Abstract
A primary initiating epitope in the NOD mouse model of Type 1 Diabetes (T1D) lies between residues 9 and 23 of the insulin B chain. The B:9–23 peptide can bind to the NOD MHC class II molecule (I-Ag7) in multiple registers, but only one, (register 3, R3), creates complexes able to stimulate the majority of pathogenic B:9–23-specific CD4+ T cells. Previously we generated a monoclonal antibody (mAb287) that targets this critical I-Ag7-B:9–23(R3) complex. When given weekly to pre-diabetic mice at either early or late stages of disease, mAb287 was able to delay or prevent T1D in the treated animals. Although the precise mechanism of action of mAb287 remains unclear, we hypothesized that it may involve deletion of antigen presenting cells (APCs) bearing the pathogenic IAg7-B:9–23(R3) complexes, and that this process might be rendered more efficient by re-directing cytotoxic T cells using a mAb287 chimeric antigen receptor (287-CAR). As anticipated, 287-CAR T cells secreted IFN-γ in response to stimulation by I-Ag7-B:9–23(R3) complexes expressed on artificial APCs, but not I-Ag7 loaded with other peptides, and killed the presenting cells in vitro. A single infusion of 287-CAR CD8+ T cells to young (5 week old) NOD mice significantly delayed the onset of overt hyperglycemia compared to untreated animals (p=0.022). None of the 287-CAR CD8+ T cell treated mice developed diabetes before 18 weeks of age, while 29% of control-CAR T cell treated mice (p=0.044) and 52% of the un-treated mice (p=0.0001) had developed T1D by this time. However, the protection provided by 287-CAR CD8+ T cells declined with time, and no significant difference in overall incidence by 30 weeks between the 3 groups was observed. Mechanistic studies indicated that the adoptively transferred 287-CAR T cells selectively homed to pancreatic lymph nodes, and in some animals could persist for at least 1–2 weeks post- transfer, but were essentially undetectable 10–15 weeks later. Our study demonstrates that CAR T cells specific for a pathogenic MHC class II:peptide complex can be effective in vivo, but that a single infusion of the current iteration can only delay, but not prevent, the development of T1D. Future studies should therefore be directed towards optimizing strategies designed to improve the longevity of the transferred cells.
Keywords: Type 1 Diabetes, Chimeric antigen receptor, CD8 T cell, monoclonal antibody, peptide/MHC
1. Introduction
A primary goal of current type 1 diabetes (T1D) research is to develop an effective antigen specific therapy for the disease. Although in theory any islet antigen might be a suitable target for tolerance induction, there is abundant evidence to suggest that T cells recognizing epitopes within (prepro)insulin may be particularly important to the islet autoimmunity that ultimately leads to T1D1, making this autoantigen a prime candidate for antigen specific intervention. In the spontaneous diabetic NOD mouse model, amino acids 9 to 23 of the insulin B chain (B:9–23 peptide) contain at least one critical epitope; mice expressing only insulin molecules with a one amino acid mutation, alanine rather than tyrosine at B16, are completely protected from the disease2. As peptide binding to MHC class II molecules is relatively promiscuous, and the binding groove is open at both ends, in many instances a single 15mer such as the B:9–23 peptide can bind in multiple registers, with distinct populations of T cells responding to the peptide bound in each register3. By trapping the peptide in each potential binding register, Kappler and colleagues demonstrated that most, if not all, islet infiltrating B:9–23 specific CD4+ T cells in NOD mice recognize complexes in which the peptide is bound in the energetically unfavorable “register 3,” which places the positive charged Arg22 in the basic pocket 94,5. Accordingly, to target the IAg7-B:9–23(R3) complex we generated a monoclonal antibody, named mAb287, that selectively binds to the complex, but not the free peptide or complexes in other registers or containing other peptides6. Administration of mAb287 to pre-diabetic NOD mice significantly reduced the severity of insulitis and delayed or prevented the development of T1D6. Moreover, mAb287 treatment inhibited islet infiltration, not only by insulin reactive CD4+ T cells, but also by CD4+ and CD8+ T cells specific to other islet antigens, suggesting that the antibody has a global impact on the function of antigen presenting cells (APCs) co- presenting the B:9–23 peptide and other islet autoantigens.
At present the precise mechanism of action of mAb287 remains unclear, but as the antibody suppresses insulitis in general, it likely involves selective deletion of the target APCs. A potential limitation of the mAb287 reagent is its modest affinity (K ~120nM6). This contrasts with other therapeutic antibodies that often have affinities that are 1–2 orders of magnitude greater than that of mAb287. Consequently, it may be necessary to maintain relatively high circulating levels of mAb287 to obtain therapeutic benefit. In contrast, there is evidence to suggest that cytotoxic T cells with receptors having similar or lower affinities to mAb287 are able to delete APCs expressing even a single copy of their cognate ligand7–9. This suggests that cytotoxic T cells re-directed with mAb287 may be more efficient in protecting recipients from diabetes than mAb287 itself. Chimeric antigen receptors (CARs, also known as artificial T cell receptors) are used to graft the specificity of a monoclonal antibody onto a T cell. CARs are recombinant receptors typically composed of an antibody-derived targeting domain fused to transmembrane and intracellular signaling domains from molecules involved in T cell activation10. CAR T cells have proven highly successful for treating multiple cancers, showing greater efficacy than that afforded by treatment with their parental monoclonal antibodies, and less toxicity than allogeneic cell transplantation11–13. Moreover, CAR T cells may also be efficacious in autoimmunity. For example, T1D in the NOD mouse was prevented by adoptive transfer of CAR T cells with peptide-MHC (pMHC) targeting domains specific to pathogenic CD8+ T cells14. We now report our initial findings that CAR T cells targeted by mAb287 maintain the specificity of mAb287, and kill antigen presenting cells in vitro. In vivo they can delay the onset of Type 1 Diabetes in a well established pre-clinical model, although the protection is eventually lost. Nonetheless, this suggests that CAR T cells targeted to APCs expressing pathogenic MHC class II:peptide complexes may be a viable therapy for T1D, and other related autoimmune conditions.
2. Methods
2.1. Animals.
Female NOD/LtJ, Thy1.1 NOD, and NOD.SCID mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained under specific pathogen-free conditions, with 12 hour light/dark cycles and food and water ad libitum, in accordance with protocols approved by the Baylor College of Medicine animal care and use committee.
