Abstract
Lysosome-related organelles comprise a diverse group of cell type-specific, membrane-bound subcellular organelles that derive at least in part from the endolysosomal system but that have unique contents, morphology, and functions to support specific physiological roles. They include melanosomes that provide pigment to our eyes and skin, alpha and dense granules in platelets and lytic granules in cytotoxic T cells and natural killer cells that release effectors to regulate hemostasis and immunity, and distinct classes of lamellar bodies in lung epithelial cells and keratinocytes that support lung plasticity and skin lubrication. The formation, maturation and/or secretion of subsets of lysosome-related organelles are dysfunctional or entirely absent in a number of hereditary syndromic disorders, including in particular the Hermansky-Pudlak syndromes. This review provides a comprehensive overview of lysosome-related organelles in humans and model organisms and presents our current understanding of how the products of genes that are defective in heritable diseases impact their formation, motility and ultimate secretion.
Keywords: Hermansky-Pudlak syndrome, Chediak-Higashi syndrome, BLOC-1, BLOC-2, BLOC-3, AP-3, RAB32, RAB38, RAB27A, Griscelli syndrome, VPS33A, VPS33B, HOPS, melanosome, Weibel-Palade body, alpha granule, dense granule, lamellar body
Introduction
The endolysosomal system in metazoans consists of a complex web of interconnected compartments and membranes that, in all cell types, serve an astoundingly large array of functions including nutrient uptake, metabolic control, signaling, pathogen destruction, innate immunity, and others.1,2 The endolysosomal system is highly plastic, allowing specific cell types to adapt it to serve their special needs. For example, phagocytic cells coopt the recycling endosomal system to provide the membrane needed to engulf large particles3,4, polarized cells diversify the endosomal system to accommodate sorting among topologically distinct plasma membrane domains5, and adipocytes and muscle cells adapt recycling endosomes to generate reservoirs from which glucose transporters can be rapidly deployed to the plasma membrane upon insulin signaling.6-8
Research over the last 15-20 years has revolutionized our understanding of endosomal system adaptation towards a distinct end - the formation of cell type-specific organelles known as lysosome-related organelles (LROs).9-11 LROs are named as such because of their primary derivation from the endosomal system, their variable content of lysosomal proteins, and often an acidic phase during their life cycle – requiring the activity of the vacuolar ATPase.12 However, they encompass a broad array of structures with distinct morphologies and functions that are unique to the physiology of their host cell (Table 1, Figure 1).9,10 These structural and functional features are conferred by the unique cell type-specific contents of each LRO, which are specifically sorted to nascent LROs during their formation. While some LROs, such as immature cytolytic granules of resting cytotoxic T cells (CTLs), double as the host’s lysosomes13 (T cell activation induces granule maturation by fusion with recycling endosomes14), others such as melanosomes, Weibel-Palade bodies and platelet dense and alpha granules coexist with bona fide endolysosomes in their particular cell type.15,16 The host cell types for the latter LRO class therefore require a dedicated system(s) by which LRO contents are segregated from cargoes destined to classical endolysosomes. In addition, most (if not all) LROs are regulated secretory organelles, and secretion at the proper time is critical for LRO function. For example, the contents of cytolytic granules must only be directed towards a target cell following immune recognition, and contents of platelet alpha and dense granules must only be released upon platelet activation at sites of vascular damage. Thus, proper regulation of the biogenesis, maintenance/protection from degradation, and secretion of LROs is necessary for normal physiological function.
Table 1.
LROs and their associated disease models
| Lysosome-Related Organelles |
Cell Types | Description | LRO disease model |
|---|---|---|---|
| LROs-Vertebrate | |||
| Melanosomes | Melanocytes or melanophores in skin, retinal pigment epithelia and other eye pigment cells | Site of melanin synthesis and storage for photoprotection and visual acuity | HPS, CHS, GS |
| Lamellar bodies (mammals) or swimbladder surfactant storage organelles (teleosts) | Alveolar type II (AT2) cells | Organelles for synthesis, storage and secretion of pulmonary surfactant | HPS |
| Lamellar bodies / Lamellar granules | Keratinocytes | Enriched in lipids that are secreted by keratinocytes into extracellular spaces of the epidermis to form a permeability barrier | ARC |
| Cytolytic granules | T cells, Natural killer cells | Store granzymes, perforin and other cytotoxic proteins that induce target cell death upon secretion | FHL, GS type 2, CHS, HPS (AP-3) |
| Weibel-Palade bodies | Endothelial cells | Cigar-shaped secretory granules containing von Willebrand Factor and other proteins that mediate hemostasis and inflammation upon secretion | HPS (BLOC-2 and AP-3), FHL3 |
| Alpha granules | Platelets, Megakaryocytes | Store a variety of protein factors that upon secretion mediate blood clotting, platelet adhesion, hemostasis, inflammation and vascularization | GPS, ARC |
| Dense granules | Platelets, Megakaryocytes (?) | Contain small molecules (e.g. serotonin, calcium, ADP and others) and polyphosphate that upon secretion enhance platelet adhesion and activation | HPS, FHL |
| MHC class II compartments | Activated dendritic cells, B lymphocytes, macrophages, Langerhans cells | Non-terminal late endosomes and lysosomes that are enriched in MHC class II molecules assembling with peptides | CHS |
| Basophilic granules | Mast cells, likely basophils | Store granzymes, heparin, histamine, serotonin, prostaglandin, and leukotrienes for secretion at sites of damage or infection to increase vasodilation | CHS, FHL |
| Azurophilic (primary) granules | Neutrophils, Eosinophils | Store lysosomal enzymes and anti-microbial peptides for release directly into phagosomes | HPS (AP-3), CHS |
| Phagosomes | Macrophages, neutrophils, dendritic cells | Surround phagocytosed microorganisms, apoptotic cells or other large particles; kill and digest the contents and initiate signal transduction cascades | HPS (AP-3), CHS, ARC in insects |
| NOX2+ inhibitory lysosomes | Conventional dendritic cells | Contain the NADPH oxidase NOX2, which negatively regulates proteolysis upon fusion with phagosomes to facilitate cross-presentation | GS2 |
| IRF7 signaling lysosomes | Plasmacytoid dendritic cells | The site of an IRF7 signaling cascade from nucleic acid sensing toll like receptors to initiate production of type I interferon | HPS |
| Acrosomes | Sperm cells | Organelles storing hydrolytic and glycolytic proteins that are secreted by sperm to reach the egg prior to fertilization | GS2 |
| Notochord vacuoles | Notochord inner cells | Organelles required for body axis elongation and spine morphogenesis | HPS |
| LROs-invertebrate | |||
| Pigment granules | Drosophila melanogaster retinal cells | Contain red and brown pigments that are necessary for light insulation in order to prevent the loss or spread of light throughout the eye | HPS |
| Gut granules | Caenorhabditis elegans intestinal cells | Storage compartment putatively containing zinc, anthranilic acid, and lipofuscin | HPS, CHS |
| Zinc storage granules | Drosophila melanogaster Malpighian tubule epithelial cells | Storage compartment that collectively contains the total body pool of chelatable zinc | HPS |
| Post-lysosomes | Dictyostelium discoideum | Deacidified secretory compartments that mature from lysosomes and constitutively secrete undigested material into the extracellular space | CHS |
| Mucocysts | Tetrahymena thermophila | Secretory granules containing peptides that, upon stimulated exocytosis, surround the cell in a thick mucus layer as a method of cellular defense | HPS |
| Riboflavin granules | Bombyx mori Malpighian tubules | Needle-shaped yellow granules that store riboflavin | HPS |
| Integument urate granules | Bombyx mori epidermal cells | Crystal form of the uric acid-containing fat body that make the larval skin opaque for protection from ultraviolet radiation | HPS |
| Putative LROs-vertebrate | |||
| Large dense-core vesicles | Specialized secretory cells (e.g. adrenal chromaffin cells) | Secretion of hormones and neuropeptides | HPS |
| Specific (secondary) granules | Neutrophils | Secretory granules containing cytotoxic proteins involved in the initiation of the inflammatory response | |
| Gelatinase (tertiary) granules | Neutrophils | Secretory granules containing gelatinase, receptors, adhesion molecules and other proteins to mediate cell adhesion to the endothelium | |
| Presynaptic vesicles | Neuron synaptic cleft | Secretory vesicles containing neurotransmitters that are released at the synapse upon stimulation | HPS (AP-3 and BLOC-1) |
| Osteoclast secretory lysosome | Osteoclasts | Secretory lysosomes involved in bone resorption and remodeling | GS2 |
| Fusiform vesicles | Urothelium | Compartment mediating the storage and transport of urothelial plaques for bladder expansion | |
| Putative LROs-invertebrate | |||
| Glue granules | Drosophila melanogaster larval salivary gland epithelial cells | Secretory granules containing highly glycosylated glue proteins required for pupal case adhesion to a solid substrate during metamorphosis | |
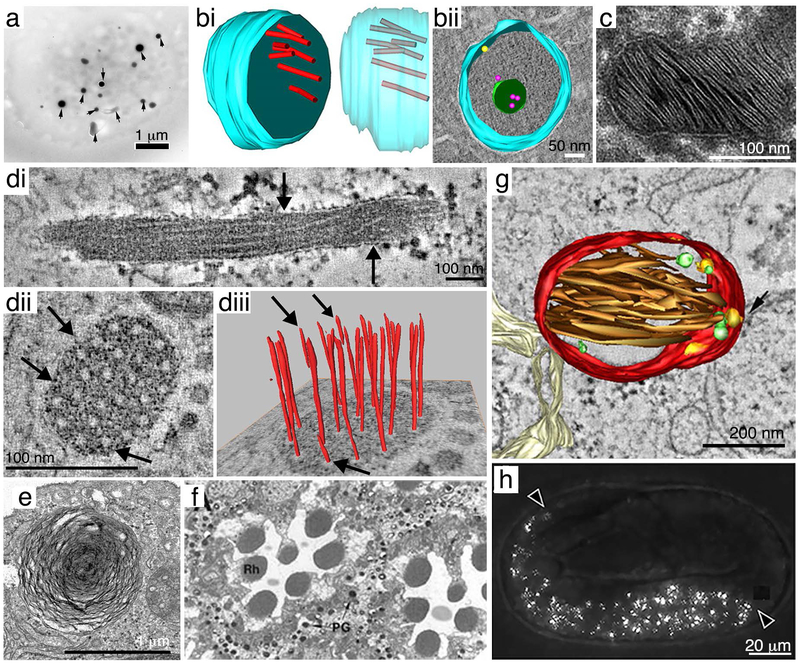
Figure 1: Examples of lysosome-related organelles.
(a) Dense granules (arrows) in a human platelet observed by whole mount electron microscopy from ref. 447. Scale bar, 1 μm. (bi – bii) 3D reconstructions of α-granules in chemically fixed human platelets analyzed by electron tomography from ref. 448. The limiting membrane is in blue. Scale bar, 50 nm. (bi) Transverse view (left) and side view with transparent membrane (right) emphasizing the arrangement of VWF tubules (red on left, red-gray on right) within the organelle. (bii) Transverse view of an alpha granule that is immunogold labeled for P-selectin (yellow) and CD63 (magenta), emphasizing an intraluminal vesicle (green). (c) A lamellar body from ex vivo human skin analyzed by thin section electron microscopy (from ref. 449). Scale bar, 100 nm. (di – diii) Electron tomography of WPBs in human umbilical vein endothelial cells from ref. 450. (di) A longitudinal tomographic slice of a WPB emphasizing vWF tubules (arrows) along the length. (dii) A transverse tomographic section of a WPB emphasizing the diameter of vWF tubules. (diii) 3D reconstruction of (dii) showing the extension of individual VWF tubules. Arrows point to VWF tubules that end halfway through the WPB. Scale bars, 100 nm. (e) A lung lamellar body from a rat type II alveolar cell analyzed by thin section electron microscopy (from ref. 451). Scale bar, 1 μm. (f) Transverse section of a wild-type D. melanogaster eye analyzed by thin section electron microscopy from ref. 77. Note the pigment granules (PG) of secondary pigment cells surrounding photoreceptor cell rhabdomeres (Rh). (g) 3D reconstruction of a stage II melanosome from electron tomography analysis of a human MNT-1 melanoma cell (from ref. 452). Red, melanosome membrane; brown, intraluminal fibrils; intralumenal vesicles are in yellow (membrane-associated) or green (free). Scale bar, 200 nm. (h) Birefringent material in gut granules (arrowheads) in a C. elegans embryo observed by polarization microscopy (from ref. 279). Scale bar, 20 μm. All panels reprinted by permission of: (a) Taylor and Francis from Platelets, ref. 447, copyright 2018; (b) American Society of Hematology from Blood, ref. 448, copyright 2010; (c) Elsevier from the Journal of Dermatological Science, ref. 449, copyright 2018; (d) Elsevier from the Journal of Structural Biology, ref. 450, copyright 2008; (e) Springer Nature from Pediatric Research, ref. 451, copyright 2012; (f) John Wiley and Sons from EMBO Journal, ref. 77,451, copyright 1997; (g) National Academy of Sciences, U.S.A from Proceedings of the National Academy of Sciences U.S.A., ref. 452, copyright 2008; and (h) PLoS Genetics, ref. 279.
Progress in understanding both the formation and secretion of LROs has been greatly accelerated by the study of syndromic human genetic disorders – and their animal models – in which these processes are disrupted in many LRO-generating cell types. Biogenetic disorders include the Hermansky-Pudlak syndromes (HPS), Chediak-Higashi syndrome (CHS), the arthrogryposis, renal dysfunction and cholestasis (ARC) syndromes, and gray platelet syndrome (GPS). Secretory disorders include the Griscelli syndromes (GS) and familial hemophagocytic lymphohistiocytosis (FLH) types 3-5. Each of these monogenic disorders impacts the function of a group of LROsa, resulting in loss of function in such diverse physiological systems as immunity, neurology, pigmentation, hemostasis, and others. For example, oculocutaneous albinism and excessive bleeding and bruising in HPS patients are due to impaired biogenesis of pigment cell melanosomes and platelet dense granules, respectively.17,18
Over the last 10-15 years, functional analyses of HPS genes and their products in particular have enlightened molecular pathways required for content delivery and function of melanosomes, dense granules, lung lamellar bodies, and several organelles in innate and adaptive immune cell types. This review will focus primarily on the roles of HPS gene products and their associated proteins in LRO biogenesis at the level of human disease, model organisms, and cell culture systems. We will briefly touch upon how the other syndromic diseases mentioned above are similarly providing new insights into LRO biogenesis, positioning and secretion, and then provide some perspectives on future studies.
LROs vs. secretory granules and secretory lysosomes
How are LROs defined and distinguished from classical secretory granules? Most experts agree that LROs derive a substantial component of their contents from the endolysosomal system, including either late endosomes, early endosomes, or both (Figure 2). By contrast, secretory granules derive most of their contents from the Golgi complex. However, the line between LROs and classical secretory granules can be blurred. For example, Weibel-Palade bodies - cigar-shaped regulated secretory organelles in endothelial cells that package and store von Willebrand factor (vWF) for stimulated secretion – have long been considered LROs10, but immature Weibel-Palade bodies bearing polymerized vWF bud directly from the trans Golgi network19 and later fuse with endosomal membranes bearing CD63 and P-selectin.20 By contrast, large dense core granules have long been considered classical secretory granules but have some features of LROs, such as the requirement for complexes that are defective in several HPS subtypes for their proper formation21-23 and the recruitment of ectopically expressed LRO cargos.24 In this review, we define LROs broadly as cell type-specific organelles for which at least some functionally or structurally significant components derive from the endolysosomal system, and/or for which their biogenesis, motility or secretion requires effectors that are disrupted in LRO diseases described here (HPS, GPS, GS, ARC or FLH) or their homologues in model systems (Table 1). This inclusive definition would encompass organelles such as cytolytic granules that have been referred to as “secretory lysosomes”25,26, but would exclude actual lysosomes (which can also be secreted under some conditions but which are not cell type-specific27,28). Note that this definition might also encompass certain types of synaptic vesicles in neurons (Table 1), but due to the complexity of the neuronal system we have chosen not to cover this topic extensively in this review.
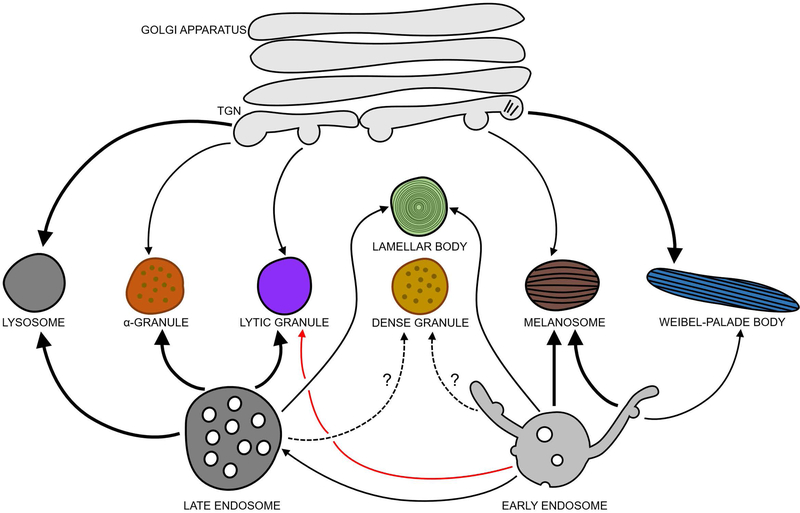
Figure 2. Biogenesis models for mammalian LROs.
Lytic granules in CTLs and natural killer cells may derive from late endosomes that fuse with secretory vesicles from the TGN, and also function as the cells’ lysosomes under basal conditions. Lytic granule maturation (by fusion with early endosomal membranes; red arrow) and secretion are triggered by immune stimulation. The other indicated mammalian LROs co-exist with endolysosomes but derive from late endosomes, early endosomes, and/or the TGN as indicated. Question marks denote LRO biogenesis pathways that are not well-characterized, and thick solid lines denote pathways, where known, by which the majority of material is targeted to maturing LROs. Alpha granules obtain material from multivesicular late endosomes in platelets and likely derive from these compartments, but also receive vWF from the TGN. Platelet dense granules and lung AT2 lamellar bodies may derive components from both late endosomes and early endosomes during maturation. In pigment cell melanocytes, immature melanosomes emerge from maturing endosomes and receive transmembrane cargo via early endosomal tubule carriers, endosome-derived vesicles, and the Golgi as they mature. In endothelial cells, immature Weibel-Palade bodies harboring vWF tubules that form in the Golgi bud from the TGN and mature by addition of cargoes derived from endosomes.
The Hermansky-Pudlak syndromes
HPS is a group of syndromic disorders characterized in all cases by varying degrees of oculocutaneous albinism, with concomitant visual impairment and susceptibility to skin cancer, and by excessive bleeding and bruising that can, under some conditions such as childbirth or surgery, become life-threatening.29 HPS is rare in the general population (incidence estimated at 1:1,000,000), but is particularly prevalent in certain populations due to founder effects; for example, HPS1 is estimated to afflict ~1:1800 individuals in Puerto Rico.29 Albinism reflects defects in the biogenesis of melanosomes in melanocytes of the skin, hair and choroid of the eye and in pigment epithelial cells of the retina, iris and ciliary body of the eye. Bleeding and bruising reflects absence of detectable dense granules in platelets. Patients with the most common HPS subtypes (HPS1 and HPS4) additionally suffer from a progressive lung fibrosis that is typically lethal without a lung transplant within the 4th or 5th decade of life. The primary insult responsible for lung fibrosis appears to be defects in the biogenesis of lamellar bodies, which are LROs in alveolar type II (AT2) cells responsible for surfactant synthesis and secretion.30-32 Consequent inflammatory sequelae, which likely result from reduced surfactant levels, initiate and expand the fibrotic response.33,34 A subset of HPS1 and 4 patients also suffer from granulomatous colitis (a type of inflammatory bowel disease), the etiology of which is currently unknown. HPS2 and HPS10 patients also suffer from lung disease35,36, but additionally suffer from recurrent bacterial and viral infections due to impaired immune responses.36-40 The molecular basis for the impaired immune response will be discussed later in this review.
HPS can be caused by inactivating mutations in any of at least 10 different genes in humans. All of these genes encode subunits of four obligate multi-subunit protein complexes: adaptor protein-3 (AP-3) and the biogenesis of lysosome-related organelles complex (BLOC)-1, −2 and −3 (Table 2, highlighted in gray, yellow, green and blue). LRO biogenesis in model organisms is also disrupted by mutations in the same genes and in genes encoding additional BLOC-1 subunits, as well as additional genes; these include genes encoding the Rab GTPase targets of BLOC-3 function (RAB32 and RAB38) and subunits of the homotypic fusion and vacuole protein sorting (HOPS) and the class c core vacuole/endosome tethering (CORVET) complexes (Table 2; HOPS/CORVET subunits highlighted in orange). The phenotype in mice with BLOC or AP-3 subunit mutations is remarkably similar to that of HPS patients. In almost all cases, disease results from nonsense mutations that ablate production of an intact subunit and that thereby (1) disrupt formation of the entire complex with which the subunit is associated, and (2) destabilize the remaining subunits. For example, mutations in HPS4 destabilize both HPS4 and HPS1 and result in a loss of BLOC-3 function.41 A substantial fraction of HPS patients lack identifiable mutations within these 10 genes, suggesting that mutations in additional genes may also cause the disease.29
TABLE 2.
