Abstract
Plasma membrane forms the barrier between the cytoplasm and the environment. Cells constantly and selectively transport molecules across their plasma membrane without disrupting it. Any disruption in the plasma membrane compromises its selective permeability and is lethal, if not rapidly repaired. There is a growing understanding of the organelles, proteins, lipids, and small molecules that help cells signal and efficiently coordinate plasma membrane repair. This review aims to summarize how these subcellular responses are coordinated and how cellular signals generated due to plasma membrane injury interact with each other to spatially and temporally coordinate repair. With the involvement of calcium and redox signaling in single cell and tissue repair, we will discuss how these and other related signals extend from single cell repair to tissue level repair. These signals link repair processes that are activated immediately after plasma membrane injury with longer term processes regulating repair and regeneration of the damaged tissue. We propose that investigating cell and tissue repair as part of a continuum of wound repair mechanisms would be of value in treating degenerative diseases.
Keywords: Injury, Calcium, Lipids, Redox, Cell membrane, Tissue repair
Introduction
Work at the turn of the twentieth century by Charles Overton established that a lipid membrane bounds the protoplasm in all living cells [1]. This barrier membrane, referred to as the cell membrane, plasmalemma, or plasma membrane has enabled the creation of living cells by physically separating the protoplasm from the surrounding primordial soup. However, this 10 nm thick membrane is quite fragile [2] and its rupture allows extracellular and intracellular milieu to mix, causing chemical imbalances in the cell that can lead to cell death. Thus, a cell’s survival depends on its ability to rapidly restore the integrity of a ruptured plasma membrane. This explains the need for a ubiquitous pathway to repair plasma membrane injury in all cells. Plasma membrane repair has been studied in a wide variety of organisms and cell types and defects in plasma membrane repair have been identified in a growing number of diseases associated with tissue degeneration (reviewed in [3–5]). Despite the capacity of all cells to repair their plasma membrane, all cells do not experience similar injuries. For example, the plasma membrane of cells in mechanically active tissues, such as muscle and lungs, experience greater damage from shear stress while membranes of cells in the gut and other epithelia exposed to pathogens are more prone to injury by pore-forming toxins.
Specialized signaling mechanisms and cellular machinery help cells determine the nature and location of the plasma membrane injury and orchestrate the appropriate repair response. There are additional features of plasma membrane injury that highlight the need to coordinate the repair response. First, the cell cannot predict where or when plasma membrane damage will occur and thus it needs to rapidly detect damage and orient the repair response based on the location of the injury. Second, the repair response must be controlled based on the nature and size of the wound. Third, repair of the injured membrane occurs in seconds to minutes—a time scale that is conducive to changes in protein and lipid interactions, but not to de novo protein synthesis. Thus, prior to injury the cell must possess all the required components of the plasma membrane repair machinery and should be able to readily activate and coordinate a response to effectively repair the membrane. With such requirements, it is not a surprise that many of the proteins that facilitate plasma membrane repair have additional roles in cell physiology. The way in which these proteins contribute to plasma membrane repair changes somewhat based on the type and size of the injury, and more than one mechanism can contribute to the repair of a single wound. These mechanisms are the subject of many reviews and a dedicated special journal issue [3, 4, 6]. Thus, instead of detailing the repair mechanisms (Fig. 1), we will instead focus on our current understanding of how these cellular mechanisms and signals are orchestrated in space and time to allow efficient repair of wounded plasma membrane.
Fig. 1.
Cellular mechanisms for plasma membrane repair. Injured plasma membrane, represented by dark blue color, is repaired by various cellular mechanisms working in tight spatial and temporal concert with one another. The various mechanisms that enable plasma membrane repair include: clot formation: organelles, vesicles, cytoskeletal proteins, as well as calcium-binding proteins aggregate around the wound site, plugging the wound and rapidly creating a barrier to prevent the free exchange of materials between the cytoplasm and the extracellular environment. Membrane internalization: endocytosis of the injured plasma membrane can remove holes in the plasma membrane formed by pore forming toxins. In the case of larger membrane injuries, endocytosis of vesicles adjacent to the site of membrane damage may offer a source of recycled membrane that can form clots or fuse back with the plasma membrane to remodel the damage site and help close the wound. Membrane shedding: similar to membrane internalization, ectocytosis can remove holes in the plasma membrane caused by pore forming toxins, as well as damaged (oxidized) lipids from membrane adjacent to the site of a large injury. The resulting vesicles (ectosomes) can then participate in extracellular signaling that potentiates tissue-level repair responses in neighboring cells. Membrane fusion: fusion of intracellular vesicles with the plasma membrane adds new membrane that is required to replace the damaged membrane lost due to injury. Additionally, the fusing vesicles can release small molecules or enzymes that can alter the extracellular matrix and membrane as well as signal to neighboring cells to facilitate both cell and tissue repair
Cellular mechanisms of repair
Clot formation
Early studies of plasma membrane injury by Victor Heilbrunn noted that injured cells can seal a torn membrane by a calcium ion-dependent process that he called the ‘surface precipitation reaction’ [7]. He hypothesized this to be a basic colloidal reaction of protoplasm that affects the protoplasm in a manner similar to clotting of blood [7]. Subsequent work has lent support to this clotting-like mechanism by showing that proteins (e.g. annexins) organelles (eg: mitohondria), or vesicles (endosomes) aggregate at the site of injury in a calcium-dependent manner [8–11]. Aggregation of calcium-sensitive proteins at the injury site occurs rapidly and is suggested to be a potential mechanism to slow the exchange of material between the cytosol and extracellular space [8, 12]. Cells are also capable of plugging large holes caused by severe plasma membrane damage, such as due to the transection of axons or skeletal myofibers. Transected axons accumulate vesicles at the cut site, which functions to protect the injured axon [10]. Similarly, localized injury of skeletal myofibers results in rapid accumulation of mitochondria at the site of injury [9]. Skeletal myofiber injury can result in a ‘stump’ where myofibrils and mitochondria accumulate in a clotting-like manner. These stumps containing the clotted material allow the myofibers to survive for several hours in vivo without a plasma membrane that is observable by electron microscopy [13]. Thus, clot formation may be a part of the repair mechanism where proteins and organelles form scaffolds to allow the cell time to build new plasma membrane, following which the scaffolds are removed.
Wound constriction
An immediate result of plasma membrane injury is disassembly of the underlying cortical cytoskeleton [14–16]. Membrane tension provided by the cortical cytoskeleton inhibits spontaneous resealing of small wounds [17], and acutely relaxing membrane tension helps pull the damaged membrane together. Depolymerization of densely packed cortical F-actin is thought to free space for vesicle fusion events to occur near the site of injury [14]. Although early disassembly of the cytoskeleton is beneficial, accumulation of F-actin in the later stages of resealing is required for successful repair by aiding in membrane trafficking, wound closure, and providing structural support to the newly formed membrane [11, 16, 18, 19]. In Xenopus and Drosophila models, actin forms a ring structure around the wound area and aids in closure via a purse-string mechanism involving the motor function of myosin [20, 21].
Membrane fusion
Early studies of invertebrate eggs implicated vesicle fusion in the process of repairing injured plasma membrane [22]. This was confirmed in studies with oocytes, which identified cortical granules as the vesicles that provide new membrane to repair the damaged plasma membrane [23]. Involvement of synaptic vesicle-like machinery in repairing invertebrate and mammalian cells provided further support to vesicle-mediated membrane repair [24, 25]. In oocytes, vesicle-mediated repair appears to involve formation of a ‘membrane patch’ to close the wound [26, 27]. While mammalian cells lack cortical granules, lysosomes have been identified as the ubiquitous calcium-dependent exocytic compartment [28, 29]. Lysosome fusion facilitates repair of plasma membrane injured by mechanical as well as toxin-induced damage [30, 31]. Lysosomal exocytosis was proposed to facilitate plasma membrane repair in a manner similar to cortical granules, where internal membrane provides a patch for the plasma membrane wound [15, 30]. However, imaging lysosomes present at the plasma membrane showed that instead of coalescing into a patch, these lysosomes respond to the calcium increase and plasma membrane injury by fusing at the site where they are present on the plasma membrane [32]. Instead of patch formation, lysosome exocytosis contributes to plasma membrane repair by releasing the lysosomal enzyme acid sphingomyelinase (ASM) [31–33]. This mechanism of lysosome-mediated repair is supported by the ability of the extracellular ASM treatment to reverse plasma membrane repair defects in cells that show reduced injury-triggered lysosomal exocytosis [32].
Membrane internalization
Plasma membrane endocytosis was first observed in cells treated with pore forming protein released by killer T cells [34]. Later on, this endocytic process was shown to depend on clathrin and dynamin and aid in the repair of injured plasma membrane, helping protect the tissue from inflammation that would result from the release of cellular proteins if necrotic cell death ensued [35]. Endocytosis was also shown to facilitate repair of plasma membrane injured by bacterial pore forming toxin streptolysin O (SLO) or injured due to cell sqeezing [31]. Injury-triggered endocytosis of SLO pores has also been shown to depend on the release of lysosomal ASM, which converts local sphingomyelin into ceramides and subsequently induces endocytosis [31, 33]. This suggests the possibility that intracellular vesicles needed to reseal the membrane may be derived from the plasma membrane itself [18].
Membrane shedding
In addition to endocytosis, repair of plasma membrane injured by SLO and other pore forming toxins has been shown to rely on microvesicle-mediated shedding of toxin pores in the plasma membrane [36, 37]. While vesicle shedding is suggested to be a physical effect of the toxin on the plasma membrane, more recent studies have implicated the endosomal sorting complex required for transport (ESCRT) machinery in shedding of SLO pores [38]. The requirement of ESCRTs has also been identified in repairing larger (micron scale) focal plasma membrane wounds as well as by mechanical injury [39]. In the case of the larger wounds, F-actin deploymerization and ESCRT assembly both facilitate shedding of damaged edges of the injured plasma membrane [16, 39]. This enables merging of the wound edges through the constriction of the wound by membrane fusion and cytoskeletal contraction.
Instead of any one of the above mechanisms for plasma membrane repair, cells employ a combination of many of the above mechanisms to work cooperatively to aid with wound closure and remodeling the wounded plasma membrane for efficient plasma membrane repair.
Signals and effectors of plasma membrane repair
Calcium and calcium-binding proteins
With an over 10,000-fold calcium gradient across the plasma membrane, injury results in a large and rapid calcium influx into the cytosol [40]. This large influx of calcium is recognized for its ability to trigger cell death [41]. However, nearly a century ago the requirement of calcium for repairing injured cells was first reported by way of a “surface precipitation reaction” [7, 42]. Excessive calcium entry across the plasma membrane is a universal signal to trigger the plasma membrane repair response [3–5]. A critical element of the calcium-triggered repair response is the cell’s ability to switch it off by removing excess cytosolic calcium. Chelation by calcium-binding proteins can temporarily curb the excess calcium overload in the moments immediately following injury. However, other compartments, such as mitochondria, can selectively take up calcium at the site of injury and help in stably buffering the calcium increase [19].