2.2. Generation of CAR expression constructs.
Total RNA was extracted from hybridoma cells expressing mAb287, and cDNAs encoding the heavy and light chains amplified by 5’ RACE (Clontech). Sequencing indicated that mAb287 is an IgG1k with parental IGHV5–6/IGHJ1 and IGKV12–44/IGKJ1 gene segments respectively. The basic design of the CARs is summarized in Figure 1A. They comprise the entire antibody light chain joined via a semi-rigid 33 amino acid linker derived from camel IgG15 to the mature variable and CH1 domains of the IgG heavy chain, and fused sequentially to the stalk, transmembrane and cytoplasmic domains of mouse CD28 (residues 116–218), and cytoplasmic domain of mouse CD3ζ (CD247; residues 52–164). The 3rd generation CAR also contains the cytoplasmic tail of mouse 4– 1BB (CD137; residues 212–256) placed between the other two signaling domains. To create the CAR expression constructs the individual domains were first amplified separately by PCR from appropriate templates, assembled by 2 or 3 rounds of splice overlap PCR, and finally introduced into the retroviral expression vector pMIG-II16 (generous gift of Dr D. Vignali, St Judes Hospital). Tofacilitate folding and surface expression the cysteine residue at the C terminus of the k light chain was mutated to glycine. The control CAR (24.1-CAR) contains the variable regions from the heavy and light chains of mAb24–1 (ATCC HB-11947). This antibody recognizes an intracellular epitope from the human cystic fibrosis transmembrane conductance regulator (residues 1477–1480).
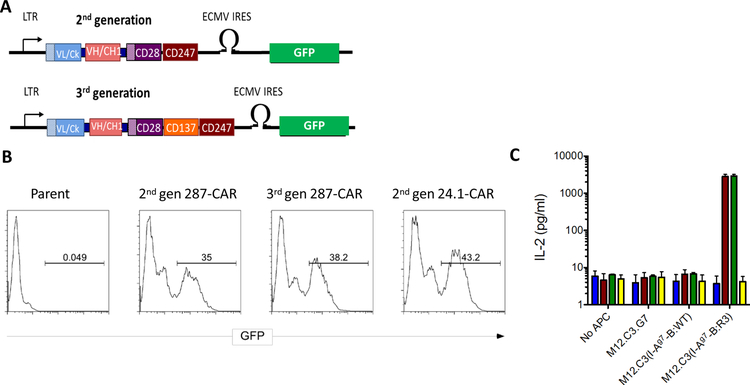
Figure 1:
Expression of CARs in 5KC thymomas. A: Cartoon illustrating the main features of the retroviral CAR constructs. They encode the antibody light chains (blue boxes) including their native leader peptides (shaded box) joined by a flexible linker (black bar) to the VH and CH1 domains (red boxes) and fused to the transmembrane (shaded) and signaling domains of CD28 (purple boxes) and cytosolic domain of CD247 [CD3ζ] (brown boxes). The 3rd generation CAR has an additional CD137 [4–1BB] signaling domain (orange box). The construct also contains an IRES driven GFP to allow detection of transduced cells. Expression is driven by the viral LTR. B: 5KC cells were transduced with replication defective retroviruses as described in methods. Expanded cells were analyzed by flow cytometry. C: Expanded cells (105) were co-cultured in triplicate with an equal number of the indicated aAPCs for 20h and secreted IL-2 quantified by ELISA. Means and SD of co-cultures containing parental 5KC cells (blue bars), or cells expressing the 2nd generation 287-CAR (brown bars), 3rd generation 287-CAR (green bars), or 2nd generation 24.1-CAR (yellow bars) from one representative experiment are shown.
2.3. Primary CAR-T cell generation.
Replication defective retroviruses encoding the CARs were generated in Phoenix-ECO cells (ATCC CRL- 3214) according to established procedures17. In brief, cells were co-transfected with the pMIGII-CAR and pCL-Eco plasmids at a ratio of 4:1 using Lipofectamine 2000 (Life Technologies). After ~48h the supernatant containing virus was collected, the media replaced, and a second viral supernatant collected 24–36h later. Supernatants were filtered through a 0.45μm PES membrane immediately prior to use. To initially validate the CARs, 5KC TCR− hybridoma cells18 were transduced by 2 rounds of spin infection19. To generate primary CAR-T cells, mouse CD8+ T cells were isolated from splenocytes of 4- week NOD female mice by negative selection (Miltenyi Biotec) on day 0. Purified cells were activated in vitro using plate bound CD3/CD28 antibodies (pre-coated at concentration of 1 μg/ml) and expanded for 48h in RPMI containing 10% heat-inactivated fetal bovine serum, 1% ITS (Life Technologies), mouse IL-7 (0.5 ng/ml; R & D Systems) and human IL-2 (100 IU/ml; PeproTech). Cells (0.5×106/well) were then transferred to retronectin (Clontech) coated 24-well plates, filtered retrovirus containing supernatant added, and the cells transduced by spinning at 2000g for 90 min at 37°C. A second transduction was performed on day 3, and the transduced cells then expanded in medium containing IL-2 + IL-7. CAR expressing cells were purified on day 5 or day 6 by FACS based on the expression of GFP from the IRES in the vector (Fig 1A; supplemental Fig 1).
2.4. Flow cytometry analysis of primary CAR-T cells
For phenotypic analysis the sorted CAR- T cells (2 × 105) were stained with APC/Fire™ 750 anti-mouse CD8a, APC-anti-mouse KLRG1, Brilliant Violet 421™ anti-mouse CD152(CTLA-4), Brilliant Violet 711™ anti-mouse CD69, Brilliant Violet 650™ anti-mouse CD25, PE/Cy7 anti-mouse CD11a/CD18 (LFA-1), and APC-anti-mouse CD215 in 3 different panels. Cells were then washed, fixed and permeabilized (BD Bioscience, CytoFix/CytoPerm Buffer), and stained with Brilliant Violet 421™ anti-mouse IFN-γ, and Brilliant Violet 650™ anti-mouse TNF-α. All antibodies were from eBioscience or BioLegend. Samples were run on an LSR Foretessa (BD) and data analyzed using FlowJo software (Tree Star).
2.5. Antigen specific functional assays of primary CAR-T cells
Antigen specificity of CAR T cells was evaluated by measuring their cytokine secretion. Four artificial antigen presenting cells (aAPCs) were used as stimulators. M12.C3.G720 was a generous gift from Dr E Unanue (Washington University). M12.C3(I-Ag7-B:WT), M12.C3(I-Ag7-B:R3) and M12.C3(TfR-MBP-DTRL) were generated by transduction of M12.C3 cells21 respectively with retroviruses encoding I-Ag7 with the native B:9–23 peptide (HLVEALYLVCGERG) or B:R3-mimotope (HLVERLYLVCGEEG5) fused to the N- terminus of the beta chain22, and a fusion protein comprising the transmembrane and endocytosis deficient truncated cytoplasmic domain of the human transferrin receptor23 and maltose binding protein (excised from pMal-c2x; NEB) linked at the C terminus to a peptide containing 3 copies of the 24.1 epitope separated by Gly-Ser linkers (DTRLGSDTRLGSDTRL). 5KC transductants (105) were co- cultured with an equal number of aAPCs for 20h and secreted mouse IL-2 quantified by ELISA24. For primary CAR-T cells, 4×104 cells were cultured with increasing numbers of aAPCs. After 16h the secreted IFNγ was quantified by ELISA (eBioscience). In vitro cytotoxicity was assessed by co-culturing primary CAR T cells (4 × 104) with aAPCs at an effector:target (E:T) ratio of 2:1 for 4 hours. The cells were then harvested and co-stained with PE anti-mouse CD19 and propidium iodide (PI). Killing was calculated as the percentage of CD19+ cells that co-stained with PI.