Disease-associated genes in humans and their model systems.
| zHuman gene | Alternative human gene names |
Affected protein complex/subunit |
Human disease |
Rodent model | Drosophila model |
Other models |
|---|---|---|---|---|---|---|
| BLOC1S1 | BLOS1, BORCS1, RT14, GCN5L1 | BLOC-1 and BORC subunit BLOS1 | Blos1Ell-Cre/loxp; Blos1nestin-Cre/loxp | blos1 |
bloc1s1ihb815 (z) blos-1(-) (c) |
|
| BLOC1S2 | BLOS2, BORCS2, CEAP, CEAP11 | BLOC-1 and BORC subunit BLOS2 | Bloc1s2−/− |
bloc1s2ihb818(z) p-Translucent (op) (d) |
||
| BLOC1S3 | Reduced Pigmentation (RP), BLOS3, HPS8 | BLOC-1 subunit BLOS3 | HPS8 | reduced pigmentation | ||
| BLOC1S4 | CNO, BLOS4, BCAS4L | BLOC-1 subunit Cappuccino | cappuccino | |||
| BLOC1S5 | MUTED, BLOS5, MU | BLOC-1 subunit Muted | muted | Tanaka’s mottled translucent (otm) (b) | ||
| BLOC1S6 |
PLDN, PALLID, BLOS6, HPS9 |
BLOC-1 subunit Pallidin | HPS9 | pallid | glo-2(-) (c) | |
| SNAPIN | BLOC1S7, BLOS7, BORCS3, SNAPAP | BLOC-1 and BORC subunit Snapin | snapin−/− | snpn-1 (c) | ||
| DTNBP1 | BLOC1S8, DBND, SNDY, HPS7 | BLOC-1 subunit Dysbindin | HPS7 | sandy | Mottled translucent of var (ov) (d) | |
| HPS3 | BLOC2S1, SUTAL | BLOC-2 subunit | HPS3 | cocoa | ||
| HPS5 | BLOC2S2, AIBP63 | BLOC-2 subunit | HPS5 | ruby-eye 2 | pink |
snow white (z) Aojuku translucent (oa) (d) |
| HPS6 | BLOC2S3 | BLOC-2 subunit | HPS6 | ruby-eye | no privacy (x) | |
| HPS1 | BLOC3S1, HPS | BLOC-3 subunit | HPS1 | pale ear | ||
| HPS4 | BLOC3S2, LE | BLOC-3 subunit | HPS4 | light ear | ||
| AP3B1 | ADTB3A, HPS2, PE | AP-3 β3A subunit | HPS2 | pearl; Ap3b1LN | ruby | apt-6(-) (c) |
| AP3D1 | ADTD, HPS10 | AP-3 δ subunit | HPS10 | mocha | garnet | apt-5(-) (c) |
| AP3M1 | AP-3 μ3A subunit | carmine | apt-7(-) (c) | |||
| AP3M2 | P47B, AP47B, CLA20 | AP-3 μ2 subunit | ||||
| AP3S1 | CLAPS3 | AP-3 σ3A subunit | orange | |||
| AP3S2 | AP3S3 | AP-3 σ2 subunit | ||||
| VPS33A | MPSPS | HOPS/CORVET subunit VPS33A | buff | carnation | vps-33.1(-) (c) | |
| VPS11 | END1, PEP5, RNF108, HLD12 | HOPS/CORVET subunit VPS11 |
vps-11(-) (c) platinum (z) |
|||
| VPS16 | HOPS/CORVET subunit VPS16A | maroon | vps-16(-) (c) | |||
| VPS18 | PEP3 | HOPS/CORVET subunit VPS18 | deep orange |
vps-18(-) (c) vps18(hi2499A) (z) |
||
| VPS41 | HOPS subunit VPS41 | light | vps-41(-) (c) | |||
| VPS39 | VAM6, TLP | HOPS subunit VPS39 |
vps-39(-) (c) lbk/vam6 (z) |
|||
| RAB38 | NY-MEL1 | RAB38 | Chocolate mouse; Ruby rat | lightoid | glo-1(-) (c) | |
| RAB32 | RAB32 | lightoid | glo-1(-) (c) | |||
| RABGGTA | PTAR3, RGGTA | Rab geranylgeranyltransferase II subunit α | gunmetal | |||
| SLC7A11 | XCT, CCBR1 | SLC7A11 | subtle gray | |||
| LYST | CHS1 | LYST | CHS | beige | mauve |
lvsA, lvsB (d) lyst-1(-) (c) |
| VPS33B | CHEVI complex subunit VPS33B | ARC | Vps33bfl/fl |
vps33.2(-) (c) vps33b MO (z) |
||
| VIPAS39 | VIPAR, VPS16B, SPE-39, C14orf133 | CHEVI complex subunit VPS16B | ARC | Vipas39fl/fl | full of bacteria |
spe39(-) (c) vipar MO (z) |
| NBEAL2 | GPS, BDPLT4 | NBEAL2 | GPS | nbeal2−/− | ||
| MYOVA | MYH12, MYR12, MYOV | Myosin Va | GS1 | dilute | ||
| RAB27A | RAM | Rab GTPase RAB27A | GS2 | ashen | ||
| MLPH | SLC2-A | Melanophilin | GS3 | leaden | ||
| UNC13D | Munc13-4 | Munc13-4 | FHL3 | Jinx | ||
| STX11 | Syntaxin-11 | FHL4 | Stx11−/− | |||
| STXBP2 | Munc18-2, UNC18-2, UNC18B | Munc18-2 (Munc18b) | FHL5 | Munc18b+/− |
Key:
(z): zebrafish; (c): C. elegans; (x): Xenopus laevis; (d): D. discoideum; (b): Bombyx mori.
Colored highlights indicate subunits of the same multisubunit protein complex.
Targeted gene knockouts in mice are indicated as −/−; mice with conditional knockouts in specific cell types through Cre/loxp technology are indicated as fl/fl.
As discussed further below, the protein complexes that are disrupted in HPS each participate in a discrete step of membrane trafficking required for LRO biogenesis or for additional functions in cell types that lack LROs (Figure 3). Not all steps appear to be equally required for minimally functional LRO biogenesis in each cell type, resulting in the differences in the disease spectrum of patients with mutations in distinct HPS genes. However, the disease spectrum is nearly identical in patients with mutations in different components of the same protein complex - e.g. the disease spectrum in HPS3, HPS5 and HPS6 patients, all with mutations in BLOC-2 subunits, is very similar.42-44 For this reason, HPS is best understood in the context of BLOC-1, BLOC-2, BLOC-3 or AP-3 disease rather than in the context of each individual gene product.
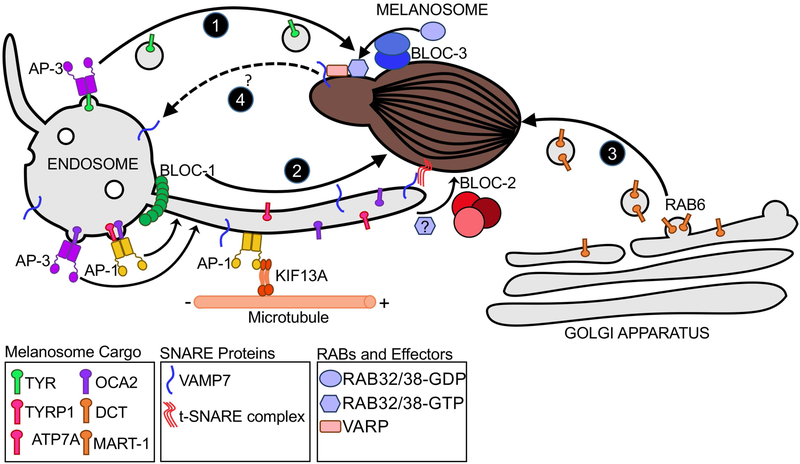
Figure 3. HPS complexes and mechanisms of cargo delivery to melanosomes.
Melanosome-destined transmembrane protein cargoes are concentrated on early endosomes by AP-3 and AP-1. The majority of TYR (green) transits a vesicular pathway that requires AP-3 (Pathway 1). Other proteins such as TYRP1, OCA2, and ATP7A, transit endosome-derived membrane tubules en route to melanosomes (Pathway 2). BLOC-1 and the KIF13A kinesin motor are required to generate the tubules along microtubules, and KIF13A is recruited to endosomes by AP-1. Cargo sorting on this pathway is mediated by AP-1 and/or AP-3; TYRP1 and ATP7A engage only AP-1, while both AP-1 and AP-3 facilitate OCA2 transport on this pathway. BLOC-2 is required to direct the tubular transport carriers to melanosome membranes, and RAB32 and/or RAB38 might also play a role in this step. Cargo delivery requires transient fusion of the tubular transport carriers with melanosome membranes mediated by the v-SNARE, VAMP7, and an unidentified t-SNARE complex. DCT and MART1 are transported to melanosomes from the Golgi Apparatus in a separate vesicular pathway that requires RAB6 (Pathway 3). VAMP7 and perhaps other cargoes are recycled from melanosomes via tubules that derive from melanosome membranes in a BLOC-3- and RAB38/RAB32-dependent manner (Pathway 4). The scaffolding protein VARP is present on these tubules and likely supports the incorporation of VAMP7 into them. The destination of these tubules is not yet known, but VAMP7 is likely ultimately returned to early endosomes.
Model systems for LRO biogenesis
Rodents.
The laboratory mouse has been perhaps the most informative for the study of HPS and LRO biogenesis, in large measure due to both (1) the similarity of HPS disease in humans to the phenotype of orthologous mutations in mice45,46, and (2) the availability of models for all HPS subtypes. The latter reflects the impact of HPS on mouse coat color and the ease with which spontaneous coat color mutants can be detected and propagated.47,48 Such spontaneous mutations have given rise to at least 15 HPS models in the mouse including correlates of all of the human HPS variants and mutants in genes encoding two additional BLOC-1 subunits (Muted49,50 and Cappuccino51), the HOPS/ CORVET subunit VPS33A52, the Rab geranylgeranyltransferase II subunit RGGTA53, and the plasma membrane cysteine/glutamate transporter SLC7A11.54 In addition, the Chocolate mouse bears a mutation in Rab38 and has pigmentation and lung defects – but curiously no platelet defect.55-57 By contrast, the Ruby mutation in Rab38 in Fawn-hooded and Tester-Moriyama rats is associated with classical hypopigmentation, bleeding diathesis and lung dysfunction.58,59 Notably, immortalized melanocyte cell lines60,61 from each of the mouse HPS models (see http://www.sgul.ac.uk/depts/anatomy/pages/Dot/Cell%20bank%20holdings.htm) and engineered HPS models in an AT2 cell line62 provide excellent tools for analyses of cell type-specific effects of HPS subtypes.
Zebrafish.
The zebrafish Danio rerio harbors a number of unique LROs, including melanosomes in skin melanophores and retinal pigment epithelia, notochord vacuoles, and lamellar body-like surfactant storage organelles at the caudal tip epithelium of the swimbladder. Melanophores are epidermal cells in fish and amphibians that, like melanocytes in mammals, generate melanosomes in which melanin is synthesized. However, unlike mammalian melanocytes, they respond more rapidly to external stimuli either by accumulating pigment granules in the cell center or by dispersing them to the cell periphery.63,64 The retinal pigment epithelia in zebrafish is structured similarly to that in mammals and also harbors melanosomes, and defects in their melanization are easy to spot even in embryos. Zebrafish and mammals share most of the genes known to regulate melanosome biogenesis and motility, and altered pigmentation has been observed in zebrafish with mutations in genes encoding subunits of BLOC-1, BLOC-2, and HOPS65-69, as well as an unidentified gene in the fade mutant.70 Mutations in numerous additional pigment cell-specific genes, such as PMEL (responsible for the amyloid matrix upon which melanins deposit in melanosomes) and SLC24A5 (a transporter that is defective in oculocutaneous albinism type 6) have also been identified in zebrafish, but will not be discussed further here.
The notochord vacuole is a fluid-filled organelle that takes up most of the volume of the inner cells at the center of the notochord and that provides rigidity to the notochord during development. As for other LROs, notochord vacuole biogenesis and maintenance require the HOPS complex, the small GTPase RAB32A, and the vacuolar proton-ATPase71, as well as the homologue of the mammalian lysosomal scavenger receptor LIMP2/ SCARB2.72 The swimbladder is an air-filled organ that inflates and deflates to regulate buoyancy, and is a valued model system for lung development and surfactant formation. Mutations in the BLOC-1 and BORC subunit, BLOS1, result in a defective swimbladder. This appears to reflect a requirement for BLOS1 in activating a transcriptional network for the surfactant system, which is critical to swimbladder function.65 It is not clear whether this reflects a primary defect in BLOC-1, BORC or both (see discussion of BLOC-1 and BORC below).
Xenopus tropicalis.
X. tropicalis harbors retinal pigment epithelia and melanophores similar to those in zebrafish. Recent identification of an HPS6 (BLOC-2 subunit) mutant with reduced pigmentation in the retina and body suggests that this organism may provide another model for LRO biogenesis.73
Drosophila melanogaster.
The D. melanogaster eye (as for eyes of other insects) consists of approximately 800 individual units called ommatidia, within which pigment granules are generated in primary pigment cells near the cornea and secondary pigment cells that surround the retinular cells along the length of the ommatidia.74 These cells make two classes of pigment: brown ommochrome and red pteridines. Defects in the formation of pigment granules lead to changes in eye color from the normal brownish red to a variety of colors. Eye color mutants in D. melanogaster have been described for over a century, and many reflect mutations in conserved genes required for pigment granule biogenesis. For example, mutations in any of the four subunits of AP-375-79 result in a similar phenotype with a reduction in the number and size of the pigment granules and loss of both ommochrome and pteridine pigments. RNAi depletion of BLOC-1 subunits80, the pink mutation in the orthologue to the HPS5 subunit of BLOC-281, the lightoid mutation in a Rab32/38 orthologue and the claret mutation in its putative guanine nucleotide exchange factor82, and mutations in the Vps16, Vps33, Vps18 and Vps41 subunits of HOPS83-87 all cause altered eye color due to malformation of pigment granules. The availability of these models allows for relatively easy testing of genetic interactions among these and other genes. For example, pigmentation defects due to BLOC-1 deficiency could be partially offset by overexpression of Rab11, a Rab GTPase that controls endosomal recycling.80
D. melanogaster and other insects may have additional LROs in other tissues. The zinc concentration in zinc storage granules of principal cells in the Malpighian tubules, the major zinc reservoir in D. melanogaster, is sensitive to mutations in genes encoding subunits of AP-3, BLOC-2, and HOPS as well as the Rab32/38 orthologue lightoid88, suggesting that the zinc storage granules are LROs. In addition, the mucin-containing glue granules of larval salivary glands have features of LROs and are sensitive to mutations in the AP-3-associated type II phosphatidylinositol-4-kinase and in AP-1, a complex similar to AP-3.89,90
Caenorhabditis elegans.
Gut granules in C. elegans - and likely in other nematodes - are LROs that are sites of fat storage in intestinal cells and contain birefringent, autofluorescent material.91,92 The accumulation of this material requires a number of gut granule membrane proteins, including the ABC transporters PGP-292, MRP4 (a homologue of mammalian MRP4 that might localize to platelet dense granules) and WHT-2.93 Like many LROs in mammalian cells, gut granules contain some lysosomal membrane proteins and co-exist with lysosomes.91 Proper gut granule formation requires homologues to components of the mammalian HPS complexes AP-3, BLOC-1 and HOPS, as well as the RAB32/38 family member, GLO-1.91,94,95
The biogenesis of gut granules in C. elegans and of LROs such as melanosomes in mammals share striking features but a few intriguing differences. Like for melanosomes in melanocytes, distinct cargoes show differential requirements for BLOC-1 and AP-3 for sorting to gut granules.95 Moreover, a homologue of the LYST (Lysosomal Trafficking regulator) protein that is deficient in CHS, LYST-1, is required for proper gut granule morphogenesis, although the effects of lyst-1(−) mutations (increased number but smaller gut granules) are opposite to that observed for LROs from CHS models.96 On the other hand, in C. elegans lacking BLOC-1 subunits, gut granule cargoes are mislocalized to the plasma membrane and lysosomes rather than primarily to early endosomes like in BLOC-1-deficient melanocytes.95 In addition, the BORC subunit, KXD1, which is partially required for melanosome and platelet dense granule biogenesis, is not required for gut granule formation. However, another gene product with a similar KxDL motif may function in gut granule formation95, suggesting that differential protein components in different cell types, tissues, or organisms may function in the biogenesis of distinct LROs.
Dictyostelium discoideum.
The slime mold D. discoideum is a simple eukaryote with a small genome and well-developed secretory and endocytic pathways.97 Because it is highly phagocytic and easy to manipulate genetically, D. discoideum has been used as a model system to dissect lysosomal secretion defects such as those in CHS.98 D. discoideum harbor LROs called post-lysosomes that are equivalent to secretory lysosomes in mammalian cells and that undergo regulated secretion in response to stimuli such as starvation. Post-lysosomes derive from classical lysosomes that are accessed by internalized extracellular material, are enriched in the vacuolar proton ATPase, and are highly acidic.99 Over time, however, they lose their acidity and mature into post-lysosomes that can be stimulated to fuse with the plasma membrane, releasing undigested material to the extracellular space.100 The post-lysosomes are distinguished from traditional lysosomes by their neutral pH, absence of vacuolar proton ATPase99,101, enrichment in cargoes P80 (a transmembrane protein that mediates copper transport)102 and AmtA (Ammonium transporter A)103, and association with cytoskeleton markers such as vacuolin104, coronin104-106, WASH105, and F-actin.104,107 The biogenesis of post-lysosomes in D. discoideum shares similarities with the biogenesis of mammalian LROs. For example, D. discoideum cells require AP-3 for post-lysosome secretion, for proper endosomal recycling of membrane proteins, and for endosomal sorting of the vSNARE, VAMP7.108-110 More strikingly, D. discoideum has served as an outstanding model to understand the function of LYST. Two LYST homologues, lvsA and lvsB, regulate the endocytic pathway at different stages. lvsA is necessary for the proper organization of the early endocytic and phagocytic system and for phagocytosis, whereas lvsB is necessary for the maturation of post-lysosomes; lvsB mutants have fewer, larger post-lysosomes that are more acidic and constitutively secrete lysosomal enzymes. There is debate as to whether this reflects a role for lvsB in negatively regulating fusion of post-lysosomes with early endosomes or in supporting transfer of endocytosed material from lysosomes to post-lysosomes.111-113 Supporting the former, lvsB appears to dampen Rab14 function in lysosomal fusion. The D. discoidium Rab14 homologue - which is normally restricted to lysosomes - was also present in post-lysosomes in lvsB mutant cells, and expression of a dominant negative Rab14 suppressed the lvsB phenotype.114,115 These observations support the notion that lvsB and LYST function to prevent fusion among endolysosomal organelles and LROs.
Tetrahymena thermophila.
The ciliated protist T. thermophila harbors a secretory organelle called the mucocyst, which stores mucins in paracrystalline arrays that are secreted in response to extracellular stimuli.116,117 Mucocysts have some functional similarities to dense core secretory vesicles in animals, and mucocyst formation requires effectors that are similar to components required for mammalian lysosome and LRO biogenesis. For example, cargo sorting to mucocysts and consequent mucocyst formation, maturation, and secretion require SOR4 (a Vps10/sortillin family member)118, AP-3119 and the Qa SNARE protein, STX7L, a homologue of the mammalian Syntaxins 7 and 13 that are involved in lysosome and LRO formation, respectively.119 Mucocyst formation also requires some components of the T. thermophila CORVET complex120, which in other organisms shares its core subunits with HOPS. Interestingly, HOPS-specific subunits of yeast and mammalian HOPS and CORVET complexes were lost during evolution to T. thermophila, whereas genes encoding the CORVET VPS8 subunit expanded to six paralogs. One of these paralogs is required for mucocyst formation and colocalizes with the Rab7 late endosome/lysosome marker, rather than endosomes like CORVET120, suggesting that the specificity of these complexes for different organelles has changed throughout evolution.
Bombyx mori.
If LROs are defined by the requirement for HPS-associated protein complexes, the silkworm appears to have two LROs. The B. mori larval integument is opaque due to the presence of urate granules, and impairment of urate granule biogenesis results in translucency. B. mori larvae with mutations in subunits of the HPS-associated BLOC-1 and BLOC-2 are translucent121-124, suggesting that these granules are LROs. In addition, riboflavin accumulation in needle-like granules in the Malpighian tubules has been observed to be reduced in mutants with translucent skin, including mutants in BLOC1 and BLOC2 subunits and in ABC transporters w-3 and Bm-brown that are homologous to the Drosophila white gene125 and brown gene126, respectively. These organelles and their control by BLOC-1 subunits are conserved in other silkworms such as Samia ricini.127
HPS-associated protein complexes
AP-3.
The heterotetrameric adaptor proteins (AP) are a family of related complexes that function as coats for sorting of integral membrane protein cargoes within the endomembrane system. They engage and accumulate cargoes on a source membrane by binding to sorting signals located in the cargo cytoplasmic domains, and recruit an outer coat and/or other accessory proteins to generate transport carriers that facilitate cargo delivery to their destinations. Five AP complexes are known128; AP-1, AP-2 and at least a cohort of AP-3 employ clathrin as an outer coat, while AP-4 and AP-5 do not. AP-2 primarily functions at the plasma membrane and plays an important role in clathrin-mediated endocytosis, whereas the other APs mediate trafficking among the TGN and endolysosomal compartments.128-130 Genetic analyses in multiple organisms show that AP-3 plays a unique role in cargo sorting to LROs, and mutations in the genes encoding two AP-3 subunits in humans cause rare forms of HPS (Table 2).131,132
AP-3 subunits
AP-3, like the other APs, is a stable heterotetramer and consists of the four adaptin subunits δ, β3, μ3, and σ3.133 The genes that encode these subunits are highly conserved throughout eukaryotic evolution with orthologues from yeast to humans.133,134,135,136 In mammals, each of the β3, μ3, and σ3 subunits has two variants. The δ, β3A and μ3A subunits are ubiquitously expressed in most/all tissues and cells, and assemble with either of the ubiquitously expressed and functionally redundant σ3A or σ3B subunits to form the AP-3A complex.137 Expression of the β3B and μ3B subunits is restricted to neuronal cells, forming (with δ and either σ3A or σ3B) the neuronal AP-3B complex that functions in cargo sorting to synaptic vesicles.138 Mutations in the genes encoding the ubiquitous δ or β3A subunit in humans lead to HPS10 or HPS2, respectively, and mutations in orthologous subunits in numerous model organisms lead to defects in LRO biogenesis and, in some cases, in cargo sorting to lysosomes.36,139 Indeed, the identification of mutations in the AP3B1 gene (encoding β3A) in HPS2 and the pearl mouse, and in the Ap3d gene (encoding δ) in the mocha mouse represented the first link between HPS and defective membrane trafficking in the endolysosomal system in mammals.131,132,140
AP-3 is a coat protein.
Although the yeast AP-3 appears to function in protein sorting from the Golgi141 and initial observations suggested a similar function for mammalian AP-3142,143, it is now well-accepted that AP-3 functions primarily from early endosomes in mammals.144,145 AP-3 is recruited to membranes at least in part by association with the small GTPase Arf1142,146,147 and possibly phosphatidylinositol-3-phosphate.148 The μ3A subunit of AP-3 recognizes tyrosine-based sorting signals in the cytoplasmic domain of cargo proteins destined for lysosomes or LROs149,150, while the interface of the σ and δ subunit hemicomplex binds to dileucine-based sorting signals151-153; the corresponding components of AP-1 and AP-2 recognize similar signals, with some complex-specific preferences based on sequence and context.135,149,154,155 Like AP-1 and AP-2, AP-3 associates with clathrin and is enriched in clathrin-coated buds on endosomes.144,145,147,156 This cohort of AP-3 is necessary for transport of specific cargoes to late endosomes or lysosomes in cells that lack LROs and to melanosomes in melanocytes.144,145 Unlike AP-1 and AP-2, however, AP-3 is not enriched in clathrin-coated vesicle fractions from cells142, and a β3A chain lacking a clathrin binding site is able to fully restore AP-3 function in diverting LAMP1 from the plasma membrane.157 While these data can be explained by a weak association with clathrin mediated by multiple binding sites, a pool of membrane-associated AP-3 lacking clathrin is readily detectable by microscopy142-145, and acute depletion of clathrin light chains did not disrupt AP-3 vesicle formation in PC12 cells.158 This suggests that AP-3 might function in both clathrin-dependent and -independent pathways.
AP-3-dependent cargo sorting in LRO biogenesis.
The function of AP-3 in LRO biogenesis is best exemplified by its role in protein sorting to maturing melanosomes (Figure 3, step 1). A critical cargo for AP-3 in pigment cells is tyrosinase (TYR), an integral membrane protein that catalyzes the limiting steps in melanin synthesis. TYR bears a di-leucine-based sorting signal in its C-terminal cytoplasmic domain that is necessary for transport to maturing melanosomes (or to lysosomes upon ectopic expression in other cell types24,159,160) and is capable of binding AP-3 as well as AP-1 and AP-2.145,161 TYR is enriched in early endosome-derived vesicles that are coated with AP-3 and clathrin, and is depleted from melanosomes and enriched in late endosomes and lysosomes of AP-3-deficient melanocytes from HPS-2 patients or pearl mice.145,162 These data suggest that TYR is sorted by AP-3 from early endosomes into vesicles destined for melanosomes (Figure 3). Intriguingly, a superficially similar dileucine-based sorting signal in another melanosomal protein, TYR related protein 1 (TYRP1)163, does not engage AP-3 and TYRP1 is not as grossly missorted in AP-3-deficient melanocytes.145,162 Moreover, a small cohort of TYR is properly sorted to melanosomes in AP-3-deficient melanocytes, likely by engaging with AP-1.145,164 This suggests that there are multiple pathways by which cargoes are sorted to melanosomes, including AP-3-dependent and -independent pathways (Figure 3). Lastly, a cohort of AP-3 interacts physically with BLOC-1165,166, which – as discussed further below – is essential for a second cargo transport pathway. In neurons, BLOC-1 and AP-3 appear to function largely in tandem to sort specific cargoes into synaptic vesicles138,166, and accordingly, melanosome sorting of another pigment cell-specific protein, OCA2, requires both AP-3 and BLOC-1.154,167 This implies a potential dual role for AP-3 in multiple sorting pathways in melanocytes.
