Abstract
Autoimmune diseases (ADs) are associated with an increased risk not only of lymphoproliferative disorders but also of myeloid malignancies. The excess risk of myelodysplastic syndromes and/or acute myeloid leukemia is observed across several AD types, including systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disorders, multiple sclerosis, among others. The risk of developing myeloid neoplasms (MNs) is dependent on several variables, including the specific AD type, chronicity and severity of the AD, type and duration of exposure of disease modifying anti-rheumatic drugs or cytotoxics/immunosuppressives, and genetic predisposition among others. Putative triggering factors linking AD to elevated MN risk include AD-directed medications, shared genetic susceptibilities between the two disease entities, and chronic immune stimulation or bone marrow infiltration by the AD. Molecular mechanisms underpinning leukemogenesis remain largely speculative and warrant further investigation. Leukemias arising in patients with AD are not always ‘therapy-related’ in that MNs may develop in certain AD subtypes even among patients with no prior therapy exposure. Only a few studies have attempted to determine factors associated with MN development in AD but failed to demonstrate consistent characteristic clinical or paraclinical features. These reports have failed to demonstrate a clear correlation between individual agent exposure and subsequent leukemia development due to the low rates of therapy exposure compounded by the rarity of MN occurrence. Notwithstanding, the leukemogenic potential is best documented with agents such as azathioprine, cyclophosphamide, and mitoxantrone; this risk of MN development does not appear to be shared by biologic approaches such as anti-tumor necrosis factors-alpha inhibitors. In this article, we discuss plausible biologic mechanisms underpinning MN pathogenesis in AD and review the data available on the development of MNs in AD patients.
Keywords: autoimmune, myeloid, leukemia, myelodysplastic syndromes, SLE, RA, IBD, azathioprine
INTRODUCTION:
Myeloid neoplasms (MNs) comprise a heterogeneous group of clonal hematopoietic malignancies characterized by their origin from precursors of the myeloid lineages with dysregulated proliferation and impaired differentiation. Myeloid neoplasms are broadly organized into acute myeloid leukemia (AML) and chronic myeloid neoplasms (cMNs) based on the clinical behavior and peripheral blood and bone marrow (BM) blast percentage. The cMNs are further categorized into myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), MDS/MPN overlap disorders, and myeloid/lymphoid neoplasms of PDGFRA, PDGFRB or FGFR1.(1) While MN classification is based primarily on blast percentages and degree of dysplasia, the availability of rapidly accumulating molecular genetic data in parallel with advances in molecular technologies has led to their incorporation in the most recent WHO 2016 revision in an attempt to better define disease entities of prognostic significance.(2),(3)
In 2018, 19,520 and 13,980 new AML and cMN cases, respectively, are projected to occur in the United States.(4) Over the past decade of data (2009-2018), this represents an approximate 52% and 30% increase in incidence rates of AML and cMN, respectively.(4, 5) The varying incidence patterns and prevalence rates according to the disease sub-type support their distinct aetiopathogenesis and/or susceptible populations(6).
Therapy-related myeloid neoplasms (t-MN) represent a distinct clinical sub-group of myeloid neoplasms (MNs) that compromise t-AML or t-MDS (or t-MDS/MPN) depending on the blast count.(7, 8) t-MNs arise as a late effect of chemotherapy and/or radiation administered for a primary condition, often a malignant disease, solid organ transplant, or autoimmune disease (AD).(9) More recent data suggests that this term should be used selectively to describe the evolution of AML/MDS, but not myelofibrosis.(10) Although the development of t-MDS/t-AML has often been linked to previous exposure to topoisomerase II inhibitors, alkylating agents, or radiation, this distinction is no longer recommended. (1, 8)The diagnosis of t-MDS/t-AML connotes uniformly poor outcomes and is often associated with specific cytogenetic abnormalities involving chromosomes 5, 7, 11q23, or 21q22.(11–13) The global incidence rates of AML and MDS, as a result of treatment for another cancer, range from 0.06-2.6 per 100,000 and 0.06-0.26 per 100,000, respectively.(14) These estimates are expected to rise with an increase in the number of cancer survivors.(15, 16)
Chemotherapy exposure for a prior malignancy increases t-MN risk by 4.7-fold compared to population baseline, with certain factors such as the age at exposure, and/or tumor type and chemotherapy regimen influencing that risk(17-20). While the decremental use of alkylating agents has resulted in a decreased incidence of t-AML in certain malignancies such as ovarian cancer and multiple myeloma,(17) a reciprocal trend of increased t-AML incidence has been observed in certain other cancer populations due to growing use of solid organ and stem cell transplant strategies. The association of limited exposure to therapeutic radiation in the absence of chemotherapy (e.g. for localized breast cancer) with subsequent development of t-MN is more controversial.(16, 21, 22)
Patients with AD who develop a secondary leukemia typically develop AML rather than acute lymphoblastic leukemia (ALL). An increased incidence of AML has been reported across several AD types such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), polymyalgia rheumatica, ankylosing spondylitis, autoimmune hemolytic anemia, systemic vasculitides, pernicious anemia, multiple sclerosis (MS), and inflammatory bowel disease (IBD).(23-25)(26-28) Interestingly, autoimmune diseases develop in as many as 10% of patients with MDS and MPNs.(29) MDS/MPN-associated AD entities include the vasculitides, Behcet’s disease, IBD, glomerulonephritides, neutrophilic dermatoses, hemolytic anemia, and immune thrombocytopenia.(30) A few large-scale studies have explored the risk association of MNs with AD. In a SEER population-based study, by Anderson et al,(23) it was observed that having an AD was associated with a significantly increased AML and MDS risk of 1.29 (95% CI, 1.2-1.39) and 1.5 (CI, 1.35-1.66), respectively. A subsequent population-based registry study in Sweden showed similar results; the risk of AML and MDS was 1.7 (95% CI, 1.5-1.9) and 2.1 (95% CI, 1.72.6) fold higher among patients with a previous history of AD.(31) The risk of developing MDS is especially high among those with AD for 10 years or longer.(32)
In this article, we review the data available on the development of MNs in AD patients. This topic gains relevance in the era of expanding treatment options, as there occurs a paradigm shift in the treatment of AD from the use of conventional immunosuppressive agents to more selective and targeted approaches such as immunotherapy-based, biologic, and molecular therapies. In addition, we will review some of the autoimmune manifestations of hematologic disease which may precede or occur concurrently with the AML or MDS
Factors contributing to MN development in AD:
From both a clinical and molecular perspective, myeloid neoplasms are etiologically heterogeneous. A delineation of the leukemogenic mechanisms underlying MNs in patients with AD will not only enhance our understanding of the association between the two entities but also help identify patients at risk of post-AD MNs who may benefit from pre-emptive strategies. Furthermore, understanding MN pathogenesis will be a crucial step toward the management of both the AD as well as the MN.
Shared genetic predisposition:
Molecular/genetic factors predisposing to AML in AD patients remain to be elucidated and are a subject of active investigation. Mutations in the tumor suppressor geneTP53, phosphatidylinositol 3-kinase/protein kinase B/mammalian Target Of Rapamycin (PI3K/mTOR) cellular pathway signaling, and cellular apoptosis such as involving the death receptor Fas, are shared by both ADs and hematologic malignancies (HMs).(33-36) Certain AD conditions may share common genetic predispositions with MNs. The occurrence of acute leukemia among patients who did not receive treatment for underlying AD suggests an intrinsic HLA associated predisposition.(37) Genes in the HLA-B region of the major histocompatibility complex (MHC) influence susceptibility to AML and response to chemotherapy.(38) For example, HLA-B27 carriers are predisposed to both ADs as well as AML.(37) The influence of HLA on the development of malignancies is even stronger for class II MHC genes.(39)
IL-1 plays a pathogenic role in a variety of HMs, particularly those involving the myeloid lineage, and may provide a pathogenetic link between hematopoietic malignancies and ADs.(40) IL-1β has been shown to regulate AML blast proliferation, leukemic cell tissue invasion, and apoptosis resistance.(41, 42) Polymorphisms within the interleukin 1 receptor antagonist gene are associated with both AD and secondary AML.(43, 44)
Chronic immune stimulation and immunologic dysregulation:
Immunologic dysregulation is a common feature to both MNs and ADs. Inflammatory cells in the immediate tumor microenvironment may be co-opted into the neoplastic process leading to activation of several pro-survival signaling pathways.(45) Pro-inflammatory chemokines and cytokines secreted by the inflammatory cells contribute to cytotoxicity, angiogenesis, and tumor progression, invasion, and metastases. The NF-kB is a central mediator of pro-inflammatory gene induction and is implicated in both ADs(46) and leukemias.(47) NF-kB contributes to tumor progression by influencing several cellular processes involving survival, proliferation(48), apoptosis(49) as well promoting tumor angiogenesis(50) and metastasis.(51) NF-kB signaling activation in tumors may be achieved either intrinsically or by extrinsic factors such as through the increased cytokine release from the tumor microenvironment.(52) Persistence of NF-κB activating stimuli in chronic inflammatory conditions may eventually outperform inhibitory feedback circuits leading to an elevated constitutive activity of NF-κB.(53) The higher incidence of cancer in patients with chronic inflammatory conditions may be explained in part by the constitutive activity of NF-κB exerting a pro-tumorigenic effect. Another important molecular mechanism triggering acute myeloid leukemogenesis involves the generation of highly reactive oxygen species by activated leukocytes and phagocytes.(54)
A key difference between the dysregulated immune responses in ADs and cancer is the disruption of immunological tolerance in the former and maintenance of immunological tolerance in the latter.(55-57) T-cells play a fundamental role in immune surveillance constraining the development of neoplastic lesions.(58) Transformed myeloid cells can develop a variety of immune escape mechanisms to induce potent tolerance to T-cells, including immunoediting, upregulating negative regulatory pathways, altering the T-cell repertoire, T-cell deletion, among others.(59) In this context, immunosuppressive treatment may further impair an already hampered immune surveillance facilitating immune escape and promoting tumor emergence.(60) Alternatively, active self-reactive cytotoxic T-cells(61) or cytotoxic exposure(62) may lead to the depletion and contraction of the hematopoietic stem cell pool potentially leading to the recruitment of genetically defective hematopoietic clones harboring genetic abnormalities.(61) Their gradual expansion and clonal dominance incurred by selection in a contracted stem cell compartment may eventually manifest as a leukemia. On the other hand, immunosuppressive therapies have been used to treat some forms of lower risk MDS.(63, 64)
Medications used to treat AD:
The therapeutic armamentarium for ADs includes several classes of drugs including antimetabolites such as methotrexate, 6-mercaptopurine, and azathioprine; alkylator agents such as cyclophosphamide, and less frequently DNA-topoisomerase II inhibitors such as mitoxantrone. Therapy related MNs have been described extensively with the use of alkylating agents and topoisomerase II inhibitors, and to a lesser extent following the use of anti-metabolites.(11, 65, 66) There does not appear to be an association between duration of drug exposure with the incidence in development of MNs.(67)
In a large population-based study with primary AD, prior azathioprine exposure was associated with a 7-fold increased risk of MNs compared to the population baseline(67). Notably, this risk was not shared by other metabolite agents such as methotrexate, mercaptopurine, or tumor necrosis factor inhibitors. While predisposition to malignancies resulting from treatment with immunosuppressive agents is primarily related to impaired immune surveillance, azathioprine may have direct mutagenic effects by inducing nonrepaired, DNA double-strand breaks to form mutagenic DNA bases.(68, 69) In addition, azathioprine has been shown to induce defective DNA-mismatch repair.(70) Chromosome 5 and/or 7 deletions are typical of alkylator-induced AML, while balanced translocations involving chromosome bands 11q23 and 21q22 have been related to previous therapy with DNA-topoisomerase II inhibitors. The effect of genotoxic agent exposure in secondary leukemogenesis is heavily influenced by individual predisposing factors, including genetic polymorphisms in drug metabolism and DNA repair processes.(70) Increased susceptibility to leukemogenesis has been linked to deficiency of drug-metabolizing enzymes(71) and genetic variants in DNA repair(72) resulting in the increased production of potentially DNA-damaging reactive intermediates and inefficient drug induced DNA damage, respectively.
