Abstract
Background:
Benign ethnic neutropenia (BEN), defined by neutrophil count less than 1·5 k/uL in the absence of other causes, is an asymptomatic condition more commonly observed in individuals of African ancestry. However, the natural history of this condition has been less well described.
Methods:
Individuals with BEN were retrospectively identified by chart review or referral to hematology clinics. They were then invited to enroll in a prospective natural history study. Retrospective and prospective clinical and laboratory data were combined for descriptive analyses.
Findings:
46 participants, younger and older adults from 2 institutions, had BEN. Hypertension was reported in 30%, musculoskeletal disorders in 15%, and upper respiratory infection in 33% of these adults. Their leukopenia resulted from isolated neutropenia, ranging from 1000 and 1500 cells/uL. The severity of infections was mild and the frequency was similar to other healthy individuals in the ambulatory clinic.
Interpretation:
In this group of BEN participants, their leukopenia was stable over time, and they had low rates of infections or common medical disorders, confirming the benign nature of this condition. The presence of BEN in children, younger adults, and older adults suggest a hereditary pattern for BEN.
Keywords: Benign Ethnic Neutropenia BEN, DARC, Leukopenia
Introduction
Complete blood counts (CBC) are a routine part of annual health maintenance testing for many adults. The components of CBC include leukocyte counts (including neutrophils, lymphocytes, eosinophils, and basophils), hemoglobin levels, and platelet counts. Asymptomatic reduction in neutrophil counts has been observed in all individuals of all ethnic backgrounds but is more commonly observed in those of African ancestry.
We previously analyzed age, sex, and ethnic characteristics of neutrophil count, using data from National Health and Nutritional Examination Survey (NHANES, Centers for Disease Control and Prevention) from 1999 to 2004. African-Americans have lower mean leukocyte counts of 3.5 k/uL, compared to 4·3 k/uL in European-Americans and 4·4 k/uL in Mexican-Americans. Using neutrophil count cutoff of <1·5 k/uL, African-Americans have a prevalence of neutropenia of 4·5%, compared to 0·74% in European-Americans and 0·48% in Mexican-Americans.1 Additionally, younger age and men have even higher prevalence. This higher prevalence of neutropenia in African-Americans has been commonly referred as BEN (or ethnic neutropenia, ENP).
The search for the cause for BEN began with admixture mapping studies, which identified a chromosome 1q22 polymorphism containing the Duffy antigen and receptor for chemokine (DARC, also known as atypical chemokine receptor 1, ACKR1) gene that strongly influenced leukocyte counts in African-Americans.2 Subsequent genome wide association studies (GWAS), focused on leukocyte and neutrophil counts, showed a single nucleotide polymorphism (SNP), rs2814778 identifying the DARC, to be strongly associated with low leukocyte and neutrophil counts.3,4 Our own GWAS replication effort, using a case-control design with only African-Americans, also showed rs2814778 to be highly associated with low leukocyte/neutrophil counts.5
However, the mechanism for DARC null state leading to leukopenia or neutropenia was unknown until recently. DARC is broadly expressed on red, kidney, brain, and endothelial cells, but not leukocytes.6 Rs2814778 is associated with DARC null on red cells only; DARC expression in other tissues are preserved. DARC on red cells is expressed a transmembrane glycoprotein as Duffy blood group antigen, Fy. Because this antigen is a receptor for Plasmodium vivax and Plasmodium knowlesi, DARC null red cells are less susceptible to malaria infections, and thereby gaining their survival advantage. Duchene et al showed that transplanting the bone marrow of DARC −/− mice into DARC +/+ mice recapitulated the BEN phenotype. Their careful lineage specific analyses showed DARC null hematopoietic cells differentiated to DARC null myeloid progenitors, and later mature activated neutrophils. These activated neutrophils in circulation, together with normal DARC expression on endothelial cells, led to their egress to the spleen, causing relative neutropenia.7
With the mechanism of BEN sufficiently addressed, the attention has turned to characterizing the natural history. We previously showed that the BEN phenotype in a cohort of children having a mild clinical course, with average neutrophil counts of near 900 cells/uL, and no increase in typical infections in the respiratory tract.8 However, similar reports in adults with BEN are currently lacking. Many of the prior descriptions of BEN described cross-sectional rates of neutropenia, or did not contain sufficient length of follow-up.1,9–11 Hence, we wrote this clinical protocol across two institutions to retrospectively identify individuals with BEN and prospectively collect their clinical data to describe the natural history for this condition.
