Abstract
Objective:
To test the hypothesis that HbA1c variability, as measured by standard deviation (SD), is associated with increased risk for incident microalbuminuria and persistent microalbuminuria in pediatric type 1 diabetes (T1D).
Methods:
A retrospective analysis using data from electronic health records was performed on 1195 patients from a pediatric diabetes clinic network in the Midwest USA from 1993 to 2009 with ≥1 yr of T1D, ≥4 total HbA1c values, ≥2 HbA1c values/yr, ≥1 urine microalbumin. Microalbuminuria, the main outcome was defined as albumin excretion rate ≥20 mcg/min or 2 of 3 consecutive urine microalbumin/creatinine ≥30 mg/gm. Patients who had persistently high microalbumin or who were treated with an angiotensin-converting-enzyme inhibitor within 1 yr were considered to have persistent microalbuminuria. Sex, race, age, diagnosis age, and duration were covariates.
Results:
Median numbers of per-patient HbA1c and microalbumin results were 14 and 3, respectively. Median intrapersonal mean HbA1c and SD were 8.62% (70.72 mol/mol) and 1.47% (16.07 mmol/mol), respectively. The median interquartile range (IQR) of diagnosis age was 9.4 yr (6.26–12.02) and diabetes duration was 4.97 yr (2.93–7.64). A total of 172 patients (14.4%) developed microalbuminuria; 55 (4.6%) had persistent microalbuminuria. Patients with higher SD of HbA1c had shorter time to microalbuminuria. In time-dependent Cox Proportional Hazard models, updated SD of HbA1c was significantly associated with microalbuminuria [univariate hazard ratio (HR) 1.48 (1.25–1.76); multivariable HR 1.28 (1.04–1.58)], whereas updated mean HbA1c was not [univariate HR 1.08 (0.97–1.22); multivariable HR 1.05 (0.92–1.2)]. Patients with persistent microalbuminuria had similar HRs.
Conclusions:
HbA1c variability is independently associated with development of microalbuminuria in children with T1D, highlighting the importance of maintaining stable glycemic control in pediatric patients.
Keywords: children, HbA1c variability, microalbuminuria, nephropathy, type 1 diabetes mellitus
Children with type-1 diabetes mellitus (T1D) experience significant risk for micro- and macrovascular complications (1–4). Mean glycemic control (HbA1c) is a major determinant of outcomes, but explains only 11% of the population variation in risk (5). Patients with the same HbA1c can have significantly divergent risk for complication (6). Furthermore, evidence suggests that poor glucose control early in the course of diabetes has long-lasting effects on the risk for diabetes-related complications (7–9). Incidence of pediatric T1D is increasing worldwide (10–14), making its sequelae a significant public health concern. Advances in insulin pharmacotherapy, insulin delivery technology, and blood glucose monitoring have led to improvements in glycemic control overall. However, many patients still develop long-term complications (15, 16). Therefore, there is a critical need to identify additional risk factors for diabetes-related complications, so that providers can better identify those patients at high risk and offer targeted intensive interventions before significant damage occurs.
Thus, we sought to determine whether variability in chronic glycemic control, as measured by HbA1c variability, is independently associated with microalbuminuria in a large population of children with T1D. Finding an association between greater HbA1c variability and early diabetic nephropathy that is independent of mean HbA1c can identify an additional target for therapy; namely, more stable glycemic control over time.
Methods
Data source
Data were extracted from the Children’s Mercy database on Type One Diabetes in Pediatrics (the ‘Mercy on TODP’ database) after getting approval from the institutional review board. The Mercy on TODP database is a longitudinal database containing demographic, clinical, and laboratory data extracted from the electronic health records of patients with T1D cared for since 1 June 1993 at the Children’s Mercy Hospital (Kansas City, MO, USA) at its main location, three metropolitan branches, three satellite cities, and six rural locations.
Inclusion/exclusion criteria for data source.
All patients diagnosed with T1D who had at least one appointment in the diabetes clinic were included. Patients with other types of diabetes (such as type 2, monogenic, cystic-fibrosis-related, or iatrogenic diabetes) or with other co-morbid diagnoses that might impact their diabetes care or complications (e.g., sickle cell disease, leukemia, congenital syndromes and heart disease) were excluded. In total, 3423 individuals were included in the database. Review of a random sample of 100 charts indicated 100% accuracy of all extracted data.
Variable definitions for data source.
Age at T1D diagnosis.
Date of diagnosis was extracted from the clinic notes and used to calculate age at diagnosis to the nearest one-tenth year. When not documented, date of diagnosis was determined as the first date at which (1) the patient met American Diabetes Association (ADA) criteria for a diagnosis of diabetes, and (2) C peptide and/or auto-antibody screening indicated T1D.
