Abstract
The lower food webs of Lake Huron and Lake Michigan have experienced similar reductions in the spring phytoplankton bloom and summer populations of Diporeia and cladocerans since the early 2000s. At the same time phosphorus concentrations have decreased and water clarity and silica concentrations have increased. Key periods of change, identified by using a method based on sequential t-tests, were 2003–2005 (Huron) and 2004–2006 (Michigan). Estimated filtration capacity suggests that dreissenid grazing would have been insufficient to directly impact phytoplankton in the deeper waters of either lake by this time (mid 2000s). Despite some evidence of decreased chlorophyll:TP ratios, consistent with grazing limitation of phytoplankton, the main impact of dreissenids on the offshore waters was probably remote, e.g., through interception of nutrients by nearshore populations. A mass balance model indicates that decreased phosphorus loading could not account for observed in-lake phosphorus declines. However, model-inferred internal phosphorus dynamics were strongly correlated between the lakes, with periods of increased internal loading in the 1990s, and increased phosphorus loss starting in 2000 in Lake Michigan and 2003 in Lake Huron, prior to dreissenid expansion into deep water of both lakes. This suggests a limited role for deep populations of dreissenids in the initial phosphorus declines in the lakes, and also suggests a role for meteorological influence on phosphorus dynamics. The high synchrony in lower trophic level changes between Lake Michigan and Lake Huron suggests that both lakes should be considered when investigating underlying causal factors of these changes.
Keywords: phosphorus, chlorophyll, zooplankton, Diporeia, Lake Huron, Lake Michigan
Introduction
Lake Huron and Lake Michigan are arguably the two most similar of the five Laurentian Great Lakes. Connected by the 8 km wide Straits of Mackinac, through which water flows in either direction, albeit primarily east (Saylor and Sloss, 1976), and with the same surface elevation, hydrologically the two water bodies can be considered a single lake (Beeton and Saylor, 1995). Differences do exist between the two lakes; most notably, the human population of the Lake Michigan basin is several times that of Lake Huron, making the former more susceptible to anthropogenic influences, while the dilutive influence of inputs from Lake Superior to Lake Huron has contributed to lower concentrations of many dissolved constituents in the latter lake (Beeton, 1965; Hough, 1958). Consequently, Lake Michigan has historically been the more productive of the two lakes, with higher chlorophyll a and primary productivity, and lower water clarity (Schelske and Roth, 1973). Several notable physical differences also exist between the two lakes, most importantly the greater residence time of Lake Michigan (62 years compared to 22 years; Quinn, 1992) and its greater average depth (85 m compared to 59 m).
The two lakes share largely congruent histories of biological invasions. Invasions and introductions of non-native fish have occurred in parallel in the two lakes, with the parasitic sea lamprey (Petromyzon marinus) becoming widespread in both lakes by the 1940s, and the non-native planktivore alewife (Alosa pseudoharengus) reaching substantial numbers slightly later (Berst and Spengler, 1972; Wells and McLain, 1972). Both species have had dramatic impacts on the food webs of the two lakes (Berst and Spangler, 1972; Wells and McLain, 1972). Similarly, invasions of non-native invertebrates have largely coincided in the two lakes. The predatory cladoceran Bythotrephes longimanus (hereafter Bythotrephes) established populations in both lakes in the mid 1980s (Bur et al., 1986; Evans, 1988), resulting in marked shifts in the crustacean zooplankton communities (Barbiero and Tuchman, 2004; Lehman and Cáceres, 1993). By 1990, zebra mussels (Dreissena polymorpha) had been found in both lakes (Griffiths et al., 1991), and the congeneric Dreissena rostriformis bugensis, or quagga mussel, was first found in the Straits of Mackinac region in 1997 and in the main bodies of both lakes by 2000 (Nalepa et al., 2007, 2001).
Since the 2000s the food webs of both Lake Huron and Lake Michigan have experienced substantial changes at multiple trophic levels. Both lakes have undergone declines in spring total phosphorus (TP) concentrations (Barbiero et al., 2012, 2011a), dramatic reductions in the size of the spring phytoplankton bloom (Barbiero et al., 2011a, Fahnenstiel et al., 2010b), increases in water clarity (Barbiero et al., 2011a, 2009b; Vanderploeg et al., 2012), declines in the abundance of benthic amphipod Diporeia (Barbiero et al. 2011b; Nalepa et al., 2009, 2007) and a biomass reduction and relative shift in summer crustacean communities away from cladocerans and cyclopoid copepods towards calanoid copepods (Barbiero et al., 2012, 2009a, 2009b; Vanderploeg et al. 2012).
In Lake Michigan, the changes in water quality, primary production and phytoplankton community structure, at least in the southern basin, have been largely ascribed to direct filtration impacts by dreissenid mussels (Fahnenstiel et al., 2010a; Kerfoot et al., 2010; Vanderploeg et al., 2010; Yousef et al., 2014; see, though, Warner and Lesht, 2015), due at least in part to the rapid expansion of D. r. bugensis into the profundal zone of that lake in the 2000s (Nalepa et al., 2010), a period during which the production declines were observed. While some of the changes seen in Lake Huron have been more pronounced than those in Lake Michigan, e.g., the near complete collapse of cladocerans in 2003 (Barbiero et al., 2009a), dreissenid expansion in Lake Huron has lagged substantially behind that in Lake Michigan (Nalepa et al., 2009, 2007), raising questions about the adequacy of dreissenid filtration as the main causal mechanism behind these changes (Barbiero et al., 2012; Bunnell et al. 2014).
Given the similarities in changes in so many lower trophic level variables in lakes Michigan and Huron within the past twenty years, we believe that a comparative examination of the magnitude and timing of these changes in the two lakes, particularly in the context of differences in dreissenid population development, can give additional insights into potential causal mechanisms. Up to now there have been few comparative assessments of recent ecosystem changes in the two lakes (see, though, Bunnell et al., 2014; Dove and Chapra, 2015; Warner and Lesht, 2015), primarily because research activities conducted by most institutions are generally limited to one lake, or indeed to geographically restricted portions of one lake. The US EPA’s Great Lakes Water Quality Survey, on the other hand, generates comparable data from Lake Huron and Lake Michigan on a range of lower food web variables, thus permitting comparative studies.
In this study we make use of GLNPO’s unique dataset to compare the magnitude and timing of changes in a suite of key lower trophic level variables from the open water of the two lakes. These include a suite of spring water quality variables as well as summer crustacean zooplankton composition and Diporeia abundances. We were specifically interested first in quantifying the degree of synchrony of lower trophic level changes between the two lakes, and in objectively determining the timing of those changes. We assess the possibility of bottom-up impacts on invertebrate populations through correlations with chlorophyll a and both crustacean zooplankton and Diporeia, and assess the possibility of more direct dreissenid impacts on Diporeia by comparing temporal and spatial distributions of the two species. To evaluate the potential impact of direct filtration by Dreissena on offshore phytoplankton, we compare Dreissena filtration rates and chlorophyll/TP ratios between the two lakes. Reductions in chlorophyll/TP ratios are indicative of control of phytoplankton by grazing, rather than nutrient limitation (Mazumder, 1994a, 1994b), and such reductions have been seen in nearshore areas of the Great Lakes in association with the establishment of dreissenids (Nicholls et al., 1999). We distinguish here between direct filtration, i.e. direct removal of phytoplankton by dreissenid grazing, from other potential dreissenid impacts, such as remote interception and sequestration of nutrients. Finally, we utilize a mass balance model to assess the extent to which changes in phosphorus loading might have contributed to reductions in offshore phosphorus concentrations in the two lakes, and by extension, to what extent changes in internal processes, including phosphorus sequestration by dreissenids, might have impacted in-lake phosphorus concentrations.
Methods
Field Sampling
Most of the data used for this study were drawn from the U.S. EPA’s Great Lakes National Program Office (GLNPO) biannual off-shore monitoring program, which began sampling lakes Huron and Michigan in 1983. A total of 35 stations were initially visited in the main basins of the lakes for water column sampling, although from 1996 onward this was reduced to 25. Benthos sampling was initiated in 1997 and has been conducted at 19 stations, some of which coincide with plankton sampling sites. Further details on locations and characteristics of stations are available in Barbiero et al. (this issue) and Lesht et al. (this issue).
Sampling surveys were conducted during the spring isothermal period and summer stratified period. Spring surveys were conducted as early as possible after ice out (typically April) to provide estimates of initial growing season concentrations of nutrients, while summer surveys were conducted during the period of stable thermal stratification (generally August). From 1983 to 1991 multiple sampling runs were often undertaken for one or both of the seasonal surveys. While sometimes separated by several weeks, no consistent differences were found in the data generated by multiple runs (Rockwell et al., 1989), so data from all runs were included in annual survey averages.
At each water column station, samples for nutrients were taken at discrete depths throughout the water column with Niskin bottles mounted on a SeaBird Carousel Water Sampler. For the present study, station averages were calculated from all water column samples except the deepest sample to avoid contamination from sediments or the benthic nepheloid layer. Spring phytoplankton samples were composited from discrete samples taken from surface, 5 m, 10 m and 20 m. Crustacean zooplankton were collected by vertical tows taken from depths of 100 m or 2 m from the bottom, whichever was shallower, using a 0.5-m diameter, 153-μm mesh conical net (D:L = 1:3) equipped with a calibrated Tsurumi-Seiki Co. (TSK) flow meter. Prior to 1997, zooplankton tows were taken to a depth of 20 m, which would miss a substantial portion of the zooplankton community. Only data from deeper tows collected during August surveys were used in this study, therefore our zooplankton time series begins in 1997. Triplicate samples for benthos density estimates were collected using a Ponar grab during August surveys from 1997–2015 except in 2004 when four replicates were taken. Secchi depth readings were taken at all stations visited between one hour after sunrise and one hour before sunset.
Analytical methods
Samples for soluble silica (Si) were filtered in the field through 0.45 μm Sartorius filters and stored at 4 °C. Samples for total dissolved phosphorus (TDP) were filtered in the field through a 0.45 μm membrane filter and along with samples for TP were preserved with H2SO4 for later analysis in the lab. TDP and TP were measured on a Lachat QuikChem AE autoanalyzer by the ascorbic acid method after acid persulfate digestion (APHA 1985). Soluble Si was determined as SiO2 by the molybdate method on a Lachat QuikChem AE autoanalyzer (APHA, 1985). Samples for particulate nutrients were collected from three representative ‘master’ stations (see Barbiero et al., this issue) in each of the two lakes. Samples for particulate organic carbon (POC) were filtered in the field onto 0.7-μm ashed glass fiber filters (GF/F) and rinsed with 0.2N HCl to remove carbonates. Samples for particulate phosphorus (PP) were filtered in the field onto 0.45-μm Sartorius filters. All particulate nutrient samples were frozen for later analysis. POC was measured by combustion in a Carlo Erba Elemental Analyzer 1108. PP was measured on a Lachat QuikChemAE autoanalyzer by the ascorbic acid method after acid persulfate digestion.
For phytoplankton, samples were split and analyzed separately for the whole phytoplankton assemblage (i.e., “soft” algae) and diatoms. All counting included measurements of cell dimensions so that algal biovolumes could be calculated. Further details of sample preparation and taxonomic analysis are provided by Reavie et al. (2014).
After collection, zooplankton samples were narcotized with soda water and preserved with 5% formalin solution. Samples were split in the laboratory using a Folsom plankton splitter, and for crustaceans, four stratified aliquots were examined per sample using a stereoscopic microscope such that rare species were enumerated from the less dilute aliquots. Abundance calculations incorporated a correction for net efficiency derived from flowmeter counts. On average, about 600 individuals were identified per sample. Length measurements were made on the first 20 individuals of each species encountered per sample, and biomass (as dry weight) was calculated for each of these measurements using length-weight relationships derived from the literature and then averaged to arrive at a taxon biomass for each sample (U.S. EPA, 2017). Data are reported as lake-wide average biomass for the major crustacean groups cladocerans, cyclopoid copepods and calanoids copepods.
Samples for benthic invertebrates were washed through a 500 μm mesh sieve and preserved with formaldehyde with Rose Bengal stain to a final concentration of 5–10%. Organisms were picked out of samples under low magnification using a dissecting microscope. Only abundances of the benthic amphipod Diporeia spp. and Dreissena spp. are reported here; the latter taxon was not enumerated in our samples until 2003. Details on benthos methods are in Burlakova et al. (this volume).
Remote Sensing Chlorophyll Estimation
Chlorophyll concentrations were estimated from SeaWiFS (1998–2007) and MODIS (2008–2016) imagery, obtained from the Ocean Color Data archive (http://oceancolor.gsfc.nasa.gov), using the Great Lakes Fit (GLF) band ratio algorithm (Lesht et al., 2016, 2013). The use of remote sensing estimates of chlorophyll allowed us to use a wider temporal range of values than would have been possible with GLNPO’s extracted chlorophyll time series, and also obviated previously documented problems with bias (see Barbiero et al., 2011a) in the GLNPO chlorophyll data series that might have compromised interpretation at the low concentrations seen in this study. Chlorophyll values were extracted from 5 pixel by 5 pixel (roughly 5 km x 5 km) boxes centered on each of the GLNPO stations and the average of the individual pixel values were used to represent the concentration at the GLNPO station. Only values obtained from boxes with a majority (> 12) of cloud-free pixels were used. Analyses which focused on the open water used data from GLNPO’s open water stations; for correlations between chlorophyll a and Diporeia, data corresponding to the appropriate GLNPO benthos stations were used. Average May concentrations were used to assess changes in spring chlorophyll a, while average April-July chlorophyll a was used to provide an index of production up to the point of our August surveys to enable comparisons with our crustacean and Diporeia data. Values were first averaged by month, then averaged across months. To assess chlorophyll:TP ratios for spring and summer cruises, average April and August values were used, respectively.
