Abstract
Background/Aim:
Monotherapy with interferon or nucleoside analog is generally not recommended during the immune tolerant (IT) phase of chronic hepatitis B virus (HBV) infection. Recognition that high HBV DNA levels are associated with hepatocellular carcinoma has increased interest in treating HBV in the IT phase. Small pediatric studies reported efficacy with combination nucleoside analog and interferon therapy.
Aim:
To evaluate the safety and efficacy of the combination of entecavir and peginterferon in adults in the IT phase of chronic HBV infection.
Methods:
HBeAg-positive adults with HBV DNA>107 IU/mL and ALT≤1.5x ULN: (M≤45, F≤30 U/L) received entecavir 0.5 mg daily for 8 weeks followed by addition of peginterferon alfa-2a 180 μg/week to entecavir for an additional 40 weeks. The primary endpoint was HBeAg loss and HBV DNA≤1000 IU/mL 48 weeks post-end-of-treatment (EOT).
Results:
Among 28 participants from 11 sites, median age was 37.2 (range 22–61) years, 54% were male and 96% were Asian. Nearly all were infected with genotype C (64%) or B (32%). Median baseline HBV DNA was 8.2 log10 IU/mL and ALT 0.9xULN. While one (4%) participant cleared HBeAg, none met the primary endpoint of both HBeAg loss AND HBV DNA ≤1,000 IU/mL 48 weeks post-EOT. ALT elevations >5xULN occurred in 8 (29%) participants, none associated with icterus. 48 weeks post-treatment, HBV DNA rebounded to baseline levels in all participants including the participant who lost HBeAg, and ALT values returned to near-baseline levels in all but 4 participants.
Conclusion:
A lead-in strategy of 8 weeks of entecavir followed by combination peginterferon and entecavir therapy for 40 weeks had limited efficacy in adults in the IT phase of chronic HBV infection and cannot be recommended.
Keywords: HBV, immunetolerant, peginterferon, entecavir, antiviral therapy
Hepatitis B virus (HBV) infection remains a significant global public health problem with roughly 250 million persons chronically infected worldwide1. Chronic HBV infection may result in progressive liver disease that leads to cirrhosis, end-stage liver disease and hepatocellular carcinoma (HCC). The majority of individuals with chronic HBV acquire infection at birth or in early childhood. The course of chronic infection has been divided into clinical phases based on virological characteristics, liver enzyme levels and hepatic histology2. The immune tolerant (IT) phase is characterized by the presence of hepatitis B e antigen (HBeAg), very high HBV DNA levels (>107 IU/mL) but normal or near-normal aminotransferases and minimal or no inflammation on liver biopsy, suggesting that despite active viral replication there is no immune response to the virus3. Most individuals in the IT phase are children or young adults who acquired infection by vertical transmission. The age of transition to the immune active (IA) phase is variable depending on HBV genotype and possibly race, but typically occurs in adolescence or early adulthood3. Entry into the IA phase is characterized by a rise in alanine aminotransferase (ALT) levels and active inflammation on biopsy with fluctuating levels of HBV DNA. Repeated ALT flares and progressive liver injury may occur with eventual clearance of HBeAg, often with the development of antibody to HBeAg (anti-HBe). After HBeAg clearance, patients may enter the immune control or inactive carrier (IC) phase of infection with low or undetectable levels of HBV DNA and normal ALT values or they may progress to HBeAg-negative IA disease with ongoing active hepatitis and fluctuating HBV DNA levels despite clearance of HBeAg3.
National and international clinical practice guidelines recommend treatment for HBV infection during the IA phases (HBeAg-positive or HBeAg-negative) of infection and for patients with cirrhosis4,5. Treatment during the IT phase has not been recommended due to the presence of minimal liver disease and limited efficacy of interferon or nucleoside analog monotherapy4,5. With recognition that high HBV DNA levels and persistence of HBeAg are associated with an increased risk of HCC, and that during the IT phase HBV-specific T cells are present and able to proliferate and produce cytokines6, there has been increased interest in treating HBV during the IT phase. A pilot study of 23 children in the IT phase treated with lamivudine for 8 weeks followed by the addition of interferon for 44 weeks showed HBeAg seroconversion in 5 (22%) and HBsAg loss in 4 (17%) children7. Based upon these promising results, the Hepatitis B Research Network developed a clinical trial to assess combination therapy using entecavir, a more potent antiviral agent than lamivudine, followed by the addition of peginterferon in adult patients in the IT phase of chronic HBV infection.
Methods
HBRN
The HBRN is an NIDDK-funded multicenter, prospective study of chronic hepatitis B in both children and adults followed at 28 clinical sites in the United States and Canada, the goals of which are to better define the natural history, pathogenesis and therapy of chronic hepatitis B. The HBRN includes both a pediatric and an adult cohort study and has initiated 3 therapy trials: two trials of entecavir and peginterferon (one in children and one in adults) with immune-tolerant chronic hepatitis B and one trial of tenofovir and peginterferon in adults with immune-active chronic hepatitis B. This report is a description of the design and results of the open-label adult immune-tolerant study carried out at 11 adult clinical sites in the United States and one in Canada. The Data Coordinating Center for the HBRN was located at the University of Pittsburgh, Pittsburgh, Pennsylvania.