AP-3 functions in cargo sorting not only for melanosome biogenesis, but also for the formation of other LROs. In model organisms, AP-3 is required for the generation of gut granules in C. elegans91, mucocysts in T. thermophila119, post-lysosomes in D. discoideum109, and eye pigment granules in D. melanogaster77,143. In mammals, AP-3 plays an essential role in sorting cargoes such as the zinc transporter ZnT-3, the GABA transporter and SNARE proteins into synaptic vesicles in neurons.132,138,168,169 Consequently, animals or individuals lacking both AP-3A and AP-3B (e.g. by loss of the δ chain in mocha mice or HPS10 patients) suffer from neuronal deficiencies.36,169-171 Excessive bleeding and bruising due to platelet storage pool deficiency, which results from defective or absent dense granules in platelets, is also associated with loss of AP-3 in HPS2 and 10 and their mouse models172,173, but relevant cargos have not yet been identified.
AP-3 function in the lung and hematopoietic cells.
Besides albinism and excessive bleeding, HPS2 patients uniquely suffer from a number of additional serious symptoms. Like patients with BLOC-3 disease, HPS2 patients suffer from interstitial lung disease and pulmonary fibrosis, likely due to an initial defect in the maturation of lamellar bodies in AT2 cells.30,33,174-176 To date, one AP-3-dependent cargo of lamellar bodies has been identified: PRDX6, a non-integral membrane member of the peroxiredoxin family of redox proteins, which is targeted to lamellar bodies in association with the AP-3 engaged transmembrane protein LIMPII/SCARB2.177 Additional AP-3-dependent lamellar body cargoes are likely to exist. AP-3-deficient patients and the pearl and mocha mouse models also suffer from recurrent bacterial and viral infections36,39,40,173,178-181 due to critical roles of AP-3 in various immune responses. Increased susceptibility to viral infections likely reflects impaired natural killer cell and cytolytic T cell activity due to defective polarization and decreased perforin content of cytolytic granules36,37,39, as well as impaired type I interferon responses from plasmacytoid dendritic cells (pDCs) due to a failure to deliver viral nucleotide sensing toll-like receptors, TLR7 and TLR9, to a signaling LRO in these cells (Table 1).182,183 AP-3 controls a different TLR trafficking step in conventional DCs (cDCs), facilitating the recruitment of TLR4 and likely other TLRs to maturing phagosomes following uptake of bacteria and other large particles. Reduced TLR recruitment to phagosomes in AP-3-deficient mice and HPS2 patients results in impaired proinflammatory signaling by bacterial stimuli and reduced antigen presentation of phagocytosed antigens to CD4+ T cells, with consequent skewing of CD4+ T cell responses toward the Th2 lineage and altered cDC maturation and chemokine responses.184,185 Additionally, AP-3 plays a role in dampening autophagic responses to pathogenic bacteria in cDCs. This has an important impact on clearance of inflammasomes, which are large cytoplasmic complexes that are assembled following a variety of cytoplasmic inflammatory stimuli and consist of many copies of nucleotide binding domain leucine rich repeat containing proteins (NLRs), inflammatory caspases, and the scaffold protein ASC. Inflammasomes process pro-IL-1 family cytokines to their mature form. Inflammasomes in AP-3-deficient cDCs are more rapidly consumed by autophagy than in control cDCs, leading to impaired inflammasome activity, consequent reduced local IL-1β and IL-18 secretion, and increased susceptibility of AP-3-deficient mice to pathogenic bacterial infection.186 AP-3 also regulates the trafficking of CD1b (in humans) and CD1d (in mice) to lysosomal compartments to acquire glycolipid antigens. Consequently, presentation of glycolipid antigens to NKT cells is impaired and NKT cell numbers are reduced in HPS2 patients and mouse models, also contributing to susceptibility to certain bacterial infections.181,187-189 Lastly, cyclic neutropenia has been observed in HPS2 patients and a dog model of AP-3 deficiency, and sorting of neutrophil elastase to primary granules and proper processing to the mature form require AP-3.190,191 Whether this reflects a direct interaction of a cytoplasmic sequence on a transmembrane form of elastase as proposed190 or an effect of AP-3 on a transmembrane carrier of elastase, as for PRDX6 in lung AT2 cells, remains unclear.
BLOC-1.
BLOC-1 subunits and HPS models.
BLOC-1 contains eight subunits: BLOS1, BLOS2, BLOS3192,193, cappucino51,194, muted50, pallidin50,195,196, snapin193,197, and dysbindin.198-200 The human gene names for each subunit are referred to as BLOC1S1-8. Each of the subunits is small (136 to 351 amino acids) and lacks obvious homology domains other than predicted short coiled coil regions. Mutations in the genes that encode dysbindin, BLOS3 and pallidin have been identified in patients with HPS subtypes HPS7, HPS8 and HPS9, respectively.200-208 These patients tend to have mild HPS disease with no obvious immunologic or lung impairment. Five HPS mouse models exist49,193,194,209-211 and are the most severely hypopigmented of the HPS models212 (by contrast, skin and hair pigmentation in human HPS7-9 patients is less obvious than in other HPS variants). BLOC-1 homologues have also been described in non-mammalian model organisms. Gut granule formation in C. elegans and synaptic vesicle recycling in D. melanogaster neurons require BLOC-180,95,213, and in the silkworm, Bombyx mori, a mutation in the muted ortholog causes translucent larval skin.124 In addition, in zebrafish BLOS1 regulates formation of melanophore and iridiphore pigment organelles and surfactant storage organelles in the swimbladder.65 Interestingly, the BLOS1, BLOS2 and snapin subunits are shared with the distinct BLOC One Related Complex (BORC)214, which will be briefly discussed later. Some of the effects of mutations in the genes encoding these subunits might reflect defects in BLOC-1, BORC, or both.
BLOC-1 function in cargo transport carrier formation.
The role of BLOC-1 in LRO biogenesis has been most clearly elucidated in cultured melanocytes from mouse HPS models. BLOC-1 is required to deliver a cohort of transmembrane protein cargoes that are involved in melanin synthesis, including TYRP1, OCA2, ATP7A and a small subset of TYR, from early endosomes to melanosomes via tubular transport carriers154,164,165,215,216 (Figure 3, step 2). These transport carriers have features of recycling endosomes164,217-219, and BLOC-1 regulates the formation of similar tubular carriers for endosomal recycling in HeLa cells.217,218 The fact that these carriers in melanocytes are co-opted specifically for melanosome cargo delivery suggests that they reflect a cell type specialization of the recycling endosomal machinery, perhaps through regulation by as yet undefined cell type-specific components such as Rab proteins. Because TYR is primarily trafficked to melanosomes via a separate AP-3-dependent vesicular pathway145,162,215,216, TYR is only mildly mislocalized in BLOC-1 deficient melanocytes. However, TYR activity in melanosomes is essentially absent in BLOC-1-deficient cells due at least in part to depletion of ATP7A, an ATP-dependent copper transporter that is required for import of the essential TYR cofactor copper into melanosomes216, and of OCA2, a pigment cell-specific chloride channel that is required to neutralize acidic early stage melanosomes, both of which are prerequisites for TYR activity.154,220 Similarly, BLOC-1 physically interacts with ATP7A and affects copper homeostasis in neuronal cells.221 A third set of cargoes, MART1 and dopachrome tautomerase (DCT), are delivered directly to melanosomes from the Golgi apparatus through a BLOC-1- and AP-3-independent but RAB6-dependent pathway222 (Figure 3, step 3). BLOC-1 is ubiquitously expressed, and roles for BLOC-1 in endosomal trafficking have been identified in other cell types. For example, in HeLa cells BLOC-1 facilitates transferrin cycling through classical recycling endosomes218, and in neurons BLOC-1 and AP-3 collaborate to mediate cycling of cargoes between endosomes and synaptic vesicles and may differentially regulate synaptic vesicle trafficking in different brain regions.138,166,170,223
BLOC-1 and cytoskeletal interactions.
BLOC-1 localizes to and is required for formation of the endosomal tubular transport carriers that fuse with melanosomes to deliver cargo164,165,218,224 (Figure 3). Negative stain EM structures of recombinant BLOC-1 suggest that BLOC-1 subunits form a flexible linear chain225, which could be consistent with BLOC-1 interactions with curved, tubulating membranes. In melanocytes and HeLa cells, BLOC-1 associates with the kinesin-3 motor, KIF13A, perhaps linking membrane tubules to the microtubule cytoskeleton, and promotes actin rearrangements to stabilize and elongate tubules, and in HeLa cells (but perhaps not in melanocytes) to sever them.217,218 Some of these interactions may be coordinated by the small GTPase RAB22.226 Consistent with a role for BLOC-1 in regulating the actin cytoskeleton, comparative proteomic analyses of brains from wild-type or BLOC-1-deficient mice suggest that loss of BLOC-1 function results in depletion of the actin nucleation complex, Arp2/3, and that this interaction is important for actin dynamics in HEK293 cells and neuronal plasticity at D. melanogaster synapses.227 Similarly, BLOC-1 interacts with the WASH complex, an Arp2/3 activator, to regulate endosomal sorting of phosphatidylinositol-4-kinase type IIɑ (PI4KIIɑ) in several cell types.228 Together, these data suggest a role for BLOC-1 in linking cargo sorting to the actin and microtubule cytoskeletons with potential implications for neuronal function. Indeed, BLOC-1-deficient mice with an inactivating dysbindin mutation show defects in the kinetics of neurotransmitter release229, and several studies have suggested that variations in the genes encoding dysbindin and other BLOC-1 subunits correlate with increased schizophrenia risk in humans, although others dispute this conclusion.230-232
BLOC-1 and SNARE interactions.
In addition to its role in regulating the cytoskeleton, BLOC-1 might also regulate SNARE-mediated membrane fusion in an as yet undefined way. The pallidin subunit interacts with the endosomal Qa t-SNARE subunit, syntaxin 13196,199, and snapin interacts with the Qbc t-SNARE subunit, SNAP25.197,199,233 Interestingly, syntaxin 13 labels the tubular endosomal transport carriers through which melanosomal cargoes are delivered.218,219 Furthermore, the endolysosomal R-SNARE, VAMP7 (a.k.a. TI-VAMP – the vSNARE required for fusion of BLOC-1-dependent tubules with melanosomes224) – localizes to AP-3-containing vesicles in neuronal cells in a BLOC-1-dependent manner166, suggesting a wider role for BLOC-1 in SNARE sorting. In addition to these direct effects on SNARE proteins, the dysbindin subunit of BLOC-1 can bind directly to N-ethylmaleimide-sensitive factor (NSF) to regulate neuronal activity.213 How these different binding events are coordinated is not yet understood.
BLOC-1 vs. BORC.
Three BLOC-1 subunits, BLOS1, BLOS2, and snapin, were recently identified as members of a separate complex called BORC. BORC also contains five additional subunits: KXD1, myrlysin, lyspersin, diaskedin, and MEF2BNB214; the human genes encoding the five BORC-specific subunits are named BORCS4-8. BORC functions on lysosomes, where it facilitates lysosome motility on distinct microtubule tracks toward the cell periphery by recruiting the Ras-like GTPase, ARL8. Active ARL8, via the adaptor SKIP, then recruits the KIF5B-containing kinesin-1 or the KIF1A-containing kinesin-3 microtubule motors.214,234 Interestingly, BORC also interacts with the Ragulator, a lysosomal complex that controls mTORC1 activation and downstream autophagy induction in response to low amino acid levels. The Ragulator-BORC interaction suppresses BORC and kinesin-mediated movement of lysosomes, causing sequestration of lysosomes at the perinuclear region in response to low nutrient levels.235,236 BORC also regulates fusion of lysosomes with autophagosomes, and is required for autophagosome transport and clearance in distal axons.237,238 Consistent with this, BORC regulates axonal transport of synaptic vesicle precursors in C. elegans, also in an Arl8-dependent manner.239 Moreover, a mouse mutant in the gene encoding the diaskedin subunit causes axonal dystrophy, motor defects, and early death in mice, suggesting a role for BORC-dependent lysosome transport in proper motor neuron function.240 Whether BORC controls LRO positioning, functions, or effector localization is not known, although gene targeting of the KXD1 subunit was reported to produce mild melanosome and platelet dense granule defects.241
The facts that BORC and BLOC-1 share three subunits and that BORC was only recently discovered have caused some confusion in the literature regarding the function of each complex. For example, knockout of the gene encoding snapin disrupts retrograde transport of synaptic vesicles, endosomes, and late endosomes, and impairs lysosomal function in neurons of embryonic mice.242-245 Expression of dynein-binding mutants of snapin cause synaptic vesicles to be trapped in synaptic terminals.246 Further, depletion of BLOS1 impairs lysosomal degradation of the epidermal growth factor receptor (EGFR).247 These functional requirements for snapin and BLOS1 were attributed in part to BLOC-1, but are more likely attributable to the role of BORC in lysosome motility and function. Of note, while functional gene knockouts of BLOC-1-specific subunits are tolerated in all animal systems analyzed, knockout of shared BLOC-1/BORC subunits BLOS1, BLOS2 or snapin or of the BORC-specific BORCS7 subunit are embryonic or perinatal lethal in mice or zebrafish65,240,242,247,248 (but not in C. elegans239, and curiously, knockout of the BORC-specific KXD1 gene is tolerated in mice241). Also confusing is the relationship of BORC and BLOC-1 to a yeast complex referred to as BLOC-1.249 Yeast BLOC-1 was identified by bioinformatics analysis of S. cerevisiae proteins and consists of homologues of BLOS1, snapin and cappuccino, as well as three other alpha helical, coiled-coil proteins similar in size to mammalian BLOC-1 subunits, Vab2b, YGL079Wp, and YKL061Wp.250 YKL061Wp and Vab2b have homologues only in fungi, but YGL079Wp contains a KxDL (Lys-x-Asp-Leu) domain and is a homologue of the mammalian BORC subunit, KxDL.250 The yeast BLOC-1 localizes to endosomes, and depletion of its subunits caused redistribution of yeast Rab5, Vps21, from endosomes to the vacuole, but did not affect sorting of AP-3 cargo.249 Therefore, it is unclear if this complex corresponds to mammalian BLOC-1, BORC, or a hybrid complex.
BLOC-2.
BLOC-2 subunits and HPS models.
BLOC-2 is comprised of three large subunits, HPS3, HPS5, and HPS6, the genes for which are mutated in corresponding HPS variants and mouse models of the disease.251-255 HPS patients with BLOC-2 mutations tend to have a mild form of the disease with no lung or immune involvement, although bleeding tendency can be severe.42-44,256 Genes encoding BLOC-2 subunits are conserved in vertebrates, and the Xenopus tropicalis HPS6 homologue is required for normal melanophore and iridophore formation.73 Additionally, a Drosophila HPS5 homologue is required for normal eye pigmentation.81,257 BLOC-2 mouse mutants have milder hypopigmentation phenotypes than BLOC-1 mutant mice, and choroidal melanosomes are aberrantly clustered.172,252,253,255 Excessive bleeding in BLOC-2-deficient mice and HPS patients might be exacerbated by defects in the maturation and secretion of Weibel-Palade bodies in endothelial cells.258,259 Upon endothelial cell damage, vWF is released from Weibel-Palade bodies into long strings that capture platelets in the blood circulation260; hence, the combined defects in endothelial vWF packaging and release and in platelet dense granule formation and release may impact bleeding susceptibility more in BLOC-2 mutants than in other HPS variants.
BLOC-2 function in tubular cargo transport carrier targeting.
The molecular function of BLOC-2 during LRO biogenesis is incompletely known, but is best understood in the context of melanosome maturation. Like BLOC-1, BLOC-2 in melanocytic cells localizes to tubular endosomes, and a cohort of BLOC-1 and BLOC-2 physically interact165, suggesting that BLOC-1 and BLOC-2 function in the same pathway. Consistently, BLOC-2 influences the melanosomal delivery of BLOC-1-dependent cargoes, including TYRP1, OCA2, ATP7A, and a cohort of TYR, from early endosomes in mouse melanocytes165,219 (Figure 3, step 2), and labeling patterns for TYRP1 and TYR are altered in melanocytes from patients with HPS3, 5 or 6.44,261-263 The cargoes are not uniformly trapped in early endosomes, however, and rather are widely distributed among endosomal compartments, melanosomes, the Golgi and the plasma membrane.215,219 Dynamic analyses of endosomal transport in BLOC-2-deficient mouse melanocytes support a role for BLOC-2 in directing the BLOC-1-dependent endosomal tubules specifically to maturing melanosomes (Figure 3). Live cell imaging analyses show that BLOC-2 is required for melanosome-destined tubular carriers to make stable contacts with maturing melanosomes; in the absence of BLOC-2 these tubules form at the same rate, but they are shorter-lived, make fewer contacts with melanosomes, and the contacts they make are of shorter duration.219 As a consequence, BLOC-1-dependent cargoes enter the recycling endosome-like tubules, but are delivered to classical targets of such endosomes – the Golgi and the plasma membrane – resulting in increased cycling through these organelles.219 Whether BLOC-2 functions in a similar capacity in other cell types is not yet known.
How BLOC-2 promotes the contact of recycling endosomal tubules with melanosomes is unknown, but might reflect either a classical membrane tethering function264 or a stabilizing function for the membrane tubules along microtubules or with the KIF13A kinesin-3 motor. Consistently, pull-down experiments suggest that the stalk domain of KIF13A might interact with BLOC-2, perhaps in association with RAB22.226 When overexpressed in HEK293 cells, the HPS6 subunit was found to coprecipitate with the dynactin p150glued subunit265, but it is not clear if this reflects a physiological function of intact BLOC-2. Moreover, depletion of HPS6 in HeLa cells or fibroblasts associated with lysosomal dispersal265, contrary to the effects on the remaining pigment granules in BLOC-2-deficient melanocytes.219 Clearly, more work needs to be done to resolve the molecular mechanism underlying BLOC-2 function.
BLOC-3, RAB32 and RAB38.
BLOC-3 subunits, activity, and HPS models.
BLOC-3 is a two-subunit complex consisting of the products of the HPS1 and HPS4 genes41,266,267. HPS1 and HPS4 have limited homology to the MON1 and CCZ1 subunits of the guanine nucleotide exchange factor (GEF) for the small GTPase, RAB7.268,269 Accordingly, Gerendopoulos et al. found that BLOC-3 has GEF activity for two highly related small GTPases, RAB32 and RAB38270, that had been previously implicated in the biogenesis of melanosomes271-273, platelet dense granules274,275, AT2 lamellar bodies59,276, D. melanogaster eye pigment granules82,277 and C. elegans gut granules.91,94 Indeed, Rab38 mutations underlie the pigmentation and vision defects in chocolate mice55,56,273 and an HPS-like disorder in Fawn-hooded and Tester Moriyama rats.58,278 Depletion of HPS1 or HPS4 in melanocytic cells resulted in mislocalization270 or cytoplasmic displacement224 of RAB32 and/or RAB38-GFP, and Hps1 gene knockout in an AT2 cell line resulted in RAB38 mislocalization62, suggesting that BLOC-3 is the major GEF for RAB32/RAB38 in these cell types. Interestingly, in C. elegans the RAB7 and RAB32 homologues are activated by different GEFs that share the same CCZ1 subunit but distinct MON1 orthologues and that function respectively in lysosome and gut granule (LRO) biogenesis.94,279
BLOC-3 and RAB38/32 function in melanosome biogenesis.
A cellular function for BLOC-3 and its target Rabs has been best characterized in melanocytes. Depletion of both RAB32 and RAB38 from melanocytes led to mislocalization of melanosome cargoes such as TYRP1 and TYR271, and the data suggested that RAB32 and RAB38 function largely redundantly in this system. Consistent with a role in forward trafficking to melanosomes, immunofluorescence and immunoelectron microscopy analyses of endogenous or epitope-tagged RAB32 and RAB38 isoforms suggested that these Rab proteins localize largely to melanosomes, as well as to tubulovesicular structures in proximity to melanosomes and the Golgi.224,270-272 Such a function in cargo trafficking to melanosomes would explain how HPS1 and HPS4 patients suffer from oculocutaneous albinism, and how depletion of HPS1, HPS4 or RAB32 from a human melanoma cell line resulted in a loss of pigmentation.270 However, unlike AP-3, BLOC-1 and BLOC-2, BLOC-3 is not absolutely required for pigmentation in mice. For example, pale ear and light ear mice that bear inactivating mutations in Hps1 and Hps4, respectively, have – as the name suggests – pigment dilution in the skin and eyes but not in the hair.280 Indeed, melanosomes in melanocytes from the hair bulb or choroid of these mice are normally pigmented and enlarged281-283, as are those in immortalized melanocytes from these lines224, whereas melanosomes in the retinal pigment epithelia and interfollicular melanocytes of the skin are small, poorly melanized, and largely depleted.282-284 The intact pigmentation and enlargement of melanosomes in some pigment cell types lacking BLOC-3 are inconsistent with a primary requisite function in anterograde cargo transport to melanosomes.
Rather, BLOC-3 and its target Rabs seem to play a primary role in retrograde transport from melanosomes224 (Figure 3, step 4). Melanosomes from “wild-type” black mice emit short, motile tubules that are enriched in VAMP7, a vesicular SNARE fusion protein required for BLOC-1-dependent anterograde cargo traffic.224,285 These tubules are also enriched in RAB38 and its effector, VARP224, a scaffolding protein that engages VAMP7 and plays a role in melanosome biogenesis286 (Figure 3, step 4). RAB38 and VARP recruitment to melanosomes - and consequent formation of the VAMP7-containing tubules - is drastically reduced in BLOC-3-deficient pale ear or light ear melanocytes, despite only a minor reduction in melanosome components.224 These data suggest that RAB38 and BLOC-3 function directly in recycling from melanosomes and perhaps not in anterograde traffic via the BLOC-1- or AP-3-dependent pathways. The large melanosome phenotype might result from the accumulation of uninhibited VAMP7 on melanosomes, driving dysregulated fusion with other melanosomes and perhaps with autophagosomes and/or lysosomes.287-290 By contrast, hypopigmentation in other BLOC-3-deficient melanocytes might reflect the loss of VAMP7 recycling. We speculate that the ultimate destinations for the retrograde VAMP7-containing tubules are early endosomes; if this is true, then the failure to recycle VAMP7 in some cells might deplete VAMP7 from early endosomes, the source of anterograde cargoes, and thus secondarily impair anterograde transport. A differential requirement for this recycling pathway to supply endosomal VAMP7 might therefore explain the differing phenotypes in distinct pigment cell types in pale ear and light ear mice and in HPS1 and 4 patients.
RAB32 and RAB38 might have additional functions in melanocytes. Both Rab proteins have been shown to associate physically with AP-3, AP-1 and BLOC-2 in melanocyte extracts272, suggesting potential roles in anterograde trafficking. Another effector of both Rabs is Myosin Vc, knockdown of which also impacts anterograde trafficking of melanosome cargoes in a human melanoma line.291 Moreover, depletion of RAB32 alone or of Myosin Vc results in destabilization of DCT272,291, a cargo of a distinct RAB6-dependent trafficking pathway from the TGN/Golgi to melanosomes222, suggesting that RAB32 might specifically tether RAB6-dependent cargo carriers to melanosomes in an anterograde pathway. It is possible that RAB32 and/or RAB38 coordinate traffic into and out of melanosomes, such that retrograde tubules emanate only at sites of input from one or more of the anterograde routes. This would provide a homeostatic mechanism to maintain a constant amount of melanosome limiting membrane, accounting for the maintenance of melanosome size during maturation. Interestingly, another myosin, Myosin VI, functions in collaboration with the branched actin regulators WASH and Arp2/3 in severing retrograde tubules from melanosomes. As a consequence, melanocytes depleted of Myosin VI are impaired in pigment transfer to keratinocytes, perhaps due to a lack of maturation.292 Whether Myosin VI is recruited to melanosomes by RAB32 or RAB38 is not yet known; if so, it might suggest that BLOC-3 and its target Rabs facilitate melanosome maturation to a secretory organelle in epidermal melanocytes.
BLOC-3 and RAB32/38 function in the lung.