2.6. Analysis of CAR T cells following adoptive transfer
Sorted GFP+CD8+ T cells were expanded in complete medium containing IL-2 as described above. Without additional re-stimulation cells continued to proliferate in vitro until day 8–10 post-transduction. Accordingly, for in vivo studies, cells were adoptively transferred cells to hosts on day 6 post transduction. To assess survival/proliferation in vivo, lymphoid tissues (spleen, pancreatic lymph nodes, and inguinal lymph nodes) were collected 3, 5, 9, and 14 days post transfer. GFP+CD8+ T cells were quantified by flow cytometry analysis. To further analyze the distribution of the transferred cells, 5 ×106 CAR-T cells from congenic Thy1.1 NOD donors were transferred to 8-week old female NOD (Thy1.2) mice. On day 5 post-transfer, pancreata and pancreatic lymph nodes (PLNs) were collected and fixed with 10% formalin, then CAR-T cells and endogenous B cells were identified by immunohistochemistry. Briefly, paraffin sections were prepared and stained as described previously25. Sections were incubated with primary antisera including mouse anti-Thy1.1 (#202508; Biolengend, San Diego, CA), monoclonal mouse anti-CD3 (#ab17143; Abcam, Cambridge, MA), rat anti-mouse B220 (#557390; BD Biosciences, San Jose, CA), followed by secondary antisera conjugated to Cy3 or Cy5 (Jackson ImmunoResearch, West Grove, PA) and DAPI (Molecular Probes, Eugene, OR, USA) as previously26. Sequential staining was performed with T cell specific antibodies followed by B cell specific antibodies to avoid cross reactivity. Images were captured with a Zeiss AxioImager M1 (Carl Zeiss, Thornwood, NY) and Volocity 6.1.1 software (PerkinElmer, Waltham, MA, USA).
2.7. Diabetes Protection by 287-CAR T cells
Sorted and expanded CAR T cells (3–5 × 106/mouse) were transferred via the tail vein to 5 week old female NOD mice. Beginning at 10 weeks of age blood glucose levels were monitored weekly with an OneTouch Ultra2 monitor (LifeScan Inc.). Animals with values of ≥250 mg/dL were re-tested the following day. Diabetes was diagnosed after two consecutive blood glucose values ≥250 mg/dL6. Mice were euthanized either following diagnosis of diabetes, or at 25 weeks of age, and the spleens, pancreatic lymph nodes (PLNs) and pancreata were harvested for flow cytometric analysis or for insulitis evaluation. Serum was collected at 4 weeks (prior to the treatment), and at 10, 12 and 14 weeks of age for analysis of serum insulin, TNF α and IFN γ by ELISA and insulin auto-antibodies (IAA) by radioimmunoassay27. In a second experiment, 5 × 106 diabetic splenocytes were transferred alone or together with 5 × 106 CAR T cells into groups of 5–6 week old NOD.SCID mice. Animals were monitored daily for signs of diabetes and followed for up to 7 weeks post-transfer.
2.8. Statistical analysis
Survival curves were analyzed using PRISM7 software (Graphpad, San Diego, CA), and p-values ≤0.05 considered statistically significant. The Generalized Gehan-Breslow-Wilcoxon test was used for analysis of time to diabetes data because it tends to be more powerful than the log rank test when the assumption of proportional hazards is violated, as is often the case in mouse models. To analyze the effect of treatment on the onset of diabetes at a certain time point, a global significance test (Pearson’s Chi-squared test) was performed initially; then pairwise comparisons between groups analyzed by Fisher’s Exact Test. The frequencies of IAAs were analyzed by Fisher’s exact test, and titers by 2 tailed Mann Whitney U test.
3. Results
3.1. Single chain Fab CARs maintain the binding specificity of the parent antibodies
The design of the 2nd and 3rd generation CARs is shown in Fig 1A. The interaction between pMHCs and T cell receptors (TCRs) takes place in areas of close contact in which the adjacent membranes are spaced ~13 nm apart28. This spacing is critical, particularly for low affinity ligands29. Consequently, to approximate the dimensions of a TCR, we fused single chain Fab fragments (scFabs) directly to the CD28 stalk. To validate the binding specificity of the CARs, we initially expressed them in 5KC cells; a murine thymoma that lacks expression of endogenous TCRa or b chains18. As shown in Figure 1B, after transduction, 35–45% of the 5KC cells express GFP from the IRES in the retroviral construct. Staining with a polyclonal anti-mouse IgG confirmed that GFP and CAR surface expression were directly proportional (data not shown). As anticipated, co-culture of 287-CAR expressing 5KC cells with aAPCs expressing I-Ag7-B:R3, but not I-Ag7 loaded with peptides from endogenous mouse proteins, resulted in a robust IL-2 response (Fig 1C). In contrast, 287-CAR expressing 5KC cells responded very weakly to I-Ag7-B:WT, consistent with the majority of the native peptide being bound in registers 1 or 2. Similarly, 5KC cells expressing the 24.1-CAR did not respond to any of the I-Ag7 aAPCs, but responded robustly to M12-C3 cells expressing the TfR-MBP-DTRL fusion protein (data now shown). These data confirm that the scFabs maintain the binding specificity of the parent antibodies.
3.2. Primary CAR T cells secrete IFN-γ in response to their cognate ligands, and lyse the antigen presenting cells
In NOD mice, insulin autoantibodies (the first sign of islet autoimmunity) rarely appear before 6 weeks of age. Thus, to ensure a predominantly naïve starting population we isolated CD8+ T cells from the splenocytes of 4-week old female NOD mice. After in vitro activation 150–240 fold expansion of the CD8 T cells was observed by day 8 of culture. After two rounds of transduction, 60%−90% of the CD8+ cells co- expressed GFP analyzed at the time of cell sorting (supplementary Figure 1).
To begin to characterize the sorted and expanded primary CAR T cells, we first analyzed the expression of selected activation markers by flow cytometry on days 8 or 9. These results are summarized in Table 1. Approximately 98% of the viable GFP+ cells retained expression of CD8, and as expected, robust expression of activation markers such as LFA-1, CD25, and CD69, but not CD152 (CTLA-4), was also observed. Approximately 29% of the cells expressed KLRG1, a marker of memory cells, whereas more than 90% co-expressed the pro-inflammatory cytokines IFN-γ and TNF-α.
