Abstract
Mitophagy (mitochondrial autophagy) in hepatocytes is an essential quality control mechanism that removes for lysosomal digestion damaged, effete and superfluous mitochondria. Mitophagy has distinct variants. In type 1 mitophagy, typical of nutrient deprivation, cup-shaped sequestration membranes (phagophores) grow, surround and sequester individual mitochondria into mitophagosomes, often in coordination with mitochondrial fission. After sequestration, the outer compartment of the mitophagosome acidifies and the entrapped mitochondrion depolarizes, followed by fusion with lysosomes. By contrast, mitochondrial depolarization stimulates type 2 mitophagy, which is characterized by coalescence of autophagic microtubule-associated protein 1A/1B-light chain 3 (LC3)-containing structures on mitochondrial surfaces without the formation of a phagophore or mitochondrial fission. Oppositely to type 1 mitophagy, the inhibition of phosphoinositide-3-kinase (PI3K) does not block type 2 mitophagy. In type 3 mitophagy, or micromitophagy, mitochondria-derived vesicles (MDVs) enriched in oxidized proteins bud off from mitochondrial inner and outer membranes and incorporate into multivesicular bodies by vesicle scission into the lumen. In response to ethanol feeding, widespread ethanol-induced hepatocellular mitochondrial depolarization occurs to facilitate hepatic ethanol metabolism. As a consequence, type 2 mitophagy develops in response to the mitochondrial depolarization. After chronic high ethanol feeding, processing of depolarized mitochondria by mitophagy becomes compromised, leading to release of mitochondrial damage-associated molecular patterns (mtDAMPs) that promote inflammatory and profibrogenic responses. We propose that the persistence of mitochondrial responses for acute ethanol metabolism links initial adaptive ethanol metabolism to mitophagy and then to chronic maladaptive changes initiating onset and the progression of alcoholic liver disease (ALD).
Keywords: Mitophagy, Alcoholic liver disease (ALD), Ethanol, Green fluorescent protein-light chain 3 (GFP-LC3), Hepatocytes, Mitochondrial damage-associated molecular patterns (mtDAMPs)
1. Introduction
Mitochondrial autophagy, or mitophagy, is the process of autophagic sequestration and lysosomal degradation of mitochondria that occurs in response to nutrient deprivation, mitochondrial injury and the need for cytoplasmic remodeling as bioenergetic demands change.1–3 Timely and well-orchestrated removal of damaged, effete and superfluous mitochondria by mitophagy is crucial for cellular homeostasis and function. Inadequate or disordered mitophagy promotes oxidative stress, cell injury, cytolethality and the pathogenesis of many diseases.4–7 By contrast, overactive mitophagy can produce mitochondrial depletion and bioenergetic deficits, as described for the hepatotoxicity of cadmium.8
2. Type 1 mitophagy
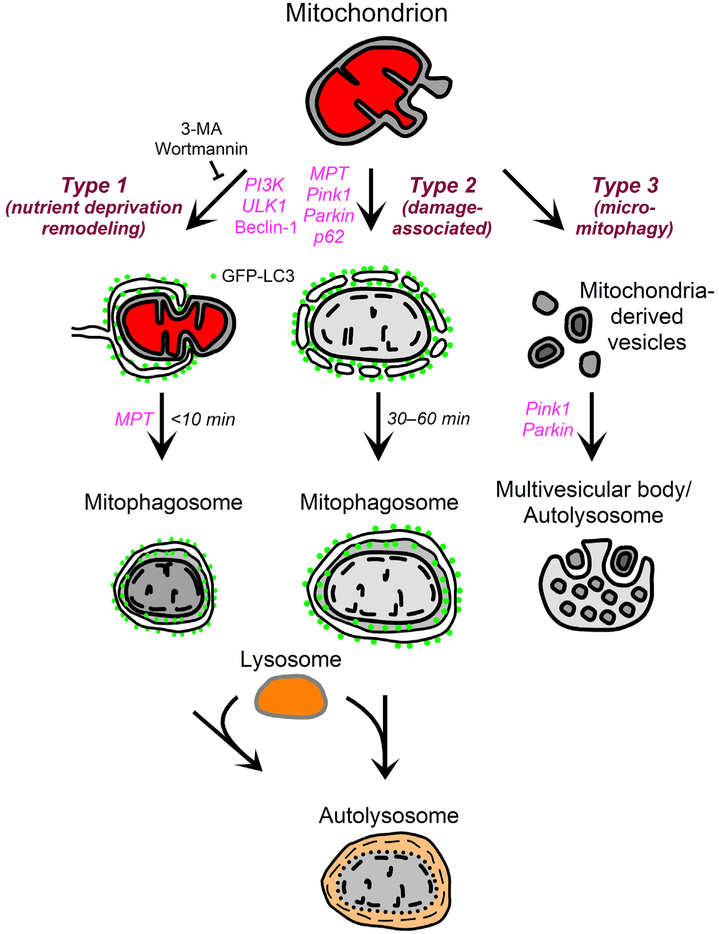