The unique chemistry of the calcium ion [40] is integral to its role in plasma membrane repair. This is demonstrated by experiments showing that reduction in the level of extracellular calcium or increasing the level of a competing divalent ion, such as magnesium, compromises the kinetics and the ability of cells to repair [24, 43]. Calcium entry from the site of injury creates a gradient starting from the wound site, and the myriad cellular mechanisms (chelators, pumps, etc.) that remove excess cytosolic calcium add to the rapid generation of a calcium gradient. This gradient activates different calcium-sensitive responses depending on the local calcium level and the relative affinity of calcium binding proteins [44]. Cells have a variety of calcium sensing proteins that exhibit a wide range of calcium binding affinities, which enables them to mount a diversity of spatially and temporally localized responses to calcium increase (Fig. 2) [45]. However, the magnitude and timing for which calcium increase needs to occur for triggering an efficient repair response are variable. While the repair of micropuncture injury of oocytes requires 1.2 mM extracellular calcium, this threshold is 400 µM for 3T3 fibroblast injury [24]. Following pore forming toxin injury, intracellular calcium increase in the low micromolar range (5–10 µM) can facilitate repair, while increase over 10 µM can cause the repair to fail [46]. Different cell types also exhibit different sensitivity to calcium increase following membrane injury for their survival [47]. Thus, there appears to be a fine line between calcium signaling and toxicity during membrane repair, necessitating a tight control over free calcium following injury.
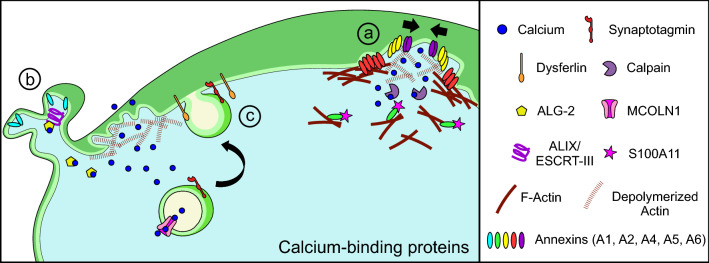
Fig. 2.
Cellular calcium-binding proteins activate plasma membrane repair machinery. Plasma membrane injury leads to entry of extracellular calcium into the cell, which activates different calcium-dependent repair mechanisms. (a) Clotting and wound constriction: various calcium-binding proteins such as annexins respond to the local high calcium and the presence of phosphatidylserine-containing membranes, which results in local aggregation of proteins and vesicles. Through activation of calcium-activated proteases such as calpains, structural and signaling proteins undergo local proteolysis. This is accompanied by re-assembly of new cytoskeleton, which is also facilitated by annexins and other calcium-binding proteins. F-actin remodeling facilitates local trafficking and trapping of vesicles at the wound site to form a clot. (b) Membrane shedding: calcium binding to ALG-2 initiates assembly of the ALIX/ESCRT-III complex. This enables cleavage of the damaged membrane edges, which are then shed as extracellular vesicles. Additionally, loss of actin at the injury site allows for the membrane to become floppy and to be easily detached from the adjacent undamaged part of the plasma membrane. Shedding of these membrane regions allows for efficient closure of the wound edges while the shed vesicles signal neighboring cells to facilitate tissue repair. (c) Vesicle fusion and calcium homeostasis: vesicles such as lysosomes contribute to local calcium signaling by sequestering or releasing their luminal calcium through transporters such as MCOLN1. Other calcium-sensitive proteins such as synaptotagmin and dysferlin facilitate tethering and fusion of vesicles with the injured plasma membrane. This provides new membrane for remodeling and allows for delivery of signaling molecules and proteins, which aid in repair
Annexins
The annexin family of calcium-binding proteins has a wide range of calcium sensitivities [48]. This allows annexins to respond to plasma membrane injury in a calcium-concentration-dependent fashion [45]. This diversity in calcium sensitivity is matched by the diversity of roles annexins have been proposed to play in processes implicated in plasma membrane repair, including clot formation, membrane trafficking, and cytoskeleton reorganization [48]. The ability of annexin family members to perform a wide array of tasks during plasma membrane repair may be due to their different binding affinities to intracellular calcium [44]. For example, annexins with the greatest calcium sensitivity respond faster to plasma membrane injury, at a time when their function is dictated by the substrates (e.g. lipids or cytoskeleton) available for binding [49, 50]. While annexins with lower calcium sensitivity respond later and can involve binding partners such as S100 proteins [16]. The difference in response to calcium signaling may determine how largely similar annexin proteins including Annexin A1, A2, A4, A5 and A6 can accumulate and potentially facilitate different aspects of plasma membrane repair (Fig. 2a) [51, 52]. However, annexin accumulation at the injury site does not necessarily indicate a role in repair. For example, Annexin A1, which accumulates at the site of myofiber injury [51], is not required for myofiber repair following focal or mechanical injury [53]. Another annexin, Annexin A5, which accumulates at the site of plasma membrane injury facilitates repair by forming two-dimensional protein arrays. These arrays may play a structural role in repair by stabilizing the damaged plasma membrane and limiting wound expansion [8]. In addition, Annexin A5—like other annexins—binds phosphatidylserine (PS) enriched at the site near injury (see discussion on lipid patterning below). In contrast to the structural lattice formed by Annexin A5, the Annexins A4 and A6 play complimentary roles in membrane curvature and constriction after injury [52]. Calcium-dependent Annexin A4 also binds preferentially to PS-rich membrane regions and curves the wounded plasma membrane edges, which is subsequently constricted inward by Annexin A6 to aid in closing the wound. This proposed mechanism seems to fit with the wound stabilizing role of Annexin A5, which is recruited to the injury site within seconds [8], while later arrival of Annexins A4 and A6 facilitates wound closure [52]. Annexin A6 has been reported to localize to the extracellular face of the plasma membrane in skeletal muscle [11, 51], which is consistent with the role of Annexin A6 in constricting the damaged free edges of the wound site. Annexin A2 is involved in repair as a complex, which it forms with the calcium-binding protein S100A11 [16] and this Annexin A2/S100A11 complex is required for actin reorganization and the membrane scission mechanism of repair described above. In contrast to the rapid annexin response described above, the Annexin A2/S100A11 complex is recruited relatively slowly (tens of seconds), which aligns with the time scale of F-actin recruitment during repair (Fig. 2a) [16]. Interestingly, Annexin A1 was also rapidly recruited in cancer cells independently of S100A11, but it accumulated in the region of damaged membrane that was eventually shed off. Unlike the in vivo role of Annexin A1 in facilitating myoblast fusion during muscle regeneration [53], but not in myofiber plasma membrane repair, in cultured cells Annexin A1 is suggested to facilitate plasma membrane repair by promoting membrane trafficking and fusion [46, 54]. The diversity of kinetics and localization of annexin recruitment at the injury site, for example the faster accumulation of Annexins A1 and A6 compared to Annexin A2, is conserved in some [11, 55], but not other systems. For example, in injured muscle fibers of Zebrafish, Annexin A2 accumulates before Annexin A1 [51]. This suggests that annexins either perform different functions in repair depending on the system/cell type, or that the timing of accumulation is not a strict requirement for the role of annexins in repair. Future studies may help address such questions related to the role of annexin complexes in plasma membrane repair.
Calpains
Similar to annexins, upon binding micromolar to millimolar level of calcium, calpains participate in plasma membrane repair in multiple cell types [56–60]. Unlike the direct effects of annexins on membrane dynamics and vesicle fusion, calpains work by calcium-dependent proteolysis of membrane and cytoskeletal proteins [59–61]. Disassembly of the cortical cytoskeleton not only helps reduce membrane tension that can inhibit resealing, but also improves access of vesicles to fuse with the plasma membrane [14, 62]. Calpains have also been implicated in the control of vesicle fusion machinery itself [63]. Thus, calpains may facilitate repair of injured plasma membrane through their contribution to cytoskeleton remodeling and action on vesicle fusion machinery. However, unlike annexins, an optimal level of calpain activity, perhaps through regulation of intracellular calcium level, is required for efficient plasma membrane repair, and excessive calpain activation can cause cell death [64].
MCOLN1 and ALG-2
Intracellular compartments contribute to cellular calcium homeostasis both by facilitating clearance and controlled release of calcium into the cytosol. These organelle-mediated secondary calcium release mechanisms allow for delayed and localized increase in calcium independent of the injury site. For example, calcium released by lysosomes is used by pyramidal neurons for long-term structural plasticity of dendritic spines, which is mediated by lysosome exocytosis [65]. Lysosomal calcium release is also relevant for membrane repair as loss of the lysosomal calcium channel MCOLN1 (TRPML1) in muscle fibers compromises injury-triggered lysosomal exocytosis and plasma membrane repair, resulting in muscular dystrophy [66]. Additionally, association of MCOLN1 with the calcium-binding protein Apoptosis-linked gene-2 (ALG-2) also facilitates calcium-dependent retrograde movement of lysosomes [67]. ALG-2 itself has been shown to be essential for plasma membrane repair as it is responsible for membrane shedding through recruitment of the ESCRT complex at the injured plasma membrane (Fig. 2b) [38, 39]. ESCRT proteins are well known for their role in membrane deformation and scission at the late endosome/lysosomes to form exosomes within the multivesicular body [68]. However, in response to plasma membrane injury, ALG-2 facilitates accumulation of ESCRT-III at the damaged plasma membrane by way of its binding partner—ALG-2 interacting protein X (ALIX). The ESCRT-III complex works together with the ATPase Vps4b to cause shedding of the damaged membrane producing extracellular vesicles called ectosomes [39]. While suggested to be relevant for small injuries (< 100 nm), ESCRT machinery is also required for shedding of ectosomes following micron-sized plasma membrane damage [38, 39]. Given the lack of lipid binding domain in ALG-2, the mechanism by which it associates with the injured plasma membrane in response to injury-triggered calcium increase requires further investigation.
Synaptotagmins
Synaptotagmins (Syt) are a family of C2 domain containing proteins that, similar to the annexin family, show a graded calcium sensitivity for the process of calcium-triggered exocytosis [69]. Early work had recognized the role of synaptic vesicle like exocytic machinery in plasma membrane repair [24, 25]. In support of this, Syt have been recognized for their role in the repair of axons in mammalian and invertebrate neurons as well as in the plasma membrane repair in astroglial cells [70, 71]. Syt facilitate calcium-triggered lysosome fusion, which is implicated in repair of neuronal and non-neuronal cells. Syt-VII is a major lysosomal synaptotagmin isoform that controls fusion pore expansion during calcium-triggered lysosome exocytosis and its absence compromises plasma membrane repair (Fig. 2c) [30, 72–75]. In cells such as astrocytes, which lack lysosomal Syt VII, another synaptotagmin isoform, Syt XI, facilitates calcium-triggered lysosome exocytosis and is required for plasma membrane repair [75]. In contrast to these Syt, lysosome exocytosis is negatively regulated by Syt II [75, 76]. Such positive and negative regulation of lysosome exocytosis may be crucial for the graded calcium dependence that regulates the extent of calcium-triggered lysosome fusion—high calcium (as experienced after membrane injury) leads to full fusion of lysosomes, while smaller increases in calcium induced by receptor stimulation lead to partial fusion of the lysosome [75]. This calcium dependence is lost in the absence of Syt [74, 75]. Furthermore, the rate of exocytosis and the number of lysosomes that exocytose are directly tied to the level of calcium increase. After plasma membrane injury, peak lysosome exocytosis occurs within 10 s while receptor stimulation-induced calcium increase causes the peak fusion to occur by 30 s [74, 75]. Thus, Syt are calcium sensors that help tune lysosome fusion based on the strength and/or duration of the calcium signal. Aside from Syt, other proteins including synaptotagmin-like protein 4a are also implicated in plasma membrane repair by its interaction with the GTPase Rab3a to facilitate lysosome trafficking to the cell periphery [77].