Table1:
Characterization of expanded CAR T cells.
| Marker | % positive cells (mean ± SD)* |
|---|---|
| CD8 | 97±1.5 |
| KLRG1(MAFA) | 29±1.7 |
| CD152(CTLA-4) | 2.5±1.5 |
| CD69 | 91±1.8 |
| CD25 | 65±9.8 |
| CD11a/CD18(LFA-1) | 94±0.8 |
| IFNγ | 91±3.1 |
| TNFα | 94±2.2 |
Mean values of 4 CAR T cell lines from repeated experiments.
Expanded CAR T cells were stained with antibodies to the indicated markers on day 8 or 9 and analyzed by flow cytometry. Results are expressed as the percentage of viable GFP+ cells.
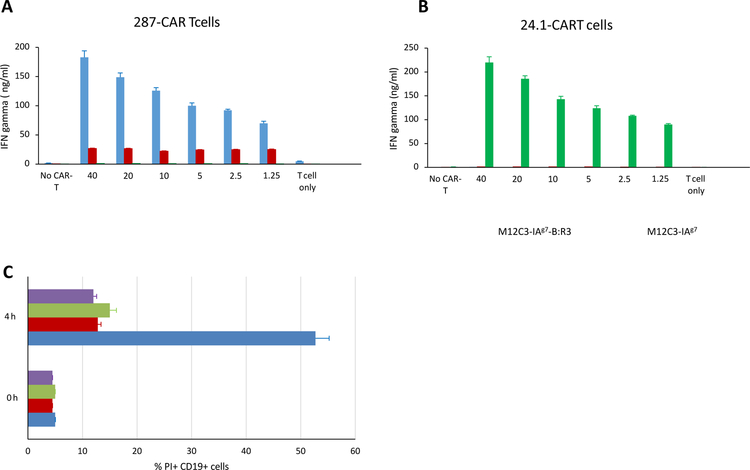
We next analyzed the ability of primary CAR-T cells to respond to their cognate antigens. Day 8 CAR T cells were co-cultured for 16h with increasing numbers of aAPCs cells. As expected, 287-CAR T cells secreted IFN-γ in response to IAg7-B:R3 stimulation in a dose dependent manner, but did not respond to aAPCs expressing native I-Ag7 or the 24.1 ligand (Figure 2A). In contrast, 24.1-CAR T cells only responded to the M12 cells displaying the CFTR peptide (Figure 2B). To determine if the CAR T cells were able to kill the aAPCs, in a second experiment we stained the cells with propidium iodide (PI) and anti-CD19 after co-culture for 4 hours at an effector/target ratio of 2:1. Dead M12 cells will stain with both markers while viable cells will exclude the nuclear dye (Supplementary Figure 2A). As shown in figure 2C (blue bars), after 4 hours co-culture with 287-CAR T cells, less than 50% of the M12.C3 (I-Ag7-B:R3) cells remained viable, while >85% excluded PI after co-culture with 24.1-CAR T cells (Figure 2C, green bars). The ~10% increase in PI positivity from baseline observed in the co-cultures of M12.C3(I-Ag7-B:R3) and 24.1-CAR T cells was also seen in 4h cultures of aAPCs without T cells (not shown) and co-cultures of M12.C3.G7 cells with either 287 or 24.1 CAR T cells (Figure 2C, purple and brown bars) suggesting that this rate of cell death is intrinsic to the lymphoma cell lines under the conditions used.
Figure 2:
Functional analysis of primary CD8+ CAR T cells. A, B: FACS purified CAR T cells (4 × 104) were co-cultured with increasing numbers of M12.C3(IAg7-B:R3) cells (blue bars), M12.C3 (IAg7) cells (red bars), or M12.C3(TFR-MBP-DTRL) cells (green bars) for 16h and secreted IFNγ quantified by ELISA. C: 287-CAR T cells (blue and brown bars) or 24.1-CAR T cells (green and purple bars) were co-cultured with M12.C3 (IAg7-B:R3) cells (blue and green bars) or M12.C3 (IAg7) cells (brown and purple bars) at an effector to target ratio of 2:1 for 0 or 4 hours. Cells were then co-stained with Allophycocyanin conjugated anti- B220 and propidium iodide and immediately analyzed by flow cytometry. The percentages of PI positive B220+ cells are shown.
3.3. 287-CAR T cells home to pancreatic lymph nodes
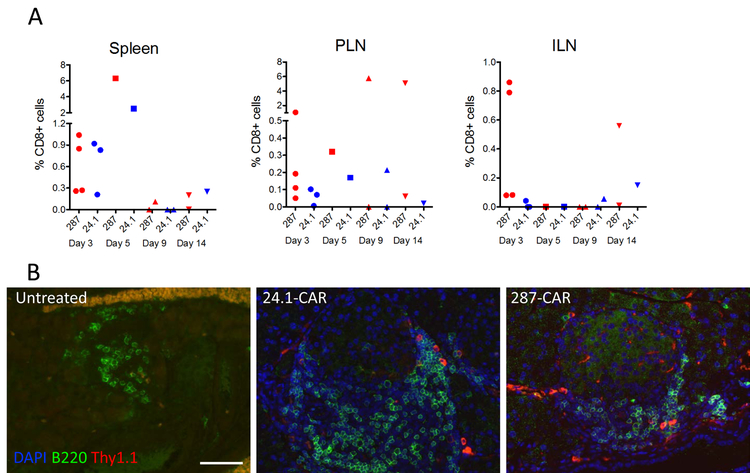
To deplete APCs that express I-Ag7-B:R3 complexes 287-CAR T cells must enter PLNs where the insulin peptides are presented. To examine their homing capacity we measured the accumulation of GFP+ T cells in PLNs by flow cytometry on days 3, 5, 9, and 14 post-transfer (Supplementary Fig 2B). These results are summarized in Figure 3A. At early time points (days 3 and 5) with one exception the highest levels of transferred cells were observed in the spleen, with similar proportions of antigen specific (red symbols) and control (blue symbols) CAR T cells evident in this tissue. However, the CARs did not appear to proliferate or survive in this location, and few GFP positive cells could be detected in spleens harvested from either test or control animals 9 or 14 days post-transfer. A similar result was observed with control (inguinal) lymph nodes. In contrast, in 50% of animals given 287-CAR T cells GFP+ cells were clearly evident in the PLNs 9 and 14 days post-transfer, comprising >5% of the total CD8+ cells present in those animals (Figure 3A, red symbols). This was not observed in mice given the control 24.1-CAR (Figure 3, blue symbols), suggesting preferential homing/expansion of the 287-CAR T cells in PLNs containing APCs expressing their cognate antigens. The difference in expansion/survival of the 287-CAR T cells at later time points likely reflects the beneficial role of the CD137 signaling domain, with high levels of GFP+ cells seen only in animals given the 3rd generation reagent. The transferred cells retained an activated phenotype in the PLNs, with >90% expressing CD69 at day 5 post transfer.