Different signaling pathways initiate mitochondrial autophagy in hepatocytes, which led to the proposal for the existence of three major types of mitophagy (Fig. 1).2,3 In type 1 mitophagy, Vps34 (Class III phosphoinositide-3-kinase (PI3K)), beclin-1, ULK1 (unc-51 like autophagy activating kinase 1) and other proteins initiate the formation of cup-shaped sequestration membranes (phagophores) that recruit microtubule-associated protein 1A/1B-light chain 3 (LC3), the mammalian ortholog of the yeast autophagy-associated protein 8 (Atg8). Phagophore formation involves growth of small LC3-containing preautophagic structures (PAS) already in association with mitochondria (Fig. 2). Growing phagophores wrap around individual mitochondria to fuse and form mitophagosomes, frequently in coordination with mitochondrial fission (Fig. 2).9 Type 1 mitophagy is characteristic of nutrient deprivation and cytoplasmic remodeling, and PI3K inhibitors like wortmannin and 3-methyladenine (3-MA) completely block type 1 mitophagy, although PAS in association with mitochondria persist (Fig. 2, arrows, lower right panel).9–11 Also in type 1 mitophagy, mitochondria remain polarized until after their sequestration into mitophagosomes. After sequestration is completed, the outer compartment of mitophagosomes (space between the inner and outer autophagosomal membranes) acidifies, and mitochondrial depolarization occurs.2 Ultimately, mitophagosomes containing depolarized mitochondria fuse with lysosomes (or late endosomes) to form autolysosomes in which hydrolytic digestion of mitochondria takes place. The entire process from initial phagophore formation to lysosomal degradation of target mitochondria occurs in as little as 15 min. In general, type 1 mitophagy degrades functional mitochondria during nutrient deprivation in order to recycle metabolic precursors or culls mitochondria that are in excess of metabolic needs.2,3,9–11
Fig. 1. Scheme of mitophagy types.
See text for details. Abbreviations: GFP-LC3, green fluorescent protein-microtubule-associated protein 1A/1B-light chain 3; 3-MA, 3-methyladenine; MPT, mitochondrial permeability transition; PI3K, phosphoinositide-3-kinase; Pink1, PTEN-induced putative kinase 1; ULK1, unc-51 like autophagy activating kinase 1.
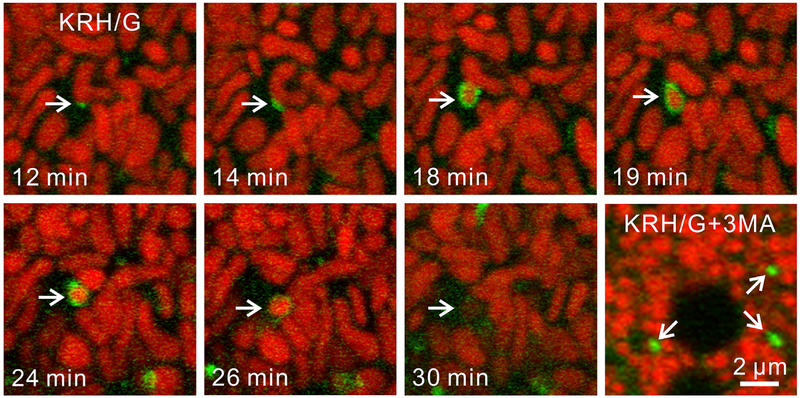
Fig. 2. Type 1 mitophagic sequestration of a polarized mitochondrion during nutrient deprivation.
GFP-LC3 transgenic hepatocytes were labeled with ΔΨ-indicating TMRM. Panels labeled 12 through 30 min represent a single experiment. After the incubation of hepatocytes in nutrient deficient Krebs-Ringer-HEPES medium containing 1 μM glucagon (KRH/G), a PAS associates with a TMRM-labeled mitochondrion (12 min) and grows into a phagophore that surrounds and sequesters a portion of the target organelle (arrows, 14–19 min). Note coordinate mitochondrial fission (18–19 min) and that mitochondrial depolarization (loss of TMRM fluorescence) does not occur until after sequestration (30 min). In a separate experiment under the same conditions but in the presence of the PI3K inhibitor 3-methyladenine (3 MA) that blocks type 1 mitophagy, PAS persist in apparent association with mitochondria (arrows, lower right panel). Adapted from references 9,13. Abbreviations: GFP-LC3, green fluorescent protein-microtubule-associated protein 1A/1B-light chain 3; ΔΨ, membrane potential; TMRM, tetramethylrhodamine methyester; PAS, preautophagic structure; PI3K, phosphoinositide-3-kinase.