P loading analyses
To assess interannual changes in internal phosphorus dynamics a modified version of the 21-segment total phosphorus mass balance model presented by Chapra and Dolan (2012) was used. The model was modified by merging the segments representing the northern and southern basins of lakes Huron and Michigan into single segments for each lake. Thus, the resulting model was based on 19 segments.
Our main goal was to determine how much the observed changes in total phosphorus concentration in lakes Huron and Michigan could be explained by changes in phosphorus loading, and by extension, the extent to which internal processes (e.g., internal loading, P sequestration by dreissenids) would have to be invoked to explain phosphorus concentrations during this period. To accomplish this, the average settling velocity for each lake that minimized the overall difference, expressed as the mean absolute error (MAE), between the predicted (Cpred ) and observed (Cobs ) phosphorus concentrations in the two model segments representing the two main lake basins for each observation in the study period was determined. Observed values were lake-wide average spring TP concentrations from GLNPO cruises.
Observed 1983 spring TP values in lakes Huron and Michigan were used as initial conditions for these segments; initial conditions in the other segments, as well as the annual loadings and outflows for all segments, were obtained from Chapra and Dolan (2011). The exchange coefficients and settling velocities in the segments not being studied (i.e. all but northern and southern Huron and Michigan) were taken from Chapra and Dolan (2012). The optimal values of settling velocities in lakes Michigan and Huron were obtained by using a downhill simplex algorithm (Press et al., 2007) and the observed spring phosphorus concentrations for the period 1984–2009. To estimate pre-1983 TP values in the lake, initial (1972) values were chosen and the model run forward, using the specified loads, inter-segment flows, and estimated settling velocities determined above, until the MAE between the predicted and observed values was minimized. Since our interest was in changes occurring in the main basins of the lakes, we present loading based on both phosphorus directly input to the main basins of the lakes as well as phosphorus that enters from the adjacent segments.
Data analysis
We analyzed the data for trends over time using the non-parametric Spearman rank correlations with year as a variable. Since this test is run on ranks, it detects successive increasing or decreasing trends, but is not sensitive to the magnitude of interannual change. Temporal coherence between the lakes was quantified with Pearson product-moment correlations between lake-wide annual averages of individual variables. Variables tested included spring TP, TDP, PP, POC, Si, and Secchi depth, May chlorophyll, summer biomass of cladocerans, cyclopoids, and calanoids, and Diporeia abundances for 30–90 m and > 90 m. In all cases, significance was assessed at α = 0.05. To assess the possibility of bottom-up impacts on invertebrate populations, correlations were run between average April-July chlorophyll a and biomass of the main crustacean groups and abundances of Diporeia, a key benthic macroinvertebrate food web component
To provide an objective assessment of the timing of changes in lower trophic level variables, we used an exploratory procedure for detecting and determining the significance of shifts in time-series data based on sequential application of Student’s t-test (Rodionov, 2004; Rodionov and Overland, 2005). This technique produces a statistic, the regime shift index (RSI), which represents the cumulative sum of normalized anomalies relative to a critical level corresponding to the mean of the previous regime. Because time-series are normalized, it can be applied to multiple variables, in which case, the final RSI is the average of the RSI for each variable in the set. Since this analysis does not allow missing data, missing values were assigned the means of immediately adjacent years; no time series had more than three missing values. Significance was assessed at α = 0.05 for a cutoff length of 10 and a Huber parameter of 1. The RSI was calculated using an Excel add-in (v 2.1) available at www.beringclimate.noaa.gov/regimes. A number of variables which captured the changes in the lower trophic levels were incorporated into the analysis. These included the spring nutrient variables TP, TDP, POC, PP, Si, as well as spring Secchi depth. Biological variables included were May chlorophyll a, August profundal Diporeia abundances, and, as a simple measure of change in the crustacean zooplankton community, the ratio of cladoceran and cyclopoid copepod to calanoid copepod biomass.
Dreissena filtration
The ability of dreissenid filtration to impact phytoplankton in the overlying water in the two lakes was estimated using dreissenid data from GLNPO benthos sites. Data were available for 2003–2015; as noted, enumeration of dreissenids began in 2003, with the two species (D. polymorpha, D. r. bugensis) first distinguished in our samples in 2007, by which time D. polymorpha had virtually disappeared from our sites in the two lakes. From 2003 to 2011, only densities were reported; beginning in 2012, total wet weight (TWW) was also determined. To calculate filtration capacity of mussels, TWW was converted to ash free dry weight (AFDW) using the equation TWW = 50.09*AFDW (Nalepa et al., 2017), and an average AFDW individual−1 was calculated for the depth categories 30–50 m, 50–90 m and > 90 m for each of the two lakes, using pooled data from 2012–2015. These average values were then multiplied by areal abundances in their respective depth categories on a per-lake basis for all years (i.e., 2003–2015) to arrive at AFDW m−2. Filtration rate (mL AFDW−1 hr−1) was calculated based on clearance rates of Lake Michigan seston assuming a temperature of 3° C using coefficients from Vanderploeg et al. (2010). The fraction of the water column cleared (FC) per day was then calculated as filtration rate*AFDW m−2 *24 based on a mean water column depth derived for each depth interval from hypsographic curves of the two lakes for the depth intervals 30–50 m, 50–90 m and > 90 m.
Since our sampling for dreissenids is limited, we supplemented our filtration estimates with those estimated using dreissenid data from more extensive surveys conducted both by NOAA and also by multiple agencies and universities as part of the Cooperative Science and Monitoring Initiative (hereafter NOAA/CSMI). Estimates of dreissenid AFDW m−2 in Lake Michigan for 2000, 2005 and 2010 were taken from Nalepa et al. (2014); AFDW m−2 for 2015 were taken from Nalepa et al. (2017). Abundances for Lake Huron for 2000 and 2003 were taken from Nalepa et al., (2007); abundances for 2007 were taken from Barbiero et al. (2013), AFDW m−2 for 2012 were provided by T. Nalepa (University of Michigan, 7 August, 2017), and estimates of AFDW individual−1 for each depth stratum from this latter year were applied to previous years to arrive at AFDW m−2. Subsequent calculations were carried out as for the GLNPO station data. It should be noted that substantial variation was seen in AFDW individual−1 estimates between lakes, years, depth strata and data sources (Table 1).
Table 1.
Values for mg AFDW individual−1 used for filtration capacity (FC) calculations.
| Lake | Year | Depth Stratum (m) |
Source | ||
|---|---|---|---|---|---|
| 31–50 | 51–90 | > 90 | |||
| Michigan | 2000 | 0.72 | 7.50 | ND | Nalepa et al., 2014 |
| 2005 | 1.60 | 1.73 | 3.08 | Nalepa et al., 2014 | |
| 2010 | 1.91 | 1.72 | 0.84 | Nalepa et al., 2014 | |
| 2015 | 3.70 | 3.20 | 1.20 | Nalepa et al., 2017 | |
| 2012–2015 | 4.42 | 4.24 | 3.70 | GLNPO data | |
| Huron | 2012 | 6.45 | 5.06 | 5.78 | Nalepa unpubl. data |
| 2012–2015 | 5.51 | 0.96 | 2.15 | GLNPO data | |
As an indication of the potential of dreissenid filtration to impact the phytoplankton community, FC was compared to an average phytoplankton growth rate of 0.06 day−1, the value used by Vanderploeg et al. (2010) for similar analyses and derived from a study of growth rates during isothermal conditions in lakes Michigan, Huron, Erie and Ontario (Fahnenstiel et al., 2000). Note that while dreissenid densities were taken from August surveys, the phytoplankton growth rate, as well as the assumption of a fully mixed water column, would correspond to spring conditions.
Results
Water Quality Variables
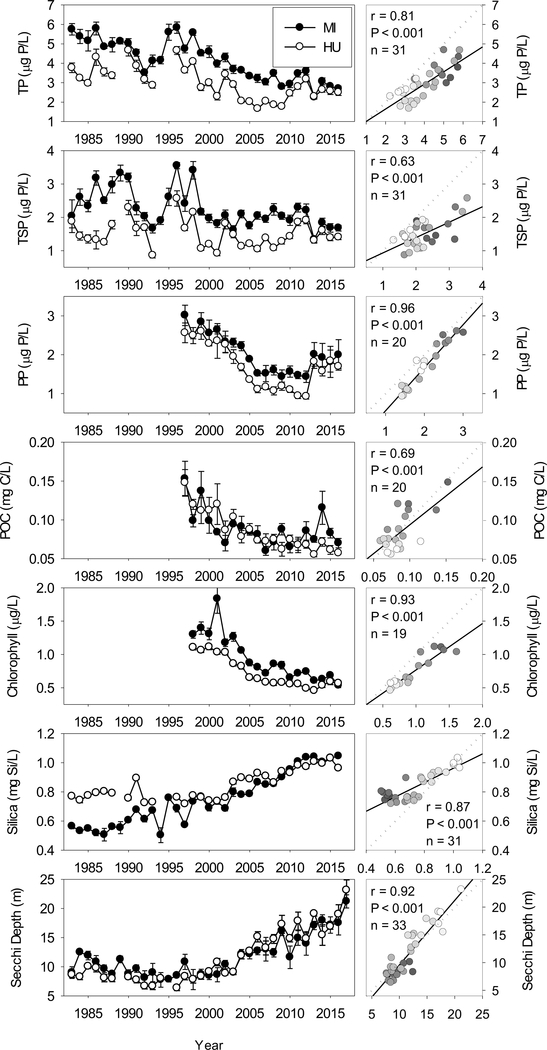
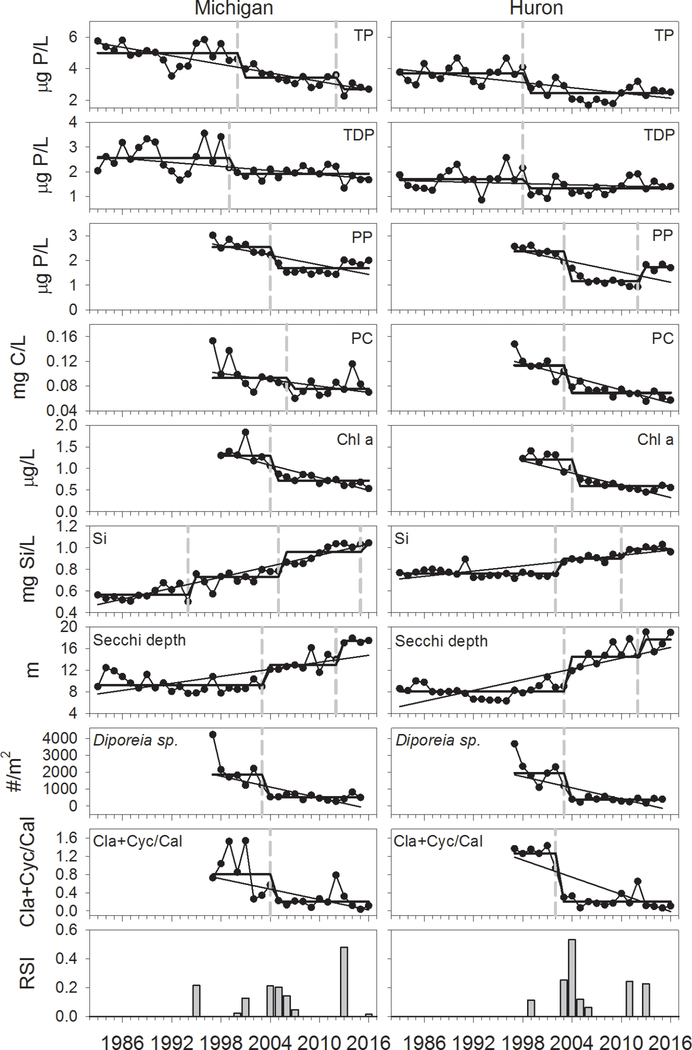
Spring TP decreased significantly in both lakes over the course of our study (Table 2). Concentrations in Lake Michigan were higher than in Lake Huron, although patterns were similar in the two lakes (r = 0.81), with elevated concentrations in the mid 1990s followed by over a decade of declines (Fig. 1). Declines apparently moderated in the mid to late 2000s, with concentrations increasing since 2010 in Lake Huron and thus converging with P levels in Lake Michigan in the last few years of our data set. TDP did not show the same rate of decline as TP; in fact there was no significant trend in Lake Huron (Table 2). This suggests that the decline in TP was disproportionately due to particulate, rather than soluble, forms of phosphorus. This was confirmed by examination of spring PP, which declined precipitously between 2000 and 2005. Spring concentrations of POC also showed declines in both lakes during the same period (Fig. 1). Since 2013, PP exhibited marked increases at all six stations examined in both lakes with the exception of the station in southern Lake Michigan. In spite of these recent increases, there were statistically significant overall declines in PP in both lakes, as was also the case for POC (Table 2). Particulate phosphorus concentrations in the two lakes were remarkably correlated (Fig 1).