Dysferlin
Similar to Syt, ferlin proteins also contain C2 domains and are implicated in plasma membrane repair by regulating injury-triggered vesicle fusion [78]. The absence of dysferlin-mediated vesicle fusion results in poor plasma membrane repair and muscular dystrophy [32, 79]. Like Syt VII and XI, dysferlin also regulates calcium-triggered lysosome exocytosis [32, 80]. However, unlike synaptotagmin, instead of triggering exocytosis, dysferlin facilitates tethering/docking of lysosomes to the plasma membrane (Fig. 2c). This enables rapid fusion of lysosomes following plasma membrane injury. While the exact mechanism for this mode of action of dysferlin has not been elucidated, lack of dysferlin has been shown to reduce the number of lysosomes present at the plasma membrane as well as cause an overall reduction in the kinetics and number of lysosomes that undergo injury-triggered exocytosis [32]. Dysferlin-mediated lysosome exocytosis releases the lysosomal enzyme ASM. Secreted ASM facilitates injury-triggered plasma membrane endocytosis required for repair [33, 81]. Interestingly, muscle fiber injury causes endocytosis of dysferlin into vesicles that can fuse with the plasma membrane in an F-actin dependent process [18]. This suggests a model in which dysferlin-mediated ASM secretion allows for the formation of endocytic vesicles to facilitate myofiber repair [81]. Extracellular application of ASM to dysferlin-deficient muscle rescues the plasma membrane repair defect in dysferlin-deficient mice and establishes the requirement of secreted ASM for plasma membrane repair [32]. Injury-triggered calcium increase also activates calpain, which has been shown in astroglial and muscle cells to cleave dysferlin at the site of membrane injury to form a truncated dysferlin protein (mini-dysferlin) that resembles synaptotagmin [60, 78]. Mini-dysferlin is detectable at the injury site in < 10 s, which is consistent with the rapid time course of lysosome exocytosis in muscle and glial cells [32, 75]. Given that dysferlin in skeletal muscle with the calpain cleavage site is limited to 10–15% of the total dysferlin protein [60], it remains to be seen how these mini-dysferlin molecules facilitate plasma membrane repair.
Calcium-dependent signaling
Calcium is a ubiquitous second messenger that regulates a variety of signaling processes by interacting with calcium-binding proteins and modifying their function by changing their charge, molecular conformation, or interaction with other proteins [40, 82]. These calcium-sensitive proteins are damage sensors and can act rapidly to facilitate plugging of the membrane wound. In addition to such a direct role, calcium signals also propagate through downstream effector proteins and compartments, which upon activation lead to prolonged signaling (Fig. 3). We will next discuss these proteins and their mode of action.
Fig. 3.
Calcium-dependent signaling coordinates the repair response (a) GTPase signaling: Rho-family GTPases regulate the assembly of F-actin cytoskeleton that facilitates the closure of plasma membrane wounds. GTPase activity is controlled by regulatory GEF and GAPs, which allow GTPases to function in distinct spatial regions relative to the wound site. (b) Lipid patterning: the site of injury undergoes local modification of lipids after injury, due to local influx and efflux of specific lipids, delivery of new lipids by vesicle fusion, and de novo generation of lipids caused by the activation of calcium-dependent phospholipases or localized secretion of enzymes such as acid sphingomyelinase (ASM). This local change in lipid species provides a platform for downstream signaling events carried out by GTPases and protein kinases. (c) Potentiation of repair: localized activation and recruitment of protein kinase C to DAG-rich membrane regions near the site of injury triggers a CREB-mediated gene transcription pathway that potentiates the repair response for repeat injuries
Rho GTPases
One mechanism by which injury-triggered calcium increase results in downstream structural changes to the cell membrane is through the activation of Rho-family GTPases, which regulate the assembly of transient contractile cytoskeletal arrays [83, 84]. A brief injury-triggered increase in calcium leads to the activation of Rho and/or Cdc42 proteins. These GTPases in turn regulate the formation well-defined zones of actin and myosin-2 in a manner that mediates constriction of the wounded edges of the plasma membrane in a purse string-like mechanism (Fig. 3a) [83, 85]. Calcium-dependent activation of PKC β and η isoforms following injury causes PKC β-mediated activation and PKC η-mediated inhibition of GTPase activity, which creates the positive feedback underlying the acute and coordinated amplification of Rho and Cdc42 leading to closure of the plasma membrane wound [86, 87]. RhoA also exhibits activation by calcium-triggered mitochondrial redox signaling and this facilitates F-actin reorganization required for repair [19]. In the sections below, we will discuss the interplay of calcium, ROS and other regulators in coordinating Rho GTPases during wound repair.
Phospholipases
Phospholipases contribute to the modification of lipid composition of the injured plasma membrane, causing downstream signaling to facilitate repair. Injury-triggered calcium increase activates phospholipase C (PLC) [88], causing production of diacylglycerol (DAG) and inositol trisphosphate (IP3) (Fig. 3b). DAG localizes to the region adjacent to the wound site and provides a key signaling platform that determines the spatial localization of downstream repair effectors such as protein kinase C (PKC) and Rho GTPases [86]. In astrocytes, IP3 increase has been shown to last for hours, well beyond the time frame for intracellular calcium increase [88]. While the mechanism of PLC-dependent IP3 production in the repair of injured cells is yet to be elucidated, repaired cells secrete IP3, suggesting a role for injury-triggered extracellular IP3 signaling. Plasma membrane injury also causes the increased activity of phospholipase D (PLD) [89]. PLD activity results in the formation of phosphatidic acid (PA), which occurs in a spatially confined concentric ring around the injury site, mirroring the kinetics of DAG formation [86]. PA has been implicated in SNARE-mediated vesicle fusion [90]. Further, PLD is involved in the synthesis of phosphatidylglycerol (PG). In response to keratinocyte wounding in vitro, PLD inhibitors prevented repair, while addition of exogenous PG aided in epithelial wound repair in mice [89]. Given the role of vesicle fusion in plasma membrane repair and the ability of PLD to catalyze PG synthesis by the transphosphatidylation reaction [91], it is possible that PLD plays multiple roles in plasma membrane and tissue repair.
Protein kinases
Protein kinase C isozymes are recruited to the plasma membrane by DAG and regulate downstream signaling pathways that facilitate repair. While not all PKC isozymes require direct calcium-binding for activation, binding to DAG indirectly requires the calcium-activated PLC. PKC signaling, through the family members PKC η and θ, regulates vesicle fusion after membrane injury via the downstream effector Munc13-1 [86, 92]. This pathway is propagated by a feed-forward mechanism in which the PKC target MARCKS mediates synthesis of phosphatidylinositol 4,5-bisphosphate (PIP2), which may allow for PLC-mediated DAG formation. Protein kinase A (PKA) activity is increased by binding of cAMP, which itself increases in the minutes following plasma membrane injury [93]. PKA activity is required for membrane resealing, but its role in this process is less well understood [94].
PKC and PKA activity is critical not only to initial resealing of damaged plasma membrane, but also for the longer-term adaptation to injury. Cells, especially those in mechanically active tissues, often undergo repeat injuries. To better deal with this membrane stress, cells have developed adaptive mechanisms to improve their plasma membrane repair ability. This process, referred to as potentiation, can reduce the time needed for resealing by twofold, thus improving survival in the face of repeated injuries [17]. Increase in PKC and PKA activity after an initial plasma membrane wound is required for short-term potentiation of repair from a repeat injury that occurs in the minutes after initial damage (Fig. 3c). These kinases initiate signaling pathways that facilitate vesicle trafficking to the cell periphery in a manner dependent on new microtubule growth [17, 95].
Long-term potentiation, which provides improved repair ability for hours or even days after an initial membrane injury, requires CREB-mediated gene expression (Fig. 3c) [96]. Phosphorylation of CREB occurs through a PKC and p38-MAPK-dependent pathway [96]. Additionally, plasma membrane repair in neighboring uninjured cells can be potentiated by extracellular signaling, which increases protein kinase G (PKG) activity in these cells. PKG activity causes CREB phosphorylation, leading to gene expression that potentiates repair. This pathway involves intracellular nitric oxide signaling [97]; however, the precise mechanism is yet to be resolved.
Purinergic signaling
Extracellular purinergic signaling helps potentiate repair of neighboring cells that are not in physical contact [98]. Release of ATP by the injured cells is sensed by neighboring cells, which initiates short-term repair potentiation. Although ATP can be passively released from injured cells, this does not appear to be a major contributor to signaling that regulates potentiation [98]. Instead, ATP secretion occurs by calcium-triggered exocytosis following plasma membrane injury [98–100]. In addition to facilitating lysosome exocytosis, dysferlin can also facilitate injury-triggered exocytic ATP secretion [98]. Since lysosome exocytosis can facilitate ATP secretion [101], ATP secretion-mediated potentiation of repair may be another role of injury-triggered lysosome exocytosis. Secreted ATP can bind purinoreceptors on neighboring cells, causing increase in intracellular calcium and cAMP [102]. This in turn can stimulate PKC and PKA activity to regulate downstream short-term potentiation pathways. Purinergic signaling also regulates Akt (protein kinase B) activity, which increases several minutes after plasma membrane injury [103]. Given that Akt activation occurs after membrane resealing is well underway, it seems likely to control longer-term processes such as lysosome biogenesis [104, 105].
Redox signaling
There is growing evidence that supports a beneficial role of reactive oxygen species (ROS) in a variety of physiological processes through activation of redox signaling [106]. An abundant source of ROS is the superoxide produced by mitochondria and in the cytosol by NADPH oxidases. These species are then rapidly dismutated to hydrogen peroxide (H2O2), which can act as a signaling molecule [107]. Redox modifications chemically alter proteins and lipids and can change their function. While uncontrolled redox modification can damage cells, the specificity, reversibility, and highly localized nature of this process allow it to facilitate signaling [108]. A feature of redox signaling enabled by endogenous antioxidants is its transient nature. Compared to calcium increase after plasma membrane injury, the lifetime of reactive oxygen generating moieties is measured in fractions of seconds. Thus, ROS increase after plasma membrane damage is transient and highly localized to the site of injury where it can facilitate repair by locally activating proteins such as MG53 and RhoA, which facilitate vesicle fusion and cytoskeletal remodeling, respectively [19, 109–111]. Chronic ROS increase appears to prevent plasma membrane repair [112]. Because ROS increase may be beneficial or detrimental to cells based on its duration, ROS increase following membrane injury needs to be tightly regulated in amplitude, space, and time. There is growing interest in understanding the machinery that produces the short-lived ROS signal and the sensors that help respond to this signal to repair the injured plasma membrane.
Protein oxidation
Similar to calcium, ROS act as an early signal alerting the cell of damage to the plasma membrane (Fig. 4). This may occur by the entry of extracellular oxidants, which has been proposed to cause the oxidation and subsequent oligomerization of the TRIM-family protein MG53, leading to its rapid localization at the site of injury [109]. MG53 is also capable of binding the negatively charged PS. PS binding in conjunction with oxidation-dependent oligomerization allows MG53 to aid in trafficking of dysferlin-containing vesicles to the plasma membrane for fusion (Fig. 4a) [109, 113]. However, exogenous MG53 exposure can also improve membrane repair in dysferlin-null mice [114]. Therefore, instead of oxidation-dependent vesicle fusion, oxidized MG53 may aid in little explored extracellular pathways for plasma membrane repair [115]. Thus, the mechanism of action of MG53 in membrane repair needs to be elucidated further.
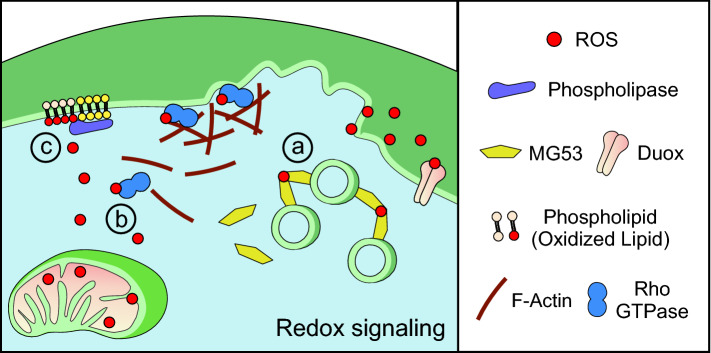
Fig. 4.