Autoimmune Disorders associated with an increased risk of Myeloid Neoplasms:
Systemic Lupus Erythematosus and Myeloid Neoplasms:
SLE is a chronic and complex multi-system autoimmune disorder in which the body’s immune system attacks its own tissues, causing widespread inflammation and tissue damage. The reported prevalence of SLE in the US is 20-150 cases per 100,000.(73, 74) This disorder occurs predominantly in the reproductive age women, with the female-to-male ratio in this group ranging 7:1 – 15:1.(73, 75) Clinical course can be highly varied, with disease spectrum ranging from a benign illness to fulminant, progressive disease-causing organ failure and death. The pharmacologic therapeutic approach in the treatment of SLE is guided by disease severity and predominant clinical manifestations. While patients with mild to moderate lupus involvement usually respond to hydroxychloroquine, short-term glucocorticoids, and/or non-steroidal inflammatory agents, steroid-sparing immunosuppressive agents such as azathioprine, methotrexate, mycophenolate, cyclosporine, cyclophosphamide, or rituximab(76, 77) are required to treat severe SLE. Several factors including early diagnosis, judicious therapy choices, prompt treatment of disease and complications have significantly improved the five-year survival rate in SLE to greater than 90%(78). Major risks of death in SLE are due to cardiovascular disease, infection, and renal disease complications.(79) Although the overall risk of death is not increased due to malignancy, certain hematologic cancers (non-Hodgkins lymphoma (NHL), leukemia) and solid tumor malignancies such as breast, gynecologic, thyroid, lung, and liver cancers are substantially increased in SLE compared to population baseline.(80-84) Among the HMs, the elevated malignancy risk is strongest for NHL.(80, 82, 85) SLE patients have a higher risk of not only developing NHL but are also predisposed to other hematologic malignancies such as Hodgkin lymphoma (HL), leukemia, and possibly myeloma(86). In addition to their elevated incidence risk in SLE, hematologic cancer-related mortality is significantly higher in SLE when compared to the general population.(87, 88) It remains to be clarified whether the poorer prognosis in SLE-related hematologic malignancies is related to a more aggressive biology, decreased survival related to associated SLE, or due to other factors.(89)
Several case reports and case series have reported the association between SLE and AML with a few suggesting a link to prior cytotoxic/immunosuppressive drug exposure.(68, 90-97) This SLE and AML association has been reported across all French-American-British morphologic subtypes.(24) While the post-AML outcomes in this setting are generally poor, long term remissions may be achieved with the use of stem cell transplantation.(96, 98, 99) It is difficult to draw conclusions from these reports whether the association of AML with SLE is secondary to innate/genetic factors or exogenous exposures.
A few large-scale studies have estimated the risk of HMs in SLE patients [table 1]. In a multisite international SLE cohort involving 16409 patients observed for 7.4 patient years, 111 HMs were observed, including 18 cases of leukemia.(80) For leukemias, the standardized incidence ratio (SIR) estimate was 1.75 (1.04-2.76). Similarly, other studies(88)’(100) have reported a higher than expected incidence of HMs in SLE compared to the general population. In a study, by Tarr et al,(88) no association was observed between exposure to a specific therapy and the development of malignancy.
Table 1.
SLE and hematologic malignancy risk
| Ref | No. of patients | Cases | Malignancy type | Calculated risk (95% CI) |
|---|---|---|---|---|
| Lofstrom et al(96) | 6438 patients | 8 | AML | For Azathioprine: 0.8(0.1-4.1) For leukopenia: 14 (1.4-141) |
| Bernatsky et al(80) | 16409 patients over 7.4 person-years | 18 | leukemia | For hematologic malignancies: SIR 3.02(2.48-3.63) For leukemia: SIR 1.75 (1.04-2.76) |
| Tarr et al(88) | 860 patients | 5 | hematologic malignancies | For hematologic malignancies: SIR of 1.31, 95% CI 0.424–3.071 |
| Bjornadal et al(84) | 5715 patients over 30-year observation period | Not available | myeloid leukemia | For acute myeloid leukemia, SIR for 1–5 years, 5–10 years and 10–15 years SLE latency were 6.1 (95% CI 2.0–14.1), 1.3 (95% CI 0.0–7.4) and 4.1 (95 CI 0.5–14.6), |
| Parikh-Patel et al(101) | 30,478 patients observed for 157,969 person-years | 29 | myeloid leukemia | For myeloid leukemia, SIR 2.96 (1.99-4.26). |
| Chen et al(100) | 11,763 patients | 7 | leukemia | For hematologic malignancies: SIR 4.96; 95% CI 4.79-5.14 For leukemia: SIR 2.64 (2.45-2.84) |
In a recent meta-analysis, by Apor et al, SLE was shown to be associated with increased SIR of leukemia (SIR 2.3, 95% CI 1.9–2.7).(86) The increased incidence of HMs was seen regardless of age, sex and geographical region, with some variations in strength of association. Importantly, the meta-analysis was exclusively of prospective cohort studies to avoid the possibility of reverse causality (i.e. hematologic malignancies preceding the SLE) as a reason for the detection of association.(86)
Studies specifically designed to assess myeloid leukemia risk in SLE are few. Bjornadal et al(84) and Parikh-Patel et al(101) reported an elevated risk of myeloid leukemia in SLE, with an SIR of 3.4 (95% CI 2.2–5.1) and 2.96 (1.99 – 4.26), respectively. The low rate of cytotoxic/immunosuppressive agent exposure combined with the low frequency of occurrence of myeloid neoplasms has made it difficult to establish the effect of prior treatment exposure on MN incidence.
Although no studies have attempted to evaluate the significance of these agents in SLE-related myeloid malignancies, one large population-based study specifically investigated the relationship between AML and SLE controlling for possible risk factors for leukemia development.(96) Lofstrom et al reported an increased risk of AML in a Swedish national cohort of 6438 SLE patients(96). The leukemia risk was confined to the subset of patients with preceding prolonged cytopenias’, particularly leukopenia. The median age at diagnosis, SLE-leukemia diagnosis period, and survival post leukemia diagnosis was 60 years, 5 years, and 6.5 months, respectively. The risk of myeloid leukemia was confined to the subgroup characterized by more men and higher age at onset of SLE. The study did not identify a difference in the frequency of cytotoxic exposure between the case and control cohorts suggesting that prior cytotoxic exposure is not a major cause for AML development in SLE.(96)
The effect of SLE latency in acute myeloid leukemia development was reported on one study.(84) The SIR in patients with SLE diagnosis for 1–5 years, 5–10 years and 10–15 years were 6.1 (95% CI 2.0–14.1), 1.3 (95% CI 0.0–7.4) and 4.1 (95 CI 0.5–14.6), respectively, suggesting a decreasing risk for myeloid leukemias with longer SLE latency. Further research is needed to investigate the biological association between SLE and myeloid leukemias’ and the role that SLE therapy might play on MN carcinogenesis.