Demographic characteristics.
Sex (male/female) and race/ethnicity (Caucasian, African-American, Hispanic, Asian, American-Indian or Alaska Native, Native Hawaiian or Pacific Islander, multi-racial, or other) were self-reported by the patient or family at the first encounter with the institution.
Hemoglobin A1c (HbA1c).
HbA1c was measured in the CMH endocrine laboratory using one of the following assays: QuikSep manual ion exchange column (1980–1999), BioRad-diaStat HPLC (1999–2001), Biorad-Variant-II HPLC (2001–2004), Primus-PDQ Boronate Affinity (2004–2008), or Tosoh-G8 HPLC (2008–present). HbA1c assays used beyond 1999 were all aligned to Diabetes Control and Complications Trial (DCCT) standards. Some of the measures obtained after 2009 were performed using In2it point-of-care instruments (Boronate Affinity, contemporary with the Tosh-G8 HPLC) when patients were seen in certain off-campus locations. Results were reported as percentages (%; NGSP standard) and SI units (mmol/mol; IFCC standard). The standard of care was to obtain HbA1c at every routine clinic appointment, typically every 3 months.
Urine microalbumin.
Urine microalbumin excretion was assessed using spot urine albumin/creatinine ratio (ACR) or timed urine albumin-excretion rate (AER), reported in mg/gm of creatinine or mcg/min, respectively. Microalbuminuria was defined as either a single abnormal AER ≥20 mcg/min, or abnormal ACR ≥30 mg/gm in two out of three consecutive samples. When an abnormal ACR or AER was followed by another abnormal result of either type, the patient was defined to have microalbuminuria at the time of the first abnormal result. Patients were considered to have persistent microalbuminuria if microalbumin levels remained elevated on subsequent collections for 1 yr, or if they were started on an angiotensin-converting-enzyme inhibitor within 1 yr of microalbuminuria.
Cohort selection for this study
From the 3423 patients in the database, patients were excluded if they: had an unknown age at diagnosis (n = 438), did not have at least one documented urine microalbumin result (n = 900), did not have at least four HbA1c measures (n = 488), did not have at least two HbA1c measures/yr (n = 391), or had T1D duration <1 yr (n = 11). Using these criteria, 1195 subjects qualified for this study.
Statistical methods
Intrapersonal mean of HbA1c levels (mean-A1c) were calculated using all HbA1c results between diagnosis and either the detection of microalbuminuria (i.e., the ‘event’) or censoring at the end of the observation period. To measure HbA1c variability, standard deviation of HbA1c (SD-A1c) was calculated similarly.
All data are expressed as mean ± SD or median interquartile range (IQR). Characteristics of patients with and without microalbuminuria were compared using chi-square test and t-tests for categorical and continuous variables, respectively. Patients were stratified into groups based on their mean-A1c and SD-A1c. Kaplan–Meier survival curves were generated for each stratum. Strata based solely on SD-A1c included those ≥50th, 75th and 90th percentiles, and those within the 50–74th and 75–89th percentiles. Strata based on both mean-A1c and SD-A1c included low mean/low SD, high mean/low SD, high mean/low SD, and high mean/high SD, with ≥50th percentile indicating ‘high’ for each stratum. The log-rank test was used to compare survival curves across strata.
Furthermore, Cox proportional hazard (CPH) analysis was used to assess the association between HbA1c variability and microalbuminuria using two models: (i) Primary (Time-Dependent-Variable) model – where for every patient, a series of updated mean-A1c and SD-A1c were calculated annually from T1D diagnosis until the event or censoring using all HbA1c measures within each year. (ii) Secondary (Fixed-Variable) model – where mean-A1c and SD-A1c were calculated using all HbA1c values from diagnosis until event or censoring. The former approach provides a more accurate assessment of glycemic control and variability over time. To complete the CPH time-dependent-variable analyses, three models were generated: Model 1A – with mean-A1c alone; Model 1B – with SD-A1c alone; and Model 1C – multivariable model with both mean-A1c and SD-A1c included, as well the covariates: sex, race/ethnicity, and age at T1D diagnosis. Similarly, for the CPH fixed-variable analyses, three such models were developed: Model 2A – with mean-A1c alone; Model 2B – with SD-A1c alone; and Model 2C – a multivariable model with mean-A1c and SD-A1c, along with covariates.
All the analyses were performed using SAS 9.2 (Cary, NC, USA) and SPSS 20. Statistical significance was set at the 95% confidence level (p < 0.05).
