Abstract
In contrast to temporal coding by synaptically acting neurotransmitters such as glutamate, neuromodulators such as monoamines signal changes in firing rate. The two modes of signaling have been thought to reflect differences in release by different cells. We now find that midbrain dopamine neurons release glutamate and dopamine with different properties that reflect storage in different synaptic vesicles. The vesicles differ in release probability, coupling to presynaptic Ca++ channels and frequency dependence. Although previous work has attributed variation in these properties to differences in location or cytoskeletal association of synaptic vesicles, the release of different transmitters shows that intrinsic differences in vesicle identity drive different modes of release. Indeed, dopamine but not glutamate vesicles depend on the adaptor protein AP-3, revealing an unrecognized linkage between the pathway of synaptic vesicle recycling and the properties of exocytosis. Storage of the two transmitters in different vesicles enables the transmission of distinct signals.
Signaling by neuromodulators such as monoamines differs in multiple ways from signaling by the classical neurotransmitters glutamate and GABA. Release of glutamate and GABA at synaptic contacts activates fast ionotropic receptors that provide precise information about timing. In contrast, monoamines activate slower G protein-coupled receptors, and dopamine neurons form en passant presynaptic boutons, often without clear postsynaptic element (Hattori et al., 1991). As a result, these cells have been thought to signal through changes in the extracellular concentration of dopamine, which generally reflects firing rate rather than timing. The difference between synaptic and volume transmission has thus been considered to reflect the properties of the receptors (ionotropic versus metabotropic) and their distance from the release site. However, differences in the mode of transmission also impose distinct requirements on the properties of release. Temporal coding requires fast, synchronous release. In contrast, frequency coding requires sustained release at rates proportionate to firing.
Midbrain dopamine neurons fire in two patterns, tonic (4–5 Hz) and bursting (~20 Hz), and the dopamine release that signals unexpected reward and promotes movement depends on burst firing (Howe and Dombeck, 2016; Schultz et al., 1997). Indeed, dopamine release increases greatly with stimulation frequency (Koranda et al., 2014; Sulzer et al., 2016), suggesting a low release probability that minimizes vesicle depletion and enables release to follow firing rate (Dittman et al., 2000). In midbrain dopamine neurons, the Ca++-binding protein calbindin indeed reduces the coupling of synaptic vesicles to presynaptic Ca++ channels (Pan and Ryan, 2012), which reduces release probability. In principle, this mechanism would be expected to impair the regulated exocytic release of any classical transmitter by these same cells.
However, many neurons including dopamine neurons corelease at least two different classical transmitters (El Mestikawy et al., 2011; Hnasko and Edwards, 2012; Tritsch et al., 2016). In some cases, release of both occurs from the same synaptic vesicles (Chaudhry et al., 1998; Jonas et al., 1998; Ren et al., 2011; Tritsch et al., 2012) and hence with the same properties. One transmitter can promote packaging of the other: vesicular glutamate transporter VGLUT2 promotes the storage of dopamine by mesolimbic dopamine projections (Birgner et al., 2010; Hnasko et al., 2010) and VGLUT3 the storage of acetylcholine (ACh) by cholinergic interneurons in the striatum (Gras et al., 2008; Nelson et al., 2014). This phenomenon requires expression of the two transporters on the same synaptic vesicles. In this case, the two transmitters activate different receptors (Lamotte d’Incamps et al., 2017; Ren et al., 2011; Sengupta et al., 2017) but convey the same information about neural activity. Ultrastructural analysis of the striatum has suggested segregation of dopamine and glutamate release sites (Zhang et al., 2015), but mesolimbic dopamine neurons appear to release glutamate and dopamine with very similar properties (Adrover et al., 2014). On the other hand, starburst amacrine cells in the retina corelease ACh and GABA, and their signaling shows a differential dependence on Ca++, suggesting a presynaptic mechanism for the differences in response (Lee et al., 2010). Recent work has suggested a similar difference between ACh and GABA release by basal forebrain neurons (Takacs et al., 2018).
Previous work has attributed variation in the properties of release to extrinsic factors such as the association of synaptic vesicles with the cytoskeleton or their proximity to Ca++ channels (Chi et al., 2001; Eggermann et al., 2012; Midorikawa et al., 2007). However, these and other extrinsic factors such as Ca++ buffering cannot alone account for differences in the release of two transmitters by the same cell. Alternatively, synaptic vesicles may differ in their intrinsic properties, reflecting differences in composition and hence identity. The phenomenon of corelease provides an entrypoint to test this possibility.
Results
Glutamate and Dopamine Release from VTA Dopamine Neurons Differ in Short-Term Plasticity
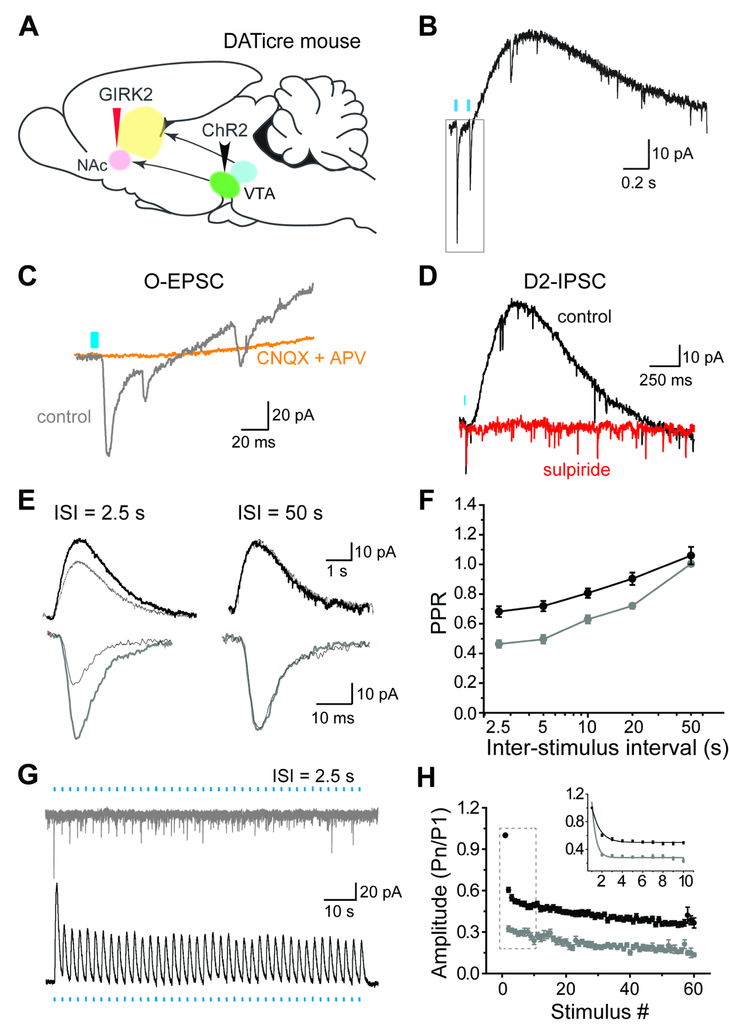
To assess the relationship between glutamate and dopamine release by ventral tegmental area (VTA) dopamine neurons, we used postsynaptic recording in brain slices. Since dopamine does not usually produce detectable postsynaptic currents, we took advantage of a G protein-coupled inwardly rectifying K+ channel (GIRK2) that couples to D2 dopamine receptors when expressed in striatal spiny projection neurons (SPNs) (Marcott et al., 2014), enabling direct comparison of postsynaptic glutamate and dopamine responses. After injection of recombinant AAV encoding GIRK2 into the ventral striatum, we expressed channelrhodopsin specifically in dopamine neurons by introducing the conditional AAV-EF1a-DIO-hChR2-mCherry into the VTA of mice expressing cre recombinase under the control of the dopamine transporter (DATicre). Using striatal slices from these mice, light evokes both rapid EPSCs and slow, inhibitory postsynaptic currents (IPSCs) in SPNs (Figure 1A,B). As expected, glutamate receptor antagonists CNQX and APV block the EPSC, and D2 dopamine receptor antagonist sulpiride completely abolishes the slow IPSC (Figure 1C,D). In addition, expression of GIRK2 was shown not to affect basic membrane properties, and the D2-IPSCs recorded here are smaller (in amplitude and duration) than those reported previously (Marcott et al., 2014).
Figure 1. Glutamate and dopamine release by dopamine neurons depress with different kinetics. See also Figure S1.
(A) DATiCre+/− mice were injected with AAV2/9.hSyn.tdTomato.T2A.GIRK2.WPRE into the medial shell of the NAc and AAV5-EF1α-DIO-hChR2-mCherry into the VTA.
(B) Representative whole cell recording from a GIRK2-expressing SPN shows light-evoked EPSC (O-EPSC) and D2 dopamine receptor-mediated inhibitory current (D2-IPSC) in response to successive stimulation with blue light at 0.4 Hz (blue bar).
(C) The AMPA receptor antagonist CNQX and NMDA receptor antagonist APV (orange) block the O-EPSC (gray).
(D) The D2 receptor antagonist sulpiride (1 μM, red) eliminates the D2-IPSC (black).
(E) Representative O-EPSC (gray) and D2-IPSC (black) responses to paired optogenetic stimulation at 2.5 s and 50 s inter-stimulus intervals (ISI). The overlapping traces show two successive responses, with the first represented by a thick line and second by a thin.
(F) Quantification of the paired-pulse response for O-EPSC (gray) and D2-IPSC (black) at different ISI. p < 0.01 by two-way ANOVA; n = 5–10 cells for each interval
(G) O-EPSCs and D2-IPSCs in response to repetitive optogenetic stimulation (60 pulses at 0.4 Hz).
(H) Summary of O-EPSC and D2-IPSC amplitude in response to repetitive stimulation at 0.4 Hz. Amplitudes were normalized to the first response in each recording. p < 10−10 by one-way ANOVA; n = 19 cells for each
The ability of vesicular glutamate transport to promote monoamine and ACh uptake requires storage of the two transmitters in at least some of the same synaptic vesicles (Gras et al., 2008; Hnasko et al., 2010; Nelson et al., 2014). Entry of the anion glutamate dissipates the lumen-positive membrane potential, thereby disinhibiting the H+-ATPase to create the low internal pH that drives monoamine and ACh uptake. Since dissipation of the pH gradient can also create the membrane potential that drives glutamate transport (Goh et al., 2011) (Figure S1A) and vesicular monoamine transport involves H+ exchange, we further assessed costorage by inhibiting VMAT2. As anticipated, the VMAT2 inhibitor TBZ (5 μM) blocks the D2-IPSC, but it also reduces the amplitude of light-evoked EPSCs (Figure S1A,B). Uptake of dopamine by VMAT2 thus promotes glutamate storage and release, indicating at least some storage of both transmitters in the same vesicles. However, the extent of costorage remains unclear.
Although previous work has not indicated a difference in the release of glutamate and dopamine by mesolimbic dopamine neurons, dopamine release was measured by electrochemical detection with a carbon fiber electrode, which reflects dopamine spillover into the bulk extracellular space (Adrover et al., 2014). Taking advantage of GIRK2 expression to compare the postsynaptic responses to dopamine and glutamate, we observe a clear difference in paired pulse ratio (PPR) between the light-evoked D2-IPSC and the EPSC (Figure 1E,F). Due to the duration of the D2-IPSC, we stimulated at relatively long intervals (2.5 s to 50 s). In slices, however, dopamine release exhibits long-lasting depression independent of D2 autoreceptors (Kennedy et al., 1992; Phillips et al., 2002; Shin et al., 2017) that enables us to compare the extent of depression even at this low stimulation frequency. The EPSC depresses more than the D2-IPSC and the difference is greatest at 2.5 s but remains throughout recovery (Figure 1E,F). In addition, prolonged stimulation at 0.4 Hz shows that the difference between D2-IPSC and EPSC persists throughout trains: the D2-IPSC remains easily detectable whereas the EPSC almost disappears (Figure 1G,H). We used the D2 antagonist sulpiride to isolate the glutamate-mediated currents, but the blockade of autoreceptors does not affect the extent of depression at this low stimulation frequency (Benoit-Marand et al., 2001; Shin et al., 2017) (Figure S1C). We also blocked GABAA, GABAB, muscarinic and D1 dopamine receptors, supporting direct rather than indirect, network effects of the transmitters released by dopamine neurons.
