Abstract
BACKGROUND
Patients with alcohol use disorder (AUD) are at an increased risk of developing Wernicke’s encephalopathy (WE), a devastating and difficult diagnosis caused by thiamine deficiency. Even as AUD is present in up to 25% of hospitalized patients on medical floors, appropriate thiamine supplementation in the hospital setting remains inadequate. These patients are particularly susceptible to thiamine deficiency and subsequent WE due to both their alcohol use and active medical illnesses. The electronic medical record (EMR) has become ubiquitous in health care systems and can be used as a tool to improve the care of hospitalized patients.
METHODS
As a quality improvement initiative, we implemented a medication order panel in the EMR with autopopulated orders for thiamine dosing to increase the appropriate use of high-dose parenteral thiamine (HPT) for hospitalized patients with AUD. We conducted a retrospective cohort study of all inpatients with AUD who received an Addiction Psychiatry Consult Service consult three months before and after the EMR change. We compared the proportion of patients receiving HPT prior to consultation (primary outcome) and the length of stay (secondary outcome) between the historical control group and the EMR intervention group.
RESULTS
Patients in the EMR intervention group were significantly more likely to receive HPT than the historical control group (20.2% vs. 2.7%, p < 0.0001). This difference remained statistically significant when adjusted for potential confounders (OR: 9.89, 95% CI: [2.77, 35.34], p = 0.0004). There was a trend towards statistical significance that the intervention group had a higher likelihood of being prescribed any thiamine (76.6% vs. 64.6%, p = 0.06) and had a shorter length of stay (median (IQR): 3.8 (2.4, 7.0) vs. 4.6 (2.9, 7.8) days, p = 0.06).
CONCLUSION
These results indicate that providing autopopulated thiamine order panels for patients with AUD can be an effective method for specialty services to increase appropriate care practices without additional education or training for providers. Further research should consider the clinical outcomes of increasing HPT for patients with AUD.
Keywords: Alcohol use disorder, thiamine deficiency, Wernicke’s encephalopathy, quality improvement, electronic medical record, decision support
1. Introduction1
Alcohol use disorder (AUD) is a common diagnosis among hospitalized patients and is present in up to 25% of patients on hospital medical floors (Moore et al., 1989; Saitz, Freedner, Palfai, Horton, & Samet, 2006; Smothers, Yahr, & Ruhl, 2004). Patients with AUD have a 15-80% prevalence of thiamine deficiency (Li, Jacob, Feng, & Kulkarni, 2008; Thomson & Marshall, 2006), and have been found to have evidence of Wernicke’s encephalopathy (WE) in up to 12.5% of autopsies (Torvik, Lindboe, & Rogde, 1982). WE is a neurologic emergency caused by thiamine deficiency (Galvin et al., 2010; Thomson & Marshall, 2006) and requires immediate high-dose parenteral thiamine (HPT) replacement to prevent or cease permanent neurologic damage (Latt & Dore, 2014). Despite the catastrophic consequences of this syndrome, it is a difficult diagnosis to make, especially in patients with AUD who may have a variety of other potential causes for neurologic changes. Vulnerable populations with AUD continue to suffer from WE and interventions are needed for its prevention and treatment.
WE is classically characterized by confusion, ophthalmoplegia, and gait ataxia. It is usually diagnosed clinically by using the Caine criteria (Caine, Halliday, Kril, & Harper, 1997), which includes the classic WE symptoms and evidence of malnutrition. Although often associated with AUD, it may also occur with other medical conditions causing malnutrition (Isenberg-Grzeda et al., 2017; Scalzo, Bowden, Ambrose, Whelan, & Cook, 2015). Hospitalized patients with AUD are particularly susceptible to complications from thiamine deficiency due to the decreased nutritional intake and impaired metabolism of thiamine that accompanies both AUD and medical illnesses (Isenberg-Grzeda, Rahane, DeRosa, Ellis, & Nicolson, 2016; Li et al., 2008; Scalzo et al., 2015). Many patients do not receive HPT until the neurologic damage has begun or has progressed and never receive a diagnosis of WE (Harper, Giles, & Finlay-Jones, 1986; Harper, 1983). Up to 59% of patients with alcohol-related deaths have been found to have histologically confirmed WE despite no obvious macroscopic changes or a prior clinical diagnosis (Naidoo, Bramdev, & Cooper, 1996).
Despite significant advances in understanding and research for AUD, utilization of medical treatments for WE is lacking. Even patients who are admitted to the hospital for treatment of an alcohol-related illness often do not receive recommended care (Moore et al., 1989). A previous study performed at our institution of 217 medical inpatients with AUD found that only 1.5% initially received the recommended ≥200mg intravenous/intramuscular (IV/IM) thiamine daily, and 17.7% did not initially receive any thiamine at all (Isenberg-Grzeda, Chabon, & Nicolson, 2014). Although there have been concerns of anaphylaxis with administration of parenteral thiamine, thiamine has been found to be safe with a lower rate of allergic reactions than many commonly administered medications, such as penicillin (Wrenn, Murphy, & Slovis, 1989).
