Abstract
Background:
In patients with biliary atresia (BA), the extent of intrahepatic biliary fibrosis negatively correlates with successful surgical bypass of the congenital cholangiopathy as well as subsequent transplant-free survival. We recently linked the expansion of a population of Prominin-1 (Prom1)-expressing hepatic progenitor cells to biliary fibrogenesis. Herein, we hypothesized that Prom1-expressing progenitor cells play a role in BA-associated fibrosis.
Methods:
Rhesus rotavirus (RRV)-mediated experimental BA was induced in newborn mice homozygous for the transgene Prom1cre-ert2-nlacz, which was knocked in to the Prom1 gene locus, thus creating functional Prom1 knockout (KO) mice, and their wildtype (WT) littermates. Clinical data and tissue samples from BA infants from the Childhood Liver Disease Research Consortium were analyzed.
Results:
Extrahepatic biliary obliteration was present in both WT and KO mice; there was no difference in serum total bilirubin (TBili) levels. The intrahepatic periportal expansion of the PROM1pos cell population, typically observed in RRV-induced BA, was absent in KO mice. RRV-treated KO mice demonstrated significantly fewer CK19pos ductular reactions (p=0.0004) and significantly less periportal collagen deposition (p=0.0001) compared to WT. RRV-treated KO mice expressed significantly less Integrin-β6, which encodes a key biliary-specific subunit of a Transforming Growth Factor-β activator (p=0.0004). Infants with successful biliary drainage (Tbili ≤1.5 mg/dL within 3 months postoperatively), which is highly predictive of increased transplant-free survival, expressed significantly less hepatic PROM1, CK19, and COLLAGEN-1α compared to those with TBili >1.5 (p<0.05).
Conclusion:
Prom1 plays an important role in biliary fibrogenesis, in part via Integrin-mediated TGF pathway activation.
Introduction:
Biliary atresia (BA), a congenital fibro-obliterative cholangiopathy, is the most common cause of pediatric end-stage liver failure and the leading indication for liver transplant in children (1–8). The extent of intrahepatic biliary fibrosis at the time of Kasai hepatoportoenterostomy, an operation designed to bypass obstructed extrahepatic bile ducts, negatively correlates with successful surgical drainage, defined as serum total bilirubin levels <1.5 mg/dL within 3 months of surgery, as well as transplant-free survival (8). Even with successful surgical drainage, most patients experience progressive intrahepatic fibrosis and still progress to end-stage liver failure and cirrhosis. Hence, greater understanding of the pathogenesis of biliary fibrosis is critical to developing novel therapeutic interventions to improve outcomes for infants with BA.
We previously demonstrated the expansion of collagen-producing cells expressing the stem cell marker Prominin-1 (Prom1) within regions of evolving biliary fibrosis both in human BA and in experimental BA induced by Rhesus rotavirus (RRV) in mouse pups (9). Prom1, also known as CD133, is a penta-span transmembrane glycoprotein expressed by stem cells and cancer stem cells in a variety of organs, including the liver (10, 11). Prom1-expressing hepatic progenitor cells (HPCs) possess significant generative capacity, particularly in the newborn (12). Cultured Prom1-expressing HPCs, clonally expanded from Mat1a null mice treated with the hepatotoxin 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), undergo myofibroblastic differentiation and express Collagen-1α1 when treated with pro-fibrogenic Transforming Growth Factor-β (TGFβ). Recent cell lineage tracing analyses indicate that biliary cells involved in ductular reactions derive from Prom1-expressing progenitor cells in association with cholestatic injury (13, 14).
The Integrin family, which comprises a large number of hetero-dimeric cell surface mechanoreceptors that transmit information between cells and the extracellular matrix, play a role in liver fibrosis. A key function of Integrins is the conversion of latent TGFβ to active TGFβ (15). Inhibition of biliary-specific Integrin-αvβ6 abrogates HPC trans-differentiation into biliary ductular reactions during cholestatic liver injury (16, 17). In both human BA and RRV-induced experimental BA, expression of Integrin-αvβ6 is increased and present within ductular reactions (18).
Thus, we hypothesize that Prom1-expressing progenitor cells promote biliary fibrosis associated with BA via the expansion of ductular reactions. Herein, we show that null mutation of Prom1 is associated with decreased ductular reactions, decreased Integrin expression and decreased collagen deposition in experimental BA.
Methods:
Mouse Model of BA.