Methods
Individuals with BEN were retrospectively identified by referrals to hematology clinic for neutropenia or having ICD-9 coding for neutropenia (288·0), then invited to enroll in a prospective natural history study at the National Institutes of Health (NIH; Bethesda, MD) or the Veterans Administration Hospital, hematology-oncology clinic (Washington, DC). BEN was defined as individuals of African ancestry with neutrophil counts <1500 cells/uL on at least 2 laboratory testing, separated by at least 1 month, in the absence of infection (including negative testing for human immunodeficiency virus, hepatitis B or C, human T lymphotropic virus), nutritional deficiency, autoimmune disorders, or invasive cancer. Both studies were approved by local institutional review boards, and all patients gave informed consent (clinicaltrials.gov, NCT00059423). Retrospective and prospective laboratory data, testing results, and clinical history were then combined for descriptive analysis. Common toxicity criteria for adverse events (CTCAE) classify neutrophil counts between 1500 cells/uL and lower limit of normal to be grade 1, and between 1500 to 1000 cells/uL grade 2.12
Results
Demographic characteristics
The NIH and VA cohorts consisted of 28 and 18 patients respectively (Table 1). The average duration of available data was 6·2 and 7·7 years; the average prospective portion of data collection was 3·1 (0·25–8·5) and 1·1 years (0·25–2·5), respectively. The median age of the NIH cohort was substantially younger at 32 years (range 8–56). In both cohorts there was a male predominance, and most never smoked cigarettes. There was also a low rate of medical disorders commonly encountered in the primary care office. Hypertension was reported in 14 individuals (30%) who were taking medications, followed by musculoskeletal conditions in 7 individuals (15%).
Table 1.
Demographic and clinical characteristics of participants
| NIH cohort | VA cohort | Total (%) | ||
|---|---|---|---|---|
| Number of participants | 28 | 18 | 46 | |
| Duration of available data | Average 6·2 years (<1–22) | Average 7·7 years (<1–27) | ||
| Sex | Male | 18 | 12 | 30 (65%) |
| Female | 10 | 6 | 16 (35%) | |
| Median age, years | 32 (8–56) | 52 (31–75) | ||
| Smoking status | Current | 2 | 2 | 4 (8·7%) |
| Past | 1 | 1 | 2 (4·3%) | |
| Never | 25 | 15 | 40 (87%) | |
| Concurrent medical conditions | Hypertension | 4 | 3 | 7 (15%) |
| Infections, non URI | hepatitis B, 1 eye infection, 1 HSV, 1 finger abscess, 1 viral gastroenteritis, 1 | HSV, 1 | 6 (13%) | |
| Infections, URI | 12* | 3 | 15 (33%) | |
| Musculoskeletal (LBP, rotator cuff, OA, TMJD, synovitis) | 5 | 2 | 7 (15%) | |
| Sickle cell trait | 3 | 1 | 4 (8·7%) | |
| Skin conditions (rash, acne, psoriasis) | 3 | 1 | 3 (6·5%) | |
| Major depression | 2 | 0 | 2 (4·3%) | |
| Others | 1 hypercholesteremia | localized prostate cancer, 1 Crohn’s disease, 1 asthma, 1 sleep apnea, 3 impaired glucose tolerance, 1 | 8 (17%) | |
| Medications | Diuretics | 3 | 4 | 7 (15%) |
| Beta-blockers | 1 | 1 | 2 (4·3%) | |
| Calcium channel blockers | 1 | 0 | 1 (2·2%) | |
| Other antihypertensives | 2 | 2 | 4 (8·7%) | |
| NSAID | 6 | 6 | 12 (26%) | |
| Antidiabetic medication | 1 | 0 | 1 (2·2%) | |
| Hormonal contraception | 3 | 0 | 3 (6·5%) | |
| Iron supplementation | 2 | 0 | 2 (4·3%) | |
| Vitamin/Mineral supplementation | 3 | 0 | 3 (6·5%) | |
| Antibiotics | 0 | 1 | 1 (2·2%) | |
| Others (sertraline, simvastatin, topical steroid cream) | 5 | 0 | 5 (11%) | |
| Allergies | Penicillin | 2 | 2 | 4 (8·7%) |
| Other antibiotics (chloroquine, sulfa, or others) | 2 | 1 | 3 (6·5%) | |
| Seasonal | 2 | 1 | 3 (6·5%) | |
| Shellfish | 1 | 1 | 2 (4·3%) |
NIH, National Institutes of Health; VA, Veteran’s Administration; URI, upper respiratory infection; LBP, low back pain; OA, osteoarthritis; TMJD, temporal mandibular joint disorder; HSV, herpes simplex virus; NSAID, non steroidal anti-inflammatory drug
most of these episodes were self-reported.