Table 2.
Results of trend analyses, using Spearman rank sum correlations with year, for spring water quality variables and summer crustacean zooplankton biomass from Lake Michigan and Lake Huron. Significant results, assessed at α = 0.05, are in bold.
| Variable | Time Series | Michigan |
Huron |
||||
|---|---|---|---|---|---|---|---|
| r | p | n | r | p | n | ||
| TP | 1983–2016 | −0.86 | <0.001 | 33 | −0.69 | <0.001 | 31 |
| TDP | 1983–2016 | −0.59 | <0.001 | 33 | −0.15 | 0.421 | 31 |
| PP | 1997–2016 | −0.94 | <0.001 | 16 | −0.96 | <0.001 | 16 |
| POC | 1997–2016 | −0.69 | 0.003 | 16 | −0.92 | <0.001 | 16 |
| May Chlorophyll a | 1998–2016 | −0.94 | <0.001 | 19 | −0.91 | <0.001 | 19 |
| Silica | 1983–2016 | 0.96 | <0.001 | 33 | 0.73 | <0.001 | 31 |
| Secchi Depth | 1983–2017 | 0.64 | <0.001 | 35 | 0.82 | <0.001 | 33 |
| Cladocerans | 1997–2016 | −0.76 | <0.001 | 20 | −0.79 | <0.001 | 20 |
| Cyclopoids | 1997–2016 | −0.25 | 0.284 | 20 | −0.71 | <0.001 | 20 |
| Calanoids | 1997–2016 | −0.24 | 0.317 | 20 | −0.64 | 0.002 | 20 |
| Total Crustaceans | 1997–2016 | −0.70 | <0.001 | 20 | −0.77 | <0.001 | 20 |
| Chl:TP Spring | 1998–2016 | −0.51 | 0.025 | 19 | −0.78 | <0.001 | 19 |
| Chl:TP Summer | 1998–2015 | 0.06 | 0.805 | 18 | −0.19 | 0.446 | 18 |
| TDP:TP Spring | 1998–2016 | 0.53 | 0.019 | 19 | 0.58 | 0.010 | 19 |
| TDP:TP Summer | 1998–2015 | 0.12 | 0.621 | 18 | 0.56 | 0.015 | 18 |
Figure 1.
Lake-wide average spring total phosphorus, total dissolved phosphorus, particulate phosphorus, particulate organic carbon, May chlorophyll a, spring soluble silica and spring Secchi depth for Lake Michigan and Lake Huron, 1983–2016. Bars indicate one standard error. Right hand plots show correlations of respective variables between Lake Michigan (x axis) and Lake Huron (y axis). Dotted line indicates 1:1 relationship; solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years. Correlation results are provided when significant (α = 0.05). Note that particulate phosphorus times series begins in 1996, chlorophyll a time series begins in 1998, Secchi depth time series extends to 2017.
Average May chlorophyll a concentrations for the period 1998–2016, as estimated by remote sensing, showed marked declines in both lakes which started soon after the initiation of the data series (Table 2, Fig. 1). In both lakes declines were most pronounced between 2002 and 2005, with subsequent concentrations remaining relatively constant. Between the first five years and the last five years of the data series, chlorophyll a concentrations were reduced by more than half in both lakes, declining from 1.40 μg/L to 0.64 μg/L in Lake Michigan, and from 1.28 μg/L to 0.53 μg/L in Lake Huron. Trends in chlorophyll were highly correlated between the lakes, and as was the case with TP, deviated from a 1:1 relationship, indicating an overall higher rate of change in Lake Michigan compared to Lake Huron, although this difference appeared slight (Fig. 1).
Spring soluble silica in the two lakes showed an inverse relationship to chlorophyll a. This would be expected given the high proportion of diatoms in the spring phytoplankton community and thus the implied decreasing demand for silica with decreasing chlorophyll (note, though, that silica was measured in April, while chlorophyll a for May is presented). Highly significant increases were seen in both lakes over the course of the time series (Table 2). The increase in Lake Michigan was relatively linear throughout this time, while a step-change occurred in Lake Huron, coinciding with the initial steep decline in chlorophyll a in 2003. In both lakes, highly significant increases were seen since 2003 (r = 0.92, 0.85, for Michigan and Huron, respectively; P < 0.001, n = 14); only in Lake Michigan, however, were increases seen prior to 2003 (r = 0.74, P < 0.001, n = 20). While silica was highly correlated between the two lakes (Fig. 1), the correlation deviated substantially from a 1:1 relationship due to the declines seen in Lake Michigan during the early part of our time series when concentrations were stable in Lake Huron. This was most likely a consequence of the earlier and more dramatic decreases in P loading in Lake Michigan promoting silica recharge in the lake through long-term reductions in diatom production/silica sedimentation (Barbiero et al., 2002). As with TP and chlorophyll, Si concentrations converged with later values in the time series.
Spring transparency, as measured by Secchi depth, exhibited remarkably similar trends in both lakes, with relatively low transparencies from the late 1980s through the early 2000s followed by increases in the mid-2000s (Fig. 1). Overall increases in transparency were highly significant in both lakes (Table 2), with average transparencies in both lakes exceeding 20 m in our most recent year of data (2017). When comparing average values from 1998–2002 with those from 2012–2016, the percent increase in transparency in both lakes was identical (90%). The two data sets were highly correlated (r = 0.92), and exhibited close to a 1:1 relationship.
Spring phytoplankton
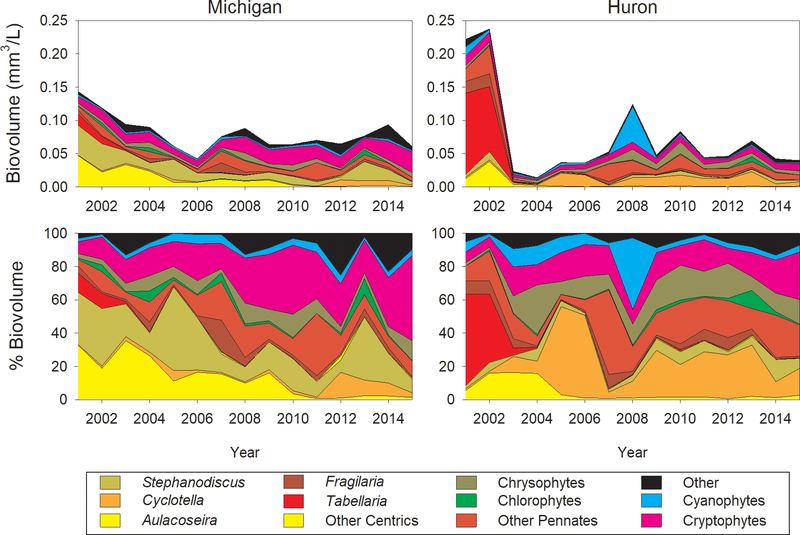
At the beginning of this sample series, both lakes were dominated by diatoms in spring, but by different assemblages. A gradual decline in spring phytoplankton from 2001 to 2006 in Lake Michigan was largely due to a loss of centric diatoms including Aulacoseira and Stephanodiscus (Fig. 2). A more abrupt decline occurred in Lake Huron from 2002 to 2003, largely due to the loss of pennate diatoms such as Tabellaria and some centrics including Aulacoseira. Since approximately 2006, phytoplankton biovolumes have remained relatively consistent. Lake Michigan has shifted to an assemblage with more flagellated cryptophytes, while Huron has shifted to an assemblage with higher relative abundances of the small, centric diatom Cyclotella and flagellated chrysophytes.
Figure 2.
Absolute and relative biovolume of spring (April) phytoplankton in Lake Michigan and Lake Huron, by dominant taxa and major taxonomic group, 2001–2015.
Summer crustacean zooplankton
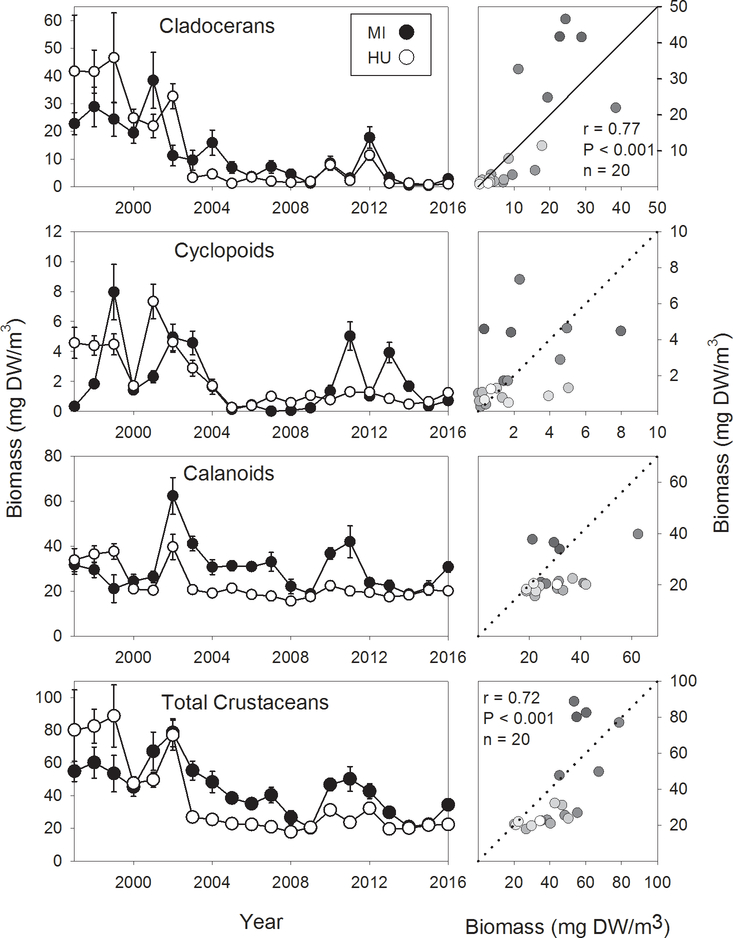
Total summer crustacean biomass exhibited significant declines in both lakes over the course of our time series (Table 2; Fig. 3). In Lake Michigan, these declines appeared to be due mostly to reduced cladoceran biomass, while in Lake Huron significant declines were seen in all three major groups of crustaceans. The decline in cladocerans was sudden in Lake Huron, occurring in 2003, and populations have remained low since then, while in Lake Michigan the decline has been more gradual and less marked. Interestingly, two recent years, 2010 and 2012, have seen somewhat elevated cladoceran biomass in both lakes. Both cladoceran biomass and total crustacean biomass were significantly correlated between the two lakes (Fig. 3). Cyclopoid biomass exhibited overall declines in both lakes, although the correlation between them was not significant (P = 0.056). Calanoid biomass showed no correlation between the two lakes, which was probably due in part to the increase in calanoid, and specifically Limnocalanus macrurus, biomass in the northern basin of Lake Michigan (Barbiero, unpublished data).
Figure 3.
Summer (August) biomass of cladocerans, cyclopoids, calanoids, and total crustacean zooplankton for Lake Michigan and Lake Huron, 1997–2016. Right hand plots show correlations of respective variables between Lake Michigan (x axis) and Lake Huron (y axis). Dotted line indicates 1:1 relationship; solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years. Correlation results are provided when significant (α = 0.05).
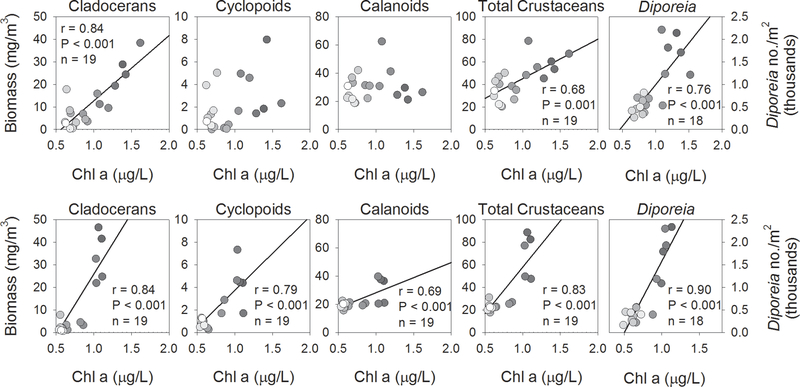
To shed light on the extent to which crustacean biomass might be impacted by resource availability, correlations were run between seasonal (April through July) chlorophyll a, estimated by remote sensing, and biomass of the main crustacean groups. Strong positive correlations were found between chlorophyll a concentration and cladoceran biomass, as well as total crustacean biomass, in both lakes (Fig. 4). In both cases, the chlorophyll/biomass relationship was steeper in Lake Huron, indicating that greater levels of crustacean biomass were associated with lower chlorophyll concentrations. In addition, a high degree of variability in both cladoceran and total crustacean biomass was associated with relatively little variation in chlorophyll in Lake Huron during the earlier years of the data series, suggesting the importance of factors other than chlorophyll in determining crustacean biomass. Neither cyclopoid nor calanoid biomass were correlated with chlorophyll in Lake Michigan, while relationships were found between these variables in Lake Huron.
Figure 4.
Correlations between April-July chlorophyll a, estimated by remote sensing, and August zooplankton biomass, by major taxonomic group, and Diporeia from stations > 90 m, for Lake Michigan (top panels) and Lake Huron (bottom panels), 1997–2016. Statistical results are shown where correlations were significant (α = 0.05); solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years. Correlation results are provided when significant (α = 0.05).