Figure 3:
Distribution of CAR T cells in secondary lymphoid tissues and pancreas after adoptive transfer. Expanded CAR T cells (3–5 × 106) were transferred to 5-week old (A) or 8 week old (B) NOD mice. A. At the indicated times post transfer individual mice were euthanized and spleens, PLNs and ILNs harvested for flow cytometric analysis. Data represents the percentage of viable CD8+ cells that co-express GFP for individual animals from 4 separate experiments. Animals given 287-CARs are indicated with red symbols and those given 24.1-CARs with blue symbols. Background signals determined using the equivalent gating strategy for tissues harvested from un-manipulated littermates have been subtracted. B. On day 5 post transfer pancreata were harvested, fixed with 10 % formalin, and embedded in paraffin. Slides were stained with anti-Thy1.1 (red), B220 (green), and DAPI (blue). Representative immunofluorescence images are shown.
To determine if the transferred cells could home to inflamed islets, in a second experiment we adoptively transferred CAR-T cells generated from congenic Thy1.1 NOD donors to 8 week old female NOD (Thy1.2) recipients. As shown in figure 3B, the transferred cells (red) were readily detectable in islets that had significant levels of B lymphocyte infiltration (green), confirming that they can home to the target tissue. Consistent with the previous flow cytometric analyses (Fig 3A), the transferred cells could also be detected in PLNs by histological staining (data not shown).
3.4. A single CAR-T cell infusion can delay the onset of T1D in NOD mice, but the protection is lost with time.
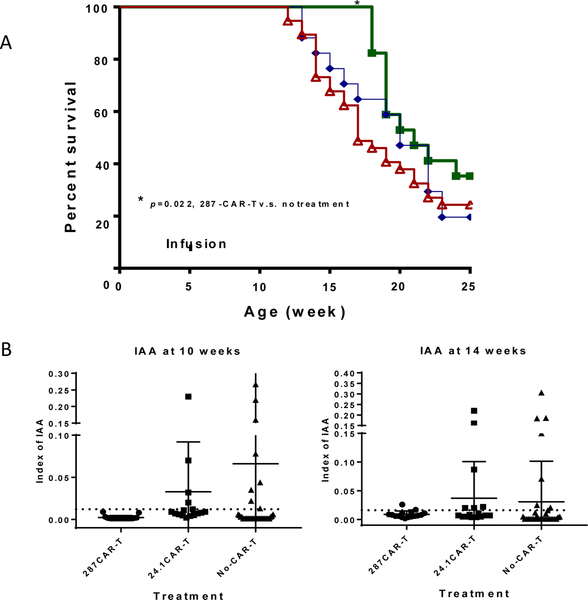
To determine whether 287-CAR T cells can mimic the protection provided by the parent antibody, we infused expanded cells into 5 week old female NOD mice and monitored the development of T1D in the treated animals. Three batches of mice were treated using CAR T cells from three separate transductions. The pooled results from this experiment are shown in figure 4. Overall, treatment with 287-CAR T cells showed modest protection from disease compared to untreated mice (p=0.022 by Gehan-Breslow-Wilcoxon test) but no significant difference when compared to the 24.1-CAR T cell treatment (p=0.27). In mice given the control 24–1 CAR T cells (diamonds) T1D first appeared after 13 weeks, 1 week later than in the untreated cohort (triangles). In contrast, no animal given 287-CAR T cells developed diabetes before 18 weeks of age (squares). Thus, while 5/17 (29%) 24.1-CAR T cell treated mice and 20/38 (52%) of untreated mice had developed T1D by 18 weeks of age, 17/17 287-CAR T cell treated mice remained normoglycemic at this time point (p=0.044 v 24–1 CAR; p=0.0001 v untreated; Table 2). However, protection was not maintained as animals started developing T1D after 18 weeks of age, and only 6/17 (35%) remained diabetes free by 25 weeks when the experiment was terminated (compared to 4/17 (23%) 24.1-CAR T cell treated mice and 9/38 (24%) untreated animals). Histological analysis of pancreata from non-diabetic 287-CAR treated mice at 25 weeks (n=3) revealed both profound peri-insulitis and intact islets (Supplementary Figure3). In contrast, all of the diabetic mice, whether they received CAR-T cells or not, had severe insulitis, and very few intact islets could be found.
Figure 4:
Development of spontaneous T1D following adoptive transfer of CAR T cells. Groups of 5 week old female NOD mice were infused with 3–5×106 CAR T cells and monitored for persistent hyperglycemia for up to 20 additional weeks. Animals diagnosed with T1D were euthanized. A. Survival curves of unmanipulated animals (n=38; red triangles), and those given 287-CAR T cells (n=17; green squares), or 24.1-CAR T cells (n=17; blue diamonds), are shown. Data is pooled from 3 separate control- and 287- CAR T cell preparations. *p<0.05. Right table: the contingency table shows the number of diabetic and non-diabetic mice at 18 weeks. B. Insulin autoantibodies were measured in serum from 10 week old (left panel) and 14 week old (right panel) animals.
Table 2:
T1D development in 18 week old treated and control mice
| Treatment | T1D mice number | Non-TID number | Total number |
|---|---|---|---|
| 287-CAR-T | 0 | 17 | 17 |
| 24.1-CAR-T | 5 | 12 | 17 |
| No-CAR-T | 20 | 18 | 38 |
The proportions of animals who had developed T1D by 18 weeks are shown. Data was analyzed by Fisher’s exact test. 287-CAR-T v.s. No-CAR-T, p=0.0001; 287-CAR-T v.s. 24.1-CAR-T, p=0.044; 24.1-CAR-T v.s. No-CAR-T, p=0.147.
Like humans, the first evidence of islet autoimmunity in NOD mice is the appearance of autoantibodies to insulin (IAA), which in mice typically peak at around 8 to 10 weeks of age. To investigate if CAR-T cell treatment inhibits the development of autoantibodies, we measured serum IAA levels at 4, 10 and 14 weeks. As expected, no IAAs were detected at 4 weeks (1 week prior to cell transfer). In contrast, at 10 weeks IAA could be detected in both control groups, but remarkably, not in the mice treated with 287- CAR T cells (p<0.01 vs 24.1-CAR T; p<0.05 vs non-treated mice) (Fig 4B). By 14 weeks, 5 of the 287-CAR T cell treated mice had developed IAA, and at this time point no significant difference was detected between the frequencies of IAA positivity in the surviving members of the three groups. However, whilst there was no difference in overall frequency, there was an apparent difference in the titers of the positive sera. The median index of the test group was 0.013 (range 0.011 – 0.026). This contrasted with 0.071 (range 0.012–0.306) in the untreated animals (p=0.0453) and 0.022 (range 0.011–0.220) in the 24.1-CAR treated animals (p=0.1013). Together this data suggests that a single infusion of 287-CAR T cells can transiently suppress islet autoimmunity, but that the control-CAR T cells cannot.