Infection and higher acuity medical visits
Among the participants in the NIH cohort, many were healthy volunteers and enrolled in other NIH studies. Twelve participants reported that they had one or two upper respiratory infection (URI) in the previous year; 2 of them also had distant history of urinary tract infections (UTIs). Prior to enrolment, one had two hospitalizations for malaria (Table 2), which occurred outside of the United States. There was one other individual who had a history of a finger abscess and another individual with eye infection. There were no other reported emergency department (ED) visits or inpatient admissions due to infections during the study period.
Table 2.
Higher acuity medical conditions
| NIH cohort | VA cohort | Total | |
|---|---|---|---|
| Emergency department visit | Viral gastroenteritis, 1 Eye infection, 1 Finger abscess, 1 |
URI, 3 Musculoskeletal, 6 Contact dermatitis, 1 Lip bleeding, 1 Pneumonia, 1 |
7 (15%)* |
| Surgery | Synovitis, 1 Splenectomy, 1 |
ICA stenosis, 1 | 3 (6·5%) |
| Hospitalizations | Malaria, 1 | ICA stenosis surgery, 1 | 2 (4·3%) |
4 individuals in the VA cohort accounted for all the emergency department visits.
Among the VA cohort of BEN participants, 4 subjects (7, 10, 12, and 14) had a total of 12 ED visits; two subjects (10 and 14) accounted for 5 visits each. Subject 10 had two ED visits (1 pneumonia and 1 URI), a year apart, due to infections requiring antibiotics; subject 7 had one ED visit for URI and was treated with antibiotics. None of these ED visits led to inpatient hospitalizations.
There was a total of 3 participants that underwent surgery and two were hospitalized (Table 2).
Leukocyte counts
All BEN individuals had mild leukopenia, which was mostly attributable to neutropenia. Neutrophil counts were <1500 cells/uL on almost half of the lab testing in the NIH cohort, and almost three-quarters in the VA cohort. Most of the neutrophil counts were between 1000–1500 cells/uL; neutrophil counts <500 were rare. In the NIH cohort, there was only one ANC of 480 cells/uL, which occurred in an otherwise healthy 8 year-old girl. The neutropenia was an isolated event without symptoms. Follow-up blood counts 2 and 4 years after this low ANC showed an increase to near 1000 cells/uL.
In the VA cohort, 3 individuals had ANC <500 cells/uL. Two subjects (31 and 51 year-old) had one event each of asymptomatic isolated neutropenia, 427 and 31 cells/uL respectively. Both recovered their neutrophil counts later. The third individual was 75 year-old and had fluctuating neutrophil counts from 288 cells/uL to 1553 cells/uL over 7 years. A bone marrow biopsy showed cellularity ranging from 30% to 50%, myeloid hypoplasia and mild megaloblastoid erythroid changes, and otherwise normal hematopoietic maturation. At the latest follow-up, this individual continued to have stable leukocyte and neutrophil counts, and had not progressed to myelodysplastic syndrome (MDS).
When neutrophil counts were graphed vs time, both NIH and VA cohort showed fluctuations of counts between 1000 and 2000 cells/uL (Fig 1A and 1B). ANC occasionally spiked to higher levels but returned to near the baseline levels for the individuals. Overall, the pattern of neutrophil counts over time demonstrate stability of counts over many years.
Figure 1A.
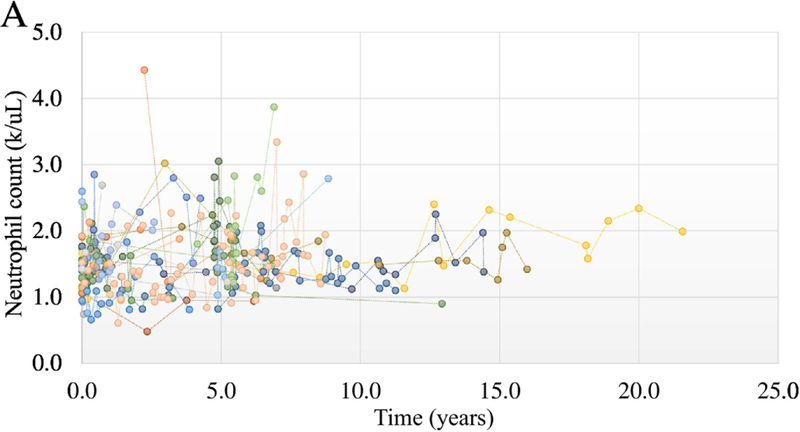
Chronicity of neutrophil counts in the NIH cohort
Figure 1B.