Use of GIRK channels to measure receptor activation has suggested that D2 dopamine receptors exhibit lower affinity than previously recognized (Marcott et al., 2014), but differences in receptor saturation might nonetheless account for the differences in synaptic depression between glutamate and dopamine. The amounts of dopamine released might decline as rapidly as the amounts of glutamate but show less apparent depression due to saturation of the first response. To assess this possibility, we reduced dopamine biosynthesis and stores by systemic injection of the tyrosine hydroxylase (TH) inhibitor α-methyl-p-tyrosine (AMPT). AMPT reduces the dopamine content in the nucleus accumbens (Figure S1D), indicating that the compound has reduced vesicular stores (Fon et al., 1997). In addition, AMPT slightly reduces EPSC amplitude (Figure S1E), as expected for the subset of synaptic vesicles storing both transmitters. However, AMPT has no effect on the synaptic depression observed with prolonged stimulation (Figure S1F), indicating that receptor saturation cannot account for the difference in depression from the EPSC.
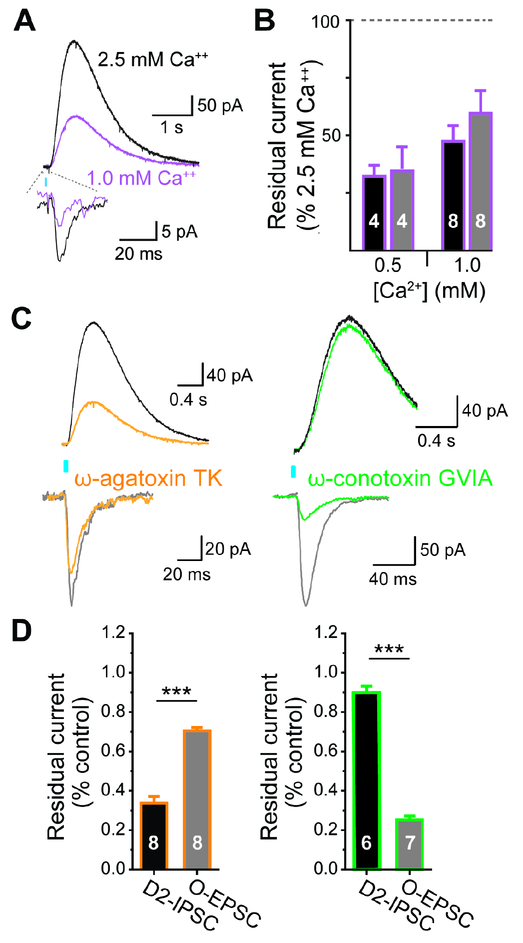
The differences in short-term plasticity between glutamate and dopamine release by VTA dopamine neurons suggest a difference in sensitivity to Ca++. Relative to 2.5 mM external Ca++, however, optogenetic activation of mesostriatal terminals produces EPSCs and D2-IPSCs of similar magnitude in 0.5 and 1.0 mM Ca++ (Figure 2A,B). Despite the lack of difference in Ca++ sensitivity, we also tested the coupling to different Ca++ channel subtypes. The P-/Q-type Ca++ channel inhibitor ω-agatoxin TK inhibits the D2-IPSC more than the EPSC (Figure 2C,D). Remarkably, the N-type ω-conotoxin GVIA inhibits the EPSC much more strongly than the D2-IPSC (Figure 2C,D). Thus, glutamate release by dopamine neurons couples preferentially to N-type channels, and dopamine release to P-/Q-type channels (Phillips and Stamford, 2000), consistent with the storage of transmitter in different vesicle populations and perhaps release at distinct sites. Since these vesicle populations contain different transmitters, intrinsic differences in molecular composition must drive the differences in release.
Figure 2. Glutamate and dopamine release by dopamine neurons couple to different presynaptic Ca++ channels.
(A) Representative recordings show O-EPSCs and D2-IPSCs in control (2.5 mM, black) and low (1.0 mM, purple) extracellular Ca++.
(B) Quantification of remaining current for the O-EPSC and D2-IPSC in 0.5 and 1.0 mM Ca++, relative to control (2.5 mM Ca++). The O-EPSC and D2-IPSC are reduced to a similar degree in both 0.5 and 1.0 mM Ca++. n = 4 for 0.5 mM Ca++ and 8 for 1.0 mM Ca++
(C) Representative recordings show O-EPSCs and D2-IPSCs recorded in control, the P/Q-type Ca++ channel blocker ω-agatoxin TK (300 nM, orange) or the N-type Ca++ channel blocker ω-conotoxin GVIA (200 nM, green).
(D) Quantification shows the per cent residual O-EPSC and D2-IPSC in ω-agatoxin TK (orange) or ω-conotoxin GVIA (green) normalized to control without drug. *** p < 0.001 by student’s t-test. Bar graphs indicate mean ± SEM. n = 8 for ω-agatoxin TK, 6–7 for ω-conotoxin GVIA
Partial Segregation of VGLUT2 and VMAT2 Within the Mesolimbic Dopamine Projection
The vesicular neurotransmitter transporters define the vesicles capable of exocytic release, and can therefore be used to identify the sites of glutamate and dopamine storage. However, the striatum receives an extensive VGLUT2+ projection from the thalamus (Hartig et al., 2003) in addition to the sparse VGLUT2 originating in midbrain dopamine neurons. Indeed, striatal VGLUT2 immunoreactivity shows remarkably little colocalization with TH (Figure S2A,D). VGLUT2 and TH have been suggested to segregate within the processes of dopamine neurons (Berube-Carriere et al., 2009; Kawano et al., 2006; Sulzer et al., 1998; Zhang et al., 2015) but VGLUT2 shows the same low colocalization with soluble fluorescent protein TdTomato expressed in dopamine neurons (Figure S2B), consistent with the predominant expression of striatal VGLUT2 by thalamic input.
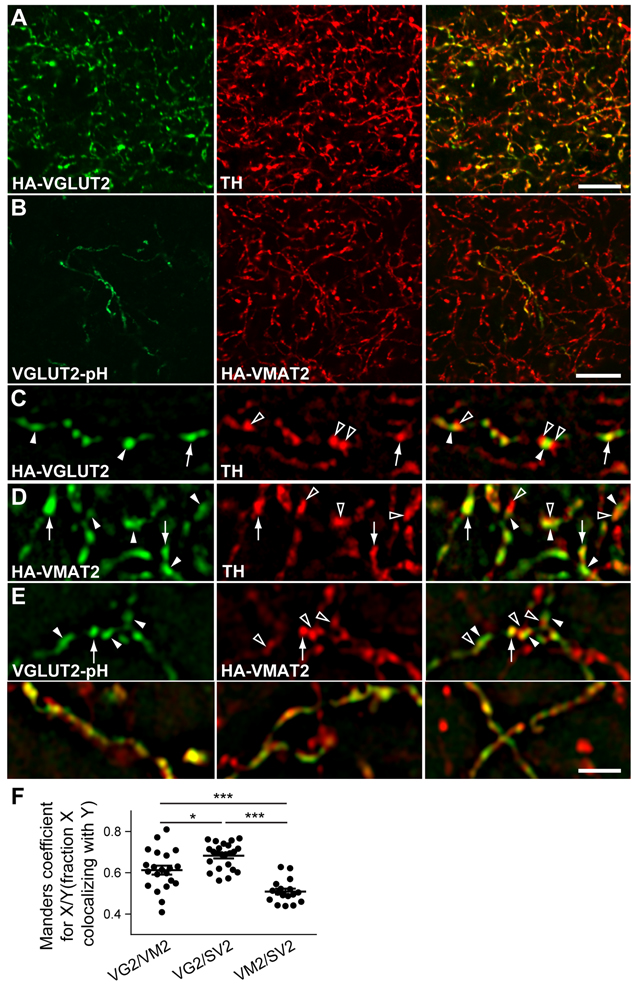
To focus on the small proportion of striatal VGLUT2+ processes made by midbrain dopamine neurons, we used adeno-associated virus (AAV) to express a cre-dependent allele of HA-tagged VGLUT2 (AAV-EF1a-DiO-VGLUT2-HA) specifically in the dopamine neurons of DATicre mice. Figure 3 (A) shows that a large fraction of the processes expressing HA-VGLUT2 double label for TH. However, the higher resolution provided by structured illumination microscopy (SIM) (Schermelleh et al., 2008) reveals that HA-VGLUT2 segregates from TH at many but not all presynaptic sites (Figure 3C). Since the available VMAT2 antibodies are not sensitive or specific (Zhang et al., 2015), we also produced HA-VMAT2 BAC transgenic mice that show appropriate localization of the transgene in monoamine neurons and their projections (Figure S3). Like TH, HA-VMAT2 shows little colocalization with total VGLUT2 (Figure S2C). However, HA-VMAT2 also differs in localization from TH (Figure 3D) even though both proteins contribute to the release of dopamine. Presumably, synaptic vesicles (containing VMAT2) simply displace the soluble biosynthetic enzyme (Figure S2A,D). To compare localization of the two transporters, we therefore produced a recombinant AAV encoding a conditional allele of VGLUT2 fused to the ecliptic pHluorin, a modified form of GFP (VGLUT2-pH) (Foss et al., 2013). Injected into the VTA of HA-VMAT2;DATicre mice, this construct colocalizes with HA-VMAT2 by standard confocal microscopy (Figure 3B). By SIM, however, HA-VMAT2 and GFP-VGLUT2 frequently segregate into adjacent but distinct punctae (Figure 3E). Quantitation confirms the reduced colocalization of VGLUT2 with VMAT2 relative to the canonical synaptic vesicle protein SV2 (Figure 3F). Consistent with this difference, SV2 colocalizes better with VGLUT2 than VMAT2 (Figure 3F). Since only dopamine neurons can express the epitope-tagged VGLUT2, these differences indicate that despite over-expression, only a fraction of the introduced VGLUT2 colocalizes with VMAT2 (Hnasko et al., 2010), consistent with the difference between glutamate and dopamine release. At the sites where they colocalize, however, this analysis does not indicate whether the two transporters colocalize on the same vesicles.
Figure 3. Epitope-tagged VGLUT2 expressed specifically in DAT+ neurons partially colocalizes with proteins involved in dopamine release. See also Figures S2 and S3.
The ventral tegmental area (VTA) of DATicre (A,C) or DATicre;HA-VMAT2 BAC transgenic mice (B,E) was injected with recombinant AAV encoding cre-dependent HA-tagged VGLUT2 (AAV-DiO-HA-VGLUT2) (a,c) or cre-dependent pHluorin-tagged VGLUT2 (AAV-DiO-VGLUT2-pH) (B,E). Four weeks later, sections through the ventral striatum were double stained for HA and TH (A,C,D) or GFP and HA (B,E).
(A) Representative confocal image of the ventral striatum from DATicre mice injected with AAV-DiO-HA-VGLUT2 and double stained for the conditional HA-VGLUT2 (green) and TH (red).
(B) Representative confocal image of DAT iCre;VMAT2-HA mice injected with AAV-DIO-VGLUT2-pHluorin and double stained for GFP and HA-VMAT2. Scale bars in A and B indicate 10 μm.
(C) Representative image acquired by structured illumination microscopy (SIM) of ventral striatum double stained for conditional HA-VGLUT2 and TH.
(D) SIM image of the ventral striatum from HA-VMAT2 BAC transgenic mice double stained for HA and TH. Filled arrowheads in C and D indicate HA immunoreactivity alone, open arrowheads TH alone and arrows double labeled punctae.
(E) Image of the ventral striatum from DATiCre/VMAT2-HA mice injected with AAV-DIO-VGLUT2-pHluorin double stained for GFP and HA-VMAT2. Filled arrowheads indicate GFP alone, open arrowheads HA alone and arrows double labeling for both. Scale bar indicates 2 μm (C-E).