Participants
Participants who met the following criteria were eligible for enrollment: at least 18 years old, HBsAg-positive, HBeAg-positive, HBV DNA >107 IU/mL on 2 occasions greater than 12 weeks apart and within 6 weeks of enrollment, and 3 documented ALT values less than 1.5 times upper limit of normal (ULN) over the year prior to study entry. The ULN differed for men (30 U/L) and women (20 U/L). Initially men with ALT ≤60 U/L and women with ALT ≤40 U/L were eligible; however, the study was amended to ensure all participants were truly in the IT phase to include only men with ALT ≤45 U/L and women with ALT ≤30 U/L. Participants more than 50 years old were required to have had a liver biopsy within 96 weeks of enrollment documenting histologic activity index (HAI) score <3 and fibrosis stage ≤1. Key exclusion criteria included: a past or current history of hepatic decompensation (ascites, variceal bleeding, hepatic encephalopathy), HCC, solid organ or bone marrow transplantation, platelet count <120,000/mm3, direct bilirubin >0.5 mg/dL, albumin <3.5 g/L, INR >1.5, creatinine clearance <50 mL/minute, HIV, hepatitis D or hepatitis C virus co-infection, pre-existing psychiatric or autoimmune disease or any other medical condition potentially exacerbated by interferon therapy. A full list of inclusion and exclusion criteria is provided in Supplemental Tables 1 and 2.
Originally designed as a randomized trial with a control arm, the protocol was revised to a single-arm pilot treatment study due to low enrollment. Participants previously randomized to the control group were offered the option of enrolling in the revised protocol provided that they still met trial eligibility criteria. The protocol was approved by the HBRN Steering Committee and the Institutional Review Boards (Research Ethics Board in the case of the Toronto site) of the participating sites, and all participants provided written informed consent. The study was overseen by an independent data safety monitoring board (DSMB) appointed by the NIDDK to monitor the clinical studies of the HBRN. The ClinicalTrials.gov identifier number was NCT01369199.
After amendment to a single arm pilot treatment study, the sample size was re-estimated based on desired precision of the point estimate of the primary outcome, i.e., assuming 25% would meet the primary endpoint and a 95% Clopper-Pearson confidence limit within +/− 15% of the estimated percentage, the revised sample size was 40 participants.
Treatment
In the initial protocol, participants were randomized to treatment or observation. After the protocol was revised, those in the control arm were offered to move to the treatment arm and subsequent enrollees received open-label therapy. Treatment consisted of open-label entecavir 0.5 mg/day for 8 weeks followed by entecavir combined with peginterferon alfa-2a 180 μg/week for an additional 40 weeks. After starting therapy, study visits occurred at weeks 8, 12, 16, 20, 24, 30, 36, 42 and 48 at which point all treatment was stopped and participants were followed for an additional 48 weeks with visits at weeks 52, 60, 72 and 96 (Figure 1). Entecavir was provided by Bristol-Myers-Squibb and peginterferon alfa-2a by Genentech under separate Clinical Trial Agreements with the NIDDK.
Figure 1. Trial Design:
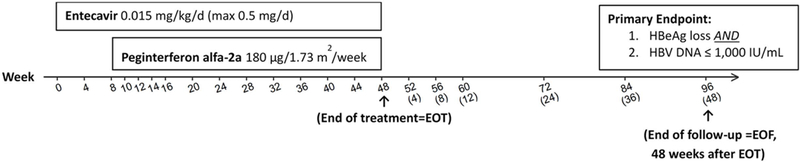
Entecavir 0.5 mg daily alone for 8 weeks followed by peginterferon alfa-2a 180 μg/week combined with entecavir for an additional 40 weeks. Endpoints were evaluated at the end of treatment (week 48) and 48 weeks after treatment completion (week 96). Weeks 52, 60, 72, and 96 correspond to 4, 12, 24, and 48 weeks after end of treatment, respectively, for those who discontinued treatment early.
Endpoints
The primary efficacy endpoint was the loss of HBeAg AND HBV DNA ≤1,000 IU/mL at the end of follow-up (48 weeks following the end of treatment). The primary safety endpoint was the frequency of adverse events (AE) and serious adverse events (SAE). Secondary efficacy endpoints were assessed at the end of treatment (EOT), i.e., week 48 for those who completed treatment per protocol, and at end of follow-up (EOF) i.e. week 96 for those who completed the full 48 weeks of treatment. Secondary endpoints included: loss of HBsAg, HBsAg seroconversion, loss of HBeAg, HBeAg seroconversion, ALT <1.5 times ULN, ALT normalization (M: <30 U/L, F: <20 U/L), HBV DNA ≤1,000 IU/mL, and HBV DNA <20 IU/mL. Other post hoc endpoints included change in ALT and quantitative HBsAg from baseline. ALT elevations ≥2-fold baseline were categorized by the height of elevation compared to the ULN using standard hepatotoxicity grading: Grade 1 as >1 to 3 times ULN, Grade 2 as >3 to 5 times ULN and Grade 3 as >5 times ULN. Icteric ALT elevations are those associated with a bilirubin >2.5 mg/dL. Participants’ maximum decline in quantitative HBsAg and HBeAg during therapy were calculated and categorized as ≤0.5 vs >0.5 log10 IU/mL and ≤1.0 vs >1.0 log10 IU/mL.
