Abstract
Scientists have long studied the actions that impact basic survival in various domains of life, such as defense, foraging, reproduction, thermoregulation, and so on, as if such actions will reveal the nature of emotion. Each domain of survival came to be characterized by a repertoire of distinct actions, and each action was thought to be caused by a dedicated neural circuit, called a survival circuit. Survival circuits are thought to be triggered by sensory events in the world, quickly producing obligatory, stereotypic reflexes as well as more flexible, deliberate responses. In this paper, we consider recent evidence from behavioral ecology that even so-called “reflexes” are better understood as purposeful, flexible actions that unfold across a range of temporal trajectories. They are highly context-dependent and tailored to the requirements of the situation. We then consider evidence from the neuroscience of motor control that motor actions are assembled by neural populations, not triggered by simple circuits. We end by considering the value of these suggestions for understanding the species-general vs. species-specific contributions to emotion.
What kinds of knowledge can inform us about the nature of human emotion? The motivational states and functional organization of behaviors closely linked to survival across species would seem to be natural entry point to this complex question. Accordingly, some scientists have proposed that emotions evolved long ago to ensure survival during defense, foraging, reproduction, thermoregulation, and fluid intake, and that humans and many non-human animals should share the neural circuits for emotion (or at least some types of emotion [1]. That is, emotions are assumed to be species-general -- fundamentally conserved states that cause species-specific actions. In such views, rats are fearful when they protect themselves from a predator. Flies are angry when they attack each other. Each emotion state is thought to trigger one of several distinct actions, and each action is thought to be caused by a dedicated neuronal apparatus. In this view, discovering the brain basis of emotions in human and nonhuman animals de facto means carefully mapping the circuitry that controls survival-related behaviors. Consider the domain of defense (i.e., fear), for example, when an animal must protect itself or its offspring from a potential threat. Various taxonomies of defensive behaviors (i.e., fear) have been proposed, organized by type of threat [2], proximity of the threat [3], or proposed computational details [4], These taxonomies differ in various ways, but share a common assumption: that a mammalian brain contains some number of innate, dedicated circuits – fear circuits -- each of which triggers a fixed reaction pattern such as freezing, flight or defensive aggression when activated by the sensory features of a threat, such as a predator. From this perspective, fear is a specific adaptation associated with a specific state caused by specific neural circuitry.
An alternative account proposes that the circuitry for emotional instances is built by a brain, as needed, by the interplay of evolved mechanisms, some of which are species-general and others which are species-specific. The circuitry that controls survival-related actions – survival circuits -- is assumed to be only one ingredient in making human emotions and therefore understanding the brain basis of emotion requires more than just the careful mapping the circuitry that supports survival-related action: it also requires an understanding the neurobiology of how these actions and their sensory consequences are made meaningful in a human brain (e.g., [5-10]. Taxonomies make an appearance in some versions of this approach, as well: one influential taxonomy, for example, assumes that defensive behaviors can be organized along a continuum of flexibility and control, anchored at one end by defensive reflexes, which are said to be executed in an obligatory, rapid manner, with little variation from instance to instance, and at the other end by flexible, goal-direct actions that result from forecasting future outcomes, with fixed action patterns and defensive habits falling somewhere in between [11]. In this view, animals (including humans) deal with threats flexibly because they have a repertoire of behavioral options to choose from, each with its own specific circuitry. From this perspective, the specific circuit that produces each defensive behavior is thought to be a necessary but not sufficient ingredient for human fear.
In this paper, we suggest several friendly amendments to this survival-circuits perspective. First, we consider recent evidence from behavioral ecology and related fields that even “reflexes” are purposeful, flexible responses that are context-dependent and take shape within various time frames. In contrast to other approaches that take an ethological approach (e.g., Mobbs, 2018 #24754}, our approach is more holistic (i.e., less atomistic) because it draws insights from a broader range of species. Second, we suggest a revision to the view survival behaviors are triggered by dedicated neural circuits by bringing those behaviors into alignment with current research on the neural basis of purposeful motor control, which suggests that motor control is achieved by neural populations that are flexibly constructed in a context sensitive way. We end the paper by considering whether our suggestions offer new opportunities to consider both the species-general and species-specific contributions to emotional events. For practical reasons of word count, we focus our discussion of defensive behaviors.
What is a Reflex?