There is insufficient evidence from clinical studies to establish the optimal dosing and frequency of thiamine for the prophylaxis or treatment of WE in patients with AUD (Day, Bentham, Callaghan, Kuruvilla, & George, 2013). However, experts and many guidelines most commonly recommend that thiamine be given parenterally and at high doses (≥200mg daily) as prophylaxis for patients at risk for WE (Galvin et al., 2010; Latt & Dore, 2014), with actual treatment of WE requiring parenteral doses of up to 500mg three times daily. Physiologic and preclinical studies provide evidence that for treatment of WE, thiamine must be given parenterally at high doses in order to promote passive diffusion across the blood-brain barrier (Cook, Hallwood, & Thomson, 1998) and bypass the active rate-limited process that normally occurs at lower blood concentrations (Butterworth, 1995; Thomson & Leevy, 1972).
To encourage timely HPT prescribing, a clinical decision support order panel with autopopulated medication orders for patients with AUD was embedded in our EMR at Montefiore Medical Center in the Bronx. Our intervention was based on computerized decision support, which minimized required clinician effort as this has been shown to improve patient care (Kawamoto, Houlihan, Balas, & Lobach, 2005). We hypothesized that this intervention would increase the likelihood of HPT being prescribed to patients with AUD prior to involvement of specialty consultation. The primary goal of this study was to assess the intervention’s effect on HPT prescribing. As a secondary goal, we tested whether the intervention had an effect on the length of stay, as a longer length of stay has been associated with worse clinical outcomes (Thomas, Guire, & Horvat, 1997).
2. Material and methods
2.1. Study Population
This study was conducted at Montefiore Medical Center Moses Division, a large urban academic medical center that serves as the university hospital for the Albert Einstein College of Medicine situated in the Bronx, NY. The EMR medication ordering workflow intervention went live on April 30th, 2015 throughout the entire hospital system. The EMR intervention group included all AUD patients who received an Addiction Psychiatry Consultation Service (APS) consult in the 89 days after the EMR change. The historical control comparison group included all AUD patients who received an APS consult in the 89 days before the EMR change. All patients with AUD who received an APS consult as requested by the primary service teams were included, with no exclusion criteria. For patients with multiple APS consults during the study period, only the first APS consult of the study period was included. There was not a dedicated detoxification unit in the hospital. This study was approved by the Albert Einstein College of Medicine Institutional Review Board.
The Bronx is New York City’s poorest borough, with 30.3% of its 1.4 million residents living below the poverty level and a higher mortality rate per 100,000 than all other counties in the city as of 2015 (“Socio-Economic Status and General Health Indicators - Bronx County,” 2017). The Bronx also has the highest proportion of adults who are diabetic, obese, actively smoke, and have poor mental health of any county in New York State (Robert Wood Johnson Foundation, 2014). These health burdens underscore the importance of implementing effective preventive treatments for diseases that, if left untreated, could have long-term and costly sequelae.
2.2. The EMR Intervention
The intervention was the adoption of an autopopulated order panel in the EMR within the usual medication ordering workflow that, when chosen, defaulted an appropriate dosing protocol of HPT for patients with AUD (Figure 1). Two pathways with different thiamine dosing protocols were created (screenshots in Figure 1B), depending on the presence or absence of altered mental status or confusion; both pathways autopopulated orders for HPT. This order panel was created in conjunction with Pharmacy Informatics, Inpatient Medicine Quality Improvement, and the APS. No additional formal education or training was given during the study period. The dosage regimen was created using a synthesis of dosing recommendations from the literature and national medical societies (Day et al., 2013; Latt & Dore, 2014). As per these guidelines, we considered thiamine orders ≥200mg IV/IM to be HPT and thus the optimal effective dose for the treatment or prevention of WE in the inpatient setting.
Figure 1.
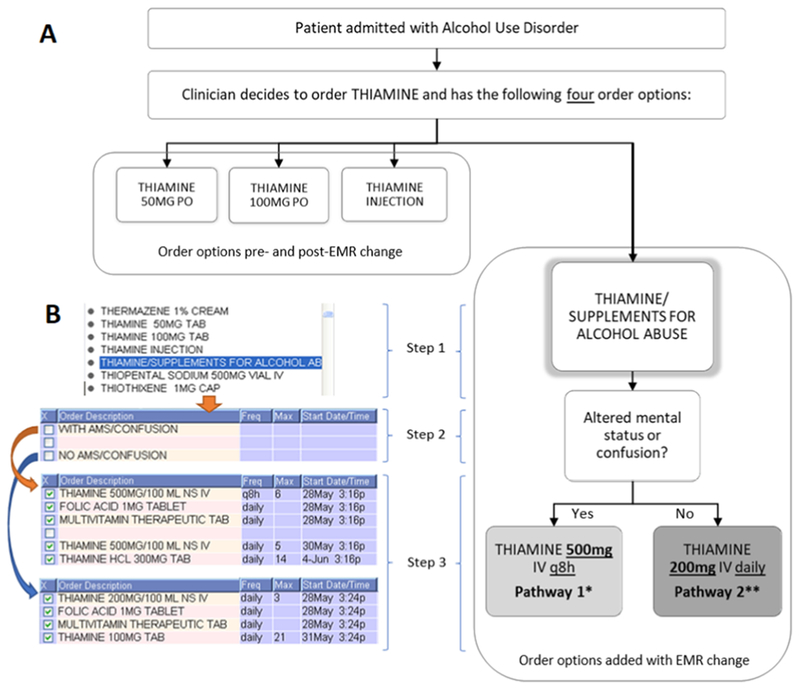
Schematic and screenshots of the changes to the electronic medical record ordering interface.
A: Schematic of the ordering workflow.