(F) Colocalization was quantified by Manders coefficient (fraction X colocalizing with Y for X/Y = VGLUT2/VMAT2, VGLUT2/SV2 and VMAT2/SV2). *, p < 0.05, ***, p < 0.001 by one-way ANOVA with post hoc Bonferroni’s test. In 3 mice, n = 21 fields for VGLUT2/VMAT2, 22 for VGLUT2/SV2 and 17 for VMAT2/SV2.
Direct Imaging of VGLUT2 and VMAT2 Reveals Differences in the Kinetics of Exocytosis
Since differences in the properties or location of receptors might account for the observed differences in signaling by glutamate and dopamine, we sought direct evidence for differences in the regulated fusion of synaptic vesicles releasing the two transmitters. To identify the two vesicle populations, we took advantage of the ecliptic pHluorin, a pH-sensitive form of GFP, that we previously fused to the lumenal domains of VMAT2 and VGLUT2 (Foss et al., 2013; Onoa et al., 2010; Voglmaier et al., 2006). Quenched at the low pH of synaptic vesicles, the fluorescence of these fusion proteins increases with exposure to higher pH at exocytosis, then declines again after endocytosis, when the vesicle reacidifies (Miesenböck et al., 1998). We previously found that a substantially smaller fraction of VMAT2 resides on vesicles responsive to stimulation (recycling pool) than for other synaptic vesicle proteins including the VGLUTs, and the difference persisted in cultured hippocampal as well as midbrain dopamine neurons (Onoa et al., 2010). However, this may simply reflect the localization of VMAT2 to other membranes, such as endosomes, from which it cannot be mobilized by stimulation, rather than a difference in the kinetics of release by vesicles that do respond to stimulation.
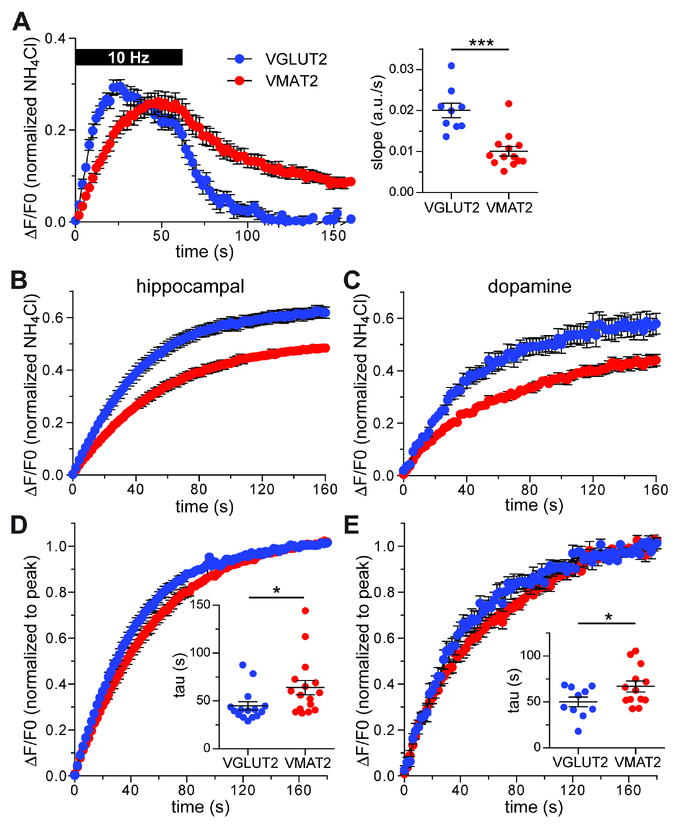
To examine the kinetics of exocytosis, we introduced the pHluorin-tagged transporters by lentivirus into hippocampal and midbrain cultures, stimulating at 10 Hz for 60 s and normalizing the response to total reporter detected by alkalinization in NH4Cl. In both hippocampal neurons and dopamine neurons (identified using the cre-dependent tdTomato reporter in midbrain cultures from DATicre mice), the fluorescence increases considerably faster for VGLUT2- than VMAT2-pHluorin (Figure 4A). Since endocytosis also occurs during stimulation, we used the H+ pump inhibitor bafilomycin A1, which prevents vesicle reacidification and hence focuses on the delivery of new synaptic vesicles. Stimulating at 10 Hz for 3 minutes to empty the recycling pool, a larger proportion of VGLUT2 than of VMAT2 responds to stimulation in both hippocampal and dopamine neurons (Figure 4B,C), consistent with previous observations (Onoa et al., 2010). To determine whether the rates of release differ, we then normalized the fluorescence response to the peak at the end of stimulation. Even when normalized to peak response, VGLUT2-pHluorin responds more rapidly than VMAT2-pHluorin, again in both hippocampal and midbrain dopamine neurons (Figure 4D,E). Independent of recycling pool size, the imaging thus shows an intrinsic difference in the release rate of glutamate and monoamine vesicles, providing direct functional evidence for storage of the two transmitters in distinct vesicles, both of which respond to stimulation. The faster release of VGLUT2 than VMAT2 is consistent with the higher intrinsic release probability of glutamate predicted by the increased short-term depression of EPSCs relative to D2-IPSCs by slice physiology. Differences in behavior of the two transporters thus correspond to the differences in transmitter release.
Figure 4. VGLUT2 and VMAT2 differ in response to stimulation in hippocampal as well as dopamine neurons.
(A) Mean response of VGLUT2-pHluorin (blue) and VMAT2-pHluorin (red) to 10 Hz stimulation for 60 s in hippocampal neurons from wild type mice (left panel). The fluorescence is normalized to that observed after alkalinization in 50 mM NH4Cl. The right panel shows the average initial slope for each coverslip. ***, p = 0.0003 by Mann-Whitney test. n = 9 coverslips for VGLUT2 and 13 coverslips for VMAT2
(B,C) Mean response of VGLUT2- and VMAT2-pHluorin in hippocampal (B) and dopamine (C) neurons to 10 Hz stimulation for 3 minutes in 600 nM bafliomycin, normalized to fluorescence in NH4Cl.
(D,E) Mean response of VGLUT2- and VMAT2-pHluorin to 10 Hz stimulation for 3 minutes in bafilomycin, normalized to the peak response at the end of stimulation in hippocampal (D) and dopamine (E) neurons. Insets show the quantification of exocytic time constant per coverslip.
Data in all panels indicate mean ± SEM. n = 15 coverslips for VGLUT2 and 16 coverslips for VMAT2 in hippocampal neurons. *, p = 0.0208 by Mann-Whitney test.
n = 10 coverslips for VGLUT2 and 13 for VMAT2 in dopamine neurons. *, p = 0.0453 by student’s t-test.
Dual Imaging Reveals Bouton-Specific Properties of VGLUT2 and VMAT2 Exocytosis
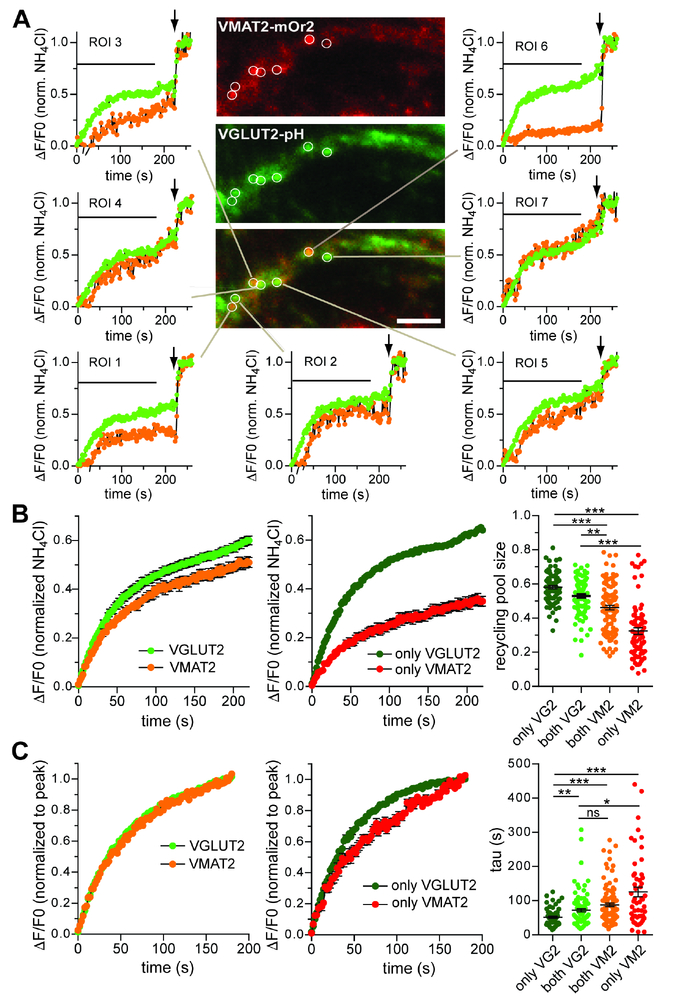
Intrinsic differences in synaptic vesicles apparently drive variation in release but differences in location (Figure 3) (Zhang et al., 2015) may contribute to the expression of these differences. To address this possibility, we simultaneously imaged the behavior of both transporters. Replacing pHluorin in the lumenal loop of VMAT2 by the red-shifted pH-sensitive fluorescent protein mOrange2, we introduced this construct and VGLUT2-pHluorin by lentivirus, conferring expression in essentially all cells. For these experiments, we used hippocampal neurons because the mOr2 reporter precludes the use of tdTomato to identify midbrain dopamine neurons and because hippocampal neurons show the same difference between transporters as the dopamine neurons (Figure 4). Using NH4Cl to reveal the total expression of each transporter (Figure 5A), we found a moderate correlation between VMAT2-mOr2 and VGLUT2-pHluorin (r = 0.54) (Figure S4A). However, many boutons express low levels of one reporter despite high levels of the other, and the data appear skewed toward a higher incidence of boutons with more VGLUT2 and less VMAT2, regardless of whether they were identified using GFP or mOr2 (data not shown). The two proteins can thus differ in location, consistent with distinct as well as overlapping release sites suggested by the analysis of dopamine projections in vivo.
Figure 5. The exocytosis of VGLUT2 and VMAT2 differs at individual boutons. See also Figure S4.
(A) Representative image from a hippocampal culture transduced with lentiviruses encoding VGLUT2-pHluorin (VGLUT2-pH, green) and VMAT2-mOrange2 (VMAT2-mOr2, red) and incubated in NH4Cl (50 mM) to alkalinize acidic intracellular compartments. Circles indicate individual boutons expressing variable amounts of the two fluorescent proteins and corresponding curves the fluorescence response to 10 Hz stimulation for 3 minutes in bafilomycin (600 nM). Horizontal lines indicate 10 Hz stimulation and the arrows indicate addition of 50 mM NH4Cl.
(B) VGLUT2-pH and VMAT2-mOr2 exhibit a similar recycling pool size when analyzed at boutons expressing both proteins but differ at boutons that express only one of the two fluorescent transporters (p < 0.0001 by Kruskal-Wallis one-way ANOVA with Dunn’s post hoc test). **, p < 0.01; ***, p < 0.001
(C) Normalization to the peak response shows a similar rate of fluorescence increase for VGLUT2-pH and VMAT2-mOr2 at boutons expressing both proteins but an increased rate for VGLUT2 and a reduced rate for VMAT2 when comparing boutons that express only one of the transporters (p < 0.0001 by Kruskal-Wallis one-way ANOVA with Dunn’s post hoc test). **, p < 0.01; ***, p < 0.001
Data in panels B and C indicate mean ± SEM. n = 104 VGLUT2+/VMAT2+, 83 VGLUT2+ and 75 VMAT2+ boutons from 10 coverslips and 3 independent cultures.