In behavioral ecology, behavioral choices (including those that allow an animal to avoid or escape a predator) are regarded as economic choices about energetics and other biological resources (e.g., [12]). From our point of view as an apex predator, cushioned by culture, this might seem a trivial observation. But for most animals in wild, such as a sparrow or mouse, the calculations that balance fleeing, fighting, feeding, copulation, and caregiving penetrate every moment of life. Incorrect calculations are consequential, and can mean the difference of life and death for an individual animal, and can even risk the survival of a species. (Incorrect calculations are consequential for humans, as well, and likely contribute to the growing incidence of metabolic-related illnesses such as diabetes, heart-disease, depression, and Alzheimer’s disease, but that is beyond the scope of our paper).
All potential actions have an energy cost, and an animal’s brain weighs these against potential rewards and revenues in the service of balancing its global energy budget. Economic choices about actions, therefore, are necessarily influenced by a number of situation-specific considerations about an animal’s state and the state of the environment. A partial list of these situated influences includes the animal’s immediate and long-term goals, the animal’s current physiological condition (e.g., parasite load, pregnancy, etc.), predator type, alarm calls from conspecifics, social group size, and the environmental context such as ambient temperature, habitat density, their ability to influence the risk of being preyed upon, and even landscape features such as the amount of grass on the ground (e.g., [13-17]. These factors not only influence which defensive action is executed (as suggested by some taxonomies of defensive behaviors (e.g., as suggested by [2, 4]), but also how any given action is implemented. When defensive actions are considered in their broader ethological contexts, it becomes clear that the nature of the animal’s current state and its developmental and evolutionary history provide a context for any response.
Recent research from evolutionary robotics reinforces these observations. This research, which use “genetic” algorithms to select successful survival strategies in virtual, real, and hybrid environments, has revealed novel insights about the ways in which co-evolving predators and prey interact with one another, and with environmental variations to shape defensive behaviors (e.g., [18, 19]). In this approach, behavior is understood as emerging from a non-linear, dynamical process that involves the agent’s body, its control system (i.e., it’s “brain”, including past experiences of interactions with particular predators in specific environments), and the conditions of the immediate environment [20]. The contributions from different influences must be studied holistically because they cannot be separated in a reductionist way, implying that defensive actions do not deterministically issue from simple neural circuits: even complete knowledge of the elements governing the interactions provides little insight into the behavior emerging from these interactions.
With these observations in mind, it becomes clear that the degree of flexibility and context-dependence in naturally occurring defensive actions is vastly under-estimated by current laboratory research (for a notable and important exception, see [21, 22]). Defensive behaviors arising from threat in typical laboratory settings arise in contexts that have intentionally removed the variation that is inherently present in normal ecological contexts. A rodent who is isolated in a featureless box is without the myriad of defensive choices that animals in the wild normal seek out. This context is not only spatial impoverished but is also temporal artificial: the animal is removed from its normal social setting (so conspecifics cannot signal the presence of a predator) and is exposed to threats such as loud noises or shocks that offer no obvious contingent response (the way a real predator might). This typically laboratory experiment is also biologically impoverished: an animal’s current physical state and the integration of other energetic concerns (such as normal “foraging”) are rarely considered. The consequence of stripping away this multidimensional context is that defensive behaviors – even those that are now called “reflexes” – will be more immediate and stereotyped than those that are studied within their natural ecological contexts. This makes organizing a taxonomy much easier, because researchers are better able to categorize behaviors as all or none. These artificially constrained, laboratory- evoked responses are then mapped on to neural circuits without opportunity to observe the graded, contingent and goal-directed nature of natural avoidance and escape behaviors, features which make the term “reflex” all but useless.
To illustrate, consider the defensive responses of perhaps the simplest extant vertebrate nervous system: the larval zebrafish. These creatures are approximately 5mm long, virtually experience-free, and are heavily preyed upon. When faced with a predator, a freely-swimming larval zebrafish is capable of producing a suite of overlaid escape behaviors, modulating its response according to predator nearness inferred from several sensory sources with different temporal dynamics, the quickest being changes in the electrosensory environment and longer processing times for the amount and rate of visual field occlusion [23, 24]. Zebrafish, like most fish, have “Mauthner neurons” in a premotor system within their brainstem, which integrates multiple sources of sensory information to produce an extremely rapid escape flip opposite the direction of occlusion (called a C-bend escape maneuver); in response to looming stimuli, zebrafish execute a C-bend flip in under 25msec. This behavior would quickly become ineffective if it was the only escape behavior available to the larval zebrafish: predators thrive on the predictability of their prey; they learn to anticipate their prey’s responses, either during lived experience or via natural selection [25-27]. As a consequence, if the fish has a leisurely additional 50 msec to organize itself, its Mauthner neuron will be progressively eliminated from the computation, producing escape behaviors that are more spatially random. At longer durations (up to 200msec), places to hide and other environmental affordances can be integrated into the decision, with the locus of computation now extended throughout the brain. The escape behavior of the larval zebrafish illustrates that even “reflexes” in the most minimal vertebrate brains vary in their movements and temporal dynamics and at times their neural mechanisms extend through the entire brain. Words like “reflexive”, “considered” and so forth are, at best, of little mechanistic help, and are, at worst, confusing with their unfounded connotations of innateness, source of volition and conscious control.