* Pathway 1: THIAMINE 500mg IV q8h × 6 doses → THIAMINE 500MG IV daily × 5 doses → THIAMINE 300mg PO daily × 14 doses
** Pathway 2: THIAMINE 200mg IV daily × 3 doses → THIAMINE 100mg PO daily × 21 doses
B: Modified screenshots of the EMR intervention workflow. In Step 1, prescribers must choose the thiamine order from a list of medication orders. In Step 2, they specify if the patient is with or without altered mental status/confusion, and in Step 3, they can view the autopopulated orders prior to processing.
2.3. Data Collection
Data were collected from a retrospective review of the medical record. Participants were identified from the patient logs of the APS and all clinical and demographic data were obtained via EMR chart review. Race/ethnicity was self-identified in the EMR including the categories of Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic other (Asian and mixed race combined), and declined. Homelessness was a documented demographic category in the EMR. Patients designated as “high risk” for WE, as characterized in a similar study by Isenberg-Grzeda et al. (2014), were in a state of alcohol intoxication, alcohol withdrawal, or delirium tremens during their hospitalization. Medical comorbidity data were collected from the medical record via chart review.
2.4. Outcome Measures
The primary outcome measure was the prescription of HPT prior to APS consultation, where the effective dose was considered as ≥200mg IV/IM (HPT) in this inpatient setting.
The initial thiamine order status was assessed for each patient and grouped into the following categories based on the highest total daily dosing observed before APS consultation: none, ≤100mg orally (PO), 200mg PO, 100mg IV/IM, and ≥200mg IV/IM. IV/IM dosing was always considered as a higher dose versus PO dosing because of its increased bioavailability. Only thiamine dosing ordered prior to APS consultation was considered for inclusion in the study.
The secondary outcome measure was length of stay. It was extracted from the electronic medical record system using healthcare surveillance software (Clinical Looking Glass; Emerging Health Information Technology, Yonkers, NY) and was verified by chart review.
2.5. Statistical Analysis
Wilcoxon rank-sum test (for continuous variables) and Pearson’s chi-square test or Fisher’s exact test (for categorical variables) were used to compare patient characteristics between the EMR intervention group and the historical control group. Multivariable logistic regression was used to assess whether the likelihood of HPT prescription was different between the two groups while adjusting for potential confounders such as age, gender, race/ethnicity, homelessness, high risk status, and comorbidities. Only variables with a significant or borderline significant effect on the outcome in the univariable logistic regression analysis were included in the multivariable model. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient characteristics
During the study period from January 31st to July 27th in 2015, the APS performed consults for 207 patients who had active AUD. A summary of patient characteristics is displayed in Table 1.
Table 1.
Patient characteristics for the whole sample and for the stratified groups.
| All (n=207) | Historical control (n=113) | EMR intervention (n=94) | p-valuea | |
|---|---|---|---|---|
| Age, years, median (IQR) | 52 (44, 58) | 51 (43, 59) | 54 (46, 58) | 0.30 |
| Male, n (%) | 150 (72.5) | 81 (71.7) | 69 (73.4) | 0.78 |
| Race/ethnicity, n (%) | 0.12 | |||
| Hispanic | 73 (35.3) | 42 (37.2) | 31 (33.0) | |
| Non-Hispanic white | 37 (17.9) | 17 (15.0) | 20 (21.3) | |
| Non-Hispanic black | 71 (34.3) | 35 (31.0) | 36 (38.3) | |
| Non-Hispanic other | 13 (6.3) | 11 (9.7) | 2 | Order options added with EMR change |
| Declined | 13 (6.3) | 8 (7.1) | 5 (5.3) | |
| Homeless, n (%) | 18 (8.7) | 9 (8.0) | 9 (9.6) | 0.68 |
| High Riskb, n (%) | 129 (62.3) | 70 (62.0) | 59 (62.8) | 0.90 |
| Total number of comorbidities, median (IQR) | 2 (2, 3) | 2 (2, 3) | 2 (1, 3) | 0.73 |
| Comorbidities, n (%) | ||||
| Cardiovascular | 87 (42.0) | 57 (50.4) | 30 (31.9) | 0.007 |
| Gastrointestinal (non-hepatic) | 73 (35.3) | 40 (35.4) | 33 (35.1) | 0.97 |
| Hepatic | 55 (26.6) | 29 (25.7) | 26 (27.7) | 0.75 |
| Neuro/Psychiatric | 69 (33.3) | 34 (30.1) | 35 (37.2) | 0.28 |
| Respiratory | 45 (21.7) | 27 (23.9) | 18 (19.2) | 0.41 |
| Metabolic | 45 (21.7) | 28 (24.8) | 17 (18.1) | 0.25 |
| Infectious (non-HIV) | 39 (18.8) | 19 (16.8) | 20 (21.3) | 0.41 |
| HIV/AIDS | 7 (3.4) | 5 (4.4) | 2 (2.1) | 0.46 |
| Hematology/Oncology | 36 (17.4) | 16 (14.2) | 20 (21.3) | 0.18 |
| Renal | 26 (12.6) | 12 (10.6) | 14 (14.9) | 0.36 |
| Otherc | 35 (16.9) | 15 (13.3) | 20 (21.3) | 0.13 |
Wilcoxon rank sum test was used to compare continuous variables while Pearson’s chi-square test or Fisher’s exact test was used to compare categorical variables.