As the development of IAAs were suppressed in the test animals we also investigated whether serum insulin levels were affected. As shown in supplementary Figure 4A, no significant difference in pancreatic tissue insulin levels was observed in any of the treated or nice treaded mice on day 5 post transfer, and no significant difference in circulating insulin levels was observed either at 12 weeks of age in the euglycemic mice either. Thus, CAR-T cell treatment did not appear to have any adverse metabolic side effects. Similarly, at the same time point no significant differences in circulating IFN-γ or TNF-α concentrations were seen (supplementary Fig 4B).
To determine the long term survival of the transferred cells PLNs were analyzed by flow cytometry, either upon diagnosis of T1D, or in normoglycemic animals sacrificed at 25 weeks of age (n=3 for 287- CAR-T treated mice, n=2 for 24.1 CAR-T treated mice, n=3 for non-treated mice); no detectable GFP+ T cells could be found at these time points (data not shown).
Adoptive transfer of “diabetic” splenocytes to NOD.SCID mice induces disease in the recipients. To determine if CAR T cells can also impact this model we transferred 5×106 splenocytes from recently diabetic NOD mice to a small group of NOD.SCID recipients either alone, or together with an equal number of 287-CAR or 24.1-CAR T cells. As shown in supplementary figure 5, 287-CAR T cells could not suppress disease in this model, with all mice developing T1D by 7 weeks post-transfer, irrespective of treatment group. However, like the previous experiment, T1D developed first in the control animals with 2/5 animals given splenocytes alone (circles) and 1/5 animals given splenocytes plus 24.1-CAR T cells (triangles) becoming hyperglycemic by 28d post transfer, a time point when 4/4 animals given splenocytes plus 287-CAR T cells (squares) remained normo-glycemic. Similarly, 10/10 control animals, but only 3/4 test animals, developed T1D by 40d post transfer. Thus, although the small group sizes preclude any firm conclusions from being drawn, these data are also suggestive that the antigen specific therapy may have provided some marginal benefit.
3.5. CAR-T cell transfer does not grossly change the immune system.
To determine if the infusion of CAR-T cells had systemic side effects on the whole immune system, we evaluated the splenic populations of CD4+, CD8+, B and NK cells by flow cytometry on day-6 post transfer to. As shown in supplementary Figure 6, there were no significant difference of the percentages of CD4, CD8, B cells or NK cells among three groups. Immune cells of the mice in disease prevention study were evaluated at the age of 25 weeks, treatment did not cause significant change of immune cells (data not shown).
4. Discussion:
There is an urgent need to develop safe and effective therapies for T1D. Although several agents that cause global immunomodulation have shown promise1,30 none have so far provided durable protection from disease, suggesting that more sophisticated approaches will likely be required. Recent advances in ex vivo cell culture and cellular engineering have led to the development of innovative adoptive cell therapies (ACTs) that are transforming immunotherapy for many human cancers10,31. For example, redirection of T cells with CARs specific for CD19 has achieved impressive results in cases of refractory pre-B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma, and is now FDA approved10,31. However, application of this technology to autoimmunity is currently lagging behind. One potential reason for this delay is a lack of suitable reagents to selectively target only those immune cells that are involved in the pathogenic process, while sparing those that are required for protective immunity. Our data described above provide a potential solution to this critical problem.
Research by our group and others has shown that the presentation of the insulin B:9–23 peptide in register 3 by I-Ag7 is critical for the activation of a key population of pathogenic T cells, and the concurrent initiation of islet autoimmunity, in NOD mice4,5,6. Moreover, T cells recognizing HLA- DQ8:B:11–23(R3) have also been isolated from the peripheral blood of humans with T1D32, suggesting that there may be shared mechanism between mice and humans. Previously, we demonstrated that immunization with an antibody specific for the pathogenic I-Ag7:B:R3 complex (mAb287) could delay or prevent T1D in half of the treated mice6. We now report that an scFab variant of this antibody can also function in the context of a chimeric antigen receptor, both in vitro and in vivo, creating an alternative method to selectively re-engineer the immune system that may ultimately overcome some of the limitations of our previous protocol.
A potential drawback of our previous studies with the parental antibody was the need for weekly injections throughout the pre-diabetic period to achieve efficacy6. Such a dosing regimen is unlikely to be suitable for direct translation to the clinic, especially given the long prodromal phase between stage1 T1D and the development of overt hyperglycemia in many individuals30. At present the precise mechanism of action of mAb287 is uncertain. Given that treatment causes a global decrease in insulitis, simple blockade of T cells specific for the target pMHC complex seems unlikely to be the major factor. One possible mechanism for the observed protection is that binding of mAb287 may induce antibody dependent cellular cytotoxicity resulting in selective deletion of APCs expressing I-Ag7:B:R3 complexes. This led us to the hypothesis that reprogramming cytotoxic T cells with a CAR based on mAb287 might achieve the same result, and if correct, provide proof of concept that this form of ACT could represent a viable treatment modality for T1D. The results described above support this idea. Specifically, in this study we demonstrate that a single dose of the 287-CAR T cells was sufficient to delay the onset of T1D in otherwise un-manipulated animals for approximately 6 weeks. This data suggests that CAR-T cells are likely a superior therapeutic modality to the parent mAb287, as a one-time treatment with mAb287 could neither prevent nor delay the onset of T1D (our unpublished data). Consistent with the observed delayed onset, 287-CAR T cells could home to, and survive in, PLNs, a primary site for activation of diabetogenic T cells. It is highly likely that the same APCs that present insulin B:R3 will also present other insulin peptides, as well as peptides from other ß-cell components, and consequently that their selective deletion will lead to a significant reduction in islet autoimmunity to multiple autoantigenic targets by a process analogous to linked suppression. As discussed elsewhere4,5, binding of the B:9–23 peptide in register 3 is highly unfavorable, and it is likely that the formation of such complexes is essentially restricted to APCs in the pancreatic draining lymph nodes and islet infiltrates where the high levels of antigen can compensate for the low affinity of binding. Like all other CAR-T cells, the 287 CAR-T cells retain their endogenous T cell receptors (TCRs), which may allow them to have specificities in addition to I-Ag7-B:R3 complexes. Research from other laboratories suggests that concomitant activation of the CAR and TCR can diminish the in vivo efficacy of CAR-CD8 T cells but not CAR-CD4 T cells33. To attempt to minimize interference from endogenous TCRs we used naïve T cells to generate the CAR-T cells. This will likely ensure that the majority of the transferred CAR T cells will express functionally irrelevant TCRs, although we cannot eliminate the possibility that a few may target islet antigens, and contribute to autoimmunity. Nonetheless, given that disease was not accelerated by either the control or test CAR T cells, we do not believe that the potential presence of autoreactive TCRs within the transferred population represents a major concern for this form of ACT. Moreover, we believe that improved CAR- CD8 T cells with longer survival time in vivo, or CAR-CD4 regulatory T cells, which we are actively working on, will increase the efficacy of T1D prevention using our antigen specific immune therapy.