3. Type 2 mitophagy
In type 2 mitophagy, mitochondrial damage and depolarization precede and initiate autophagic sequestration (Fig. 1). In normally polarized mitochondria, PTEN-induced putative kinase 1 (Pink1) binds to mitochondria but then is proteolytically degraded in a membrane potential (ΔΨ)-dependent fashion.4,12 After mitochondrial depolarization, Pink1 fails to degrade and instead accumulates on mitochondria to promote Parkin binding to mitochondria. Parkin is an E3 ligase, and the ubiquitination of mitochondrial proteins leads to recruitment of autophagy receptor proteins like p62/sequestosome-1 (SQSTM-1), followed by the association of LC3-containing membranes that form an autophagosome enveloping the depolarized mitochondrion. By contrast to type 1 mitophagy of polarized mitochondria, PI3K inhibitors fail to block type 2 mitophagy.13 Moreover, growth of cup-shaped phagophores around target mitochondria with coordinate mitochondrial fission is absent in type 2 mitophagy. A variety of other autophagy receptors, including BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (Bnip3), Nix, optineurin and double FYVE-containing protein 1 (DFCP1), also associate with depolarized mitochondria to promote LC3 binding and autophagic sequestration.14–17
4. Micromitophagy (type 3 mitophagy)
In a third type of mitophagy, vesicles bud off from mitochondria that originate from both the inner and outer membranes and contain oxidized proteins (Fig. 1).18–20 These mitochondria-derived vesicles (MDVs) then transit to and are internalized into multivesicular bodies, a type of secondary lysosome. Topologically, internalization of MDVs followed by vesicle scission into the lumen of multivesicular bodies is a form of microautophagy, as described in yeast for internalization of vesicles into the digestive vacuole.21 Such type 3 mitophagy, or micromitophagy, is Pink1/Parkin-dependent, confirming the link to autophagy.22
5. Mitochondrial depolarization in adaptive ethanol metabolism
Ethanol undergoes a two-step detoxifying oxidation first to acetaldehyde (AcAld) and then to acetate, a process occurring chiefly in the liver. Alcohol dehydrogenase (ADH) and to a lesser extent cytochrome P450 2E1 (CYP2E1) and catalase catalyze the first oxidation step, which converts ethanol to AcAld. Aldehyde dehydrogenase-2 (ALDH2) in the mitochondrial matrix further oxidizes AcAld to acetate.23–25 Together, ADH and ALDH2 form two mol of nicotinamide adenine dinucleotide (reduced) (NADH) for each mol of ethanol oxidized to acetate. For ethanol metabolism to continue, this NADH must be re-oxidized to NAD+ by the mitochondrial respiratory chain. Indeed, after acute ethanol ingestion, hepatic ethanol metabolism and oxygen consumption nearly double within 2–3 h. This swift increase in alcohol metabolism (SIAM) acts to metabolize and thereby detoxify ethanol and especially its more toxic metabolite, AcAld, more quickly.24,26
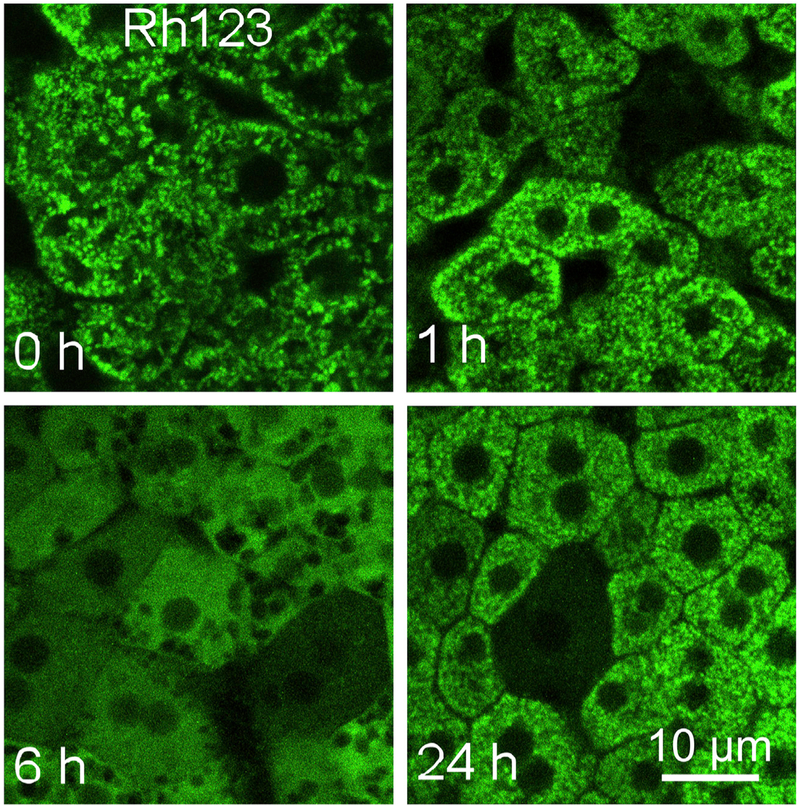