Rheumatoid Arthritis and Myeloid Neoplasms:
Rheumatoid arthritis (RA) is a multifactorial, immune-mediated disease characterized by chronic polyarticular joint inflammation and damage, as well as extra-articular involvement(102). Extra-articular disease manifestations occur in up to 40% of patients and is a marker associated with increased mortality in RA.(103, 104) Since RA is characterized by acute and chronic synovial inflammation which is associated with a destructive process in joints, early disease recognition and prompt institution of disease modifying antirheumatic drugs (DMARDs) aimed at reducing inflammation and lowering disease activity remains a cornerstone in RA management.(105, 106) DMARDs may include non-biologic agents such as hydroxychloroquine, sulfasalazine, methotrexate or biologic therapies such as TNF-alpha inhibitors, etanercept, infliximab; IL-1 receptor antagonist, anakinra; pegylated Fab fragment such as certolizumab; IL-6 receptor antagonist, tocilizumab; anti-CD20 antibodies, rituximab, among others.(107) Compared to those in the general population, patients with RA are at a modestly increased risk in overall malignancy, likely due to increased risk of lymphoma and lung cancer.(108-110) The incidence is highest in the first five years following diagnosis of RA.(111) Risk factors associated with increased risk for malignancy development include patient characteristics such as male sex, increased age, and disease features such as inflammatory activity and increased white blood cell count.(110) Data linking hematologic malignancies to higher mortality in patients with RA is conflicting, with one study reporting a worse cancer mortality over general population(112) while such a rise was not observed in a later report.(113)
There do not appear to be major differences based on the type of approach (non-biologic or biologic/TNF) in terms of cancer risk.(114) However, there is conflicting data suggesting that patients with RA who have been treated with anti-TNF therapy may be at an increased risk of development of certain cancer types such as invasive melanoma,(115) non-melanomatous skin cancers,(116) and lymphomas(117) due to their effects on the immune function.(110) One of the confounding findings in these studies(117) was that majority of the patients treated with anti-TNF-α therapy were ones who had severe RA and hence were already at an increased risk of lymphoma development. There is no substantial increase in the risk of leukemias in patients treated with anti-TNF therapy as compared to those treated with any non-biological DMARDs.(118-120)
Several early case reports and case series have reported on the association between AML/MDS and RA, among patients exposed to azathioprine,(68) methotrexate,(121) cyclophosphamide,(122) anti-TNF agents.(123) This excess leukemia risk was further confirmed in several population-based cancer registry studies.(101, 124-126) A few controlled studies have specifically evaluated DMARD use and risk of hematologic malignant neoplasms, and found a significant, albeit weak, association with azathioprine and cyclophosphamide therapies.(127-129) In large-scale prospective cohort study, by Cibere et al, RA was associated with a significant excess of leukemia SIR 2.47 (95% CI 1.12-4.69). Only 2 of 12 leukemias had a prior exposure to cyclophosphamide or azathioprine suggesting a link to persistent immune stimulation associated with RA itself.(130)
Two population-based studies reported specifically on the relative risk of myeloid leukemias in RA [table 2]. In one study, by Askling et al, the risk of myeloid leukemia was assessed in three cohorts of rheumatoid arthritis patients retrieved from the Swedish Cancer Registry.(131) Patients were segregated into three categories, one of patients recruited within one year of RA onset, one of hospitalized patients with advanced disease, and another, of patients treated with anti-TNF agents. A significant association between RA and AML risk was observed only in the inpatient advanced [SIR 2.4 (1.9-3.0)] and early-arthritis [SIR 4.3 (1.2-10.9)] cohorts but not in the TNF-blocker group, which argues for DMARD approach as a critical risk factor in AML development.(131) The SIRs in patients with RA diagnosis, among the inpatient cohort, for 1–4 years, 5–9 years and 10+ years were 2.2 (1.3–3.2), 1.8 (0.9–3.2) and 2.1 (1.3–3.2), respectively, suggesting no effect of RA latency on the risk for AMLs.(131)
Table 2.
Rheumatoid arthritis and AML risk
| Ref | No. of patients | Malignancy type | Cases | Calculated risk (95% CI) |
|---|---|---|---|---|
| Askling et al(131) | Early arthritis=3703 | leukemias | AML = 4 CML = 0 |
SIR 4.3 (1.2-10.9) SIR 0.0 (0.0-17.7) |
| Advanced RA=53067 | AML = 68 CML = 13 |
SIR 2.4 (1.9-3.0) SIR 2.4 (1.3-4.1) |
||
| TNF blockers=4160 | AML = 68 CML = 13 |
SIR 0.0 (0.0-7.4) SIR 2.4 (0.0-27.0) |
||
| Hemminki et al(132) | 42262 patients followed over 25 years | leukemias | AML = 52 | For AML, SIR 2.4 (1.79-3.15) 2000-04: SIR 6.90 (95% CI 2.95-13.66) 1990–99: SIR 2.51 (95% CI 1.14-4.80) |
Hemminki et al analyzed the temporal trends in RA-related cancer using a nation-wide Swedish Cancer registry database of 42262 RA patients who were followed from 1980 to 2004.(132) There was an overall increase in AML risk, with further risk in patients diagnosed after 1999 and those under the age of 50 years. The risk of AML was increased during the 1-4-year period following RA diagnosis.
Inflammatory Bowel Disease and Myeloid Neoplasms:
Inflammatory bowel disease (IBD) is an umbrella term for two conditions-Crohn’s disease (CD) and Ulcerative Colitis (UC)- and is characterized by chronic inflammation of the digestive tract. Both these disorders carry distinct clinicopathologic characteristics and differentiation between CD and UC hinges upon a combination of endoscopy, imaging, and histopathologic features.(133) The peak incidence for onset of IBD is between 15-30 years of age.(134) Extra-intestinal manifestations, most commonly involving the mouth, skin, joints, liver, and eyes, occurs in up to 30% of IBD patients and correlate with increased disease severity at baseline.(135) Treatment of IBD is individualized based on the disease severity and extent. Established drug categories in the treatment of IBD include anti-inflammatory drugs (e.g. mesalamine, corticosteroids), immunomodulators (e.g. azathioprine, 6-MP, methotrexate, cyclosporine), antibiotics (metronidazole, rifaximin), and biologics (anti-TNF agents).(136) Mild-moderate UC can usually be managed with low dose 5-amionsalicylate and/or glucocorticoids while patients with severe/fulminant UC require therapy rescue with agents such as cyclosporine, azathioprine, and anti-TNF agents such as infliximab.(137) Anti-TNF drugs (such as infliximab or adalimumab) in combination with an immunomodulator, usually a thiopurine (such as azathioprine and 6-mercaptopurine), are recommended for induction of remission in moderately severe CD followed by long-term maintenance with anti-TNF therapy and thiopurines or methotrexate.(138)
Population-based studies evaluating the leukemia risk in IBD are summarized in table 3. In one study, by Askling et al, the risk of leukemia was assessed in 47,679 IBD Swedish patients, retrieved from four population-based data sources, followed for up to 40 years. Patients were grouped into two cohorts: a) regional and population-based cohort, and b) a nationwide and population based inpatient registry. Myeloid leukemia (acute and chronic) in UC occurred more often than expected, with relative risks between 1.5 and 2.9 in the two cohorts. There was a non-significant trend toward higher relative risks of myeloid leukemia among men and during the 1990s (v 1980–1989), with the excess number of cases occurring between sixth and tenth year of follow up. In contrast, the relative risk of myeloid leukemias in CD was not elevated in the regional cohort but there was an increased occurrence of CML in the inpatient cohort. There was little variation of myeloid leukemia in CD with sex, age, or time. The relative significant excess of AML specifically in UC, has been observed in other studies.(139, 140) One series of patients with IBD(140) treated with sulfasalazine and steroids showed an excess in AML risk, particularly of the promyelocytic subtype (M3) which is highly curable and associated with relatively favorable outcomes if promptly diagnosed and treated. A meta-analysis comprising eight population-based cohort studies, by Pedersen et al,(141) confirmed these observations of an increased risk of leukemias with UC (SIR 2.00, 95% CI 1.31–3.06), but not CD.
Table 3.
Inflammatory Bowel Disease and leukemia risk
| Reference | No. of patients | Malignancy type |
Cases | Calculated risk (95% CI) |
|---|---|---|---|---|
| Askling et al(202) | 47 679 patients, UC/regional = 4467, UC/inpatient 20036; CD/regional = 3561, CD/inpatient = 19024 |
Myeloid leukemias | UC/regional: AML = 9 UC/inpatient:AML = 19 CD/regional: AML = 2 CD/inpatient: AML = 84 |
RR for AML in UC/regional, 2.53 (1.2 – 4.8) RR for AML in UC/inpatient, 1.53 (0.9 - 2.4) RR for AML in CD/regional, 0.9 (0.1 – 3.2) RR for AML in CD/inpatient,0.51 (0.1-1.3) |
| Wang et al(142) | 3348 patients, CD = 685 UC = 2663 |
Leukemias | CD = 5 UC = 4 |
For CD, SIR 19.23 (6.2–44.9) For UC, SIR 2.92 (0.8–7.5) |
| Greenstein et al(140) | 1961 patients, UC = 734 CD = 1227 |
leukemias | UC, AML = 6 CD, AML = 1 |
For UC, RR for AML 8.7 For CD, RR for AML 0.76 |
| Madjlessi et al(139) | 1248 patients | leukemias | UC, AML = 3 | For UC, RR for AML 11.4 (2.3 to 24.9) |
| Pederson et al(141) | 17,052 patients | leukemias | - | For CD, SIR 0.99 (0.5–1.99) For UC, SIR 2.00, 95% CI 1.31–3.06) |
In another study, by Wong et al,(142) SIR of HMs was significantly elevated to 14.1 in CD patients and 2.5 in UC patients, and the SIRs of leukemia in the CD and UC population were 19.2 (p <0.01) and 2.92 (p = 0.1), respectively. It must be noted here that the study does not report the lineage (myeloid/lymphoid or both) and acuity/chronicity of the leukemia population. In this study, the incidence of HMs was highest in the first year of diagnosis both in UC (SIR 4.8) and CD (SIR 10.2) patients, but not for other cancers. The early appearance of HMs argues for shared genetic susceptibility or trigger factors. No significant association was found between effects of the more often used drugs (i.e., ASA, immunomodulators, thiopurine) on HM risk(142).
The role of IBD itself vs. the role of treatment in the development of hematological disorders still needs further exploration.(143) Yadav et al(144) estimated the overall risk of cancer by medications used to treat IBD in a cohort of 839 IBD patients followed-up for a median 18 years. Patients treated with immunomodulators and biologic agents were at a numerically, but not statistically, higher risk of hematologic malignant tumors.(144) The leukemia risk was not specifically evaluated. A meta-analysis of 18 studies including 8808 RA patients did not find an increased incidence of lymphoma or any non-hematologic cancer in patients treated with anti-TNF therapy (etanercept, infliximab, and adalimumab).(145) However, the meta-analysis could not speculate about malignancies which developed after 1 year of follow up.