High Risk was defined as patients who were in a state of alcohol intoxication, alcohol withdrawal, or delirium tremens during their hospitalization.
Other types of comorbidities included urologic, endocrine, trauma, rhabdomyolysis, dermatologic, and arthritic.
For the whole sample, the median (IQR) age was 52 (44, 58) years old. The patients were predominantly male (72.5%), and more than half (62.3%) were high risk. The EMR intervention group and the historical control group were comparable except for cardiovascular comorbidity, where the proportion of this comorbidity was higher in the historical control group compared to the EMR intervention group (50.4% vs. 31.9%, p = 0.007).
3.2. Primary Outcome: Thiamine Prescribing
As shown in the unadjusted analyses in Table 2, the proportion of patients receiving HPT was significantly higher in the EMR intervention group compared to the historical control group (20.2% vs. 2.7%, p < 0.0001). There was also a trend towards statistical significance that the EMR intervention group was more likely to be prescribed any dose of thiamine (versus no thiamine prescribed) compared to the historical control group (76.6% vs. 64.6%, p = 0.06).
Table 2.
Thiamine dosage and length of stay.
| All (n=207) | Historical control (n=113) | EMR intervention (n=94) | p-valuea | |
|---|---|---|---|---|
| Thiamine Dosage, n (%) | 0.0003 | |||
| ≥200mg IM/IV (HPT) | 22 (10.6) | 3 (2.7) | 19 (20.2) | <.0001 |
| 100mg IM/IV | 37 (17.9) | 19 (16.8) | 18 (19.2) | 0.66 |
| 200mg PO | 1 (0.5) | 1 (0.9) | 0 | 1.00 |
| ≤100mg PO | 85 (41.1) | 50 (44.3) | 35 (37.2) | 0.31 |
| None | 62 (30.0) | 40 (35.4) | 22 (23.4) | 0.06 |
| Length of stay, days, median (IQR) | 4.1 (2.6, 7.7) | 4.6 (2.9, 7.8) | 3.8 (2.4, 7.0) | 0.06 |
EMR = electronic medical record, HPT = high-dose parenteral thiamine
P-value comparing historical control to EMR intervention group (bold indicates p<0.05).
In the univariable logistic regression analyses (Table 3), we assessed the association of each potential confounder from Table 1 with prescription of HPT. High risk status was borderline significant versus low risk in increasing the likelihood of prescribing HPT (OR = 3.0, 95% CI: [0.98, 9.2], p = 0.055). The total number of comorbidities was associated with a higher likelihood of prescribing HPT (OR = 1.38, 95% CI: [1.04, 1.82], p = 0.02). When examining each individual comorbidity, hematology/oncology comorbidity and renal comorbidity were also associated with a higher likelihood of prescribing HPT (OR = 3.2, 95% CI: [1.23, 8.3], p = 0.02; OR = 4.1, 95% CI: [1.48,11.3], p = 0.007, respectively).
Table 3.
Univariate analyses for the outcome: “≥200mg IM/IV” vs. other dosage.
| p-value* | Odds Ratio (95% CI) | |
|---|---|---|
| EMR intervention vs. control | 0.0005 | 9.29 (2.66, 32.50) |
| Age, years | 0.85 | 1.004 (0.965, 1.044) |
| Male vs. female | 0.98 | 1.015 (0.38, 2.74) |
| Race/ethnicity | 0.58 | |
| Hispanic vs non-Hispanic white | 0.47 | 1.57 (0.30, 8.17) |
| Non-Hispanic black vs non-Hispanic white | 0.45 | 2.87 (0.59, 13.84) |
| Non-Hispanic other vs non-Hispanic white | 0.55 | 3.18 (0.40, 25.31) |
| Declined vs non-Hispanic white | 0.55 | 3.18 (0.40, 25.31) |
| Homeless | 0.94 | 1.06 (0.23, 4.93) |
| High Risk | 0.055 | 3.00 (0.98, 9.22) |
| Comorbidities | ||
| Total number of comorbidities | 0.02 | 1.38 (1.04, 1.82) |
| Cardiovascular | 0.14 | 0.48 (0.18, 1.29) |
| Gastrointestinal | 0.56 | 1.31 (0.53, 3.23) |
| Hepatic | 0.11 | 2.09 (0.84, 5.21) |
| Neuro/Psychiatric | 0.87 | 0.93 (0.36, 2.39) |
| Respiratory | 0.91 | 1.066 (0.371, 3.067) |
| Metabolic | 0.67 | 0.78 (0.25, 2.44) |
| Infectious | 0.11 | 2.23 (0.84, 5.91) |
| Hepatic | 0.11 | 2.09 (0.84, 5.21) |
| HIV/AIDS | 1.00 | NA |
| Hematology/Oncology | 0.02 | 3.21 (1.23, 8.35) |
| Renal | 0.007 | 4.08 (1.48, 11.25) |
| Other | 0.18 | 2.02 (0.73, 5.59) |
OR = Odds Ratio, CI = Confidence Interval
NA = Not available. All 7 patients with HIV/AIDS did not get the ≥200mg dosage and OR could not be estimated using logistic regression. This P-value is from Fisher’s exact test.
Bold indicates p<0.05.
Based on these univariable logistic regression model results, two multivariable logistic regression models were fitted (Table 4). The main model, Model 1, included group, high risk, and total number of comorbidities and Model 2 included group, high risk, hematology/oncology and renal comorbidities. In both models, the odds of being prescribed HPT was higher in the EMR intervention group than in the control group (Model 1: OR = 9.89, 95% CI: [2.77, 35.34], p = 0.0004; Model 2: OR = 8.35, 95% CI: [2.31, 30.21], p = 0.001).
