Abstract
C-type inactivation is a process by which ion flux through a voltage gated K+ channel is regulated at the selectivity filter. A recent structure of the Kv1.2 channel provides a view into the structural changes at the selectivity filter during C-type inactivation.
Voltage gated K+ (Kv) channels contain a central pore domain, which houses the pathway for ions across the membrane, and peripheral voltage-sensor domains that regulate the opening of the pore domain in response to the membrane potential.1 The flux of K+ through a Kv channel is governed by the processes of activation and inactivation. Activation initiates the flux of K+ through the pore and involves the opening of the cytoplasmic gate in the pore domain. The cytoplasmic gate is formed by the crossing of the inner helices of the pore domain. Opening of the cytoplasmic gate is coupled to voltage sensor movement in response to changes in the membrane potential. Activation of Kv channels is generally followed by inactivation processes, which turn off the flux of K+ through the pore.
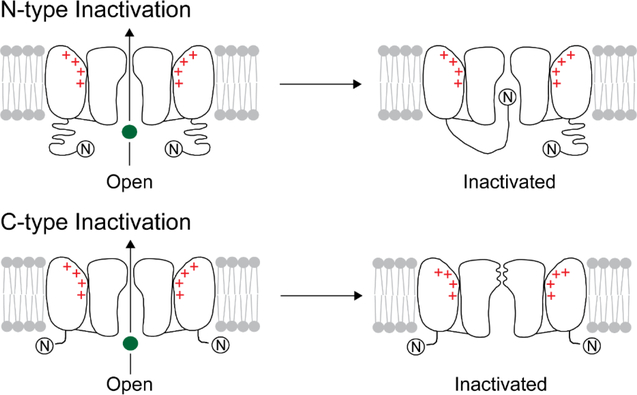
Inactivation in a Kv channel takes place through two distinct mechanisms that are commonly referred to as N-type and C-type (Fig. 1).2,3 In N-type inactivation, the N-terminus of the channel binds to the open pore domain and blocks the flow of ions through the pore while in C-type inactivation, ion flow is blocked by conformational changes at the selectivity filter, which is present towards the extracellular side of the pore. The selectivity filter is the narrowest part of the ion pathway and is the site at which the channel discriminates K+ from Na+.1 The selectivity filter consists of four K+ binding sites (called S1 to S4, extracellular to intracellular) that are built using mainly the main chain carbonyl oxygen atoms (Fig. 2A).4,5 The structure of the selectivity filter is very highly conserved in K+ channels. While there is ample evidence for the selectivity filter being the locus for C-type inactivation, the molecular details of the conformational change were not known.2,3 In this issue Zhe Lu and colleagues report the structure of a mutant Kv channel that potentially captures the C-type inactivated conformation of the selectivity filter.
Figure 1).
Inactivation in a voltage gated K+ channel. A cartoon illustration of N-type (A) and C-type (B) inactivation in a Kv channel. The K+ ion is depicted as a green sphere.
Figure 2).
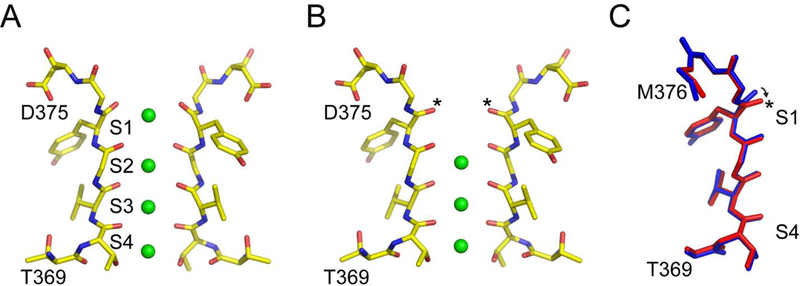
Confirmations for the K+ channel selectivity filter. A) The structure of the selectivity filter of the Kv1.2–2.1 chimeric channel in the conductive state (pdb: 2R9R). The K+ ions are depicted as green spheres and the ion binding sites are labelled S1-S4. B) The structure of the selectivity filter of the V406W-Kv1.2–2.1 channel (pdb: 5WIE). The Y373 carbonyl groups, marked with an asterisk, move inward to distort the S1 site. C) Superposition of the selectivity filter region of a single subunit of the wild type (blue) and the V406W-Kv1.2–2.1 channel (red). The major structural difference in the selectivity filter between the conductive and the putative C-type inactivated conformation is at the Y373 carbonyl group and is indicated by an arrow.