The important insight here is that defensive behaviors -- whether they are executed within milliseconds or minutes of a threat -- are, fundamentally, purposeful motor actions. Duration should not be confused with control (Malcolm MacIver, personal communication). The current scientific consensus about the neural control of purposeful motor behavior is increasingly at odds with the idea of fixed action (or reaction) survival circuits. We turn to this topic next.
Assemblies of Neural Populations, Not Pre-Set Motor Programs in Simple Circuits
A detailed discussion of the neuroscience of motor control is beyond the scope of this article, but a general sketch will serve our purpose: there is widespread agreement that motor movements are assembled compositionally from a large number of neural elements to create a much larger variety of actions [28, 29]. That is, actions are constructed, they are not simply triggered by fixed, preprogrammed circuits. Similarly complex combinatorial systems in biology include language, genes, the retina, and the autonomic nervous system.
The distributed nature of motor control is apparent both in its command structure and in the complexity of its real-world execution. A single behavior, like running, requires a configuration of muscle contractions within the limbs and trunk that is specific to the physical conditions of the immediate environment. Is the running surface smooth or bumpy? Uphill, downhill or level? Hard or soft and pliable? Must obstacles be avoided or met? How fatigued or energized are the muscles? How much salt and water are available in the animal’s body? An animal’s nervous system has to deal with these varying physical features when preparing the specifics of the muscle, joint and tendon movements that constitute the motor action. The sensory features of the environment are also integrated as part of the neural representation of motor actions [30] in a way that takes into account the current sensory state of the animal’s body (e.g., [31, 32]), because these, too, are in the service of motor control. In addition, the mechanical implications of executing a specific set of movements are not always perfectly predictable in a novel environment, making stored patterns of neuromuscular activity arising from fixed neural circuits ill-suited to the task of motor control. Instead, movements are assembled by selection from various levels of a representation hierarchy that spans cortex to spinal cord. An intention to run is represented in pre-motor cortices. The initiation of forward motion is influenced by midbrain computations, with basal ganglia input. The alternating limb movements of running engages spinal cord modules, further implemented by the joint-angle arrays and the “muscle synergies” of cross-body co-activations of spinal and brainstem origin (see Figure 1). Together, this hierarchy assembles motor movements in a generative way that is more flexible and functional than what could be accomplished with pre-set motor programs for specific muscle contractions and joint movements alone.
Figure 1. A schematic depicting a motor hierarchy.
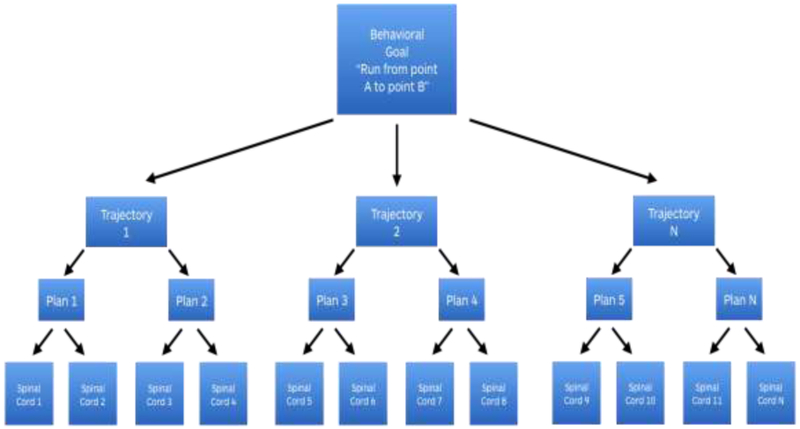
The goal “to run from point A to point B” can be accomplished via movement along multiple trajectories. Any given trajectory can be implemented by various combinations of hip, knee and ankle joint movements. And any joint movement can be achieved with a variety of muscle movement combinations because there are many muscle fibers around each joint. Therefore, as a motor control signal is assembled, each level of motor control can separate into multiple lower-level patterns, with the potential to become specified in more than one higher dimensional pattern; that is, a multimodal summary supervenes on, or can be entailed by, more than one lower level pattern of motor signals. In this way, behavioral goal is transformed into a particular set of motor commands until reaches the bottom of the motor hierarchy, occupied by the spinal cord pattern generators and simple motor circuits (modules for specific patterns of muscle fiber activity and joint movements).