Virological Assays
Study eligibility was based on qualitative HBeAg and HBsAg results performed at the individual sites. Efficacy endpoints were based on quantitative HBeAg and HBsAg (Elecsys, Roche Molecular Systems, Branchburg, NJ) assays performed at the HBRN central virology laboratory at the University of Washington, with lower limits of detection (LLOD) of 0.3 IU/mL for HBeAg and 0.05 IU/mL for HBsAg. HBV DNA testing was performed centrally using a real-time PCR assay (COBAS Ampliprep/COBAS TaqMan Test, v.2.0; Roche Molecular Systems) with a lower limit of quantification (LLOQ) of 20 IU/mL and a lower limit of detection of 10 IU/mL. HBV genotyping was performed at the Molecular Epidemiology and Bioinformatics Laboratory in the Division of Viral Hepatitis at the Centers for Disease Control and Prevention using mass spectrometry8.
Statistical Analysis
Descriptive statistics (median, range) and bootstrapped 95% confidence intervals about medians are reported for continuous variables. Frequencies and percentages are reported for categorical variables. The primary efficacy analysis was performed including all enrolled participants. Participants who did not have primary endpoint data were considered as treatment failures. The percentage of participants achieving the efficacy endpoints was summarized with the point estimate and the 95% exact binomial confidence interval. For safety endpoints, rates per person-year and 95% confidence intervals about a Poisson variable are shown. For post-hoc calculations of aggregate treatment response for HBV DNA, HBsAg, HBeAg and ALT, participants who stopped therapy prior to the given time-point or who did not have lab results were excluded (i.e. per-protocol analysis). One participant who was never treated with study medications was excluded for post-hoc analyses. SAS 9.3 (SAS Institute, Cary NC, USA) and R 3.3.1 were used for statistical analysis and graphical display.
Results
Participant Characteristics
Of 58 patients screened, 29 met eligibility criteria and were enrolled in the trial. Three of these participants were randomized to the control arm and 2 agreed to move to the treatment arm after the protocol amendment (both still met eligibility criteria), while the other participant refused consent, leaving 28 participants in the treatment arm. Reasons for exclusion included: refused consent (n=16), lab exclusion for HBV DNA, ALT level, platelet, hemoglobin, or neutrophil count (n=13), and other medical condition (n=1) (Supplemental Figure 1). Of the 28 who were to start treatment, 15 (54%) were male, 27 (96%) were Asian, the median age was 37.2 years (range 22–61 years) and median body mass index was 22.4 (range 18.0–28.3) kg/m2. There were 18 (64%) individuals with genotype C, 9 (32%) with genotype B and one (4%) with genotype E infection. At baseline, the median HBV DNA level was 8.2 (range 7.2–8.8) log10 IU/mL and the median quantitative HBsAg level was 4.7 (range 4.2–5.1) log10 IU/mL. Baseline ALT values ranged from 9 to 47 U/L or 0.5 to 1.6 times ULN (median=0.9 times ULN) and 19 (68%) had values at or below the ULN. The median FIB-4 score was 0.7 (range 0.4–2.3). One participant who was 61 years of age underwent liver biopsy and had HAI=1 and Ishak fibrosis=0. Other baseline laboratory parameters are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristics | Total N=28 n (%) or median (range) |
|---|---|
| Demographics | |
| Sex | |
| Male | 15 (54) |
| Female | 13 (46) |
| Race | |
| Black/African American | 1 (4) |
| Asian | 27 (96) |
| Age (years) | 37.2 (22.2 – 61.2) |
| BMI (kg/m2) | 22.4 (18.0 – 28.3) |
| Virology and serology | |
| HBV Genotype | |
| B | 9 (32) |
| C | 18 (64) |
| E | 1 (4) |
| HBeAg (+) | 28 (100) |
| HBV DNA (log10 IU/mL) - Screening | 8.3 (7.4–8.8) |
| HBV DNA (log10 IU/mL) - Baseline | 8.2 (7.2 – 8.8) |
| Quantitative HBeAg (log10 IU/mL) | 3.3 (2.3 – 3.6) |
| Quantitative HBsAg (log10 IU/mL) | 4.7 (4.2 – 5.1) |
| ALT (U/L) - Screening | 21 (9 – 47*) |
| Males | 32 (16 – 47*) |
| Females | 18 (9 – 26) |
| ALT (U/L) - Baseline | 21 (9 – 47) |
| Males | 27 (14 – 47) |
| Females | 15 (9 – 30) |
| AST (U/L) | 20 (15 – 30) |
| FIB-4 | 0.7 (0.4 – 2.3) |
| INR | 1.0 (0.9 – 1.1) |
| Total Bilirubin (mg/dL) | 0.6 (0.3 – 2.0) |
| Albumin (g/dL) | 4.2 (3.5 – 4.8) |
| Platelets (x103/mm3) | 242.5 (137 – 325) |
| White blood cells (x103/mm3) | 5.0 (3.0 – 9.1) |
A male participant with ALT 47 was eligible enrolled under the study protocol v6.0 (ALT ≤60 for males, ≤40 for females). The study protocol v7.0 was revised as ALT ≤45 for males, ≤30 for females.