Precise pathway functions for BLOC-3 or RAB32/38 in other cell types are less clear. BLOC-3 and RAB38 play critical functions in the maturation of lamellar bodies in AT2 cells, but how is not known. RAB38 localizes to lamellar bodies in AT2 cells276, and AT2 cells lacking either RAB38 or BLOC-3 subunits have greatly enlarged lamellar bodies with altered surfactant contents.32,59,276 A long-term consequence of this effect on AT2 cells in HPS1 and HPS4 patients is a progressive lung fibrosis that is typically lethal in the 5th decade of life.29 Modeling of the disease in double Hps1/AP3b1-deficient mice31,176 – and more recently in single Hps1- or Ap3b1-deficient mice treated with bleomycin30,174 – indicates that the affected lung epithelium hypersecretes nitric oxide synthase and the chemokine MCP-1.31,34 Both of these factors activate alveolar macrophages to hypersecrete additional chemokines, inflammatory cytokines and TGFβ, all of which contribute to AT2 apoptosis and fibrotic macrophage infiltration.33,34,174 Increased circulating levels of galectin-3 and of chitinase 3-like-1 protein, a cytokine that stimulates fibroproliferative repair in the lung, also contribute to enhanced fibrosis and AT2 apoptosis.293,294 The molecular mechanisms by which BLOC-3 or its target Rabs might enhance chemokine secretion by AT2 cells are not clear, but recent development of an HPS1 model in an AT2 cell line that recapitulates phenotypes of primary AT2 cells, including increased MCP-1 production62, will likely accelerate discovery in this area.
BLOC-3 and RAB32/38 function in inflammation.
Although the primary effect of BLOC-3 and RAB32 in lung fibrosis (at least in mouse models) is mediated by AT2 cells174, their depletion impacts macrophages and monocytes directly in their ability to restrict bacterial infections. RAB32 is highly expressed in macrophages and monocytes295, and both RAB32 and RAB38 accumulate on phagosomes or vacuoles harboring several pathogenic bacteria following phagocytosis.296-298 Intriguingly, RAB32 and RAB38 are targets of a bacterial effector protease, GtgE, secreted into the cytosol of infected macrophages by the type III secretion system of Salmonella Typhimurium, a pathogen of both mice and humans. S. typhi, a related pathogen, lacks GtgE and is thus unable to establish a productive infection in wild-type mice but effectively infects Hps4-deficient mice.297 S. Typhimurium also secretes a RAB32 GAP, and loss of both the protease and the GAP impairs virulence in wild-type but not Hps4-deficient mice.299 The mechanism by which this restriction occurs is not clear, but overexpression of a dominant negative form of RAB32 in a macrophage cell line impaired the recruitment of cathepsin D to latex bead phagosomes296, suggesting a possible role for RAB32 in facilitating phagosome/lysosome fusion in this cell type.
In addition to oculocutaneous albinism, bleeding diathesis and lung fibrosis, a subset of HPS1 and HPS4 patients (~20-30%) suffer from a debilitating form of inflammatory bowel disease, referred to as granulomatous colitis.29 The incidence of this symptom does not appear to be related to genotype and likely reflects heightened sensitivity of HPS1 and 4 patients to an environmental trigger. The cellular and molecular basis for this susceptibility is not yet known, but might reflect a unique role for BLOC-3 and RAB32/38 in epithelial cells or secretory cells of the gut. Alternatively or additionally, since heritable forms of inflammatory bowel syndrome are often associated with innate immune defects300,301, the impact of BLOC-3 and RAB32 in macrophages and monocytes might underlie the susceptibility. Consistent with the latter, conditional knockout of Rab32 in CD11c+ cells (dendritic cells, monocytes and other innate immune cells) causes increased susceptibility to dextran sodium sulfate-induced colitis and enhanced production of proinflammatory cytokines and chemokines.302 It will be important to validate this finding in the human system and to determine if the inflammatory response underlies the susceptibility to colitis in HPS1 and 4 patients.
HOPS and CORVET.
HOPS and CORVET subunits and model systems.
The HOPS and CORVET complexes play critical roles in membrane tethering and fusion within the early endosomal and endolysosomal pathways, respectively.303-307 Both complexes contain the class c core complex (VPS-C) comprised of VPS11, VPS16, VPS18 and VPS33A.303-305,308,309 Complex specificity is dictated by the CORVET-specific subunits VPS3 and VPS8305 and the HOPS-specific subunits VPS39 and VPS41.308,309 The genes encoding all HOPS and CORVET subunits are highly conserved across species310,311 with a few exceptions. Of the two complexes, HOPS has been better studied in both yeast and higher eukaryotic model systems. HOPS functions to bind and tether apposing membranes through interactions between its HOPS-specific subunits and either ras-like GTPases or adaptor proteins.312-314
The HOPS subunits were initially discovered in screens to identify genetic mutants that interfered with protein sorting to the vacuole (vacuole protein sorting or vps mutants) in the yeast, Saccharomyces cerevisiae.315,316 Null mutations in the genes encoding any of the four VPS-C subunits, Vps11p, Vps16p, Vps18p, or Vps33p, were sufficient to cause major defects resulting in loss of recognizable vacuoles, an accumulation of small vesicular structures, and decreased processing of vacuolar proteins.315-317 Unsurprisingly, most studies of HOPS and CORVET that have been done in yeast as null mutations are lethal in higher eukaryotes.85,86,306,318 However, many interesting questions about the functions of HOPS and CORVET have been raised by studying hypomorphic alleles of subunit genes. In particular, several HOPS or CORVET subunit mutations cause LRO defects in Drosophila, C. elegans, Tetrahymena or vertebrate models52,84-87,91,94,120,306,319,320, suggesting a possible specific role for VPS-C in LRO biogenesis.
HOPS and CORVET function as endosomal tethers and SNARE regulators.
The architecture of the HOPS complex has been most clearly defined using single particle negative stain electron microscopy analysis of HOPS purified from yeast. These studies reveal a seahorse shape with a large head region, a smaller tail region321, and the HOPS-specific subunits Vps39p and Vps41p at either end, thereby providing a model of how HOPS facilitates tethering of apposing membranes.321,322 In yeast models, Vps39p and Vps41p both tether membranes through direct interactions with the RAB7 homologue.308,309,322,323 By contrast, mammalian VPS39 and VPS41 have not been shown to directly engage RAB7, but rather bind to late endosomes, lysosomes, and autophagosomes through the adaptor proteins RILP313 and PLEKHM1312 and the small GTPase ARL8b.312 Accordingly, knockdown of VPS-C subunits in mammalian cells results in endosome-lysosome and autophagosome-lysosome fusion defects307,324,325, and a point mutation in VPS11 appears to cause an autosomal recessive form of leukoencephalopathy associated with dysfunctional autophagosome: lysosome fusion.326
Whereas the major role of the VPS-C proteins VPS11, VPS16A, and VPS18 seems to be in organizing the complex for tethering function321,323,327, core protein VPS33A is a member of the Sec1/Munc18 (SM) family of proteins308,327-331; hence, it binds syntaxin family SNAREs328,332, facilitates SNARE complex assembly in order to overcome the energy barrier for membrane fusion329,330,333, and protects SNARE complexes from premature disassembly.334 Upon VPS41- and VPS39-dependent membrane tethering, VPS33A binds lysosomal and autophagosomal SNARES307 to catalyze SNARE complex formation and fusion; it is as yet unclear whether VPS33A can bind a single syntaxin t-SNARE or prefers 3- and/or 4-helical SNARE bundles.321,328,335,336 Structural models suggest how VPS33A binds t- and v-SNAREs.330,331 Overlapping data on the structure of C. thermophilum Vps33 with the syntaxin/Qa-SNARE orthologue Vam3 bound to domain 1 or with the v-SNARE Nyv1 bound to domain 3a suggest that both SNAREs can bind simultaneously, aligning the two SNAREs such that they are in register to form a partially zippered SNARE pair.330 Vps33 mutations that are predicted to impact SNARE binding disrupt proper fusion and maturation in the yeast endolysosomal pathway330, demonstrating that SNARE binding is critical for Vps33p function. Specifically, Vps33 mutations along the predicted v-SNARE binding region reduced v-SNARE binding and induced accumulation of small vacuoles, whereas introduction of a Vps33 deletion predicted to disrupt both v-SNARE and t-SNARE binding phenocopied complete loss of Vps33p expression.330 Thus, binding to both vSNARE and tSNARE appears to be critical for VPS33 function.
HOPS and CORVET functions in LRO biogenesis.
A number of HOPS subunit mutations in animal models cause HPS-like phenotypes, implicating HOPS in LRO biogenesis. In C. elegans, knockdown or mutation of HOPS subunits results in a loss of gut granules.91,94 Null mutations in vps18 and vps11 in zebrafish319 and medaka320, respectively, cause hypopigmentation due to fewer melanosomes in epidermal and retinal pigment epithelia, suggesting a role for VPS-C in the biogenesis of melanosomes. In D. melanogaster, HOPS subunit genes – including those encoding homologs of VPS1885, VPS4187, VPS16A83,84 and VPS33A85,306,337 – are among the granule group of genes that function in the formation of eye pigment granules.83,85,87 Deletion or loss of function alleles of the VPS1885,86, VPS4187, or VPS33A306 homologs are lethal as these genes are required for normal trafficking to lysosomes; however, hypomorphic alleles and clonal knockouts of these genes or the VPS16A homolog in the eye cause changes in eye color due to defective protein trafficking to pigment granules.83,84,306,338 Flies with eye-specific knockdowns of HOPS subunits have strong retinal degeneration characterized by defective trafficking to lysosomes, aberrant autophagosome accumulation, and loss of Type I and Type II pigment granules.84,306,338 Interestingly, null mutations of Syntaxin 17, encoding the autophagosomal SNARE conserved in flies and mammals, result in an accumulation of autophagosomes339 but no change in eye color338, suggesting that the LRO defect in HOPS mutants is not a consequence of defective autophagosome-lysosome fusion and must involve a distinct tSNARE.
VPS33A mutations and HPS.
The mouse model buff (bf) most closely links a HOPS subunit mutation to HPS.52 Buff mice harbor a point mutation in Vps33a, and have an autosomal recessive phenotype that is similar to other mouse HPS models characterized by coat hypopigmentation52,340, decreased melanosome number and size in the RPE52, and prolonged bleeding times associated with decreased ATP secretion and dense granule count in platelets.52,172 The causative mutation, a missense mutation of a conserved residue of VPS33A (D251E)52,340, lies in a region predicted to interact with the v-SNARE – most likely VAMP7.327 Therefore, the HPS phenotypes observed may be due to altered v-SNARE binding to VPS33A, impairing the ability of CORVET and HOPS to properly couple tethering and SM activity in the biogenesis of melanosomes and platelet dense granules. However, whether the coat color defect reflects a defect in melanosome biogenesis per sé is not completely clear. Two reports suggest that cultured or in situ buff melanocytes are hypopigmented and harbor more immature melanosomes52,341, whereas another report suggests that cultured buff melanocytes are normally pigmented.342 Hypopigmentation in the eye is also only partial52,340,343, perhaps reflecting the hypomorphic nature of the buff mutation. Interestingly, fusiform vesicles (FVs) - a secretory granule-like putative LRO found in urothelium - accumulate as multivesicular bodies in buff mice344, suggesting a possible role for VPS33A in their trafficking or maturation as well.
Several groups have attempted to uncover the effect of the buff mutation on VPS33A function.328,345 Unlike vps33 null mutations, introduction of an orthologous bf mutation in yeast Vps33p (D300E) has no effect on growth rate, vacuole morphology, or protein trafficking to vacuoles.328 However, a more dramatic change at this residue, D300G, causes Vps33p mislocalization, zinc-dependent growth defects at non-permissive temperatures, and partial defects in protein trafficking to the vacuole.328 These data support an important function for this conserved residue and suggest that its mutagenesis might impact function under specific conditions. Zhen and Wei proposed that the buff mutation increases the affinity of VPS33A for v-SNAREs, hypothesizing that this decreases the rate of v-SNARE release and disrupts fusion by limiting the concentrations of available VPS33A and v-SNARE.345 However, additional work is needed to validate these conclusions and to understand the full effect of the buff mutation on VPS33A function and its role in LRO biogenesis.
Several human disease variants of VPS33A have been reported but not carefully studied. Suzuki et al report one HPS patient with no mutations in HPS1, HPS2, HPS3, and HPS4 but a mutation in VPS33A resulting in a I256L replacement52, which falls within domain 3a adjacent to D251; in fact, the buff mutation is predicted to disrupt the normal interaction between D251 and I256.327 However, it is unclear if the conservative I256L replacement is causative for the disease, and verification of this or other VPS33A mutations in HPS patients has not been reported. Another homozygous VPS33A mutation – R498W, predicted within the VPS16-interacting region of VPS33A – was identified in patients with systemic effects that appear to stem from lysosomal dysfunction346,347, but these patients showed no obvious signs of HPS. The mechanisms underlying the different functional effects of distinct VPS33A mutations warrant further experimental attention.
Additional disorders of LRO biogenesis, secretion and motility
Chediak-Higashi syndrome.
Like HPS, CHS is characterized by a variable, often mild oculocutaneous albinism and excessive bleeding and bruising. However, the major symptom is immune system dysregulation that often leads to a lethal hemophagocytic lymphohistiocytosis (HLH) in an “accelerated phase” due to impaired cytotoxic T cell and NK cell activity and consequent lymphoproliferation and hyperinflammation.348 Surviving patients suffer from a late-onset neurological impairment.349 The symptoms reflect defects in lysosome and phagosome maturation350, concomitant with the formation of giant enlarged lysosomes and LROs that cannot be secreted.351,352 The defective gene, LYST or CHS1, encodes a ~4000 amino acid protein with several identifiable protein domains including BEACH, pleckstrin homology, WD40 repeats, perilipin-like, lectin-like, and HEAT/Armadillo-like repeats.353-358 LYST is thought to either promote fission of endolysosomal organelles or prevent their fusion115,359,360, as the giant organelles in cytotoxic T cells, NK cells and other cell types from CHS patients or its beige mouse model contain markers of both late endosomes/ lysosomes and early endosomes.361,362 However, the mechanism by which LYST does so is not yet clear. In a Dictyostelium model, the LYST orthologue LvsB appears to function by antagonizing homotypic lysosomal fusion mediated by RAB14.115 Accordingly, knock down of RAB14 in a human LYST-deficient NK cell line restored normal lytic granule number, size and agonist-stimulated secretion.363 The latter study also suggested that the large size of the granules in LYST-deficient NK cells impeded their secretion through the cortical actin network at the immunological synapse, and that granule secretion in these cells was normalized by actin depolymerization.363 LYST also plays a role in phagolysosomal maturation, resulting in defective TRIF/TRAM-dependent signaling by toll-like receptors 3 and 4 in LYST-deficient macrophages and dendritic cells and consequent impaired innate immune responses to bacterial pathogens.350 Similarly, the D. melanogaster LYST orthologue prevents fusion of incompletely matured phagosomes.364 LYST’s enormous size has impeded mechanistic analysis of its mode of action, but analyses of cells from an increasing cohort of identified patients with missense mutations might provide key insights into the function of specific domains.362
Arthrogryposis, renal dysfunction, and cholestasis syndrome (ARC).
Arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome is a heritable autosomal recessive multisystem disorder characterized by arthrogryposis (congenital joint contracture), kidney tubule acidosis, cholestasis (failure of the liver to secrete bile), bleeding diathesis, and failure to thrive, among other characteristics.365-368 Most patients do not survive past their first year due to metabolic decompensation, recurrent infections, and/or uncontrolled bleeding.367,369 ARC is caused by loss-of-function mutations in the genes encoding either VPS33B or VIPAR (a.k.a. VPS16B or SPE-39).367,368,370-376 Like VPS33A, VPS33B is a homolog of yeast Vps33p and is an SM protein.310,377 However, VPS33A and B are functionally distinct and cannot compensate for each other.306,378 Moreover, neither VPS33B nor VIPAR is incorporated into the CORVET or HOPS complexes.84,324,327,379 Instead, VPS33B and VIPAR together form the class c homologs in endosome-vesicle interaction (CHEVI) complex.84,324,327,374,379,380 Data from C. elegans381,382, D. melanogaster376,383, and zebrafish372,374 with null alleles, mice with loss-of-function mutations369,383-385, and ARC patient samples370,373,374,386 indicate that the CHEVI complex functions in specific, context-dependent trafficking pathways that differ among specialized cell types. For example, the cholestasis observed in ARC patients likely reflects a role for the CHEVI complex in apical recycling in polarized epithelial cells.370,374,384 Resident membrane proteins in hepatocytes that are continuously recycled to apical membranes via apical recycling endosomes (e.g. bile salt export pump and claudin-1) are mislocalized throughout the plasma membrane in CHEVI-deficient cells, but other resident apical membrane proteins that do not undergo recycling (e.g. multiple drug resistance-associated protein 2) are not.370,374,384 In other cell types, the CHEVI complex functions in the biogenesis and/or trafficking of LROs such as platelet α-granules369,371,386 and skin lamellar bodies373,385, explaining the excessive bleeding and ichthyosis, respectively, in ARC patients. In Drosophila hemocytes and mammalian macrophages, the CHEVI complex is necessary for the fusion of phagosomes with lysosomes during phagosome maturation and for clearance of internalized toll-like receptors from endosomes376,383; the latter underlies hyperstimulation of inflammatory signaling in VPS33B-deficient cells.383 Lastly, in C. elegans, the CHEVI subunit orthologues are required for formation of an LRO in spermatocytes called the membranous organelle.381,382 How CHEVI regulates these events is not entirely clear, but a recent report on the VPS33B protein interactome provides some potential clues.379 Specifically, VPS33B interacts with CCDC22, a member of the CCC complex that functions together with the WASH complex to effect endosomal recycling387, and with VPS53, a subunit of the Golgi-associated retrograde protein (GARP) complex and endosome-associated recycling complex (EARP)380, both of which function as tethers during endosomal recycling. Overall, these studies suggest that the CHEVI complex functions in context-dependent, tissue-specific endosomal trafficking pathways, perhaps by regulating endosomal recycling.
Gray platelet syndrome.
GPS is a heritable disorder in which platelets from hematoxylin stained blood smears appear gray because they lack contents in their alpha granules.388 This is accompanied by macrothrombocytopenia and typically mild but occasionally severe excessive bleeding and bruising. Most GPS patients389 bear loss-of-function mutations in the gene encoding NBEAL2, a ~2800 amino acid protein with many of the same structural features as LYST, the causative agent of CHS.390-392 Mice lacking a functional NBEAL2 gene phenocopy the human disease.393-395 Like for LYST, a molecular understanding of NBEAL2 function is lacking. A recent study identified several potential binding partners for the NBEAL2 BEACH domain including the putative ER exit site regulator SEC16A, the scaffolding protein VAC14 for the phosphatidylinositol-3-phosphate 5-kinase and phosphatase, and the RAC1/CDC42 GTPase exchange factor DOCK7396, suggesting potential roles in late endosomal maturation required for alpha granule biogenesis397 and/or for actin-dependent signaling processes. Intriguingly, RAC1 has been implicated in platelet spreading and secretion of factors stored in alpha granules398-401, although many of these studies rely on inhibitors with potential off-target effects.402
Griscelli syndrome.
GS types 1-3 and the corresponding mouse mutants reflect defects in the three components of a complex required for melanosome positioning within skin melanocytes: the unconventional myosin Va (MYOVA), the Rab GTPase RAB27A, and a linker between them, melanophilin (MLPH).403 RAB27A associates directly with mature melanosomes and, via the linker MLPH, engages a melanocyte-specific spliced isoform of MYOVA to allow for peripheral melanosome capture and motility on actin filaments.404-411 Consequently, melanocytes deficient in any of these components accumulate melanosomes near the microtubule organizing center and fail to efficiently transfer melanosomes to keratinocytes. This results in hypopigmentation of the skin and hair in GS1, 2 and 3 patients and corresponding mouse models.412-414 Interestingly, MLPH that has not been phosphorylated by protein kinase A can bind to microtubules in vitro and relocate the associated MYOVA and RAB27A from actin filaments to microtubules; this might provide a mechanism for protein kinase A-dependent regulation of melanosome localization in vivo.415 MLPH function in RAB27A-dependent organelle motility appears to be limited to skin melanocytes, and hence GS3 patients display pigment dilution in the skin and hair, but not in the eye.413 RAB27A-dependent melanosome motility in retinal pigment epithelium employs a distinct adaptor, MYRIP, and a different myosin, MYOVIIA.416,417
By contrast to MLPH, MYOVA and RAB27A function in other physiologically critical venues, and hence most GS1 and GS2 patients suffer more severe consequences. MYOVA plays a number of important roles in development, secretion, plasticity, and signaling in the neuronal system.418 Therefore, GS1 patients suffer from neurological impairment of varying severity (depending on the site of the mutation - for example, patients with mutations in the exon encoding the melanocyte-specific tail region of MYOVA resemble GS3 patients413). Among many other functions, RAB27A plays a critical role in the final steps of cytolytic granule secretion in cytotoxic T cells and NK cells14,419,420, and thus GS2 patients suffer from both immunodeficiency and accelerated phase HLH due to lymphoproliferation and an ensuing cytokine storm. In these cell types, RAB27A engages a distinct group of effector proteins including Munc13-4 (see section on FHL3 below)14,421,422, the synaptotagmin-like proteins Slp1 and Slp2a423,424 for tethering of lytic granules to the plasma membrane, and Slp3 at an earlier stage for kinesin-1-dependent motility of lytic granules to the plasma membrane.425 RAB27A and the homologous RAB27B function in regulated secretion in many other cell types, but their physiological importance is not clear. For example, although RAB27A appears to function in docking insulin granules to the pancreatic β cell plasma membrane426 and the RAB27A/MLPH/MYOVA complex plays an inhibitory role for mast cell granule secretion427, reports of any clinical consequence in GS patients are lacking.
Familial hemophagocytic lymphohistiocytosis types 3-5.
FHL, like HLH in GS2 and CHS, is characterized by defective cytolytic granule exocytosis, leading to defects in T cell and NK cell cytolytic activity and consequent lymphoproliferation, hyperinflammation, and a life-threatening cytokine storm. In addition, patients have a bleeding and bruising tendency due to defective platelet granule secretion. Whereas the gene underlying FHL1 has not been identified, and mutations in the gene encoding the cytolytic effector perforin underlie FHL2428, FHL3-5 are due to mutations in genes encoding the machinery required for secretion of lytic granules, platelet alpha and dense granules, and other regulated secretory organelles – specifically UNC13D (encoding munc13-4, mutated in FHL3)429, STX11 (encoding syntaxin-11, mutated in FHL4430,431) and STXBP2 (encoding munc18-2, mutated in FHL5432,433). STX11 is a Qa SNARE that is part of the tSNARE complex at the plasma membrane required for cytolytic granule and platelet granule secretion.434-437 Munc18-2 is a SM family member that specifically stabilizes STX11 in platelets and cytotoxic T cells and is required for efficient STX11 expression at the plasma membrane438 and for granule fusion.433,434,439 It is particularly critical to stabilize full fusion intermediates mediated by the lipid-anchored STX11.437 Consistent with its function, a number of FHL5 mutations disrupt the binding sites on munc18-2 for STX11 or αSNAP440 or impede release of the N-terminal STX11 peptide and thus block subsequent munc18-2 function in facilitating SNARE complex assembly.434 Munc13 family members are thought to function in tethering and/or regulating tSNARE conformational changes required for SNARE complex assembly.441 Accordingly, the RAB27A effector, munc13-4, functions during granule tethering to the plasma membrane in CTLs and platelets.421,422,442 This step appears to require RAB27A, as GS2 patients with RAB27A mutations that impair its interaction with munc13-4 suffer from HLH but not pigment dilution.443 In CTLs, munc13-4 also appears to function independently of RAB27A at an earlier step required for cytolytic granule maturation.14 Intriguingly, HLH is not a spontaneous feature of the mouse FHL3 model but is induced by infection444, suggesting that FHL in humans might similarly be triggered by infectious agents.