For their structural studies, they used a variant of the Kv1.2 channel, referred to as Kv1.2–2.1 chimeric channel, for which a high resolution structure had previously been reported by the MacKinnon group.6 The original structure of the Kv1.2–2.1 chimeric channel was determined under conditions that favor the inactivated state (at 0 mV) but showed the selectivity filter in a conductive conformation (Fig. 2A). To trap the selectivity filter in the C-type inactivated conformation, the authors used a V406W substitution in the inner helix of the pore domain. The corresponding mutation in the Shaker K+ channel (V478W) was previously reported by the Swartz group and shows very rapid C-type inactivation.7 The overall structure of the Kv1.2–2.1 chimeric channel with the V406W substitution is very similar to the wild type except for changes in S6 and the selectivity filter. The changes in S6 are mainly local to the site of the substitution and due to the replacement of Val with the bulkier Trp. The surprising finding in the structure was the effect on the selectivity filter. The structure shows that the outer carbonyls of the S1 site (indicated by an asterisk, Fig 2B, 2C) move slightly in the inward direction. This change in the outer carbonyls at the S1 site has the effect of distorting the S1 site and no ion occupancy is observed at this site. Given the low resolution of the structural data, the authors do a thorough analysis of the data to confirm that the lack of ion occupancy observed at the S1 site is correct and not due to the lack of high resolution structural data. The authors suggest that the disruption in the S1 site will render the channels non-conductive and propose that the structure of the selectivity filter in this mutant channel corresponds to the conformation of the selectivity filter in the C-type inactivated state.
Is this the C-type inactivated conformation of the selectivity filter? A defining characteristic of C-type inactivation is a dependence on the extracellular K+ concentration and the structure provides a straight forward explanation for this effect.8–10 High extracellular K+ will increase the ion occupancy at the outermost site, S1 and therefore hinder C-type inactivation. A direct analysis of the effect of ion occupancy at the various sites in the selectivity filter on C-type inactivation has been carried out for the KcsA channel and this study ruled out any role for the ion occupancy at the S1 site on the inactivation process.11 This discrepancy may however be due to structural differences in the inactivated states of the KcsA and Kv1.2 channels.
The changes observed in the selectivity filter at the S1 site in the mutant channel compared to the wild type are quite small (less than 1 Å, Fig. 2C) and fall within the range of fluctuations predicted by molecular dynamics simulations for the selectivity filter.12 Further, the change at the S1 site is only observed in one of the molecules in the asymmetric unit, while the other molecule showed the selectivity filter in the conductive state.
It has been shown that on C-type inactivation, residues in the outer vestibule (448–450) in the Shaker K+ channel show a change in environment, an observation based on changes in site directed labelling and metal mediated cross-linking on C-type inactivation.13,14 Metal mediated crosslinking of Cys residues substituted at Y82 (equivalent to T449 in Shaker) is also observed on inactivation in the KcsA channel.15 Fluorescent probes introduced at various sites in the vestibule or the turret region of the Shaker channel show a change in fluorescence on C-type inactivation which suggests a structural change in this region.16–18 The structure shows only marginal changes in these regions (Fig. 2C) compared to the conductive state and is therefore not consistent with these studies.