Redox signaling facilitates plasma membrane repair. Reactive oxygen species (ROS) present at the site of injury may be produced by mitochondria or the NADPH oxidase Duox. Redox signaling results in protein and lipid modification that initiates repair pathways in parallel with calcium-dependent mechanisms. (a) Vesicle aggregation: oxidation of the TRIM-family protein MG53 causes its oligomerization. This promotes vesicle aggregation near the site of injury and may promote repair by participating in clotting or membrane fusion. (b) Cytoskeletal rearrangement: redox regulation of Rho-family GTPases facilitates cytoskeletal rearrangement. Oxidation increases the activity of Rho GTPase, which mediates F-actin assembly at the site of injury. (c) Lipid modification: redox signaling provides additional diversity in the spatial and temporal patterning of lipids. In conjunction with calcium-dependent patterning, phospholipases interact with oxidized lipids to provide signaling platforms to activate downstream repair pathways
Independent of MG53, oxidation of signaling proteins regulates downstream mechanisms to facilitate plasma membrane repair. Oxidation of PKA increases its activity, which increases the response of SNAP-25 and NSF—components of SNARE-mediated vesicle fusion machinery [110]. This ROS-mediated PKA activation is independent of cAMP and is suggested to be due to direct oxidation of the regulatory subunits of the PKA holoenzyme [116]. In particular, the regulatory subunits of Type I PKA, but not Type II, are capable of forming intramolecular disulfide bonds in response to oxidation that causes dissociation and activation of the catalytic PKA subunits [116]. Regardless of the precise mechanism, low levels of oxidation as well as acute exposure to high oxidation promote the phosphorylation of downstream PKA targets [117]. In contrast, chronic exposure to oxidation abolishes PKA signaling. Similar to PKA, Rho GTPase signaling regulates repair by influencing its downstream targets. Oxidation of RhoA, which contains a redox-sensitive motif, increases its activity [118]. Redox-dependent activation of RhoA facilitates repair by mediating F-actin reorganization (Fig. 4b) [19]. Redox-regulation of Rho GTPases may also occur via different mechanisms. For example, ROS inhibition of Cdc42GAP in smooth muscle cells results in the indirect increase of Cdc42 [119]. The role of ROS in regulating protein kinase and Rho GTPase signaling suggests that redox signaling may facilitate repair in parallel with the conventional calcium-dependent signaling pathways.
Lipid oxidation
The role of ROS as a signaling molecule depends on its level as well as its spatial and temporal compartmentalization. Therefore, the location of ROS production after plasma membrane injury as well as the extent to which it is produced can have seemingly contradictory effects on the ability of cells to repair membrane damage. Of interest in this regard is the observation that plasma membrane-directed glutathione peroxidase 4 (Gpx4) aids in repair, suggesting that plasma membrane lipid peroxidation inhibits repair [120]. This observation is supported by the ability of vitamin E, a membrane localized antioxidant, to improve plasma membrane repair, while the cytosolic antioxidant N-acetyl cysteine (NAC) does not affect repair [112]. In contrast, phospholipases, which are required for injury-induced lipid patterning (described in the section below) are also regulated by ROS such that PLC preferentially cleaves peroxidized phospholipids, and acute treatment with hydrogen peroxide increases PLC-mediated lipid cleavage [121, 122]. After stretch induced injury in astrocytes, increased membrane oxidation was observed along with a concomitant increase in PLC activity and phosphatidylcholine (PC) biosynthesis [123]. This, and the fact that lipid peroxidation is reversible [124], suggests that the oxidation of membrane lipids may constitute a signaling process to direct the precise spatial patterning of lipid species, such as DAG, after injury (Fig. 4c). Oxidants near the site of injury may modify lipids, thus directing PLC to generate DAG adjacent to the injury site. PLC can also generate IP3, which is secreted after injury and can stimulate calcium release from intracellular stores, thus participating in intercellular membrane repair signaling [88]. This potentially ties ROS signaling back into calcium-dependent repair signaling. Other studies investigating more general, globally acting antioxidants, such as DTT and melatonin, conclude they are detrimental to repair [109, 110, 120]. Thus, involvement of lipid oxidation in membrane repair does not appear to be straightforward. Collectively, these studies suggest that the role of ROS in plasma membrane repair needs to take into consideration the magnitude, the spatial and temporal localization of ROS production, and the nature of the oxidizing species.
Calcium and redox crosstalk
Calcium and redox signaling pathways regulate plasma membrane repair by interacting with the repair machinery described above. However, calcium and ROS signaling also impact on one another at different levels, which affect how signals are generated and propagated in the cell [125]. Injury-triggered increase in intracellular calcium can induce ROS production by NADPH oxidases (Nox) and mitochondria. While the Nox isoform Duox plays an important role in tissue repair (discussed below), calcium-dependent mitochondrial ROS production facilitates plasma membrane repair by activation of RhoA, which mediates F-actin accumulation at the site of injury [19].
Similarly, ROS activation of plasma membrane and organellar calcium channels modulates calcium signaling by controlling calcium entry and release into cellular compartments. The activity of plasma membrane voltage-gated calcium channels (VGCC) is regulated by ROS, and calcium influx mediated by VGCCs has been implicated in both cell death as well as plasma membrane repair in neurons [126–128]. Blocking L-type calcium channels in muscle cells causes poor repair, possibly by preventing translocation of dysferlin to the injury site [78]. ROS can also modulate calcium entry and release from intracellular organelles such as ER, mitochondria, and lysosomes [125, 129]. Mitochondrial ROS activation of the lysosomal calcium channel MCOLN1 causes calcium release from lysosomes [130], and this may regulate lysosome exocytosis to facilitate repair. Such a crosstalk between mitochondria and lysosomes could allow for lysosomal exocytosis to continue beyond the initial calcium increase caused by plasma membrane injury. However, chronic elevation of ROS is detrimental to mitochondrial and lysosomal function [131, 132]. This highlights the delicate balance needed in calcium and ROS homeostasis during repair. Crosstalk between these signaling pathways increases the diversity possible in generating new signals and, thus, allows for the complex spatiotemporal organization of machinery that is necessary for plasma membrane repair.
Spatiotemporal organization of signaling elements and effectors
The focal nature of plasma membrane injury demands a localized response. Successful repair also depends on a series of sequential steps including stabilization of the damaged membrane, vesicle movement and fusion, and cytoskeletal reorganization. Therefore, cellular machinery must be targeted to the correct location and arrive at the appropriate time in order to enable plasma membrane repair. The calcium and redox signaling pathways described above control the activation of repair machinery. However, the organization of this machinery and how the precise spatial and temporal coordination is achieved in response to plasma membrane damage is critical to the ability of a cell to repair (Fig. 5).
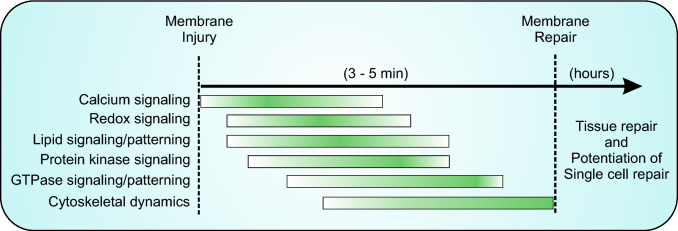
Fig. 5.
Timeline of events coordinating repair. Injured cells undergo a set of overlapping events that occur in a sequential manner to enable successful membrane repair. The membrane repair process includes events required to reseal the wounded membrane, as well as the cytoskeletal reassembly and other events required to return the cell membrane to its pre-injury state. The events involved in the repair response do not have a discreet start and stop time as they depend on the nature of injury and the cell type involved in the repair response. However, these steps proceed in a stereotypical order illustrated here, which ensures rapid (< 5 min) repair of plasma membrane injury. Similar stereotypical events can occur at a slower (minutes to hours) timescale in the injured and surrounding cells to potentiate repair from a subsequent membrane injury, as well as repair of damage to the tissue caused by single cell injury. The details of these events are discussed in the text
Lipid patterning
Lipids are the basic constituent of cellular membranes. Recent studies have identified that organization of plasma membrane lipids at the wound site changes in response to injury [27, 86]. Plasma membrane injury triggers lipid patterning that can occur rapidly (< 20 s) or slowly, and results in pattern formation by lipids at the wound site. These lipid movements form distinct and sometimes overlapping zones around the wound site. Lipid changes rely both on movement of specific lipids to the site of injury, and de novo generation of lipids. These re-organized lipids then facilitate temporal and spatial control of protein localization and function at the wound site (Fig. 3).
One of the lipids that reorganizes at the wound site is PS. PS is a negatively charged lipid with the ability to bind proteins such as annexins, dysferlin, and MG53, which are all involved in plasma membrane repair [133]). Following plasma membrane injury in Xenopus oocytes and zebrafish skeletal muscle, PS localizes directly adjacent to the wound site [86]. PS translocation in zebrafish muscle is guided by the arginine-rich PS binding region of dysferlin [133]. However, the details of how dysferlin itself translocates to the injury site and how it helps localize PS require further investigation. This model, in which PS binding proteins may regulate PS translocation, also invokes a potential role of annexin and MG53 localization in PS translocation. PS patterning at the wound edge helps annexins to localize and stabilize the wound edge and induce folding of the wounded membrane edge that aids in repair [52].
Beyond forming distinct spatial zones, lipid signaling also provides a temporal element of regulation. This is achieved by de novo synthesis of lipids in response to plasma membrane injury. Ceramide formation from sphingomyelin is an example of this. Ceramide is formed directly as a result of injury-triggered release of ASM due to dysferlin and synaptotagmin-mediated lysosome exocytosis (see discussion above) [32, 75]. Locally high concentrations of ASM catalyze local formation of ceramide from plasma membrane sphingomyelin [134]). Ceramide clusters may facilitate repair by inducing removal of the injured membrane by endocytosis or by ectocytic shedding [31, 135]. Ceramide-rich regions are also implicated in production of ROS, although a role for this has not been investigated in plasma membrane repair [136]. Interestingly, ceramide, along with phosphatidylcholine (PC), are the primary sources of injury-triggered diacylglycerol (DAG) synthesis, which is catalyzed by PLC [86, 137]. Rapid DAG patterning adjacent to the injury site gives it the ability to tightly control downstream effectors, such as PKC family members in a tightly controlled window of space and time [137]. DAG recruits and activates the conventional PKC (cPKC) β in addition to novel PKC (nPKC) η [137]. In response to plasma membrane damage, PKC β is recruited to the injury site by DAG, where it participates in activating the GTPases Rho and Cdc42, thus contributing to local actin assembly [138]. This lipid patterning is critical for repair with absence of DAG patterning after injury resulting in reduced Rho GTPase activation and failure to close membrane wounds [86]. Within this ring, PKC η localizes in a narrower band directly adjacent to the free plasma membrane edge and inhibits GTPase activity. Thus, mutually opposing activities of PKC family members, recruited by DAG, result in GTPase patterning (Fig. 3a, discussed below). How a single lipid species, DAG, could recruit PKC β and η to distinct and partially overlapping spatial compartments is unclear. It may in part be due to the activation of nPKCs (e.g. η) only requiring binding of DAG, while cPKCs (e.g. β) require both DAG and calcium binding [92]. Therefore, a combinatorial calcium and lipid signaling component may determine the spatiotemporal organization and activation of PKCs after injury. Another lipid—phosphatidylinositol 4,5-bisphosphate (PIP2)—localizes into a distinct ring 20-40 s after membrane injury in Xenopus oocytes and zebrafish skeletal muscle, albeit with slower kinetics [86, 133]. PIP2 distribution determines the location of actin assembly at the plasma membrane and is required for vesicle fusion in secretory cells [139]. Interestingly, Rho GTPases can also stimulate PIP2 formation by activating PI(4)P-5 kinase, which could help in F-actin accumulation at the plasma membrane [61].