In a given instance, then, even a single motor action arises from the assembly of widely distributed populations embedded in synchronized neural activity [33], stretching from association cortices (important for action planning and sensory sampling) and primary motor cortex (important for execution of motor actions) all the way down to the motor neurons in the ventral horn of the spinal cord that contain the modules which impose a specific pattern of muscle fiber activity and joint angles.
Goals and concepts.
Neurons in premotor association areas, which are positioned at the highest level of the motor hierarchy, integrate multiple sources of sensory, motor and visceromotor information to represent an action goal [30]: they represent an intention to execute an action in specific physical surroundings. Premotor cortices, for example, are heteromodal association cortices (they represent information from more than one modality) and represent multimodal summaries that are referred to as abstractions. As information is learned, neural activity propagates (in layers 2 and 3 of the cortical sheet) along a lamination gradient in the cerebral cortex from primary sensory cortices containing smaller neurons with fewer connections to cortices containing progressively larger neurons with more connections, representing shared information with progressive more efficient neural summaries [34]. The largest neurons, found in association cortices in the front of the brain including some premotor cortices, integrate across sensory modalities by summarizing their shared information (i.e., the statistical relationships in their patterns of activity) [35], effectively achieving dimensionality reduction. This functional integration suggests the hypothesis that an action goal is represented as a distributed, embodied action concept [6, 7] – an integrated summary of multimodal information about motor actions in a particular sensory context (where the context includes the state of the world and the body). Premotor cortices can be thought of as representing the more abstract features these concepts. Action concepts not only give rise to motor movements but they also allow animals to anticipate and understand the actions of others [36].
Degeneracy and the integration of distributed action components.
An action goal “to run from point A to point B in a particular context and at a particular speed” is a general plan that must be translated, via progressively more detailed instructions, into a set of specific muscle fiber contractions and joint movements. Neural signals that begin as the most abstract features of an action concept (in premotor cortices) must recruit neural populations in primary motor cortex and sensory cortices [37] as well cascade through the midbrain, brainstem and spinal cord modules that combine to specify the initiation, termination and dynamic forces that drive body movements (Figure 1 is a highly schematized depiction of the neural hierarchy that controls purposeful motor behavior). The hierarchical architecture of the motor system allows information to be translated in a one-to-many pattern, termed motor equivalence because one action goal can be implemented by more than one plans of muscle contraction and joint movement, each with some prior probability of being functional in a given situation or context (e.g., [38]). In biology, motor equivalence is described as “degeneracy”: is the ability of structurally different elements to perform the same function [39] and has been well-documented in the brain (e.g., [40-42]). Systems with degeneracy carry more information efficiently (i.e., they are high in complexity), are information-gaining (i.e., generative), and are robust to damage [43]. Degeneracy in the motor control hierarchy allows for greater movement flexibility than would be possible with fixed action circuits, thereby allowing motor actions remain functional in novel circumstances. Flexibility and robustness derive not only from which action is executed (as suggested by [2, 4]), but also how any given action is implemented in a specific pattern of muscle contractions and joint angles.
The importance of prediction.
Action concepts are not just degenerate in their execution of motor control, but they implement this control by prediction. Evidence from motor neuroscience suggests that the motor system runs a forward model, which represents the causal relationships between potential future actions and their sensory consequences [44, 45]. The model represents initial conditions (in both the body and in the environment) and constructs an situated action concept – the motor system’s best guess as to which actions will be most functional in a given context and how those actions can be most efficiently implemented in that context. The “best guess” is rooted in similarity – the brain “remembers” neural patterns from prior experiences that are similar to present conditions, which then predict the future state of the system to guide behavior. These neural signals effectively predict forward in time and space to anticipate how the motor system’s state will change as a function of the motor command, as well as anticipate the expected sensory consequences of those motor movements (based on similar experiences in the past). Action concepts, therefore, can be thought of as the neural signals that cascade from association cortices to the spinal cord circuits and pattern generators to predictively control the body and to neutrally anticipate the resulting perceptions and experiences. This is how the brain is thought to represent the causal relationship between actions and their sensory consequences [46]. In effect, prediction signals are candidates for categorizing incoming sensory inputs to make them meaningful, and the associated motor movements can be thought of as part of what makes sensations meaningful [6, 7].