We also used dual imaging to determine whether the differences in response reflect targeting of the two reporters to different vesicles at the same bouton. Focusing on boutons that express both reporters, we found that the proportion of VMAT2 responsive to stimulation varies considerably at different boutons, in contrast to the typical synaptic vesicle protein VGLUT2 which responds more consistently (Figures 5A, S4B). Thus, the reporters must localize at least in part to different vesicles even when expressed at the same bouton. Surprisingly, VMAT2 responds to a lesser extent when total VMAT2 predominates and to a greater extent at boutons where total VGLUT2 predominates. The response of VMAT2 thus correlates with the expression of VGLUT2, and raises the possibility that VGLUT2 influences the trafficking of VMAT2.
We then assessed differences in the kinetics of release, normalizing to the maximum fluorescence after 10 Hz stimulation in bafilomycin. Despite the different rates of exocytosis observed when imaging the reporters individually (Figure 4D,E), Figure 5C shows that the average time constant of exocytosis does not differ between the two reporters imaged at the same boutons. Since colocalization with VGLUT2 may determine the properties of VMAT2, the differences observed by imaging the two reporters separately may reflect those boutons which contain only one reporter.
Using alkalinization in NH4Cl to identify boutons that contain only one of the two reporters, we first examined recycling pool size. Figure 5 (B) shows that the difference between recycling pool size of VGLUT2 and VMAT2 differs much more dramatically when the transporters localize to distinct sites, and the increased difference derives from the reduced recycling pool size of VMAT2 when expressed alone, without VGLUT2. The kinetics of release also differ more dramatically at boutons containing only one reporter, in this case driven almost equally by increases in the rate of exocytosis by VGLUT2 and by decreases in VMAT2 (Figure 5C). Thus, targeting of the transporters to distinct release sites contributes to the differences in rate of release.
Glutamate and Dopamine Release Differ in Dependence on Stimulation Frequency
Previous work has shown that the slow Ca++ chelator EGTA inhibits the response of VMAT2-pHluorin in dopamine neurons to a greater extent than the response of VGLUT1-pHluorin in hippocampal neurons (Pan and Ryan, 2012), indicating that the monoamine vesicles are more loosely coupled to presynaptic Ca++ channels (Eggermann et al., 2012). This difference was attributed to the expression in dopamine neurons of the calcium-buffering protein calbindin (Pan and Ryan, 2012). However, the differential effect of conotoxins (Figure 2) now indicates that coupling to Ca++ entry can differ among different synaptic vesicle populations within a single neuron. To assess the extent of coupling to presynaptic Ca++ channels, we again used the pHluorin reporters expressed in hippocampal neurons, monitoring their sensitivity to the slow Ca++ buffer EGTA which limits the access of entering Ca++ to more distant synaptic vesicles but not those directly adjacent to the site of Ca++ entry. After loading with EGTA-AM (100 μM) or vehicle control, we examined the response of both reporters to brief, high frequency stimulation (40 Hz for 2.5 s). Even in hippocampal neurons, EGTA inhibits the VMAT2 response to a greater extent than that of VGLUT2 (Figure S5A), again consistent with the lower probability of dopamine release predicted by paired pulse stimulation.
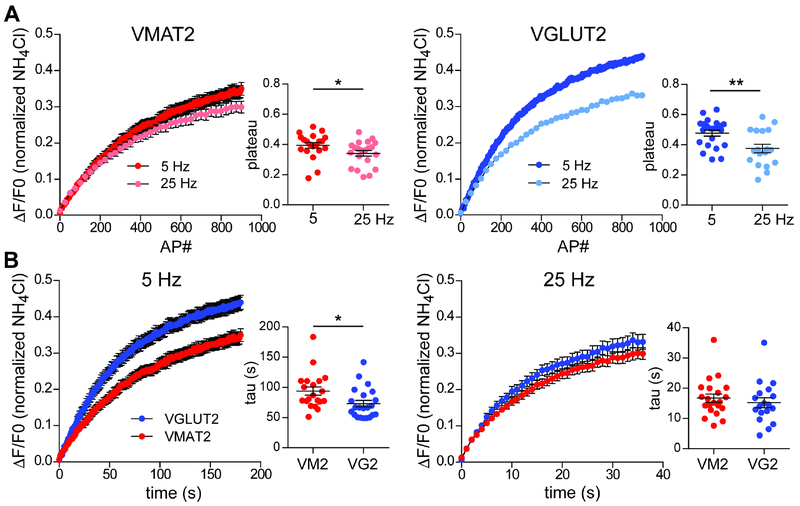
We also used the pHluorin reporters in bafilomycin to examine the frequency dependence of exocytosis by the different synaptic vesicle populations. Normalizing to stimulus number, VMAT2 shows little decrement at 25 Hz relative to 5 Hz (Figure 6A). In contrast, VGLUT2 shows a large decrement at 25 Hz, again consistent with vesicle depletion due to high release probability. Comparing the two transporters directly, VMAT2 shows less response than VGLUT2 at 5 Hz (Figure 6B), as predicted by the lower probability of dopamine release. At 25 Hz, however, the transporters show a similar response, consistent with the ability of VMAT2-containing synaptic vesicles to increase exocytosis with high frequency stimulation and of dopamine signaling to encode frequency. Further, VMAT2 exhibits the same difference from VGLUT2 in hippocampal neurons, indicating that the mechanisms responsible are not restricted to dopamine neurons (Figure S5B).
Figure 6. Exocytosis of VMAT2 and VGLUT2 differ in frequency dependence. See also Figure S5.
Dopamine neuron cultures from DATiCre/B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice were stimulated with 900 action potentials (APs) at either 5 or 25 Hz in the presence of bafilomycin, and the fluorescence normalized to that in the presence of NH4Cl.
(A) Fitting the response to a single exponential, VMAT2-pH fluorescence reaches a slightly lower plateau after 900 AP stimulation at 25 Hz than after the same number of APs at 5 Hz (*, p = 0.0294 by Mann-Whitney test). VGLUT2-pH reaches a substantially lower plateau at 25 Hz relative to 5 Hz (**, p = 0.0044 by unpaired t-test).
(B) Comparison of the two reporters stimulated at 5 Hz shows a faster response for VGLUT2-pH than VMAT2-pH (*, p = 0.0437 by unpaired t-test). At 25 Hz, the rate of response is similar for VGLUT2- and VMAT2-pH (p = 0.1643 by unpaired t-test).
Data show mean ± SEM. n = 22 coverslips for VGLUT2-pH at 5 Hz and 17 coverslips at 25 Hz; for VMAT2-pH, n = 20 coverslips for both 5 and 25 Hz stimulation.
Synaptic Vesicles Releasing Glutamate and Dopamine Form Through Different Recycling Pathways
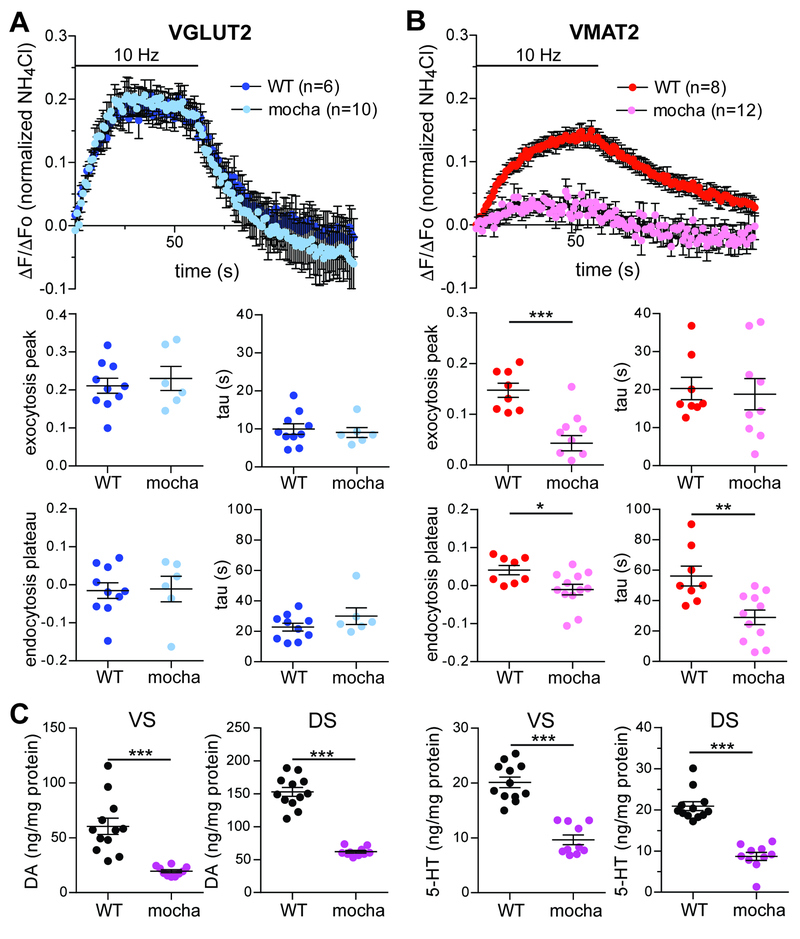
The differences in neurotransmitter content and release properties indicate that synaptic vesicles containing glutamate and dopamine differ in identity, predicting that they also differ in biogenesis. Although synaptic vesicles generally form through a clathrin-dependent mechanism that involves the heterotetrameric adaptor protein AP-2 (Kononenko et al., 2014; Saheki and De Camilli, 2012; Watanabe et al., 2014), specific proteins such as the zinc transporter ZnT3 and v-SNARE VAMP7 require the related adaptor AP-3 for targeting to synaptic vesicles (Salazar et al., 2004; Scheuber et al., 2006). Loss of AP-3 in mocha mice has little effect on synaptic transmission, but does impair asynchronous release at hippocampal mossy fiber synapses (Evstratova et al., 2014). Thus, we considered the possibility that AP-3 contributes to the formation of specific synaptic vesicles that might also differ in neurotransmitter content. To test this, we imaged the behavior of VGLUT2- and VMAT2-pHluorin in dopamine neurons from wild type and mocha mice. As predicted from the modest effects on excitatory transmission (Evstratova et al., 2014; Voglmaier et al., 2006), VGLUT2-pHluorin responds to stimulation and undergoes compensatory endocytosis with the same kinetics in both wild type and mocha neurons (Figure 7A). However, the mocha mutation greatly reduces the response of VMAT2-pHluorin to stimulation (Figure 7B). Considering the role of AP-3 in synaptic vesicle formation, the requirement for AP-3 of VMAT2 but not VGLUT2 suggests that AP-3 specifically contributes to the formation of vesicles that store dopamine, not glutamate. The rate and extent of compensatory endocytosis also appears enhanced by the mocha mutation, suggesting redistribution of the transporter to a different recycling pathway in the absence of AP-3.
Figure 7. Loss of AP-3 impairs the response of VMAT2-pHluorin to stimulation without affecting VGLUT2-pHluorin. See also Figure S6.
Dopamine neurons were identified using the fluorescent cocaine analog JHC 1–64 (30 nM), the cultures stimulated at 10 Hz for 60 s, and the fluorescence normalized to that observed after alkalinization in NH4Cl.