GTPase patterning
As mentioned above, the Rho-family GTPases, Rho and Cdc42, enable plasma membrane repair through regulation of actin cytoskeleton dynamics following injury. Similar to lipids, Rho and Cdc42, as well as another Rho-family GTPase Rac1, show distinct pattern formation in Xenopus and Drosophila models [140]. Rho1 (RhoA-homologue referred to as Rho hereafter) forms a narrow concentric ring directly adjacent to the wound site. This ring of Rho is surrounded, and partially overlapped, by a more diffuse region of Cdc42 and Rac activity (Fig. 3a) [141]. It has been proposed that this spatial organization allows for cortical actin flow by Cdc42 and Rac activity while Rho facilitates branched actin assembly and constriction of the contractile array along with myosin II [142]. Therefore, it is clear that precise spatial localization is required for GTPases to regulate actin assembly during plasma membrane repair; however, it is not obvious how these proteins achieve this. A proposed treadmilling model suggests that GTPases are selectively activated at the leading edge of their respective zone and inactivated at the trailing edge [143]. Thus, GTPase activity flux controls the constriction of the contractile array responsible for wound closure. GTPase regulatory proteins, guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) have the ability to regulate spatiotemporal activity of GTPases [144], and regulation by these proteins has been proposed to provide the basis for GTPase flux during contractile array constriction [145].
In Xenopus a dual GEF/GAP protein called Abr can regulate Rho and Cdc42 activity in mutually opposing fashion [146]. The GEF function of Abr, capable of activating GTPases, selectively activates Rho, while the GAP function, which inhibits GTPases, selectively targets Cdc42. Furthermore, active Rho is required to cause Abr translocation to the site of injury. Thus, a positive feedback loop occurs between Rho and its regulator Abr, in which Rho recruits Abr, subsequently activating more Rho. Additionally, Abr GAP activity within the Rho-zone limits Cdc42. A conceptual issue facing this type of regulation is that such a positive feedback loop should cause the Rho-zone to expand and inhibit Cdc42. Thus, there must be additional regulators that either limit Rho or selectively activate the other GTPases. In Drosophila, GEF proteins, in addition to one GAP, act specifically on a particular GTPase potentially helping to explain how Cdc42 and Rac remain active [147]. Still the question of how GTPases localize, not just activate upon injury, is less clear. Interestingly, GTPases in Drosophila rely on binding stabilized actin filaments for pattern formation. This is interesting, as cortical actin network disassembly is required for repair [148]. Actin filaments accumulate later during repair at a time when Rho GTPases are already active. This conundrum may be due to technical limitation in detecting localized actin stabilization, which may be too transient to be identified by most live imaging studies, but sufficient to allow for Rho activation. Another possibility may be that activation relies upon accumulation of additional proteins, such as Annexin B9 [147]. Like other annexins, Annexin B9 in Drosophila accumulates rapidly (< 3 s) and is required to stabilize actin. Perhaps, Annexin B9-mediated actin stabilization near the injury site leads to GEF recruitment. It seems likely that other GAP proteins are associated with each GTPase to prevent the constitutive activity of a GTPase within its own zone, thus regulating the temporal aspect of signaling. For example, the relationship between GEFs and GAPs themselves can determine whether GTPase activity will occur as a single long-lasting signal or as multiple transient spikes [144]. A final level of regulation adding to the complexity of GTPase signaling is their role in regulating phospholipases. For example, the Rho-family GTPases Cdc42 and Rac1, but not Rho, can stimulate the activity of PLC-β2 [149]. This would not only connect aspects of GTPase and lipid patterning after injury, but also be a mechanism to more precisely define the distinct spatial zones separating Rho and its family members. Here it would be possible that GTPases contribute to their own localization by regulating the synthesis of lipid species such as DAG. In the context of plasma membrane repair, more work is needed to identify regulatory proteins as well as downstream effectors (PLCs and other effectors) that allow for the functional complexity to exist between Rho, Cdc42, and Rac.
Cytoskeletal organization
Patterning of lipids and GTPases plays an important role in regulating the location of actin assembly, but the temporal organization of actin as well as microtubules can also regulate the GTPases. Actin response to plasma membrane injury is biphasic, with initial depolymerization being followed by accumulation at the site of injury [148]. Cortical actin disassembly and reassembly in response to calcium increase occurs immediately after injury and is mediated by the formin INF2 [150]. Loss of cortical actin relieves membrane tension preventing the damaged free edges of the wound site from expanding outward and provides physical space to allow vesicles to fuse with the plasma membrane [14, 62]. In addition to INF2, F-actin accumulation at the membrane is organized by Rho GTPase signaling and occurs in parallel with vesicle trafficking and fusion events [148]. Thus, the F-actin network appears to aid in vesicle trafficking as well as provide structural support to the newly resealed membrane. Unlike actin, microtubule accumulation is not critical for the wound closure [19]. However, microtubule growth is required for vesicle and organelle trafficking that facilitates longer-term potentiation of the injured cell. In the minutes following membrane injury, PKC-dependent microtubule growth aids in replenishing vesicles at the cell periphery [95]. This action improves the ability of cells to repair from subsequent injuries. Furthermore, trafficking of organelles, such as mitochondria, along microtubules is required for the regeneration of neurons, which is a longer-term process following initial axonal repair [151, 152]. Localized proteolysis of sub-membrane spectrin by calpain allows microtubule-based vesicle trap formation that aids in the accumulation and fusion of anterograde transported vesicles at the spectrin free membrane. This process facilitates the formation of new growth cones in injured neurons [62].
Shared regulators of cellular and tissue-level repair
Wound repair is a process conserved across most of our tissues and is recognized for its medical significance [153]. While there are in-depth reviews of tissue level repair pathways [154, 155], single cell plasma membrane repair has often been viewed as an event unrelated to tissue repair. In recent years, the importance of plasma membrane repair in the pathophysiology of disease in many tissues has been appreciated [4, 156]. It is also clear that overlapping mechanisms exist between the repair processes at the single cell and tissue scale [157]. Importantly, signaling elements that identify damage and initiate repair mechanisms are conserved. A result of tissue injury is damage to individual cells, which initiates signaling cascades for cell and tissue level responses. Thus, tissue repair responses are built upon the signals and repair responses that regulate single cell repair. In support of this view, we will discuss the shared regulators of single cell and tissue repair.
Extracellular vesicle signaling
Small, diffusible molecules have several advantages as extracellular signals, including that they are readily generated and transmitted by the injured cells. However, these diffusible small molecules, proteins, and peptides are prone to rapid removal from circulation by renal clearance. This is avoided by the formation of extracellular vesicles, which can transfer a bolus of proteins, lipids, or other small signaling molecules discussed above to nearby cells or neighboring tissues [158]. This type of signaling is widely implicated in tissue repair [159].
Repair machinery used to reseal plasma membrane disruptions can participate in extracellular signaling by generating extracellular vesicles at the site of injury [39]. A role for ceramide-rich regions of plasma membrane, ESCRT-machinery, Rho GTPase signaling, and actin assembly in the formation of extracellular vesicles has been identified [160–162]. These regulators are all activated or are localized near the membrane and, therefore, are capable of participating in vesicle formation. In addition to facilitating the formation of extracellular vesicles, other regulators of plasma membrane repair are utilized as signals associated with these vesicles. Annexin A1, an early responder to plasma membrane injury, accumulates within damaged portions of the membrane that are shed into the extracellular space (Fig. 6a) [16]. Extracellular vesicles containing Annexin A1 promote epithelial wound repair, and leukocytes secrete Annexin A1-containing vesicles to resolve the inflammatory phase of wound repair [163, 164]. A related annexin—Annexin A2—has been implicated in muscle tissue inflammation caused by lack of the membrane repair protein dysferlin through its role in immune signaling (Fig. 6) [165]. Macrophages, leukocytes that mediate the inflammatory response, also respond to single cell plasma membrane disruption by recognizing exposed PS at the site of membrane damage [133]. This lipid patterning response is necessary for proper spatiotemporal regulation of the plasma membrane repair response, and recruitment of macrophages to the site of injury in this manner provides a mechanism for early initiation of tissue level repair. Communication between damaged cells and macrophages is not unidirectional. Macrophages also secrete extracellular vesicles to promote regeneration of injured neurons. In this case, NADPH oxidase 2 (Nox2) containing extracellular vesicles are taken up by injured neurons leading to ROS generation that regulates a pro-regenerative gene expression program [166]. These findings demonstrate that the signals and machinery collectively regulating plasma membrane repair also contribute to tissue level repair and regeneration when transmitted appropriately across the tissue. Thus, the tissue level repair response usurps the extracellular signals that are generated after injury and used for plasma membrane repair.
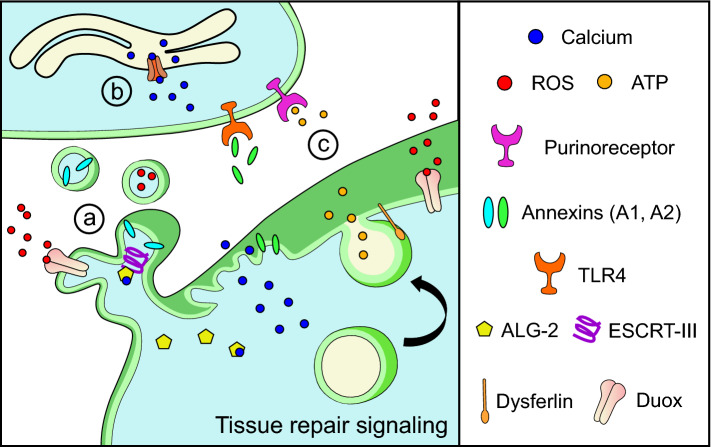
Fig. 6.
Signals released during plasma membrane repair initiate tissue repair (a) Extracellular vesicle signaling: membrane shedding after cell injury, due to cytoskeletal changes and ESCRT-III activation, generates extracellular vesicles containing molecules including Annexin A1 and ROS. These extracellular vesicles communicate with nearby cells to initiate tissue repair pathways. Release of cytosolic proteins (such as Annexin A2) from injured cells also initiates inflammatory signaling by interacting with cognate cell surface receptors (e.g. TLR4). (b) Intercellular calcium signaling: intracellular calcium increase initiates plasma membrane repair in injured cells. Calcium increase in neighboring, non-injured cells can be propagated by purinergic signaling and can improve future repair responses even in the absence of injury. (c) Purinergic signaling: cytosolic calcium increase stimulates the release of ATP by vesicle exocytosis or through plasma membrane channels. Binding of the released ATP to purinoreceptors on neighboring cells activates inflammatory and immune cells while also helping to propagate tissue-level calcium waves by triggering intracellular calcium increase
Small molecule signaling
Much of the focus on signaling during tissue repair is on the innate immune response such as those facilitated by the damaged-associated molecular patterns (DAMPs), which are molecules released by damaged cells. Typically, these molecules effect target cells by binding specific cell surface receptors to elicit longer-term responses through gene expression [167]. In contrast, injury-triggered signals that lack target specificity such as calcium, ROS, and ATP (also a classic DAMP) are rapidly generated and actively propagated to produce immediate responses that facilitate tissue repair. These signals, which as we described above can originate from damaged cells as well as neighboring undamaged cells, can also be derived from immune and other cells that infiltrate the injured areas of a tissue.