The structure proposed as the C-type inactivated state is therefore consistent with some but not all of the experimental findings on the C-type inactivated state. It is therefore quite likely that the physiologically relevant C-type inactivated conformation may involve additional changes than visualized in the present structure. This can be addressed through determining the structures of other mutants that show an enhanced rate of C-type inactivation. Prime positions to target would be the residues W362 and Y373 (corresponding to W434 and Y445 in the Shaker K+ channel).19,20
The concerns notwithstanding, the structure determined by Zhe Lu and colleagues represents an important step in understanding the process of C-type inactivation in Kv channels. A key question to be tackled in future studies is whether similar changes underlie C-type inactivation in different K+ channels. Comparison of the functional data on KcsA to the structural data of the Kv1.2 channel strongly suggests that the changes that take place on inactivation in these channels are likely different. However, a conclusive answer to this question will have to await the determination of additional channel structures.
ACKNOWLEDGEMENTS
Research in my laboratory is supported by a grant from the NIH (GM087546).
REFERENCES
- 1.MacKinnon R Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture). Angew Chem Int Ed Engl 43, 4265–4277, (2004). doi: 10.1002/anie.200400662. [DOI] [PubMed] [Google Scholar]
- 2.Kurata HT & Fedida D A structural interpretation of voltage-gated potassium channel inactivation. Prog Biophys Mol Biol 92, 185–208, (2006). doi: 10.1016/j.pbiomolbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hoshi T & Armstrong CM C-type inactivation of voltage-gated K+ channels: pore constriction or dilation? J Gen Physiol 141, 151–160, (2013). doi: 10.1085/jgp.201210888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Morais-Cabral JH, Kaufman A & MacKinnon R Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414, 43–48, (2001). doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 5.Long SB, Campbell EB & Mackinnon R Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903, (2005). doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 6.Long SB, Tao X, Campbell EB & MacKinnon R Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382, (2007). doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 7.Kitaguchi T, Sukhareva M & Swartz KJ Stabilizing the closed S6 gate in the Shaker Kv channel through modification of a hydrophobic seal. J Gen Physiol 124, 319–332, (2004). doi: 10.1085/jgp.200409098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshi T, Zagotta WN & Aldrich RW Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538, (1990). [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Barneo J, Hoshi T, Heinemann SH & Aldrich RW Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels 1, 61–71, (1993). [PubMed] [Google Scholar]
- 10.Baukrowitz T & Yellen G Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron 15, 951–960, (1995). [DOI] [PubMed] [Google Scholar]
- 11.Matulef K, Annen AW, Nix JC & Valiyaveetil FI Individual Ion Binding Sites in the K(+) Channel Play Distinct Roles in C-type Inactivation and in Recovery from Inactivation. Structure 24, 750–761, (2016). doi: 10.1016/j.str.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen TW, Andersen OS & Roux B On the importance of atomic fluctuations, protein flexibility, and solvent in ion permeation. J Gen Physiol 124, 679–690, (2004). doi: 10.1085/jgp.200409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yellen G, Sodickson D, Chen TY & Jurman ME An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J 66, 1068–1075, (1994). doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Jurman ME & Yellen G Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron 16, 859–867, (1996). [DOI] [PubMed] [Google Scholar]
- 15.Raghuraman H, Cordero-Morales JF, Jogini V, Pan AC, Kollewe A, Roux B & Perozo E Mechanism of Cd2+ coordination during slow inactivation in potassium channels. Structure 20, 1332–1342, (2012). doi: 10.1016/j.str.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha A & Bezanilla F Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 19, 1127–1140, (1997). [DOI] [PubMed] [Google Scholar]
- 17.Loots E & Isacoff EY Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J Gen Physiol 112, 377–389, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loots E & Isacoff EY Molecular coupling of S4 to a K(+) channel’s slow inactivation gate. J Gen Physiol 116, 623–636, (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Yan Y & Sigworth FJ How does the W434F mutation block current in Shaker potassium channels? J Gen Physiol 109, 779–789, (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RE, Larsson HP & Isacoff EY A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys J 74, 1808–1820, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]