Table 4.
Multivariable logistic regression results.
| Model 1 | |||
|---|---|---|---|
| Variable | OR | 95% CI | p-value |
| EMR intervention vs. control | 9.89 | 2.77-35.34 | 0.0004 |
| High risk vs. low risk | 3.20 | 0.99-10.34 | 0.052 |
| Total number of comorbidities | 1.41 | 1.03-1.92 | 0.03 |
| Model 2 | |||
| Variable | OR | 95% CI | p-value |
| EMR intervention vs. control | 8.35 | 2.31-30.21 | 0.001 |
| High risk vs. low risk | 4.38 | 1.24-15.45 | 0.02 |
| Hematology/Oncology | 2.41 | 0.79-7.35 | 0.12 |
| Renal | 4.93 | 1.41-17.22 | 0.01 |
OR = Odds Ratio, CI = Confidence Interval
As a sensitivity analysis, we further adjusted Models 1 and 2 for cardiovascular comorbidity, which was more prevalent in the control group. The likelihood of prescribing HPT remained significant in both models (Model 1: OR = 8.83, 95% CI [2.44, 31.96], p = 0.009; Model 2: OR = 7.81, 95% CI [2.14, 28.47], p = 0.002) while cardiovascular comorbidity remained nonsignificant (Model 1: p = 0.13; Model 2: p = 0.27).
3.3. Secondary Outcome: Length of Stay
As shown in the unadjusted analyses in Table 2, there was a trend towards statistical significance that length of stay was shorter in the EMR intervention group compared to the historical control (median (IQR): 3.8 (2.4, 7.0) vs. 4.6 (2.9, 7.8), P = 0.06).
4. Discussion
With the near universal adoption of electronic records into healthcare systems, interventions leveraging prescriber interaction with the EMR provide new opportunities to improve quality of care. Following the creation of an autopopulated order panel designed to increase HPT prescribing for inpatients with AUD, we observed a significant increase in the proportion of patients receiving HPT from 2.7% of patients to 20.2% of patients. This effect remained robust after adjusting for potential confounders. The EMR intervention group also had shorter lengths of stay as compared to the historical control group that approached statistical significance. In light of the many difficulties and obstacles that patients with AUD face in accessing high quality care, changing the EMR medication ordering workflow can be an effective method for specialty services to improve quality of care.
To date, there are limited studies of methodologies to increase thiamine supplementation for hospitalized patients with AUD. A prior clinical study used interventions that required educational programing (Day et al., 2010), which increased treatment with parenteral thiamine of patients who exhibited clinical features of WE, but decreased prophylaxis against WE in patients with AUD. Other studies investigated differences between different hospitals with and without specific protocols promoting parenteral thiamine (Day et al., 2015) or have not yet reported on the effects of their implemented order set (Flynn, Macaluso, D’Empaire, & Troutman, 2015). On a larger scale, many countries have increased the delivery of oral thiamine supplementation by fortifying their flour with thiamine, leading to decreased rates of WE (Harper et al., 1998; Ma & Truswell, 1995).
To our knowledge, this is the first study to examine the effect of EMR modifications alone, without a specific educational or training plan, on treatment for patients with a substance use disorder. With the shortage of mental health professionals worsening (Kakuma et al., 2011), and the even greater shortage of addiction specialists, the conventional consultation model of providing care is inadequate. Rates of alcohol-related emergency room visits are increasing faster than the rate of overall visits, with rates of thiamine prescriptions declining despite more dispensed medications and diagnostic testing for these patients (Mullins, Mazer-Amirshahi, & Pines, 2016). In order to produce self-sustaining changes, the EMR intervention in this study prioritized decreasing the workload stress on the healthcare system without a requirement for additional training. Our intervention has similarities but also important major differences with the pharmacy-driven interventions of antibiotic stewardship programs, which have been widely successful in both improving outcomes and cutting costs by better management of antibiotics (Davey et al., 2013; Ostrowsky et al., 2013). Both initiatives aim to extend the reach of specialty consultation services beyond direct patient interaction and to immediately influence medication choice. However, while antibiotic stewardship requires large expenses and labor to initiate and maintain, modifying the EMR to promote specific prescribing practices requires some effort to initiate, minimal work to maintain, and can be easily implemented over large healthcare systems.
Our intervention lacks many of the shortcomings of computerized decision support systems identified by Ash, Berg, & Coiera (2004), including excessive use of alerts and reminders. The intervention is classified as an “automatic provision of decision support as part of clinician workflow,” which Kawamoto et al. (2005) found to be most strongly associated with improved clinical outcomes. Additionally, our study is only the first step of a traditional, step-wise quality improvement project which relies on multiple interventions and review over time to ensure a continued improvement in quality of care (Taylor et al., 2014). One possible shortcoming of decision support using autopopulated orders is that it may be difficult to keep the content current (for review, see Ash, Sittig, Campbell, Guappone, & Dykstra, 2007). However, our approach of using specialty consultation services in creating and managing the decision support interventions could aid in keeping interventions up to date. Autopopulated orders have recently been shown to be effective to curb medication overuse, in contrast to prompts to increase practice adherence. To combat the opioid epidemic, Chiu et al. (2018) recently showed that lowering the default number of opioid pills prescribed in the EMR decreased the mean amount of opioids prescribed after surgical procedures by over 15%. In contrast, a systematic review by Shojania et al. (2010) found that computer prompts only increased adherence to processes of care by a median of 4.2%.