Calcium
Tissue scale calcium signaling is among the earliest damage signals, arising almost immediately after injury and spreading throughout the injured tissue in a wave-like manner similar to single cell injury [168, 169]. Notably, intercellular calcium waves appear to be dependent on intracellular calcium mobilization [170, 171]. Thus, intracellular calcium signaling is responsible for generating and propagating tissue scale calcium waves. The initial intracellular calcium increase likely occurs by direct extracellular calcium entry into physically damaged cells as in single cell injury, or via voltage-gated calcium or mechanosensory TRP channels located on non-injured cells nearby (Fig. 6b) [169, 172].
ATP
Widely recognized for its intracellular role as the energy currency of a cell, ATP is also a quintessential signaling molecule that enables cellular and tissue-level communication in our body by way of purinergic receptors (Fig. 6c) [173]. ATP can be produced by extracellular signals such as calcium and in turn help in propagation of calcium waves for minutes after the initial calcium rise. This may occur by the active release of calcium through plasma membrane calcium ATPases or through signaling by extracellular ligands including ATP [45, 170, 174]. An abundance of ectoapyrases limits the spatial range of extracellular signaling by ATP. However, purinergic signaling-dependent calcium release from intracellular stores can mirror the process of short-term potentiation of single cell repair (Fig. 6b). As a result of this, cytosolic calcium increase in injured or neighboring cells can cause subsequent ATP secretion via exocytosis or plasma membrane channels, thus resulting in a wave-like propagation of ATP signaling well beyond the immediate site of injury.
ROS
ROS signaling, particularly by hydrogen peroxide produced by NADPH oxidases or mitochondria, has been implicated in many aspects of wound repair and tissue regeneration [175–178]. Hydrogen peroxide generated from the NADPH oxidase Duox, residing in the plasma membrane of injured cells, has been shown to form tissue-scale gradients and recruit immune cells to the site of injury in zebrafish, Drosophila, and Xenopus tadpoles (Fig. 6) [168, 179, 180]. ROS signaling is delayed relative to calcium increase, but can extend across the tissue for hundreds of microns [179, 180]. Importantly, both calcium and ATP can activate ROS production by Duox, highlighting how these signaling molecules are interrelated [181], and Duox-generated ROS affects both short-term responses and long-term responses, such as inducing vimentin gene expression to control cell migration [182, 183]. In mammalian cells, treatment with exogenous ROS speeds up epithelial wound closure in vitro [184]. ROS produced within injured skeletal muscle fibers is also critical for tissue repair, as antioxidant treatment inhibits the later stages of muscle repair by affecting regeneration [178, 185]. Although more work is needed to define the precise pathways affected by calcium, ATP, and ROS during tissue repair, each has been implicated in the repair of multiple tissues across several species [155].
An unanswered question that links injury-triggered signaling in single cells and tissues is how a cell or nearby cells can distinguish between normal cell function and damage-induced signaling. Calcium, ATP, and ROS are universal second messengers and thus involved in many physiological and pathophysiological processes. However, they produce different outcomes to activate repair pathways. In both single cell and tissue repair, these and other signals may be distinguished based on intensity, spatiotemporal profile, or by the precise combination of the level of these signals after injury. Signaling by calcium, ATP and ROS all initiate at or close to a wound site and decrease further from the wound. Thus, gradients of these signals may inform nearby cells of their position relative to an injury site. Furthermore, because multiple signals exist simultaneously, but are generated and propagate with unique kinetics, cells surrounding a wound are exposed to a unique combination and intensity of signaling molecules depending on their location. For example, calcium waves are generated quickly and travel relatively large distances while ATP is limited to local signaling. ROS signaling arises slower but can travel hundreds of microns to reach distant immune cells. The variety of signals produced after injury ensures that nearby cells are exposed to a unique niche of signaling molecules, making it likely that tissue repair relies on the same injury-induced spatiotemporal patterning of signals reminiscent of this concept discussed above for the repair of single cell injury.
Conclusion
The number of signals and the multitude of signaling combinations that can be generated by spatial and temporal coding provide cells with a large variety of signals to tailor the repair process to the extent, nature, and location of the injury. Such a controlled response that involves coordinating a diversity of signals provides redundancy and robustness needed to fine tune the repair process to the magnitude and the nature of plasma membrane injury. These signals facilitate movement of proteins and lipids that enable subcellular events such as membrane fusion as well as membrane and cytoskeletal remodeling required for repairing plasma membrane injuries. However, plasma membrane repair does not stop with the closure of the wound. The process continues by way of longer term signaling, including extracellular signaling that primes successfully repaired as well as bystander cells, to repair more efficiently from future injuries. These signals also extend beyond single cell repair to tissue level repair. Here, signals derived from cells undergoing plasma membrane repair help initiate tissue-level wound repair responses. This demonstrates that single cell repair and tissue repair are related processes that are part of the same continuum, albeit occurring at different spatial and temporal scales. Overall, the continuity of wound repair responses from cells to tissues through overlapping and conserved signals shows that careful orchestration of a series of signals and resulting responses allows cells and tissues to repeatedly recover from injury throughout our lifespan. Defects in these repair processes are causative for a variety of diseases, in which dysregulated repair causes altered regeneration and tissue loss due to scarring and chronic inflammation. Understanding how cell and tissue repair proceeds efficiently is crucial to better understand these diseases and may aid in developing novel therapies to treat them.
Acknowledgements
A. H. performed this work as part of his doctoral studies at the Institute for Biomedical Sciences at the George Washington University, and this writing constitutes part of his Ph.D. dissertation. J. K. J. and A. H. acknowledge financial support by Grants from the National Institute of Arthritis and Musculoskeletal and Skin Disease (R01AR055686), National Institute of Child Health and Human Development (U54HD090257), and Clark Charitable Foundation. We thank our lab members for useful discussions and inputs during the course of writing and editing this work.
References
- 1.Lombard J. Once upon a time the cell membranes: 175 years of cell boundary research. Biol Direct. 2014;9(1):32. doi: 10.1186/s13062-014-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey EN. The tension at the surface of marine eggs, especially those of the sea urchin, Arbacia. Biol Bull. 1931;61(3):273–279. doi: 10.2307/1536947. [DOI] [Google Scholar]
- 3.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19(1):697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 4.Cooper ST, McNeil PL. Membrane repair: mechanisms and pathophysiology. Physiol Rev. 2015;95(4):1205–1240. doi: 10.1152/physrev.00037.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazek AD, Paleo BJ, Weisleder N. Plasma membrane repair: a central process for maintaining cellular homeostasis. Physiology. 2015;30(6):438–448. doi: 10.1152/physiol.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews N, Perez F (2015) The plasma membrane repair shop: fixing the damage. In: Andrews N, Perez F, Boizet-Bonhoure B (eds) Seminars in cell & developmental biology, vol 45. Elsevier, p 1 [DOI] [PubMed]
- 7.Heilbrunn L. The surface precipitation reaction of living cells. Proc Am Philos Soc. 1930;69(1):295–301. [Google Scholar]
- 8.Bouter A, Gounou C, Bérat R, Tan S, Gallois B, Granier T, d’Estaintot BL, Pöschl E, Brachvogel B, Brisson AR. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat Commun. 2011;2:270. doi: 10.1038/ncomms1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma N, Medikayala S, Defour A, Rayavarapu S, Brown KJ, Hathout Y, Jaiswal JK. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J Biol Chem. 2012;287(36):30455–30467. doi: 10.1074/jbc.M112.354415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eddleman CS, Bittner GD, Fishman HM. Barrier permeability at cut axonal ends progressively decreases until an ionic seal is formed. Biophys J. 2000;79(4):1883–1890. doi: 10.1016/S0006-3495(00)76438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demonbreun AR, Quattrocelli M, Barefield DY, Allen MV, Swanson KE, McNally EM. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J Cell Biol. 2016;213(6):705–718. doi: 10.1083/jcb.201512022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouter A, Carmeille R, Gounou C, Bouvet F, Degrelle S, Evain-Brion D, Brisson A. Annexin-A5 and cell membrane repair. Placenta. 2015;36:S43–S49. doi: 10.1016/j.placenta.2015.01.193. [DOI] [PubMed] [Google Scholar]
- 13.Papadimitriou J, Robertson T, Mitchell C, Grounds M. The process of new plasmalemma formation in focally injured skeletal muscle fibers. J Struct Biol. 1990;103(2):124–134. doi: 10.1016/1047-8477(90)90016-6. [DOI] [PubMed] [Google Scholar]
- 14.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114(19):3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 15.McNeil PL. Repairing a torn cell surface: make way, lysosomes to the rescue. J Cell Sci. 2002;115(5):873–879. doi: 10.1242/jcs.115.5.873. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, Kallunki T, Jäättelä M, Nylandsted J. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun. 2014;5:3795. doi: 10.1038/ncomms4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000;11(12):4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDade JR, Archambeau A, Michele DE. Rapid actin-cytoskeleton-dependent recruitment of plasma membrane-derived dysferlin at wounds is critical for muscle membrane repair. FASEB J. 2014;28(8):3660–3670. doi: 10.1096/fj.14-250191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn A, Van der Meulen JH, Defour A, Hogarth M, Sreetama SC, Reed A, Scheffer L, Chandel NS, Jaiswal JK. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci Signal. 2017 doi: 10.1126/scisignal.aaj1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bement WM, von Dassow G. Single cell pattern formation and transient cytoskeletal arrays. Curr Opin Cell Biol. 2014;26:51–59. doi: 10.1016/j.ceb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark AG, Miller AL, Vaughan E, Hoi-Ying EY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19(16):1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers R., Jr Microdissection studies: I. The visible structure of cell protoplasm and death changes. Am J Physiol Leg Content. 1917;43(1):1–12. doi: 10.1152/ajplegacy.1917.43.1.1. [DOI] [Google Scholar]
- 23.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle–vesicle fusion events. J Cell Biol. 1997;139(1):63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263(5145):390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 25.Bi G-Q, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131(6):1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6(6):499. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 27.Davenport NR, Sonnemann KJ, Eliceiri KW, Bement WM. Membrane dynamics during cellular wound repair. Mol Biol Cell. 2016;27(14):2272–2285. doi: 10.1091/mbc.e16-04-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159(4):625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137(1):93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106(2):157–169. doi: 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 31.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180(5):905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Defour A, Van der Meulen JH, Bhat R, Bigot A, Bashir R, Nagaraju K, Jaiswal JK. Dysferlin regulates cell membrane repair by facilitating injury-triggered acid sphingomyelinase secretion. Cell Death Dis. 2014;5(6):e1306. doi: 10.1038/cddis.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189(6):1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23(3):249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Thiery J, Keefe D, Saffarian S, Martinvalet D, Walch M, Boucrot E, Kirchhausen T, Lieberman J. Perforin activates clathrin- and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood. 2010;115(8):1582–1593. doi: 10.1182/blood-2009-10-246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyel PA, Loultcheva L, Roth R, Salter RD, Watkins SC, Yokoyama WM, Heuser JE. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J Cell Sci. 2011;124(14):2414–2423. doi: 10.1242/jcs.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero M, Keyel M, Shi G, Bhattacharjee P, Roth R, Heuser JE, Keyel PA. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 2017;24(5):798. doi: 10.1038/cdd.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science. 2014;343(6174):1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 39.Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, Jaiswal JK. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun. 2014;5:5646. doi: 10.1038/ncomms6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaiswal J. Calcium—how and why? J Biosci. 2001;26(3):357–363. doi: 10.1007/BF02703745. [DOI] [PubMed] [Google Scholar]