Results
Cohort characteristics
The study cohort included 1195 patients (53% male, 88% Caucasian, 7.6% African-American). Of these, 83% (n = 997) had an HbA1c before initiation of insulin therapy indicating that the majority of patients were either diagnosed with T1D in our center or referred shortly after diagnosis. In addition, 50% of the cohort had 14 or more HbA1c values (IQR: 8–21) and 50% had at least three urine microalbumin measures (IQR: 2–5) before developing microalbuminuria or being censored. The population median (IQR) for mean-A1c was 8.62% (8.05–9.41%) [70.72 (64.49–79.35)mmol/mol]; and that for SD–A1c was 1.47% (1.03–2.06%) [16.07 (11.26–22.52) mmol/mol]. One hundred seventy-two patients (14.4%) developed microalbuminuria. When patients with microalbuminuria were compared to those without microalbuminuria, there was no significant difference between the two groups in their mean values for age at T1D diagnosis (9.2 vs. 9.0 yr), mean-A1c (8.91 vs. 8.83%) (73.89 vs. 73.01 mmol/mol) and SD-A1c (1.72 vs. 1.66%) (18.80 vs. 18.14 mmol/mol) (p > 0.05 for all). In addition, when comparing chronological ages and T1D duration at the last microalbumin measure, patients with microalbuminuria were younger (13.8 vs. 14.9 yr, p = 0.0002) and had a shorter duration of diabetes (4.6 vs. 5.9 yr, p < 0.0001). A summary of patient characteristics and data quality measures is provided in Table 1.
Table 1.
Summary of patient characteristics and data quality measures. (1) Data presented as mean ± SD. (B) Data presented as median (IQR).
| Variable | Entire cohort (n = 1195) |
Without microalbuminuria (n = 1023) |
With microalbuminuria (n = 172) |
|---|---|---|---|
| (A) Patient characteristics | |||
| Sex (% male) | 53% | 53% | 55% |
| Race (%) | |||
| Caucasians | 88.4% | 88% | 90.7% |
| African-American | 7.6% | 8.1% | 4.7% |
| Other | 4% | 3.9% | 4.6% |
| Age at T1D diagnosis (yr) | 9.1 ± 3.9 | 9.0 ± 3.9 | 9.2 ± 3.4 |
| Duration of T1D (yr) | 5.7 ± 3.5 | 5.9 ± 3.6 | 4.6 ± 3.1 * |
| Mean-A1c (NGSP %) | 8.8 ± 1.3 | 8.8 ± 1.3 | 8.9 ± 1.3 |
| (IFCC mmol/mol) | 73.1 ± 14.1 | 73.0 ± 14.0 | 73.9 ± 14.7 |
| SD-A1c (NGSP%) | 1.67 ± 0.88 | 1.66 ± 0.86 | 1.72 ± 0.96 |
| (IFCC mmol/mol) | 18.25 ± 9.62 | 18.14 ± 9.40 | 18.80 ± 10.49 |
| Age at event or censoring (yr) | 14.7 ± 3.5 | 14.9 ± 3.6 | 13.8 ± 2.5** |
| (B) Data quality measures | |||
| No. of HbA1c per patient | 14 (8–21) | 14 (9–22) | 12 (6.5–19)* |
| No. of microalbumin measures per patient | 3 (2–5) | 3 (2–5) | 2 (1–5)* |
| Year of T1D diagnosis | 2001 (1996–1904) | 2001 (1997–1904) | 1997 (1994–1902) ** |
T1d, type 1 diabetes; IQR, interquartile range; SD, standard deviation; NGSP, national glycohemoglobin standardization program; IFCC, international federation of clinical chemistry.
p < 0.0001,
p = 0.0003 when comparing patients with and those without microalbuminuria group.
Timing of microalbuminuria onset
In order to analyze the association between HbA1c variability and microalbuminuria, we generated survival curves and CPH models.
Survival curves stratified by SD-A1c level.