Treatment Outcomes
One participant withdrew consent prior to the first dose of medication and was counted as failing to meet study efficacy endpoints, however on-treatment results for this participant were excluded from calculations of summary statistics. Two additional participants were not available for the last follow-up visit leaving 25 participants who completed 48-week post-treatment follow-up (Supplemental Figure 1). Among 28 participants, 27 adults initiated treatment and 20 (71%) completed the full 48 weeks of entecavir and 40 weeks of peginterferon treatment without dose reduction or early discontinuation. Four participants had at least one peginterferon dose reduction or interruption. Three participants discontinued one or both study medications. Two participants discontinued peginterferon early due to an AE, one at week 36 due to altered thyroid function and the other at week 35 due to a diagnosis of Graves disease. The average weekly dose was 177 μg (range 90–180 μg) for the 24 participants who did not discontinue peginterferon early, representing 98% of the total expected dose.
Primary Efficacy Endpoint
No participant met the primary endpoint of both HBeAg loss and HBV DNA ≤1000 IU/mL 48 weeks after treatment ended (week 96 for those who completed treatment) (Table 2). One participant lost HBeAg at week 48 and remained HBeAg-negative, anti-HBe positive and HBsAg-positive at week 96. However, HBV DNA levels, that were suppressed to undetectable by the end of treatment, rebounded to high levels (7.5 log10 IU/mL) by week 96 accompanied by an increase in ALT levels from 27 U/L at the end of treatment to 70 U/L at week 96. Viral sequencing confirmed the presence of the double BCP mutations (A1762T and G1764A) but the absence of the precore mutation (G1896A) at baseline and at last study follow-up. No post-trial follow-up was available for this individual.
Table 2.
Efficacy Endpoints*
| End of Treatment (EOT) | End of Follow-up (EOF) | |||
|---|---|---|---|---|
| Endpoints | n=28 (%) | 95% Confidence Intervals (%) |
n=28 (%) | 95% Confidence Intervals (%) |
| HBsAg loss | 0 (0) | (0.0–12.3) | 0 (0) | (0.0–12.3) |
| HBsAg seroconversion | 0 (0) | (0.0–12.3) | 0 (0) | (0.0–12.3) |
| HBeAg loss** | 1 (4) | (0.1–18.3) | 1 (4) | (0.1–18.3) |
| HBeAg seroconversion | 1 (4) | (0.1–18.3) | 1 (4) | (0.1–18.3) |
| HBV DNA< 20 IU/mL | 5 (18) | (6.1–36.9) | 0 (0) | (0.0–12.3) |
| HBV DNA≤ 1000 IU/mL | 26 (93) | (76.5–99.1) | 0 (0) | (0.0–12.3) |
| ALT< 1x ULN | 11 (39) | (21.5–59.4) | 13 (46) | (27.5–66.1) |
| ALT< 1.5 x ULN | 16 (57) | (24.5–62.8) | 21 (75) | (55.1–89.3) |
| Primary Endpoint: | ||||
| HBeAg loss** & HBV DNA≤ 1000 IU/mL | 1 (4) | (0.1–18.3) | 0 (0) | (0.0–12.3) |
Analyses were performed using all enrolled participants.
The individual with discrepant qualitative (positive) and quantitative (negative) HBeAg results over time was not regarded as HBeAg loss.
One other participant had discrepant qualitative and quantitative HBeAg results throughout the study. At screening, the local qualitative HBeAg was positive but the central quantitative HBeAg at baseline was below the limit of detection (<0.3 IU/mL). Multiple results from both assays were unchanged and this participant remained HBeAg-positive by local qualitative testing but HBeAg-negative by the central quantitative assay throughout the course of therapy and follow-up with a similar HBV DNA curve during and after treatment as seen in other participants. This individual with discrepant qualitative and quantitative HBeAg results was not regarded as HBeAg loss and quantitative HBeAg results were not included in aggregate summary statistics for HBeAg (Table 2, Figure 3a).
Figure 3.
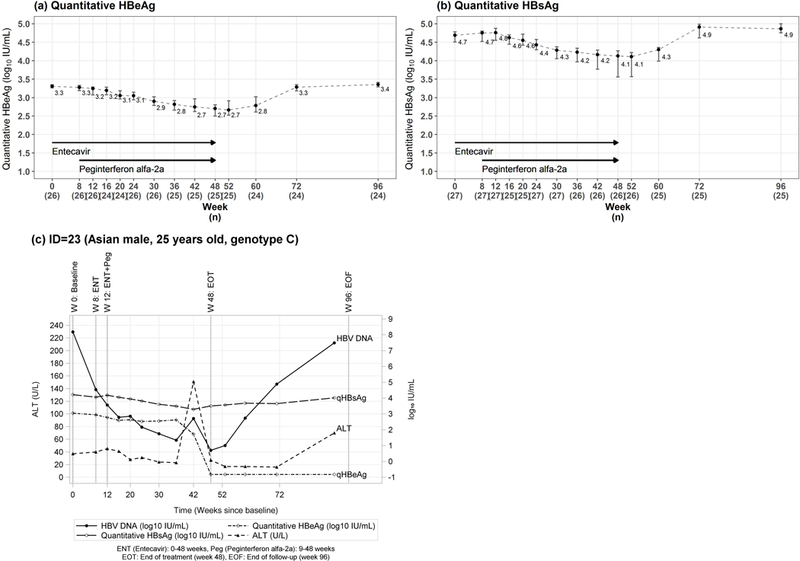
(a) Quantitative HBeAg, (b) quantitative HBsAg, and (c) labs over time in the participant who had HBeAg seroconversion quantitative (a) HBeAg and (b) HBsAg levels are shown before, during and after treatment. (c) The pattern of ALT, HBV DNA and quantitative HBsAg, HBeAg are shown in the individual who underwent HBeAg seroconversion on treatment. This patient stopped peginterferon at week 35 due to a diagnosis of Graves disease but continued entecavir until week 48. The decrease in HBeAg and HBsAg levels and sharp rise in ALT levels occurred after peginterferon was stopped and before entecavir was discontinued. The week 48 line indicates the end of entecavir treatment and the first time HBeAg was found to be negative.