- 41.Schanne F, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 42.Heilbrunn L. The colloid chemistry of protoplasm: I. General considerations II. The electrical charges of protoplasm. Am J Physiol Leg Content. 1923;64(3):481–498. doi: 10.1152/ajplegacy.1923.64.3.481. [DOI] [Google Scholar]
- 43.De Mello W. Membrane sealing in frog skeletal-muscle fibers. Proc Natl Acad Sci. 1973;70(4):982–984. doi: 10.1073/pnas.70.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potez S, Luginbühl M, Monastyrskaya K, Hostettler A, Draeger A, Babiychuk EB. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J Biol Chem. 2011;286(20):17982–17991. doi: 10.1074/jbc.M110.187625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boucher E, Mandato CA. Plasma membrane and cytoskeleton dynamics during single-cell wound healing. Biochim Biophys Acta Mol Cell Res 1853. 2015;10:2649–2661. doi: 10.1016/j.bbamcr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Babiychuk E, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009;16(8):1126. doi: 10.1038/cdd.2009.30. [DOI] [PubMed] [Google Scholar]
- 47.Scolding N, Morgan B, Campbell A, Compston D. The role of calcium in rat oligodendrocyte injury and repair. Neurosci Lett. 1992;135(1):95–98. doi: 10.1016/0304-3940(92)90144-V. [DOI] [PubMed] [Google Scholar]
- 48.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82(2):331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 49.Boye TL, Nylandsted J. Annexins in plasma membrane repair. Biol Chem. 2016;397(10):961–969. doi: 10.1515/hsz-2016-0171. [DOI] [PubMed] [Google Scholar]
- 50.Draeger A, Schoenauer R, Atanassoff AP, Wolfmeier H, Babiychuk EB. Dealing with damage: plasma membrane repair mechanisms. Biochimie. 2014;107:66–72. doi: 10.1016/j.biochi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Roostalu U, Strähle U. In vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell. 2012;22(3):515–529. doi: 10.1016/j.devcel.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Boye TL, Maeda K, Pezeshkian W, Sønder SL, Haeger SC, Gerke V, Simonsen AC, Nylandsted J. Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nat Commun. 2017;8(1):1623. doi: 10.1038/s41467-017-01743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leikina E, Defour A, Melikov K, Van der Meulen JH, Nagaraju K, Bhuvanendran S, Gebert C, Pfeifer K, Chernomordik LV, Jaiswal JK. Annexin A1 deficiency does not affect myofiber repair but delays regeneration of injured muscles. Sci Rep. 2015;5:18246. doi: 10.1038/srep18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281(46):35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 55.Koerdt SN, Gerke V. Annexin A2 is involved in Ca2+-dependent plasma membrane repair in primary human endothelial cells. Biochim Biophys Acta Mol Cell Res 1864. 2017;6:1046–1053. doi: 10.1016/j.bbamcr.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282(4):2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 57.Xie X, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci. 1991;11(10):3257–3267. doi: 10.1523/JNEUROSCI.11-10-03257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howard M, David G, Barrett J. Resealing of transected myelinated mammalian axons in vivo: evidence for involvement of calpain. Neuroscience. 1999;93(2):807–815. doi: 10.1016/S0306-4522(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 59.Gitler D, Spira ME. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron. 1998;20(6):1123–1135. doi: 10.1016/S0896-6273(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 60.Redpath G, Woolger N, Piper A, Lemckert F, Lek A, Greer P, North K, Cooper S. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol Biol Cell. 2014;25(19):3037–3048. doi: 10.1091/mbc.e14-04-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendricks BK, Shi R. Mechanisms of neuronal membrane sealing following mechanical trauma. Neurosci Bull. 2014;30(4):627–644. doi: 10.1007/s12264-013-1446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamber D, Erez H, Spira ME. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol. 2009;219(1):112–125. doi: 10.1016/j.expneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Evans JS, Turner MD. Emerging functions of the calpain superfamily of cysteine proteases in neuroendocrine secretory pathways. J Neurochem. 2007;103(3):849–859. doi: 10.1111/j.1471-4159.2007.04815.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- 65.Padamsey Z, McGuinness L, Emptage NJ. Inhibition of lysosomal Ca2+ signalling disrupts dendritic spine structure and impairs wound healing in neurons. Commun Integr Biol. 2017;10(5–6):e1344802. doi: 10.1080/19420889.2017.1344802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng X, Zhang X, Gao Q, Samie MA, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med. 2014;20(10):1187. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18(4):404. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schöneberg J, Lee I-H, Iwasa JH, Hurley JH. Reverse-topology membrane scission by the ESCRT proteins. Nat Rev Mol Cell Biol. 2017;18(1):5. doi: 10.1038/nrm.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277(10):7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 70.Detrait E, Yoo S, Eddleman C, Fukuda M, Bittner G, Fishman H. Plasmalemmal repair of severed neurites of PC12 cells requires Ca2+ and synaptotagmin. J Neurosci Res. 2000;62(4):566–573. doi: 10.1002/1097-4547(20001115)62:4<566::AID-JNR11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 71.Yoo S, Nguyen MP, Fukuda M, Bittner GD, Fishman HM. Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca2+-regulated proteins. J Neurosci Res. 2003;74(4):541–551. doi: 10.1002/jnr.10771. [DOI] [PubMed] [Google Scholar]
- 72.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148(6):1141–1150. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caler EV, Chakrabarti S, Fowler KT, Rao S, Andrews NW. The exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J Exp Med. 2001;193(9):1097–1104. doi: 10.1084/jem.193.9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2(8):e233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sreetama S, Takano T, Nedergaard M, Simon S, Jaiswal J. Injured astrocytes are repaired by Synaptotagmin XI-regulated lysosome exocytosis. Cell Death Differ. 2016;23(4):596. doi: 10.1038/cdd.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baram D, Adachi R, Medalia O, Tuvim M, Dickey BF, Mekori YA, Sagi-Eisenberg R. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J Exp Med. 1999;189(10):1649–1658. doi: 10.1084/jem.189.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Encarnação M, Espada L, Escrevente C, Mateus D, Ramalho J, Michelet X, Santarino I, Hsu VW, Brenner MB, Barral DC. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J Cell Biol. 2016;213(6):631–640. doi: 10.1083/jcb.201511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders A-K, Turnbull L, Whitchurch CB, North KN, Cooper ST. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33(12):5085–5094. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bansal D, Miyake K, Vogel SS, Groh S, Chen C-C, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423(6936):168. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 80.Han W-Q, Xia M, Xu M, Boini KM, Ritter JK, Li N-J, Li P-L. Lysosome fusion to the cell membrane is mediated by the dysferlin C2A domain in coronary arterial endothelial cells. J Cell Sci. 2012;125(5):1225–1234. doi: 10.1242/jcs.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corrotte M, Almeida PE, Tam C, Castro-Gomes T, Fernandes MC, Millis BA, Cortez M, Miller H, Song W, Maugel TK. Caveolae internalization repairs wounded cells and muscle fibers. Elife. 2013;2:e00926. doi: 10.7554/eLife.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 83.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168(3):429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bement WM, Hoi-Ying EY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19(1):95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154(4):785–798. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaughan EM, You J-S, Yu H-YE, Lasek A, Vitale N, Hornberger TA, Bement WM. Lipid domain-dependent regulation of single-cell wound repair. Mol Biol Cell. 2014;25(12):1867–1876. doi: 10.1091/mbc.e14-03-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holmes WR, Liao L, Bement W, Edelstein-Keshet L. Modeling the roles of protein kinase Cβ and η in single-cell wound repair. Mol Biol Cell. 2015;26(22):4100–4108. doi: 10.1091/mbc.e15-06-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Floyd CL, Rzigalinski BA, Weber JT, Sitterding HA, Willoughby KA, Ellis EF. Traumatic injury of cultured astrocytes alters inositol (1, 4, 5)-trisphosphate-mediated signaling. Glia. 2001;33(1):12–23. doi: 10.1002/1098-1136(20010101)33:1<12::AID-GLIA1002>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 89.Arun SN, Xie D, Howard AC, Zhong Q, Zhong X, McNeil PL, Bollag WB. Cell wounding activates phospholipase D in primary mouse keratinocytes. J Lipid Res. 2013;54(3):581–591. doi: 10.1194/jlr.M027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bader M-F, Vitale N. Phospholipase D in calcium-regulated exocytosis: lessons from chromaffin cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2009;1791(9):936–941. doi: 10.1016/j.bbalip.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Frohman MA, Sung T-C, Morris AJ. Mammalian phospholipase D structure and regulation. Biochim Biophys Acta Mol Cell Biol Lipids. 1999;1439(2):175–186. doi: 10.1016/S1388-1981(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 92.Zuzek A, Fan JD, Spaeth CS, Bittner GD. Sealing of transected neurites of rat B104 cells requires a diacylglycerol PKC-dependent pathway and a PKA-dependent pathway. Cell Mol Neurobiol. 2013;33(1):31–46. doi: 10.1007/s10571-012-9868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Togo T. Cell membrane disruption stimulates cAMP and Ca2+ signaling to potentiate cell membrane resealing in neighboring cells. Biol Open. 2017;6(12):1814–1819. doi: 10.1242/bio.028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spaeth CS, Boydston EA, Figard LR, Zuzek A, Bittner GD. A model for sealing plasmalemmal damage in neurons and other eukaryotic cells. J Neurosci. 2010;30(47):15790–15800. doi: 10.1523/JNEUROSCI.4155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci. 2006;119(13):2780–2786. doi: 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]
- 96.Togo T. Long-term potentiation of wound-induced exocytosis and plasma membrane repair is dependant on cAMP-response element-mediated transcription via a protein kinase C- and p38 MAPK-dependent pathway. J Biol Chem. 2004;279(43):44996–45003. doi: 10.1074/jbc.M406327200. [DOI] [PubMed] [Google Scholar]
- 97.Togo T. Cell membrane disruption stimulates NO/PKG signaling and potentiates cell membrane repair in neighboring cells. PLoS One. 2012;7(8):e42885. doi: 10.1371/journal.pone.0042885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Covian-Nares JF, Koushik SV, Puhl HL, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 2010;123(11):1884–1893. doi: 10.1242/jcs.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74(5):1951–1960. doi: 10.1046/j.1471-4159.2000.0741951.x. [DOI] [PubMed] [Google Scholar]
- 100.Sivaramakrishnan V, Bidula S, Campwala H, Katikaneni D, Fountain SJ. Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes. J Cell Sci. 2012;125(19):4567–4575. doi: 10.1242/jcs.107318. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, X-s Gu, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9(8):945. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 102.Togo T. Short-term potentiation of membrane resealing in neighboring cells is mediated by purinergic signaling. Purinergic Signal. 2014;10(2):283–290. doi: 10.1007/s11302-013-9387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neary JT, Kang Y, Tran M, Feld J. Traumatic injury activates protein kinase B/Akt in cultured astrocytes: role of extracellular ATP and P2 purinergic receptors. J Neurotrauma. 2005;22(4):491–500. doi: 10.1089/neu.2005.22.491. [DOI] [PubMed] [Google Scholar]
- 104.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 105.Palmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, Chaudhury A, Bajaj L, Bondar VV, Bremner L. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun. 2017;8:14338. doi: 10.1038/ncomms14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J Mol Cell Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko J-K, Lin P, Thornton A, Zhao X. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11(1):56. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spaeth C, Fan J, Spaeth E, Robison T, Wilcott R, Bittner G. Neurite transection produces cytosolic oxidation, which enhances plasmalemmal repair. J Neurosci Res. 2012;90(5):945–954. doi: 10.1002/jnr.22823. [DOI] [PubMed] [Google Scholar]