Perspectives
Research over the last two decades has enormously expanded our understanding of LROs and the heritable diseases in which their function is impaired. Our progress to date has opened up new areas of investigation into the basic biology of LROs and has illuminated molecular mechanisms by which the endosomal system is adapted to the needs of specialized cells. At the same time, new LROs are being discovered at a steady pace, suggesting that such adaptations may be more prevalent among cell types than previously thought. Importantly, our progress has also provided a new understanding into the mechanisms of heritable diseases that impact LROs and related organelle systems. Future work will likely build on these studies by discovering novel LRO-related disorders and by developing therapies for them.
Our progress to date in understanding LROs has largely been driven by the intensive study of specific model systems, particularly mammalian melanosomes, cytolytic granules, and Weibel-Palade bodies and several of the invertebrate model LROs (Figures 1, 2 and Table 1). These studies, driven by the identification of specific cargo transmembrane proteins and the availability of reagents to follow their fate under various genetic manipulations, have particularly illuminated the commonalities and diversity of LRO assembly pathways (Figure 2). We now need to build on these studies to gain specific insights into other medically relevant LROs. For example, we have only scratched the surface in understanding the contents and delivery pathways for lung lamellar bodies or platelet dense and alpha granules, defects in which underlie the lung fibrosis and excessive bleeding in HPS and GPS. Studies of these systems are likely to yield interesting surprises. For example, recent evidence suggests that dense granules do not mature until the final stages of platelet formation from megakaryocytes.445 Understanding the temporal regulation of this step will likely lead to new ideas regarding signaling pathways that can regulate LRO biogenesis in specific cell types. Similarly, understanding the complex network of protein: protein interactions among NBEAL2, the CHEVI complex, and other effectors that are essential for alpha granule formation may allow us to better understand how these enigmatic organelles segregate from the late endosomal system.446 An important contribution to our understanding will be the identification of relevant cargoes for each LRO system; availability of such cargoes and reagents to easily detect them will allow a rapid test of whether the paradigms generated in better studied systems to date apply more generally to other LROs.
Non-mammalian model systems will likely play increasingly important roles in dissecting the physiological functions of LRO biogenetic and secretion effectors. The ability in many of these systems to combine mutations and to identify gene modifiers will extend known gene interaction networks. Combining data from analyses from such tractable genetic systems with the increasingly available complex interactomes from proteomics data in mammalian systems will provide important tests of the functionality of interaction partners and their importance in the physiology of new LROs. We thus should expect more robust molecular detail in our models of LRO biogenesis, motility and secretion in the coming years.
While diseases such as HPS, CHS and GS have opened new avenues of basic science research over the last two decades, we can expect more insights to come from new diseases in the future as whole genome sequencing of patients will expand the number of candidate genes underlying LRO biogenesis and function. This has already come to light in several ways. For example, a recent publication reported screening of nearly 1000 albino patients for known albinism genes.203 Many harbored novel mutations in known genes, and nearly 28% of the patients lacked mutations in known albinism genes; sequencing of these patients is likely to identify novel genes that contribute either specifically to melanosome biology or generally to LRO biogenesis or function. On the other side of the spectrum, sequencing of individual patients with novel syndromic disorders can identify novel genes and mechanisms in LRO biology. For example, screening of a pediatric patient with a FLH-like disease led to the identification of a novel mutation in STXBP1 with a dominant negative phenotype434. Thus, we can expect many new insights from analyses of patient samples in the coming years.
In turn, future studies will allow us to build on our knowledge of LROs to devise new therapies for the diseases that have taught us so much about LRO biogenesis and secretion. New modes of gene therapy, in some cases combined with bone marrow transplant for hematologic disorders, may provide relief for some symptoms of syndromic disorders. The major challenges to be overcome in this respect are symptoms arising from non-hematopoietic cell types, such as the lung AT2 cells that are the drivers of lung fibrosis in the most common HPS patients; difficulties in specifically targeting these cells for gene therapy are exacerbated by the likely short half-life of the affected AT2 cells. In cases such as this, understanding how inflammatory sequelae contribute to disease progression might uncover other treatment options.
Synopsis.
Lysosome-related organelles are cell type-specific subcellular structures that derive from both the secretory and endolysosomal pathways and that play key roles in numerous physiological systems, in most cases following stimulus-dependent secretion of their contents. The biogenesis and/or secretion of lysosome-related organelle subsets are disrupted in hereditary syndromic disorders such as the Hermansky-Pudlak syndromes and Familial Hemophagocytic Lymphohistiocytosis. We comprehensively review lysosome-related organelles in humans and model organisms and the mechanisms by which products of disease genes regulate their formation, motility and ultimate secretion.
Acknowledgments
We thank our collaborators past and present, former lab members, and other members of the community for sharing their thoughts and data during preparation of this manuscript. This work was supported by funding from the National Institutes of Health, including R01 EY015625 from the National Eye Institute, R01 AR048155 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases, R01 HL121323 and T32 HL007439 from the National Heart, Lung and Blood Institute, and R01 GM108807 and K12 GM081259 from the National Institute of General Medical Sciences. The authors have no conflicts of interest to declare.
Footnotes
GPS is unique among these syndromes in that it appears to impact only alpha granule biogenesis in megakaryocytes and platelets.
REFERENCES
- 1.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould GW, Lippincott-Schwartz J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat Rev Mol Cell Biol. 2009;10(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149(3):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins RF, Schreiber AD, Grinstein S, Trimble WS. Syntaxins 13 and 7 function at distinct steps during phagocytosis. J Immunol. 2002;169(6):3250–3256. [DOI] [PubMed] [Google Scholar]
- 5.Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109(6 part 2):3259–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aledo JC, Lavoie L, Volchuk A, Keller SR, Klip A, Hundal HS. Identification and characterization of two distinct intracellular GLUT4 pools in rat skeletal muscle: evidence for an endosomal and an insulin-sensitive GLUT4 compartment. Biochem J. 1997;325(3):727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J Histochem Cytochem. 1997;45(8):1083–1096. [DOI] [PubMed] [Google Scholar]
- 8.Hashiramoto M, James DE. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol Cell Biol. 2000;20(1):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks MS, Heijnen HFG, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25(4):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19(4):394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol. 2014;6(9):a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14(10):1265–1278. [DOI] [PubMed] [Google Scholar]
- 13.Peters PJ, Borst J, Oorschot V, Fukuda M, Krähenbühl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173(5):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ménager MM, Ménasché G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8(3):257–267. [DOI] [PubMed] [Google Scholar]
- 15.Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152(4):809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf DJ, Nightingale TD, Zenner HL, Lui-Roberts WW, Cutler DF. Formation and function of Weibel-Palade bodies. J Cell Sci. 2008;121(1):19–27. [DOI] [PubMed] [Google Scholar]
- 17.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19(1):19–42. [DOI] [PubMed] [Google Scholar]
- 18.Wei A-H, Li W. Hermansky-Pudlak syndrome: Pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013;26(2):176–192. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro F, Kriston-Vizi J, Metcalf DJ, Martin-Martin B, Freeman J, Burden JJ, Westmoreland D, Dyer CE, Knight AE, Ketteler R, Cutler DF. A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev Cell. 2014;29(3):292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannah MJ, Williams R, Kaur J, Hewlett LJ, Cutler DF. Biogenesis of Weibel-Palade bodies. Semin Cell Dev Biol. 2002;13(4):313–324. [DOI] [PubMed] [Google Scholar]
- 21.Hao Z, Wei L, Feng Y, Chen X, Du W, Ma J, Zhou Z, Chen L, Li W. Impaired maturation of large dense-core vesicles in muted-deficient adrenal chromaffin cells. J Cell Sci. 2015;128(7):1365–1374. [DOI] [PubMed] [Google Scholar]
- 22.Asensio CS, Sirkis DW, Edwards RH. RNAi screen identifies a role for adaptor protein AP-3 in sorting to the regulated secretory pathway. The Journal of cell biology. 2010;191(6):1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirkis DW, Edwards RH, Asensio CS. Widespread dysregulation of peptide hormone release in mice lacking adaptor protein AP-3. PLoS Genet. 2013;9(9):e1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol Biol Cell. 1999;10(11):3979–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305:55–59. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths GM. Secretion from myeloid cells: secretory lysosomes. Microbiol Spectr. 2016;4(4): 10.1128/microbiolspec.MCHD-0030-2016. [DOI] [PubMed] [Google Scholar]
- 27.Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10(8):316–321. [DOI] [PubMed] [Google Scholar]
- 28.Andrews NW, Almeida PE, Corrotte M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014;24(12):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seward SL Jr., Gahl WA Hermansky-Pudlak syndrome: health care throughout life. Pediatrics. 2013;132(1):153–160. [DOI] [PubMed] [Google Scholar]
- 30.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2012;186(10):1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, Tao JQ, Gochuico BR, Gahl W, Guo CJ, Gow AJ, Beers MF, Guttentag S. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2011;184(4):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guttentag SH, Akhtar A, Tao JQ, Atochina E, Rusiniak ME, Swank RT, Bates SR. Defective surfactant secretion in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Cell Mol Biol. 2005;33(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young LR, Borchers MT, Allen HL, Gibbons RS, McCormack FX. Lung-restricted macrophage activation in the pearl mouse model of Hermansky-Pudlak syndrome. J Immunol. 2006;176(7):4361–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young LR, Gulleman PM, Short CW, Tanjore H, Sherrill T, Qi A, McBride AP, Zaynagetdinov R, Benjamin JT, Lawson WE, Novitskiy SV, Blackwell TS. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. JCI Insight. 2016;1(17):e88947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hengst M, Naehrlich L, Mahavadi P, Grosse-Onnebrink J, Terheggen-Lagro S, Skanke LH, Schuch LA, Brasch F, Guenther A, Reu S, Ley-Zaporozhan J, Griese M. Hermansky-Pudlak syndrome type 2 manifests with fibrosing lung disease early in childhood. Orphanet J Rare Dis. 2018;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ammann S, Schulz A, Krägeloh-Mann I, Dieckmann NM, Niethammer K, Fuchs S, Eckl KM, Plank R, Werner R, Altmüller J, Thiele H, Nürnberg P, Bank J, Srauss A, von BH, et al. Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood. 2016;127(8):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark RH, Stinchcombe JC, Day A, Blott E, Booth S, Bossi G, Hamblin T, Davies EG, Griffiths GM. Adaptor protein 3–dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nature Immunol. 2003;4:1111–1120. [DOI] [PubMed] [Google Scholar]
- 38.Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, Kontny U, Muller C, Nurden A, Rohr J, Henschen M, Pannicke U, Niemeyer C, Nurden P, Ehl S. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak Syndrome Type II. Blood. 2006;108(1):81–87. [DOI] [PubMed] [Google Scholar]
- 39.Fontana S, Parolini S, Vermi W, Booth S, Gallo F, Donini M, Benassi M, Gentili F, Ferrari D, Notarangelo LD, Cavadini P, Marcenaro E, Dusi S, Cassatella M, Facchetti F, et al. Innate immunity defects in Hermansky-Pudlak type 2 syndrome. Blood. 2006;107(12):4857–4864. [DOI] [PubMed] [Google Scholar]
- 40.Wenham M, Grieve S, Cummins M, Jones ML, Booth S, Kilner R, Ancliff PJ, Griffiths GM, Mumford AD. Two patients with Hermansky Pudlak syndrome type 2 and novel mutations in AP3B1. Haematologica. 2010;95(2):333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang P-W, Oiso N, Gautam R, Swank RT, Spritz RA. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS4 proteins are components of two complexes, BLOC-3 and BLOC-4, involved in the biogenesis of lysosome-related organelles. J Biol Chem. 2003;278(22):20332–20337. [DOI] [PubMed] [Google Scholar]
- 42.Huizing M, Anikster Y, Fitzpatrick DL, Jeong AB, D’Souza M, Rausche M, Toro JR, Kaiser-Kupfer MI, White JG, Gahl WA. Hermansky-Pudlak syndrome type 3 in Ashkenazi Jews and other non-Puerto Rican patients with hypopigmentation and platelet storage-pool deficiency. Am J Hum Genet. 2001;69(5):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huizing M, Hess R, Dorward H, Claassen DA, Helip-Wooley A, Kleta R, Kaiser-Kupfer MI, White JG, Gahl WA. Cellular, molecular and clinical characterization of patients with Hermansky-Pudlak syndrome type 5. Traffic. 2004;5(9):711–722. [DOI] [PubMed] [Google Scholar]
- 44.Huizing M, Pederson B, Hess RA, Griffin A, Helip-Wooley A, Westbroek W, Dorward H, O’Brien KJ, Golas G, Tsilou E, White JG, Gahl WA. Clinical and cellular characterisation of Hermansky-Pudlak syndrome type 6. J Med Genet. 2009;46(12):803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L. Mouse models of Hermansky Pudlak syndrome: a review. Pigment Cell Res. 1998;11(2):60–80. [DOI] [PubMed] [Google Scholar]
- 46.Swank RT, Novak EK, McGarry MP, Zhang Y, Li W, Zhang Q, Feng L. Abnormal vesicular trafficking in mouse models of Hermansky-Pudlak Syndrome. Pigment Cell Res. 2000;13(Suppl. 8):59–67. [DOI] [PubMed] [Google Scholar]
- 47.Silvers WK. The coat colors of mice. A model for mammalian gene action and interaction. New York: Springer-Verlag; 1979. [Google Scholar]
- 48.Lamoreux ML, Delmas V, Larue L, Bennett DC. The Colors of Mice: A Model Genetic Network. Oxford, UK: Wiley-Blackwell; 2010. [Google Scholar]
- 49.Zhang Q, Li W, Novak EK, Karim A, Mishra VS, Kingsmore SF, Roe BA, Suzuki T, Swank RT. The gene for the muted (mu) mouse, a model for Hermansky-Pudlak syndrome, defines a novel protein which regulates vesicle trafficking. Hum Mol Genet. 2002;11(6):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falcon-Perez JM, Starcevic M, Gautam R, Dell’Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet dense granules. J Biol Chem. 2002;277(31):28191–28199. [DOI] [PubMed] [Google Scholar]
- 51.Ciciotte SL, Gwynn B, Moriyama K, Huizing M, Gahl WA, Bonifacino JS, Peters LL. Cappuccino, a mouse model of Hermansky-Pudlak syndrome, encodes a novel protein that is part of the pallidin-muted complex (BLOC-1). Blood. 2003;101(11):4402–4407. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci USA. 2003;100(3):1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, McMurtrie EB, Tchernev VT, Wallace MR, Seabra MC, Swank RT, Kingsmore SF. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc Natl Acad Sci USA. 2000;97(8):4144–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci USA. 2005;102(31):10964–10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks BP, Larson DM, Chan CC, Kjellstrom S, Smith RS, Crawford MA, Lamoreux L, Huizing M, Hess R, Jiao X, Hejtmancik JF, Maminishkis A, John SW, Bush R, Pavan WJ. Analysis of ocular hypopigmentation in Rab38cht/cht mice. Invest Ophthalmol Vis Sci. 2007;48(9):3905–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen YA, Wu XS, Jiang Y, Bittner M, Hammer JA III, Pavan WJ Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA. 2002;99(7):4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osanai K, Oikawa R, Higuchi J, Kobayashi M, Tsuchihara K, Iguchi M, Jongsu H, Toga H, Voelker DR. A mutation in Rab38 small GTPase causes abnormal lung surfactant homeostasis and aberrant alveolar structure in mice. Am J Pathol. 2008;173(5):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby ( R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome. 2004;15(4):307–314. [DOI] [PubMed] [Google Scholar]
- 59.Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Lung Cell Mol Physiol. 2010;298(2):L243–L251. [DOI] [PubMed] [Google Scholar]
- 60.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39(3):414–418. [DOI] [PubMed] [Google Scholar]
- 61.Sviderskaya EV, Hill SP, Evans-Whipp TJ, Chin L, Orlow SJ, Easty DJ, Cheong SC, Beach D, DePinho RA, Bennett DC. p16Ink4a in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94(6):446–454. [DOI] [PubMed] [Google Scholar]
- 62.Kook S, Qi A, Wang P, Meng S, Gulleman P, Young LR, Guttentag SH. Gene-edited MLE-15 cells as a model for the Hermansky-Pudlak Syndromes. Am J Respir Cell Mol Biol. 2018;58(5):566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schliwa M, Bereiter-Hahn J. Pigment movements in fish melanophores: morphological and physiological studies. V. Evidence for a microtubule-independent contractile system. Cell and tissue research. 1975;158(1):61–73. [DOI] [PubMed] [Google Scholar]
- 64.Tuma MC, Gelfand VI. Molecular mechanisms of pigment transport in melanophores. Pigment cell research. 1999;12(5):283–294. [DOI] [PubMed] [Google Scholar]
- 65.Chen T, Song G, Yang H, Mao L, Cui Z, Huang K. Development of the swimbladder surfactant system and biogenesis of lysosome-related organelles is regulated by BLOS1 in zebrafish. Genetics. 2018;208(3):1131–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daly CM, Willer J, Gregg R, Gross JM. snow white, a zebrafish model of Hermansky-Pudlak Syndrome type 5. Genetics. 2013;195(2):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schonthaler HB, Fleisch VC, Biehlmaier O, Makhankov Y, Rinner O, Bahadori R, Geisler R, Schwarz H, Neuhauss SC, Dahm R. The zebrafish mutant lbk/vam6 resembles human multisystemic disorders caused by aberrant trafficking of endosomal vesicles. Development (Cambridge, England). 2008;135(2):387–399. [DOI] [PubMed] [Google Scholar]
- 68.Thomas JL, Vihtelic TS, denDekker AD, Willer G, Luo X, Murphy TR, Gregg RG, Hyde DR, Thummel R. The loss of vacuolar protein sorting 11 (vps11) causes retinal pathogenesis in a vertebrate model of syndromic albinism. Investigative ophthalmology & visual science. 2011;52(6):3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maldonado E, Hernandez F, Lozano C, Castro ME, Navarro RE. The zebrafish mutant vps18 as a model for vesicle-traffic related hypopigmentation diseases. Pigment Cell Res. 2006;19(4):315–326. [DOI] [PubMed] [Google Scholar]
- 70.Bahadori R, Rinner O, Schonthaler HB, Biehlmaier O, Makhankov YV, Rao P, Jagadeeswaran P, Neuhauss SC. The Zebrafish fade out mutant: a novel genetic model for Hermansky-Pudlak syndrome. Invest Ophthalmol Vis Sci. 2006;47(10):4523–4531. [DOI] [PubMed] [Google Scholar]
- 71.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200(5):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diaz-Tellez A, Zampedri C, Ramos-Balderas JL, García-Hernández F, Maldonado E. Zebrafish scarb2a insertional mutant reveals a novel function for the Scarb2/Limp2 receptor in notochord development. Dev Dyn. 2016;245(4):508–519. [DOI] [PubMed] [Google Scholar]
- 73.Nakayama T, Nakajima K, Cox A, Fisher M, Howell M, Fish MB, Yaoita Y, Grainger RM. no privacy, a Xenopus tropicalis mutant, is a model of human Hermansky-Pudlak Syndrome and allows visualization of internal organogenesis during tadpole development. Dev Biol. 2017;426(2):472–486. [DOI] [PubMed] [Google Scholar]
- 74.Shoup JR. The development of pigment granules in the eyes of wild type and mutant Drosophila melanogaster. J Cell Biol. 1966;29(2):223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kretzschmar D, Poeck B, Roth H, Ernst R, Keller A, Porsch M, Strauss R, Pflugfelder GO. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3beta adaptin gene ruby. Genetics. 2000;155(1):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lloyd VK, Sinclair DA, Wennberg R, Warner TS, Honda BM, Grigliatti TA. A genetic and molecular characterization of the garnet gene of Drosophila melanogaster. Genome. 1999;42(6):1183–1193. [PubMed] [Google Scholar]
- 77.Ooi CE, Moreira JE, Dell’Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. The EMBO journal. 1997;16(15):4508–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mullins C, Hartnell LM, Wassarman DA, Bonifacino JS. Defective expression of the mu3 subunit of the AP-3 adaptor complex in the Drosophila pigmentation mutant carmine. Mol Gen Genet. 1999;262(3):401–412. [DOI] [PubMed] [Google Scholar]
- 79.Mullins C, Hartnell LM, Bonifacino JS. Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol Gen Genet. 2000;263(6):1003–1014. [DOI] [PubMed] [Google Scholar]
- 80.Cheli VT, Daniels RW, Godoy R, Hoyle DJ, Kandachar V, Starcevic M, Martinez-Agosto JA, Poole S, Diantonio A, Lloyd VK, Chang HC, Krantz DE, Dell’angelica EC. Genetic modifiers of abnormal organelle biogenesis in a Drosophila model of BLOC-1 deficiency. Hum Mol Genet. 2010;19(5):861–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falcón-Pérez JM, Romero-Calderón R, Brooks ES, Krantz DE, Dell’Angelica EC. The drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky-Pudlak Syndrome 5 (HPS5). Traffic. 2007;8(2):154–168. [DOI] [PubMed] [Google Scholar]
- 82.Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc Natl Acad Sci USA. 2004;101(32):11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant P, Maga T, Loshakov A, Singhal R, Wali A, Nwankwo J, Baron K, Johnson D. An Eye on Trafficking Genes: Identification of Four Eye Color Mutations in Drosophila. G3 (Bethesda, Md). 2016;6(10):3185–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Kramer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci. 2005;118(16):3663–3673. [DOI] [PubMed] [Google Scholar]
- 85.Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol Cell. 1999;4(4):479–486. [DOI] [PubMed] [Google Scholar]
- 86.Shestopal SA, Makunin IV, Belyaeva ES, Ashburner M, Zhimulev IF. Molecular characterization of the deep orange (dor) gene of Drosophila melanogaster. Mol Gen Genet. 1997;253(5):642–648. [DOI] [PubMed] [Google Scholar]
- 87.Warner TS, Sinclair DA, Fitzpatrick KA, Singh M, Devlin RH, Honda BM. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome. 1998;41(2):236–243. [PubMed] [Google Scholar]