Diporeia
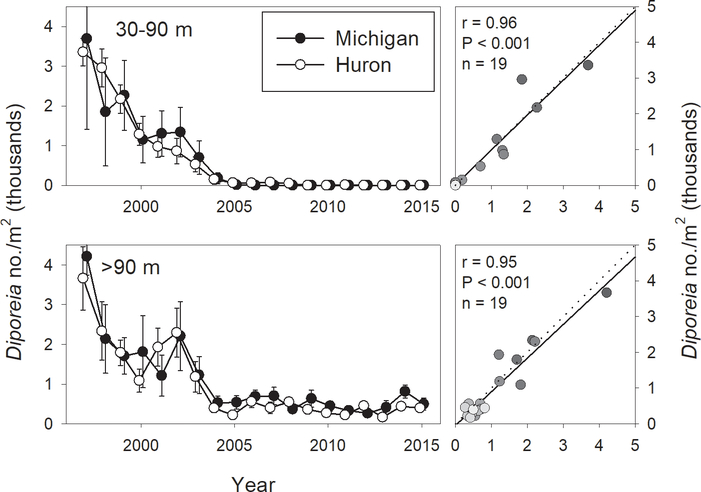
Diporeia populations in both lakes were apparently in decline by the initiation of our benthic sampling program in 1997 (Fig. 5). While Diporeia declines had been reported in Lake Michigan in the early 1990s (Nalepa et al., 1998), similar information is not available for Lake Huron. After a brief stabilization in the early 2000s, another steep decline occurred in both lakes between 2002 and 2004. Populations had virtually disappeared from shallower (30–90 m) sites by 2005 in both lakes, while populations at our deeper (> 90 m) sites in both lakes appear to have stabilized since 2004. Population densities in the two lakes showed a remarkable degree of synchronicity in both depth regions; average densities in the two depth zones were highly correlated between the lakes, with correlation coefficients similar for the two depth zones (r = 0.96, 0.95 for 30–90 m and > 90 m, respectively) (Fig. 5). Population densities at deep sites were highly correlated with corresponding chlorophyll a concentrations in both lakes (Fig. 4). Due to their disappearance from shallow sites after 2004, correlations were not run with Diporeia densities from these sites.
Figure 5.
Abundances of Diporeia in Lake Michigan and Lake Huron, 1997–2015, for 30–90 m and > 90 m. Right hand plots show correlations of respective variables between Lake Michigan (x axis) and Lake Huron (y axis). Dotted line indicates 1:1 relationship; solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years. Correlation results are provided when significant (α = 0.05).
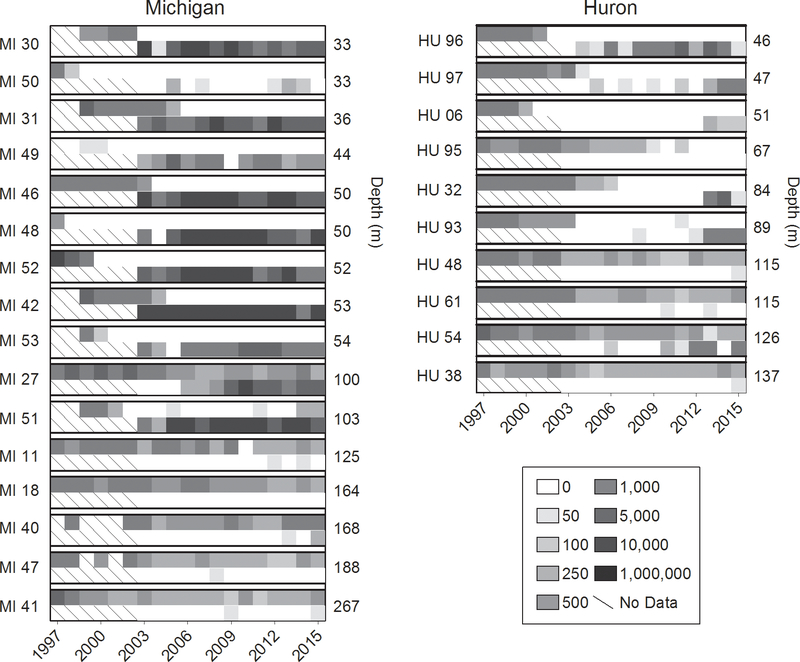
Dreissena was not counted in our samples until 2003, by which time dreissenids had already appeared at a number of stations, primarily from shallow (< 90 m) depths in Lake Michigan. At some shallow sites in Lake Michigan there appeared to be several years of overlap between the two species before Diporeia disappeared, while in others the disappearance of Diporeia preceded the appearance of Dreissena by several years (Fig. 6). In Lake Huron, where Dreissena population development occurred much later, the disappearance of Diporeia from shallow sites almost always preceded appearance of Dreissena, in some cases by a decade. Populations of Diporeia at deeper sites in both lakes had less apparent correspondence with Dreissena abundance, with declines taking place prior to, or without, the appearance of Dreissena. However, in only two cases, HU 54 and MI 27, was there extensive coexistence between the two species. It should be recognized that dreissenid distribution is extremely patchy, and may not be sampled effectively by Ponar grab (see Karatayev et al., this issue). Therefore, our data might underestimate the degree of overlap between the two taxa to a certain degree.
Figure 6.
Station-specific abundances (number/m2) of Diporeia (upper rows) and Dreissena (lower rows) in Lake Michigan and Lake Huron, 1997–2015. Hashes indicate no data. Station names are to the left of each plot, station depths to the right. Note that Dreissena was not enumerated in our samples until 2003.
Timing of changes
Sequential t-tests revealed a number of potential change points in spring water quality variables, August profundal (> 90 m) Diporeia abundances and the August zooplankton community, with the strongest signals clustered around 2003–2006 in both lakes (Fig. 7). In Lake Michigan, change points were detected between 2004 and 2006 for most variables examined, the exceptions being TP and TDP, where change points were detected in 2001 and 2000, respectively. Strong signals from single variables led to high RSI values in 1995 (Si) and 2013 (Secchi depth). The timing of potential shifts in Lake Huron was similar to, though slightly earlier than, those of Lake Michigan. Most variables exhibited signs of shifts between 2003 and 2005. TP and TDP were exceptions, with changes seen in 1999 and thus preceding those of most other variables, as was the case in Lake Michigan. Also as in Lake Michigan, Secchi depth exhibited a further indication of increase in 2013.
Figure 7.
Regime shift analysis of spring total phosphorus, total dissolved phosphorus, particulate phosphorus, particulate organic carbon, May chlorophyll a, spring soluble silica, spring Secchi depth, August profundal (> 90 m) Diporeia abundance, and ratio of August cladoceran and cyclopoid biomass to calanoid biomass for Lake Michigan and Lake Huron, 1983–2017. Dashed vertical lines indicate occurrences of shifts, straight lines indicate Sen-Thiel regressions, stepped lines indicate averages for each period. Bottom panel indicates regime shift values averaged across all variables.
Dreissena filtration
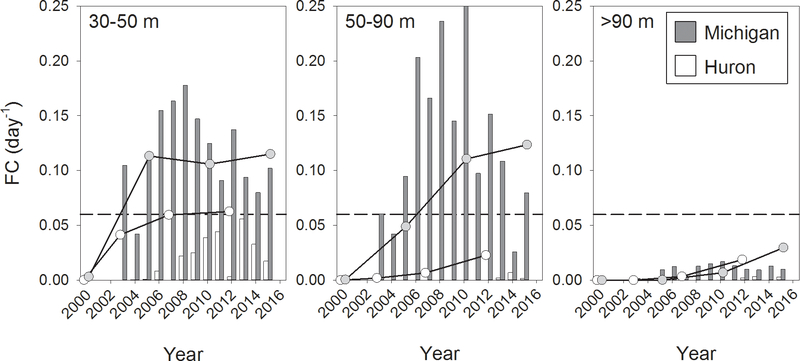
Estimated fraction of the water column cleared (FC) by dreissenids for the shallowest region examined in Lake Michigan (30–50 m) exceeded the nominal phytoplankton growth rate of 0.06 day−1 from the first year of our data series (2003) and appeared to peak between 2006 and 2010 (Fig. 8). Values from the more extensive NOAA/CSMI surveys were generally in keeping with those from GLNPO, with FC exceeding 0.06 day−1 sometime between 2000 and 2005. FC values calculated from GLNPO data for Lake Huron in this depth range were substantially lower than those in Lake Michigan, and showed a general increasing trend. Only in 2013 did they approach the nominal phytoplankton growth rate. Estimates of FC made with non-GLNPO data were higher than GLNPO values, and approximately equaled 0.06 day−1 by 2007, although showed little subsequent increase. FC values estimated from GLNPO data for the 50–90 m stratum of Lake Michigan were higher in most years than those in the shallower stratum, and increased to a peak in 2010. Values exceeded 0.06 day−1 by 2005. Values based on broader surveys, in contrast, increased steadily from 2000 through 2015, exceeding 0.06 day−1 sometime after 2005. Both sets of FC values for Lake Huron in this depth stratum were substantially below those for Lake Michigan, and neither had approached 0.06 day−1 by the end of their respective time series. Values for the deepest stratum (> 90 m), which comprised most of the open water in Lake Michigan and the northern portion of Lake Huron, never exceeded 0.03 day−1 in either lake.
Figure 8.
Estimated fraction of water column cleared (FC) by Dreissena filtration in different depth zones of Lake Michigan and Lake Huron. Bars represent FC calculated from GLNPO data; lines represent FC estimated from more extensive NOAA/CSMI surveys. Dotted line corresponds to nominal average growth phytoplankton rate of 0.06 day−1 (Fahnenstiel et al., 2000). See text for details of calculations.
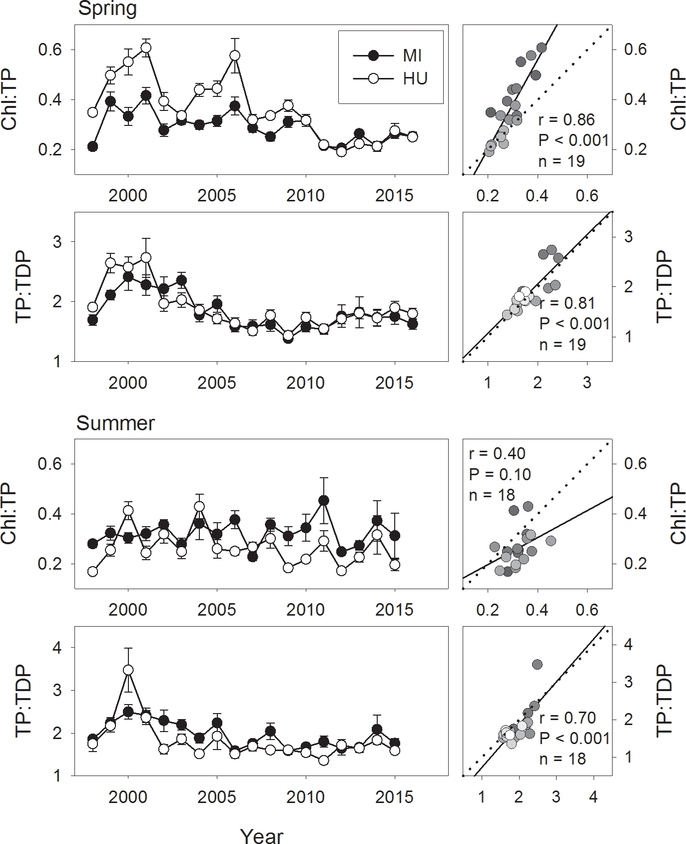
Chlorophyll:phosphorus ratios
Since direct filtration of phytoplankton by grazers such as dreissenids might be expected to disproportionately reduce chlorophyll concentrations relative to phosphorus concentrations, the ratio of chlorophyll a to total phosphorus (Chl:TP) was calculated for both spring and summer samples (Fig. 9). Spring Chl:TP declined significantly in both lakes (Table 2), with greater declines seen in Lake Huron. In both lakes the period of decline was from 2006 to 2011. Comparing the first five (1998–2002) with the last five (2012–2016) years of our time series, ratios dropped from 0.48 to 0.23 in Lake Huron, a decline of over 50%. A smaller decline (0.32 to 0.24; 27%) was seen in Lake Michigan. In spite of the difference in the magnitude of change, Chl:TP ratios were highly correlated between the two lakes (Fig. 9). Coincident significant decreases in the ratio of spring TP to TDP were also seen (Table 2), as would be expected if phytoplankton growth were being limited by grazing, rather than nutrients. Little evidence of change in Chl:TP ratios was seen in summer (Fig. 9). The ratio of summer TP to TDP showed little apparent trend in Lake Michigan, while in Lake Huron a significant decreasing trend, roughly corresponding to the decline in Chl:TP, was seen (Table 2).
Figure 9.
Spring (April) and summer (August) chlorophyll a:total phosphorus ratios and total phosphorus:total dissolved phosphorus ratios for Lake Michigan and Lake Huron, 1998–2016. Right hand plots show correlations of respective variables between Lake Michigan (x axis) and Lake Huron (y axis). Dotted line indicates 1:1 relationship; solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years. Correlation results are provided when significant (α = 0.05).