Research on forward motor models belongs to a larger mathematically-formalized neuroscience-inspired account of how a human brain works, referred to as predictive coding (e.g., [7, 47-49]). It has been hypothesized, based on both anatomical [35] and functional evidence [6, 50], that predictive coding offers a unified computational account of how a brain functions as an internal model of its body in the world [19]. Predictive coding, via an internal (forward) model, is thought to equip a brain to anticipate the needs of the body and attempt to meet those needs before they arise [19], referred to as allostasis [51], thereby allowing efficiently [52] control of purposeful motor actions in the service of a balanced energy budget.
If prediction signals are the brain’s hypotheses for future states, then incoming sensory inputs are the data used to test those hypotheses. A brain monitors errors in the exteroceptive sensory domains and in the interoceptive sensory domain (interoceptive prediction errors are called reward prediction errors and are thought to be represented in the midbrain dopamine system (this system is discussed in [53]). Discrepancies between actual and predicted sensory inputs are essential for motor control because they allow the brain to fine tune motor behaviors to avoid future mistakes. In effect, by encoding prediction errors, the brain updates its (forward) internal model to improve prediction in the future. In addition, smooth motor movements require the correction of any movement errors as they arise. A brain can process prediction error via a variety of pathways, but one important pathway for error correction involves the cerebellum. Sensory prediction errors that correct motor movements are acquired and processed too slowly to allow for fine-grained motor control. To compensate for these delays, the cerebellum estimates the sensory state of the world [45] and the body [31], in effect allowing it to estimate the sensory prediction errors that are necessary to correct its forward model. This is called an observer model [44]. Many motor movements only unfold after prediction error is sufficiently minimized, and the resulting representations serve as inferences about what caused the sensory events and associated actions in the first place [46]. In some cases, such as immediate action is required (recall the C-bend flip of the zebra fish larvae), prediction error a luxury that an animal cannot afford, and motor movements will be executed without correction.
Insights for the Neural Control of Survival-Related Behaviors
What can we learn about the neural control of survival-related behaviors from our brief peek at the behavioral ecology literature, combined with our sketch of the neural hierarchy that controls purposeful motor actions? For a start, we might question whether any defensive behavior is ever encoded in a specific neural circuit. If defensive actions and other survival- related behaviors are like other purposeful motor actions, then they are much more context- dependent and flexible than is allowed by current laboratory paradigms, even when behaviors occur in ms after the appearance of a threat. This flexibility not only arises from having a repertoire of actions (as suggested by the existing taxonomies), but also because survival-related actions are governed by an animal’s internal model – not some types of defensive behaviors (as suggested by [2, 4]), but all defensive behaviors. Research showing that motor actions are largely assembled in a flexible neural hierarchy, rather than triggered by pre-set motor programs in simple circuits, suggests that even “reflexes” that are present in spinal cord circuits and pattern generators are modulated by the brain’s internal model, and evidence supports this hypothesis [54]. Moreover, if defensive actions and other survival-related behaviors are like other purposeful motor actions, then any given action has more than one neural assembly that supports it. Motor movements are assembled from an action concept by degenerate, distributed neural populations that can implement the same action via variable low-level muscle, joint and tendon patterns. This degenerate architecture will involve much more than neurons in the amygdala, the basal ganglia, the hypothalamus and the periacquiductal gray: if survival-related behaviors are like other purposeful behaviors, then they are controlled by a flexible hierarchy involving neurons that span many brain areas, including the cortex and the cerebellum. This suggests a many (neural assemblies)-to-one (action) relationship, rather than the one-to-one relationships that populate existing taxonomies. And, indeed, a growing number of scientific studies lend some support to each of these hypotheses (e.g., in the domain of protecting against a threat [55- 64]; also see research the findings on the behavioral ecology literature discussed above). Even the most basic reflexes, such as startle responses, are not entirely influenced by centrally- generated intentions to move (i.e., behavioral goals), but neither are they completely free from the influences of those intentions. Sensory events in the world (i.e., stimuli) do not determine a specific motor response; they set the occasion for it [30].
The Nature of Emotion
Our discussion thus far leads us to suggest that the current cast of “survival circuits” are only a small part of much richer, more flexible, context-sensitive complex system for assembling and controlling survival-related behaviors. Furthermore, our discussion suggests a role for concepts and goals in the construction of those behaviors. And the degree of complexity and abstraction in the concepts and goals constructed by a brain might reveal important insights about the nature of emotion and its possible variation in humans and non-human animals. For example, the hierarchy in Figure 1 can be extended to include a functional concept (e.g., to protect against a predator), which contains various action concepts (behavioral intentions to run, to attack, to freeze, to faint, to signal conspecifics) that are similar for the purposes of meeting that function in a specific context. Each action concept supervenes on a broad array of implementation plans, which in turn can be realized by multiple combinations of muscle movements, and so on. This is a central idea motivating the theory of constructed emotion (e.g., [6, 7]). It is hypothesized that emotional events are assembled when the brain constructs emotion concepts, on the fly, as part of a forward internal model that contains a behavioral intention -- a descending cascade of potential visceromotor and motor patterns, sometimes (but not always) resulting in a survival behavior – as well as prediction signals that simulate the expected sensory consequences of the expected motor movements (called an efference copy or corollary discharge). It is further hypothesized that some of these expected sensory consequences eventually become the basis of experience [47, 65]. In humans, an experience of emotion is not always reportable: consciousness is distinct from awareness, so that it is possible to experience without awareness.