The driver of increased hepatic oxygen consumption during SIAM is widespread depolarization of hepatocellular mitochondria, as determined by intravital multiphoton microscopy of ΔΨ-indicating cationic fluorophores like rhodamine 123 and tetramethylrhodamine methyester (TMRM).27 Ethanol-induced mitochondrial depolarization occurs in an all-or-nothing fashion within individual hepatocytes. In a dose- and time-dependent manner, mitochondria in up 85%–90% of hepatocytes depolarize after single high dose intragastric ethanol feeding (5–6 g/kg body weight) (Fig. 3). After 1 g/kg ethanol, a dose producing a blood alcohol matching the legal limit for the operation of motor vehicles, depolarization occurs in 10%–15% of hepatocytes. Associated with mitochondrial depolarization is a sharp decrease of NAD(P)H autofluorescence assessed by intravital microscopy, indicating oxidation of NAD(P)H to nonfluorescent NAD(P)+.27 Together, mitochondrial NAD(P)H oxidation, depolarization and increased respiration (SIAM) signify mitochondrial uncoupling as an adaptive response to promote NAD+ regeneration in support of ADH and ALDH2-dependent alcohol metabolism. In corroboration of an uncoupling mechanism, hepatic ATP decreases ~60% after high dose ethanol.27 Ethanol-induced mitochondrial depolarization then reverses after peaking after ~6 h as ethanol is metabolically eliminated. Several mechanisms for ethanol-induced mitochondrial depolarization are possible, such as futile cycling of H+, Ca2+, K+ or other ion, but the specific mechanism remains to be elucidated. Although mitochondrial dysfunction can and frequently does cause lethal hepatocellular injury,28 liver necrosis and apoptosis as assessed by release of lactate dehydrogenase (LDH), histology and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining are minor compared to the widespread mitochondrial depolarization after single high dose ethanol feeding.27
Fig. 3. Hepatic mitochondrial depolarization during adaptive ethanol metabolism.
Mice were gavaged with 6 g/kg ethanol, and mitochondrial depolarization was visualized by intravital multiphoton microscopy of rhodamine 123 (Rh123, all panels) at 0–24 h after treatment. Adapted from reference 27.
6. Closure of voltage dependent anion channels (VDAC)
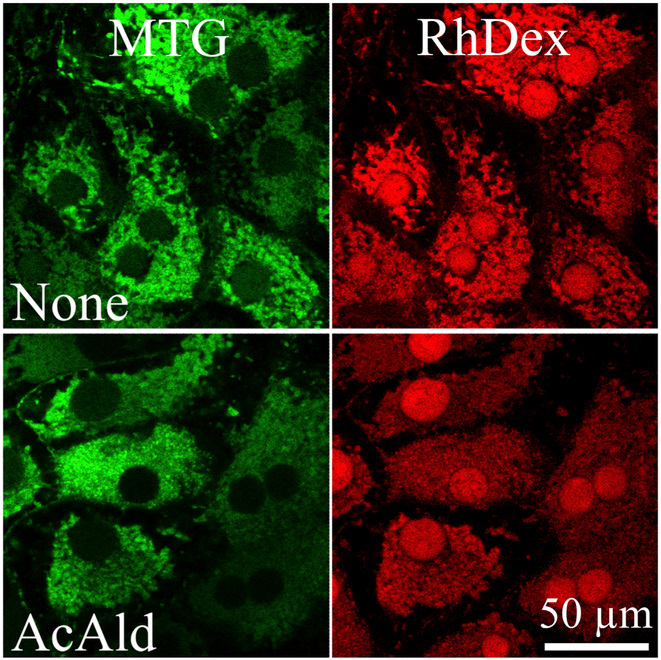
VDAC is a highly conserved 30 kDa mitochondrial protein that forms a ~2.5 nm aqueous pore in the outer membrane, which in the open state allows passage of solutes up to 5 kDa in size.29 In cultured hepatocytes, treatment with ethanol and AcAld inhibits VDAC conductance, as shown by decreased outer membrane permeability to adenine nucleotides and low molecular weight rhodamine-dextran (RhDex) (Fig. 4) and by suppression of ureagenesis, a process requiring extensive passage of metabolites through VDAC.30–33 This VDAC closure is sufficient to suppress mitochondrial entry and egress of anionic metabolites, including respiratory substrates, acyl-CoA, ATP, ADP and Pi. Since membrane-permeant AcAld does not require VDAC to enter mitochondria, VDAC closure and respiratory stimulation from mitochondrial depolarization are adaptive responses that together promote selective and more rapid hepatic ethanol and AcAld oxidation.32 Both VDAC closure in cultured hepatocytes and hepatic mitochondrial depolarization in vivo after ethanol are a response to AcAld formation, since ADH inhibition or genetic deficiency decreases VDAC closure and depolarization, whereas ALDH2 inhibition does the opposite.27,31 CYP2E1 inhibition and genetic deficiency as well as ALDH2 activation with ALDA1 also decrease ethanol-induced mitochondrial depolarization.27
Fig. 4. Decreased outer membrane permeability to 3 kDa RhDex after ethanol exposure.