Other studies have suggested an association between IBD and MDS based on the high frequency of MDS in patients with IBD.(146, 147) The simultaneous development of both entities may be explained by shared immunologic derangements.(148, 149) In one series, most UC patients were diagnosed with IBD before MDS, whereas the diagnosis of CD and MDS were simultaneous in one-half(146). Patients with MDS who were diagnosed with a concurrent IBD at an age (median 71 years) much older than expected.(146)
In regard to leukemia-related mortality in IBD, two studies reported a statistically significant increase in mortality rate in UC patients compared to the general population.(150, 151) High dose chemotherapy can effect durable remissions,(24) and allogeneic stem cell transplant should be considered not only in the management of leukemia but also for its therapeutic effects on refractory cases of CD.(152)
Multiple Sclerosis and Myeloid Neoplasms:
Multiple sclerosis (MS) is the most common immune-mediated inflammatory disease of the central nervous system characterized by focal or multifocal areas of demyelination and relapsing-remitting clinical course.(153) A range of disease modifying immunomodulatory agents including injectables such as interferon preparations and glatiramer acetate; neutralizing antibody infusion therapies such as natalizumab, alemtuzumab, ocrelizumab; oral immunomodulators such as teriflunomide, fingolimod, dimethyl fumarate, are approved for use in relapsing-remitting and progressive forms of MS.(154) Acute myeloid leukemia is well described complication in MS, particularly in the context of prior mitoxantrone. Mitoxantrone is a topoisomerase II inhibitor, anthraquinone antibiotic approved in the treatment of relapsing–remitting or progressive multiple sclerosis.(155) Case reports/series and population-studies have associated mitoxantrone treatment with the development of t-AML, particularly the promyelocytic leukemia subtype.(36, 156-159) In a 2011 report of 40 Italian centers, 30 cases of AML were observed among 3220 patients followed over a median period of 49 months.(36) The median interval from the start of therapy to AML diagnosis was 33 months (range 13-84 months), with 27% developing AML 4 years after mitoxantrone infusion. The authors observed a higher risk of AML (rate ratio = 4.89) in patients who had a cumulative mitoxantrone dose >108 mg/m2 indicating a dose dependency on AML risk. A cumulative dose of 90 mg/m2 was the cutoff point best associated with higher AML risk.(36) In a prior study, by Ghalie et al,(160) the t-AML rate was relatively low (0.5%) likely due to lower cumulative mitoxantrone exposure (mean, 60 mg/m2) and shorter follow up time (mean, 36 months). These early observations were further confirmed in a prospective study(161) which demonstrated an excess risk of AML in mitoxantrone treated MS patients. There did not appear to be an association between AML incidence and age at disease diagnosis or treatment initiation of MS, MS disease duration, gender or concomitant medications.(161)
Overall, these studies(36, 158, 160, 161) show a high incidence of AML, particularly acute promyelocytic leukemia subtype, in mitoxantrone-treated MS patients. Recent reports found that the outcome of AML in MS patients was not inferior compared to de novo cases, likely due to the high representation of AML-M3 type which is associated with favorable outcomes.(162, 163) The drug is typically reserved for patients with rapidly advancing disease who have failed other therapies due to its limited clinic benefit and potential for significant toxicities(164).
Systemic Sclerosis and Myeloid Neoplasms:
Systemic sclerosis (SSc) is a chronic multisystem autoimmune disorder characterized by widespread collagen accumulation in the skin and other visceral organs. The aim of immunosuppressive therapy (such as methotrexate, cyclophosphamide, mycophenolate) is to reduce progression and severity of SSc complications (raynaud's phenomenon, digital ulcers, pulmonary arterial hypertension, skin and lung disease, renal and gastrointestinal involvement).(165) Sporadic case reports have reported on the association between SSc and myeloid malignancies such as CML(166, 167) and AML.(168) Colaci et al(169) reviewed SSc patients with HMs who were described in world literature, from 1954 to 2017. One hundred-thirty SSc subjects were identified including 28 with leukemia and 16 with myeloproliferative disorders (4 with myelofibrosis, 1 with polycythemia vera, and 11 with CML). Median time from SSc diagnosis to identification of myelofibrosis and CML was 15 (5-17) and 3 (0-8) years, respectively(169). Notably, majority of the leukemias and CML were diagnosed in the first years of disease, when the immunosuppressors are least likely to have exerted an iatrogenic effect.(169) Detailed clinical history was available in only a few patients making it difficult to identify SSc-related myeloid leukemia risk factors.
Autoimmune Entities preceding or occurring concurrently with the Myeloid Malignancies:
As alluded to before, certain ADs may precede or occur concurrently with a myeloid neoplasm. In fact, MDS/AML may coexist with ADs or even be preceded by these autoimmune manifestations months to years.
Vasculitides and Myeloid Neoplasms:
Vasculitides may rarely complicate HMs as an early manifestation of disease, and have been described in association with MDS,(170, 171) chronic myelomonocytic leukemia (CMML),(172, 173), and AML.(174, 175) Secondary vasculitides that develop in the setting of MDS usually involve small cutaneous vessels and, less commonly, medium-sized muscular arteries(29, 30). ANCA-associated and large vessel vasculitides are rare. Fain et al(170) reported on series of 60 vasculitis patients who were followed-up of 45 months and found to develop at least one malignancy. Vasculitides diagnosed were cutaneous leukocytoclastic (LV,45%), polyarteritis nodosa (PAN,36.7%), granulomatosis with polyangiitis (GPA,6.7%), microscopic polyangiitis (MPA,5%), and Henoch-Schonlein purpura (HSP,5%). The median age of patients with associated MDS (n = 21) was 66 years, the majority of whom were male. Regardless of the type, vasculitides associated to MDS had more frequent renal involvement and a more severe disease evolution.(170) The pathogenesis of vasculitides and their relationship with myeloid malignancies remains to be clarified.
Glomerulonephritides and Myeloid Neoplasms:
As alluded to before, MDS can be associated with a host of clinical autoimmune disorders including paraneoplastic glomerulonephritides. In a series of 114 patients with MDS and 11 patients with CMML, the prevalence of paraneoplastic glomerulonephritis was reported to be 2% (n = 2) and 27% (n = 3), respectively.(176) Aberrant TNF overproduction secondary to MDS associated autoimmune T-cell response has been suggested to play an important role in the development of paraneoplastic glomerulonephritis.(177) In a post-mortem study of 94 kidneys obtained post-mortem from patients with leukemia and lymphoma, 9 cases were detected to have subclinical glomerular immune complex disease; the incidence was highest in patients with acute myelomonocytic leukemia (16%)(178). Further evaluation of these immune complexes have identified antigens related to oncornaviruses, suggesting a possible viral-related etiology in GS pathogenesis.(178) The relatively higher prevalence of nephropathy in CMML(176, 179) suggests a role for monocytosis in disease pathogenesis, based on their tendency to infiltrate visceral organs and secrete TNF alpha at high levels, although this hypothesis remains to be substantiated.(176) A variety of glomerulonephritides have been reported to be associated with MDS/CMML/AML including minimal change disease (MCD), focal segmental, mesangioproliferative, and membranoproliferative glomerulonephritis(180, 181).
Glomerulonephritis (GS) occurs in about 3.6% MPN patients (polycythemia vera, essential thrombocythemia and primary myelofibrosis) and is of focal segmental and mesangial proliferative GS type(182). Plasma derived growth factor is key factor in pathogenesis of MPN-related renal disease has been shown to enhance mesangial proliferation and fibrosis.(183) Treatment with myelosuppressives for the underlying cMPNs had achieved partial remission of GS in some cases.(182)
Rarely, membranoproliferative GS (MPGS), MCD, membranous nephropathy has been reported to be associated with CML.(180, 181) While the indolent clinical course of CML suggests that GS may occur independently of the malignancy, response of GS to the tyrosine kinase inhibitor for CML(184, 185) indicates that CML may be contributing to MPGS pathogenesis.
Behcet’s disease and myeloid neoplasms:
Behcet’s disease (BD) is a chronic, immune-mediated, inflammatory disorder characterized by oral, genital, and ocular involvement, skin lesions and a positive pathergy test.(186) Less commonly, patients can present with vascular, arthritic, gastrointestinal and central nervous system involvement. The disease is rare in the US, with an estimated prevalence of 5.2 per 100,000 people.(187) There have been rare case reports of BD associated with MDS,(188, 189) CML-chronic phase,(190, 191) and AML.(192, 193) Cytogenetic aberrations, especially trisomy 8 which occurs in 64–87% of BD-MDS cases,(194, 195) are thought to play an important role in the pathogenesis of BD associated with MDS. In a population-based study, by Jung et al,(196) patients with BD had a greater risk of MDS than the general population (SIR 65.7 [7.9–237.4] in men and 53.9 [11.1–157.4] in women), but not of hematological cancer. In regard to management of these patients, several of the cases in the aforementioned reports were treated with allogeneic stem cell transplantation which resulted in the resolution of remission of MDS/AML and BD.(188, 189, 192, 193)
Sjogren’s syndrome and myeloid neoplasms:
Sjogren’s syndrome (SS) is an inflammatory disorder most often affecting the tear and salivary glands, and may either be primary or secondary (prior rheumatologic disease such as SLE or RA) in origin. Although an association between SS and myeloid malignancies is extremely rare, cases of AML,(197) CML,(198) and CMML(199) have been described, either as a paraneoplastic syndrome or in the context of prior cyclophosphamide exposure.
Conclusions:
Most studies evaluating the risk of myeloid malignancies evaluating the risk of hematologic malignancies in autoimmune disease have found a significant excess of MDS and/or AML across several AD types, including SLE, RA, IBD, MS, among others. Notably, ADs are much more commonly associated with MDS/AML than chronic myeloid leukemia (CML) or MPNs,(23) supporting pathobiologic differences between the entities. While numerous contributory factors such as medications used to treat the AD, shared genetic predispositions between the AD and myeloid neoplasm, chronic immune stimulation or bone marrow infiltration by the AD, are possible, studies investigating mechanisms underlying leukemogenesis are lacking and hypotheses remain largely speculative. Notwithstanding, leukemias arising in patients with AD are not always ‘therapy-related’ in that myeloid neoplasms may develop among certain AD subtypes even in patients with no prior cytotoxic exposure.
As alluded to above, therapy related myeloid neoplasms arise as a delayed effect of treatment of AD. Conversely, rheumatologic syndromes may co-occur with or herald an underlying hematologic malignancy, as a paraneoplastic phenomenon, in which case resolution of the cancer usually leads to resolution of this syndrome.(200, 201) Furthermore, the early appearance of hematologic malignancies argues for shared genetic susceptibility or trigger factors.
A few large-scale studies and meta-analyses have confirmed the excess risk of hematologic malignancies, including myeloid leukemias, in SLE and RA. Among IBDs, the excess risk of myeloid leukemia appears to be confined to UC, for reasons that remain unclear. MS-related AML, which is predominantly a M3 FAB subtype, has been described in the context of mitoxantrone use. Evidence of association between myeloid neoplasms and other rarer ADs such as SSc, BD, SS, autoimmune vasculitides and glomerulonephritides, are limited to case reports and series. Allogeneic stem cell transplant, a standard approach to treating t-MNs, is increasingly being recognized as a viable option in treating severe, refractory AD.