(A) Mean response of VGLUT2-pH in dopamine neurons from wild type (dark blue) and mocha mice (light blue). The rate of fluorescence increase (p = 0.6676 by unpaired t-test) and peak fluorescence (p = 0.5920 by unpaired t-test) during stimulation as well as the rate of fluorescence decline (p = 0.4278 by unpaired t-test) and plateau (p = 0.9136 by unpaired t-test) after stimulation show no difference between wild type and mocha neurons. Data indicate mean ± SEM. n = 10 coverslips for wild type and 6 coverslips for mocha neurons
(B) Mean response of VMAT2-pH in dopamine neurons from wild type (red) and mocha mice (pink). The peak fluorescence response shows a substantial reduction in mocha neurons relative to wild type (***, p = 0.0001 by unpaired t-test) whereas exocytosis kinetics do not differ (p = 0.7747 by unpaired t-test). Compensatory endocytosis reaches a lower plateau (*, p = 0.0180 by unpaired t-test) with faster kinetics (**, p = 0.0032 by unpaired t-test) in neurons from mocha mice relative to wild type. Data indicate mean ± SEM. n = 8 coverslips for wild type and 12 coverslips for mocha neurons
(C) The mocha mutation greatly reduces dopamine (DA) and serotonin (5-HT) levels in the ventral striatum (VS) and dorsal striatum (DS). ***, p < 0.001 by unpaired t-test. n = 12 for WT and 10 for mocha
In addition to the defect in VMAT2 exocytosis, imaging of the pHluorin reporter showed a major reduction in total presynaptic VMAT2 assessed by unquenching of the intracellular protein in NH4Cl (1436 ± 71 au, n = 120 boutons for WT vs. 753 ± 33 au, n = 165 boutons for mocha, p < 0.0001 by Mann-Whitney). In contrast, VGLUT2-pHluorin showed no difference between mocha and wild-type (750 ± 40 au, n = 131 boutons for WT vs. 672 ± 35 au, n = 90 boutons for mocha, p = 0.343 by Mann-Whitney). This suggested an additional defect in axonal targeting of specifically VMAT2 that should affect the vesicular stores of dopamine. To test this possibility in vivo, we measured the tissue content of monoamine, which reflects vesicular stores (Fon et al., 1997; Takahashi et al., 1997; Wang et al., 1997). Figure 7C shows that the mocha mutation greatly reduces tissue dopamine and serotonin content in both regions. However, the metabolites of dopamine and serotonin both show either less or no reduction in the absence of AP-3 (Figure S6). The metabolite/monoamine ratio thus suggests increased monoamine turnover in mocha mice. Since the pHluorin imaging and catecholamine levels suggest reduced presynaptic localization of VMAT2 in mocha neurons, we also measured the binding of specific VMAT2 ligand [3H]-tetrabenazine to striatal homogenates, observing an ~50% reduction in mocha tissue relative to wild-type (Figure 8L). Thus, mocha mice show impaired axonal targeting of VMAT2 in vivo.
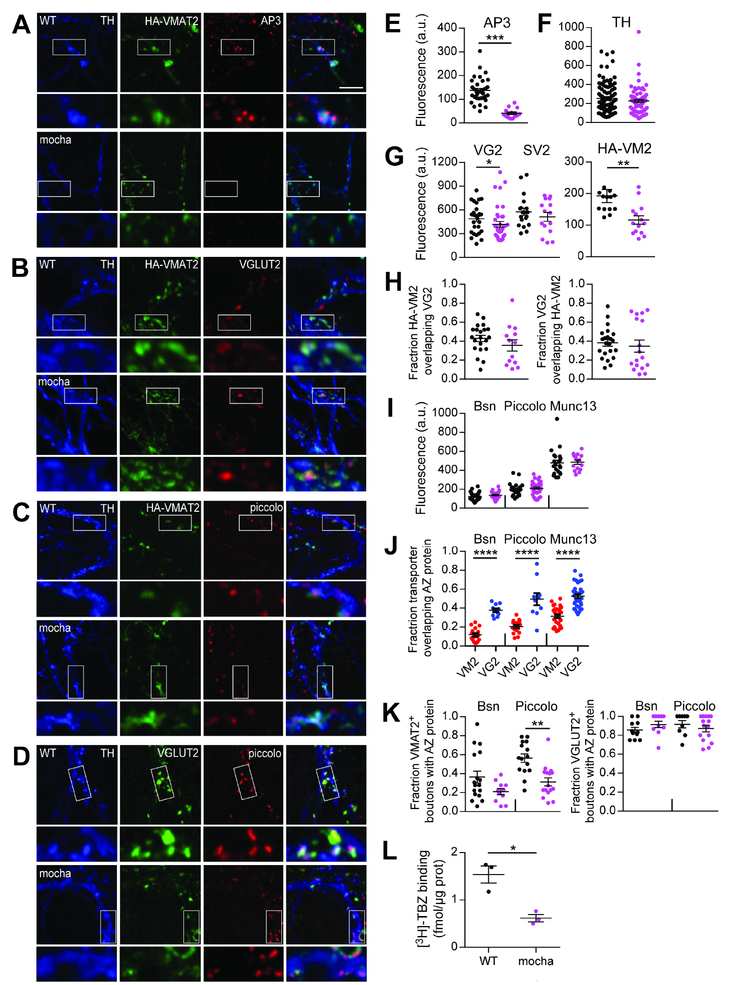
Figure 8. Loss of AP3 reduces VMAT2 localization to dopamine neuron release sites.
(A-D) Confocal images of midbrain cultures from HA-VMAT2/WT mice (above) and HA-VMAT2/mocha mice (below). (A) TH+ neurons label for AP-3 in WT but not mocha cultures (quantified in E). (B) VGLUT2 and VMAT2 colocalize in a subset of TH+ boutons in both wild type and mocha cultures (quantified in H). (C) Piccolo colocalizes with VMAT2 in a subset of TH+ boutons in both WT and mocha cultures. The mocha mutation reduces the colocalization of piccolo and VMAT2 (quantified in K). (D) Piccolo colocalizes with VGLUT2 in a subset of TH+ boutons from wild type and mocha mice (quantified in K). Outlined areas are magnified below. Scale bar, 5 um.
(E-K) Quantitation of the results shown in (A-D), with WT in black and mocha in magenta. The mocha mutation eliminates immunolabeling for AP3 (p < 0.0001 by Mann-Whitney) (E) (n = 34 images for WT and 16 images for mocha from 3 coverslips of each), and does not affect immunofluorescence intensity for TH (p = 0.4756 by Mann-Whitney) (F) (n = 90 images for WT and 79 images for mocha from 9 coverslips of each). (G) The boutons of mocha neurons show reduced labeling intensity for both VGLUT2 and VMAT2 but not SV2. p = 0.0227 by Mann-Whitney for VGLUT2 (n = 38 for WT and 47 for mocha from 6 coverslips of each), p = 0.0017 by Mann-Whitney for VMAT2 (n = 13 images for WT and 14 for mocha from 3 coverslips of each) and p = 0.3865 by Mann-Whitney for SV2 (n = 18 images for WT and 15 for mocha from three coverslips of each). (H) Colocalization in TH+ neurons of VGLUT2 and HA-VMAT2 (Manders coefficients) is unaffected by the loss of AP3. p = 0.1555 by unpaired t-test for HA-VMAT2/VGLUT2 (left) and p = 0.7220 by Mann-Whitney for VGLUT2/HA-VMAT2 (right). n = 22 images for WT and 15 for mocha. (I) The intensity of labeling for active zone proteins bassoon (Bsn), piccolo and munc13 is unaffected by the mocha mutation. p = 0.1094 for bassoon (n = 35 images for WT and 27 for mocha from 3 coverslips of each), p = 0.3369 for piccolo (n = 23 images for WT and 35 for mocha from 3 coverslips of each) and p = 0.2759 for munc13 (n = 23 images for WT and 15 for mocha from 3 coverslips of each), all by unpaired t-test. (J) VGLUT2+ punctae show greater colocalization than HA-VMAT2+ punctae with active zone proteins bassoon, piccolo and munc13. p < 0.0001 by unpaired t-test for bassoon, piccolo and munc13. n = 17 images for bassoon/VM2, 11 for bassoon/VG2, 16 for piccolo/VM2, 10 for piccolo/VG2, 31 for munc13/VM2 and 35 for munc13/VG2 (K) The mocha mutation reduces the fraction of VMAT2+ boutons colocalizing with piccolo (p = 0.0004, n = 15 images for WT, 16 for mocha) but not bassoon (p = 0.1454, n = 17 images for WT, 11 for mocha) or the fraction of VGLUT2+ boutons with either active zone protein (p = 0.1386 for VGLUT2/bassoon, n = 11 images for WT and 10 for mocha and p = 0.3983 for VGLUT2/piccolo (n = 9 images for WT and 15 for mocha), all by unpaired t-test.
(L) Specific [3H]-tetrabenazine binding is severely reduced in the striatal homogenates of mocha mice relative to WT (*, p < 0.05 by two-way ANOVA). n = 3 mice/genotype.
Scatterplots show mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001
To characterize the role of AP-3 in the structure of release sites formed by dopamine neurons, we prepared midbrain cultures from HA-VMAT2 BAC transgenic mice with and without the mocha mutation. Figure 8(A,E) shows that AP-3 colocalizes with VMAT2 at TH+ boutons, and mocha neurons do not label for AP-3, indicating antibody specificity. The mocha mutation reduces expression of HA-VMAT2 more significantly than that of VGLUT2 and does not affect labeling for SV2, consistent with a more important role for AP-3 in dopamine release (Figure 8A,F,G). Despite the reduction in transporter labeling, the extent of colocalization remains unaffected by the loss of AP-3, although the extent of colocalization varies greatly among cells (Figure 8B,H).
To determine whether the effect of mocha on release reflects a change in the localization of VMAT2+ and VGLUT2+ vesicles to the active zone, we stained for active zone proteins bassoon, piccolo and munc13. Loss of AP-3 does not affect the average fluorescence intensity of TH+ punctae that label for these proteins (Figure 8C,D,I). Consistent with recent work that indicates synaptic signaling by dopamine at active zones (Liu et al., 2018; Marcott et al., 2014), VMAT2 colocalizes with all three active zone proteins. However, VGLUT2 colocalizes with them to a greater extent than VMAT2 (Figure 8C,D,J), supporting a more synaptic mode of signaling by glutamate. Indeed, almost all VGLUT2+ boutons contain active zone proteins and this proportion is unaffected by the mocha mutation. In contrast, a much smaller proportion of VMAT2+ boutons label for an active zone protein, and the mocha mutation selectively reduces the fraction of boutons colocalizing with piccolo, not bassoon (Figure 8C,D,K). Thus, loss of AP-3 does not disrupt presynaptic architecture. Rather, it selectively influences monoamine release sites, and the reduced colocalization with piccolo presumably reflects the disturbance in monoamine vesicles.
Discussion
The results show that dopamine neurons release glutamate and dopamine from synaptic vesicles with different functional properties. The rates of synaptic depression observed for the two transmitters in striatal slices strongly suggest a presynaptic locus for the differences in signaling, and dependence on different presynaptic Ca++ channels further supports a difference in release. Taking advantage of the vesicular transporters to label the two vesicle populations, imaging in culture provides direct evidence for differences in their behavior. The slower response of VMAT2 than VGLUT2 is consistent with the low release probability inferred from slice recording. Increased sensitivity to EGTA supports the looser coupling of VMAT2+ vesicles to presynaptic Ca++ channels. On the other hand, the effect of glutamate on dopamine (and ACh) storage (Gras et al., 2008; Hnasko et al., 2010; Nelson et al., 2014; Tritsch et al., 2012) and here of dopamine on glutamate storage suggest that a subpopulation of synaptic vesicles store both transmitters. Although the transporters frequently differ in localization, they can therefore occur on the same vesicles.
Previous work has shown that differences in the properties of transmitter release reflect differences in the association of synaptic vesicles with the cytoskeleton, synaptic ribbons and coupling to presynaptic Ca++ channels (Chi et al., 2001; Eggermann et al., 2012; Midorikawa et al., 2007). However, the demonstration that synaptic vesicles differ in transmitter content, and content correlates with the properties of release, means that differences in vesicle identity drive the differences in release and may indeed dictate the differences in cytoskeletal association and localization. Further, the selective effect of the mocha mutation on trafficking of VMAT2 demonstrates that monoamine vesicles require a recycling pathway that involves AP-3 in addition to (or perhaps instead of) the canonical clathrin adaptor AP-2 which regenerates most synaptic vesicles (Kononenko et al., 2014; Saheki and De Camilli, 2012; Watanabe et al., 2014). We infer that the pathway of recycling generates synaptic vesicles with different release properties, suggesting a previously unrecognized linkage between specific endocytic and exocytic limbs of the synaptic vesicle cycle.