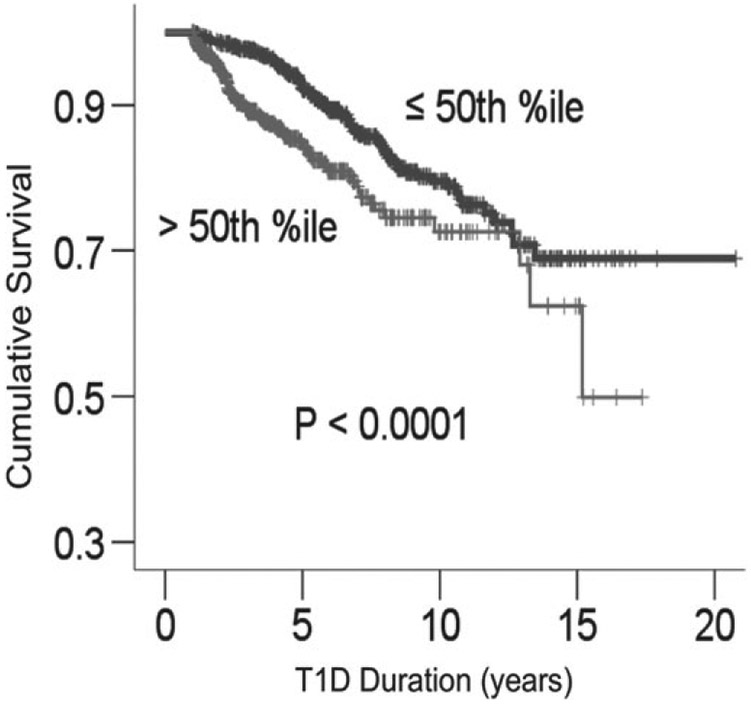
The cohort was stratified on intrapersonal SD-A1c to generate survival curves for SD-A1c above/below 1.47% (16.07 mmol/mol) (50th percentile) (Fig. 1). As shown in Fig. 1, patients with SD-A1c above 1.47% (16.07 mmol/mol) developed microalbuminuria earlier when compared to those with SD-A1c below that median. Among patients who developed microalbuminuria (n = 172), it is notable that 25% of patients with SD-A1c > 90th percentile had microalbuminuria within 1.2 yr and 50% within 1.6 yr of T1D diagnosis. Among patients with SD-A1c at 75–90th percentile, 25% had microalbuminuria within 2 yr and 50% within 2.4 yr of T1D diagnosis. In contrast, median (IQR) for time to microalbuminuria in patients with SD-A1c <50th percentile and between 50th and 75th percentile were 5.3 (4–7.8) yr and 3.2 (2.1–5.4) yr, respectively.
Fig. 1.
Survival curves for microalbuminuria based on standard deviation (SD)-A1c above/below 1.47% (16.07 mmol/mol) (50th percentile).
Survival curves stratified by mean-A1c and SD-A1c.
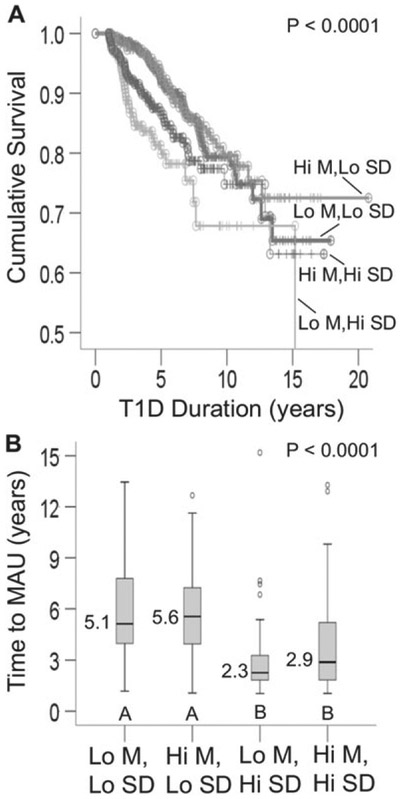
In order to compare and contrast the effects of mean-A1c and SD-A1c on microalbuminuria, we stratified the study cohort into four groups using the median mean-A1c and SD-A1c as cutoffs: (i) low mean/low SD; (ii) high mean/low SD; (iii) low mean/high SD; (iv) high mean/high SD (Fig. 2). Mean-A1c levels for both the low mean groups were 7.9 and 7.9% (62.85 and 62.85 mmol/mol), respectively; whereas mean-A1c levels for the high mean groups were 9.3 and 10% (78.15 and 85.8 mmol/mol) for low SD and high SD groups, respectively. Patients with high SD-A1c developed microalbuminuria more frequently (Fig. 2A) and almost twice as fast as those with low SD-A1c (Fig. 2B). In contrast, mean-A1c appeared to show minimal or no association with time to microalbuminuria.
Fig. 2.
Comparison among subjects stratified by their mean-A1c and standard deviation (SD)-A1c. Low mean: mean-A1c <50th percentile; high mean: mean-A1c ≥ 50th percentile. Low SD: SD-A1c <50th percentile; High SD: SD-A1c ≥ 50th percentile. (A) Survival curves for microalbuminuria (n = 1195). (B) Box plots showing time to microalbuminuria (MAU) in patients who developed MAU (n = 172). Time to MAU differed between patients with low SD (box plots labeled A) vs. high SD (box plots labeled B); p < 0.002 for all pair-wise comparisons of box plots A vs. box plots B. Open circles represent outliers.
CPH models.
Multivariable Cox models were used to define the hazard ratios (HRs) for every 1U increase in mean-A1c and SD-A1c. Using these models, in addition to comparing all patients who developed microalbuminuria (n = 172) with those who did not develop microalbuminuria (n = 1023), we also ran analyses to compare those patients who developed persistent microalbuminuria (n = 55) with those who did not develop microalbuminuria (n = 1023).