- 88.Tejeda-Guzman C, Rosas-Arellano A, Kroll T, Webb SM, Barajas-Aceves M, Osorio B, Missirlis F. Biogenesis of zinc storage granules in Drosophila melanogaster. J Exp Biol. 2018;221(Pt 6):jeb168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, Boulianne GL, Chang HC, Le Borgne R, Krämer H, Brill JA. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell. 2011;22(12):2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burgess J, Del Bel LM, Ma CI, Barylko B, Polevoy G, Rollins J, Albanesi JP, Krämer H, Brill JA. Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila. Development (Cambridge, England). 2012;139(16):3040–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, Grant BD, Priess JR. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2005;16(7):3273–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schroeder LK, Kremer S, Kramer MJ, Currie E, Kwan E, Watts JL, Lawrenson AL, Hermann GJ. Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Mol Biol Cell. 2007;18(3):995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Currie E, King B, Lawrenson AL, Schroeder LK, Kershner AM, Hermann GJ. Role of the Caenorhabditis elegans multidrug resistance gene, mrp-4, in gut granule differentiation. Genetics. 2007;177:1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delahaye JL, Foster OK, Vine A, Saxton DS, Curtin TP, Somhegyi H, Salesky R, Hermann GJ. Caenorhabditis elegans HOPS and CCZ-1 mediate trafficking to lysosome-related organelles independently of RAB-7 and SAND-1. Mol Biol Cell. 2014;25(7):1073–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hermann GJ, Scavarda E, Weis AM, Saxton DS, Thomas LL, Salesky R, Somhegyi H, Curtin TP, Barrett A, Foster OK, Vine A, Erlich K, Kwan E, Rabbitts BM, Warren K. C. elegans BLOC-1 functions in trafficking to lysosome-related gut granules. PLoS One. 2012;7(8):e43043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barrett A, Hermann GJ. A Caenorhabditis elegans homologue of LYST functions in endosome and lysosome-related organelle biogenesis. Traffic. 2016;17(5):515–535. [DOI] [PubMed] [Google Scholar]
- 97.Allen LA, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8(1):36–40. [DOI] [PubMed] [Google Scholar]
- 98.Dimond RL, Knecht DA, Jordan KB, Burns RA, Livi GP. Secretory mutants in the cellular slime mold Dictyostelium discoideum. Methods Enzymol. 1983;96:815–828. [DOI] [PubMed] [Google Scholar]
- 99.Fok AK, Clarke M, Ma L, Allen RD. Vacuolar H(+)-ATPase of Dictyostelium discoideum. A monoclonal antibody study. J Cell Sci. 1993;106 ( Pt 4):1103–1113. [DOI] [PubMed] [Google Scholar]
- 100.Padh H, Ha J, Lavasa M, Steck TL. A post-lysosomal compartment in Dictyostelium discoideum. J Biol Chem. 1993;268(9):6742–6747. [PubMed] [Google Scholar]
- 101.Clarke M, Kohler J, Arana Q, Liu T, Heuser J, Gerisch G. Dynamics of the vacuolar H(+)-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. J Cell Sci. 2002;115(Pt 14):2893–2905. [DOI] [PubMed] [Google Scholar]
- 102.Ravanel K, de Chassey B, Cornillon S, Benghezal M, Zulianello L, Gebbie L, Letourneur F, Cosson P. Membrane sorting in the endocytic and phagocytic pathway of Dictyostelium discoideum. Eur J Cell Biol. 2001;80(12):754–764. [DOI] [PubMed] [Google Scholar]
- 103.Kirsten JH, Xiong Y, Davis CT, Singleton CK. Subcellular localization of ammonium transporters in Dictyostelium discoideum. BMC Cell Biol. 2008;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rauchenberger R, Hacker U, Murphy J, Niewohner J, Maniak M. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr Biol. 1997;7(3):215–218. [DOI] [PubMed] [Google Scholar]
- 105.Carnell M, Zech T, Calaminus SD, Ura S, Hagedorn M, Johnston SA, May RC, Soldati T, Machesky LM, Insall RH. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J Cell Biol. 2011;193(5):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Hostos EL, Rehfuess C, Bradtke B, Waddell DR, Albrecht R, Murphy J, Gerisch G. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J Cell Biol. 1993;120(1):163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee E, Knecht DA. Visualization of actin dynamics during macropinocytosis and exocytosis. Traffic. 2002;3(3):186–192. [DOI] [PubMed] [Google Scholar]
- 108.Charette SJ, Mercanti V, Letourneur F, Bennett N, Cosson P. A role for Adaptor Protein-3 complex in the organization of the endocytic pathway in dictyostelium. Traffic. 2006;7(11):1528–1538. [DOI] [PubMed] [Google Scholar]
- 109.Charette SJ, Cosson P. Altered composition and secretion of lysosome-derived compartments in Dictyostelium AP-3 mutant cells. Traffic. 2008;9(4):588–596. [DOI] [PubMed] [Google Scholar]
- 110.Bennett N, Letourneur F, Ragno M, Louwagie M. Sorting of the v-SNARE VAMP7 in Dictyostelium discoideum: a role for more than one Adaptor Protein (AP) complex. Exp Cell Res. 2008;314(15):2822–2833. [DOI] [PubMed] [Google Scholar]
- 111.Cornillon S, Dubois A, Bruckert F, Lefkir Y, Marchetti A, Benghezal M, De Lozanne A, Letourneur F, Cosson P. Two members of the beige/CHS (BEACH) family are involved at different stages in the organization of the endocytic pathway in Dictyostelium. J Cell Sci. 2002;115(4):737–744. [DOI] [PubMed] [Google Scholar]
- 112.Kypri E, Schmauch C, Maniak M, De Lozanne A. The BEACH protein LvsB is localized on lysosomes and postlysosomes and limits their fusion with early endosomes. Traffic. 2007;8(6):774–783. [DOI] [PubMed] [Google Scholar]
- 113.Charette SJ, Cosson P. A LYST/beige homolog is involved in biogenesis of Dictyostelium secretory lysosomes. J Cell Sci. 2007;120(14):2338–2343. [DOI] [PubMed] [Google Scholar]
- 114.Falkenstein K, De Lozanne A. Dictyostelium LvsB has a regulatory role in endosomal vesicle fusion. J Cell Sci. 2014;127(20):4356–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kypri E, Falkenstein K, De Lozanne A. Antagonistic control of lysosomal fusion by Rab14 and the Lyst-related protein LvsB. Traffic. 2013;14(5):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turkewitz AP, Madeddu L, Kelly RB. Maturation of dense core granules in wild type and mutant Tetrahymena thermophila. EMBO J. 1991;10(8):1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tokuyasu K, Scherbaum OH. Ultrastructure of mucocysts and pellicle of Tetrahymena pyriformis. J Cell Biol. 1965;27(1):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Briguglio JS, Kumar S, Turkewitz AP. Lysosomal sorting receptors are essential for secretory granule biogenesis in Tetrahymena. J Cell Biol. 2013;203(3):537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaur H, Sparvoli D, Osakada H, Iwamoto M, Haraguchi T, Turkewitz AP. An endosomal syntaxin and the AP-3 complex are required for formation and maturation of candidate lysosome-related secretory organelles (mucocysts) in Tetrahymena thermophila. Mol Biol Cell. 2017;28(11):1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sparvoli D, Richardson E, Osakada H, Lan X, Iwamoto M, Bowman GR, Kontur C, Bourland WA, Lynn DH, Pritchard JK, Haraguchi T, Dacks JB, Turkewitz AP. Remodeling the specificity of an endosomal CORVET tether underlies formation of regulated secretory vesicles in the ciliate Tetrahymena thermophila. Curr Biol. 2018;28(5):697–710 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fujii T, Daimon T, Uchino K, Banno Y, Katsuma S, Sezutsu H, Tamura T, Shimada T. Transgenic analysis of the BmBLOS2 gene that governs the translucency of the larval integument of the silkworm, Bombyx mori. Insect Mol BIol. 2010;19(5):659–667. [DOI] [PubMed] [Google Scholar]
- 122.Fujii T, Banno Y, Abe H, Katsuma S, Shimada T. A homolog of the human Hermansky-Pudluck syndrome-5 (HPS5) gene is responsible for the oa larval translucent mutants in the silkworm, Bombyx mori. Genetica. 2012;140(10-12):463–468. [DOI] [PubMed] [Google Scholar]
- 123.Wang L, Kiuchi T, Fujii T, Daimon T, Li M, Banno Y, Katsuma S, Shimada T. Reduced expression of the dysbindin-like gene in the Bombyx mori ov mutant exhibiting mottled translucency of the larval skin. Genome. 2013;56(2):101–108. [DOI] [PubMed] [Google Scholar]
- 124.Zhang H, Kiuchi T, Wang L, Kawamoto M, Suzuki Y, Sugano S, Banno Y, Katsuma S, Shimada T. Bm-muted, orthologous to mouse muted and encoding a subunit of the BLOC-1 complex, is responsible for the otm translucent mutation of the silkworm Bombyx mori. Gene. 2017;629:92–100. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H, Kiuchi T, Hirayama C, Banno Y, Katsuma S, Shimada T. A reexamination on the deficiency of riboflavin accumulation in Malpighian tubules in larval translucent mutants of the silkworm, Bombyx mori. Genetica. 2018;146(4-5):425–431. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Kiuchi T, Hirayama C, Katsuma S, Shimada T. Bombyx ortholog of the Drosophila eye color gene brown controls riboflavin transport in Malpighian tubules. Insect Biochem Mol Biol. 2018;92:65–72. [DOI] [PubMed] [Google Scholar]
- 127.Lee J, Kiuchi T, Kawamoto M, Shimada T, Katsuma S. Accumulation of uric acid in the epidermis forms the white integument of Samia ricini larvae. PLoS One. 2018;13(10):e0205758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park SY, Guo X. Adaptor protein complexes and intracellular transport. Biosci Rep. 2014;34(4):e00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS. The fifth adaptor protein complex. PLoS Biol. 2011;9(10):e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hirst J, Itzhak DN, Antrobus R, Borner GHH, Robinson MS. Role of the AP-5 adaptor protein complex in late endosome-to-Golgi retrieval. PLoS Biol. 2018;16(1):e2004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta3A subunit of the AP-3 adaptor. Mol Cell. 1999;3(1):11–21. [DOI] [PubMed] [Google Scholar]
- 132.Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, Burmeister M. Mutation in AP-3delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21(1):111–122. [DOI] [PubMed] [Google Scholar]
- 133.Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colours. Trends in cell biology. 1998;8(7):282–288. [DOI] [PubMed] [Google Scholar]
- 134.Llinares E, Barry AO, Andre B. The AP-3 adaptor complex mediates sorting of yeast and mammalian PQ-loop-family basic amino acid transporters to the vacuolar/lysosomal membrane. Sci Rep. 2015;5:16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem. 2011;286(3):2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anand VC, Daboussi L, Lorenz TC, Payne GS. Genome-wide analysis of AP-3-dependent protein transport in yeast. Mol Biol Cell. 2009;20(5):1592–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dell’Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16(5):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell. 2009;20(5):1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dell’Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21(4):552–559. [DOI] [PubMed] [Google Scholar]
- 140.Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, Zhen L, Rusiniak ME, Eicher EM, Robinson MS, Gorin MB, Swank RT. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet. 1999;8(2):323–330. [DOI] [PubMed] [Google Scholar]
- 141.Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91(1):109–118. [DOI] [PubMed] [Google Scholar]
- 142.Simpson F, Bright NA, West MA, Newman LS, Darnell RB, Robinson MS. A novel adaptor-related protein complex. J Cell Biol. 1996;133(4):749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137(4):835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol. 2004;164(7):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16(11):5356–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ooi CE, Dell’Angelica EC, Bonifacino JS. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142(2):391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Drake MT, Zhu Y, Kornfeld S. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol Biol Cell. 2000;11(11):3723–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baust T, Anitei M, Czupalla C, Parshyna I, Bourel L, Thiele C, Krause E, Hoflack B. Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell. 2008;19(5):1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem. 1998;273(40):25915–25921. [DOI] [PubMed] [Google Scholar]
- 150.Mardones GA, Burgos PV, Lin Y, Kloer DP, Magadan JG, Hurley JH, Bonifacino JS. Structural basis for the recognition of Tyrosine-based sorting signals by the Mu3A subunit of the AP-3 adaptor complex. J Biol Chem. 2013;288(13):9563–9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim B-Y, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. 2003;163(6):1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Janvier K, Bonifacino JS. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell. 2005;16(9):4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kelly BT, McCoy AJ, Späte K, Miller SE, Evans PR, Höning S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sitaram A, Dennis MK, Chaudhuri R, De Jesus-Rojas W, Tenza D, Setty SRG, Wood CS, Sviderskaya EV, Bennett DC, Raposo G, Bonifacino JS, Marks MS. Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell. 2012;23(16):3178–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18(5):1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dell’Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. [DOI] [PubMed] [Google Scholar]
- 157.Peden AA, Rudge RE, Lui WWY, Robinson MS. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol. 2002;156(2):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zlatic SA, Grossniklaus EJ, Ryder PV, Salazar G, Mattheyses A, Peden AA, Faundez V. Chemical-genetic disruption of clathrin function spares Adaptor Complex 3-dependent endosome vesicle biogenesis. Mol Biol Cell. 2013;24(15):2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calvo PA, Frank DW, Bieler BM, Berson JF, Marks MS. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J Biol Chem. 1999;274(18):12780–12789. [DOI] [PubMed] [Google Scholar]
- 160.Simmen T, Schmidt A, Hunziker W, Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J Cell Sci. 1999;112(1):45–53. [DOI] [PubMed] [Google Scholar]
- 161.Höning S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17(5):1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huizing M, Sarangarajan R, Strovel E, Zho Y, Gahl WA, Boissy RE. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol Biol Cell. 2001;12(7):2075–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vijayasaradhi S, Xu YQ, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J Cell Biol. 1995;130(4):807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Delevoye C, Hurbain I, Tenza D, Sibarita J-B, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, Raposo G. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187(2):247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SRG, Marks MS, Raposo G, Dell’Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17(9):4027–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell’Angelica EC, Peden AA, E. Werner E, Faundez V BLOC-1 complex deficiency alters the targeting of Adaptor Protein complex-3 cargoes. Mol Biol Cell. 2006;17(9):4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hoyle DJ, Rodriguez-Fernandez IA, Dell’Angelica EC. Functional interactions between OCA2 and the protein complexes BLOC-1, BLOC-2 and AP-3 inferred from epistatic analyses of mouse coat pigmentation. Pigment Cell Melanoma Res. 2011;24(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell. 2004;15(2):575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, Takeuchi A, Miyazaki T, Watanabe M, Yuasa S, Manabe T, et al. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Biol. 2004;167(2):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Newell-Litwa K, Chintala S, Jenkins S, Pare J, McGaha L, Smith Y, Faundez V. Hermansky-Pudlak protein complexes, AP-3 and BLOC-1, differentially regulate presynaptic composition in the striatum and hippocampus. J Neurosci. 2010;30(3):820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Evstratova A, Chamberland S, Faundez V, Tóth K. Vesicles derived via AP-3-dependent recycling contribute to asynchronous release and influence information transfer. Nat Commun. 2014;5:5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Novak EK, Hui SW, Swank RT. Platelet storage pool deficiency in mouse pigment mutations associated with seven distinct genetic loci. Blood. 1984;63(3):536–544. [PubMed] [Google Scholar]
- 173.Shotelersuk V, Dell’Angelica EC, Hartnell L, Bonifacino JS, Gahl WA. A new variant of Hermansky-Pudlak syndrome due to mutations in a gene responsible for vesicle formation. Am J Med. 2000;108(5):423–427. [DOI] [PubMed] [Google Scholar]
- 174.Young LR, Pasula R, Gulleman PM, Deutsch GH, McCormack FX. Susceptibility of Hermansky-Pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. Am J Respir Cell Mol Biol. 2007;37(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gochuico BR, Huizing M, Golas GA, Scher CD, Tsokos M, Denver SD, Frei-Jones MJ, Gahl WA. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lyerla TA, Rusiniak ME, Borchers M, Jahreis G, Tan J, Ohtake PJ, Novak EK, Swank RT. Aberrant lung structure, composition, and function in a murine model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L643–653. [DOI] [PubMed] [Google Scholar]
- 177.Kook S, Wang P, Young L, Schwake M, Saftig P, Weng X, Meng Y, Neculai D, Marks MS, Gonzales L, Beers MF, Guttentag S. Impaired Lysosomal Integral Membrane Protein 2-dependent Peroxiredoxin 6 delivery to lamellar bodies accounts for altered alveolar phospholipid content in Adaptor Protein-3-deficient pearl mice. J Biol Chem. 2016;291(16):8414–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kotzot D, Richter K, Gierth-Fiebig K. Oculocutaneous albinism, immunodeficiency, hematological disorders, and minor anomalies: a new autosomal recessive syndrome? Am J Med Genet. 1994;50(3):224–227. [DOI] [PubMed] [Google Scholar]
- 179.Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, Gahl WA. Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatric Res. 2002;51(2):150–158. [DOI] [PubMed] [Google Scholar]
- 180.Chiang P-W, Spector E, Thomas M, Frei-Jones M. Novel mutation causing Hermansky–Pudlak Syndrome Type 2. Pediatric Blood & Cancer. 2010;55(7):1438. [DOI] [PubMed] [Google Scholar]
- 181.Jung J, Bohn G, Allroth A, Boztug K, Brandes G, Sandrock I, Schaffer AA, Rathinam C, Kollner I, Beger C, Schilke R, Welte K, Grimbacher B, Klein C. Identification of a homozygous deletion in the AP3B1 gene causing Hermansky-Pudlak syndrome, type 2. Blood. 2006;108(1):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107(46):19973–19978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-Like Receptor 9 signaling by Adaptor Protein 3. Science. 2010;329:1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, Shen H, Laufer TM, Marks MS. Adaptor Protein-3 in dendritic cells facilitates phagosomal Toll-like receptor signaling and antigen presentation to CD4+ T cells. Immunity. 2012;36(5):782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Prandini A, Salvi V, Colombo F, Moratto D, Lorenzi L, Vermi W, De Francesco MA, Notarangelo LD, Porta F, Plebani A, Facchetti F, Sozzani S, Badolato R. Impairment of dendritic cell functions in patients with adaptor protein-3 complex deficiency. Blood. 2016;127(26):3382–3386. [DOI] [PubMed] [Google Scholar]
- 186.Mantegazza AR, Wynosky-Dolfi MA, Casson CN, Lefkovith AJ, Shin S, Brodsky IE, Marks MS. Increased autophagic sequestration in adaptor protein-3 deficient dendritic cells limits inflammasome activity and impairs antibacterial immunity. PLOS Pathogens. 2017;13(12):e1006785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Briken V, Jackman RM, Dasgupta S, Höning S, Porcelli SA. Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J. 2002;21(4):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sugita M, Cao X, Watts GFM, Rogers RA, Bonifacino JS, Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16(5):697–706. [DOI] [PubMed] [Google Scholar]
- 189.Elewaut D, Lawton AP, Nagarajan NA, Maverakis E, Khurana A, Höning S, Benedict CA, Sercarz E, Bakke O, Kronenberg M, Prigozy TI. The adaptor protein AP-3 Is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Va14i NKT cells. J Exp Med. 2003;198(8):1133–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Benson KF, Li FQ, Person RE, Albani D, Duan Z, Wechsler J, Meade-White K, Williams K, Acland GM, Niemeyer G, Lothrop CD, Horwitz M. Mutations associated with neutropenia in dogs and humans disrupt intracellular transport of neutrophil elastase. Nat Genet. 2003;35(1):90–96. [DOI] [PubMed] [Google Scholar]
- 191.Meng R, Bridgman R, Toivio-Kinnucan M, Niemeyer GP, Vernau W, Hock T, Lothrop CD Jr. Neutrophil elastase processing defect in cyclic hematopoietic dogs. Exp Hematol. 2010;38(2):104–115. [DOI] [PubMed] [Google Scholar]
- 192.Gwynn B, Martina JA, Bonifacino JS, Sviderskaya EV, Lamoreux ML, Bennett DC, Moriyama K, Huizing M, Helip-Wooley A, Gahl WA, Webb LS, Lambert AJ, Peters LL. Reduced pigmentation (rp), a mouse model of Hermansky-Pudlak syndrome, encodes a novel component of the BLOC-1 complex. Blood. 2004;104(10):3181–3189. [DOI] [PubMed] [Google Scholar]
- 193.Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2 and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem. 2004;279(27):28393–28401. [DOI] [PubMed] [Google Scholar]
- 194.Gwynn B, Ciciotte SL, Hunter SJ, Washburn LL, Smith RS, Andersen SG, Swank RT, Dell’Angelica EC, Bonifacino JS, Eicher EM, Peters LL. Defects in the cappuccino (cno) gene on mouse chromosome 5 and human 4p cause Hermansky-Pudlak syndrome by an AP-3-independent mechanism. Blood. 2000;96(13):4227–4235. [PubMed] [Google Scholar]
- 195.Huang L, Kuo YM, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nature Genet. 1999;23(3):329–332. [DOI] [PubMed] [Google Scholar]
- 196.Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3(9):666–677. [DOI] [PubMed] [Google Scholar]
- 197.Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nature Neurosci. 1999;2(2):119–124. [DOI] [PubMed] [Google Scholar]
- 198.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276(26):24232–24241. [DOI] [PubMed] [Google Scholar]
- 199.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15(2):204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell’Angelica EC, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nature Genet. 2003;35(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bryan MM, Tolman NJ, Simon KL, Huizing M, Hufnagel RB, Brooks BP, Speransky V, Mullikin JC, Gahl WA, Malicdan MC, Gochuico BR. Clinical and molecular phenotyping of a child with Hermansky-Pudlak syndrome-7, an uncommon genetic type of HPS. Mol Genet Metab. 2017;120(4):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cullinane AR, Curry JA, Golas G, Pan J, Carmona-Rivera C, Hess RA, White JG, Huizing M, Gahl WA. A BLOC-1 mutation screen reveals a novel BLOC1S3 mutation in Hermansky–Pudlak Syndrome type 8. Pigment Cell Melanoma Res. 2012;25(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lasseaux E, Plaisant C, Michaud V, Pennamen P, Trimouille A, Gaston L, Monferme S, Lacombe D, Rooryck C, Morice-Picard F, Arveiler B. Molecular characterization of a series of 990 index patients with albinism. Pigment Cell Melanoma Res. 2018;31(4):466–474. [DOI] [PubMed] [Google Scholar]
- 204.Lowe GC, Guiu IS, Chapman O, Rivera J, Lordkipanidzé M, Dovlatova N, Wilde J, Watson SP, Morgan NV, collaborative obotUG. Microsatellite markers as a rapid approach for autozygosity mapping in Hermansky-Pudlak syndrome: Identification of the second HPS7 mutation in a patient presenting late in life. Thromb Haemost. 2013;109(4):766–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morgan NV, Pasha S, Johnson CA, Ainsworth JR, Eady RAJ, Dawood B, McKeown C, Trembath RC, Wilde J, Watson SP, Maher ER. A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak Syndrome (HPS8). Am J Hum Genet. 2006;78(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Badolato R, Prandini A, Caracciolo S, Colombo F, Tabellini G, Giacomelli M, Cantarini ME, Pession A, Bell CJ, Dinwiddie DL, Miller NA, Hateley SL, Saunders CJ, Zhang L, Schroth GP, et al. Exome sequencing reveals a pallidin mutation in a Hermansky-Pudlak-like primary immunodeficiency syndrome. Blood. 2012;119(13):3185–3187. [DOI] [PubMed] [Google Scholar]
- 207.Yousaf S, Shahzad M, Kausar T, Sheikh SA, Tariq N, Shabbir AS, Genomics UoWCfM, Ali M, Waryah AM, Shaikh RS, Riazuddin S, Ahmed ZM Identification and clinical characterization of Hermansky-Pudlak syndrome alleles in the Pakistani population. Pigment Cell Melanoma Res. 2016;29(2):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Okamura K, Abe Y, Araki Y, Wakamatsu K, Seishima M, Umetsu T, Kato A, Kawaguchi M, Hayashi M, Hozumi Y, Suzuki T. Characterization of melanosomes and melanin in Japanese patients with Hermansky-Pudlak syndrome types 1, 4, 6, and 9. Pigment Cell Melanoma Res. 2018;31(2):267–276. [DOI] [PubMed] [Google Scholar]
- 209.Lyon MF. Hereditary absence of otoliths in the house mouse. J Physiol. 1951;114(3):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Theriault LL, Hurley LS. Ultrastructure of developing melanosomes in C57 Black and pallid mice. Developmental Biol 1970;23(2):261–275. [DOI] [PubMed] [Google Scholar]
- 211.Swank RT, Sweet HO, Davisson MT, Reddington M, Novak EK. Sandy: a new mouse model for platelet storage pool deficiency. Genet Res. 1991;58(1):51–62. [DOI] [PubMed] [Google Scholar]
- 212.Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays. 2004;26(6):616–628. [DOI] [PubMed] [Google Scholar]