A previous report has suggested that midbrain dopamine neurons form entirely distinct release sites for glutamate and dopamine (Zhang et al., 2015). The analysis by super-resolution microscopy now shows that the two sites are usually adjacent. The apparent discrepancy may reflect the difference in resolution between light and electron microscopy, but we have also found that the most strongly VGLUT2+ striatal boutons derive from the thalamic projection. Expression of a conditional, epitope-tagged VGLUT2 was thus required to identify the VGLUT2 in dopaminergic boutons. Despite over-expression of VGLUT2, we observe partial segregation of the two vesicle populations. Immunoisolation has also been used to assess the colocalization of VMAT2 and VGLUT2 on striatal synaptic vesicles, showing either some or no colocalization (Hnasko et al., 2010; Zhang et al., 2015). However, the inefficiency of immunoisolation together with the expression of VGLUT2 by abundant thalamic inputs that do not express VMAT2 and the expression of VGLUT2 by only a subset of dopamine inputs make it difficult to draw conclusions from this data about segregation of the two transporters within dopamine neurons.
Why did voltammetry not detect a difference in release of glutamate and dopamine (Adrover et al., 2014)? Voltammetry measures dopamine overflow out of the synapse, and clearance by the dopamine transporter may underestimate the extent of release, increasing the apparent depression to resemble that observed for EPSCs. In contrast, the D2-IPSC measures the direct postsynaptic response. Previous work has suggested that dopamine release depends more on N-type Ca++ channels (Brimblecombe et al., 2015; Phillips and Stamford, 2000), but this may also reflect the measurement of overflow by voltammetry.
The differences in release of glutamate and dopamine provide a physiological rationale for different synaptic vesicle recycling pathways. AP-3 regenerates synaptic vesicles from endosomes (Blumstein et al., 2001; Faundez et al., 1997, 1998) and appears required for the localization of particular proteins to synaptic vesicles (Kantheti et al., 1998; Newell-Litwa et al., 2010; Scheuber et al., 2006). However, mocha mice lacking AP-3 form synapses with relatively normal excitatory transmission (Evstratova et al., 2014; Martinez-Arca et al., 2000; Voglmaier et al., 2006). The preferential effect of AP-3 loss on VMAT2 rather than VGLUT2 now provides an explanation for the modest effects previously reported—AP-3 is required specifically for the release of dopamine, not glutamate. Since VMAT2 transports norepinephrine and serotonin, we presume that this mechanism contributes to the regulated release of all monoamine transmitters. Consistent with a role for AP-3 in the recycling of monoamine synaptic vesicles, we find that the adaptor localizes to presynaptic boutons. The known role of AP-3 at endosomes further suggests that VMAT2 recycles through this compartment rather than directly from the plasma membrane.
However, loss of AP-3 also appears to reduce the axonal localization of VMAT2. Mocha mice show a large depletion of striatal monoamine content, which reflects vesicular stores and/or the activity of VMAT2. Live imaging of VMAT2-pHluorin in the presence of NH4Cl shows a reduction in total presynaptic VMAT2 in mocha neurons. Immunofluorescence for HA-VMAT2 confirms the reduction in vitro, and the effect of the mocha mutation on VGLUT2 is less significant, supporting the specificity suggested by live imaging. Further, striatal homogenates from mocha mice show greatly reduced binding to the VMAT2 ligand 3H-tetrabenazine. These results indicate a role for AP-3 in axonal targeting of VMAT2, in addition to its local, presynaptic role in vesicle recycling. Interestingly, AP-3 is required for presynaptic differentiation in C. elegans (Li et al., 2016), and the reduced axonal targeting of VMAT2 in mocha mice presumably reflects this ancestral role in neuronal polarity.
Previously, we found that AP-3 also sorts VMAT2 to peptidergic large dense core vesicles (LDCVs) (Asensio et al., 2010; Sirkis et al., 2013). Since LDCVs can deliver membrane proteins to the cell surface where they recycle into synaptic vesicles (Strasser et al., 1999), the axonal targeting of VMAT2 may involve LDCVs. The exocytosis of LDCVs also requires strong stimulation due to their distance from the sites of Ca++ entry, raising the possibility that localization to LDCVs accounts for the differences in release from glutamate. However, imaging of LDCV exocytosis with peptide-pHluorin reporters, in neurons as well as endocrine cells, shows sudden, discrete increases in fluorescence sparsely distributed throughout the axon (Logan et al., 2017; van de Bospoort et al., 2012), very different from the general, graded increase in synaptic fluorescence reported here for VMAT2-pHluorin. Despite repeated attempts, we have rarely observed abrupt, focal exocytosis of VMAT2-pHluorin in neurons (unpublished observations). In addition, although VMAT2 localizes to LDCVs in endocrine cells, the transporter targets strongly to synaptic vesicles in neurons (Zhang et al., 2015), and dopamine neurons contain very few LDCVs (Nirenberg et al., 1997, 1998). Thus, release from LDCVs cannot account for the properties of VMAT2-pHluorin reliably elicited by stimulation in neurons.
The difference in release probability for VGLUT2+ and VMAT2+ vesicles, together with the differences in coupling to presynaptic Ca channels, suggest that the two vesicle populations may also differ in their targeting to the presynaptic active zone. Indeed, VGLUT2 colocalizes to a greater extent than VMAT2 with bassoon, piccolo and munc13. Consistent with recent evidence for synaptic signaling by dopamine (Liu et al., 2018; Marcott et al., 2014), a substantial fraction of VMAT2+ boutons nonetheless contain all three active zone proteins. Interestingly, loss of AP-3 specifically reduces the presence of piccolo at VMAT2+ boutons, with no effect on bassoon or expression of either active zone protein at VGLUT2+ boutons. The trafficking mediated by AP-3 thus influences specific features of the active zone but only at VMAT2+ boutons, consistent with its role in trafficking of this transporter and presumably other proteins that recycle through the same pathway.
The results also show that VGLUT2 and VMAT2 respond differently to stimulation in hippocampal as well as midbrain dopamine neurons. This suggests the transporters target to synaptic vesicle populations that exist in many neuronal populations. In hippocampal neurons, loss of AP-3 indeed impairs asynchronous glutamate release (Evstratova et al., 2014). Thus, AP-3 may target VMAT2 to synaptic vesicles that confer asynchronous release, consistent with weak coupling to presynaptic Ca++ channels. VGLUT2 sorts to synaptic vesicles that couple more tightly, and VGLUT recycling depends primarily on the clathrin adaptor AP-2 (Foss et al., 2013; Kim and Ryan, 2009). However, other adaptors such as AP-3 may target the VGLUTs to vesicles with low release probability, conferring the observed asynchronous release (Evstratova et al., 2014; Li et al., 2017; Salazar et al., 2005).
Dual imaging of VGLUT2 and VMAT2 shows that exocytosis of the two vesicle populations can differ considerably at individual boutons, supporting the segregation to distinct vesicles even when they colocalize by light microscopy. Indeed, recent work on the corelease of GABA by cholinergic basal forebrain neurons has shown differences in release of these two transmitters despite storage at the same release site by electron microscopy (Takacs et al., 2018). The segregation of release sites in dopamine neurons suggests differences from basal forebrain neurons, and we find differences in recycling pool size and exocytosis kinetics for VMAT2 and VGLUT2 are larger when comparing sites that contain only one of the two transporters. Although vesicle identity appears to drive the differences in release, the release site thus contributes to these differences, due to the expression of different Ca++ channels or different recycling pathways. Recent work has suggested that dopamine neurons exhibit a high probability of dopamine release due to the expression of active zone components (Liu et al., 2018). Indeed, dopamine release shows prolonged short-term depression by D2-IPSC as well as voltammetry. Nonetheless, the probability of dopamine release is lower than that of glutamate release by the same cells, which may reflect association with different active zone components. Postsynaptic target neurons in the striatum may contribute to the segregation of release sites (Fortin et al., 2019), but we observe differential release in the absence of these cells, and it is possible that the transporters themselves also contribute. Indeed, VMAT2 belongs to the same family as the vesicular ACh transporter, but ACh release from basal forebrain neurons depends more on N-type channels (Takacs et al., 2018), in contrast to dopamine release from midbrain neurons which depends on P/Q-type. On the other hand, VGLUT1 has been shown to influence the endocytosis of other synaptic vesicle proteins (Pan et al., 2015), raising the possibility that the transporters influence vesicle composition and as a result, exocytosis.
The morphological homogeneity of synaptic vesicles (Peters et al., 1970) has suggested that they use a common final pathway to regulated exocytosis, with variation due to extrinsic factors that influence their mobilization (Kim and Ryan, 2010), rather than intrinsic differences in their composition (Alabi and Tsien, 2012; Rizzoli and Betz, 2005). Recent work has suggested that synaptic vesicles undergoing spontaneous release belong to a distinct pool that does not respond to stimulation (Ramirez and Kavalali, 2011; Sara et al., 2005), although this has been controversial (Bal et al., 2013; Fredj and Burrone, 2009; Groemer and Klingauf, 2007; Hua et al., 2010; Hua et al., 2011; Ramirez et al., 2012). We now provide functional evidence for intrinsic differences in synaptic vesicle composition (neurotransmitter content) within the pool that does respond to stimulation. AP-3 produces a biochemically distinct set of synaptic vesicles with lower release probability and reduced synchrony due at least in part to differences in coupling to presynaptic Ca++ channels. Corelease takes advantage of these general endocytic mechanisms to store transmitter in vesicle populations with different release properties and so deconvolve neural activity into two outputs, with distinct physiological and behavioral roles.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Robert H. Edwards (Robert.Edwards@ucsf.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Both male and female mice were used for all experiments. All mice received unlimited food and water and were exposed to 12h/12h light/dark cycle. DAT iCre/+ heterozygous mice were used for stereotactic injections of conditional AAVs at 3–4 weeks of age in animals used for electrophysiology and at 2 months of age in animals used for anatomy. Mice were allowed to recover for at least 3 weeks before further procedures. All experiments with animals were performed according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Generation of HA-VMAT2 BAC transgenic mice
Bacterial artificial chromosome (BAC) BAC23–233F7 containing the entire mouse genome was modified using a two-step procedure (Gong et al., 2002). Overlap extension PCR was used to insert the hemagglutinin (HA) epitope tag into the large lumenal loop between transmembrane domains 1 and 2 at a site previously described (Tan et al., 1998), as well as to amplify surrounding regions, and this PCR product was cloned into shuttle vector pLD53 conferring ampicillin resistance and sucrose sensitivity. The resulting shuttle vector was electroporated into host bacteria containing the BAC (conferring chloramphenicol resistance), selected with ampicillin and chloramphenicol and potential cointegrates screened by PCR. Positive clones were submitted to double selection with chloramphenicol and sucrose and potentially resolved BAC clones identified by PCR and confirmed by Southern analysis. The integrity of the modified BAC was assessed by restriction digest and compared to the original unmodified BAC. Modified BAC DNAs were then diluted into polyamine microinjection buffer and microinjected into C57/Bl6J eggs at the Gladstone Institutes Transgenic Core Facility (San Francisco). Transgenic offspring were identified by PCR for the HA tag, three founders identified and mated to C57Bl/6J mice. Two of the founders transmitting the transgene with the expected mendelian ratio expressed HA-VMAT2 at low or undetectable levels. The third founder transmitted with low frequency (1/29 offspring), suggesting chimerism, but subsequent transmission occurred at mendelian ratios with robust expression of HA-VMAT2.
Primary culture
Hippocampal neurons.
Hippocampi were dissected from P0 mice of both sexes, dissociated in 0.25% trypsin, washed three times with Hank’s Balanced Salt Solution (HBSS) containing 10 mM HEPES and 20 mM glucose, triturated and plated on poly-L-lysine coated coverslips at 350 cells/mm2. Neurons were plated in Minimal Essential Medium containing 1x B27 (Gibco, 17504–044), 2 mM glutamax (Gibco, 35050–061), 5% FBS (HyClone, defined), 21 mM glucose (Sigma, G8769) and 1x penicillin/streptomycin (Corning, 30–002-CL). After one day in vitro (DIV1), 3/4 of the medium was changed to Neurobasal (Gibco, 21103–049) with B27, glutamax and penicillin/streptomycin. Cells were infected with lentiviruses encoding VGLUT2-pHluorin, VMAT2-pHluorin or VGLUT2-pHluorin + VMAT2-mOrange2 on DIV 4. Cytosine arabinoside at 4 μM was added on DIV7 to inhibit glial proliferation.