Time-dependent-variable CPH models.
Because mean-A1c and SD-A1c can vary from quarter to quarter, we developed time-dependent CPH models (Models 1A, 1B, and 1C) as described earlier. Mean-A1c was not significantly associated with time to microalbuminuria, with HR of 1.08 (0.97–1.22), p = 0.17 (Model 1A, Table 2). On the other hand, the SD-A1c was significantly associated with time to microalbuminuria with HR of 1.48 (1.25–1.76), p < 0.0001 (Model 1B). In the multivariable analysis (Model 1C), mean-A1c remained insignificant with HR 1.05 (0.92–1.2), p = 0.44, whereas SD-A1c continued to be significant with HR of 1.28 (1.04–1.58), p = 0.02. Similar results were obtained when we compared individuals with persistent microalbuminuria to those with without microalbuminuria (Table 3).
Table 2.
Cox proportional hazard (CPH) models for microalbuminuria using time-dependent and fixed-variable calculation of mean-A1c and SD-A1c. Duration of T1D was the time variable in the CPH models. Models 1C and 2C were adjusted for sex, race, and age at T1D diagnosis. Results shown are hazard ratios (HR) for every 1-U increase in each variable
| Cox proportional hazard | |||||||
|---|---|---|---|---|---|---|---|
| Time-dependent models |
Fixed-variable models |
||||||
| Model | HR | 95% CI | p-value | Model | HR | 95% CI | p-value |
| Model 1A | Model 2A | ||||||
| Mean-A1c series | 1.08 | 0.97–1.22 | 0.17 | Mean-A1c | 1.02 | 0.90–1.15 | 0.82 |
| Model 1B | Model 2B | ||||||
| SD-A1c series | 1.48 | 1.25–1.76 | <0.0001 | SD-A1c | 1.64 | 1.41–1.91 | <0.0001 |
| Model 1C | Model 2C | ||||||
| Mean-A1c series | 1.05 | 0.92–1.20 | 0.44 | Mean-A1c | 0.92 | 0.80–1.06 | 0.23 |
| SD-A1c series | 1.28 | 1.04–1.58 | 0.02 | SD-A1c | 1.60 | 1.30–1.97 | <0.0001 |
| Age at T1D | 1.14 | 1.08–1.20 | <0.0001 | Age at T1D | 1.12 | 1.07–1.18 | <0.0001 |
| Sex (M vs. F) | 1.04 | 0.77–1.41 | 0.79 | Sex (M vs. F) | 1.04 | 0.77–1.41 | 0.79 |
| Race | Race | ||||||
| AA vs. Caucasian | 0.45 | 0.21–0.95 | 0.037 | AA vs. Caucasian | 0.46 | 0.21–0.99 | 0.049 |
| Other vs. Caucasian | 1.34 | 0.65–2.75 | 0.42 | Other vs. Caucasian | 1.35 | 0.66–2.76 | 0.42 |
CI, confidence interval; F, female; M, male; T1D, type 1 diabetes; SD, standard deviation.
Significant values of p that are < 0.05 are given in bold.
Table 3.
Cox proportional hazard (CPH) models for individuals developing persistent microalbuminuria vs. those without microalbuminuria. Models were developed using time-dependent and fixed-variable calculation of mean-A1c and SD-A1c. Duration of T1D was the time variable in the CPH models. Models 1C and 2C were adjusted for sex, race, and age at T1D diagnosis. Results shown are hazard ratios (HR) for every 1-U increase in each variable
| Cox proportional hazard | |||||||
|---|---|---|---|---|---|---|---|
| Time-dependent models |
Fixed-variable models |
||||||
| Model | HR | 95% CI | p-value | Model | HR | 95% CI | p-value |
| Model 1A | Model 2A | ||||||
| Mean-A1c series | 1.08 | 0.89–1.32 | 0.43 | Mean-A1c | 1.02 | 0.81–1.25 | 0.94 |
| Model 1B | Model 2B | ||||||
| SD-A1c series | 1.75 | 1.35–2.30 | <0.0001 | SD-A1c | 1.87 | 1.50–2.32 | <0.0001 |
| Model 1C | Model 2C | ||||||
| Mean-A1c series | 0.98 | 0.78–1.25 | 0.90 | Mean-A1c | 0.85 | 0.66–1.09 | 0.20 |
| SD-A1c series | 1.71 | 1.22–2.40 | 0.02 | SD-A1c | 2.18 | 1.58–2.99 | <0.0001 |
| Age at T1D | 1.09 | 1.00–1.19 | 0.048 | Age at T1D | 1.06 | 0.98–1.16 | 0.15 |
| Sex (M vs. F) | 0.62 | 0.36–1.07 | 0.08 | Sex (M vs. F) | 0.6 | 0.35–1.04 | 0.07 |
| Race | Race | ||||||
| AA vs. Caucasian | 0.44 | 0.13–1.50 | 0.21 | AA vs. Caucasian | 0.39 | 0.10–1.46 | 0.39 |
| Other vs. Caucasian | 0.46 | 0.07–3.40 | 0.45 | Other vs. Caucasian | 0.46 | 0.06–3.37 | 0.45 |
CI, confidence interval; F, female; M, male; SD, standard deviation.