HBV DNA response
Median log10 HBV DNA levels declined during the 8 weeks of entecavir monotherapy from 8.2 (range 7.2–8.8) log10 IU/mL at baseline to 4.9 (range 3.2–6.0) log10 IU/mL at week 8. At the end of treatment, HBV DNA levels were ≤1000 IU/mL in all 26 participants who completed therapy and <20 IU/mL in 5 participants (Table 2). Only 1 of these 5 participants had undetectable HBV DNA (<10 IU/mL). No participants experienced on-treatment virological breakthrough. After stopping therapy, median HBV DNA levels rebounded to 8.3 (range 6.9–9.0) log10 IU/mL by week 96 (Figure 2a) and levels were within 0.3 log10 IU/mL of baseline values in all but 4 participants (Figure 2b). In the subject (ID=23) who became HBeAg-negative, HBV DNA was 7.5 log10 IU/mL at week 96 compared to 8.2 log10 IU/mL at baseline (Figure 2b).
Figure 2. HBV DNA levels.
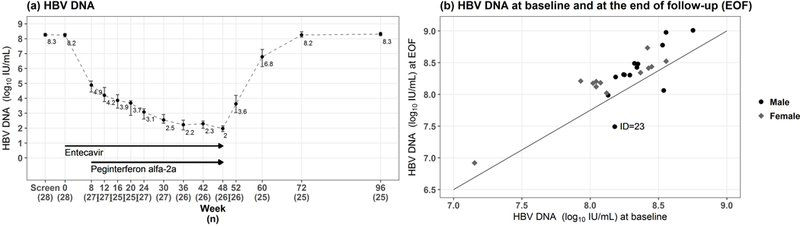
(a) Median and 95% confidence intervals (CI) of HBV DNA levels are shown at baseline, during and after treatment. (b) HBV DNA levels at baseline are plotted against HBV DNA at the end of follow-up (week 96) indicating that HBV DNA levels returned to near-baseline levels (>107 IU/mL) in all participants including the individual who cleared HBeAg at the end of follow-up.
HBeAg response
Median quantitative HBeAg levels declined from 3.3 (range 2.3–3.6) log10 IU/mL to 2.7 (range LLOD-3.2) log10 IU/mL by week 48 and rebounded to 3.4 (range LLOD-3.6) log10 IU/mL by week 96 (Figure 3a). As noted, one participant experienced HBeAg seroconversion at week 48 of treatment that persisted to the end of follow-up but HBV DNA levels rebounded to near baseline levels with accompanying increase in ALT (Figure 3c).
HBsAg response
No participants cleared HBsAg during the study. Median quantitative HBsAg levels were similar at baseline 4.7 (range 4.2 to 5.1) and week 8, 4.7 (4.1 to 5.1) log10 IU/mL, during entecavir monotherapy. After the addition of peginterferon, median HBsAg levels declined to 4.1 (1.7 to 4.9) log10 IU/mL by week 48 but rebounded to 4.9 (4.0 to 5.3) log10 IU/mL by the end of follow-up (Figure 3b). During therapy, the maximum HBsAg decline was 3.1 log10 IU/mL. Of the 27 treated participants, 19 (70%) had more than 0.5 log10 decline and 6 (22%) had a more than 1 log10 decline in HBsAg levels.
ALT responses
None of the 27 participants who started therapy experienced an ALT elevation of 2-fold baseline during lead-in entecavir monotherapy while 19 (70%) had such an elevation during combination therapy which were above 5 times ULN in 8 (30%). There were no icteric ALT elevations (bilirubin >2.5 mg/dL) and treatment was not altered due to ALT increases. All ALT elevations resolved after completing treatment and ALT levels returned to near-baseline levels in most participants. (Figure 4). In addition, 7 Grade 1 and 4 Grade 2 ALT elevations were noted during combination therapy.
Figure 4. ALT levels.
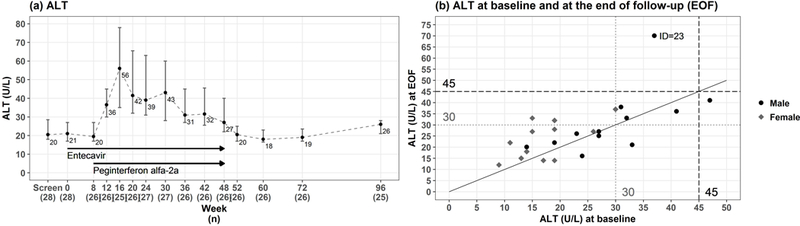
(a) Median and 95% CI of ALT levels are shown before, during and after treatment. (b) ALT levels at baseline are plotted against ALT levels at end of follow-up (week 96) indicating that ALT levels returned to near-baseline levels in most participants. ALT values of 1.5 ULN are indicated for men (45 U/L) in dashed lines and women (30 U/L) in dotted lines, respectively.