Dopamine neurons.
Ventral midbrain region containing substantia nigra and ventral tegmental area was dissected from neonatal DAT iCre/B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, wild type or mocha mice of both sexes. Mocha mice were compared to littermate controls. Tissue pieces were dissociated in papain for 15 minutes, washed with HBSS containing 10 mM HEPES and 20 mM glucose, triturated and plated on astrocyte monolayers at 1000 cells/mm2 in medium containing 60% Neurobasal-A, 30% Basal Media Eagle, 10% fetal bovine serum (HyClone, defined), 1x B27, 2 mM GlutaMAX, 10 ng/mL glial cell line-derived neurotrophic factor (Millipore, MA, USA) and 1x penicillin/streptomycin. Cells were infected with lentiviruses encoding VGLUT2-pHluorin or VMAT2-pHluorin on DIV 2.
METHOD DETAILS
Stereotactic virus injection
Anesthesia was induced and maintained with inhaled isoflurane (2%). For the slice recording, DAT-Cre/+ mice (Slc6a3cre) were injected at 200 nL/min with 400 nL recombinant adeno-associated virus (AAV) encoding double floxed channelrhodopsin-2 (ChR2) (AAV5-EF1α-DIO-ChR2-mCherry) into the ventral tegmental area (coordinates in mm relative to bregma, −3.25 anteroposterior, −4.40 dorsoventral, and ±0.5 mediolateral) and with 300 nL AAV encoding GIRK2 (AAV2/9.hSynapsin.tdTomato.T2A.mGIRK2–1-A22A.WPRE diluted 1:3 in PBS) into the medial shell of the nucleus accumbens (+1.00 anteroposterior, −4.40 dorsoventral, and ±0.75 mediolateral). For the anatomy, DAT-Cre/+ or DAT-Cre/+/HA-VMAT2 mice were injected with 500 nL AAV5-EF1α-DIO-HA-VGLUT2 or AAV9-syn-DIO-VGLUT2-pHluorin respectively in the VTA.
Immunohistochemistry
Mice were perfused with 4% PFA in PBS, the brains postfixed overnight in 4% PFA and cryoprotected in PBS containing 30% sucrose. Sections (35 μm) were cut using a Leica CM3050 S cryostat. Cultured cells were fixed for 10 minutes in 4% PFA in PBS and washed three times in PBS. Floating sections as well as cultured cells were blocked and permeabilized in PBS containing 4% normal goat serum and 0,2% Triton X-100. Primary and secondary antibodies were diluted in PBS containing 1% normal goat serum and 0.2% Triton X-100 in PBS for staining slices and in 1% normal goat serum in PBS for staining cultured cells. Primary antibodies were incubated overnight at 4° C and sec ondary antibodies at room temperature for 2 hours in the dark. Slices were mounted in Fluoromount-G (SouthernBiotech) or Vectashield (Vector Laboratories). Antibodies were used at the following dilutions: guinea pig anti-VGLUT2 1/2000, mouse and rabbit anti-TH 1/1000, all other primary antibodies at 1/500. All secondary antibodies were used at 1/500 dilution.
Images were acquired using the Nikon Ti Microscope equipped with CSU-W1 spinning disk confocal, Andor Zyla sCMOS camera and a Plan Apo VC 100x/1.4 oil objective or with N-SIM module, Andor DU-897 EMCCD camera and Apo TIRF 100x/1.49 Oil objective. Lasers (488 nm, 561 nm and 640 nm) were used for excitation and ET525/50, ET600/50 and ET700/75 filters for emission. For midbrain cultures, tyrosine hydroxylase staining was used as mask and colocalization with different markers was calculated only in TH+ cells. JACoP plugin (Bolte and Cordelieres, 2006) in ImageJ was used to determine colocalization coefficients.
Slice electrophysiology
Slice preparation.
Three to eight weeks after virus injection, mice were deeply anesthetized with intraperitoneal ketamine/xylazine and transcardially perfused with cold artificial cerebrospinal fluid (ACSF) containing (in mM) 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 26 NaHCO3, 1 NaH2PO4, and 11 D-glucose (315 Osm, pH 7.4), then decapitated. The brains were removed and cut on a vibratome (Leica) in aerated (95% O2, 5% CO2), cold slicing solution that contained (in mM): 110 choline chloride, 2.5 KCl, 7 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 D-glucose, 11.6 Na ascorbate and 3.1 Na pyruvate. Slices were incubated at 35° C for a minimum of 30 minutes in aerated ACSF. Nucleus accumbens slices were visualized with an upright infrared differential interference contrast (IR-DIC) microscope (BX50WI; Olympus) and perfused with aerated ACSF at room temperature throughout recording. Picrotoxin (100 μM), CGP55845 (300 nM), scopolamine hydrobromide (200 nM), and SCH 23390 hydrochloride (1 μM) (all obtained from Sigma) were added to the ACSF to isolate glutamate receptor- and D2 dopamine receptor-mediated currents. The D2 antagonist sulpiride (1 μM) was also added to isolate the glutamate-mediated EPSCs, but its omission did not reduce the extent of synaptic depression in response to trains (data not shown). ω-Agatoxin TK and ω-conotoxin GVIA (both from Alomone) were used to assess dependence on P-/Q-type and N-type Ca++ channels.
Whole cell recordings.
Whole cell voltage clamp recordings (Vh = −70 mV) were made from tdTomato+ spiny projection neurons (SPNs) in the shell of the nucleus accumbens. Glass electrodes (3–5 MΩ) contained 115 mM K methanesulphonate, 20 mM NaCl, 1.5 mM MgCl2, 10 mM HEPES-KOH, 10 mM tetrapotassium BAPTA, 1 mg/ml ATP, 0.1 mg/ml GTP, 1.5 mg/ml phosphocreatine and 5 mM QX314 (pH 7.4, 275 mOsm) to record glutamate receptor- and D2 receptor-mediated currents; or cesium methanesulfonate-based internal solution containing (in mM) 136 CsMeSO4, 7 CsCl, 0.25 EGTA, 10 HEPES, 2 MgATP, 0.3 Na2GTP, 8 NaCl, 0.1 spermine, 7 phosphocreatine and 5 QX314 to isolate glutamate receptor-mediated currents. Field blue light (488 nm, pulse width 2–5 ms) was applied through a 40x objective to excite ChR2. The D2-IPSC was measured by subtracting the baseline (1– 20 ms before the first stimulus) from the peak amplitude of the IPSC. Sensitivity to TBZ (Tocris) was determined before and after 30 min bath incubation.
Measurement of monoamine levels
Age- and weight-matched adult male and female mice (~25 g) were divided into two groups and injected intraperitoneally with α-methyl-p-tyrosine methyl ester hydrochloride (AMPT,100 mg/kg) or saline 24, 20, and 4 h before either slice recording or the measurement of monoamine content. To measure tissue monoamine after treatment with AMPT, the nucleus accumbens was isolated from slices prepared as above, frozen on dry ice and stored at −80° C. To measure monoamine levels in wild type and mocha mice, age-matched animals were euthanized by CO2 inhalation, decapitated, the ventral and dorsal striata dissected in HBSS and immediately frozen on dry ice. Tissue catecholamine levels were measured by HPLC with coupled electrochemical detection at the Vanderbilt Neurochemistry Core.
[3H]-Dihydrotetrabenazine binding
To dissect striatal tissue, the brains were placed into a rodent brain matrix (RBM-2000C, Protech International Inc.) and two 1 mm coronal slices collected. Cortical tissue was removed and striatal tissue collected in ice cold SHT buffer (320 mM sucrose, 10 mM HEPES/Tris pH 7.4) with 0.5 mM EDTA and Complete™ Protease Inhibitor Cocktail (Roche). Tissue from each striatum was disrupted with 12 strokes of a Dounce homogenizer at 500 rpm in ice cold SHT buffer. The sample was then sonicated for 30 seconds before sedimentation at 2000x g for 2 min. The pellet was discarded and the protein content of the supernatant determined by BCA. For the dihydrotetrabenazine binding, 50 μg membrane protein was incubated in SHT buffer with 10 nM (+)-a-dihydrotetrabenazine [9-O-methyl-3H] (ARC; 80 Ci/mmol) for 30 minutes at 30° C. The reaction was stopped by filtration through a Supor 200 0.2 μm filter (PALL) and washed 3 times in ice-cold SHT buffer with 20 mM tetrabenazine (Fluka). Binding was done in triplicate for each sample and nonspecific binding determined in the presence of 10 μM non-radioactive tetrabenazine in the binding assay. Specific binding was normalized to the amount of membrane protein added to the reaction.
Live imaging
Hippocampal cultures between 16–19 DIV and dopamine neuron cultures between 14–16 DIV were mounted in a laminar flow perfusion and stimulation chamber on an inverted Nikon TE300 fluorescence microscope. Fluorescence signals were collected under epifluorescence illumination using the following bandpass filters: 470/40 nm excitation and 525/50 nm emission for pHluorin; 545/25 nm excitation and 605/70 nm emission for mOrange2, tdTomato or JHC 1–64. Midbrain cultures from wild type and mocha mice were incubated for 10 min in fluorescent DAT ligand JHC 1–64 (30 nM) to visualize dopamine neurons (Eriksen et al., 2009). Cultures were then rinsed in imaging buffer before mounting in the chamber. All other midbrain cultures were made from DAT iCre/B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice and dopamine neurons detected by the presence of cell-filling tdTomato. Dopamine neurons were located in the red channel and a single image acquired before switching to the pHluorin channel for imaging the response to stimulation. Images were acquired at 0.5 Hz in most experiments, and at 5 Hz for EGTA application. The frequency dependence and mocha experiments were imaged at 1 Hz. Action potentials (APs) were evoked by passing 1 ms bipolar current pulses through platinum–iridium electrodes, to yield fields of 5–10 V/cm. Cells were continuously perfused with Tyrode’s buffer (119 mM NaCl, 25 mM HEPES, 2 mM CaCl2, 2 mM MgCl2, 2.5 mM KCl, and 30 mM glucose at pH 7.4) containing 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 10 μM 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (APV). Imaging was done at room temperature in most experiments except for the analysis of frequency dependence in hippocampal and dopamine neurons, which was performed at 35° C. Total cellular pHluorin or mOrange2 content was revealed by applying Tyrode’s buffer with 50 mM NH4Cl, where NaCl was reduced to 69 mM. To measure exocytosis, Tyrode’s solution with 600 nM Bafilomycin A1 (Calbiochem, 196000) was applied to cells just before starting the acquisition. To test the Ca++ coupling of VMAT2- or VGLUT2-pHluorin exocytosis, cells were incubated for 3 minutes in 100 μM EGTA-AM (Calbiochem, 324628) or vehicle (0.1% DMSO).
QUANTIFICATION AND STATISTICAL ANALYSIS
Data analysis.
Images were processed using ImageJ software. For most live imaging experiments, varicosities were manually selected based on fluorescence in buffer containing NH4Cl. In dual color pHluorin/mOrange imaging varicosities in either channel were selected automatically using the granulometric filter in ImageJ. Background was subtracted and integrated fluorescence density in the varicosities was normalized to baseline before stimulation and to unquenched pHluorin fluorescence in NH4Cl, or to maximal fluorescence after stimulation (as indicated in the text). Curves were fitted using GraphPad Prism 6 software.
Statistics.
Statistical analysis of data was performed using GraphPad Prism 6 software. The D’Agostino & Pearson omnibus test was used to assess normality. The two-tailed unpaired t-test was used for data with a normal distribution and the Mann-Whitney test for data that did not pass the test for normality. For multiple comparisons, ANOVA was used for the normally distributed data, and Kruskal-Wallis for the remainder.
