and result in leaks and difficulty maintaining asepsis. The operator must diligently inspect all components for deterioration prior to operation. Another limitation of the design is the need to maintain the medium reservoir at a temperature slightly higher than the desired temperature for the valve chamber due to heat loss. While the system does have the ability to control two separate heating elements, we opted not to independently heat the valve chamber in order to reduce complexity, minimize contamination risk, and preserve visualization of the valve. In addition, some fluctuations can be observed in the pressure waveforms at higher flow rates. These fluctuations are strongest at the beginning of diastole, indicating that pressure reflections are formed on the aortic side of the valve when it closes. Sources of non-physiological behavior may include the relief valve and the feedback-controlled peristaltic pump, but these non-physiological components are necessary for generating a range of sub-physiological conditions. Finally, while we have demonstrated cellular viability within our system, we have not yet characterized cellular function including proliferation, differentiation, and collagen production and alignment.
Supplementary Material
Supplementary Figure 1. Sensitivity analysis of bioreactor. (A) The stroke rate was varied and the resulting maximum and minimum pressures on the aortic and ventricular sides of the tissue valve were measured. (B) The resistance of the variable resistor in parallel with the check-valve on the outflow side of the pulsatile pump was varied and the minimum pressure on the ventricular side of the tissue valve was measured. (C) The resistance on the outflow side of the tissue valve was varied and the maximum pressure on the aortic side of the tissue valve was measured.
Supplementary Figure 2. Validation of bioreactor against a ViVitro Pulse Duplicator system. (A) pressure and (B) flow recordings from the bioreactor described in this study and a commercial ViVitro Pulse Duplicator system are superimposed for direct comparison. The same commercial valve (Medtronic Freestyle™ aortic bioprosthesis) was installed in both systems for the test.
Supplementary Table 1. Error analysis.
Video 1. Valve functioning in bioreactor. Video taken using the boroscope and digital camera on the outflow side of the tissue valve.
Acknowledgments
The authors gratefully acknowledge Dr. Sorin V. Pislaru, M.D., Ph.D. for assistance with echocardiographic imaging and Drs. Robert T. Tranquillo and Zeeshan Syedain for assistance with pulse duplicator validation.
This work was supported by a generous gift from HH Sheikh Hamed Bin Zayed Al-Nahyan and by the NIH (T32 HL007111, K99 HL129068).
Footnotes
Conflict of Interest: The authors disclose that Harvard Apparatus owns intellectual property filings related to this work with JB, HH, and JC listed as co-inventors. BT, JC, MY, RH, DM, DD, RS, and AL declare that they have no conflict of interest.
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Aleksieva G, Hollweck T, Thierfelder N, Haas U, Koenig F, Fano C, Dauner M, Wintermantel E, Reichart B, Schmitz C and Akra B. Use of a special bioreactor for the cultivation of a new flexible polyurethane scaffold for aortic valve tissue engineering. Biomed Eng Online 11: 92, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrollahi P and Tayebi L. Bioreactors for heart valve tissue engineering: a review. Journal of Chemical Technology and Biotechnology 91: 847–856, 2016. [Google Scholar]
- 3.Barzilla JE, McKenney AS, Cowan AE, Durst CA and Grande-Allen KJ. Design and validation of a novel splashing bioreactor system for use in mitral valve organ culture. Annals of biomedical engineering 38: 3280–3294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American C Heart Association Statistics and S. Stroke Statistics. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buse EE, Hilbert SL, Hopkins RA and Converse GL. Pulse Duplicator Hydrodynamic Testing of Bioengineered Biological Heart Valves. Cardiovasc Eng Technol 7: 352–362, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Converse GL, Buse EE, Neill KR, McFall CR, Lewis HN, VeDepo MC, Quinn RW and Hopkins RA. Design and efficacy of a single-use bioreactor for heart valve tissue engineering. J Biomed Mater Res B Appl Biomater 105: 249–259, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Dumont K, Yperman J, Verbeken E, Segers P, Meuris B, Vandenberghe S, Flameng W and Verdonck PR. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif Organs 26: 710–714, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Durst CA and Grande-Allen KJ. Design and physical characterization of a synchronous multivalve aortic valve culture system. Annals of biomedical engineering 38: 319–325, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan TC, Cornelissen C, Koch S, Tschoeke B, Sachweh JS, Schmitz-Rode T and Jockenhoevel S. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28: 3388–3397, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gandaglia A, Bagno A, Naso F, Spina M and Gerosa G. Cells, scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations. Eur J Cardiothorac Surg 39: 523–531, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Ghazanfari S, Driessen-Mol A, Sanders B, Dijkman PE, Hoerstrup SP, Baaijens FP and Bouten CV. In Vivo Collagen Remodeling in the Vascular Wall of Decellularized Stented Tissue-Engineered Heart Valves. Tissue engineering. Part A 21: 2206–2215, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand DK, Wu ZJ, Mayer JE Jr. and Sacks MS. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Annals of biomedical engineering 32: 1039–1049, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hoerstrup SP, Sodian R, Sperling JS, Vacanti JP and Mayer JE Jr. New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue engineering 6: 75–79, 2000. [DOI] [PubMed] [Google Scholar]
- 14.ISO/TC. 5840–1:2015: Cardiovascular implants -- Cardiac valve prostheses --Part 1: General requirements. 2015.