From this perspective, what distinguishes humans and non-human animals is not the computational principles that govern neural representations but the content that they give rise to. The computational role of most major brain parts remains stable across the vertebrate lineage. All brains, when in a predictive mode, can be described as automatically and effortlessly forming ad hoc concepts to categorize anticipated sensory inputs and guide action. What may differ among species is the type of concepts that a brain can construct because of general brain-scaling functions [66] and the information available in an animal’s niche. For example, the human brain has expanded association cortices in the frontal lobes, parietal cortex and inferotemporal cortex when compared to other primates, even other great apes [67, 68]. This expansion potentially allows for increased information compression and dimensionality reduction, suggesting the possibility that human brains may be able to create multimodal summaries (i.e., concepts) characterized by more abstraction. This hypothesis in no way diminishes the importance of survival-related behaviors in human emotion, nor invalidates the importance of studying survival-related behaviors in animal models for the purposes of understanding part of the biology of human emotion. This hypothesis does suggest, however, that solving the puzzle of human emotion may require creating a science of “emotion ecology” involving both species-general and species-specific processes.
Table 1.
Comparing Assumptions About the Neural Control of Motor Movements
| Motor System | Survival Circuits |
|---|---|
| Actions are assembled from more basic elements | Actions are triggered by pre-set motor programs |
| Distributed population codes | Simple circuits |
| One-to-many generative grammar | One-to-one fixed path |
| Neural architecture often functions in a predictive mode using a forward internal model | Neural architecture functions in a reactive, stimulus-driven mode |
| Computations are species-general but contents of representations contains some features that are species specific | Computations and contents are species-general |
Acknowledgements
The paper was supported by grants from the U.S. Army Research Institute for the Behavioral and Social Sciences (W911NF-16-1-019), the National Cancer Institute (U01 CA193632) and the National Institute of Mental Health (R01 MH113234 and R01 MH109464), and the National Science Foundation (CMMI 1638234). The views, opinions, and/or findings contained in this paper are those of the authors and shall not be construed as an official U.S. Department of the Army position, policy, or decision, unless so designated by other documents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scarantino A, Are LeDoux’s survival circuits basic emotions under a different name? Current Opinion in Behavioral Sciences, 2018. 24: p. 75–82. [Google Scholar]
- 2.Gross CT, and Canteras NS, The many paths to fear. Nat Rev Neurosci, 2012. 13(9): p. 651–8. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow MS, Emotion, motivation and function. Current Opinion in Behavioral Sciences, 2018. 19: p. 105–109. [Google Scholar]
- 4.Bach DR and Dayan P, Algorithms for survival: a comparative perspective on emotions. Nature Reviews Neuroscience, 2017. 18: p. 311. [DOI] [PubMed] [Google Scholar]
- 5.Barrett LF, Emotions are real. Emotion, 2012. 12(3): p. 413–29. [DOI] [PubMed] [Google Scholar]
- 6.Barrett LF, How emotions are made: The secret life the brain. 2017, New York, NY: Houghton-Mifflin-Harcourt. [Google Scholar]
- 7.Barrett LF, The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci, 2017. 12(1): p. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeDoux JE, Rethinking the emotional brain. Neuron, 2012. 73(4): p. 653–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeDoux JE, Anxious: Using the brain to understand and treat fear and anxiety. 2015: Penguin. [Google Scholar]
- 10.LeDoux JE and Brown R, A higher-order theory of emotional consciousness. Proceedings of the National Academy of Sciences, 2017. 114(10): p. E2016–E2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeDoux J and Daw ND, Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat Rev Neurosci, 2018. 19(5): p. 269–282. [DOI] [PubMed] [Google Scholar]
- 12.Bonenfant M and Kramer DL, The influence of distance to burrow on flight initiation distance in the woodchuck, Marmota monax. Behavioral Ecology, 1996. 7(3): p. 299–303. [Google Scholar]