Phosphorus loading and P dynamics
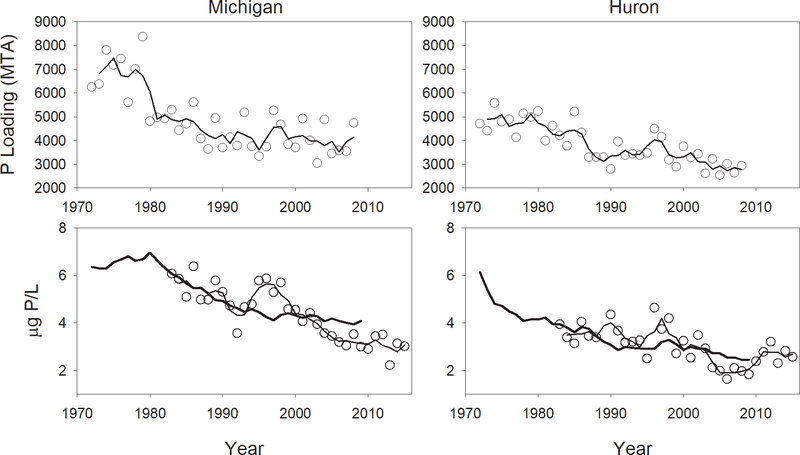
Both the timing and magnitude of changes in phosphorus loading differed markedly between the two lakes. Phosphorus loading (calculated from our modified Chapra-Dolan model) into the main basin of Lake Michigan declined rapidly in the late 1970s and early 1980s, with little change since the mid 1980s (Fig. 10). Loading in Lake Huron, in contrast, showed a more continual decline throughout the course of the time series. In both cases, declines since 1972 were statistically significant (Michigan: r = −0.73, P < 0.001; Huron: r = −0.80, P < 0.001) although only in Lake Huron did external loading show a significant decline since 1990 (Michigan: r = −0.15, P = 0.541; Huron: r = −0.50, P = 0.02).
Figure 10.
Upper panels: phosphorus loading into the main basins of Lake Michigan (left) and Lake Huron (right). Lines = 3 yr running average. Bottom panels: predicted in-lake TP based on loading (heavy lines), measured spring whole-lake averaged total phosphorus (symbols annual values, dashed line 3 yr running avg). See text for details of in-lake TP estimates.
When fit to our entire dataset (1983–2009), the apparent settling velocities required to minimize the total prediction error for in-lake TP, based on loading estimates, were 17.1 m/yr for Lake Michigan and 14.7 m/yr for Lake Huron. Predicted in-lake TP concentrations, based on loads to the main basins of the lakes and these constant settling velocities, suggest gradually moderating declines in both lakes, with relatively little change from the 1990s on (Fig. 10). Estimated reductions in in-lake TP between 2000 and 2009, based solely on loading data, amounted to 0.24 μg/L for Lake Michigan and 0.42 μg/L for Lake Huron. This compares to observed decreases over this time period of 1.55 μg/L for Lake Michigan and 1.44 μg/L for Lake Huron.
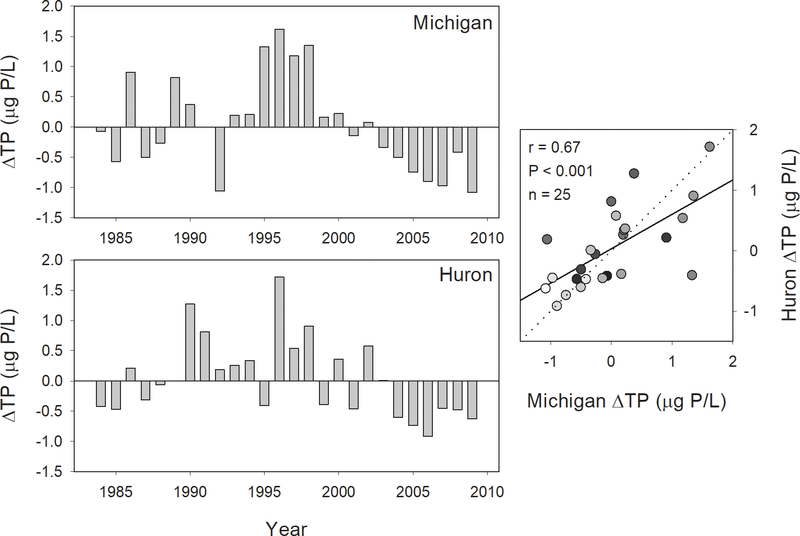
The model under-predicted in-lake TP from 1995–2000 and over predicted observed TP after 2001, suggesting relatively greater internal loading during the former period and relatively greater P loss during the latter period (Fig. 11). Patterns of these residuals were similar in the two lakes, as confirmed by a correlation of these residuals, which was highly significant (r = 0.67, P < 0.001, n = 25; Fig. 11). While loading data only allow prediction of in-lake TP up to 2009, barring any dramatic changes in external loading, it seems likely that TP concentrations in Lake Michigan would continue to be below predicted values through the end of our data series, while those in Lake Huron would converge on predicted values after 2010.
Figure 11.
Residuals of estimated and predicted total phosphorus in Lake Michigan and Lake Huron (left graphs) and correlation between residuals in Lake Michigan (x-axis) and Lake Huron (y-axis), 1983–2009 (right graph). Dotted line indicates 1:1 relationship; solid line indicates least-squares regression line, provided for reference. Gradation of symbol fills correspond to year, with lighter fills indicating more recent years.
Discussion
The lower trophic levels of both Lake Huron and Lake Michigan underwent a period of rapid change in the mid-2000s. These changes included reductions in spring particulate P, particulate C, spring chlorophyll a, increases in spring silica and Secchi depth, shifts in the composition of the August crustacean zooplankton community and accelerated declines in Diporeia populations. A remarkably high degree of temporal coherence was seen between the two lakes in most variables; the majority of correlations we have presented would be characterized as ‘high’ or ‘very high’ according to Magnuson et al. (2006). Interestingly, correlations between biological variables (May chlorophyll a, Diporeia abundances) were among the highest seen in our study, which is counter to expectations since temporal coherence is typically highest for physical variables, intermediate for chemical variables, and lowest for biological variables, given their successively decreasing responsiveness to common external drivers (Kratz et al., 1998). However, a number of studies have demonstrated the influence of extrinsic factors, e.g., climate, on planktonic community structure, in some cases over a broad regional scale (Rusak et al., 2008, 1999; Straile, 2002; Straile and Adrian, 2000; Winder and Schindler, 2004). The high synchrony that we saw in so many lower trophic level variables suggests the influence of either common external drivers or equally synchronous internal drivers. Possible common external drivers might include climatic factors such as precipitation, air temperature and extent and duration of ice cover. All of these have been associated with changes in nutrients, chlorophyll and primary productivity in lakes Huron and Michigan (Nicholls, 1998; Rockwell et al., 1980; Warner and Lesht, 2015), as will be discussed further below.
A number of potential causative factors have been posited to explain the recent changes seen in Lake Huron beginning in 2003, including invasive species, vertebrate and invertebrate predation, and anomalous weather conditions (Barbiero et al., 2009a; Bunnell et al., 2011; Dunlop and Riley, 2013; He et al., 2015). Speculation regarding causative factors for the declines in spring primary production and changes in the offshore phytoplankton and zooplankton communities in Lake Michigan, in contrast, has centered largely around direct filtration by dreissenid mussels (Fahnenstiel et al. 2010a, 2010b; Kerfoot et al., 2010; Vanderploeg et al., 2010; Yousef et al., 2014; although see also Vanderploeg et al., 2012). Part of the rationale for this was the presumed temporal coherence between documented changes and the establishment of large populations of dreissenid mussels (Fahnenstiel et al., 2010b). Using a method based on a sequential t-test, we identified 2003–2005 and 2004–2006 as pivotal periods of change for Lake Huron and Lake Michigan, respectively, although declines in TP were apparent approximately ten years earlier in both lakes. Dreissenid densities in Lake Michigan in the 51–90 m depth stratum were 16,265/m2 in 2005 (Nalepa et al., 2014), but populations were substantially lower (13/m2 at > 90 m) in the deeper regions of the lake of which our water quality data are most representative. While mussel populations increased considerably between 2005 and 2010 in southern Lake Michigan (Nalepa et al., 2010), even by 2010 lake-wide biomass was largely centered around the 50 m depth isopleth in the lake (Waples et al., 2017). Dreissenid densities in the offshore of Lake Huron were substantially lower during the period of greatest change in that lake: 88/m2 at 51–90 m and 1/m2 at > 90 m in 2003, and 305/m2 and 135/m2 in 2007 (Barbiero et al., 2013; Nalepa et al., 2007). Thus, the period of greatest change in both lakes largely preceded the main expansion of quagga mussels into the offshore. In fact, changes in most variables moderated, or even reversed, while dreissenids were still increasing in profundal regions. In Lake Huron, for instance, phytoplankton biovolume, May chlorophyll a, Diporeia abundance and crustacean community make-up changed little after 2005, while spring TP has increased since 2010.
Several studies have provided estimates of the fraction of the water column cleared (FC) by dreissenids in Lake Michigan to assess the potential importance of dreissenid grazing on offshore phytoplankton populations (Fahnenstiel et al., 2010b; Rowe et al., 2015; Vanderploeg et al., 2010). To our knowledge, FC has not previously been estimated for Lake Huron. Our estimates of dreissenid FC, based on both annual data from GLNPO benthos stations and less frequent data from more extensive surveys, suggest that dreissenid filtration could have overcome the nominal phytoplankton growth rate in waters in the 51–90 m depth zone only after 2005 in Lake Michigan, while dreissenid populations had not reached sufficient numbers as of 2015 for grazing to have impacted waters > 90 m, a stratum accounting for 70% of the volume of the lake, a conclusion also reached by Vanderploeg et al. (2010) and Rowe et al. (2015). In Lake Huron, sufficient populations had not developed sufficiently as of 2015 for grazing to have impacted phytoplankton in waters greater than 50 m, a depth zone containing 80% of the volume of the lake.
It should be recognized that there is substantial uncertainty in estimates of FC, and therefore caution should be applied in their interpretation. Filtration rates are affected by a number of factors, including mussel size, food quality and quantity, and water temperature (Berg et al., 1996; Madon et al., 1998; Sprung and Rose, 1988), about which assumptions need to be made when extrapolating lab results to the field. Mussel size in particular can have a large impact on estimates of filtration rates. We saw substantial differences in AFDW individual−1 between the two lakes, between depth regions and years within the lakes, and between the two data sets that we used for FC estimation. While some of these differences no doubt reflect actual differences in size distributions or mussel condition (Nalepa et al., 2017), discrepancies between values from different data sources for the same lake, depth stratum and year raise questions about representativeness of size data and indicate that some caution is warranted in interpreting FC results.
When extending estimates of filtration rate to water column clearance rates it is assumed that the overlying water behaves as a continuously stirred tank reactor in which mussels have continuous contact with the entire water column, and that there is no refiltration of water. Both of these assumptions are unrealistic (Boegman et al., 2008; MacIsaac et al., 1999), particularly in deeper, offshore environments, and result in an overestimation of the effects of benthic grazing (Ackerman et al., 2001). A phytoplankton growth rate of 0.06 day−1 has been used in most studies, including the present one, as a benchmark to assess the point at which dreissenid filtration capacity can negatively impact phytoplankton standing crop. This value corresponds to approximately half the maximum growth rate (μmax) under isothermal conditions in lakes Michigan, Erie, Huron, and Ontario determined by Fahnenstiel et al. (2000). Since the majority of simulated in situ growth rates in that study were near μmax, a value of 0.06 day−1 may be unrealistically low.
The reduction of phytoplankton standing crop and/or production through direct grazing by mussels, as opposed to nutrient interception/sequestration by mussels, is essentially a top-down mechanism, by which grazing pressure, rather than nutrient availability, limits phytoplankton growth. This should be apparent as a decoupling of the relationship between the limiting nutrient, i.e., P, and measures of phytoplankton biomass (Mazumder 1994a, 1994b). Declines in Chl:TP have been seen in a number of lakes in association with dreissenid invasions (Higgins et al., 2011; Mellina et al., 1995; Nicholls et al., 1999, 1997). Since a portion of the phosphorus removed from the water column as phytoplankton biomass is returned as dissolved P (Bootsma and Liao, 2014; Heath et al., 1995; James et al., 2000, 1997), an expected ancillary consequence of dreissenid-effected reductions in Chl:TP is an increase in the relative proportion of dissolved to total P, even if no overall decrease in TP is seen. Increases in dissolved phosphorus in association with Dreissena have been reported from Saginaw Bay (Johengen et al., 1995), western and eastern Lake Erie (Holland et al., 1995; Nicholls et al., 1999) and Oneida Lake (Idrisi et al., 2001), often with little change in TP. We have shown significant declines in Chl:TP in Lake Huron which coincided with an increase in TDP:TP. Similar, though less marked, results were seen in Lake Michigan. In both lakes, these changes were largely restricted to spring, when dreissenids would theoretically have access to the entire water column. Although the changes in ratios discussed above are consistent with a lake-wide grazing effect in both lakes, estimated FC at depth > 30 m appears insufficient to have accounted for the declines in chlorophyll a that we documented here, particularly during the periods of most rapid change in the two lakes.