- 15.Jana S, Tefft BJ, Spoon DB and Simari RD. Scaffolds for tissue engineering of cardiac valves. Acta biomaterialia 10: 2877–2893, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Jana S, Tranquillo RT and Lerman A. Cells for tissue engineering of cardiac valves. Journal of tissue engineering and regenerative medicine 10: 804–824, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Kaasi A, Cestari IA, Stolf NA, Leirner AA, Hassager O and Cestari IN. A new approach to heart valve tissue engineering: mimicking the heart ventricle with a ventricular assist device in a novel bioreactor. Journal of tissue engineering and regenerative medicine 5: 292–300, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Konig F, Hollweck T, Pfeifer S, Reichart B, Wintermantel E, Hagl C and Akra B. A pulsatile bioreactor for conditioning of tissue-engineered cardiovascular constructs under endoscopic visualization. J Funct Biomater 3: 480–496, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lichtenberg A, Tudorache I, Cebotari S, Ringes-Lichtenberg S, Sturz G, Hoeffler K, Hurscheler C, Brandes G, Hilfiker A and Haverich A. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials 27: 4221–4229, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Migneco F, Hollister SJ and Birla RK. Tissue-engineered heart valve prostheses: ‘state of the heart’. Regen Med 3: 399–419, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mol A, Driessen NJ, Rutten MC, Hoerstrup SP, Bouten CV and Baaijens FP. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Annals of biomedical engineering 33: 1778–1788, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mol A, Rutten MC, Driessen NJ, Bouten CV, Zund G, Baaijens FP and Hoerstrup SP. Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114: I152–158, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Morsi YS, Yang WW, Owida A and Wong CS. Development of a novel pulsatile bioreactor for tissue culture. J Artif Organs 10: 109–114, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Narita Y, Hata K, Kagami H, Usui A, Ueda M and Ueda Y. Novel pulse duplicating bioreactor system for tissue-engineered vascular construct. Tissue engineering 10: 1224–1233, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Boronyak SM, Le T, Holmes A, Sotiropoulos F and Sacks MS. A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions. J Biomech Eng 136: 121009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy S, Gottlieb D, Engelmayr GC Jr., Aikawa E, Schmidt DE, Gaitan-Leon DM, Sales VL, Mayer JE Jr. and Sacks MS. The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials 31: 1114–1125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rath S, Salinas M, Villegas AG and Ramaswamy S. Differentiation and Distribution of Marrow Stem Cells in Flex-Flow Environments Demonstrate Support of the Valvular Phenotype. PLoS One 10: e0141802, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenke-Layland K, Opitz F, Gross M, Doring C, Halbhuber KJ, Schirrmeister F, Wahlers T and Stock UA. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals-an in vitro study. Cardiovasc Res 60: 497–509, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Sierad LN, Simionescu A, Albers C, Chen J, Maivelett J, Tedder ME, Liao J and Simionescu DT. Design and Testing of a Pulsatile Conditioning System for Dynamic Endothelialization of Polyphenol-Stabilized Tissue Engineered Heart Valves. Cardiovasc Eng Technol 1: 138–153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spoon DB, Tefft BJ, Lerman A and Simari RD. Challenges of biological valve development. Interventional Cardiology 5: 319–334, 2013. [Google Scholar]
- 31.VeDepo M, Buse E, Quinn R, Hopkins R and Converse G. Extended bioreactor conditioning of mononuclear cell-seeded heart valve scaffolds. J Tissue Eng 9: 2041731418767216, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warnock JN, Konduri S, He Z and Yoganathan AP. Design of a sterile organ culture system for the ex vivo study of aortic heart valves. J Biomech Eng 127: 857–861, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Williams A, Nasim S, Salinas M, Moshkforoush A, Tsoukias N and Ramaswamy S. A “sweet-spot” for fluid-induced oscillations in the conditioning of stem cell-based engineered heart valve tissues. J Biomech 65: 40–48, 2017. [DOI] [PubMed] [Google Scholar]
- 34.Ziegelmueller JA, Zaenkert EK, Schams R, Lackermair S, Schmitz C, Reichart B and Sodian R. Optical monitoring during bioreactor conditioning of tissue-engineered heart valves. Asaio J 56: 228–231, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Sensitivity analysis of bioreactor. (A) The stroke rate was varied and the resulting maximum and minimum pressures on the aortic and ventricular sides of the tissue valve were measured. (B) The resistance of the variable resistor in parallel with the check-valve on the outflow side of the pulsatile pump was varied and the minimum pressure on the ventricular side of the tissue valve was measured. (C) The resistance on the outflow side of the tissue valve was varied and the maximum pressure on the aortic side of the tissue valve was measured.
Supplementary Figure 2. Validation of bioreactor against a ViVitro Pulse Duplicator system. (A) pressure and (B) flow recordings from the bioreactor described in this study and a commercial ViVitro Pulse Duplicator system are superimposed for direct comparison. The same commercial valve (Medtronic Freestyle™ aortic bioprosthesis) was installed in both systems for the test.
Supplementary Table 1. Error analysis.
Video 1. Valve functioning in bioreactor. Video taken using the boroscope and digital camera on the outflow side of the tissue valve.