This type of intervention may also be especially useful at encouraging care practices that prescribers often forget, or are uncomfortable prescribing because of a lack of specific knowledge, such as with buprenorphine (Cunningham, Sohler, McCoy, & Kunins, 2006). In light of the current under-utilization of medications for substance use disorders, partially due to lack of provider familiarity (Cunningham et al., 2006), changing the EMR workflow is a strategy for widespread change that warrants further study.
This study has several limitations. First, we studied a single site with a low amount of parenteral thiamine prescribed. It is possible that the increase in HPT resulted from just a few clinicians changing their practices, with a majority prescribing as usual. Previous studies on primary medical services have shown very different practices and outcomes between physicians within the same institution (Southern & Arnsten, 2015; Southern, Bellin, & Arnsten, 2011). However, this does not discount the impact that our intervention made on prescribing practices on the hospital as a whole.
Second, this study may not have accounted for seasonal changes of admission lengths and prescribing practices across the study period. Although we have not seen literature specifically on seasonal variations in hospitalized patients with AUD, patients with heart failure (Akintoye et al., 2017) and patients admitted to psychiatric units (Federman et al., 2000) generally have a longer length of stay during wintertime in hospitals in colder climates. Cardiovascular comorbidities may have been more frequent in the historical control group because it occurred during the winter, when cardiovascular diseases have a higher incidence (Fares, 2013). Studies of alcohol related visits in the emergency department have not found seasonal differences in presentation rates (Comelli, Lippi, Sanchis-Gomar, Turcato, & Cervellin, 2018), although alcohol related visits have been increasing in the United States overall (Mullins et al., 2016). An analysis of the time period 1 year before and after the EMR changes may have controlled for seasonal changes, although the magnitude of our findings suggest that seasonal variations would minimally impact our primary outcome.
Lastly, we only studied patients who had received an APS consult, which is likely a small proportion of all hospitalized patients with an AUD diagnosis. It is likely that there were many inpatients with an active AUD that did not receive an APS consult. Of note, although outcomes were not followed for the non-APS population, they were exposed to the same intervention. A follow up study to examine all patients hospitalized with an AUD would broaden the scope of this work. Another shortcoming and future area of improvement is prompting appropriate HPT prescription at the time of inappropriately low-dose or non-parenteral thiamine doses.
Improved medical care for alcohol-related disorders can be used as a form of harm-reduction to prevent the adverse health effects of AUD as well as to provide a platform to encourage treatment-seeking behaviors. Harm reduction efforts for AUD to reduce drinking have been shown to decrease morbidity from alcohol use (Charlet & Heinz, 2017). We hope that increasing appropriate thiamine supplementation will increase patients’ positive interactions with treatment for AUD, as well as help prevent some of the cognitive impairment seen with prolonged alcohol use that makes late-stage treatment more difficult. Further study is needed to determine if increasing HPT can shorten the length of stay, but it is possible that early and adequate administration can ameliorate the weakness, ataxia, and confusion of thiamine deficiency or WE that can keep patients with AUD in hospitals.
5. Conclusions
The adoption of an autopopulated EMR order panel for thiamine supplementation in patients with AUD resulted in a significant increase in the prescription of HPT and a trend towards statistical significance for decreasing the length of stay. Modifying the EMR can be an effective method for specialty services to enhance care practices without requiring direct patient care. This may be especially helpful in large health systems or in systems with few specialists available. The absence of an educational or training component in our intervention is particularly salient given the time and labor costs of such programs and the fact that they often do not have a durable impact. Further research is required to consider the clinical effects of increasing HPT for patients with AUD in this setting. In light of the many difficulties and obstacles that patients with substance use disorders face in accessing high quality care, utilizing the functionality of the EMR by embedding medication ordering workflows that encourage appropriate prescribing can be a powerful approach to improve treatment delivery.
Highlights.