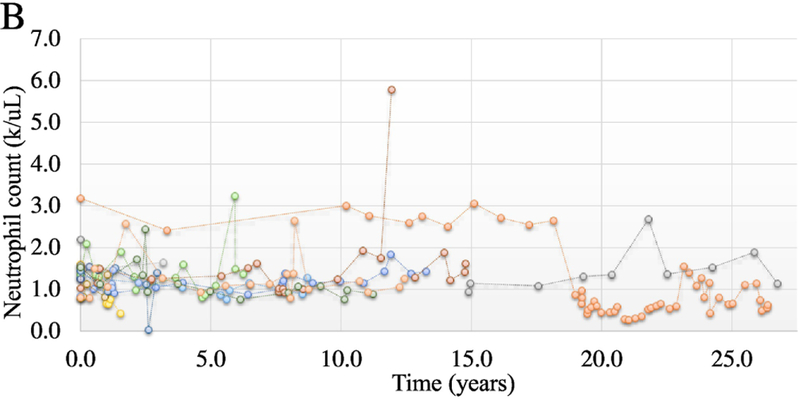
Chronicity of neutrophil counts in the VA cohort
Other blood counts and Duffy testing
Other than low neutrophil and leukocyte counts in both group of participants, the remainder of CBC were otherwise normal. Most had normal lymphocyte, monocyte, eosinophil, and basophil counts. Very low lymphocyte or monocyte counts were rare. Additionally, most had normal hemoglobin levels and platelet counts. Among the NIH cohort, Duffy antigens (Fya and Fyb) on red cells were tested, and most (23 of 24 individuals) were Fya and Fyb negative, confirming a DARC null red cell phenotype. There was one participant with Fya positive and Fyb negative expression.
Discussion
Through our previous analyses of the large NHANES data from 25,222 participants, we were only able to show the cross-sectional prevalence of neutropenia.1 Thus, these two cohorts of younger and older adults, enriched for BEN, provided very valuable natural history for the phenotype. The retrospective and prospective laboratory and clinical data collected from these cohorts allowed us to conclude with several important observations. First, the leukocyte and neutrophil counts of BEN participants remained stable over several years to decades, with neutrophil counts fluctuating mostly between 1000 and 1500 cells/uL (or 1·0 to 1·5 × 109 cells/L). Neutrophil counts <500 cells/uL were rare. Findings from these BEN participants are consistent and complementary to our prior cross-sectional data. Additionally, the neutrophil count was the only blood lineage affected; lymphopenia, monocytopenia, anemia, or thrombocytopenia were infrequent.
Equally important, we confirmed that the clinical nature for this condition was benign. Our participants had lower rates of hypertension, diabetes, or musculoskeletal disorders than the national average of 31%, 15%, and 19–55% respectively.13,14 Additionally, a minority of individuals (33%) had typical annual rates of respiratory infections, and non-respiratory infections were reported in a smaller group (13%). Utilization of emergency department or hospitalizations for urgent medical conditions was also low. Thus, these ambulatory infections could be inferred to be mild and were treated symptomatically. These infection rates could also be viewed as expected, given their counts met CTCAE grade 1 or grade 2 neutropenia. Furthermore, the gene expression pattern in the neutrophils from the NIH BEN participants was similar to that from those without BEN.5 Taken together, these findings support the notion that BEN is clinically benign.
We also performed DARC testing of the red cells, and not surprisingly, an overwhelming proportion (96%) of them were DARC negative. We had also performed DARC genotyping in a smaller subset of the NIH participants which showed that they were homozygous for the −67C/C genotype with reduced DARC mRNA expression. The neutrophil expression of the BEN participants were largely similar to the non-BEN individuals, except the BEN neutrophils had higher expression in the hematopoietic mobilization and leukocyte migration pathways.5 Recently, some have proposed using DARC genotype or DARC phenotype (Fya and Fyb) as an adjunctive testing for BEN.15 While DARC status is a better determinant of African ancestry and would capture a larger proportion of individuals with BEN, the DARC null genotype can be observed in those without neutropenia (Supplemental Table F).5 Thus, DARC null should not be viewed as diagnostic or pathognomonic for BEN at this time.