Rat hepatocytes were permeabilized with digitonin and then incubated with 3-kDa RhDex (right panels) to allow the red-fluorescing marker to enter the mitochondrial intermembrane space through VDAC. 4,4-Diisothiocyanatostilbene-2,2-disulfonic acid, a VDAC inhibitor, was then added to entrap RhDex in the intermembrane space after subsequent RhDex washout. Co-loaded MitoTracker Green (MTG, left panels) marked the mitochondrial matrix space. Note how acetaldehyde (AcAld, 500 μM) pretreatment prevented RhDex uptake. Adapted from reference 31. Abbreviations: RhDex, rhodamine-dextran; VDAC, voltage dependent anion channels.
7. Steatosis and adaptive ethanol metabolism
Mitochondrial depolarization and VDAC closure during adaptive ethanol metabolism likely underlie acute steatosis after ethanol feeding, since VDAC closure blocks mitochondrial entry of fatty acyl-CoA for β-oxidation and since fat droplets in vivo develop predominantly in hepatocytes with depolarized mitochondria.27 VDAC closure also likely prevents futile ATP consumption by the F1FO-ATP synthase working in reverse in uncoupled mitochondria. Instead, glycolysis maintains hepatic ATP at about half normal levels even in the absence of ATP formation by oxidative phosphorylation.
8. Mitophagy after ethanol
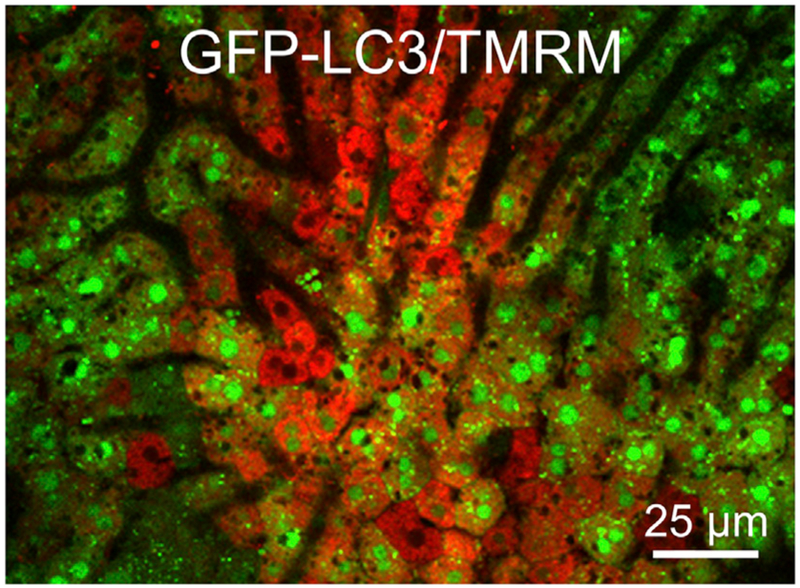
Acute ethanol treatment of rodents markedly stimulates mitophagy, and this autophagy decreases acute ethanol-induced hepatotoxicity and steatosis in a Parkin-dependent fashion.34,35 Since mitochondrial depolarization initiates type 2 mitophagy, mitochondrial depolarization likely triggers hepatocellular mitophagy after ethanol.2,9,36 In confirmation, intravital imaging experiments in ethanol-treated green fluorescent protein (GFP)-LC3 transgenic mice show that GFP-LC3 autophagic puncta arise predominantly in hepatocytes with depolarized mitochondria, consistent with the conclusion that ethanol-induced depolarization is initiating mitophagy (Fig. 5).37,38 PINK1 protein expression increases after chronic ethanol, indicating the stabilization of PINK1 on depolarized mitochondria, whereas blunting of mitochondrial depolarization by Alda-1, an activator of ALDH2, decreases PINK1 accumulation.39 Other studies also show that PINK1 and Parkin mediate mitophagy after acute and chronic-binge ethanol, consistent with type 2 mitophagy.35,40
Fig. 5. Association of hepatic ethanol-induced mitochondrial depolarization with autophagy.
A female green fluorescent protein-microtubule-associated protein 1A/1B-light chain 3 (GFP-LC3) transgenic mouse was gavaged with ethanol (4 g/kg body weight), and intravital multiphoton microscopy of the liver was performed after 2–3 h. The image shows the red fluorescence of ΔΨ-indicating TMRM, which was injected via the carotid artery, and the green fluorescence of autophagy-indicating GFP-LC3. Note increased autophagic GFP-LC3 puncta in hepatocytes with depolarized mitochondria (loss of TMRM fluorescence) (C.S. Mittler, Z. Zhong and J.J. Lemasters, unpublished). Abbreviation: TMRM, tetramethylrhodamine methylester.