Studies specifically designed to evaluate the role of AD-directed therapy in the myeloid neoplasm risk are few and have yielded conflicting results due to the low rate of cytotoxic/immunosuppressive agent exposure combined with the rarity of occurrence of myeloid neoplasms. Among the immunosuppressive therapies used in AD, the carcinogenic potential of myeloid neoplasms is best documented with azathioprine, and to a lesser extent cyclophosphamide and mitoxantrone.(67, 68, 128) This excess risk of myeloid neoplasms is not shared by anti-TNF alpha antagonist therapy in RA or IBD.
In conclusion, there appears to be an excess risk of myeloid neoplasm risk in AD, independent of cytotoxic exposure, as suggested by occurrence of MNs early in the treatment course and among patients with no prior therapy. Not all cytotoxic/immunosuppressive agents are equivalent in their leukemogenic potential, with the strongest evidence linked to certain agents such as azathioprine, cyclophosphamide, and mitoxantrone. The underlying genetic predisposition of the individual patient further compounds this assessment. A challenge with identifying patients at risk for AD-related MNs is that studies evaluating MNs in patients with AD have failed to demonstrate consistent characteristic clinical or paraclinical features. Important future research agendas include evaluation of molecular defects underlying leukemogenesis in AD and identification of risk factors associated with MN development in AD.
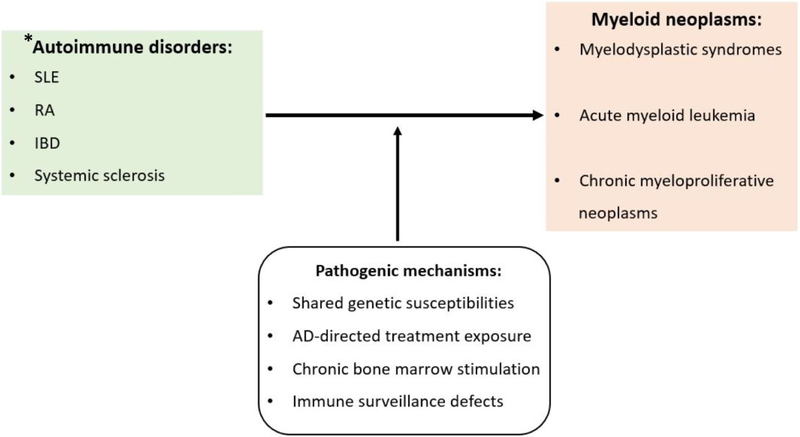
Figure 1:
Illustration of the factors contributing to development of myeloid neoplasms in autoimmune disease (AD). MNs may be therapy-related (t-MN), occurring after prior cytotoxic or immunosuppressive agent exposure. Leukemogenic potential is highest with thiopurines (azathioprine), alkylators (cyclophosphamide), and topoisomerase inhibitors (mitoxantrone). Important genetic factors influencing t-MN risk are polymorphisms in DNA-repair and drug metabolizing enzymes. Factors likely implicated in MNs arising in AD-treatment naïve patients are shared genetic susceptibilities and/or chronic immune stimulation of the bone marrow environment. * Other ADs such as vasculitides, glomerulonephritides, Behcet’s disease, Sjogren’s syndrome have shown to co-occur and are sometimes the first presenting features of MNs.
Practice points:
Myelodysplastic syndromes can coexist with or occur during the course of AD. Clinicians should maintain a low threshold for bone marrow evaluation in patients with AD and unexplained cytopenias
While there is still insufficient evidence to inform therapy choices in AD treatment based on their MN risk, certain drug classes such as thiopurines (azathioprine), alkylators (cyclophosphamide), and topoisomerase inhibitors (e.g. mitoxantrone) should be carefully considered due to their well-documented leukemogenic potential and preferably substituted with safer treatment alternatives.
Based on data from a limited number of studies, there does not appear to be an excess risk of MNs with biologic therapies, namely TNF-inhibitors.
Research agendas:
Understanding molecular and biologic mechanisms underpinning leukemogenesis in AD
Development of translational murine autoimmune models to elucidate the impact of bone marrow microenvironment in MDS/AML pathogenesis and progression.
Identification of shared genetic predispositions and pathophysiologic pathways in AD and MNs
Delineation of pre-clinical and clinical risk factors associated with AML/MDS development in AD patients with no prior therapy exposure.
Investigating the role of allogeneic stem cell transplant in the successful management of concurrent MN and AD
Acknowledgement/funding:
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicts of interest: A.M.Z. had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Ariad, Agios, Novartis, Acceleron, Astellas, Daiichi Sankyo and Takeda; and received honoraria from and was a speaker for Takeda. None of these relationships were related to the development of this manuscript.
Declaration of conflicts of interest: AMZ. received research funding (institutional) from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, ADC Therapeutics. AMZ had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Ariad, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, and Takeda. AMZ received honoraria from and was a speaker for Takeda (past). None of these relationships were related to the development of this manuscript. PB declares relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. [DOI] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 3.Abou Zahr A, Kavi AM, Mukherjee S, Zeidan AM. Therapy-related myelodysplastic syndromes, or are they? Blood Rev. 2017;31(3):119–28. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. [DOI] [PubMed] [Google Scholar]
- 6.Srour SA, Devesa SS, Morton LM, Check DP, Curtis RE, Linet MS, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol. 2016;174(3):382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeidan AM, Xu M, Steensma DP. The importance of erythroblast enumeration in myeloid neoplasia. Ann Hematol. 2017;96(2):329–30. [DOI] [PubMed] [Google Scholar]
- 8.Zeidan AM, Al Ali N, Barnard J, Padron E, Lancet JE, Sekeres MA, et al. Comparison of clinical outcomes and prognostic utility of risk stratification tools in patients with therapy-related vs de novo myelodysplastic syndromes: a report on behalf of the MDS Clinical Research Consortium. Leukemia. 2017;31(6):1391–7. [DOI] [PubMed] [Google Scholar]
- 9.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masarova L, Todisco G, Manshouri T, Newberry KJ, Cortes JE, Kantarjian HM, et al. Therapy-related myelofibrosis does not appear to exist. Blood Adv. 2017;1(14):863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. [DOI] [PubMed] [Google Scholar]
- 12.Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127(2):197–205. [DOI] [PubMed] [Google Scholar]
- 13.Slovak ML, Bedell V, Popplewell L, Arber DA, Schoch C, Slater R. 21q22 balanced chromosome aberrations in therapy-related hematopoietic disorders: report from an international workshop. Genes Chromosomes Cancer. 2002;33(4):379–94. [DOI] [PubMed] [Google Scholar]
- 14.Lubeck DP, Danese M, Jennifer D, Miller K, Richhariya A, Garfin PM. Systematic Literature Review of the Global Incidence and Prevalence of Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood. 2016;128(22):5930. [Google Scholar]
- 15.Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Zeidan AM. Risk stratification in therapy-related myelodysplastic syndromes. Oncotarget. 2017;8(46):80103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121(15):2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballinger ML, Goode DL, Ray-Coquard I, James PA, Mitchell G, Niedermayr E, et al. Monogenic and polygenic determinants of sarcoma risk: an international genetic study. Lancet Oncol. 2016;17(9):1261–71. [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Blackford AL, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armitage JO, Carbone PP, Connors JM, Levine A, Bennett JM, Kroll S. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol. 2003;21(5):897–906. [DOI] [PubMed] [Google Scholar]
- 21.Zeidan AM, Long JB, Wang R, Hu X, Yu JB, Huntington SF, et al. Risk of myeloid neoplasms after radiotherapy among older women with localized breast cancer: A population-based study. PLoS One. 2017;12(9):e0184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Zeidan AM, Yu JB, Soulos PR, Davidoff AJ, Gore SD, et al. Myelodysplastic Syndromes and Acute Myeloid Leukemia After Radiotherapy for Prostate Cancer: A Population-Based Study. Prostate. 2017;77(5):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramadan SM, Fouad TM, Summa V, Hasan S, Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97(6):805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabry TL, Sachar DB, Janowitz HD. Acute myelogenous leukemia in patients with ulcerative colitis. J Clin Gastroenterol. 1980;2(3):225–7. [DOI] [PubMed] [Google Scholar]
- 26.Weed J, Ko C, Stahl M, Much M, Witt D, Zeidan AM, et al. Reactive granulomatous dermatitis presenting as subcutaneous nodules and cords in a patient with advanced myelodysplastic syndrome. Ann Hematol. 2017;96(6):1037–9. [DOI] [PubMed] [Google Scholar]
- 27.Li AW, Yin ES, Stahl M, Kim TK, Panse G, Zeidan AM, et al. The skin as a window to the blood: Cutaneous manifestations of myeloid malignancies. Blood Rev. 2017;31(6):370–88. [DOI] [PubMed] [Google Scholar]
- 28.Wang A, Brunet CM, Zeidan AM. Rheumatologic Manifestations of Hematologic Neoplasms. Curr Rheumatol Rev. 2017;13(1):51–8. [DOI] [PubMed] [Google Scholar]
- 29.Castro M, Conn DL, Su WP, Garton JP. Rheumatic manifestations in myelodysplastic syndromes. J Rheumatol. 1991;18(5):721–7. [PubMed] [Google Scholar]
- 30.Saif MW, Hopkins JL, Gore SD. Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2002;43(11):2083–92. [DOI] [PubMed] [Google Scholar]
- 31.Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson AB, Neogi T, Prout M, Jick S. Relative risk of myelodysplastic syndromes in patients with autoimmune disorders in the General Practice Research Database. Cancer Epidemiol. 2014;38(5):544–9. [DOI] [PubMed] [Google Scholar]
- 33.Ramenghi U, Bonissoni S, Migliaretti G, DeFranco S, Bottarel F, Gambaruto C, et al. Deficiency of the Fas apoptosis pathway without Fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood. 2000;95(10):3176–82. [PubMed] [Google Scholar]