- 13.Chivers DP, et al. , At odds with the group: changes in lateralization and escape performance reveal conformity and conflict in fish schools. Proceedings of the Royal Society B: Biological Sciences, 2016. 283 (1841). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper WE and Blumstein DE, Escaping From Predators: An Integrative View of Escape Decisions. 2015: Cambridge University Press. [Google Scholar]
- 15.Fernández-Juricic JE, M. D., and Lucas E, Factors affecting intra- and inter-specific variations in the difference between alert distances and flight distances for birds in forested habitats. Canadian Journal of Zoology, 2002. 80(7): p. 1212–1220. [Google Scholar]
- 16.Heithaus MR, et al. , Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. Journal of Animal Ecology, 2009. 78(3): p. 556–562. [DOI] [PubMed] [Google Scholar]
- 17.Lima SL and Dill LM, Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology, 1990. 68(4): p. 619–640. [Google Scholar]
- 18.Nolfi S, Co-evolving predator and prey robots. Adaptive Behavior, 2012. 20: p. 10–15. [Google Scholar]
- 19.Nolfi S and Floreano D, Co-evolving predator and prey robots: Do arm races arise in artificial evolution? Artificial Life, 1998. 4(4). [DOI] [PubMed] [Google Scholar]
- 20.Nolfi S, et al. , Evolutionary robotics, in Springer Handbook of Robotics, S. B. and K. O., Editors. 2016, Springer. [Google Scholar]
- 21.Amir A, et al. , Amygdala signaling during foraging in a hazardous environment. The Journal of Neuroscience, 2015. 35(38): p. 12994–13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriazi P, Headley DB, and Pare D, Multi-dimensional coding by basolateral amygdala neurons. Neuron, 2018. 99(6): p. 1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya K, McLean DL, and MacIver MA, Visual threat assessment and reticulospinal encoding of calibrated responses in larval zebrafish. Current Biology, 2017. 27: p. 2751–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn TW, et al. , Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron, 2016. 89: p. 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catania KC, Tentacled snakes turn C-starts to their advantage and predict future prey behavior. . Proceedings of the National Academy of Sciences, 2009. 106: p. 11183–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari MCO, et al. , Daily variation in behavioural lateralization is linked to predation stress in a coral reef fish. . Animal Behaviour, 2017. 133: p. 189–193. [Google Scholar]
- 27.Nolfi S and Floreano D, Synthesis of autonomous robots through evolution.. Trends Cogn Sci, 2002. 6: p. 31–37. [DOI] [PubMed] [Google Scholar]
- 28.Flash T and Bizzi E, Cortical circuits and modules in movement generation: experiments and theories. Curr Opin Neurobiol, 2016. 41: p. 174–178. [DOI] [PubMed] [Google Scholar]
- 29.Mussa-Ivaldi FA and Bizzi E, Motor learning through the combination of primitives. Philos Trans R Soc Lond B Biol Sci, 2000. 355(1404): p. 1755–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzolatti G and Strick PL, Cognitive functions of the premotor systems, in Principles of Neural Science, Kandel ER, et al. , Editors. 2013, McGraw-Hill Medical: New York: p. 412–425. [Google Scholar]
- 31.Herzfeld DJ and Shadmehr R, Cerebellum estimates the sensory state of the body. Trends Cogn Sci, 2014. 18(2): p. 66–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shadmehr R, Huang Helen J., and Ahmed Alaa A., A Representation of Effort in Decision-Making and Motor Control. Current Biology, 2016. 26(14): p. 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deneve S, Alemi A, and Bourdoukan R, The Brain as an Efficient and Robust Adaptive Learner. Neuron, 2017. 94(5): p. 969–977. [DOI] [PubMed] [Google Scholar]
- 34.Finlay BL and Uchiyama R, Developmental mechanisms channeling cortical evolution. Trends in neurosciences, 2015. 38(2): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 35.Sepulcre J, Functional streams and cortical integration in the human brain. Neuroscientist, 2014. 20(5): p. 499–508. [DOI] [PubMed] [Google Scholar]
- 36.Cook R, et al. , Mirror neurons: from origin to function. Behav Brain Sci, 2014. 37(2): p. 177–92. [DOI] [PubMed] [Google Scholar]
- 37.Kaas JH and Stepniewska I, Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates. . Journal of Comparative Neurology, 2016. 524: p. 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallivan JP, et al. , Parallel specification of competing sensorimotor control policies for alternative action options. Nat Neurosci, 2016. 19(2): p. 320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelman GM and Gally JA, Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences, 2001. 98: p. 13763–13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gjorgjieva J, Drion G, and Marder E, Computational implications of biophysical diversity and multiple timescales in neurons and synapses for circuit performance. Curr Opin Neurobiol, 2016. 37: p. 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marder ET, A. L., Multiple models to capture the variability in biological neurons and networks. Nature Neuroscience, 2011. 14: p. 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sporns O, Networks of the Brain. 2011: MIT press. [Google Scholar]