Unfortunately, although disease onset was delayed by 287-CAR T cell infusion, the effect was not durable. At present we cannot be certain of the reason for this, but our inability to recover any GFP positive cells from the diabetic animals suggests that the most likely explanation is that the transferred cells had only a limited lifespan. Indeed, the high rate of progression between 18 and 20 weeks observed in the animals treated with 287-CAR T cells is suggestive of the acute loss of an agent that had previously been keeping the disease in check. Such results are reminiscent of those obtained in early iterations of CAR T cell therapy for cancer, and could indicate that our current CAR design and/or dosing strategy is sub-optimal. As reviewed by Schubert and colleagues31, the design of the intracellular signaling domain is a critical parameter for CAR T cell functionality in vivo. For these studies we selected two well studied variants, a “second generation” CAR having CD28 and CD3ζ domains, and a “third generation” CAR with an additional domain from CD137 (4–1BB). Both variants were well expressed (although primary cells transduced with the 2nd generation reagents tended to expand more efficiently in vitro than those transduced with the 3rd generation reagents), and all responded robustly and selectively to their cognate antigens. However, while both 2nd and 3rd generation 287-CAR T cells could be recovered from PLNs within 5 days of transfer, only cells transduced with the 3rd generation variant persisted in vivo beyond 2 weeks. Recently, June and colleagues reported that CAR T cell persistence can be enhanced through ICOS and 4–1BB co-stimulation34, raising the possibility that replacement of the CD28 transmembrane and signaling domains with those from ICOS could improve the longevity of the 287-CAR T cells.
Beyond CAR design, another critical variable that remains to be optimized is the dosing strategy. Since our long term goal is to develop a therapy that can be translated to the clinic, we believed it important to attempt to minimize the number of infusions, and so for this proof of concept study focused only on a single ACT dose given early in the disease process. Although encouraging, the results obtained clearly indicate that with our current one-time CAR-T treatment strategy was not successful. At present we are uncertain why the protection waned with time but one possibility is that the amount of target antigen present at the time of treatment was insufficient to achieve robust proliferation, with those transferred cells that did expand eventually becoming exhausted. If this is the case, additional infusions at later time points may significantly improve efficacy. Alternatively, since our previous studies with the parental antibody demonstrated that it could also be effective in animals at the stage II/stage III transition6, it may also be possible to change the initial infusion to the late pre-diabetic period35 when the increased level of insulitis will mean that the density of IAg7/B:R3 complexes on the target APCs will likely be much higher. Finally, we are also aware that our scFab might also be used to redirect other subsets of T cells, such as CD4+ T regulatory cells (Tregs). For this proof of concept study our primary goal was to mimic the likely mode of action of the parent antibody, and hence we elected to redirect cytotoxic cells. However, a possible limitation of this approach is that by targeting their cognate APCs we may have negatively impacted the generation of memory T cells necessary to sustain the response for extended periods, or the expansion of induced or “natural Tregs. Since this is less likely to occur if the target APCs are modulated, rather than killed, we are actively exploring the feasibility of utilizing other T cell subsets, possibly using a 4th generation construct to boost or stabilize the regulatory function of the transduced cells.
In summary, in this pilot study we provide the first demonstration that CAR T cells can be used to selectively target APCs presenting pathogenic T cell epitopes relevant to autoimmunity. Although many variables remain to be optimized, we believe that our study provides significant support for the hypothesis that an effective antigen-specific adoptive cell therapies for T1D that is based on targeting disease relevant epitope presentation can be developed. Such a therapy is likely to have far fewer side- effects that other treatments currently in clinical trials.
Supplementary Material
SF 1: Expression of CARs in transduced CD8± T cells. CD8+ T cells were purified by negative selection from the spleens of 5 week old NOD mice, expanded in vitro and transduced with replication deficient retroviruses as described in methods. On day 8 cells were analyzed by flow cytometry. A representative experiment is shown.
SF 2: Representative flow cytometry plots and gating strategies. A. Gating strategy for determination of CAR T cell mediated APC killing. B. Gating strategy for measuring the frequency of transferred CAR T cells in secondary lymphoid tissues.
SF 3: Histological staining of pancreas sections. Pancreas sections from non-diabetic 287-CAR-T cell treated mice (A-B), diabetic 24.1-CAR-T cell treated mice (C-D) and non-diabetic untreated NOD mice (E-F) were stained with hematoxylin and eosin. Representative images are shown.
SF 4: Levels of serum insulin and tissue cytokines in CAR-T cell treated and un-treated mice. A. Sera were collected at 12 weeks from 287-CAR T cell treated mice (black column, n=17), 24.1-CAR T cell treated mice (blank column, n=17) and non-treated mice (grey column, n=36). Insulin levels were measured using mouse insulin solid-phase sandwich ELISA kit. p>0.3 between any two groups by Mann–Whitney test. B. 3–5 ×106 CAR T cells were indeed introduced into 8 week old NOD mice. Five days post transfer mice were sacrificed and IFN-γ and TNF-α in pancreatic lysates measured by ELISA.
SF 5: Development of T1D following co-transfer of diabetic splenocytes and CAR T cells. Groups of 5–6 week old female NOD.SCID mice were infused with 5×106 diabetic splenocytes either alone (n=5; red circles) or together with 5×106 287-CAR T cells (n=4; green squares), or 5×106 24.1-CAR T cells (n=5; blue triangles). Animals were monitored for signs of diabetes for up to 7 weeks post-transfer.
SF 6: Analysis of immune sub-populations following CAR-T cell transfer. CAR T cells were transferred to 8 week old recipients. On day 5 post-transfer, the percentages of splenic CD4 T cells, CD8 T cells, B cells and NK cells were analyzed by flow cytometry.
Highlights:
CD8 T cells redirected by a single chain Fab from the I-Ag7/insulin B:9–23 (register 3) specific monoclonal antibody mAb287 are able to selectively kill antigen presenting cells expressing this peptide-MHC complex on their cell surface.
The re-directed T cells selectively expand in the pancreatic draining lymph nodes where the relevant target ligands are presented.
A single infusion of the I-Ag7-insulin specific CD8 T cells to young NOD mice, but not cells re- directed with a control antibody, suppressed the appearance of insulin autoantibody and delayed the onset of disease for at least 6 weeks.
Given the similarities between the pathogenesis of T1D in the NOD mouse and humans, this work lays the foundations for the development of similar antigen specific cell therapies for the treatment of the human disease.