- 34.Patel RK, Mohan C. PI3K/AKT signaling and systemic autoimmunity. Immunol Res. 2005;31(1):47–55. [DOI] [PubMed] [Google Scholar]
- 35.Mullauer L, Gruber P, Sebinger D, Buch J, Wohlfart S, Chott A. Mutations in apoptosis genes: a pathogenetic factor for human disease. Mutat Res. 2001;488(3):211–31. [DOI] [PubMed] [Google Scholar]
- 36.Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14(19):2009–23. [DOI] [PubMed] [Google Scholar]
- 37.Au WY, Hawkins BR, Cheng N, Lie AK, Liang R, Kwong YL. Risk of haematological malignancies in HLA-B27 carriers. Br J Haematol. 2001;115(2):320–2. [DOI] [PubMed] [Google Scholar]
- 38.Heise E, Parrish E, Cooper R. HLA-B17 and the HLA-A1, B17 haplotype in acute myelogenous leukemia. Tissue Antigens. 1979;14(2):98–104. [DOI] [PubMed] [Google Scholar]
- 39.Dorak MT, Chalmers EA, Gaffney D, Wilson DW, Galbraith I, Henderson N, et al. Human major histocompatibility complex contains several leukemia susceptibility genes. Leuk Lymphoma. 1994;12(3– 4):211–22. [DOI] [PubMed] [Google Scholar]
- 40.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3(4):330–9. [DOI] [PubMed] [Google Scholar]
- 41.Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97(7):2121–9. [DOI] [PubMed] [Google Scholar]
- 42.Turzanski J, Grundy M, Russell NH, Pallis M. Interleukin-1beta maintains an apoptosis-resistant phenotype in the blast cells of acute myeloid leukaemia via multiple pathways. Leukemia. 2004;18(10):1662–70. [DOI] [PubMed] [Google Scholar]
- 43.Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. 2002;34(2):204–9. [DOI] [PubMed] [Google Scholar]
- 44.Demeter J, Messer G, Ramisch S, Mee JB, di Giovine FS, Schmid M, et al. Polymorphism within the second intron of the IL-1 receptor antagonist gene in patients with hematopoietic malignancies. Cytokines Mol Ther. 1996;2(4):239–42. [PubMed] [Google Scholar]
- 45.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: from molecular pathogenesis to therapeutic target. Oncotarget. 2015;6(8):5490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr., NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19(8):5785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkins ND. Achieving transcriptional specificity with NF-kappa B. Int J Biochem Cell Biol. 1997;29(12):1433–48. [DOI] [PubMed] [Google Scholar]
- 50.Xie TX, Xia Z, Zhang N, Gong W, Huang S. Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23(3):725–32. [PubMed] [Google Scholar]
- 51.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114(4):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10. [DOI] [PubMed] [Google Scholar]
- 53.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou F, Shen Q, Claret FX. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J Leukoc Biol. 2013;94(3):423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeidan AM, Knaus HA, Robinson TM, Towlerton AMH, Warren EH, Zeidner JF, et al. A Multicenter Phase I Trial of Ipilimumab in Patients with Myelodysplastic Syndromes following Hypomethylating Agent Failure. Clin Cancer Res. 2018;24(15):3519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gbolahan OB, Zeidan AM, Stahl M, Abu Zaid M, Farag S, Paczesny S, et al. Immunotherapeutic Concepts to Target Acute Myeloid Leukemia: Focusing on the Role of Monoclonal Antibodies, Hypomethylating Agents and the Leukemic Microenvironment. Int J Mol Sci. 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topfer K, Kempe S, Muller N, Schmitz M, Bachmann M, Cartellieri M, et al. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boddu PC, Kadia TM. Molecular Pathogenesis of Acquired Aplastic Anemia. Eur J Haematol. 2018. [DOI] [PubMed] [Google Scholar]
- 62.Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shallis RM, Chokr N, Stahl M, Pine AB, Zeidan AM. Immunosuppressive therapy in myelodysplastic syndromes: a borrowed therapy in search of the right place. Expert Rev Hematol. 2018;11(9):715–26. [DOI] [PubMed] [Google Scholar]
- 64.Stahl M, DeVeaux M, de Witte T, Neukirchen J, Sekeres MA, Brunner AM, et al. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(14):1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400(1-3):233–55. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen-Bjergaard J, Andersen MK, Christiansen DH, Nerlov C. Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia. Blood. 2002;99(6):1909–12. [DOI] [PubMed] [Google Scholar]
- 67.Ertz-Archambault N, Kosiorek H, Taylor GE, Kelemen K, Dueck A, Castro J, et al. Association of Therapy for Autoimmune Disease With Myelodysplastic Syndromes and Acute Myeloid Leukemia. JAMA Oncol. 2017;3(7):936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with −7/7q. Cancer Genet Cytogenet. 1998;104(2):94–7. [DOI] [PubMed] [Google Scholar]
- 69.Sill H, Olipitz W, Zebisch A, Schulz E, Wolfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162(4):792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92(10):1389–98. [DOI] [PubMed] [Google Scholar]
- 71.Yenson PR, Forrest D, Schmiegelow K, Dalal BI. Azathioprine-associated acute myeloid leukemia in a patient with Crohn's disease and thiopurine S-methyltransferase deficiency. Am J Hematol. 2008;83(1):80–3. [DOI] [PubMed] [Google Scholar]
- 72.Hasan SK, Buttari F, Ottone T, Voso MT, Hohaus S, Marasco E, et al. Risk of acute promyelocytic leukemia in multiple sclerosis: coding variants of DNA repair genes. Neurology. 2011;76(12):1059–65. [DOI] [PubMed] [Google Scholar]
- 73.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56(6):2092–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11(5):352–6. [DOI] [PubMed] [Google Scholar]
- 76.Belmont HM. Treatment of systemic lupus erythematosus - 2013 update. Bull Hosp Jt Dis (2013). 2013;71(3):208–13. [PubMed] [Google Scholar]
- 77.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–8. [DOI] [PubMed] [Google Scholar]
- 78.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35(11):2152–8. [DOI] [PubMed] [Google Scholar]
- 79.Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2014;66(4):608–16. [DOI] [PubMed] [Google Scholar]
- 80.Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun. 2013;42:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–7. [DOI] [PubMed] [Google Scholar]
- 82.Abu-Shakra M, Gladman DD, Urowitz MB. Malignancy in systemic lupus erythematosus. Arthritis Rheum. 1996;39(6):1050–4. [DOI] [PubMed] [Google Scholar]
- 83.Ramsey-Goldman R, Mattai SA, Schilling E, Chiu YL, Alo CJ, Howe HL, et al. Increased risk of malignancy in patients with systemic lupus erythematosus. J Investig Med. 1998;46(5):217–22. [PubMed] [Google Scholar]
- 84.Bjornadal L, Lofstrom B, Yin L, Lundberg IE, Ekbom A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31(2):66–71. [DOI] [PubMed] [Google Scholar]
- 85.Mellemkjaer L, Andersen V, Linet MS, Gridley G, Hoover R, Olsen JH. Non-Hodgkin's lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40(4):761–8. [DOI] [PubMed] [Google Scholar]
- 86.Apor E, O'Brien J, Stephen M, Castillo JJ. Systemic lupus erythematosus is associated with increased incidence of hematologic malignancies: a meta-analysis of prospective cohort studies. Leuk Res. 2014;38(9):1067–71. [DOI] [PubMed] [Google Scholar]
- 87.Moss KE, Ioannou Y, Sultan SM, Haq I, Isenberg DA. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis. 2002;61(5):409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tarr T, Gyorfy B, Szekanecz E, Bhattoa HP, Zeher M, Szegedi G, et al. Occurrence of malignancies in Hungarian patients with systemic lupus erythematosus: results from a single center. Ann N Y Acad Sci. 2007;1108:76–82. [DOI] [PubMed] [Google Scholar]
- 89.Bernatsky S, Ramsey-Goldman R, Clarke A. Exploring the links between systemic lupus erythematosus and cancer. Rheum Dis Clin North Am. 2005;31(2):387–402, viii-ix. [DOI] [PubMed] [Google Scholar]
- 90.Lee SL. Clinical experiences with the L.E. cell test. J Mt Sinai Hosp N Y. 1955;22(2):74–8. [PubMed] [Google Scholar]
- 91.Deaton JG, Levin WC. Systemic lupus erythematosus and acute myeloblastic leukemia. Report of their coexistence and a survey of possible associating features. Arch Intern Med. 1967;120(3):345–8. [PubMed] [Google Scholar]
- 92.Vismans JJ, Briet E, Meijer K, den Ottolander GJ. Azathioprine and subacute myelomonocytic leukemia. Acta Med Scand. 1980;207(4):315–9. [DOI] [PubMed] [Google Scholar]
- 93.Ng HS, Ng HW, Sinniah R, Feng PH. A case of systemic lupus erythematosus with sideroblastic anaemia terminating in erythroleukaemia. Ann Rheum Dis. 1981;40(4):422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taguchi F, Miyoshi T, Nakajima N, Nishimura J, Nawata H. [Acute promyelocytic leukemia developed in the course of systemic lupus erythematosus: a case report]. Rinsho Ketsueki. 1990;31(12):1965–6. [PubMed] [Google Scholar]
- 95.Colovic M, Jankovic G, Lazarevic V, Novak A. Acute megakaryoblastic leukaemia in a patient with systemic lupus erythematosus. Med Oncol. 1997;14(1):31–4. [DOI] [PubMed] [Google Scholar]
- 96.Lofstrom B, Backlin C, Sundstrom C, Hellstrom-Lindberg E, Ekbom A, Lundberg IE. Myeloid leukaemia in systemic lupus erythematosus--a nested case-control study based on Swedish registers. Rheumatology (Oxford). 2009;48(10):1222–6. [DOI] [PubMed] [Google Scholar]
- 97.Vasquez S, Kavanaugh AF, Schneider NR, Wacholtz MC, Lipsky PE. Acute nonlymphocytic leukemia after treatment of systemic lupus erythematosus with immunosuppressive agents. J Rheumatol. 1992;19(10):1625–7. [PubMed] [Google Scholar]
- 98.Meloni G, Capria S, Vignetti M, Mandelli F, Modena V. Blast crisis of chronic myelogenous leukemia in long-lasting systemic lupus erythematosus: regression of both diseases after autologous bone marrow transplantation. Blood. 1997;89(12):4659. [PubMed] [Google Scholar]