At the end of treatment, ALT levels decreased and by EOF, 13 of 28 (46%) participants had normal ALT and 21 of 28 (75%) had values <1.5 times ULN (Table 2). At week 96, ALT was above 1.5 times ULN in 4 of 25 subjects but in no instance, was ALT more than twice the baseline level (Figure 4b). Three of these 4 were women with ALT values ranging from 32 to 37 U/L, compared to baseline values ranging from 15 to 30 U/L. The other participant was the person who had HBV DNA rebound despite HBeAg seroconversion and ALT of 70 U/L at week 96 compared to 37 U/L at baseline.
Safety
There were 42 treatment-emergent AEs (i.e., from first treatment to last follow-up) reported by 14 participants (Supplemental Table 3a), of which none was scored as probably or definitely related to entecavir whereas 14 (33%) were considered probably or definitely related to peginterferon (Supplemental Tables 3b and 3c). The treatment emergent AE rate was 86 per 100 person-year (95% CI: 64 to 117 per 100 person-years). The treatment emergent SAE rate was 2 per 100 person-year (95% CI: 0 to 15 per 100 person-years). Most AEs were mild (50%) or moderate (43%) in severity (Supplemental Table 3b). The most common AEs were dermatologic (24%) and urological (14%) (Table 3). Three AEs led to 3 separate treatment interruptions of peginterferon in one participant. Two participants stopped peginterferon therapy due to an AE possibly related to peginterferon, one at week 36 due to altered thyroid function and the other at week 35 due to a specific diagnosis of Graves disease. Both participants continued entecavir. One SAE was reported during the off-treatment observation period, a case of malaria, considered to be unrelated to treatment.
Table 3.
Treatment emergent adverse events by system (n=42) reported by 14 participants
| System | # of AEs (%) |
|---|---|
| Dermatologic | 10 (24) |
| Urological | 6 (14) |
| Gastrointestinal | 4 (10) |
| Constitutional symptoms | 4 (10) |
| Ear/nose/throat | 4 (10) |
| Endocrine | 3 (7) |
| Neurological | 3 (7) |
| Infection | 2 (5) |
| Musculoskeletal | 2 (5) |
| Hematological | 2 (5) |
| Ophthalmic | 1 (2) |
| Severe respiratory | 1 (2) |
Discussion
National and international guidelines do not recommend treatment during the IT phase of chronic HBV infection largely due to limited efficacy of currently available therapies4,5. This study evaluated adding peginterferon to entecavir for 40 weeks after 8 weeks of lead-in entecavir monotherapy. Only one of 28 (3.6%) participants achieved HBeAg seroconversion and this individual had rebound in HBV DNA and ALT post-treatment, transitioning to the HBeAg-negative IA phase rather than to the inactive carrier state. No patients achieved off therapy virological control or HBsAg loss.
Although the combination treatment was not effective, this trial provides some useful insights. The concept of immune tolerance was first proposed based on the observation that, despite high levels of viremia, patients in the IT phase have minimal or no hepatic inflammation suggesting a lack of immune recognition or tolerance to the virus2. However, more in depth characterization of the HBV-specific immune response in different phases of chronic HBV infection has challenged the concept of immune tolerance. Young patients in the IT phase have been shown to have similar or greater frequency and reactivity of HBV-specific T cells in the peripheral blood compared to older patients in the immune active phase of disease9. Furthermore, viral diversity in young IT patients suggests immune selection pressure, questioning the concept of true tolerance to the virus10. Bertoletti and colleagues have noted that the main difference between the IT phase and the IA phase is not immune tolerance but the presence of an inflammatory milieu in the IA phase that drives non-specific inflammation in response to recognition of HBV by T cells and have thus suggested renaming the IT phase as the ‘non-inflammatory, high viremic’ phase of disease6. This is reflected in the recent European Association for the Study of the Liver (EASL) HBV clinical practice guidelines in which the authors proposed a new nomenclature for the phases of chronic HBV infection and suggested that the IT phase be renamed ‘HBeAg-positive Chronic Infection’ to distinguish it from the IA phase, which they proposed to rename ‘HBeAg-positive Chronic Hepatitis’4. The data from our study are notable for the fact that despite a rapid rebound in HBV DNA levels after stopping entecavir and peginterferon to near baseline values there was no associated hepatitis flare with ALT values settling out at or near baseline levels. Continued follow-up showed that ALT values remained stable, indicating that the patients returned to the same ‘tolerant’ or ‘non-inflammatory’ state post-treatment. This is similar to what was seen after stopping 4 years of nucleoside analog therapy in IT patients11 and contrasts notably with studies of antiviral therapy withdrawal in HBeAg-positive patients who started therapy in the IA phase of disease in which there were reports of severe or even fatal withdrawal hepatitis12. These data suggest that the immune response to HBV is indeed different in patients in the IT and IA phase of chronic HBV infection. Studies of nucleoside analog withdrawal in HBeAg-negative patients have also reported ALT flares in 53–76% of patients in association with viral rebound13–15.