Significant values of p that are < 0.05 are given in bold.
Fixed-variable CPH models.
We also developed three CPH models using the overall-fixed Mean-A1c and SD-A1c calculated for each patient by including all available HbA1c values before the event or censoring (Models 2A, 2B, and 2C). Mean-A1c was insignificant in Model 2A with a HR of 1.02 (0.9–1.15), p = 0.82; whereas, in Model 2B, SD-A1c was significantly associated with time to microalbuminuria with HR of 1.64 (1.41–1.91), p < 0.0001 as shown in Table 2. In the multivariable model (Model 2C), mean-A1c remained insignificant [HR of 0.92 (0.8–1.06), p = 0.23]; whereas, SD-A1c continued to be significant [HR of 1.6 (1.3–1.97), p < 0.0001]. Similar results were obtained when we compared patients with persistent microalbuminuria to those without microalbuminuria (Table 3).
Among covariates, age at T1D diagnosis was significantly associated with time to microalbuminuria development (Table 2; p < 0.0001). African-American race was found to be associated with slower progression to microalbuminuria compared to non-Hispanic white race (p = 0.037 and p = 0.049, respectively; Table 2, models 1C and 2C). There were no differences in time to microalbuminuria based on sex, and no differences between other race/ethnicity groups and non-Hispanic whites.
Discussion
Poor glycemic control is known to be associated with T1D complications risk; however, we found that mean-A1c was not independently associated with microalbuminuria after adjusting for variability in HbA1c. In contrast, HbA1c variability was independently associated with the time to microalbuminuria onset. These findings were unchanged even when we analyzed only those patients who developed persistent microalbuminuria against those who did not develop microalbuminuria. In addition, we found that non-Hispanic white patients exhibited higher risk than African-American patients, suggesting a possible underlying genetic or physiologic predisposition to microalbuminuria. Finally, we validated a previous study (17) showing that age at T1D diagnosis is associated with the risk of developing microalbuminuria, with patients diagnosed at older ages experiencing a greater risk than younger patients.
Glycemic variability can be characterized as either short-term (intra- and inter-day blood glucose variability) (18, 19) or long-term (HbA1c variability) (20, 21). While previous studies have shown that acute hyperglycemia and daily glycemic variability associate with biomarkers of vascular oxidative stress and inflammation (22–25), there are none that show a relationship between daily glycemic variability and the development of diabetes microvascular complications. Our study shows a significant association between HbA1c variability and the rate of development of microalbuminuria during the first two decades after diagnosis of childhood-onset T1D. This work adds to the current body of evidence that long-term glycemic variability associates with risk for microvascular complications in patients with T1D. Previously, two studies conducted in primarily adult populations with T1D identified an association between HbA1c variability and nephropathy (20, 21). In the DCCT cohort HbA1c variability was significantly associated with time to development of microalbuminuria, nephropathy and retinopathy, independent of the mean-A1c (20). Furthermore, HbA1c variability was associated with time to microalbuminuria, progression of renal disease and incident cardiovascular events in T1D patients enrolled in the observational Finnish Diabetic Nephropathy study (FinnDiaNe) (21).
In a UK registry-based study which included pediatric patients, the authors identified a significant association between SD-A1c and the development of microalbuminuria (26). However, they additionally noted an association between mean-A1c and the risk for microalbuminuria, which was not observed in our study. These disparate findings may be explained by the fact that the UK study included only annual HbA1c measures, with 50% of patients having four or fewer total HbA1c results. In contrast, our study leverages denser longitudinal HbA1c data. Also our study is enhanced by the development of time-dependent models in which updated mean-A1c and SD-A1c were calculated annually. Several differences in population characteristics may also explain the different findings. First, the median (IQR) for T1D duration for our cohort was shorter at 4.97 (2.94–7.64) yr compared to 8.6 (5.6–11.6) yr for the UK-based study. Second, mean-A1c for this cohort (8.84%) (73.12 mmol/mol) was significantly lower than the mean-A1c for the UK-based cohort (9.5%) (80.34 mmol/mol). Finally, mean-A1c and SD-A1c were not significantly different between patients with and without microalbuminuria in this study [8.9 vs. 8.8% (73.78 vs. 72.68 mmol/mol) and 1.72 vs. 1.66% (18.80 vs. 18.14 mmol/mol), respectively]. In contrast, patients with microalbuminuria in the UK study exhibited higher mean-A1c and SD-A1c compared to those without microalbuminuria [10.4 vs. 9.4% (90.17 vs. 79.24 mmol/mol) and 1.16 vs. 0.86% (12.68 vs. 9.40 mmol/mol), respectively].