- 99.Eilertsen GO, Nossent JC. Erythroleukaemia complicating ANA-negative systemic lupus erythematosus. Scand J Rheumatol. 2007;36(6):478–80. [DOI] [PubMed] [Google Scholar]
- 100.Chen YJ, Chang YT, Wang CB, Wu CY. Malignancy in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Am J Med. 2010;123(12):1150 e1–6. [DOI] [PubMed] [Google Scholar]
- 101.Parikh-Patel A, White RH, Allen M, Cress R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control. 2008;19(8):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. [DOI] [PubMed] [Google Scholar]
- 103.Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol. 2011;38(6):983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29(1):62–7. [PubMed] [Google Scholar]
- 105.Sokka T, Pincus T. Rheumatoid arthritis: strategy more important than agent. Lancet. 2009;374(9688):430–2. [DOI] [PubMed] [Google Scholar]
- 106.Schoels M, Knevel R, Aletaha D, Bijlsma JWJ, Breedveld FC, Boumpas DT, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis. 2010;69(4):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalden JR. Emerging Therapies for Rheumatoid Arthritis. Rheumatol Ther. 2016;3(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smitten AL, Simon TA, Hochberg MC, Suissa S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilton KM, Matteson EL. Malignancy Incidence, Management, and Prevention in Patients with Rheumatoid Arthritis. Rheumatol Ther. 2017;4(2):333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen YJ, Chang YT, Wang CB, Wu CY. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63(2):352–8. [DOI] [PubMed] [Google Scholar]
- 112.Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol. 2003;30(5):958–65. [PubMed] [Google Scholar]
- 113.Abasolo L, Judez E, Descalzo MA, Gonzalez-Alvaro I, Jover JA, Carmona L, et al. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum. 2008;37(6):388–97. [DOI] [PubMed] [Google Scholar]
- 114.Michaud TL, Rho YH, Shamliyan T, Kuntz KM, Choi HK. The comparative safety of tumor necrosis factor inhibitors in rheumatoid arthritis: a meta-analysis update of 44 trials. Am J Med. 2014;127(12):1208–32. [DOI] [PubMed] [Google Scholar]
- 115.Raaschou P, Simard JF, Holmqvist M, Askling J, Group AS. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. BMJ. 2013;346:f1939. [DOI] [PubMed] [Google Scholar]
- 116.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–5. [PubMed] [Google Scholar]
- 117.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and antitumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–51. [DOI] [PubMed] [Google Scholar]
- 118.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann Rheum Dis. 2009;68(5):648–53. [DOI] [PubMed] [Google Scholar]
- 119.Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, et al. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64(5):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Setoguchi S, Solomon DH, Weinblatt ME, Katz JN, Avorn J, Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2757–64. [DOI] [PubMed] [Google Scholar]
- 121.Choi BR, Ahn MJ, Lee WS, Kim TH, Bae SC, Jun JB. Acute erythroleukemia in a rheumatoid arthritis patient during low-dose methotrexate therapy. Rheumatol Int. 2005;25(4):311–3. [DOI] [PubMed] [Google Scholar]
- 122.Balakrishnan C, Pathan E, Khodaiji S, Dasgupta A, Mangat G, Joshi VR. Myelodysplasia and acute myeloid leukaemia in a case of rheumatoid arthritis with secondary amyloidosis treated with chlorambucil. J Assoc Physicians India. 2004;52:423–5. [PubMed] [Google Scholar]
- 123.Saba NS, Kosseifi SG, Charaf EA, Hammad AN. Adalimumab-induced acute myelogenic leukemia. South Med J. 2008;101(12):1261–2. [DOI] [PubMed] [Google Scholar]
- 124.Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31(11):691–6. [DOI] [PubMed] [Google Scholar]
- 125.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A(10):1753–7. [DOI] [PubMed] [Google Scholar]
- 126.Gridley G, McLaughlin JK, Ekbom A, Klareskog L, Adami HO, Hacker DG, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85(4):307–11. [DOI] [PubMed] [Google Scholar]
- 127.Baker GL, Kahl LE, Zee BC, Stolzer BL, Agarwal AK, Medsger TA Jr. Malignancy following treatment of rheumatoid arthritis with cyclophosphamide. Long-term case-control follow-up study. Am J Med. 1987;83(1):1–9. [DOI] [PubMed] [Google Scholar]
- 128.Bernatsky S, Clarke AE, Suissa S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Intern Med. 2008;168(4):378–81. [DOI] [PubMed] [Google Scholar]
- 129.Jones M, Symmons D, Finn J, Wolfe F. Does exposure to immunosuppressive therapy increase the 10 year malignancy and mortality risks in rheumatoid arthritis? A matched cohort study. Br J Rheumatol. 1996;35(8):738–45. [DOI] [PubMed] [Google Scholar]
- 130.Cibere J, Sibley J, Haga M. Rheumatoid arthritis and the risk of malignancy. Arthritis Rheum. 1997;40(9):1580–6. [DOI] [PubMed] [Google Scholar]
- 131.Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hemminki K, Li X, Sundquist K, Sundquist J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatology (Oxford). 2008;47(5):698–701. [DOI] [PubMed] [Google Scholar]
- 133.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 134.Johnston RD, Logan RF. What is the peak age for onset of IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S4–5. [DOI] [PubMed] [Google Scholar]
- 135.Greuter T, Bertoldo F, Rechner R, Straumann A, Biedermann L, Zeitz J, et al. Extraintestinal Manifestations of Pediatric Inflammatory Bowel Disease: Prevalence, Presentation, and Anti-TNF Treatment. J Pediatr Gastroenterol Nutr. 2017;65(2):200–6. [DOI] [PubMed] [Google Scholar]
- 136.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cohen RD. How should we treat severe acute steroid-refractory ulcerative colitis? Inflamm Bowel Dis. 2009;15(1):150–1. [DOI] [PubMed] [Google Scholar]
- 138.Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT, Practice AGAIC, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145(6):1459–63. [DOI] [PubMed] [Google Scholar]
- 139.Mir Madjlessi SH, Farmer RG, Weick JK. Inflammatory bowel disease and leukemia. A report of seven cases of leukemia in ulcerative colitis and Crohn's disease and review of the literature. Dig Dis Sci. 1986;31(10):1025–31. [DOI] [PubMed] [Google Scholar]
- 140.Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, et al. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56(12):2914–21. [DOI] [PubMed] [Google Scholar]
- 141.Pedersen N, Duricova D, Elkjaer M, Gamborg M, Munkholm P, Jess T. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105(7):1480–7. [DOI] [PubMed] [Google Scholar]
- 142.Wang LH, Yang YJ, Cheng WC, Wang WM, Lin SH, Shieh CC. Higher Risk for Hematological Malignancies in Inflammatory Bowel Disease: A Nationwide Population-based Study in Taiwan. Am J Gastroenterol. 2016;111(9):1313–9. [DOI] [PubMed] [Google Scholar]
- 143.Zeidan A, Sham R, Shapiro J, Baratta A, Kouides P. Hepatosplenic T-cell lymphoma in a patient with Crohn's disease who received infliximab therapy. Leuk Lymphoma. 2007;48(7):1410–3. [DOI] [PubMed] [Google Scholar]
- 144.Yadav S, Singh S, Harmsen WS, Edakkanambeth Varayil J, Tremaine WJ, Loftus EV Jr. Effect of Medications on Risk of Cancer in Patients With Inflammatory Bowel Diseases: A Population-Based Cohort Study from Olmsted County, Minnesota. Mayo Clin Proc. 2015;90(6):738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68(7):1136–45. [DOI] [PubMed] [Google Scholar]
- 146.Harewood GC, Loftus EV Jr., Tefferi A, Tremaine WJ, Sandborn WJ. Concurrent inflammatory bowel disease and myelodysplastic syndromes. Inflamm Bowel Dis. 1999;5(2):98–103. [DOI] [PubMed] [Google Scholar]
- 147.Eng C, Farraye FA, Shulman LN, Peppercorn MA, Krauss CM, Connors JM, et al. The association between the myelodysplastic syndromes and Crohn disease. Ann Intern Med. 1992;117(8):661–2. [DOI] [PubMed] [Google Scholar]
- 148.Hamblin T Immunologic abnormalities in myelodysplastic syndromes. Hematol Oncol Clin North Am. 1992;6(3):571–86. [PubMed] [Google Scholar]
- 149.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22(4):382–9. [DOI] [PubMed] [Google Scholar]
- 150.Viscido A, Bagnardi V, Sturniolo GC, Annese V, Frieri G, D'Arienzo A, et al. Survival and causes of death in Italian patients with ulcerative colitis. A GISC nationwide study. Dig Liver Dis. 2001;33(8):686–92. [DOI] [PubMed] [Google Scholar]
- 151.Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer. 1986;58(7):1569–74. [DOI] [PubMed] [Google Scholar]
- 152.Salem GA, Selby GB. Stem cell transplant in inflammatory bowel disease: a promising modality of treatment for a complicated disease course. Stem Cell Investig. 2017;4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katz Sand IB, Lublin FD. Diagnosis and differential diagnosis of multiple sclerosis. Continuum (Minneap Minn). 2013;19(4 Multiple Sclerosis):922–43. [DOI] [PubMed] [Google Scholar]
- 154.Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88. [DOI] [PubMed] [Google Scholar]
- 155.Hartung HP, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–25. [DOI] [PubMed] [Google Scholar]
- 156.Bosca I, Pascual AM, Casanova B, Coret F, Sanz MA. Four new cases of therapy-related acute promyelocytic leukemia after mitoxantrone. Neurology. 2008;71(6):457–8. [DOI] [PubMed] [Google Scholar]
- 157.Ramkumar B, Chadha MK, Barcos M, Sait SN, Heyman MR, Baer MR. Acute promyelocytic leukemia after mitoxantrone therapy for multiple sclerosis. Cancer Genet Cytogenet. 2008;182(2):126–9. [DOI] [PubMed] [Google Scholar]