- 213.Gokhale A, Mullin AP, Zlatic SA, Easley CA 4th, Merritt ME, Raj N, Larimore J, Gordon DE, Peden AA, Sanyal S, Faundez V The N-ethylmaleimide-sensitive factor and dysbindin interact to modulate synaptic plasticity. J Neurosci. 2015;35(19):7643–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell. 2015;33(2):176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Setty SRG, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell’Angelica EC, Raposo G, Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18(3):768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Setty SRG, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep. 2014;6(3):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L, Gokhale A, Morel E, Faundez V, Marks MS, Raposo G. BLOC-1 brings together the actin and microtubule cytoskeletons to generate recycling endosomes. Curr Biol. 2016;26(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Délevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, Rux J, Sviderskaya EV, Bennett DC, G. R, Marks MS, et al. BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J Cell Biol. 2015;209(4):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bellono NW, Escobar IE, Lefkovith AJ, Marks MS, Oancea E. An intracellular anion channel critical for pigmentation. eLife. 2014;3:e04543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gokhale A, Vrailas-Mortimer A, Larimore J, Comstra HS, Zlatic SA, Werner E, Manvich DF, Iuvone PM, Weinshenker D, Faundez V. Neuronal copper homeostasis susceptibility by genetic defects in Dysbindin, a schizophrenia susceptibility factor. Hum Mol Genet. 2015;24(19):5512–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Patwardhan A, Bardin S, Miserey-Lenkei S, Larue L, Goud B, Raposo G, Delevoye C. Routing of the RAB6 secretory pathway towards the lysosome related organelle of melanocytes. Nat Commun. 2017;8:15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare J-F, Smith Y, Faundez V. The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell. 2011;22(24):4854–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dennis MK, Delevoye C, Acosta-Ruiz A, Hurbain I, Romao M, Hesketh GG, Goff PS, Sviderskaya EV, Bennett DC, Luzio JP, Galli T, Owen DJ, Raposo G, Marks MS. BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers. J Cell Biol. 2016;214(3):293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee HH, Nemecek D, Schindler C, Smith WJ, Ghirlando R, Steven AC, Bonifacino JS, Hurley JH. Assembly and architecture of Biogenesis of Lysosome-related Organelles Complex-1 (BLOC-1). J Biol Chem. 2012;287(8):5882–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shakya S, Sharma P, Bhatt AM, Jani RA, Delevoye C, Setty SR. Rab22A recruits BLOC-1 and BLOC-2 to promote the biogenesis of recycling endosomes. EMBO Rep. 2018;19(12):e45918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gokhale A, Hartwig C, Freeman AH, Das R, Zlatic SA, Vistein R, Burch A, Carrot G, Lewis AF, Nelms S, Dickman DK, Puthenveedu MA, Cox DN, Faundez V. The proteome of BLOC-1 genetic defects identifies the Arp2/3 actin polymerization complex to function downstream of the schizophrenia susceptibility factor Dysbindin at the synapse. J Neurosci. 2016;36(49):12393–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ryder PV, Vistein R, Gokhale A, Seaman MN, Puthenveedu M, Faundez V. The WASH complex, and endosomal Arp2/3 activator, interacts with the Hermansky-Pudlak syndrome complex BLOC-1 and its cargo phosphatidylinositol-4 kinase type II alpha. Mol Biol Cell. 2013;24(14):2269–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181(5):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O’Neill FA, Walsh D, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ghiani CA, Dell’Angelica EC. Dysbindin-containing complexes and their proposed functions in brain: from zero to (too) many in a decade. ASN Neuro. 2011;3(2):e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Morris DW, Murphy K, Kenny N, Purcell SM, McGhee KA, Schwaiger S, Nangle JM, Donohoe G, Clarke S, Scully P, Quinn J, Meagher D, Baldwin P, Crumlish N, O’Callaghan E, et al. Dysbindin (DTNBP1) and the biogenesis of lysosome-related organelles complex 1 (BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. Biol Psychiatry. 2008;63(1):24–31. [DOI] [PubMed] [Google Scholar]
- 233.Vites O, Rhee JS, Schwarz M, Rosenmund C, Jahn R. Reinvestigation of the role of snapin in neurotransmitter release. J Biol Chem. 2004;279(25):26251–26256. [DOI] [PubMed] [Google Scholar]
- 234.Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS. BORC functions upstream of Kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 2016;17(8):1950–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Filipek PA, de Araujo MEG, Vogel GF, De Smet CH, Eberharter D, Rebsamen M, Rudashevskaya EL, Kremser L, Yordanov T, Tschaikner P, Fürnrohr BG, Lechner S, Dunzendorfer-Matt T, Scheffzek K, Bennett LL, et al. LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning. J Cell Biol. 2017;216(12):4199–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pu J, Keren-Kaplan T, Bonifacino JS. A Ragulator–BORC interaction controls lysosome positioning in response to amino acid availability. J Cell Biol. 2017;216(2):4183–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jia R, Guardia CM, Pu J, Chen Y, Bonifacino JS. BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy. 2017;13(10):1648–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Farías GG, Guardia CM, De Pace R, Britt DJ, Bonifacino JS. BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc Natl Acad Sci USA. 2017;114(14):E2955–E2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Niwa S, Tao L, Lu SY, Liew GM, Feng W, Nachury MV, Shen K. BORC regulates the axonal transport of synaptic vesicle precursors by activating ARL-8. Curr Biol. 2017;27(17):2569–2578 e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Snouwaert JN, Church RJ, Jania L, Nguyen M, Wheeler ML, Saintsing A, Mieczkowski P, Manuel de Villena FP, Armao D, Moy SS, Lorenzo DN, Koller BH. A mutation in the Borcs7 subunit of the lysosome regulatory BORC complex results in motor deficits and dystrophic axonopathy in mice. Cell Rep. 2018;24(5):1254–1265. [DOI] [PubMed] [Google Scholar]
- 241.Yang Q, He X, Yang L, Zhou Z, Cullinane AR, Wei A, Zhang Z, Hao Z, Zhang A, He M, Feng Y, Gao X, Gahl WA, Huizing M, Li W. The BLOS1-interacting protein KXD1 is involved in the biogenesis of lysosome-related organelles. Traffic. 2012;13(8):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tian JH, Wu ZX, Unzicker M, Lu L, Cai Q, Li C, Schirra C, Matti U, Stevens D, Deng C, Rettig J, Sheng ZH. The role of Snapin in neurosecretion: snapin knock-out mice exhibit impaired calcium-dependent exocytosis of large dense-core vesicles in chromaffin cells. J Neurosci. 2005;25(45):10546–10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhou B, Cai Q, Xie Y, Sheng ZH. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Rep. 2012;2(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron. 2010;68(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J Cell Biol. 2015;209(3):377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Di Giovanni J, Sheng ZH. Regulation of synaptic activity by snapin-mediated endolysosomal transport and sorting. EMBO J. 2015;34(15):2059–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang A, He X, Zhang L, Yang L, Woodman P, Li W. Biogenesis of lysosome-related organelles complex-1 subunit 1 (BLOS1) interacts with sorting nexin 2 and the endosomal sorting complex required for transport-I (ESCRT-I) component TSG101 to mediate the sorting of epidermal growth factor receptor into endosomal compartments. J Biol Chem. 2014;289(42):29180–29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou W, He Q, Zhang C, He X, Cui Z, Liu F, Li W. BLOS2 negatively regulates Notch signaling during neural and hematopoietic stem and progenitor cell development. Elife. 2016;5:e18108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.John Peter AT, Lachmann J, Rana M, Bunge M, Cabrera M, Ungermann C. The BLOC-1 complex promotes endosomal maturation by recruiting the Rab5 GTPase-activating protein Msb3. J Cell Biol. 2013;201(1):97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hayes MJ, Bryon K, Satkurunathan J, Levine TP. Yeast homologues of three BLOC-1 subunits highlight KxDL proteins as conserved interactors of BLOC-1. Traffic. 2011;12(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Anikster Y, Huizing M, White J, Shevchenko YO, Fitzpatrick DL, Touchman JW, Compton JG, Bale SJ, Swank RT, Gahl WA, Toro JR. Mutation of a new gene causes a unique form of Hermansky–Pudlak syndrome in a genetic isolate of central Puerto Rico. Nature Genet. 2001;28(4):376–380. [DOI] [PubMed] [Google Scholar]
- 252.Gautam R, Chintala S, Li W, Zhang Q, Tan J, Novak EK, Di Pietro SM, Dell’Angelica EC, Swank RT. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2). J Biol Chem. 2004;279(13):12935–12942. [DOI] [PubMed] [Google Scholar]
- 253.Suzuki T, Li W, Zhang Q, Novak EK, Sviderskaya EV, Wilson A, Bennett DC, Roe BA, Swank RT, Spritz RA. The gene mutated in cocoa mice, carrying a defect of organelle biogenesis, is a homologue of the human hermansky-pudlak syndrome-3 gene. Genomics. 2001;78(1/2):30–37. [DOI] [PubMed] [Google Scholar]
- 254.Di Pietro SM, Falcon-Perez JM, Dell’Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic. 2004;5(4):276–283. [DOI] [PubMed] [Google Scholar]
- 255.Zhang Q, Zhao B, Li W, Oiso N, Novak EK, Rusiniak ME, Gautam R, Chintala S, O’Brien EP, Zhang Y, Roe BA, Elliott RW, Eicher EM, Liang P, Kratz C, et al. Ru2 and Ru encode mouse orthologs of the genes mutated in human Hermansky-Pudlak syndrome types 5 and 6. Nature Genet. 2003;33(2):145–153. [DOI] [PubMed] [Google Scholar]
- 256.Michaud V, Lasseaux E, Plaisant C, Verloes A, Perdomo-Trujillo Y, Hamel C, Elcioglu NH, Leroy B, Kaplan J, Jouk PS, Lacombe D, Fergelot P, Morice-Picard F, Arveiler B. Clinico-molecular analysis of eleven patients with Hermansky-Pudlak type 5 syndrome, a mild form of HPS. Pigment Cell Melanoma Res. 2017;30(6):563–570. [DOI] [PubMed] [Google Scholar]
- 257.Syrzycka M, McEachern LA, Kinneard J, Prabhu K, Fitzpatrick K, Schulze S, Rawls JM, Lloyd VK, Sinclair DA, Honda BM. The pink gene encodes the Drosophila orthologue of the human Hermansky-Pudlak syndrome 5 (HPS5) gene. Genome. 2007;50(6):548–556. [DOI] [PubMed] [Google Scholar]
- 258.Sharda A, Kim SH, Jasuja R, Gopal S, Flaumenhaft R, Furie BC, Furie B. Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125(10):1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ma J, Zhang Z, Yang L, Kriston-Vizi J, Cutler DF, Li W. BLOC-2 subunit HPS6 deficiency affects the tubulation and secretion of von Willebrand factor from mouse endothelial cells. J Genet Genomics. 2016;43(12):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Michaux G, Abbitt KB, Collinson LM, Haberichter SL, Norman KE, Cutler DF. The physiological function of von Willebrand’s factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev Cell. 2006;10(2):223–232. [DOI] [PubMed] [Google Scholar]
- 261.Boissy RE, Richmond B, Huizing M, Helip-Wooley A, Zhao Y, Koshoffer A, Gahl WA. Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3. Am J Pathol. 2005;166(1):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J Invest Dermatol. 2007;127(6):1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Richmond B, Huizing M, Knapp J, Koshoffer A, Zhao Y, Gahl WA, Boissy RE. Melanocytes derived from patients with HermanskyPudlak Syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J Invest Dermatol. 2005;124(2):420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. [DOI] [PubMed] [Google Scholar]
- 265.Li K, Yang L, Zhang C, Niu Y, Li W, Liu JJ. HPS6 interacts with dynactin p150Glued to mediate retrograde trafficking and maturation of lysosomes. J Cell Sci. 2014;127(21):4574–4588. [DOI] [PubMed] [Google Scholar]
- 266.Martina JA, Moriyama K, Bonifacino JS. BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J Biol Chem. 2003;278(31):29376–29384. [DOI] [PubMed] [Google Scholar]
- 267.Nazarian R, Falcón-Pérez JM, Dell’Angelica EC. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): A complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. Proc Natl Acad Sci USA. 2003;100(15):8770–8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kinch LN, Grishin NV. Longin-like folds identified in CHiPS and DUF254 proteins: vesicle trafficking complexes conserved in eukaryotic evolution. Protein Sci. 2006;15(11):2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cheli VT, Dell’angelica EC. Early origin of genes encoding subunits of biogenesis of lysosome-related organelles complex-1, −2 and −3. Traffic. 2010;11(5):579–586. [DOI] [PubMed] [Google Scholar]
- 270.Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22(22):2135–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 function in concert with Rab38 and Rab32 to mediate protein trafficking to lysosome-related organelles. J Biol Chem. 2012;287(23):19550–19563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell. 2007;18(10):3914–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ninkovic I, White JG, Rangel-Filho A, Datta YH. The role of Rab38 in platelet dense granule defects. J Thromb Haemost. 2008;6(12):2143–2151. [DOI] [PubMed] [Google Scholar]
- 275.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120(19):4072–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang C, Liu Z, Huang X. Rab32 is important for autophagy and lipid storage in Drosophila. PLoS One. 2012;7(2):e32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol. 2005;16(4):852–856. [DOI] [PubMed] [Google Scholar]
- 279.Morris C, Foster OK, Handa S, Peloza K, Voss L, Somhegyi H, Jian Y, Vo MV, Harp M, Rambo FM, Yang C, Hermann GJ. Function and regulation of the C. elegans Rab32 family member GLO-1 in lysosome-related organelle biogenesis. PLoS Genet. 2018;14(11):e1007772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lane PW, Green EL. Pale ear and light ear in the house mouse: mimic mutations in linkage groups XII and XVII. J Hered. 1967;58(1):17–20. [DOI] [PubMed] [Google Scholar]
- 281.Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J Invest Dermatol. 2002;119(5):1156–1164. [DOI] [PubMed] [Google Scholar]
- 282.Suzuki T, Li W, Zhang Q, Karim A, Novak EK, Sviderskaya EV, Hill SP, Bennett DC, Levin AV, Nieuwenhuis H,K, Fong C-T, Castellan C, Miterski B, Swank RT, Spritz RA Hermansky-Pudlak syndrome is caused by mutations in HPS4, the human homolog of the mouse light-ear gene. Nature Genet. 2002;30(3):321–324. [DOI] [PubMed] [Google Scholar]
- 283.Gardner JM, Wildenberg SC, Keiper NM, Novak EK, Rusiniak ME, Swank RT, Puri N, Finger JN, Hagiwara N, Lehman AL, Gales TL, Bayer ME, King RA, Brilliant MH. The mouse pale ear (ep) mutation is the homologue of human Hermansky-Pudlak syndrome. Proc Natl Acad Sci USA. 1997;94(17):9238–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J Invest Dermatol. 2007;127(2):421–428. [DOI] [PubMed] [Google Scholar]
- 285.Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J Biol Chem. 2011;286(9):7507–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell. 2009;20(12):2900–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pryor PR, Mullock BM, Bright NA, Lindsay MR, Gray SR, Richardson SC, Stewart A, James DE, Piper RC, Luzio JP. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5(6):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pols MS, van Meel E, Oorschot V, Ten Brink C, Fukuda M, Swetha MG, Mayor S, Klumperman J. hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat Commun. 2013;4:1361. [DOI] [PubMed] [Google Scholar]
- 289.Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793(12):1901–1916. [DOI] [PubMed] [Google Scholar]
- 290.Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146(2):303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bultema JJ, Boyle JA, Malenke PB, Martin FE, Dell’Angelica EC, Cheney RE, Di Pietro SM. Myosin Vc interacts with rab32 and rab38 proteins and works in the biogenesis and secretion of melanosomes. J Biol Chem. 2014;289(48):33513–33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ripoll L, Heiligenstein X, Hurbain I, Domingues L, Figon F, Petersen KJ, Dennis MK, Houdusse A, Marks MS, Raposo G, Delevoye C. Myosin VI and branched actin filaments mediate membrane constriction and fission of melanosomal tubule carriers. J Cell Biol. 2018;217(8):2709–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhou Y, He CH, Herzog EL, Peng X, Lee C-M, Nguyen TH, Gulati M, R. B, Gahl WA, L. M, Lee CG, Elias JA Chitinase 3–like–1 and its receptors in Hermansky-Pudlak syndrome–associated lung disease. J Clin Invest. 2015;125(8):3178–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhou Y, He CH, Yang DS, Nguyen T, Cao Y, Kamle S, Lee CM, Gochuico BR, Gahl WA, Shea BS, Lee CG, Elias JA. Galectin-3 interacts with the CHI3L1 axis and contributes to Hermansky-Pudlak Syndrome lung disease. J Immunol. 2018;200(6):2140–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gurkan C, Lapp H, Alory C, Su AI, Hogenesch J, Balch WE. Large-scale profiling of rab GTPase trafficking networks: the Membrome. Mol Biol Cell. 2005;16(8):3847–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Seto S, Tsujimura K, Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic. 2011;12(4):407–420. [DOI] [PubMed] [Google Scholar]
- 297.Spano S, Galan JE. A Rab32-dependent pathway contributes to Salmonella typhi host restriction. Science. 2012;338(6109):960–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hoffmann C, Finsel I, Otto A, Pfaffinger G, Rothmeier E, Hecker M, Becher D, Hilbi H. Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol. 2014;16(7):1034–1052. [DOI] [PubMed] [Google Scholar]
- 299.Spano S, Gao X, Hannemann S, Lara-Tejero M, Galan JE. A bacterial pathogen targets a host Rab-family GTPase defense pathway with a GAP. Cell Host Microbe. 2016;19(2):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Karki R, Man SM, Malireddi RK, Kesavardhana S, Zhu Q, Burton A, Sharma BR, Qi X, Pelletier S, Vogel P, Rosenstiel P, Kanneganti TD. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature. 2016;540:583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Romberg N, Al M,K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, West B, Podoltsev NA, Boggon TJ, Kazmierczak BI, Lifton RP Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46(10):1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Xie X, Ni Q, Zhou D, Wan Y. Rab32-related antimicrobial pathway is involved in the progression of dextran sodium sulfate-induced colitis. FEBS Open Bio. 2018;8(10):1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Peterson MR, Emr SD. The class C Vps complex functions at multiple stages of the vacuolar transport pathway. Traffic. 2001;2(7):476–486. [DOI] [PubMed] [Google Scholar]
- 304.Srivastava A, Woolford CA, Jones EW. Pep3p/Pep5p complex: a putative docking factor at multiple steps of vesicular transport to the vacuole of Saccharomyces cerevisiae. Genetics. 2000;156(1):105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12(5):739–750. [DOI] [PubMed] [Google Scholar]
- 306.Akbar MA, Ray S, Krämer H. The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol Biol Cell. 2009;20(6):1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25(8):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97(17):9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. The Journal of cell biology. 2000;151(3):551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pevsner J, Hsu SC, Hyde PS, Scheller RH. Mammalian homologues of yeast vacuolar protein sorting (vps) genes implicated in Golgi-to-lysosome trafficking. Gene. 1996;183(1-2):7–14. [DOI] [PubMed] [Google Scholar]
- 311.Gissen P, Johnson CA, Gentle D, Hurst LD, Doherty AJ, O’Kane CJ, Kelly DA, Maher ER. Comparative evolutionary analysis of VPS33 homologues: genetic and functional insights. Hum Mol Genet. 2005;14(10):1261–1270. [DOI] [PubMed] [Google Scholar]
- 312.Marwaha R, Arya SB, Jagga D, Kaur H, Tuli A, Sharma M. The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes. J Cell Biol. 2017;216(4):1051–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Amaya C, Militello RD, Calligaris SD, Colombo MI. Rab24 interacts with the Rab7/Rab interacting lysosomal protein complex to regulate endosomal degradation. 2016;17(11):1181–1196. [DOI] [PubMed] [Google Scholar]
- 314.Khatter D, Raina VB, Dwivedi D, Sindhwani A, Bahl S, Sharma M. The small GTPase Arl8b regulates assembly of the mammalian HOPS complex on lysosomes. 2015;128(9):1746–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8(11):4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Banta LM, Robinson JS, Klionsky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107(4):1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Banta LM, Vida TA, Herman PK, Emr SD. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol Cell Biol. 1990;10(9):4638–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lorincz P, Lakatos Z, Varga A, Maruzs T, Simon-Vecsei Z, Darula Z, Benko P, Csordas G, Lippai M, Ando I, Hegedus K, Medzihradszky KF, Takats S, Juhasz G. MiniCORVET is a Vps8-containing early endosomal tether in Drosophila. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development (Cambridge, England). 2005;132(15):3561–3572. [DOI] [PubMed] [Google Scholar]
- 320.Yu JF, Fukamachi S, Mitani H, Hori H, Kanamori A. Reduced expression of vps11 causes less pigmentation in medaka, Oryzias latipes. Pigment Cell Res. 2006;19(6):628–634. [DOI] [PubMed] [Google Scholar]
- 321.Brocker C, Kuhlee A, Gatsogiannis C, Balderhaar HJ, Honscher C, Engelbrecht-Vandre S, Ungermann C, Raunser S. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci U S A. 2012;109(6):1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ho R, Stroupe C. The HOPS/class C Vps complex tethers membranes by binding to one Rab GTPase in each apposed membrane. Mol Biol Cell. 2015;26(14):2655–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ostrowicz CW, Brocker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandre S, Ungermann C. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11(10):1334–1346. [DOI] [PubMed] [Google Scholar]
- 324.Wartosch L, Günesdogan U, Graham SC, Luzio JP. Recruitment of VPS33A to HOPS by VPS16 is required for lysosome fusion with endosomes and autophagosomes. Traffic. 2015;16(7):727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chirivino D, Del Maestro L, Formstecher E, Hupe P, Raposo G, Louvard D, Arpin M. The ERM proteins interact with the HOPS complex to regulate the maturation of endosomes. Mol Biol Cell. 2011;22(3):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang J, Lachance V, Schaffner A, Li X, Fedick A, Kaye LE, Liao J, Rosenfeld J, Yachelevich N, Chu ML, Mitchell WG, Boles RG, Moran E, Tokita M, Gorman E, et al. A founder mutation in VPS11 causes an autosomal recessive leukoencephalopathy linked to autophagic defects. PLoS Genet. 2016;12(4):e1005848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Graham SC, Wartosch L, Gray SR, Scourfield EJ, Deane JE, Luzio JP, Owen DJ. Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc Natl Acad Sci USA. 2013;110(33):13345–13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lobingier BT, Merz AJ. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol Biol Cell. 2012;23(23):4611–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nature structural & molecular biology. 2010;17(6):710–717. [DOI] [PubMed] [Google Scholar]
- 330.Baker RW, Jeffrey PD, Zick M, Phillips BP, Wickner WT, Hughson FM. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science. 2015;349(6252):1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Baker RW, Jeffrey PD, Hughson FM. Crystal structures of the Sec1/Munc18 (SM) protein Vps33, alone and bound to the homotypic fusion and vacuolar protein sorting (HOPS) subunit Vps16. PLoS One. 2013;8(6):e67409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25(8):1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19(6):2500–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lobingier BT, Nickerson DP, Lo SY, Merz AJ. SM proteins Sly1 and Vps33 co-assemble with Sec17 and SNARE complexes to oppose SNARE disassembly by Sec18. Elife. 2014;3:e02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kramer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011;22(14):2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lurick A, Kuhlee A, Brocker C, Kummel D, Raunser S, Ungermann C. The Habc domain of the SNARE Vam3 interacts with the HOPS tethering complex to facilitate vacuole fusion. J Biol Chem. 2015;290(9):5405–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Huizing M, Didier A, Walenta J, Anikster Y, Gahl WA, Kramer H. Molecular cloning and characterization of human VPS18, VPS 11, VPS16, and VPS33. Gene. 2001;264(2):241–247. [DOI] [PubMed] [Google Scholar]