DATA AND SOFTWARE AVAILABILITY
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Acknowledgements
We thank members of the Edwards lab for helpful discussion, the lab of Anatol Kreitzer for guidance in the recording from striatal slices, DeLaine Larsen for assistance with the structured illumination microscopy, Susan Voglmaier for constructs, Barry Calagui and Samir Batarni for technical support. This work was supported by a fellowship from the Fondation Fyssen and support from the Feldman Foundation (to K.S.), F30 DA40996 (to P.F.M.), R01 DA35821 and NS95809 (to C.P.F.), P01 DA10154 (to R.H.E.), the UCSF Kavli Institute for Fundamental Neuroscience, and the UCSF Weill Institute for Neurosciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Authors declare no competing interests.
References
- Adrover MF, Shin JH, and Alvarez VA (2014). Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci 34, 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi AA, and Tsien RW (2012). Synaptic vesicle pools and dynamics. Cold Spring Harbor Persp Biol 4, a013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio CS, Sirkis DW, and Edwards RH (2010). RNAi screen identifies a role for adaptor protein AP-3 in sorting to the regulated secretory pathway. J Cell Biol 191, 1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal M, Leitz J, Reese AL, Ramirez DM, Durakoglugil M, Herz J, Monteggia LM, and Kavalali ET (2013). Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron 80, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, and Gonon F (2001). Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci 21, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, and Descarries L (2009). The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol 517, 873–891. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, et al. (2010). VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A 107, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein J, Faundez V, Nakatsu F, Saito T, Ohno H, and Kelly RB (2001). The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes. J Neurosci 21, 8034–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, and Cordelieres FP (2006). A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224, 213–232. [DOI] [PubMed] [Google Scholar]
- Brimblecombe KR, Gracie CJ, Platt NJ, and Cragg SJ (2015). Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J Physiol 593, 929–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, and Storm-Mathisen J (1998). The vesicular GABA transporter VGAT localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18, 9733–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, and Ryan TA (2001). Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4, 1187–1193. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, and Regehr WG (2000). Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci 20, 1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, and Jonas P (2012). Nanodomain coupling between Ca(2)(+) channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, and Trudeau LE (2011). From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci 12, 204–216. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Rasmussen SG, Rasmussen TN, Vaegter CB, Cha JH, Zou MF, Newman AH, and Gether U (2009). Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci 29, 6794–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstratova A, Chamberland S, Faundez V, and Toth K (2014). Vesicles derived via AP-3-dependent recycling contribute to asynchronous release and influence information transfer. Nat Commun 5, 5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng JT, and Kelly RB (1997). ADP ribosylation factor 1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol 138, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng JT, and Kelly RB (1998). A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93, 423–432. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun B-C, Killeen N, Sulzer D, and Edwards RH (1997). Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron 19, 1271–1283. [DOI] [PubMed] [Google Scholar]
- Fortin GM, Ducrot C, Giguere N, Kouwenhoven WM, Bourque MJ, Pacelli C, Varaschin RK, Brill M, Singh S, Wiseman PW, et al. (2019). Segregation of dopamine and glutamate release sites in dopamine neuron axons: regulation by striatal target cells. FASEB J 33, 400–417. [DOI] [PubMed] [Google Scholar]
- Foss SM, Li H, Santos MS, Edwards RH, and Voglmaier SM (2013). Multiple dileucine-like motifs direct VGLUT1 trafficking. J Neurosci 33, 10647–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredj NB, and Burrone J (2009). A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci 12, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh GY, Huang H, Ullman J, Borre L, Hnasko TS, Trussell LO, and Edwards RH (2011). Presynaptic regulation of quantal size: K(+)/H(+) exchange stimulates vesicular glutamate transport. Nat Neurosci 14, 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Yang XW, Li C, and Heintz N (2002). Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res 12, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, et al. (2008). The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci 11, 292–300. [DOI] [PubMed] [Google Scholar]
- Groemer TW, and Klingauf J (2007). Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci 10, 145–147. [DOI] [PubMed] [Google Scholar]
- Hartig W, Riedel A, Grosche J, Edwards RH, Fremeau RT Jr., Harkany T, Brauer K, and Arendt T (2003). Complementary distribution of vesicular glutamate transporters 1 and 2 in the nucleus accumbens of rat: Relationship to calretinin-containing extrinsic innervation and calbindin-immunoreactive neurons. J Comp Neurol 465, 1–10. [DOI] [PubMed] [Google Scholar]
- Hattori T, Takada M, Moriizumi T, and van der Kooy D (1991). Single dopaminergic nigrostriatal neurons form two chemically distinct synaptic types: possible transmitter segregation within neurons. J Comp Neurol 309, 391–401. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, and Edwards RH (2010). Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65, 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, and Edwards RH (2012). Neurotransmitter corelease: mechanism and physiological role. Ann Rev Physiol 74, 225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, and Dombeck DA (2016). Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Sinha R, Martineau M, Kahms M, and Klingauf J (2010). A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat Neurosci 13, 1451–1453. [DOI] [PubMed] [Google Scholar]
- Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, and Edwards RH (2011). v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71, 474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, and Sandkuhler J (1998). Corelease of two fast neurotransmitters at a central synapse. Science 281, 419–424. [DOI] [PubMed] [Google Scholar]
- Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, et al. (1998). Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, microsomes and synaptic vesicles. Neuron 21, 111–122. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, and Hisano S (2006). Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol 498, 581–592. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, and Wightman RM (1992). Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem 59, 449–455. [DOI] [PubMed] [Google Scholar]
- Kim SH, and Ryan TA (2009). A distributed set of interactions controls {micro}2 functionality in AP-2’s role as a sorting adaptor in synaptic vesicle endocytosis. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, and Ryan TA (2010). CDK5 serves as a major control point in neurotransmitter release. Neuron 67, 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, et al. (2014). Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82, 981–988. [DOI] [PubMed] [Google Scholar]
- Koranda JL, Cone JJ, McGehee DS, Roitman MF, Beeler JA, and Zhuang X (2014). Nicotinic receptors regulate the dynamic range of dopamine release in vivo. J Neurophysiol 111, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte d’Incamps B, Bhumbra GS, Foster JD, Beato M, and Ascher P (2017). Segregation of glutamatergic and cholinergic transmission at the mixed motoneuron Renshaw cell synapse. Sci Rep 7, 4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, and Zhou ZJ (2010). Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Santos MS, Park CK, Dobry Y, and Voglmaier SM (2017). VGLUT2 Trafficking Is Differentially Regulated by Adaptor Proteins AP-1 and AP-3. Front Cell Neurosci 11, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Merrill SA, Jorgensen EM, and Shen K (2016). Two clathrin adaptor protein complexes instruct axon-dendrite polarity. Neuron 90, 564–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kershberg L, Wang J, Schneeberger S, and Kaeser PS (2018). Dopamine secretion Is mediated by sparse active zone-like release sites. Cell 172, 706–718 e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan T, Bendor J, Toupin C, Thorn K, and Edwards RH (2017). alpha-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, and Ford CP (2014). Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron 84, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, and Galli T (2000). Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol 149, 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa M, Tsukamoto Y, Berglund K, Ishii M, and Tachibana M (2007). Different roles of ribbon-associated and ribbon-free active zones in retinal bipolar cells. Nat Neurosci 10, 1268–1276. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, and Rothman JE (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Bussert TG, Kreitzer AC, and Seal RP (2014). Striatal cholinergic neurotransmission requires VGLUT3. J Neurosci 34, 8772–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Chintala S, Jenkins S, Pare JF, McGaha L, Smith Y, and Faundez V (2010). Hermansky-Pudlak protein complexes, AP-3 and BLOC-1, differentially regulate presynaptic composition in the striatum and hippocampus. J Neurosci 30, 820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, and Pickel VM (1997). Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse 26, 194–198. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, and Pickel VM (1998). Ultrastructural localization of the vesicular monoamine transporter 2 in mesolimbic and nigrostriatal dopaminergic neurons. Adv Pharmacol 42, 240–243. [DOI] [PubMed] [Google Scholar]
- Onoa B, Li H, Gagnon-Bartsch JA, Elias LA, and Edwards RH (2010). Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J Neurosci 30, 7917–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Marrs J, and Ryan TA (2015). Vesicular glutamate transporter 1 orchestrates recruitment of other synaptic vesicle cargo proteins during synaptic vesicle recycling. J Biol Chem 290, 22593–22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, and Ryan TA (2012). Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat Neurosci 15, 813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, and Webster H.d. (1970). Chapter 5 Synapses In The Fine Structure of the Nervous System Neurons and their Supporting Cells (Oxford Univ. Press; ), pp. 138–211. [Google Scholar]
- Phillips PE, Hancock PJ, and Stamford JA (2002). Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44, 15–22. [DOI] [PubMed] [Google Scholar]
- Phillips PE, and Stamford JA (2000). Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing. Brain Res 884, 139–146. [DOI] [PubMed] [Google Scholar]
- Ramirez DM, and Kavalali ET (2011). Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol 21, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Khvotchev M, Trauterman B, and Kavalali ET (2012). Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron 73, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, and Luo M (2011). Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69, 445–452. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, and Betz WJ (2005). Synaptic vesicle pools. Nat Rev Neurosci 6, 57–69. [DOI] [PubMed] [Google Scholar]
- Saheki Y, and De Camilli P (2012). Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 4, a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Craige B, Love R, Kalman D, and Faundez V (2005). Vglut1 and ZnT3 co-targeting mechanisms regulate vesicular zinc stores in PC12 cells. J Cell Sci 118, 1911–1921. [DOI] [PubMed] [Google Scholar]
- Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, and Faundez V (2004). The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell 15, 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, and Kavalali ET (2005). An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563–573. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, et al. (2008). Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, and Galli T (2006). Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A 103, 16562–16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, and Montague PR (1997). A neural substrate of prediction and reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Bocchio M, Bannerman DM, Sharp T, and Capogna M (2017). Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J Neurosci 37, 1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Adrover MF, and Alvarez VA (2017). Distinctive modulation of dopamine release in the nucleus accumbens shell mediated by dopamine and acetylcholine receptors. J Neurosci 37, 11166–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkis DW, Edwards RH, and Asensio CS (2013). Widespread dysregulation of peptide hormone release in mice lacking adaptor protein AP-3. PLoS Gen 9, e1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser JE, Arribas M, Blagoveshchenskaya AD, and Cutler DF (1999). Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles. Mol Biol Cell 10, 2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, and Rice ME (2016). Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 6, 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, and Rayport S (1998). Dopamine neurons make glutamatergic synapses in vitro. J Neurosci 18, 4588–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs VT, Cserep C, Schlingloff D, Posfai B, Szonyi A, Sos KE, Kornyei Z, Denes A, Gulyas AI, Freund TF, et al. (2018). Co-transmission of acetylcholine and GABA regulates hippocampal states. Nat Comm 9, 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, and Uhl GR (1997). VMAT2 knockout mice: heterozygous display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion and enhanced MPTP toxicity. Proc Natl Acad Sci USA 94, 9938–9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PK, Waites C, Liu Y, Krantz DE, and Edwards RH (1998). A leucine-based motif mediates the endocytosis of vesicular monoamine and acetylcholine transporters. J Biol Chem 273, 17351–17360. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, and Sabatini BL (2012). Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, and Sabatini BL (2016). Mechanisms and functions of GABA co-release. Nat Rev Neurosci 17, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bospoort R, Farina M, Schmitz SK, de Jong A, de Wit H, Verhage M, and Toonen RF (2012). Munc13 controls the location and efficiency of dense-core vesicle release in neurons. J Cell Biol 199, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, and Edwards RH (2006). Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84. [DOI] [PubMed] [Google Scholar]
- Wang Y-M, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, and Caron MG (1997). Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 19, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Trimbuch T, Camacho-Perez M, Rost BR, Brokowski B, Sohl-Kielczynski B, Felies A, Davis MW, Rosenmund C, and Jorgensen EM (2014). Clathrin regenerates synaptic vesicles from endosomes. Nature 515, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, and Morales M (2015). Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci 18, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.