Given the low estimated FC values for the profundal regions of the two lakes during key periods of change, and also considering the large discrepancy in dreissenid densities and estimated FC between the two lakes in the 50–90 m depth stratum in view of similar magnitudes of change in most variables, it seems unlikely that direct grazing by dreissenids in > 50 m regions of the lakes was the primary driver of changes in offshore variables. In lieu of, or in addition to, direct filtration, dreissenids might impact the offshore ecosystem remotely through several mechanisms. Increased interception, retention and recycling of nutrients by the littoral benthic community, driven by dreissenids in the nearshore, are thought to be capable of reducing nutrient transport to the offshore (Hecky et al., 2004). This hypothesis, termed the nearshore shunt, was formulated in large part to explain dreissenid-associated reductions in nutrients in offshore regions thought too deep to be directly impacted by filtration. Indeed, until recently impacts of dreissenids on water column parameters have been thought to be restricted to shallow systems with high population densities (Hecky et al., 2004; MacIsaac et al., 1999); quagga mussel populations in hypolimnetic regions of the Great Lakes were assumed to be incapable of exerting substantial grazing pressure on the algal community because of the depth of the lakes (Vanderploeg et al., 2002). Vanderploeg et al. (2010) extended the nearshore shunt hypothesis to the mid-depth (30–50 m), positing a role for dreissenid populations at these depths in intercepting phosphorus and carbon that would normally move from inshore to offshore and thus providing a mechanism for remote dreissenid impacts on deeper regions where mussel densities were insufficient to directly reduce phytoplankton populations through filtration. Mosely and Bootsma (2015) have, in fact, shown substantial dreissenid impacts on P cycling at a 55 m site in southwestern Lake Michigan in 2013, though the extent to which this might impact whole-lake concentrations or fractions of P was not explicitly addressed in their study.
Phosphorus intercepted by mussels can become incorporated into mussel shells/biomass, be excreted, or be egested as feces or pseudofeces. The P incorporated into mussel shells/biomass, the portion of excreted P retained by benthic biota such as Cladophora, and the percentage of feces/pseudofeces permanently lost to the sediments, represent a potential net loss to the offshore pelagic. Rowe et al. (2017) estimated the P contained in mussel biomass in 2010 in Lake Michigan to be equivalent to 1 μg/L, on a lake wide basis, which they compared to an observed decline in offshore TP of 1.6 μg/L between 2000 and 2010. Applying the same constants given in Rowe et al. (2017) to data from Nalepa et al. (2007) and Barbiero et al. (2013), we calculated an accumulation of P in mussel biomass in Lake Huron equivalent to 0.43 μg/L, on a whole lake basis, between 2000 and 2007, during which time TP declined in the offshore by 1.1 μg/L. According to our P mass balance model, the reduction in P attributable solely to reductions in loading between 2000 and 2007 was slightly greater than 0.3 μg/L for both lakes. This suggests that P accumulated in mussel biomass could have contributed more to the decline in phosphorus in both of these lakes than decreased loading during this period. To this would be added P contained in biodeposits that are not returned to the water column, as well as any excreted P subsequently taken up and retained by benthic biota such as Cladophora. The magnitude of dreissenid P sequestration due to incorporation into biomass should be most pronounced during periods of rapid population expansion, and should moderate as populations reach steady state (Bootsma and Liao, 2014). Such a dynamic was seen in alkalinity, used as a surrogate for calcium, in Lake Erie, where an initial rapid decline during a period of rapid dreissenid increase was followed by stabilization and then recovery (Barbiero et al., 2006). If lake-wide dreissenid populations are in fact approaching steady state, this could help explain the moderation in rates of change that we observed in a number of trophic level variables after an initial period of more dramatic change, and in particular the tendency towards increased TP in Lake Huron in the last years of our dataset.
An increased loss of phosphorus from the water column by any of the mechanisms outlined above should be apparent as a divergence between observed in-lake TP and that predicted on the basis of loading, with lower than predicted in-lake TP indicative of increased sedimentation/sequestration. We found such divergences starting in approximately 2000 in Lake Michigan and 2003 in Lake Huron. While this predates the major expansion of mussels into the profundal zones of the lakes, dreissenids had in fact been in both lakes for at least a decade by that time. Detailed information on nearshore dreissenid population development during the 1990s in the two lakes does not seem to be available, so it is difficult to quantitatively assess potential nearshore P sequestration. Nonetheless, the timing of changes in internal P dynamics lends support to a role for nearshore dreissenid populations in enhancing P loss in the two lakes, as outlined above. In a calibration of a P model for the entire Great Lakes, Chapra and Dolan (2012) found a similar divergence between predicted and observed phosphorus, which they accommodated by increasing settling velocity for all lakes except Lake Superior after 1990. The choice of 1990, though, appeared to be dictated largely by discrepancies between predicted and observed values in Lake Ontario. In fact, we saw an underestimation of P in the 1990s, which indicates increased internal loading of P, rather than increased loss.
Changes in internal loading, while consistent with dreissenid-related P sequestration, can also result from a number of other mechanisms, including interannual differences in sediment resuspension (Cotner et al., 2000; Eadie et al., 2002;) and ice cover (Rockwell et al., 1980; Rodgers and Salisbury, 1981). Warner and Lesht (2015) found both ice cover and spring precipitation to be predictors of spring TP concentrations in lakes Huron and Michigan; invasive mussel densities, in contrast, were not. Nicholls (1998) found a strong relationship between P in the outflow of Lake Huron and both percent ice cover and lake level; no relationship was found between P and increased loading/inflows. He suggested that during relatively ice-free winters, e.g., due to El Niño years, P is kept in suspension by wind and can thus result in substantially higher in-lake concentrations. These meteorological impacts can be geographically extensive; during the particularly strong El Niño year of 1983, unusually high P concentrations were seen from Lake Superior to Lake Ontario (Nicholls, 1998). The temporal coherence in implied periods of high and low internal P loading that we observed between Lake Huron and Lake Michigan suggests that internal P dynamics might be due at least in part to such broad-scale climactic factors. A controlling influence of meteorology on P dynamics in the two lakes seems most likely during the period of increased apparent internal loading in the mid-1990s, in which dreissenids would presumably have played no role. Support for this is provided by recent work identifying periods of high and low wave action in Lake Michigan which correspond to the periods identified in our analyses as indicative of high and low internal loading, respectively (Cary Troy, Purdue University, Nov., 20 2017, pers. comm.). The potential importance of meteorology in influencing primary productivity was also demonstrated by a recent model simulation of Lake Michigan by Rowe et al. (2017). There is ample suggestive evidence of the influence of meteorology on interannual P dynamics to warrant further exploration of this relationship.
Higher trophic levels
The timing of shifts in both crustacean zooplankton community size and composition and Diporeia densities were similar between the two lakes and tied to varying degrees to the changes seen in chlorophyll a. This close association between changes in these two groups of invertebrates suggests responses, direct or indirect, to common factors, perhaps involving resource levels, although the exact mechanisms are difficult to resolve.
This synchrony between lakes was particularly striking in the case of Diporeia, as had been noted in a previous analysis of a shorter time series (Barbiero et al., 2011b), and contrasts with the dyssynchrony in Dreissena abundances. Nalepa et al. (2007) had previously pointed out the similarity in rates of Diporeia decrease in the two lakes in spite of far lower densities of Dreissena in Lake Huron. The Diporeia declines seen across the Great Lakes have been assumed to be due to interactions with Dreissena (Nalepa et al., 2007; Watkins et al., 2007), although the lack of spatial overlap between these taxa in regions of Diporeia decline (Nalepa et al., 2010; Watkins et al., 2013) have posed challenges to formulating a compelling causal mechanism. Food limitation, as mediated by competition with D. polymorpha, was originally hypothesized to be responsible for the declines (Dermott and Munawar, 1993; Nalepa et al., 1998), although declining populations have not exhibited physiological indications of starvation (Nalepa et al., 2006). Even so, the strong correlation between chlorophyll a and profundal Diporeia populations reported here for both lakes do support a contributory role for food limitation in the declines of Diporeia. The period of greatest Diporeia decline in Lake Huron in this time series corresponded with marked decreases in spring populations of large diatoms, which would settle quickly to the bottom and represent an important food source for Diporeia (Gardner et al., 1990). A similar, but more gradual, shift in phytoplankton was seen in Lake Michigan.
Direct negative interactions between Diporeia and Dreissena is less likely. As has been seen in past studies (Dermott, 2001; Nalepa et al., 2003; Watkins et al., 2013), Diporeia declines often preceded the appearance of Dreissena at our sites. Dreissena was largely absent from deeper sites in Lake Michigan between 1997 and 2005 when Diporeia populations had declined dramatically at those sites, while in Lake Huron, Diporeia had disappeared from shallow sites before the arrival of Dreissena. In some cases its disappearance preceded the arrival of Dreissena by ten years. Declines at deeper sites in Lake Huron had stabilized long before Dreissena was apparent at those sites. Our data cannot resolve the causes for the Diporeia declines, but the extremely high degree of synchrony in population dynamics between the two lakes, in light of similar patterns in chlorophyll a but dissimilar patterns of Dreissena distribution, would lend more weight to the importance of food limitation, perhaps mediated on a broad geographic scale and in a lake-specific way by Dreissena, and less to direct interactions with Dreissena.
Total August crustacean zooplankton biomass declined in both lakes, driven in large part by declines in cladocerans (predominantly Daphnia mendotae), resulting in a zooplankton community dominated by calanoid copepods. The striking coincidence between the decline of the spring bloom in Lake Huron and the collapse of the cladoceran population there led to speculation of a bottom-up effect (Barbiero et al., 2011a), although the limited seasonal overlap between the spring bloom and cladoceran populations made simple acceptance of that relationship problematic. However, the size of the spring bloom has been shown to be highly correlated with that of the deep chlorophyll layer (DCL) (Pothoven and Fahnenstiel, 2013), and it may be the DCL which is having a greater impact on cladoceran population size. Pothoven and Fahnenstiel (2013) reported declines in the DCL in southeastern Lake Michigan, which they attributed to a reduction in the spring bloom, and thus a reduction in the initial inoculum for the DCL, and to a disruption of horizontal transport of nutrients to the offshore, both caused by dreissenids. This could be particularly important considering the deeper vertical distribution of the main cladoceran, D. mendotae, seen in both lakes in recent years (Bourdeau et al., 2015; Nowicki et al., 2017).
Vanderploeg et al. (2012) also saw decreases in cladocerans at their site in southeast Lake Michigan, which they attributed to decreased vertebrate planktivory allowing increases in the invertebrate predator Bythotrephes. The main vertebrate planktivore in the lakes, alewife, has declined over the course of the study in both lakes, albeit much more dramatically in Lake Huron (He et al. 2015; Riley et al., 2008) than in Lake Michigan (Madenjian et al., 2015). While the lower populations of alewife in Lake Huron, compared to Lake Michigan, and the greater loss of cladocerans in Lake Huron might seem to support Vanderploeg et al.’s hypothesis, we have seen no increase in Bythotrephes in either lake in the GLNPO long-term data set (Barbiero, unpublished data). A more detailed look at zooplankton communities in these two lakes will be deferred for a separate treatment. Regardless of the cause, though, the decline of the highly efficient cladoceran grazers in both lakes could have consequences for nutrient dynamics given their important role in P cycling in the lakes (Korstad, 1983; Scavia and Fahnenstiel, 1987).
Conclusions
We have shown remarkable synchrony between a number of lower food web variables in Lake Huron and Lake Michigan, this in marked contrast to the substantial differences between the two lakes in the time course of dreissenid colonization of the offshore. While we have presented data suggestive of grazing control of spring phytoplankton in the offshore, including decreases in Chl:TP and increases in TDP:TP, these occurred when calculated filtration impacts of dreissenid populations in the offshore were low in both lakes, and particularly in Lake Huron. Similarly, a suite of offshore lower trophic level variables exhibited major shifts which preceded the main period of dreissenid expansion into the profundal zone in both lakes, and in some cases stabilized while profundal dreissenid populations were expanding. Finally, the changes in lower trophic level variables in Lake Huron were more pronounced, and often earlier, than those in Lake Michigan, in spite of vastly lower profundal dreissenid populations in the former lake, arguing against direct grazing impacts by offshore dreissenids as a primary mechanism. Our data, viewed in toto, point to a greater likelihood that any dreissenid impacts on the offshore waters were remote, through nearshore sequestration of nutrients, as proposed by Hecky et al. (2004) and Vanderploeg et al. (2010), before the main expansion of D. bugensis into the profundal zone. Our analyses also suggest that reductions in P loading have probably contributed little to the declines in TP seen in the lakes. The striking degree of synchrony among many of the variables we examined suggest that broad climatic factors, such as interannual differences in wave action, could also be playing a substantial role in governing nutrient and lower food web dynamics. It is important to note that none of these factors are mutually exclusive, and it is possible, if not likely, that multiple factors are additively and/or cumulatively contributing in varying degrees to the changes seen. Finally, given the high degree of concordance in lower trophic level variables in the two lakes, we believe that more robust conclusions can be reached by employing a comparative approach when formulating hypotheses of potential causal factors to explain the changes that both lakes are experiencing.