Thiamine deficiency continues to affect patients with alcohol use disorder
Most hospitalized patients are not prescribed appropriate thiamine regimens for AUD
Changes to the EMR ordering workflow can be effective in improving care
EMR edits increased high-dose parenteral thiamine prescribing (≥200mg IM/IV)
Acknowledgments
Funding: This work was supported by NIDA T32 DA007294-25 (Wai).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Abbreviations: APS – Addiction Psychiatry Consultation Service, AUD – Alcohol Use Disorder, EMR – Electronic Medical Record, HPT – High-Dose Parenteral Thiamine, WE – Wernicke’s encephalopathy
References
- Akintoye E, Briasoulis A, Egbe A, Adegbala O, Alliu S, Sheikh M, … Levine D. (2017). Seasonal variation in hospitalization outcomes in patients admitted for heart failure in the United States. Clinical Cardiology, 40(11), 1105–1111. 10.1002/clc.22784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash JS, Berg M, & Coiera E (2004). Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. Journal of the American Medical Informatics Association, 11(2), 104–112. 10.1197/jamia.M1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash JS, Sittig DF, Campbell EM, Guappone KP, & Dykstra RH (2007). Some unintended consequences of clinical decision support systems In AMIA Annual Symposium Proceedings (Vol. 2007, p. 26). American Medical Informatics Association. [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF (1995). Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metabolic Brain Disease, 10(1), 1–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7596324 [DOI] [PubMed] [Google Scholar]
- Caine D, Halliday GM, Kril JJ, & Harper CG (1997). Operational criteria for the classification of chronic alcoholics: Identification of Wernicke’s encephalopathy. Journal of Neurology Neurosurgery and Psychiatry, 62(1), 51–60. 10.1136/jnnp.62.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, & Heinz A (2017). Harm reduction -a systematic review on effects of alcohol reduction on physical and mental symptoms. Addiction Biology, 22(5), 1119–1159. 10.1111/adb.12414 [DOI] [PubMed] [Google Scholar]
- Chiu AS, Jean RA, Hoag JR, Freedman-Weiss M, Healy JM, & Pei KY (2018). Association of Lowering Default Pill Counts in Electronic Medical Record Systems With Postoperative Opioid Prescribing. JAMA Surgery 10.1001/jamasurg.2018.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli I, Lippi G, Sanchis-Gomar F, Turcato G, & Cervellin G (2018). Visits for alcohol-related problems in a large urban Emergency Department. Results of a 15-year survey. Acta Bio Medica Atenei Parmensis, 88(4), 514–518. 10.23750/abm.v88i4.6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CCH, Hallwood PM, & Thomson AD (1998). B vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol and Alcoholism 10.1093/oxfordjoumals.alcalc.a008400 [DOI] [PubMed] [Google Scholar]
- Cunningham CO, Sohler NL, McCoy K, & Kunins HV (2006). Attending physicians’ and residents’ attitudes and beliefs about prescribing buprenorphine at an urban teaching hospital. Family Medicine, 38(5), 336–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16673195 [PubMed] [Google Scholar]
- Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, … Wilcox M. (2013, April 30). Interventions to improve antibiotic prescribing practices for hospital inpatients (Davey P, Ed.), Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 10.1002/14651858.CD003543.pub3 [DOI] [PubMed] [Google Scholar]
- Day E, Bentham PW, Callaghan R, Kuruvilla T, & George S (2013). Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol. Cochrane Database of Systematic Reviews 10.1002/14651858.CD004033.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day E, Callaghan R, Kuruvilla T, George S, Webb K, & Bentham P (2010). Pharmacy-based intervention in Wernicke’s encephalopathy. The Psychiatrist, 34(06), 234–238. 10.1192/pb.bp.109.025775 [DOI] [Google Scholar]
- Day GS, Ladak S, Curley K, Farb NAS, Masiowski P, Pringsheim T, … del Campo C Martin. (2015). Thiamine prescribing practices within university-affiliated hospitals: A multicenter retrospective review. Journal of Hospital Medicine, 10(4), 246–253. 10.1002/jhm.2324 [DOI] [PubMed] [Google Scholar]
- Fares A (2013). Winter cardiovascular diseases phenomenon. North American Journal of Medical Sciences, 5(4), 266–79. 10.4103/1947-2714.110430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federman EJ, Drebing CE, Boisvert C, Penk W, Binus G, & Rosenheck R (2000). Relationship Between Climate and Psychiatric Inpatient Length of Stay in Veterans Health Administration Hospitals. American Journal of Psychiatry, 157(10), 1669–1673. 10.1176/appi.ajp.157.10.1669 [DOI] [PubMed] [Google Scholar]
- Flynn A, Macaluso M, D’Empaire I, & Troutman MM (2015). Wernicke’s Encephalopathy: Increasing Clinician Awareness of This Serious, Enigmatic, Yet Treatable Disease. The Primary Care Companion for CNS Disorders, 17(3). 10.4088/PCC.14r01738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, & Leone MA (2010). EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. European Journal of Neurology. 10.1111/j.1468-1331.2010.03153.x [DOI] [PubMed] [Google Scholar]