After chronic ethanol, some studies show enhanced autophagic flux and increased numbers of autophagosomes and autolysosomes, whereas others indicate that a disruption of autophagosomal processing and lysosomal function leads to the accumulation of autophagosomes.40–43 Transcription factor EB (TFEB) is the master regulator of lysosomal biogenesis, which increases after acute ethanol but decreases after chronic ethanol in mice and in patients with alcoholic hepatitis, implying suppressed lysosomal biogenesis and autophagy after chronic ethanol.43 Suppression of lysosomal processing contributes to ethanol-dependent injury, since overexpression of TFEB decreases liver injury after chronic ethanol plus binge drinking.44
9. Mitochondrial damage-associated molecular patterns (mtDAMPs)
Mitochondrial injury can lead to release of mtDAMPs, which stimulate immune responses.45–47 In immune cells, impaired autophagy leads to release of autophagosomal/autolysosomal contents as inflammatory mediators.48–50 During chronic ethanol feeding as autophagic processing of depolarized mitochondria and mitophagosomes becomes compromised, we proposed that mtDAMPs are released directly to the cytosol and to the extracellular space by the formation of exosomes and the fusion of depolarized mitochondria, mitophagosomes and autolysosomes with the plasma membrane.37,38 In support of this hypothesis, mtDAMPs in the serum, such as ATP, cardiolipin and mitochondrial DNA (mtDNA), increase after ethanol to promote injury.39,51–53
mtDAMPs released as a consequence of disordered mitophagy may also stimulate fibrosis. For example, the mtDAMP, mtDNA, binds to and activates toll-like receptor 9 (TLR9), which is expressed on collagen-producing hepatic stellate cells (HSC), and direct activation of TLR9 causes HSC activation and fibrosis.54–56 Taken together, the sequence of ethanol-induced mitochondrial depolarization, mitophagy and mtDAMP release form a chain of causation linking early adaptive ethanol metabolism to chronic maladaptive pro-inflammatory and pro-fibrotic changes initiating the onset and progression of alcoholic liver disease (ALD). Nonetheless, more information is needed regarding the profile and time course of mtDAMP release in ALD, the relation of mtDAMPs to the severity of ALD, and which mtDAMPs are most critical for ALD pathogenesis.
10. Mitophagy and the mitochondrial permeability transition
In the mitochondrial permeability transition (MPT), high conductance permeability transition (PT) pores open in the mitochondrial inner membrane that nonselectively conduct solutes of molecular weight up to about 1500 Da.57–59 Ca2+, oxidative stress, and various reactive chemicals induce PT pore opening, whereas cyclosporin A and some of its non-immunosuppressive analogs like N-methyl-4-isoleucine cyclosporin (NIM811) inhibit the MPT.59,60 Patch clamping shows that conductance through PT pores is so great that opening of a single PT pore in the inner membrane may be sufficient to cause mitochondrial depolarization.61 Overall, onset of the MPT causes near immediate mitochondrial depolarization, and the depolarization of even a single mitochondria induces selective type 2 mitophagy, as shown in laser scanning confocal microscope experiments in which photoirradiation to individual mitochondria leads to sustained and irreversible depolarization accompanied by inner membrane permeabilization akin to the MPT.1,13,62 Nonetheless, MPT blockers like cyclosporin A and NIM811 inhibit type 1 mitophagy after nutrient deprivation and during cytoplasmic remodeling, apparently by preventing mitochondrial depolarization after mitophagic sequestration and the further processing of mitophagosomes into acidic autolysosomes.10,11,63–65 Why MPT onset is needed for further mitophagic processing remains unknown. However, the MPT is not responsible for in vivo mitochondrial depolarization induced by ethanol, since cyclosporin A does not block depolarization, calcein does not re-distribute from the cytosol into the depolarized mitochondria, and ethanol-induced mitochondrial depolarization is reversible.27
11. Can mitophagy be reversible?
An unanswered question is when mitophagy become irreversible. Since early mitophagosomes during type 1 mitophagy contain polarized and apparently functional mitochondria, is it still possible for entrapped functional mitochondrion to be released from sequestration, for example, after the restoration of nutrient-replete conditions? Ethanol induces profound mitochondrial depolarization and extensive mitophagy. However, when mitochondrial polarization returns as ethanol becomes metabolically eliminated, no obvious deficit in mitochondrial number and mass is apparent upon recovery (Fig. 3, compare 24 h to 0 and 1 h). This suggests that some mitophagic sequestration may reverse as ethanol is eliminated and mitochondrial coupling is restored. The purpose of reversible mitophagy would then be to sequester temporarily uncoupled mitochondria that are futilely hydrolyzing ATP and generating excess reactive oxygen species (ROS), as occurs during adaptive ethanol metabolism.66–69 Nonetheless, ethanol also causes oxidative mtDNA damage and depletion, as well as a compensatory enhancement of mitochondrial biogenesis documented by increases of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), the master regulator of mitochondrial biogenesis, and mitochondrial transcription factor A (TFAM), an activator of mtDNA transcription and replication.40,70–74 Such observations show progression to lysosomal digestion of at least some depolarized mitochondria after ethanol feeding.