Acknowledgements
We gratefully acknowledge the support of Julie Lietz in the preparation of this manuscript. This work was supported by the USEPA Great Lakes National Program Office as part of EPA Contract No. EP-C-15-012, Scientific and Technical Support with CSRA, LLC under the direction of Louis Blume, Project Manager as well as by agreements with Cornell University, Department of Natural Resources and Buffalo State College under Prime Agreement Award GL-00E01184 from the U.S. EPA “Great Lakes Long Term Biological Monitoring of Zooplankton, Benthos, and Chlorophyll a” and with Regents of the University of Minnesota (to E. Reavie) from the U.S. Environmental Protection Agency under Prime Agreement Awards GL-00E23101-2 and GL-00E01980 “Great Lakes Biological Monitoring: Phytoplankton”. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the USEPA.
References
- Ackerman JD, Loewen MR, Hamblin PF, 2001. Benthic–pelagic coupling over a zebra mussel reef in western Lake Erie. Limnol. Oceanogr 46, 892–904. [Google Scholar]
- APHA (American Public Health Association), 1985. Standard Methods for the Examination of Water and Wastewater. 16th ed. Washington, D.C.: American Public Health Association. [Google Scholar]
- Barbiero RP, Rockwell DC, Warren GJ, Tuchman ML, 2006. Changes in spring phytoplankton communities and nutrient dynamics in the eastern basin of Lake Erie since the invasion of Dreissena spp. Can. J Fish. Aquat. Sci 63: 1549–1563. [Google Scholar]
- Barbiero RP, Balcer M, Rockwell DC, Tuchman ML, 2009a. Recent shifts in the crustacean zooplankton community of Lake Huron. Can. J. Fish. Aquat. Sci 66, 816–828. [Google Scholar]
- Barbiero RP, Bunnell DB, Rockwell DC, Tuchman ML, 2009b. Recent increases in the large glacial-relict calanoid Limnocalanus macrurus in Lake Michigan. J. Great Lakes Res 35, 285–292. [Google Scholar]
- Barbiero RP, Lesht BM, Warren GJ, 2011a. Evidence for bottom-up control of recent shifts in the pelagic food web of Lake Huron. J. Great Lakes Res 37, 78–85. [Google Scholar]
- Barbiero RP, Schmude K, Lesht BM, Riseng CM, Warren GJ, Tuchman ML, 2011b. Trends in Diporeia populations across the Laurentian Great Lakes, 1997–2009. J. Great Lakes Res 37, 9–17. [Google Scholar]
- Barbiero RP, Lesht BM, Warren GJ, 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. J. Great Lakes Res 38, 368–380. [Google Scholar]
- Barbiero RP, Nalepa TF, Lesht BM, Warren GJ, 2013. Status of phytoplankton, zooplankton and benthos, in: Riley SC (Ed.), The State of Lake Huron in 2010. Great Lakes Fish. Comm. Spec. Pub 13–01, pp. 10–20. [Google Scholar]
- Barbiero RP, Tuchman ML, 2004. Changes in the crustacean communities of Lakes Michigan, Huron and Erie following the invasion of the predatory cladoceran Bythotrephes longimanus. Can. J. Fish. Aquat. Sci 61, 2111–2125. [Google Scholar]
- Barbiero RP, Tuchman ML, Warren GJ, Rockwell DC, 2002. Evidence of recovery from phosphorous enrichment in Lake Michigan. Can. J. Fish. Aquat. Sci 59, 1639–1647. [Google Scholar]
- Barbiero RP, Lesht BM, Hinchey EK, Nettsheim TG, 2018. A brief history of the U.S. EPA Great Lakes National Program Office’s Water Quality Surveys. J. Great Lakes Res, this issue. [Google Scholar]
- Beeton AM, 1965. Eutrophication of the St. Lawrence Great Lakes. Limnol. Oceanogr 10, 240–254. [Google Scholar]
- Beeton AM, Saylor JH, 1995. Limnology of Lake Huron, in: Munawar M, Edsall T, Leach J (Eds.), The Lake Huron Ecosystem: Ecology, Fisheries and Management. SPB Academic Publishing, Amsterdam, pp. 1–37. [Google Scholar]
- Berg DJ, Fisher SW, Landrum PF, 1996. Clearance and processing of algal particles by zebra mussels (Dreissena polymorpha). J. Great Lakes Res 22, 779–788. [Google Scholar]
- Berst AH, Spangler GR, 1972. Lake Huron: effects of exploitation, introductions, and eutrophication on the salmonid community. J. Fish. Res. Board Can 29, 877–887. [Google Scholar]
- Boegman L, Loewen MR, Hamblin PF, Culver DA, 2008. Vertical mixing and weak stratification over zebra mussel colonies in western Lake Erie. Limnol. Oceanogr 53: 1093–1110. [Google Scholar]
- Bootsma HA, Liao Q, 2014. Nutrient cycling by dreissenid mussels: controlling factors and ecosystem response In: Nalepa TF, Schloesser DW (Eds.), Quagga and Zebra Mussels: Biology, Impacts and Control, 2nd ed. CRC Press, Boca Raton, FL, pp. 555–574. [Google Scholar]
- Bourdeau PE, Pangle KL, Peacor SD, 2015. Factors affecting the vertical distribution of the zooplankton assemblage in Lake Michigan: The role of the invasive predator Bythotrephes longimanus. J. Great Lakes Res 41, 115–124. [Google Scholar]
- Bunnell DB, Barbiero RP, Ludsin SA, Madenjian CP, Warren GJ, Dolan DM, Brenden TO, Briland R, Gorman OT, He JX, Johengen TH, Lantry BF, Lesht BM, Nalepa TF, Riley SC, Riseng CM, Treska TJ, Tsehaye I, Walsh MG, Warner DM, Weidel BC, 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. BioSci. 64, 26–39. [Google Scholar]
- Bunnell DB, Davis BM, Warner DM, Chrisinske MA, Roseman EF, 2011. Planktivory in the changing Lake Huron zooplankton community: Bythotrephes consumption exceeds that of Mysis and fish. Freshw. Biol 56, 1281–1296. [Google Scholar]
- Bur MT, Klarer DM, Krieger KA, 1986. First records of a European cladoceran, Bythotrephes cederstroemi, in Lakes Erie and Huron. J. Great Lakes Res 12, 144–146. [Google Scholar]
- Burlakova LE, Barbiero RP, Karatayev AY, Daniel SE, Hinchey EK, Warren GJ, 2017. The benthic community of the Laurentian Great Lakes: analysis of spatial gradients and temporal trends from 1998–2014. J. Great Lakes Res, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapra SC, Dolan DM, 2011. GL2K: A Mass-Balance Modeling Framework for Simulating Long-Term Trends of Great Lakes Water Quality (Version 1.3): Documentation and Users Manual Civil and Environmental Engineering Dept., Tufts University, Medford, MA., [Google Scholar]
- Chapra SC, Dolan DM, 2012. Great Lakes total phosphorus revisited: 2. mass balance modeling. J. Great Lakes Res 38, 740–754. [Google Scholar]
- Cotner JB, Johengen TH, Biddanda BA, 2000. Intense winter heterotrophic production stimulated by benthic resuspension. Limnol. Oceanogr 45, 1672–1676. [Google Scholar]
- Dermott R, 2001. Sudden disappearance of the amphipod Diporeia from eastern Lake Ontario, 1993–1995. J. Great Lakes Res 27, 423–433. [Google Scholar]
- Dermott R, Munawar M, 1993. Invasion of Lake Erie offshore sediments by Dreissena, and its ecological implications. Can. J. Fish. Aquat. Sci 50, 2298–2304. [Google Scholar]
- Dove A, Chapra SC, 2015. Long-term nutrients and trophic response variables for the Great Lakes. Limnol. Oceanogr 60, 696–721. [Google Scholar]
- Dunlop ES, Riley SC, 2013. The contribution of cold winter temperatures to the 2003 alewife population collapse in Lake Huron. J. Great Lakes Res 39, 682–689. [Google Scholar]
- Eadie BJ, Schwab DJ, Johengen TH, Lavrentyev PJ, Miller GS, Holland RE, Leshkevich GA, Lansing MB, Morehead NR, Robbins JA, Hawley N, Edgington DN, Van Hoof PL, 2002. Particle transport, nutrient cycling, and algal community structure associated with a major winter-spring sediment resuspension event in southern Lake Michigan. J. Great Lakes Res 28, 324–337. [Google Scholar]
- Evans MS, 1988. Bythotrephes cederstroemi: its new appearance in Lake Michigan. J. Great Lakes Res 14, 234–240. [Google Scholar]
- Fahnenstiel GL, Nalepa TF, Pothoven SA, Carrick HJ, Scavia D, 2010a. Lake Michigan lower food web: long-term observations and Dreissena impact. J. Great Lakes Res 36, 1–4. [Google Scholar]
- Fahnenstiel GL, Pothoven S, Vanderploeg H, Klarer D, Nalepa T, Scavia D, 2010b. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. J. Great Lakes Res 36, 20–29. [Google Scholar]
- Fahnenstiel GL, Stone RA, McCormick MJ, Schelske CL, Lohrenz SE, 2000. Spring isothermal mixing in the Great Lakes: evidence of nutrient limitation and nutrient-light interactions in a suboptimal light environment. Can. J. Fish. Aquat. Sci 57, 1901–1910. [Google Scholar]
- Gardner WS, Quigley MA, Fahnenstiel GL, Scavia D, Frez W, 1990. Pontoporeia hoyi - a direct trophic link between spring diatoms and fish in Lake Michigan, in: Tilzer MM, Serruya C (Eds.), Large Lakes: Ecological Structure and Function. Springer-Verlag, New York, pp. 632–644. [Google Scholar]
- Griffiths RW, Schloesser DW, Leach JH, Kovalak WP, 1991. Distribution and dispersal of the zebra mussel (Dreissena polymorpha) in the Great Lakes region. Can. J. Fish. Aquat. Sci 48, 1381–1388. [Google Scholar]
- He JX, Bence JR, Madenjian CP, Pothoven SA, Dobiesz NE, Fielder DG, Johnson JE, Ebener MP, Cottrill RA, Mohr LC, Koproski SR, 2015. Coupling age-structured stock assessment and fish bioenergetics models: a system of time-varying models for quantifying piscivory patterns during the rapid trophic shift in the main basin of Lake Huron. Can. J. Fish. Aquat. Sci 72, 7–23. [Google Scholar]
- Heath RT, Fahnenstiel GL, Gardner WS, Cavaletto JF, Hwang S-J, 1995. Ecosystem level effects of zebra mussels (Dreissena polymorpha): An enclosure experiment in Saginaw Bay, Lake Huron. J Great Lakes Res. 21, 501–516. [Google Scholar]
- Hecky RE, Smith REH, Barton DR, Guildford SJ, Taylor WD, Charlton MN, Howell T, 2004. The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci 61, 1285–1293. [Google Scholar]
- Higgins SN, Vander Zanden MJ, Joppa LN, Vadeboncoeur Y, 2011. The effect of dreissenid invasion on chlorophyll and the chlorophyll: total phosphorus ratio in north temperate lakes. Can. J. Fish. Aquat. Sci 68, 319–329. [Google Scholar]
- Holland RE, Johengen TH, Beeton AM, 1995. Trends in nutrient concentrations in Hatchery Bay, western Lake Erie, before and after Dreissena polymorpha. Can. J. Fish. Aquat. Sci 52, 1202–1209. [Google Scholar]
- Hough JL, 1958. Geology of the Great Lakes. University of Illinois Press, Urbana, Illinois. [Google Scholar]
- Idrisi N, Mills EL, Rudstam LG, Stewart DJ, 2001. Impact of zebra mussels (Dreissena polymorpha) on the pelagic lower trophic levels of Oneida Lake, New York. Can. J. Fish. Aquat. Sci 58, 1430–1441. [Google Scholar]
- James WF, Barko JW, Davis M, Eakin HL, Rogala JT, Miller AC, 2000. Filtration and excretion by zebra mussels: implications for water quality impacts in Lake Pepin, Upper Mississippi River. J. Freshw. Ecol 15, 429–437. [Google Scholar]
- James WF, Barko JW, Eaking HL, 1997. Nutrient regeneration by the zebra mussel (Dreissena polymorpha). J. Freshw. Ecol 12, 209–216. [Google Scholar]
- Johengen TH, Nalepa TF, Fahnenstiel GL, Goudy G, 1995. Nutrient changes in Saginaw Bay, Lake Huron, after the establishment of the zebra mussel (Dreissena polymorpha). J. Great Lakes Res 21, 449–464. [Google Scholar]
- Karatayev AY, Mehler K, Burlakova LE, Hinchey EK, Warren GJ, 2018. Benthic video image analysis facilitates monitoring of Dreissena populations across spatial scales. J. Great Lakes Res, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot WC, Yousef F, Green SA, Budd JW, Schwab DJ, Vanderploeg HA, 2010. Approaching storm: disappearing winter bloom in Lake Michigan. J. Great Lakes Res 36, 30–41. [Google Scholar]
- Korstad J, 1983. Nutrient regeneration by zooplankton in southern Lake Huron. J. Great Lakes Res 9, 374–388. [Google Scholar]
- Kratz TK, Soranno PA, Baines SB, Benson BJ, 1998. Interannual synchronous dynamics in north temperate lakes in Wisconsin, USA, in: George DG, Jones JG, Puncochar CS, Reynolds CS, Sutcliffe DW (Eds.), Management of lakes and reservoirs during global climate change. Kluwer Academic Publishers, pp. 273–287. [Google Scholar]