Despite the lack of hepatitis flares after stopping treatment, ALT elevations to more than twice baseline occurred in 19 patients during peginterferon therapy and levels rose to above 5 times ULN in 8 (30%) participants. Declines in quantitative HBsAg and HBeAg levels of at least 0.5 log IU/mL were also seen during combination therapy and were more common in those with ALT elevations, possibly suggesting that the ALT elevations were associated with viral control. Neither ALT flares nor decline in quantitative HBsAg or HBeAg levels occurred during the 8 weeks of entecavir monotherapy. These results are in keeping with previous data showing that HBsAg decline rarely occurs during nucleoside analog therapy but is commonly observed during peginterferon treatment16.
Previous studies of interferon therapy for adult IT patients showed negligible rates of HBeAg or HBsAg loss17. Trials of nucleoside therapy in the IT patients have shown that HBV DNA can be suppressed but HBeAg and HBsAg loss are rare18. However, small trials in children of interferon treatment after a lead-in of 8 weeks of lamivudine reported HBeAg seroconversion rates of up to 39% and rates of HBsAg loss of up to 21%7,19. Part of the rationale for combining a nucleoside analog with interferon is the observation that viral suppression may lead to some degree of restoration of HBV-specific immune responses, which may then promote a more robust immune response driven by interferon20–22. We hypothesized that by using a more potent agent such as entecavir, we might achieve similar results in adult patients to those seen in children. A parallel HBRN study in children found that 3% had durable HBeAg and HBsAg clearance with undetectable HBV DNA and normal ALT. Although there were responses in children but none in adults, the confidence intervals around the response rates were similar. Notably, in the one adult participant who cleared HBeAg and developed anti-HBe in our study, HBV DNA levels increased after stopping therapy and ALT levels rose to 70 U/L at last follow-up. While it is difficult to make strong inferences from one individual, it raises the possibility that therapy was actually harmful to this participant, leading to a loss of ‘tolerance’ and the development of HBeAg-negative immune active disease. Similarly, in a study of nucleoside analog monotherapy in 20 adult IT patients, HBeAg loss occurred in 3 patients after treatment cessation and 1 developed HBeAg-negative active disease23.
This study has important limitations, most notably the lack of a control arm. The original protocol was designed to include an untreated control arm, however recruitment was lower than anticipated, largely due to a low frequency of eligible IT patients in the HBRN sites, demonstrating the challenge of studying this population in North America. To gain some insight into the effect of therapy compared to the natural history of the IT phase, we evaluated the virological and clinical parameters of study participants prior to entering this trial while they were followed as part of the prospective HBRN cohort study. Because of variable duration of follow-up, it is difficult to make formal comparisons, but there were little to no changes in HBV DNA and HBsAg levels among the participants prior to starting therapy. Second, the overall sample size was smaller than planned, however the very limited response rate makes it unlikely that a significant therapeutic effect was missed. Based on the confidence intervals, a maximum of a 12% response rate could have been missed. Third, the study consisted of almost exclusively Asian patients with genotypes B and C, however the IT phase is most frequently observed in Asians who acquire HBV perinatally. Fourth, the median age of participants was 37.2 years and 8 (29%) were above 40 years of age, older than is often seen in the IT phase. Age was not an apparent determinant of response but whether younger patients may have responded differently as suggested by the two responders in the HBRN trial in children is unknown. Fifth, it is possible that a longer duration of combination therapy may have led to greater responses given that in many individuals quantitative HBsAg levels were trending downwards at the end of therapy, however tolerability of peginterferon-based therapy for greater than a year is not likely to be a clinically attractive treatment strategy.
In conclusion, an 8-week lead-in of entecavir followed by addition of 40 weeks of peginterferon was ineffective in adults in the IT phase of chronic HBV infection. The lack of hepatitis flare upon rebound viremia after stopping antiviral therapy suggests that the immune response to HBV in the IT phase is truly distinct from that in the IA phase of disease. Understanding the mechanisms leading to tolerance or at least lack of inflammation may be key to developing therapeutic strategies for the IT phase of chronic HBV.
Supplementary Material
Acknowledgments
Financial Support/Funding:
The HBRN was funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases to the following investigators Lewis R. Roberts, MB, ChB, PhD (DK 082843), Anna Suk-Fong Lok, MD (U01 DK082863), Steven H. Belle, PhD, MScHyg (U01 DK082864), Kyong-Mi Chang, MD (U01 DK082866), Michael W. Fried, MD (U01 DK082867), Adrian M. Di Bisceglie, MD (U01 DK082871), William M. Lee, MD (U01 DK082872), Harry L. A. Janssen, MD, PhD (U01 DK082874), Daryl T-Y Lau, MD, MPH (U01 DK082919), Richard K. Sterling, MD, MSc (U01 DK082923), Steven-Huy B. Han, MD (U01 DK082927), Robert C. Carithers, MD (U01 DK082943), Norah A. Terrault, MD, MPH (U01 DK082944), an interagency agreement with NIDDK: Lilia M. Ganova-Raeva, PhD (A-DK-3002–001) and support from the intramural program, NIDDK, NIH: Marc G. Ghany, MD. Additional funding to support this study was provided to Kyong-Mi Chang, MD, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling, MD, MSc (UL1 TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Norah A. Terrault, MD, MPH (CTSA Grant Number UL1 TR000004), Michael W. Fried, MD (CTSA Grant Number UL1T R001111), and Anna Suk-Fong Lok (CTSA Grant Number UL1 RR024986 and U54 TR001959.) Additional support was provided by Bristol-Myers-Squibb and Genentech via Clinical Trial Agreements with NIDDK and Roche Molecular Systems via a Cooperative Research and Development Agreement (CRADA) through the NIDDK.