12. Conclusions
Overall, mitophagy is an essential quality control mechanism that removes dysfunctional mitochondria that might otherwise promote ATP depletion, oxidative stress and ultimately cell death. Mitophagy is also an important survival strategy in nutrient deprivation and a remodeling mechanism. Such remodeling together with mitochondrial biogenesis adjusts mitochondrial content to changing bioenergetic needs. In adaptive ethanol metabolism, mitochondrial uncoupling and depolarization together with VDAC closure act to oxidize toxic AcAld to non-toxic acetate selectively and more rapidly, but another consequence of mitochondrial depolarization is instigation of mitophagy. After chronic alcohol, excessive and sustained mitophagic burden leads to disordered autophagic processing and likely the release of mtDAMPs intracellularly and extracellularly to activate inflammasomes and other receptors that mediate inflammatory and profibrotic responses, which we propose to be a primary proinflammatory and profibrotic event in ALD. In this way, acute adaptive alcohol metabolism by initiating mitophagy promotes chronic maladaptive consequences causing the pathogenesis of ALD.
Acknowledgements
This work was supported, in part, by Grants AA025379, AA021191, AA022815, and DK073336 from the USA National Institutes of Health (NIH). Imaging facilities were supported, in part, by P30 CA138313 and 1S10OD018113.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemasters JJ. Variants of mitochondrial autophagy: types 1 and 2 mitophagy and micromitophagy (Type 3). Redox Biol. 2014;2:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czaja MJ, Ding WX, Donohue TM Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CT. Mechanisms of selective autophagy and mitophagy: implications for neurodegenerative diseases. Neurobiol Dis. 2018. pii: S0969-9961(18)30275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Culp ML, Craver JG, Darley-Usmar V. Mitochondrial function and autophagy: integrating proteotoxic, redox, and metabolic stress in Parkinson’s disease. J Neurochem. 2018;144:691–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann Med. 2018;50:121–127. [DOI] [PubMed] [Google Scholar]
- 8.Pi H, Xu S, Zhang L, et al. Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy. 2013;9:1780–1800. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Lemasters JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol. 2011;300:C308–C317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006;2:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin RT, Lemasters JJ. Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy. 2009;5:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal. 2011;14:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I, Lemasters JJ. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid Redox Signal. 2011;14:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubli DA, Gustafsson AB. Unbreak my heart: targeting mitochondrial autophagy in diabetic cardiomyopathy. Antioxid Redox Signal. 2015;22:1527–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweers RL, Zhang J, Randall MS, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104:19500–19505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong YC, Holzbaur EL. Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria. Autophagy. 2015;11:422–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci U S A. 2014;111:E4439–E4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soubannier V, McLelland GL, Zunino R, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135–141. [DOI] [PubMed] [Google Scholar]
- 19.Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One. 2012;7 e52830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadete VJ, Deschênes S, Cuillerier A, et al. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J Physiol. 2016;594:5343–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissová I, Salin B, Schaeffer J, Bhatia S, Manon S, Camougrand N. Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy. 2007;3:329–336. [DOI] [PubMed] [Google Scholar]
- 22.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Cederbaum AI. Cytochrome P450s and alcoholic liver disease. Curr Pharmaceut Des. 2018;24:1502–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford BU, Rusyn I. Swift increase in alcohol metabolism (SIAM): Understanding the phenomenon of hypermetabolism in liver. Alcohol. 2005;35:13–17. [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1–35. [DOI] [PubMed] [Google Scholar]
- 26.Yuki T, Thurman RG. The swift increase in alcohol metabolism. Time course for the increase in hepatic oxygen uptake and the involvement of glycolysis. Biochem J. 1980;186:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Z, Ramshesh VK, Rehman H, et al. Acute ethanol causes hepatic mitochondrial depolarization in mice: role of ethanol metabolism. PLoS One. 2014;9, e91308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemasters JJ. Chapter 5-hepatotoxicity due to Mitochondrial Injury Drug-induced Liver Disease. third ed. Academic Press; 2013:85–100. [Google Scholar]
- 29.Colombini M VDAC structure, selectivity, and dynamics. Biochim Biophys Acta. 2012;1818:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmuhamedov E, Lemasters JJ. Ethanol exposure decreases mitochondrial outer membrane permeability in cultured rat hepatocytes. Arch Biochem Biophys. 2009;481:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmuhamedov EL, Czerny C, Beeson CC, Lemasters JJ. Ethanol suppresses ureagenesis in rat hepatocytes: role of acetaldehyde. J Biol Chem. 2012;287:7692–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator-thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. [DOI] [PubMed] [Google Scholar]