These groups of healthy young and older adults, along with the asymptomatic children reported in another prior report, represented the largest collection of individuals with BEN to date.8 With BEN being consistently described across different age groups, this finding suggested the heritability of BEN. This observation is further supported by earlier small family studies, where BEN was clearly observed in several family pedigrees.16,17 Additionally, a large GWAS showed that other than DARC, there were other genes that contributed to the heritability of neutrophil counts: C-X-C motif chemokine ligand 2 (CXCL2) on chromosome 4q13, and cyclin dependent kinase 6 (CDK6) on7q21. The polygenic heritability for neutrophil phenotypes was estimated at 20–25%.3
Although sample size of these two cohorts of BEN individuals were relatively small, they still represented the largest group reported in the literature to date. The NIH group, which was younger and likely more similar to individuals being followed in the ambulatory clinic, may be different than the VA group. Combining them together likely made the group more clinically heterogenous. Also, bone marrow biopsy was performed in only one individual – the oldest person of the VA cohort. There was one other individual in their 70’s, 3 in their 60’s, and 7 in their 50’s. While more bone marrow biopsies would help to rule out myelodysplasia, the stable and isolated neutrophil counts in all over many years and their lack of symptoms suggested the likelihood of MDS was low. Prior reports of showing normal bone marrow in BEN further support this notion.18–21
In summary, with BEN being identified at about 4% of adults, this work represented screening over 1000 individuals to collect this group of BEN individuals. This group of younger and older adults with BEN had leukopenia resulted from isolated neutropenia. Most had neutrophil counts between 1000 and 1500 cells/uL. They had low rates of infections or common medical disorders, confirming the benign nature of this condition. The presence of BEN in children, younger adults, and older adults suggest a hereditary pattern for BEN. The stability of blood counts over decades, including leukocyte subsets, normal hemoglobin levels, and normal platelet counts, indicate that myelodysplasia is unlikely even in the older adults. Recent murine experiments, GWAS, mRNA microarray, and DARC genetic work also showed that the mechanism for BEN is biologically benign.
Table 3.
Mean blood count laboratory values
| Laboratory parameter (normal range, units) | NIH cohort (range of values) | VA cohort (range of values) |
|---|---|---|
| WBC (4·0–10·0, k/uL) | 3·66 (1·9–6·9) <3·0: 21·6% <2·0: 0·3% |
3·10 (1·4–7·4) <3·0: 54% <2·0: 4·3% |
| Hemoglobin (11–17·5, g/dL) | 14·0 (men) 12·1 (women) (10–16·6) |
14·4 (men) 12·6 (women) (11·1–16·6) |
| MCV (79–92, fL) | 88 (80–100) | 90 (79–103) |
| Platelet Count (161–369, k/uL) | 231 (70–462) | 199 (122–331) |
| Neutrophil (34–75, %) | 43 (19–67) | 39 (1–86) |
| Lymphocytes (11–53, %) | 44 (16–75) | 43 (5–71) |
| Monocytes (4–13, %) | 9·7 (1·5–34) | 13 (1–37) |
| Eosinophil % (0–7, %) | 2·5 (0·4–28) | 5·1 (0·1–20) |
| Basophil % (0–1, %) | 0·69 (0–2·8) | 0·8 (0–2·1) |
| Neutrophil count (1·3–6·2, k/uL) | 1·58 (0·48–4·4) <1·5: 46%* <1·0: 8·4%* <0·5: 0·3%* |
1·26 (0·03–5·8) <1·5: 78%* <1·0: 35%* <0·5: 6·1%* |
| Lymphocytes count (0·8–3·6, k/uL) | 1·6 (0·6–3·7) <1·0: 33% <0·75: 0·6% |
1·3 (0·35–3·6) <1·0: 29% <0·75: 3·5% |
| Monocytes count (0·13–0·83, k/uL) | 0·35 (0·06–0·95) <0·2: 3·3% |
0·39 (0·04–1·03) <0·2: 6·5% |
| Eosinophil count (0·0–0·54, k/uL) | 0·09 (0·01–1·2) | 0·12 (0–0·52) |
| Basophil count (0·0–0·1, k/uL) | 0·02 (0–0·11) | 0 |
all the values <0·5 were captured in the <1·0; similarly, the values <1·0 and <0·5 were captured in the <1·5.