- 43.Whitacre J and Bender A, Degeneracy: a design principle for achieving robustness and evolvability. Journal of Theoretical Biology, 2010. 263(1): p. 143–153. [DOI] [PubMed] [Google Scholar]
- 44.Wolpert DM, Pearson KG, and Ghez CPJ, The organization and planning of movement, in Principles of Neural Science., Kandel ER, et al. , Editors. 2013, McGraw-Hill Medical: New York: p. 743–752. [Google Scholar]
- 45.Shadmehr R, Smith MA, and Krakauer JW, Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci, 2010. 33: p. 89–108. [DOI] [PubMed] [Google Scholar]
- 46.Lochmann T and Deneve S, Neural processing as causal inference. Current Opinion in Neurobiology, 2011. 21(5): p. 774–781. [DOI] [PubMed] [Google Scholar]
- 47.Barrett LF and Simmons WK, Interoceptive predictions in the brain. Nature Reviews Neuroscience, 2015. 16: p. 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friston K, et al. , Active Inference: A Process Theory. Neural Comput, 2017. 29(1): p. 1–49. [DOI] [PubMed] [Google Scholar]
- 49.Kleckner IR, et al. , Evidence for a Large-Scale Brain System Supporting Allostasis and Interoception in Humans. Nat Hum Behav, 2017. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbas H, General cortical and special prefrontal connections: principles from structure to function. Annual review of neuroscience, 2015. 38: p. 269–289. [DOI] [PubMed] [Google Scholar]
- 51.Sterling P, Allostasis: a model of predictive regulation. Physiology & behavior, 2012. 106(1): p. 5–15. [DOI] [PubMed] [Google Scholar]
- 52.Sterling P and Laughlin S, Principles of neural design. 2015: MIT Press. [Google Scholar]
- 53.Schultz W, Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci, 2016. 17(3): p. 183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearson KG and Gordon JE, Spinal reflexes, in Principles of Neural Science, Kandel ER, et al. , Editors. 2013, McGraw-Hill Medical: New York: p. 790–811. [Google Scholar]
- 55.Adhikari A, et al. , Basomedial amygdala mediates top-down control of anxiety and fear. Nature, 2015. 527(7577): p. 179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen WE, et al. , Global Representations of Goal-Directed Behavior in Distinct Cell Types of Mouse Neocortex. Neuron, 2017. 94(4): p. 891–907 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duvarci S and Pare D, Amygdala microcircuits controlling learned fear. Neuron, 2014. 82(5): p. 966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herry C and Johansen JP, Encoding of fear learning and memory in distributed neuronal circuits. Nature neuroscience, 2014. 17(12): p. 1644–1654. [DOI] [PubMed] [Google Scholar]
- 59.Kim SY, et al. , Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 2013. 496(7444): p. 219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim CK, et al. , Molecular and Circuit-Dynamical Identification of Top-Down Neural Mechanisms for Restraint of Reward Seeking. Cell, 2017. 170(5): p. 1013–1027 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li SSY and McNally GP, The conditions that promote fear learning: prediction error and Pavlovian fear conditioning. Neurobiology of learning and memory, 2014. 108: p. 14–21. [DOI] [PubMed] [Google Scholar]
- 62.McHugh SB, et al. , Aversive prediction error signals in the amygdala. J Neurosci, 2014. 34(27): p. 9024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tovote P, Fadok JP, and Ldthi A, Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 2015. 16(6): p. 317–331. [DOI] [PubMed] [Google Scholar]
- 64.Ye L, et al. , Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell, 2016. 165(7): p. 1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chanes L and Barrett LF, Redefining the Role of Limbic Areas in Cortical Processing. Trends in Cognitive Sciences, 2016. 20(2): p. 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Workman A, et al. , Modeling transformations of neurodevelopmental sequences across mammalian species. Journal of Neuroscience, 2013. 17: p. 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherwood CC, et al. , Human brain evolution writ large and small. Prog Brain Res, 2012. 195: p. 237–54. [DOI] [PubMed] [Google Scholar]
- 68.Sherwood CC, et al. , Evolutionary Specializations of Human Brain Microstructure, in Evolution of Nervous Systems. 2017. p. 121–139. [Google Scholar]