List of Abbreviations:
- HBV
Hepatitis B virus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HCC
hepatocellular carcinoma
- HBRN
Hepatitis B Research Network
- HBsAg
hepatitis B surface antigen
- HIV
human immunodeficiency virus
- HBeAg
hepatitis B e antigen
- Anti-HBs
antibody to HBsAg
- Anti-HBe
antibody to HBeAg
- ULN
upper limit of the normal range
- IT
immune tolerant
- IA
immune active
- IC
inactive carrier or immune control
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45(4):1056–1075. [DOI] [PubMed] [Google Scholar]
- 3.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–2063. [DOI] [PubMed] [Google Scholar]
- 4.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology. 2017;67(2):370–398. [DOI] [PubMed] [Google Scholar]
- 5.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cellular & molecular immunology. 2015;12(3):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Antiga L, Aw M, Atkins M, Moorat A, Vergani D, Mieli-Vergani G. Combined lamivudine/interferon-alpha treatment in “immunotolerant” children perinatally infected with hepatitis B: a pilot study. The Journal of pediatrics. 2006;148(2):228–233. [DOI] [PubMed] [Google Scholar]
- 8.Ganova-Raeva L, Ramachandran S, Honisch C, Forbi JC, Zhai X, Khudyakov Y. Robust hepatitis B virus genotyping by mass spectrometry. Journal of clinical microbiology. 2010;48(11):4161–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy PTF, Sandalova E, Jo J, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143(3):637–645. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Chien MH, Huang HP, et al. Distinct hepatitis B virus dynamics in the immunotolerant and early immunoclearance phases. Journal of virology. 2010;84(7):3454–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong VW, Hui AJ, Wong GL, et al. Four-year Outcomes After Cessation of Tenofovir in Immune-tolerant Chronic Hepatitis B Patients. J Clin Gastroenterol. 2018;52(4):347–352. [DOI] [PubMed] [Google Scholar]
- 12.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–2695. [DOI] [PubMed] [Google Scholar]
- 13.Cao J, Chi H, Yu T, et al. Off-Treatment Hepatitis B Virus (HBV) DNA Levels and the Prediction of Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B: A Prospective Stop Study. The Journal of infectious diseases. 2017;215(4):581–589. [DOI] [PubMed] [Google Scholar]
- 14.Berg T, Simon KG, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. Journal of hepatology. 2017;67(5):918–924. [DOI] [PubMed] [Google Scholar]
- 15.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143(3):629–636 e621. [DOI] [PubMed] [Google Scholar]
- 16.Reijnders JG, Rijckborst V, Sonneveld MJ, et al. Kinetics of hepatitis B surface antigen differ between treatment with peginterferon and entecavir. Journal of hepatology. 2011;54(3):449–454. [DOI] [PubMed] [Google Scholar]
- 17.Tseng TC, Kao JH. Treating Immune-tolerant Hepatitis B. Journal of viral hepatitis. 2015;22(2):77–84. [DOI] [PubMed] [Google Scholar]
- 18.Chan HL, Chan CK, Hui AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology. 2014;146(5):1240–1248. [DOI] [PubMed] [Google Scholar]
- 19.Poddar U, Yachha SK, Agarwal J, Krishnani N. Cure for immune-tolerant hepatitis B in children: is it an achievable target with sequential combo therapy with lamivudine and interferon? Journal of viral hepatitis. 2013;20(5):311–316. [DOI] [PubMed] [Google Scholar]
- 20.Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963–973 e969. [DOI] [PubMed] [Google Scholar]
- 21.Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. Journal of hepatology. 2011;54(2):209–218. [DOI] [PubMed] [Google Scholar]
- 22.Tan AT, Hoang LT, Chin D, et al. Reduction of HBV replication prolongs the early immunological response to IFNalpha therapy. Journal of hepatology. 2014;60(1):54–61. [DOI] [PubMed] [Google Scholar]
- 23.Wong VW, Hui AJ, Wong GL, et al. Four-year Outcomes After Cessation of Tenofovir in Immune-tolerant Chronic Hepatitis B Patients. Journal of clinical gastroenterology. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Reid E, Juleff N, Windsor M, et al. Type I and III IFNs Produced by Plasmacytoid Dendritic Cells in Response to a Member of the Flaviviridae Suppress Cellular Immune Responses. J Immunol. 2016;196(10):4214–4226. [DOI] [PubMed] [Google Scholar]
- 25.Taleb K, Auffray C, Villefroy P, et al. Chronic Type I IFN Is Sufficient To Promote Immunosuppression through Accumulation of Myeloid-Derived Suppressor Cells. J Immunol. 2017;198(3):1156–1163. [DOI] [PubMed] [Google Scholar]
- 26.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