Acknowledgment
This work is supported in part by the intramural research program of the National Institute of Diabetes, Digestive, and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Rahul Lakhotia - none
Anita Aggarwal - none
Mary E Link - none
Griffin P Rodgers - none
Matthew M Hsieh - none
References
- 1.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med 2007;146(7):486–92. [DOI] [PubMed] [Google Scholar]
- 2.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet 2009;5(1):e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiner AP, Lettre G, Nalls MA, et al. Genome-wide association study of white blood cell count in 16,388 African Americans: the continental origins and genetic epidemiology network (COGENT). PLoS Genet 2011;7(6):e1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Glessner JT, Zhang H, et al. GWAS of blood cell traits identifies novel associated loci and epistatic interactions in Caucasian and African-American children. Hum Mol Genet 2013;22(7):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles BA, Hsieh MM, Adeyemo AA, et al. Analyses of genome wide association data, cytokines, and gene expression in African-Americans with benign ethnic neutropenia. PLoS One 2018;13(3):e0194400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Van Kim C, Tournamille C, Kroviarski Y, Cartron JP, Colin Y. The 1.35-kb and 7.5-kb Duffy mRNA isoforms are differently regulated in various regions of brain, differ by the length of their 5’ untranslated sequence, but encode the same polypeptide. Blood 1997;90(7):2851–3. [PubMed] [Google Scholar]
- 7.Duchene J, Novitzky-Basso I, Thiriot A, et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nat Immunol 2017;18(7):753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz MV, Meier ER, Hsieh MM. Identification and Clinical Characterization of Children With Benign Ethnic Neutropenia. J Pediatr Hematol Oncol 2016;38(3):e140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rana SR, Castro OL, Haddy TB. Leukocyte counts in 7,739 healthy black persons: effects of age and sex. Ann Clin Lab Sci 1985;15(1):51–4. [PubMed] [Google Scholar]
- 10.Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch Intern Med 1991;151(3):501–5. [PubMed] [Google Scholar]
- 11.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol 1996;49(8):664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Common terminology criteria for adverse events: Cancer Therapy Evaluation Program, National Cancer Institute; 2017. [version 5.0:[December 18th, 2018. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf.
- 13.Selected health conditions and risk factors, by age: United States, selected years 1988–1994 through 2015–2016: NCHS, National Health and Nutrition Examination Survey; 2017. [December 18th, 2018. Available from: https://www.cdc.gov/nchs/data/hus/2017/053.pdf.
- 14.Clarke TC, Nahin RL, Barnes PM, Stussman BJ. Use of complementary health approaches for musculoskeletal pain disorders among adults: United States, 2012. National Health Statistics Reports2016 [PubMed]
- 15.Dinardo CL, Kerbauy MN, Santos TC, et al. Duffy null genotype or Fy(a-b-) phenotype are more accurate than self-declared race for diagnosing benign ethnic neutropenia in Brazilian population. Int J Lab Hematol 2017;39(6):e144–e6. [DOI] [PubMed] [Google Scholar]
- 16.Shoenfeld Y, Alkan ML, Asaly A, Carmeli Y, Katz M. Benign familial leukopenia and neutropenia in different ethnic groups. Eur J Haematol 1988;41(3):273–7. [DOI] [PubMed] [Google Scholar]
- 17.Denic S, Showqi S, Klein C, Takala M, Nagelkerke N, Agarwal MM. Prevalence, phenotype and inheritance of benign neutropenia in Arabs. BMC Blood Disord 2009;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mintz U, Sachs L. Normal granulocyte colony-forming cells in the bone marrow of Yemenite Jews with genetic neutropenia. Blood 1973;41(6):745–51. [PubMed] [Google Scholar]
- 19.Mason BA, Lessin L, Schechter GP. Marrow granulocyte reserves in black Americans. Hydrocortisone-induced granulocytosis in the “benign” neutropenia of the black. Am J Med 1979;67(2):201–5. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien HA, Horton MA. Granulopoiesis in the neutropenia of negroes. Scand J Haematol 1983;31(5):424–6. [DOI] [PubMed] [Google Scholar]
- 21.Rezvani K, Flanagan AM, Sarma U, Constantinovici N, Bain BJ. Investigation of ethnic neutropenia by assessment of bone marrow colony-forming cells. Acta Haematol 2001;105(1):32–7. [DOI] [PubMed] [Google Scholar]