The finding that mean-A1c is associated with neither the risk for, nor the time to development of, microalbuminuria in our cohort is novel and suggests that the influences of glycemic risk factors for diabetes-related complications might be impacted by their temporal relationship to ontogeny. Alternatively, glycemic risk factors could differentially influence various outcomes, with fluctuating glycemic control exhibiting a greater influence on the rate of development of early-stage nephropathy and mean glycemic control exerting a dominant influence on the rate of development of later-stage nephropathy.
The results of this study are clinically important. If validated in prospective studies, the next important step is to evaluate whether interventions that cause reductions in HbA1c variability can decrease risk for microalbuminuria. Patients with microalbuminuria are at increased risk for developing progressive diabetic nephropathy and chronic kidney disease (27). Furthermore, the FinnDiaNe study and Pittsburgh Epidemiology of Diabetes Complications study revealed that the presence and severity of diabetic kidney disease in patients with T1D increases their all-cause mortality (28, 29). Recent publications have noted that, in Swedish (30), Scottish (31) and North-American (32) cohorts, patients with T1D have increased risk for all-cause mortality and premature cardiovascular-related deaths; presence of renal disease further increases these mortality risks. Hence efforts to minimize the onset and progression of diabetic nephropathy will be vital to decreasing the long-term morbidity and mortality for patients with T1D.
A significant strength of our study is the large size of the pediatric cohort, the majority of whom had been followed since T1D diagnosis, with longitudinally dense HbA1c and urine microalbumin data. Other strengths include robust statistical analyses that include time-dependent models evaluating mean-A1c/SD-A1c series as continuous variables and stratification of the cohort into different groups based on both their mean-A1c and SD-A1c. Limitations of this study include using data from a single network of clinical centers in a retrospective study; retrospective data collection has the potential to introduce bias into an investigation. In addition, because the study uses routine clinical care data, we cannot control for various assay methods used for HbA1c, although all assay methods were aligned to DCCT standards starting in 1999. Similarly, assays for urine microalbumin and creatinine have changed over time and the authors were unable to control for assay changes longitudinally; if laboratories in the future report the specific assay utilized with each result, health outcomes studies utilizing electronic health record data would benefit significantly. This investigation is also limited by the lack of availability of measures of blood pressure, body mass index (BMI), and insulin sensitivity (e.g., waist circumference, waist-to-hip ratio, estimated insulin sensitivity, HDL/TGLC), which each may influence risk for diabetes-related complications. Finally, this study is limited by its focus on incident microalbuminuria and persistent microalbuminuria as outcomes; recent studies have suggested that the occurrence of regression to normoalbuminuria in some patients limits the utility of microalbuminuria as a marker of nephropathy (33). Others have suggested that changes in estimated glomerular filtration rate (GFR) and the presence of incident renal hyperfiltration represent better surrogate outcomes for incident early nephropathy in pediatric diabetes (34). In fact, the ADA has recently recommended the routine measurement of eGFR in pediatric patients with T1D (35).
This study underscores that children and adolescents with T1D should be strongly encouraged to have good and stable glycemic control during the first decade after diagnosis of T1D to minimize their risk for developing microalbuminuria. Interventions that specifically impact the degree of HbA1c variability should be developed and tested. Because HbA1c is obtained quarterly in these patients, using HbA1c variability as a biomarker for long-term glycemic variability seems reasonable; if validated, HbA1c variability could easily be calculated and reported in electronic health records. Additionally, the relationship between daily glycemic variability and HbA1c variability remains undefined. Further studies are needed to evaluate how HbA1c variability can be used to risk-stratify pediatric patients with T1D and to evaluate the relationship between HbA1c variability and both intra- and inter-day glycemic variability. Finally, future investigations designed to evaluate HbA1c variability as a risk factor for incident early-stage nephropathy should include eGFR-derived measures as outcome variables.