- 111.Duan X, Chan KT, Lee KK, Mak AF. Oxidative stress and plasma membrane repair in single myoblasts after femtosecond laser photoporation. Ann Biomed Eng. 2015;43(11):2735–2744. doi: 10.1007/s10439-015-1341-4. [DOI] [PubMed] [Google Scholar]
- 112.Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nat Commun. 2011;2:597. doi: 10.1038/ncomms1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang M, J-k Ko, Weisleder N, Takeshima H, Ma J. Redox-dependent oligomerization through a leucine zipper motif is essential for MG53-mediated cell membrane repair. Am J Physiol Cell Physiol. 2011;301(1):C106–C114. doi: 10.1152/ajpcell.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gushchina LV, Bhattacharya S, McElhanon KE, Choi JH, Manring H, Beck EX, Alloush J, Weisleder N. Treatment with recombinant human MG53 protein increases membrane integrity in a mouse model of limb girdle muscular dystrophy 2B. Mol Ther. 2017;25(10):2360–2371. doi: 10.1016/j.ymthe.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andrews NW, Corrotte M, Castro-Gomes T. Above the fray: surface remodeling by secreted lysosomal enzymes leads to endocytosis-mediated plasma membrane repair. Semin Cell Dev Biol. 2015;45:10–17. doi: 10.1016/j.semcdb.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, Begum S, Kentish JC, Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281(31):21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 117.Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling KINASE VERSUS PHOSPHATASE INACTIVATION. J Biol Chem. 2007;282(30):22072–22079. doi: 10.1074/jbc.M702582200. [DOI] [PubMed] [Google Scholar]
- 118.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4(11):e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Q-F, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol. 2009;297(2):C299–C309. doi: 10.1152/ajpcell.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Labazi M, McNeil AK, Kurtz T, Lee TC, Pegg RB, Angeli JPF, Conrad M, McNeil PL. The antioxidant requirement for plasma membrane repair in skeletal muscle. Free Radic Biol Med. 2015;84:246–253. doi: 10.1016/j.freeradbiomed.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Angelova PR, Abramov AY. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 122.Domijan A-M, Kovac S, Abramov AY. Lipid peroxidation is essential for phospholipase C activity and the inositol-trisphosphate-related Ca2+ signal. J Cell Sci. 2014;127(1):21–26. doi: 10.1242/jcs.138370. [DOI] [PubMed] [Google Scholar]
- 123.Lamb RG, Harper CC, McKinney JS, Rzigalinski BA, Ellis EF. Alterations in phosphatidylcholine metabolism of stretch-injured cultured rat astrocytes. J Neurochem. 1997;68(5):1904–1910. doi: 10.1046/j.1471-4159.1997.68051904.x. [DOI] [PubMed] [Google Scholar]
- 124.Fisher AB, Vasquez-Medina JP, Dodia C, Sorokina EM, Tao J-Q, Feinstein SI. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018;14:41–46. doi: 10.1016/j.redox.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gurkoff G, Shahlaie K, Lyeth B, Berman R. Voltage-gated calcium channel antagonists and traumatic brain injury. Pharmaceuticals. 2013;6(7):788–812. doi: 10.3390/ph6070788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium. 2011;50(5):407–423. doi: 10.1016/j.ceca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 128.Nehrt A, Rodgers R, Shapiro S, Borgens R, Shi R. The critical role of voltage-dependent calcium channel in axonal repair following mechanical trauma. Neuroscience. 2007;146(4):1504–1512. doi: 10.1016/j.neuroscience.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong Z, Shanmughapriya S, Tomar D, Siddiqui N, Lynch S, Nemani N, Breves SL, Zhang X, Tripathi A, Palaniappan P. Mitochondrial Ca2+ uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell. 2017;65(6):1014–1028. doi: 10.1016/j.molcel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Demers-Lamarche J, Guillebaud G, Tlili M, Todkar K, Bélanger N, Grondin M, Nguyen AP, Michel J, Germain M. Loss of mitochondrial function impairs lysosomes. J Biol Chem. 2016;291(19):10263–10276. doi: 10.1074/jbc.M115.695825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coblentz J, Croix CS, Kiselyov K. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem J. 2014;457(2):361–368. doi: 10.1042/BJ20130647. [DOI] [PubMed] [Google Scholar]
- 133.Middel V, Zhou L, Takamiya M, Beil T, Shahid M, Roostalu U, Grabher C, Rastegar S, Reischl M, Nienhaus GU. Dysferlin-mediated phosphatidylserine sorting engages macrophages in sarcolemma repair. Nat Commun. 2016;7:12875. doi: 10.1038/ncomms12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Castro-Gomes T, Corrotte M, Tam C, Andrews NW. Plasma membrane repair is regulated extracellularly by proteases released from lysosomes. PLoS One. 2016;11(3):e0152583. doi: 10.1371/journal.pone.0152583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28(8):1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X, Becker KA, Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem. 2010;26(1):41–48. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- 137.Asaoka Y, S-i Nakamura, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17(10):414–417. doi: 10.1016/0968-0004(92)90011-W. [DOI] [PubMed] [Google Scholar]
- 138.Hoi-Ying EY, Bement WM. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat Cell Biol. 2007;9(2):149. doi: 10.1038/ncb1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tran DT, Masedunskas A, Weigert R, Ten Hagen KG. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat Commun. 2015;6:10098. doi: 10.1038/ncomms10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verboon JM, Parkhurst SM. Rho family GTPases bring a familiar ring to cell wound repair. Small GTPases. 2015;6(1):1–7. doi: 10.4161/21541248.2014.992262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Simon CM, Vaughan EM, Bement WM, Edelstein-Keshet L. Pattern formation of Rho GTPases in single cell wound healing. Mol Biol Cell. 2013;24(3):421–432. doi: 10.1091/mbc.e12-08-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol. 2014;24(2):144–155. doi: 10.1016/j.cub.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burkel BM, Benink HA, Vaughan EM, von Dassow G, Bement WM. A Rho GTPase signal treadmill backs a contractile array. Dev Cell. 2012;23(2):384–396. doi: 10.1016/j.devcel.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duman JG, Mulherkar S, Tu Y-K, Cheng JX, Tolias KF. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci Lett. 2015;601:4–10. doi: 10.1016/j.neulet.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. BioEssays. 2006;28(10):983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vaughan EM, Miller AL, Hoi-Ying EY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol. 2011;21(4):270–277. doi: 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nakamura M, Verboon JM, Parkhurst SM. Prepatterning by RhoGEFs governs Rho GTPase spatiotemporal dynamics during wound repair. J Cell Biol. 2017;216:3959–3969. doi: 10.1083/jcb.201704145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Godin LM, Vergen J, Prakash Y, Pagano RE, Hubmayr RD. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol Lung Cell Mol Physiol. 2011;300(4):L615–L623. doi: 10.1152/ajplung.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-β2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 1998;17(21):6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wales P, Schuberth CE, Aufschnaiter R, Fels J, García-Aguilar I, Janning A, Dlugos CP, Schäfer-Herte M, Klingner C, Wälte M. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. Elife. 2016;5:e19850. doi: 10.7554/eLife.19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou B, Yu P, Lin M-Y, Sun T, Chen Y, Sheng Z-H. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han SM, Baig HS, Hammarlund M. Mitochondria localize to injured axons to support regeneration. Neuron. 2016;92(6):1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 154.Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14(4):249. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- 155.Enyedi B, Niethammer P. Mechanisms of epithelial wound detection. Trends Cell Biol. 2015;25(7):398–407. doi: 10.1016/j.tcb.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cong X, Hubmayr RD, Li C, Zhao X. Plasma membrane wounding and repair in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L371–L391. doi: 10.1152/ajplung.00486.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sonnemann KJ, Bement WM. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu Rev Cell Dev Biol. 2011;27:237–263. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 159.Than UTT, Guanzon D, Leavesley D, Parker T. Association of extracellular membrane vesicles with cutaneous wound healing. Int J Mol Sci. 2017;18(5):956. doi: 10.3390/ijms18050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34(19):2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 162.Jaiswal JK, Nylandsted J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle. 2015;14(4):502–509. doi: 10.1080/15384101.2014.995495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dalli J, Norling LV, Renshaw D, Cooper D, Leung K-Y, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112(6):2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 164.Leoni G, Neumann P-A, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G. Annexin A1—containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Investig. 2015;125(3):1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Defour A, Medikayala S, Van der Meulen JH, Hogarth MW, Holdreith N, Malatras A, Duddy W, Boehler J, Nagaraju K, Jaiswal JK. Annexin A2 links poor myofiber repair with inflammation and adipogenic replacement of the injured muscle. Hum Mol Genet. 2017;26(11):1979–1991. doi: 10.1093/hmg/ddx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB-T, Santos CX. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol. 2018;20(3):307–319. doi: 10.1038/s41556-018-0039-x. [DOI] [PubMed] [Google Scholar]
- 167.Niethammer P. The early wound signals. Curr Opin Genet Dev. 2016;40:17–22. doi: 10.1016/j.gde.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23(5):424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shannon EK, Stevens A, Edrington W, Zhao Y, Jayasinghe AK, Page-McCaw A, Hutson MS. Multiple mechanisms drive calcium signal dynamics around laser-induced epithelial wounds. Biophys J. 2017;113(7):1623–1635. doi: 10.1016/j.bpj.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aihara E, Hentz CL, Korman AM, Perry NP, Prasad V, Shull GE, Montrose MH. In vivo epithelial wound repair requires mobilization of endogenous intracellular and extracellular calcium. J Biol Chem. 2013;288(47):33585–33597. doi: 10.1074/jbc.M113.488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Restrepo S, Basler K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP 3 R-mediated intercellular calcium waves. Nat Commun. 2016;7:12450. doi: 10.1038/ncomms12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu S, Chisholm AD. A Gα q-Ca2+ signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol. 2011;21(23):1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Khakh BS, Burnstock G. The double life of ATP. Sci Am. 2009;301(6):84–92. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boucher I, Rich C, Lee A, Marcincin M, Trinkaus-Randall V. The P2Y2 receptor mediates the epithelial injury response and cell migration. Am J Physiol Cell Physiol. 2010;299(2):C411–C421. doi: 10.1152/ajpcell.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta Gen Subj. 2008;1780(11):1348–1361. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 177.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, Mounier R. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27(5):276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, Gallop JL, Dorey K, Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013;15(2):222. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Oliveira S, López-Muñoz A, Candel S, Pelegrín P, Calado Â, Mulero V. ATP modulates acute inflammation in vivo through dual oxidase 1-derived H2O2 production and NF-κB activation. J Immunol. 2014;192(12):5710–5719. doi: 10.4049/jimmunol.1302902. [DOI] [PubMed] [Google Scholar]
- 182.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.LeBert D, Squirrell JM, Freisinger C, Rindy J, Golenberg N, Frecentese G, Gibson A, Eliceiri KW, Huttenlocher A. Damage-induced reactive oxygen species regulate vimentin and dynamic collagen-based projections to mediate wound repair. Elife. 2018;7:e30703. doi: 10.7554/eLife.30703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Loo AEK, Halliwell B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem Biophys Res Commun. 2012;423(2):253–258. doi: 10.1016/j.bbrc.2012.05.100. [DOI] [PubMed] [Google Scholar]
- 185.Vezzoli M, Castellani P, Corna G, Castiglioni A, Bosurgi L, Monno A, Brunelli S, Manfredi AA, Rubartelli A, Rovere-Querini P. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid Redox Signal. 2011;15(8):2161–2174. doi: 10.1089/ars.2010.3341. [DOI] [PubMed] [Google Scholar]