- 158.Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW, Therapeutics, Technology Assessment Subcommittee of the American Academy of N. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74(18):1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chan A, Lo-Coco F. Mitoxantrone-related acute leukemia in MS: an open or closed book? Neurology. 2013;80(16):1529–33. [DOI] [PubMed] [Google Scholar]
- 160.Ghalie RG, Mauch E, Edan G, Hartung HP, Gonsette RE, Eisenmann S, et al. A study of therapy-related acute leukaemia after mitoxantrone therapy for multiple sclerosis. Mult Scler. 2002;8(5):441–5. [DOI] [PubMed] [Google Scholar]
- 161.Pascual AM, Tellez N, Bosca I, Mallada J, Belenguer A, Abellan I, et al. Revision of the risk of secondary leukaemia after mitoxantrone in multiple sclerosis populations is required. Mult Scler. 2009;15(11):1303–10. [DOI] [PubMed] [Google Scholar]
- 162.Ammatuna E, Montesinos P, Hasan SK, Ramadan SM, Esteve J, Hubmann M, et al. Presenting features and treatment outcome of acute promyelocytic leukemia arising after multiple sclerosis. Haematologica. 2011;96(4):621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ellis R, Boggild M. Therapy-related acute leukaemia with Mitoxantrone: what is the risk and can we minimise it? Mult Scler. 2009;15(4):505–8. [DOI] [PubMed] [Google Scholar]
- 164.Goodin DS, Arnason BG, Coyle PK, Frohman EM, Paty DW, Therapeutics, et al. The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;61(10):1332–8. [DOI] [PubMed] [Google Scholar]
- 165.Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–39. [DOI] [PubMed] [Google Scholar]
- 166.Kasifoglu T, Korkmaz C, Yasar S, Gulbas Z. Scleroderma and chronic myeloid leukemia: a sheer coincidence, a consequence of long lasting D-penicillamine therapy or a plausible relationship of both diseases? Rheumatol Int. 2006;27(2):175–7. [DOI] [PubMed] [Google Scholar]
- 167.Watanabe S, Sugihara T, Takahashi M, Ata K, Kanzaki A, Yamada O, et al. [Concordant improvement of progressive systemic sclerosis and chronic myelogenous leukemia with interferon-alpha treatment]. Rinsho Ketsueki. 1994;35(9):895–7. [PubMed] [Google Scholar]
- 168.Dupond JL, Humbert P, Fest T, de Wazieres B. [Scleroderma, Gougerot-Sjogren syndrome and myelomonocytic leukemia]. Rev Rhum Mal Osteoartic. 1989;56(5):425–6. [PubMed] [Google Scholar]
- 169.Colaci M, Giuggioli D, Vacchi C, Ferri C. Haematological Malignancies in Systemic Sclerosis Patients: Case Reports and Review of the World Literature. Case Rep Rheumatol. 2017;2017:6230138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fain O, Hamidou M, Cacoub P, Godeau B, Stirnemann J, Guillevin L, et al. Association between Vasculitides and Myelodysplastic Syndrome (MDS): A Report on 21 Cases. Blood. 2004;104(11):4714. [Google Scholar]
- 171.Odell ID, Zeidan AM, Parker TL, Colegio OR, Subtil A. Leukaemic vasculitis with myelodysplastic syndrome. Lancet. 2015;386(9992):501–2. [DOI] [PubMed] [Google Scholar]
- 172.Park JK, Gelber AC, Zheng G, McDevitt MA, Gocke CD, Baer AN. Large-vessel vasculitis as an early manifestation of chronic myelomonocytic leukemia. J Clin Oncol. 2011;29(20):e601–3. [DOI] [PubMed] [Google Scholar]
- 173.Mori M, Togami K, Fujita H, Inoue D, Kimura T, Shimoji S, et al. Successful allogeneic bone marrow transplantation for chronic myelomonocytic leukemia complicated by refractory aortitis. Bone Marrow Transplant. 2010;45(4):796–7. [DOI] [PubMed] [Google Scholar]
- 174.Chandratilleke D, Anantharajah A, Vicaretti M, Benson W, Berglund LJ. Migratory large vessel vasculitis preceding acute myeloid leukemia: a case report. J Med Case Rep. 2017;11(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Warrington KJ, Scheithauer BW, Michet CJ. Acute myeloid leukemia associated with necrotizing temporal arteritis. J Rheumatol. 2003;30(4):846–8. [PubMed] [Google Scholar]
- 176.Saitoh T, Murakami H, Uchiumi H, Moridaira K, Maehara T, Matsushima T, et al. Myelodysplastic syndromes with nephrotic syndrome. Am J Hematol. 1999;60(3):200–4. [DOI] [PubMed] [Google Scholar]
- 177.Voulgarelis M, Giannouli S, Ritis K, Tzioufas AG. Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest. 2004;34(10):690–700. [DOI] [PubMed] [Google Scholar]
- 178.Sutherland JC, Mardiney MR Jr, . Immune complex disease in the kidneys of lymphoma-leukemia patients: the presence of an oncornavirus-related antigen. J Natl Cancer Inst. 1973;50(3):633–44. [DOI] [PubMed] [Google Scholar]
- 179.Morschhauser F, Wattel E, Pagniez D, Lovi V, Rose C, Bauters F, et al. Glomerular injury in chronic myelomonocytic leukemia. Leuk Lymphoma. 1995;18(5–6):479–83. [DOI] [PubMed] [Google Scholar]
- 180.Lien YH, Lai LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7(2):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;21(1):27–35. [DOI] [PubMed] [Google Scholar]
- 182.Au WY, Chan KW, Lui SL, Lam CC, Kwong YL. Focal segmental glomerulosclerosis and mesangial sclerosis associated with myeloproliferative disorders. Am J Kidney Dis. 1999;34(5):889–93. [DOI] [PubMed] [Google Scholar]
- 183.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19(1):12–23. [DOI] [PubMed] [Google Scholar]
- 184.Dwyer JP, Yates KM, Sumner EL, Stone WJ, Wang Y, Koury MJ, et al. Chronic myeloid leukemia-associated membranoproliferative glomerulonephritis that responded to imatinib mesylate therapy. Clin Nephrol. 2007;67(3):176–81. [DOI] [PubMed] [Google Scholar]
- 185.Subramanian M, Kilara N, Manjunath R, Mysorekar V. Membranoproliferative glomerulonephritis secondary to chronic myeloid leukemia. Saudi J Kidney Dis Transpl. 2010;21(4):738–41. [PubMed] [Google Scholar]
- 186.Criteria for diagnosis of Behcet's disease. International Study Group for Behcet’s Disease. Lancet. 1990;335(8697):1078–80. [PubMed] [Google Scholar]
- 187.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behcet’s disease in the US: a population-based study. Arthritis Rheum. 2009;61(5):600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yamato K Successful cord blood stem cell transplantation for myelodysplastic syndrome with Behcet disease. Int J Hematol. 2003;77(1):82–5. [DOI] [PubMed] [Google Scholar]
- 189.Tomonari A, Tojo A, Takahashi T, Iseki T, Ooi J, Takahashi S, et al. Resolution of Behcet’s disease after HLA-mismatched unrelated cord blood transplantation for myelodysplastic syndrome. Ann Hematol. 2004;83(7):464–6. [DOI] [PubMed] [Google Scholar]
- 190.Budak-Alpdogan T, Demircay Z, Alpdogan O, Direskeneli H, Ergun T, Bayik M, et al. Behcet's disease in patients with chronic myelogenous leukemia: possible role of interferon-alpha treatment in the occurrence of Behcet's symptoms. Ann Hematol. 1997;74(1):45–8. [DOI] [PubMed] [Google Scholar]
- 191.Vaiopoulos G, Terpos E, Viniou N, Nodaros K, Rombos J, Loukopoulos D. Behcet's disease in a patient with chronic myelogenous leukemia under hydroxyurea treatment: a case report and review of the literature. Am J Hematol. 2001;66(1):57–8. [DOI] [PubMed] [Google Scholar]
- 192.Nakamura Y, Matsuguma M, Tokunaga Y, Yamamoto K, Tanaka M, Tanaka Y, et al. Successful Treatment of Behcet's Disease Associated with Acute Myeloid Leukemia with Myelodysplasia-related Changes Using Azacitidine and Tacrolimus before Allogeneic Hematopoietic Stem Cell Transplantation. Intern Med. 2017;56(10):1199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lim SH, Hulsey M, Esler WV. Resolution of Behcet's disease after non-myeloablative allogeneic stem cell transplant for acute myeloid leukaemia. Rheumatology (Oxford). 2009;48(1):88–9. [DOI] [PubMed] [Google Scholar]
- 194.Ahn JK, Cha HS, Koh EM, Kim SH, Kim YG, Lee CK, et al. Behcet's disease associated with bone marrow failure in Korean patients: clinical characteristics and the association of intestinal ulceration and trisomy 8. Rheumatology (Oxford). 2008;47(8):1228–30. [DOI] [PubMed] [Google Scholar]
- 195.Tada Y, Koarada S, Haruta Y, Mitamura M, Ohta A, Nagasawa K. The association of Behcet's disease with myelodysplastic syndrome in Japan: a review of the literature. Clin Exp Rheumatol. 2006;24(5 Suppl 42):S115–9. [PubMed] [Google Scholar]
- 196.Jung YS, Han M, Kim DY, Cheon JH, Park S. Cancer risk in Korean patients with Behcet's disease: A nationwide population-based study. PLoS One. 2017;12(12):e0190182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wakita H, Asai T, Nakamura M, Endoh N, Hiruma K, Igarashi T, et al. [Development of acute myelocytic leukemia in the course of Sjogren's syndrome]. Rinsho Ketsueki. 1989;30(1):56–60. [PubMed] [Google Scholar]
- 198.Minowa R, Miyagawa S, Fukumoto T, Majima T, Shimoyama T, Fujimura Y, et al. Primary Sjogren’s syndrome followed by chronic myelogenous leukemia: a case report with a ten year history. J Dermatol. 1998;25(7):460–4. [DOI] [PubMed] [Google Scholar]
- 199.Ponge T, Champetier de Ribes F, Ponge A, Garand R, Cottin S. Chronic myelomonocytic leukemia and primary Sjogren's syndrome. Clin Rheumatol. 1988;7(1):110–3. [DOI] [PubMed] [Google Scholar]
- 200.Manger B, Schett G. Rheumatic paraneoplastic syndromes - A clinical link between malignancy and autoimmunity. Clin Immunol. 2018;186:67–70. [DOI] [PubMed] [Google Scholar]
- 201.Andras C, Csiki Z, Ponyi A, Illes A, Danko K. Paraneoplastic rheumatic syndromes. Rheumatol Int. 2006;26(5):376–82. [DOI] [PubMed] [Google Scholar]
- 202.Askling J, Brandt L, Lapidus A, Karlen P, Bjorkholm M, Lofberg R, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54(5):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]