- Lehman JT, Cáceres CE, 1993. Food-web responses to species invasion by a predatory invertebrate: Bythotrephes in Lake Michigan. Limnol. Oceanogr 38, 879–891. [Google Scholar]
- Lesht BM, Barbiero RP, Warren GJ, 2013. A band-ration algorithm for retrieving open lake chlorophyll values from satellite observations of the Great Lakes. J. Great Lakes Res 39, 138–152. [Google Scholar]
- Lesht BM, Barbiero RP, Warren GJ, 2018. Using satellite observations to assess the spatial representativeness of the GLNPO water quality monitoring program. J. Great Lakes Res, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesht BM, Barbiero RP, Warren GJ, 2016. Verification of a simple band ratio algorithm for retrieving Great Lakes open water surface chlorophyll concentrations from satellite observations. J. Great Lakes Res 42, 448–454. [Google Scholar]
- MacIsaac HJ, Johannsson OE, Ye J, Sprules WG, Leach JH, McCorquodale JA, Grigorovich IA, 1999. Filtering impacts of an introduced bivalve (Dreissena polymorpha) in a shallow lake: application of a hydrodynamic model. Ecosystems 2, 338–350. [Google Scholar]
- Madenjian CP, Bunnell DB, Warner DM, Pothoven SA, Fahnenstiel GL, Nalepa TF, Vanderploeg HA, Tsehaye I, Claramunt RM, Clark RD Jr., 2015. Changes in the Lake Michigan food web following dreissenid mussel invasions: A synthesis. J. Great Lakes Res 41, 217–231. [Google Scholar]
- Madon SP, Schneider DW, Stoechel JA, Sparks RE, 1998. Effects of inorganic sediment and food concentrations on energetic processes of the zebra mussel, Dreissena polymorpha: implications for growth in turbid rivers. Can. J. Fish. Aquat. Sci 55, 401–413. [Google Scholar]
- Magnuson JJ, Kratz TK, Benson BJ, 2006. Long-Term Dynamics of Lakes in the Landscape: Long-Term Ecological Research on North Temperate Lakes. Oxford University Press, New York. [Google Scholar]
- Mazumder A, 1994a. Phosphorus-chlorophyll relationships under contrasting herbivory and thermal stratification: predictions and patterns. Can. J. Fish. Aquat. Sci 51, 390–400. [Google Scholar]
- Mazumder A, 1994b. Phosphorus-chlorophyll relationships under contrasting zooplankton community structure: potential mechanisms. Can. J. Fish. Aquat. Sci 51, 401–407. [Google Scholar]
- Mellina E, Rasmussen JB, Mills EL, 1995. Impact of zebra mussel (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Can. J. Fish. Aquat. Sci 52, 2553–2573. [Google Scholar]
- Mosley CM, Bootsma HA, 2015. Phosphorus cycling and grazing by profunda quagga mussels in Lake Michigan. J. Great Lakes Res 41, 38–48. [Google Scholar]
- Nalepa TF, Burlakova LE, Elgin AK, Karatayev AY, Lang GA, Mehler K, 2017. Major Findings from the CSMI Benthic Macroinvertebrate Survey in Lake Michigan in 2015 with an Emphasis on Temporal Trends Chapter 3, in: Lake Erie and Lake Michigan Benthos: Cooperative Science and Monitoring Initiative. Final Report. USGS-GLRI G14AC00263. Great Lakes Center, SUNY Buffalo State, Buffalo, NY. [Google Scholar]
- Nalepa TF, Fanslow DL, Foley AJ III, Lang GA, Eadie BJ, Quigley MA, 2006. Continued disappearance of the benthic amphipod Diporeia spp. in Lake Michigan: is there evidence of food limitation? Can. J. Fish. Aquat. Sci 63, 872–890. [Google Scholar]
- Nalepa TF, Fanslow DL, Lang GA, 2009. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to the invasive mussel Dreissena rostriformis bugensis. Freshw. Biol 54, 466–479. [Google Scholar]
- Nalepa TF, Fanslow DL, Lang GA, Mabrey K, Rowe M, 2014. Lake-wide benthic surveys in Lake Michigan in 1994–95, 2000, 2005, and 2010: abundances of the amphipod Diporeia spp. and abundances and biomass of the mussels Dreissena polymorpha and Dreissena rostriformis bugensis NOAA Technical Memorandum GLERL-164. [Google Scholar]
- Nalepa TF, Fanslow DL, Lansing MB, Lang GA, 2003. Trends in the benthic macroinvertebrate community of Saginaw Bay, Lake Huron, 1987 to 1996: responses to phosphorus abatement and the zebra mussel, Dreissena polymorpha. J. Great Lakes Res 29, 14–33. [Google Scholar]
- Nalepa TP, Fanslow DL, Pothoven SA, 2010. Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. J. Great Lakes Res 36, 5–19. [Google Scholar]
- Nalepa TF, Fanslow DL, Pothoven SA, Foley AJ III, Lang GA, 2007. Long-term trends in benthic macroinvertebrate populations in Lake Huron over the past four decades. J. Great Lakes Res 33, 421–436. [Google Scholar]
- Nalepa TF, Hartson DJ, Fanslow DL, Lang GA, Lozano SJ, 1998. Declines in benthic macroinvertebrate populations in southern Lake Michigan, 1980–1993. Can. J. Fish. Aquat. Sci 55, 2402–2413. [Google Scholar]
- Nalepa TF, Schloesser DW, Pothoven SA, Hondorp DW, Fanslow DL, Tuchman ML, Fleischer GQ, 2001. First finding of the amphipod Echinogammarus ischnus and the mussel Dreissena bugensis in Lake Michigan. J. Great Lakes Res 27, 384–391. [Google Scholar]
- Nicholls KH, 1998. El Niño, ice cover, and Great Lakes phosphorus: implications for climate warming. Limnol. Oceanogr 43, 715–719. [Google Scholar]
- Nicholls KH, Hopkins GJ, Standke SJ, 1997. Effects of zebra mussels on chlorophyll, nitrogen, phosphorus and silica in north shore waters of Lake Erie. Ministry of Environment and Energy, Toronto: ISBN 0–7778- 6707–9, PIBS 3856E. [Google Scholar]
- Nicholls KH, Hopkins GJ, Standke SJ, 1999. Reduced chlorophyll to phosphorus ratios in nearshore Great Lakes waters coincide with the establishment of dreissenid mussels. Can. J. Fish. Aquat. Sci 56, 153–161. [Google Scholar]
- Nowicki CJ, Bunnell DB, Armenion PM, Warner DM, Vanderploeg HA, Cavaletto JF, Mayer CM, Adams JV, 2017. Biotic and abiotic factors influencing zooplankton vertical distribution in Lake Huron. J. Great Lakes Res 42, 1044–1054. [Google Scholar]
- Pothoven SA, Fahnenstiel GL, 2013. Recent changes in summer chlorophyll a dynamics of southeastern Lake Michigan. J. Great Lakes Res 39, 287–294. [Google Scholar]
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP, 2007. Section 10.5. Downhill Simplex Method in Multidimensions Numerical Recipes: The Art of Scientific Computing, third ed. Cambridge University Press, New York. [Google Scholar]
- Quinn FH, 1992. Hydraulic residence times for the Laurentian Great Lakes. J. Great Lakes Res 18, 22–28. [Google Scholar]
- Reavie ED, Barbiero RP, Allinger LE, Warren GJ, 2014. Phytoplankton trends in the Great Lakes: 2001–2011. J. Great Lakes Res 40, 618–639. [Google Scholar]
- Riley SC, Roseman EF, Nichols SJ, O’Brien TP, Kiley CS, Schaeffer JS, 2008. Deepwater demersal fish community collapse in Lake Huron. Trans. Am. Fish. Soc 137, 1879–1890. [Google Scholar]
- Rockwell DC, DeVault DS III, Palmer MF, Marion CV, Bowden RJ, 1980. Lake Michigan intensive survey, 1976–1977. U.S. Environmental Protection Agency. Great Lakes National Program Office, Chicago, IL, EPA-905/4–80-003-A. [Google Scholar]
- Rockwell DC, Salisbury DK, Lesht BM, 1989. Water quality in the middle Great Lakes: results of the 1985 USEPA survey of Lakes Erie, Huron and Michigan. U.S. Environmental Protection Agency. Great Lakes National Program Office, Chicago, IL, EPA-905/6/89–001. [Google Scholar]
- Rodionov SN, 2004. A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett 31, L09204. [Google Scholar]
- Rodionov SN, Overland JE, 2005. Application of a sequential regime shift detection method to the Bering Sea ecosystem. ICES J. Mar. Sci 62, 328–332. [Google Scholar]
- Rodgers PW, Salisbury DK, 1981. Consideration of the anomalous ice cover of 1976–1977. J. Great Lakes Res 7, 467–480. [Google Scholar]
- Rowe MD, Anderson EJ, Vanderploeg HA, Pothoven SA, Elgin AK, Wang J, Yousef F, 2017. Influence of invasive quagga mussels, phosphorus loads, and climate on spatial and temporal patterns of productivity in Lake Michigan: A biophysical modeling study. Limnol. Oceanogr 62, 2629–2649. [Google Scholar]
- Rowe MD, Obenour DR, Nalepa TF, Vanderploeg HA, Yousef F, Kerfoot WC, 2015. Mapping the spatial distribution of the biomass and filter-feeding effect of invasive dreissenid mussels on the winter–spring phytoplankton bloom in Lake Michigan. Freshw. Biol 60, 2270–2285. [Google Scholar]
- Rusak JA, Yan ND, Somers KM, McQueen DJ, 1999. The temporal coherence of zooplankton population abundances in neighboring north-temperate lakes. Am. Nat 153, 46–58. [DOI] [PubMed] [Google Scholar]
- Rusak JA, Yan ND, Somers KM, 2008. Regional climatic drivers of synchronous zooplankton dynamics in north-temperate lakes. Can. J. Fish. Aquat. Sci 65, 878–889. [Google Scholar]
- Saylor JH, Sloss PW, 1976. Water volume transport and oscillatory current flow through the Straits of Mackinac. J. Phys. Oceanogr 6, 229–237. [Google Scholar]
- Scavia D, Fahnenstiel GL, 1987. Dynamics of Lake Michigan phytoplankton: mechanisms controlling epilimnetic communities. J. Great Lakes Res 13, 103–120. [Google Scholar]
- Schelske CL, Roth JC, 1973. Limnological survey of lakes Michigan, Superior, Huron and Erie. Univ. Mich. Great Lakes Res. Div No. 17. [Google Scholar]
- Sprung M, Rose U, 1988. Influence of food size and food quantity on the feeding of the mussel Dreissena polymorpha. Oecologia. 77, 526–532. [DOI] [PubMed] [Google Scholar]
- Straile D, 2002. North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc. R. Soc. Lond. Biol 269, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straile D, Adrian R, 2000. The North Atlantic Oscillation and plankton dynamics in two European lakes – two variations on a general theme. Global Change Biol. 6, 663–670. [Google Scholar]
- U.S. EPA. 2017. SOP LG403. Standard Operating Procedure for Zooplankton Analysis. Revision 08, February 2017. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL. [Google Scholar]
- Vanderploeg HA, Liebig JR, Nalepa TF, Fahnenstiel GL, Pothoven SA, 2010. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. J. Great Lakes Res 36, 50–59. [Google Scholar]
- Vanderploeg HA, Nalepa TF, Jude DJ, Mills EL, Holeck KT, Liebig JR, Grigorovich IA, Ojaveer H, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci 59, 1209–1228. [Google Scholar]
- Vanderploeg HA, Pothoven SA, Fahnenstiel GL, Cavaletto JF, Liebig JR, Stow CA, Nalepa TF, Madenjian CP, Bunnell DB, 2012. Seasonal zooplankton dynamics in Lake Michigan: Disentangling impacts of resource limitation, ecosystem engineering, and predation during a critical ecosystem transition. J. Great Lakes Res 38, 336–352. [Google Scholar]
- Waples JT, Bootsma HA, Klump JV, 2017. How are coastal benthos fed? Limnol. Oceanogr. Lett 2, 18–28. [Google Scholar]
- Warner DM, Lesht BM, 2015. Relative importance of phosphorus, invasive mussels and climate for patterns in chlorophyll a and primary production in Lakes Michigan and Huron. Freshw. Biol 60, 1029–1043. [Google Scholar]
- Watkins JM, Dermott R, Lozano SJ, Mills EL, Rudstam LG, Scharold JV, 2007. Evidence for remote effects of dreissenid mussels on the amphipod Diporeia: analysis of Lake Ontario benthic surveys, 1972–2003. J. Great Lakes Res 33, 642–657. [Google Scholar]
- Watkins JM, Rudstam LG, Crabtree DL, Walsh MG, 2013. Is reduced benthic flux related to the Diporeia decline? Analysis of spring blooms and whiting events in Lake Ontario. J. Great Lakes Res 39, 395–403. [Google Scholar]
- Wells L, McLain AL, 1972. Lake Michigan: effects of exploitation, introductions, and eutrophication on the salmonid community. J. Fish. Res. Board Can 29, 889–898. [Google Scholar]
- Winder M, Schindler DE, 2004. Climatic effects on the phenology of lake processes. Global. Change Biol 10, 1844–1856. [Google Scholar]
- Yousef F, Kerfoot WC, Shuchman R, Fahnenstiel G, 2014. Bio-optical properties and primary production of Lake Michigan: insights from 13-years of SeaWiFS imagery. J. Great Lakes Res 40, 317–324. [Google Scholar]