- Harper C (1983). The incidence of Wernicke’s encephalopathy in Australia- A neuropathological study of 131 cases. Journal of Neurology, Neurosurgery and Psychiatry, 46(7), 593–598. https://doi.Org/10.1136/jnnp.46.7.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Sheedy DL, Lara AI, Garrick TM, Hilton JM, & Raisanen J (1998). Prevalence of Wernicke-Korsakoff syndrome in Australia: Has thiamine fortification made a difference? Medical Journal of Australia, 168(11), 542–545. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9640303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Giles M, & Finlay-Jones R (1986). Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. Journal of Neurology Neurosurgery, and Psychiatry, 49, 341–345. https://doi.Org/10.1136/jnnp.49.4.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Chabon B, & Nicolson SE (2014). Prescribing Thiamine to Inpatients With Alcohol Use Disorders. Journal of Addiction Medicine, 8(1), 1–5. 10.1097/01.ADM.0000435320.72857.c8 [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Rahane S, DeRosa AP, Ellis J, & Nicolson SE (2016). Wernicke-Korsakoff syndrome in patients with cancer: a systematic review. The Lancet Oncology 10.1016/S1470-2045(16)00037-1 [DOI] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Shen MJ, Alici Y, Wills J, Nelson C, & Breitbart W (2017). High rate of thiamine deficiency among inpatients with cancer referred for psychiatric consultation: results of a single site prevalence study. Psycho-Oncology, 26(9), 1384–1389. 10.1002/pon.4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuma R, Minas H, van Ginneken N, Dal Poz MR, Desiraju K, Morris JE, … Scheffler RM. (2011). Human resources for mental health care: current situation and strategies for action. The Lancet, 378(9803), 1654–1663. 10.1016/S0140-6736(11)61093-3 [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Houlihan CA, Balas EA, & Lobach DF (2005). Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. Bmj, 330(7494), 765 10.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt N, & Dore G (2014). Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Internal Medicine Journal, 44(9), 911–915. 10.1111/imj.12522 [DOI] [PubMed] [Google Scholar]
- Li SF, Jacob J, Feng J, & Kulkami M (2008). Vitamin deficiencies in acutely intoxicated patients in the ED. The American Journal of Emergency Medicine, 26(7), 792–795. https://doi.Org/10.1016/J.AJEM.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Ma JJ, & Truswell AS (1995). Wernicke-Korsakoff syndrome in Sydney hospitals: before and after thiamine enrichment of flour. The Medical Journal of Australia, 163(10), 531–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8538524 [DOI] [PubMed] [Google Scholar]
- Moore RD, Bone LR, Geller G, Mamon JA, Stokes EJ, & Levine DM (1989). Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA, 261(3), 403–407. [PubMed] [Google Scholar]
- Mullins PM, Mazer-Amirshahi M, & Pines JM (2016). Alcohol-related visits to US emergency departments, 2001–2011. Alcohol and alcoholism, 52(1), 119–125. 10.1093/alcalc/agw074 [DOI] [PubMed] [Google Scholar]
- Naidoo DP, Bramdev A, & Cooper K (1996). Autopsy prevalence of Wernicke’s encephalopathy in alcohol-related disease. South African Medical Journal = Suid-Afrikaanse Tydskrif Vir Geneeskunde, 86(9), 1110–2. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8888781 [PubMed] [Google Scholar]
- Ostrowsky B, Sharma S, DeFino M, Guo Y, Shah P, McAllen S, … Bhalla R. (2013). Antimicrobial Stewardship and Automated Pharmacy Technology Improve Antibiotic Appropriateness for Community-Acquired Pneumonia. Infection Control & Hospital Epidemiology, 34(06), 566–572. 10.1086/670623 [DOI] [PubMed] [Google Scholar]
- Robert Wood Johnson Foundation. (2014). County Health Rankings Retrieved June 4, 2018, from http://www.countyhealthrankings.org/
- Saitz R, Freedner N, Palfai TP, Horton NJ, & Samet JH (2006). The severity of unhealthy alcohol use in hospitalized medical patients. Journal of General Internal Medicine, 21(4), 381–385. https://doi.Org/10.1111/j.1525-1497.2006.00405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, & Cook MJ (2015). Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. Journal of Neurology, Neurosurgery & Psychiatry, 86(12), jnnp-2014-309598 10.1136/jnnp-2014-309598 [DOI] [PubMed] [Google Scholar]
- Shojania KG, Jennings A, Mayhew A, Ramsay C, Eccles M, & Grimshaw J (2010). Effect of point-of-care computer reminders on physician behaviour: a systematic review. Canadian Medical Association Journal, 182(5), E216–E225. 10.1503/cmaj.090578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers BA, Yahr HT, & Ruhl CE (2004). Detection of Alcohol Use Disorders in General Hospital Admissions in the United States. Archives of Internal Medicine, 164(7), 749 https://doi.Org/10.1001/archinte.164.7.749 [DOI] [PubMed] [Google Scholar]
- Socio-Economic Status and General Health Indicators - Bronx County. (2017, August). Retrieved June 4, 2018, from https://www.health.ny.gov/statistics/chac/chai/docs/ses_58.htm
- Southern WN, & Arnsten JH (2015). Increased Risk of Mortality among Patients Cared for by Physicians with Short Length-of-Stay Tendencies. Journal of General Internal Medicine, 30(6), 712–718. 10.1007/sll606-014-3155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern WN, Bellin EY, & Arnsten JH (2011). Longer lengths of stay and higher risk of mortality among inpatients of physicians with more years in practice. American Journal of Medicine, 124(9), 868–874. https://doi.Org/10.1016/j.amjmed.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, & Reed JE (2014). Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Quality and Safety 10.1136/bmjqs-2013-001862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JW, Guire KE, & Horvat GG (1997). Is patient length of stay related to quality of care? Hospital & Health Services Administration, 42(4), 489–507. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10174462 [PubMed] [Google Scholar]
- Thomson AD, & Leevy CM (1972). Observations on the mechanism of thiamine hydrochloride absorption in man. Clinical Science, 43(2), 153–163. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/5048302 [DOI] [PubMed] [Google Scholar]
- Thomson AD, & Marshall EJ (2006). The natural history and pathophysiology of Wernicke’s encephalopathy and Korsakoff’s psychosis. Alcohol and Alcoholism 10.1093/alcalc/agh249 [DOI] [PubMed] [Google Scholar]
- Torvik A, Lindboe CF, & Rogde S (1982). Brain lesions in alcoholics. A neuropathological study with clinical correlations. Journal of the Neurological Sciences, 56(2–3), 233–248. 10.1016/0022-510X(82)90145-9 [DOI] [PubMed] [Google Scholar]
- Wrenn KD, Murphy F, & Slovis CM (1989). A toxicity study of parenteral thiamine hydrochloride. Annals of Emergency Medicine. 10.1016/S0196-0644(89)80215-X [DOI] [PubMed] [Google Scholar]