- 338.Takats S, Pircs K, Nagy P, Varga A, Karpati M, Hegedus K, Kramer H, Kovacs AL, Sass M, Juhasz G. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell. 2014;25(8):1338–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Takats S, Nagy P, Varga A, Pircs K, Karpati M, Varga K, Kovacs AL, Hegedus K, Juhasz G. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J Cell Biol. 2013;201(4):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Dickie MM. Buff. Mouse News Lett. 1964;30:30. [Google Scholar]
- 341.Nguyen T, Wei ML. Characterization of Melanosomes in Murine Hermansky–Pudlak Syndrome: Mechanisms of Hypopigmentation. Journal of Investigative Dermatology. 2004;122(2):452–460. [DOI] [PubMed] [Google Scholar]
- 342.Samaraweera P, Donatien PD, Qazi S, Kobayashi T, Hearing VJ, Panthier JJ, Orlow SJ. Identification and characterization of a melanocyte-specific novel 65-kDa peripheral membrane protein. Eur J Biochem. 1999;266(3):924–934. [DOI] [PubMed] [Google Scholar]
- 343.Anderson MG, Hawes NL, Trantow CM, Chang B, John SWM. Iris phenotypes and pigment dispersion caused by genes influencing pigmentation. Pigment Cell Melanoma Res. 2008;21(5):565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Guo X, Tu L, Gumper I, Plesken H, Novak EK, Chintala S, Swank RT, Pastores G, Torres P, Izumi T, Sun TT, Sabatini DD, Kreibich G. Involvement of vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic. 2009;10(9):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhen Y, Li W. Impairment of autophagosome-lysosome fusion in the buff mutant mice with the VPS33A(D251E) mutation. Autophagy. 2015;11(9):1608–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Dursun A, Yalnizoglu D, Gerdan OF, Yucel-Yilmaz D, Sagiroglu MS, Yuksel B, Gucer S, Sivri S, Ozgul RK. A probable new syndrome with the storage disease phenotype caused by the VPS33A gene mutation. 2017;26(1):1–12. [DOI] [PubMed] [Google Scholar]
- 347.Kondo H, Maksimova N, Otomo T, Kato H, Imai A, Asano Y, Kobayashi K, Nojima S, Nakaya A, Hamada Y, Irahara K, Gurinova E, Sukhomyasova A, Nogovicina A, Savvina M, et al. Mutation in VPS33A affects metabolism of glycosaminoglycans: a new type of mucopolysaccharidosis with severe systemic symptoms. Hum Mol Genet. 2017;26(1):173–183. [DOI] [PubMed] [Google Scholar]
- 348.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi Syndrome. Mol Genet Metabolism. 1999;68(2):283–303. [DOI] [PubMed] [Google Scholar]
- 349.Introne WJ, Westbroek W, Groden CA, Bhambhani V, Golas GA, Baker EH, Lehky TJ, Snow J, Ziegler SG, Malicdan MC, Adams DR, Dorward HM, Hess RA, Huizing M, Gahl WA, et al. Neurologic involvement in patients with atypical Chediak-Higashi disease. Neurology. 2017;88(7):e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Westphal A, Cheng W, Yu J, Grassl G, Krautkrämer M, Holst O, Föger N, Lee KH. Lysosomal trafficking regulator Lyst links membrane trafficking to toll-like receptor-mediated inflammatory responses. J Exp Med. 2017;214(1):227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Brandt EJ, Elliott RW, Swank RT. Defective lysosomal enzyme secretion in kidneys of Chediak-Higashi (beige) mice. J Cell Biol. 1975;67(3):774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Chiang SCC, Wood SM, Tesi B, Akar HH, Al-Herz W, Ammann S, Belen FB, Caliskan U, Kaya Z, Lehmberg K, Patiroglu T, Tokgoz H, Ünüvar A, Introne WJ, Henter JI, et al. Differences in granule morphology yet equally impaired exocytosis among cytotoxic T cells and NK cells from Chediak-Higashi syndrome patients. Front Immunol. 2017;8:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ Jr., Perou CM, Boissy RE, Duyk GM, Spritz RA, Moore KJ Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14(3):307–311. [DOI] [PubMed] [Google Scholar]
- 354.Ward DM, Shiflett SL, Huynh D, Vaughn MB, Prestwich G, Kaplan J. Use of expression constructs to dissect the functional domains of the CHS/beige protein: identification of multiple phenotypes. Traffic. 2003;4(6):403–415. [DOI] [PubMed] [Google Scholar]
- 355.Cullinane AR, Schäffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14(7):749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, Lovett M, Kingsmore SF. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Perou CM, Moore KJ, Nagle DL, Misumi DJ, Woolf EA, McGrail SH, Holmgren L, Brody TH, Dussault BJ Jr., Monroe CA, Duyk GM, Pryor RJ, Li L, Justice MJ, Kaplan J Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet. 1996;13(3):303–308. [DOI] [PubMed] [Google Scholar]
- 358.Burgess A, Mornon JP, de Saint Basile G, Callebaut I. A concanavalin A-like lectin domain in the CHS1/LYST protein, shared by members of the BEACH family. Bioinformatics. 2009;25(10):1219–1222. [DOI] [PubMed] [Google Scholar]
- 359.Durchfort N, Verhoef S, Vaughn MB, Shrestha R, Adam D, Kaplan J, Ward DM. The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion. Traffic. 2012;13(1):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Holland P, Torgersen ML, Sandvig K, Simonsen A. LYST affects lysosome size and quantity, but not trafficking or degradation through autophagy or endocytosis. Traffic. 2014;15(12):1390–1405. [DOI] [PubMed] [Google Scholar]
- 361.Sepulveda FE, Burgess A, Heiligenstein X, Goudin N, Ménager MM, Romao M, Côte M, Mahlaoui N, Fischer A, Raposo G, Ménasché G, de Saint Basile G. LYST controls the biogenesis of the endosomal compartment required for secretory lysosome function. Traffic. 2015;16(2):191–203. [DOI] [PubMed] [Google Scholar]
- 362.Gil-Krzewska A, Wood SM, Murakami Y, Nguyen V, Chiang SCC, Cullinane AR, Peruzzi G, Gahl WA, Coligan JE, Introne WJ, Bryceson YT, Krzewski K. Chediak-Higashi syndrome: Lysosomal trafficking regulator domains regulate exocytosis of lytic granules but not cytokine secretion by natural killer cells. J Allergy Clin Immunol. 2016;137(4):1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Gil-Krzewska A, Saeed MB, Oszmiana A, Fischer ER, Lagrue K, Gahl WA, Introne WJ, Coligan JE, Davis DM, Krzewski K. An actin cytoskeletal barrier inhibits lytic granule release from natural killer cells in patients with Chediak-Higashi syndrome. J Allergy Clin Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Rahman M, Haberman A, Tracy C, Ray S, Krämer H. Drosophila mauve mutants reveal a role of LYST homologs late in the maturation of phagosomes and autophagosomes. Traffic. 2012;13(12):1680–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Nezelof C, Dupart MC, Jaubert F, Eliachar E. A lethal familial syndrome associating arthrogryposis multiplex congenita, renal dysfunction, and a cholestatic and pigmentary liver disease. J Pediatr. 1979;94(2):258–260. [DOI] [PubMed] [Google Scholar]
- 366.Smith H, Galmes R, Gogolina E, Straatman-Iwanowska A, Reay K, Banushi B, Bruce CK, Cullinane AR, Romero R, Chang R, Ackermann O, Baumann C, Cangul H, Cakmak C,F, Aygun C, et al. Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome. Hum Mutat. 2012;33(12):1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M, Fischler B, FitzPatrick D, Garcia-Cazorla A, Guyot D, Jacquemont S, Koletzko S, et al. Clinical and molecular genetic features of ARC syndrome. Hum Genet. 2006;120(3):396–409. [DOI] [PubMed] [Google Scholar]
- 368.Jang JY, Kim KM, Kim GH, Yu E, Lee JJ, Park YS, Yoo HW. Clinical characteristics and VPS33B mutations in patients with ARC syndrome. Journal of pediatric gastroenterology and nutrition. 2009;48(3):348–354. [DOI] [PubMed] [Google Scholar]
- 369.Bem D, Smith H, Banushi B, Burden JJ, White IJ, Hanley J, Jeremiah N, Rieux-Laucat F, Bettels R, Ariceta G, Mumford AD, Thomas SG, Watson SP, Gissen P. VPS33B regulates protein sorting into and maturation of alpha-granule progenitor organelles in mouse megakaryocytes. Blood. 2015;126(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, McClean P, Lynch SA, Thompson RJ, Lo B, Quarrell OW, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;363(4):400–404. [DOI] [PubMed] [Google Scholar]
- 371.Lo B, Li L, Gissen P, Christensen H, McKiernan PJ, Ye C, Abdelhaleem M, Hayes JA, Williams MD, Chitayat D, Kahr WH. Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106(13):4159–4166. [DOI] [PubMed] [Google Scholar]
- 372.Matthews RP, Plumb-Rudewiez N, Lorent K, Gissen P, Johnson CA, Lemaigre F, Pack M. Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6. Development (Cambridge, England). 2005;132(23):5295–5306. [DOI] [PubMed] [Google Scholar]
- 373.Hershkovitz D, Mandel H, Ishida-Yamamoto A, Chefetz I, Hino B, Luder A, Indelman M, Bergman R, Sprecher E. Defective lamellar granule secretion in arthrogryposis, renal dysfunction, and cholestasis syndrome caused by a mutation in VPS33B. Arch Dermatol. 2008;144(3):334–340. [DOI] [PubMed] [Google Scholar]
- 374.Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gürakan F, Utine E, Ozkan TB, Denecke J, Vukovic J, Di R,M, Mandel H, Cangul H, et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42(4):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cullinane AR, Straatman-Iwanowska A, Seo JK, Ko JS, Song KS, Gizewska M, Gruszfeld D, Gliwicz D, Tuysuz B, Erdemir G, Sougrat R, Wakabayashi Y, Hinds R, Barnicoat A, Mandel H, et al. Molecular investigations to improve diagnostic accuracy in patients with ARC syndrome. Hum Mutat. 2009;30(2):E330–E337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Akbar MA, Tracy C, Kahr WHA, Krämer H. The full-of-bacteria gene is required for phagosome maturation during immune defense in Drosophila. J Cell Biol. 2011;192(3):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Carim L, Sumoy L, Andreu N, Estivill X, Escarceller M. Cloning, mapping and expression analysis of VPS33B, the human orthologue of rat Vps33b. Cytogenet Cell Genet. 2000;89(1-2):92–95. [DOI] [PubMed] [Google Scholar]
- 378.Galmes R, ten Brink C, Oorschot V, Veenendaal T, Jonker C, van der Sluijs P, Klumperman J. Vps33B is required for delivery of endocytosed cargo to lysosomes. Traffic. 2015;16(12):1288–1305. [DOI] [PubMed] [Google Scholar]
- 379.Hunter MR, Hesketh GG, Benedyk TH, Gingras AC, Graham SC. Proteomic and biochemical comparison of the cellular interaction partners of human VPS33A and VPS33B. J Mol Biol. 2018;430(14):2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Spang A Membrane tethering complexes in the endosomal system. Front Cell Dev Biol. 2016;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Zhu GD, L’Hernault SW. The Caenorhabditis elegans spe-39 gene is required for intracellular membrane reorganization during spermatogenesis. Genetics. 2003;165(1):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gengyo-Ando K, Kage-Nakadai E, Yoshina S, Otori M, Kagawa-Nagamura Y, Nakai J, Mitani S. Distinct roles of the two VPS33 proteins in the endolysosomal system in Caenorhabditis elegans. Traffic. 2016;17(11):1197–1213. [DOI] [PubMed] [Google Scholar]
- 383.Akbar MA, Mandraju R, Tracy C, Hu W, Pasare C, Kramer H. ARC syndrome-linked Vps33B protein is required for inflammatory endosomal maturation and signal termination. Immunity. 2016;45(2):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hanley J, Dhar DK, Mazzacuva F, Fiadeiro R, Burden JJ, Lyne AM, Smith H, Straatman-Iwanowska A, Banushi B, Virasami A, Mills K, Lemaigre FP, Knisely AS, Howe S, Sebire N, et al. Vps33b is crucial for structural and functional hepatocyte polarity. J Hepatol. 2017;66(5):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rogerson C, Gissen P. VPS33B and VIPAR are essential for epidermal lamellar body biogenesis and function. Biochim Biophys Acta. 2018;1864(5 Pt A):1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Urban D, Li L, Christensen H, Pluthero FG, Chen SZ, Puhacz M, Garg PM, Lanka KK, Cummings JJ, Kramer H, Wasmuth JD, Parkinson J, Kahr WH. The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet α-granule biogenesis. Blood. 2012;120(25):5032–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Phillips-Krawczak CA, Singla A, Starokadomskyy P, Deng Z, Osborne DG, Li H, Dick CJ, Gomez TS, Koenecke M, Zhang JS, Dai H, Sifuentes-Dominguez LF, Geng LN, Kaufmann SH, Hein MY, et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol Biol Cell. 2015;26(1):91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Chen CH, Lo RW, Urban D, Pluthero FG, Kahr WH. Alpha-granule biogenesis: from disease to discovery. Platelets. 2017;28(2):147–154. [DOI] [PubMed] [Google Scholar]
- 389.Bottega R, Pecci A, De Candia E, Pujol-Moix N, Heller PG, Noris P, De Rocco D, Podda GM, Glembotsky AC, Cattaneo M, Balduini CL, Savoia A. Correlation between platelet phenotype and NBEAL2 genotype in patients with congenital thrombocytopenia and α-granule deficiency. Haematologica. 2013;98(6):868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi MC, Bertone P, Jordan G, Kettleborough RN, Kiddle G, Kostadima M, Read RJ, Sipos B, Sivapalaratnam S, Smethurst PA, Stephens J, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43(8):735–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, Zivony-Elboum Y, Gumruk F, Cetin M, Khayat M, Boerkoel CF, Kfir N, Huang Y, Maynard D, Dorward H, Berger K, Kleta R, Anikster Y, et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet α-granules. Nat Genet. 2011;43(8):732–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kahr WH, Hinckley J, Li L, Schwertz H, Christensen H, Rowley JW, Pluthero FG, Urban D, Fabbro S, Nixon B, Gadzinski R, Storck M, Wang K, Ryu GY, Jobe SM, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43(8):738–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Deppermann C, Cherpokova D, Nurden P, Schulz JN, Thielmann I, Kraft P, Vögtle T, Kleinschnitz C, Dütting S, Krohne G, Eming SA, Nurden AT, B. E, Stoll G, Stegner D, et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J Clin Invest. 2013;123(8):3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Deppermann C, Nurden P, Nurden AT, Nieswandt B, Stegner D. the Nbeal2(−/−) mouse as a model for the gray platelet syndrome. Rare Dis. 2013;1:e26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kahr WH, Lo RW, Li L, Pluthero FG, Christensen H, Ni R, Vaezzadeh N, Hawkins CE, Weyrich AS, Di Paola J, Landolt-Marticorena C, Gross PL. Abnormal megakaryocyte development and platelet function in Nbeal2(−/−) mice. Blood. 2013;122(19):3349–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Mayer L, Jasztal M, Pardo M, Aguera de Haro S, Collins J, Bariana TK, Smethurst PA, Grassi L, Petersen R, Nurden P, Favier R, Yu L, Meacham S, Astle WJ, Choudhary J, et al. Nbeal2 interacts with Dock7, Sec16a, and Vac14. Blood. 2018;131(9):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91(7):2313–2325. [PubMed] [Google Scholar]
- 398.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci U S A. 2014;111(40):14430–14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hwaiz R, Rahman M, Syk I, Zhang E, Thorlacius H. Rac1-dependent secretion of platelet-derived CCL5 regulates neutrophil recruitment via activation of alveolar macrophages in septic lung injury. J Leukoc Biol. 2015;97(5):975–984. [DOI] [PubMed] [Google Scholar]
- 400.Sakurai Y, Fitch-Tewfik JL, Qiu Y, Ahn B, Myers DR, Tran R, Fay ME, Ding L, Spearman PW, Michelson AD, Flaumenhaft R, Lam WA. Platelet geometry sensing spatially regulates alpha-granule secretion to enable matrix self-deposition. Blood. 2015;126(4):531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Hwaiz R, Rahman M, Zhang E, Thorlacius H. Platelet secretion of CXCL4 is Rac1-dependent and regulates neutrophil infiltration and tissue damage in septic lung damage. Br J Pharmacol. 2015;172(22):5347–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Dutting S, Heidenreich J, Cherpokova D, Amin E, Zhang SC, Ahmadian MR, Brakebusch C, Nieswandt B Critical off-target effects of the widely used Rac1 inhibitors NSC23766 and EHT1864 in mouse platelets. J Thromb Haemost. 2015;13(5):827–838. [DOI] [PubMed] [Google Scholar]
- 403.Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res. 2009;22(3):268–282. [DOI] [PubMed] [Google Scholar]
- 404.Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152(4):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC. The leaden gene product is required with rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 2002;3(3):193–202. [DOI] [PubMed] [Google Scholar]
- 406.Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci. 2001;114(6):1091–1100. [DOI] [PubMed] [Google Scholar]
- 407.Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JAr. Identification of an organelle receptor for myosin-Va. Nature Cell Biol. 2002;4(4):271–278. [DOI] [PubMed] [Google Scholar]
- 408.Wu X, Wang F, Rao K, Sellers JR, Hammer JA III Rab27a is an essential component of melanosome receptor for myosin Va. Mol Biol Cell. 2002;13(5):1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va. Implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277(14):12432–12436. [DOI] [PubMed] [Google Scholar]
- 410.Provance DWJ, James TL, Mercer JA. Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic. 2002;3(2):124-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277(28):25423–25430. [DOI] [PubMed] [Google Scholar]
- 412.Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the Myosin-Va gene. Nature Genet. 1997;16:289–292. [DOI] [PubMed] [Google Scholar]
- 413.Ménasché G, Ho CH, Sanal O, Feldmann J, Tezcan I, Ersoy F, Houdusse A, Fischer A, de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest. 2003;112(3):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nature Genet. 2000;25(2):173–176. [DOI] [PubMed] [Google Scholar]
- 415.Oberhofer A, Spieler P, Rosenfeld Y, Stepp WL, Cleetus A, Hume AN, Mueller-Planitz F, Okten Z. Myosin Va’s adaptor protein melanophilin enforces track selection on the microtubule and actin networks in vitro. Proc Natl Acad Sci USA. 2017;114(24):E4714–E4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell. 2004;15(5):2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, Futter CE, Seabra MC. The ternary Rab27a-Myrip-myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic. 2007;8(5):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hammer JA 3rd., Wagner W Functions of class V myosins in neurons. J Biol Chem. 2013;288(40):28428–28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152(4):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Haddad EK, Wu X, Hammer JAr, Henkart PA Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152(4):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CHG, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, Heck AJR, Brose N, Kleijmeer M, van der Sluijs P. Munc13–4 Is an effector of Rab27a and controls secretion of lysosomes in haematopoietic cells. Mol Biol Cell. 2005;16(2):731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Elstak ED, Neeft M, Nehme NT, Voortman J, ., Cheung M, Goodarzifard M, Gerritsen HC, van Bergen En Henegouwen PM, Callebaut I, de Saint Basile G, van der Sluijs P The munc13–4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118(6):1570–1578. [DOI] [PubMed] [Google Scholar]
- 423.Holt O, Kanno E, Bossi G, Booth S, Daniele T, Santoro A, Arico M, Saegusa C, Fukuda M, Griffiths GM. Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic. 2008;9(4):446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Menasche G, Menager MM, Lefebvre JM, Deutsch E, Athman R, Lambert N, Mahlaoui N, Court M, Garin J, Fischer A, de Saint Basile G. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood. 2008;112(13):5052–5062. [DOI] [PubMed] [Google Scholar]
- 425.Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint Basile G, Ménasché G. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119(17):3879–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115(2):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Singh RK, Mizuno K, Wasmeier C, Wavre-Shapton ST, Recchi C, Catz SD, Futter C, Tolmachova T, Hume AN, Seabra MC. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13–4 in mast cell secretion. FEBS J. 2013;280(3):892–903. [DOI] [PubMed] [Google Scholar]
- 428.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–1959. [DOI] [PubMed] [Google Scholar]
- 429.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115:461–473. [DOI] [PubMed] [Google Scholar]
- 430.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nürnberg P, Janka G, Hennies HC. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14(6):827–834. [DOI] [PubMed] [Google Scholar]
- 431.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjöld M, Henter J-I, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11–deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, Al-Harbi M, Alangari A, Le D,F, Gennery AR, Prince N, Cariou A, Nitschke P, Blank U, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Spessott WA, Sanmillan ML, McCormick ME, Patel N, Villanueva J, Zhang K, Nichols KE, Giraudo CG. Hemophagocytic lymphohistiocytosis caused by dominant negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood. 2015;125(10):1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Halimani M, Pattu V, Marshall MR, Chang HF, Matti U, Jung M, Becherer U, Krause E, Hoth M, Schwarz EC, Rettig J. Syntaxin11 serves as a t-SNARE for the fusion of lytic granules in human cytotoxic T lymphocytes. Eur J Immunol. 2014;44(2). [DOI] [PubMed] [Google Scholar]
- 437.Spessott WA, Sanmillan ML, McCormick ME, Kulkarni VV, Giraudo CG. SM protein Munc18-2 facilitates transition of Syntaxin 11-mediated lipid mixing to complete fusion for T-lymphocyte cytotoxicity. Proc Natl Acad Sci U S A. 2017;114(11):E2176–E2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Dieckmann NM, Hackmann Y, Aricò M, Griffiths GM. Munc18–2 is required for Syntaxin 11 localization on the plasma membrane in cytotoxic T-lymphocytes. Traffic. 2015;16(12):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120(12):2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Hackmann Y, Graham SC, S. E, Höning S, Lehmberg K, Aricò M, Owen DJ, Griffiths GM Syntaxin binding mechanism and disease-causing mutations in Munc18-2. Proc Natl Acad Sci USA. 2013;110(47):E4482–E4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Rizo J Mechanism of neurotransmitter release coming into focus. Protein Sci. 2018;27(8):1364–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116(6):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cetica V, Hackmann Y, Grieve S, Sieni E, Ciambotti B, Coniglio ML, Pende D, Gilmour K, Romagnoli P, Griffiths GM, Aricò M. Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13-4 binding. J Allergy Clin Immunol. 2015;135(5):1310–1318.e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Crozat K, Hoebe K, Ugolini S, Hong NA, Janssen E, Rutschmann S, Mudd S, Sovath S, Vivier E, Beutler B. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. J Exp Med. 2007;204(4):853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Hanby HA, Bao J, Noh J-Y, Jarocha D, Poncz M, Weiss MJ, Marks MS. Platelet dense granules begin to selectively accumulate mepacrine during proplatelet formation. Blood Adv. 2017;1:1478–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Lo RW, Li L, Leung R, Pluthero FG, Kahr WHA. NBEAL2 (Neurobeachin-Like 2) is required for retention of cargo proteins by alpha-hranules during Their production by megakaryocytes. Arterioscler Thromb Vasc Biol. 2018;38(10):2435–2447. [DOI] [PubMed] [Google Scholar]
- 447.Chen D, Uhl CB, Bryant SC, Krumwiede M, Barness RL, Olson MC, Gossman SC, Erdogan Damgard S, Gamb SI, Cummins LA, Charlesworth JE, Wood-Wentz CM, Salisbury JL, Plumhoff EA, Van Cott EM, et al. Diagnostic laboratory standardization and validation of platelet transmission electron microscopy. Platelets. 2018;29(6):574–582. [DOI] [PubMed] [Google Scholar]
- 448.van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–1156. [DOI] [PubMed] [Google Scholar]
- 449.Menon GK, Lee SE, Lee SH. An overview of epidermal lamellar bodies: novel roles in biological adaptations and secondary barriers. J Dermatol Sci. 2018;92(1):10–17. [DOI] [PubMed] [Google Scholar]
- 450.Valentijn KM, Valentijn JA, Jansen KA, Koster AJ. A new look at Weibel-Palade body structure in endothelial cells using electron tomography. J Struct Biol. 2008;161(3):447–458. [DOI] [PubMed] [Google Scholar]
- 451.Watanabe Y, Tsuda H, Kotani T, Sumigama S, Mano Y, Hayakawa M, Sato Y, Kikkawa F. Amniotic lamellar body count and congenital diaphragmatic hernia in humans and in a rat model. Pediatr Res. 2013;73(3):344–348. [DOI] [PubMed] [Google Scholar]
- 452.Hurbain I, Geerts WJC, Boudier T, Marco S, Verkleij A, Marks MS, Raposo G. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci USA. 2008;105(50):19726–19731. [DOI] [PMC free article] [PubMed] [Google Scholar]