Acknowledgements
Database development/maintenance and other informational technology support were kindly provided by Mitchell S. Barnes, Gary R. Krueger, Eric J. Wiedmer, Kaleb T. Wade, and other members of the IT team at CMH. Also, we would like to acknowledge Nimisha Verma, Syed Mohiuddin, and Adam Van Mason for their support during their medical school years. This study was supported by funding awarded to S. R., in part by an NIH Clinical and Translational Science Award Grant (UL1 TR000001, formerly UL1RR033179). M. A. C. received support from a Physician Scientist Award made by the Children’s Mercy Hospital. The rest was by a Young Investigator Award made to S.R. by the Children’s Mercy Hospital. These were unrestricted grants; thus the funding sources did not have direct influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. M. L. was supported by a travel grant from the Anna Cederberg Foundation to aid his participation in this study.
Footnotes
Prior presentations: Parts of this study were presented in abstract form at: (i) 71st Scientific Sessions of the American Diabetes Association, San Diego, CA, USA, 24–28 June 2011, (ii) Pediatric Academic Societies Annual Meeting, Vancouver, Canada, 3–6 May 2014, and (iii) the ISPAD 2014 Annual Meeting, Toronto, Canada, 3–6 September 2014.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993: 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005: 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy the Epidemiology of Diabetes Interventions and Complications (EDIC) study: JAMA 2003: 290: 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995: 75: 894–903. [DOI] [PubMed] [Google Scholar]
- 5.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes 2008: 57: 995–1001. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995: 44: 968–983. [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000: 342: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaghue KC, Wadwa RP, Dimeglio LA et al. ISPAD Clinical Practice Consensus Guidelines 2014. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 2014: 15 Suppl 20: 257–269. [DOI] [PubMed] [Google Scholar]
- 9.Samuelsson U, Steineck I, Gubbjornsdottir S. A high mean-HbA1c value 3–15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood--a pilot study using two nation-wide population based quality registries. Pediatr Diabetes 2014: 15: 229–235. [DOI] [PubMed] [Google Scholar]
- 10.Vehik K, Hamman RF, Lezotte D et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007: 30: 503–509. [DOI] [PubMed] [Google Scholar]
- 11.Karvonen M, Viik-Kajander M, Moltchanova E et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Care 2000: 23: 1516–1526. [DOI] [PubMed] [Google Scholar]
- 12.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes – the analysis of the data on published incidence trends. Diabetologia 1999: 42: 1395–1403. [DOI] [PubMed] [Google Scholar]
- 13.Diamond Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 2006: 23: 857–866. [DOI] [PubMed] [Google Scholar]
- 14.Dokheel TM, Pennsylvania Pittsburgh Diabetes Epidemiology Research Group. An epidemic of childhood diabetes in the United States? Evidence from Allegheny County. Diabetes Care 1993: 16: 1606–1611. [DOI] [PubMed] [Google Scholar]
- 15.PambiancO G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006: 55: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 16.Miller RG, Secrest AM, Ellis D, Becker DJ, Orchard TJ. Changing impact of modifiable risk factors on the incidence of major outcomes of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2013: 36: 3999–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego PH, Bulsara MK, Frazer F, Lafferty AR, Davis EA, Jones TW. Prevalence and risk factors for microalbuminuria in a population-based sample of children and adolescents with T1DM in Western Australia. Pediatr Diabetes 2006: 7: 165–172. [DOI] [PubMed] [Google Scholar]
- 18.Rodbard D New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther 2009: 11: 551–565. [DOI] [PubMed] [Google Scholar]
- 19.Rodbard D Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther 2009:11 (Suppl 1): S55–S67. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 2008: 31: 2198–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waden J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009: 58: 2649–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monnier L, Mas E, Ginet C et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006: 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 23.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003: 52: 2795–2804. [DOI] [PubMed] [Google Scholar]
- 24.Quagliaro L, Piconi L, Assaloni R et al. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 2005: 183: 259–267. [DOI] [PubMed] [Google Scholar]
- 25.Gordin D, Forsblom C, Ronnback M et al. Acute hyperglycaemia induces an inflammatory response in young patients with type 1 diabetes. Ann Med 2008: 40: 627–633. [DOI] [PubMed] [Google Scholar]
- 26.Marcovecchio ML, Dalton RN, Chiarelli F, Dunger DB. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care 2011: 34: 1011–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 1996: 7: 930–937. [DOI] [PubMed] [Google Scholar]
- 28.Groop PH, Thomas MC, Moran JL et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009: 58: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010: 53: 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind M, Svensson AM, Kosiborod M et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014: 371: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 31.Livingstone SJ, Levin D, Looker HC et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 2015: 313: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Writing Group for the DERG, Orchard TJ, Nathan DM et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015: 313: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003: 348: 2285–2293. [DOI] [PubMed] [Google Scholar]
- 34.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes 2014: 21: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association (11). Children and adolescents. Diabetes Care 2015: 38 (Suppl): S70–S76. [DOI] [PubMed] [Google Scholar]