Acknowledgements
The authors would like to thank Dr Susan G Hilsenbeck for help in statistical analysis and Dr Matthew Bettini for imaging equipment. This study was supported by grants from the JDRF (1-INO-2015–74-S-B to HWD & LZ and 2-SRA-2016–238-S-B to LZ), the Caroline Wiess Law Fund for Research in Molecular Medicine at Baylor College of Medicine (to LZ), the Children’s Diabetes Foundation at Denver (to HWD), and the Robert and Janice McNair Foundation (to MP). Cell sorting was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (CA125123 and RR024574) and the expert assistance of Joel M. Sederstrom. RC and SH were supported by R01DK114356. LZ and HWD conceived the study, designed experiments and interpreted data. LZ, TS, HWD, JRC, NS, RC, DM and LY performed experiments. LZ, HWD, and MP wrote and edited the manuscript. LZ is the guarantor of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankovich AJ, Girvin AT, Moesta AK, Garcia KC. Peptide register shifting within the MHC groove: theory becomes reality. Mol Immunol 2004;40:1033–9. [DOI] [PubMed] [Google Scholar]
- 4.Crawford F, Stadinski B, Jin N, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. 2011:16729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. 2010:10978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Crawford F, Yu L, et al. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. 2014:2656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. 2004:524–30. [DOI] [PubMed] [Google Scholar]
- 8.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. 2003:973–83. [DOI] [PubMed] [Google Scholar]
- 9.Irvine DJ, Purbhoo MA, krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature 2002:845–9. [DOI] [PubMed] [Google Scholar]
- 10.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018;359:1361–5. [DOI] [PubMed] [Google Scholar]
- 11.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. 2014:333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 2010:3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. 2013:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman S, Lewis MD, Siew LK, et al. Adoptive Transfer of mRNA-Transfected T Cells Redirected against Diabetogenic CD8 T Cells Can Prevent Diabetes. Mol Ther 2017;25:456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terskikh AV, Le Doussal JM, Crameri R, Fisch I, Mach JP, Kajava AV. “Peptabody”: a new type of high avidity binding protein. Proc Natl Acad Sci U S A 1997;94:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc 2006;1:406–17. [DOI] [PubMed] [Google Scholar]
- 17.Bettini ML, Bettini M, Nakayama M, Guy CS, Vignali DA. Generation of T cell receptor-retrogenic mice: improved retroviral-mediated stem cell gene transfer. 2013:1837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature 2009;458:1043–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michels AW, Landry LG, McDaniel KA, et al. Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes. Diabetes 2017;66:722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrasco-Marin E, Shimizu J, Kanagawa O, Unanue ER. The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J Immunol 1996;156:450–8. [PubMed] [Google Scholar]
- 21.Griffith IJ, Nabavi N, Ghogawala Z, et al. Structural mutation affecting intracellular transport and cell surface expression of murine class II molecules. J Exp Med 1988;167:541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature 1994;369:151–4. [DOI] [PubMed] [Google Scholar]
- 23.Collawn JF, Stangel M, Kuhn LA, et al. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 1990;63:1061–72. [DOI] [PubMed] [Google Scholar]
- 24.Allicotti G, Borras E, Pinilla C. A time-resolved fluorescence immunoassay (DELFIA) increases the sensitivity of antigen-driven cytokine detection. J Immunoassay Immunochem 2003;24:345–58. [DOI] [PubMed] [Google Scholar]
- 25.Tuttle AH, Rankin MM, Teta M, et al. Immunofluorescent detection of two thymidine analogues (CldU and IdU) in primary tissue. J Vis Exp 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox AR, Barrandon O, Cai EP, et al. Resolving Discrepant Findings on ANGPTL8 in beta-Cell Proliferation: A Collaborative Approach to Resolving the Betatrophin Controversy. PLoS One 2016;11:e0159276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaya N, Liu E, Miao D, Li M, Yu L, Eisenbarth GS. Murine high specificity/sensitivity competitive europium insulin autoantibody assay. Diabetes TechnolTher 2009;11:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer TA. Adhesion receptors of the immune system. Nature 1990;346:425–34. [DOI] [PubMed] [Google Scholar]
- 29.Chen BM, Al-Aghbar MA, Lee CH, et al. The Affinity of Elongated Membrane-Tethered Ligands Determines Potency of T Cell Receptor Triggering. Front Immunol 2017;8:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenbaum C, Lord S, VanBuecken D. Emerging Concepts on Disease-Modifying Therapies in Type 1 Diabetes. Curr Diab Rep 2017;17:119. [DOI] [PubMed] [Google Scholar]
- 31.Schubert ML, Hoffmann JM, Dreger P, Muller-Tidow C, Schmitt M. Chimeric antigen receptor transduced T cells: Tuning up for the next generation. Int J Cancer 2018;142:1738–47. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Chow IT, Sosinowski T, et al. Autoreactive T cells specific for insulin B:11–23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc Natl Acad Sci U S A 2014;111:14840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Kohler ME, Chien CD, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guedan S, Posey AD Jr., Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4–1BB costimulation. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis MD, de Leenheer E, Fishman S, Siew LK, Gross G, Wong FS. A reproducible method for the expansion of mouse CD8+ T lymphocytes. J Immunol Methods 2015;417:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SF 1: Expression of CARs in transduced CD8± T cells. CD8+ T cells were purified by negative selection from the spleens of 5 week old NOD mice, expanded in vitro and transduced with replication deficient retroviruses as described in methods. On day 8 cells were analyzed by flow cytometry. A representative experiment is shown.
SF 2: Representative flow cytometry plots and gating strategies. A. Gating strategy for determination of CAR T cell mediated APC killing. B. Gating strategy for measuring the frequency of transferred CAR T cells in secondary lymphoid tissues.
SF 3: Histological staining of pancreas sections. Pancreas sections from non-diabetic 287-CAR-T cell treated mice (A-B), diabetic 24.1-CAR-T cell treated mice (C-D) and non-diabetic untreated NOD mice (E-F) were stained with hematoxylin and eosin. Representative images are shown.
SF 4: Levels of serum insulin and tissue cytokines in CAR-T cell treated and un-treated mice. A. Sera were collected at 12 weeks from 287-CAR T cell treated mice (black column, n=17), 24.1-CAR T cell treated mice (blank column, n=17) and non-treated mice (grey column, n=36). Insulin levels were measured using mouse insulin solid-phase sandwich ELISA kit. p>0.3 between any two groups by Mann–Whitney test. B. 3–5 ×106 CAR T cells were indeed introduced into 8 week old NOD mice. Five days post transfer mice were sacrificed and IFN-γ and TNF-α in pancreatic lysates measured by ELISA.
SF 5: Development of T1D following co-transfer of diabetic splenocytes and CAR T cells. Groups of 5–6 week old female NOD.SCID mice were infused with 5×106 diabetic splenocytes either alone (n=5; red circles) or together with 5×106 287-CAR T cells (n=4; green squares), or 5×106 24.1-CAR T cells (n=5; blue triangles). Animals were monitored for signs of diabetes for up to 7 weeks post-transfer.
SF 6: Analysis of immune sub-populations following CAR-T cell transfer. CAR T cells were transferred to 8 week old recipients. On day 5 post-transfer, the percentages of splenic CD4 T cells, CD8 T cells, B cells and NK cells were analyzed by flow cytometry.