- 33.Lemasters JJ. Evolution of voltage-dependent anion channgel function: From molecular sieve to governator to actuator of ferroptosis. Front Oncol. 2017;7:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JA, Ni HM, Ding Y, Ding WX. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G324–G340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Z, Rehman H, Liu Q, et al. A unifying hypothesis linking ethanol-induced mitochondrial depolarization to mitophagy and the proinflammatory and early profibrotic events in alcoholic liver disease. Program and Abstracts of ISBRA/ ESBRA Congress on Alcohol and Alcoholism, Berlin, Germany. http://isbraesbra-2016.org/essential_grid/a-unifying-hypothesis-linking-ethanol-induced-mitochondrial-depolarization-to-mitophagy-and-the-proinflammatory-and-early-profibrotic-events-in-alcoholic-liver-disease/; 2016. [Google Scholar]
- 38.Zhong Z, Lemasters JJ. A unifying hypothesis linking hepatic adaptations for ethanol metabolism to the proinflammatory and profibrotic events of alcoholic liver disease. Alcohol Clin Exp Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudler H, Parlier A, Martin-Vaca B, et al. Activation of aldehyde dehydrogenase-2 attenuates chronic ethanol-induced steatohepatitis. Gastroenterology. 2015;148:S989–S990. [Google Scholar]
- 40.Eid N, Ito Y, Maemura K, Otsuki Y. Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol-treated rats: an immunohistochemical and electron microscopic study. J Mol Histol. 2013;44:311–326. [DOI] [PubMed] [Google Scholar]
- 41.Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM Jr. Ethanol consumption reduces the proteolytic capacity and protease activities of hepatic lysosomes. Biochim Biophys Acta. 1995;1245:421–429. [DOI] [PubMed] [Google Scholar]
- 43.Thomes PG, Trambly CS, Fox HS, Tuma DJ, Donohue TM Jr. Acute and chronic ethanol administration differentially modulate hepatic autophagy and transcription factor EB. Alcohol Clin Exp Res. 2015;39:2354–2363. [DOI] [PubMed] [Google Scholar]
- 44.Chao X, Wang S, Zhao K, et al. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology. 2018;155:865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23:1329–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1332. discussion 1332-1324. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Prakash YS, Eissa NT. Secretory function of autophagy in innate immune cells. Cell Microbiol. 2014;16:1637–1645. [DOI] [PubMed] [Google Scholar]
- 49.Lapaquette P, Guzzo J, Bretillon L, Bringer MA. Cellular and molecular connections between autophagy and inflammation. Mediat Inflamm. 2015;2015:398483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fésüs L, Demény MÁ, Petrovski G. Autophagy shapes inflammation. Antioxid Redox Signal. 2011;14:2233–2243. [DOI] [PubMed] [Google Scholar]
- 51.Iracheta-Vellve A, Petrasek J, Satishchandran A, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol. 2015;63:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrasek J, Iracheta-Vellve A, Saha B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolla R, Vay D, Mottaran E, et al. Antiphospholipid antibodies associated with alcoholic liver disease specifically recognise oxidised phospholipids. Gut. 2001;49:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gäbele E, Mühlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. [DOI] [PubMed] [Google Scholar]
- 55.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010. pii:192543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28(Suppl 1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 58.Biasutto L, Azzolini M, Szabo I, Zoratti M. The mitochondrial permeability transition pore in AD 2016: an update. Biochim Biophys Acta. 2016;1863:2515–2530. [DOI] [PubMed] [Google Scholar]
- 59.Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium. 2018;70:56–63. [DOI] [PubMed] [Google Scholar]
- 60.Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. [DOI] [PubMed] [Google Scholar]
- 61.Szabó I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 62.Aggarwal BB, Quintanilha AT, Cammack R, Packer L. Damage to mitochondrial electron transport and energy coupling by visible light. Biochim Biophys Acta. 1978;502:367–382. [DOI] [PubMed] [Google Scholar]
- 63.Cui T, Fan C, Gu L, et al. Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Res. 2011;1394:1–13. [DOI] [PubMed] [Google Scholar]
- 64.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. [DOI] [PubMed] [Google Scholar]
- 65.Carreira RS, Lee Y, Ghochani M, Gustafsson ÅB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Z, Zhang Y, Li Q Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1alpha expression and mitochondrial ROS production. PLoS One. 2017;12:e0183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teplova VV, Kruglov AG, Kovalyov LI, Nikiforova AB, Fedotcheva NI, Lemasters JJ. Glutamate contributes to alcohol hepatotoxicity by enhancing oxidative stress in mitochondria. J Bioenerg Biomembr. 2017;49:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andringa KK, Udoh US, Landar A, Bailey SM. Proteomic analysis of 4-hydroxynonenal (4-HNE) modified proteins in liver mitochondria from chronic ethanol-fed rats. Redox Biol. 2014;2:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han D, Ybanez MD, Johnson HS, et al. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: Biogenesis, remodeling, and functional alterations. J Biol Chem. 2012;287:42165–42179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han D, Johnson HS, Rao MP, et al. Mitochondrial remodeling in the liver following chronic alcohol feeding to rats. Free Radic Biol Med. 2017;102:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–743. [PubMed] [Google Scholar]
- 73.Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. [DOI] [PubMed] [Google Scholar]
- 74.Mansouri A, Gaou I, De Kerguenec C, et al. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–190. [DOI] [PubMed] [Google Scholar]