Prom1cre-ert2-nlacz transgenic mice (Jackson Laboratory, Bar Harbor, ME), in which a Cre recombinase and nuclear β-galactosidase (LacZ) cassette was knocked into the Prominin-1 gene locus (11), were backcrossed into a BALB/c background over at least seven generations (Charles River Laboratories, Wilmington, MA). Thus, mice homozygous for Prom1cre-ert2-nlacz are functionally Prom1 knockouts (KO), whereas mice lacking the Prom1cre-ert2-nlacz transgene are wildtype (WT). Notably, Prom1 KO mice are viable and live to adulthood without gross phenotypic differences to WT littermates. Additionally, mouse body weight, liver size, gross histologic appearance are not different between WT and KO mice (Supplemental Fig 1). Newborn pups were inoculated intraperitoneally (1.5×106 colony forming units RRV or saline control) within the 1st day of life to induce experimental BA (jaundice, acholic stool, obliterated extrahepatic bile ducts) as previously described (19). At two weeks of life, serum and liver were analyzed for bilirubin level, gene expression (quantitative polymerase chain reaction (qPCR) and western blot), and histology (immunofluorescence, Sirius red, and hematoxylin/eosin). All mouse experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Children’s Hospital Los Angeles.
Immunofluorescence and Immunohistochemistry Imaging.
Livers were fixed in 4% paraformaldehyde (Polysciences, Inc., Warrington, PA) and embedded in paraffin or Tissue-Tek® Optimum Cutting Temperature (OCT) Sakura Finetek, Torrance, CA) for sectioning. Immunofluorescence (IF) staining signals for PROM1 (1:50 dilution, eBiosciences, San Diego, CA), CYTOKERATIN-19 (1:100 dilution, gift from JR Friedman, University of Pennsylvania, Philadelphia. PA), and LacZ (1:400, Bioss, Woburn, MA) were detected by secondary antibodies conjugated either with anti-mouse cyanine (Cy) 3, Cy5, anti-rat Cy3/Cy5, or anti-rabbit Cy3/Cy5 (1:200 dilution, Jackson ImmunoResearch Labs, West Grove, PA) (9, 10, 20). Images were then acquired by a Leica DM5500B IF microscope using Leica Suite Advanced Fluorescence (LAS AF) 6000 software (Leica Microsystems, Wetzlar, Germany). Immunohistochemistry was performed using 2.1 μg/mL anti-INTEGRIN-β6 (gift from Shelia Violette, Biogen, Inc). Bright-field images were captured using a Leica DM1000 (DFC290) transmitted light microscope (Leica Microsystems AG, Heerbrugg, Switzerland) with Leica Application Suite (version 2.7.1R1).
Western Blotting Analysis.
Total protein lysates were prepared and western blotting analyses were performed as previously described (9, 10). Please refer to the Supplemental Table for a full list of antibodies.
Polymerase Chain Reaction.
Total RNA was isolated from snap-frozen mouse liver tissues. Complementary DNA synthesis, reverse-transcriptase polymerase chain reaction (RT-PCR), and qPCR were performed, as previously described, using intron-spanning and gene-specific primers (9, 10). See the Supplemental Table for the full list of primers and probes.
Determination of Tissue Fibrosis.
Sirius Red staining was performed as previously described (20) and quantified densitometrically using ImageJ (National Institutes of Health [NIH], Bethesda, MD) software to determine the extent of fibrosis by calculated collagen proportionate area (CPA) (9, 21, 22).
Serum Bilirubin Assay.
Whole mouse blood was obtained at time of euthanasia by direct cardiac puncture. BD Microtainer tubes with Lithium Heparin (BD Bioscience, San Diego, CA) were used to separate serum from whole blood. Total serum bilirubin was determined using a Total Bilirubin Reagent Set (Pointe Scientific, Canton, MI) and standardized using a Bilirubin Calibrator Set (Pointe Scientific, Canton, MI).
Human BA Analyses.
Serum from 30 infants with the diagnosis of BA and 10 with the diagnosis of indeterminate cholestasis (IC) were obtained along with corresponding prospectively collected clinical and available hepatic gene expression data (23) from the Childhood Liver Disease Research Network (ChiLDReN), a multicenter consortium focused on pediatric liver diseases, under a network approved ancillary study. Enzyme-linked immunosorbent assays (ELISA) were performed to determine the amount of PROM1 protein present in each serum specimen (MyBioSource, San Diego, CA). Correlations with hepatic gene expression profiles were obtained via microarray analyses previously performed as described, based on liver samples collected at time of Kasai operation (23). Complete microarray and clinical data sets were available for 18 of the BA patients. All analyses of human data were performed in accordance with a protocol approved by the Institutional Review Board of Children’s Hospital Los Angeles.
Statistical Analysis.
Unpaired t-test, analysis of variance with post-Hoc Tukey’s, Spearman correlation, and Mann-Whitney tests were used for statistical analyses to calculate statistical significance (p<0.05) using GraphPad Prism (GraphPad, La Jolla, CA).
Results:
Null mutation of Prom1 does not lead to changes in extrahepatic biliary obstruction.
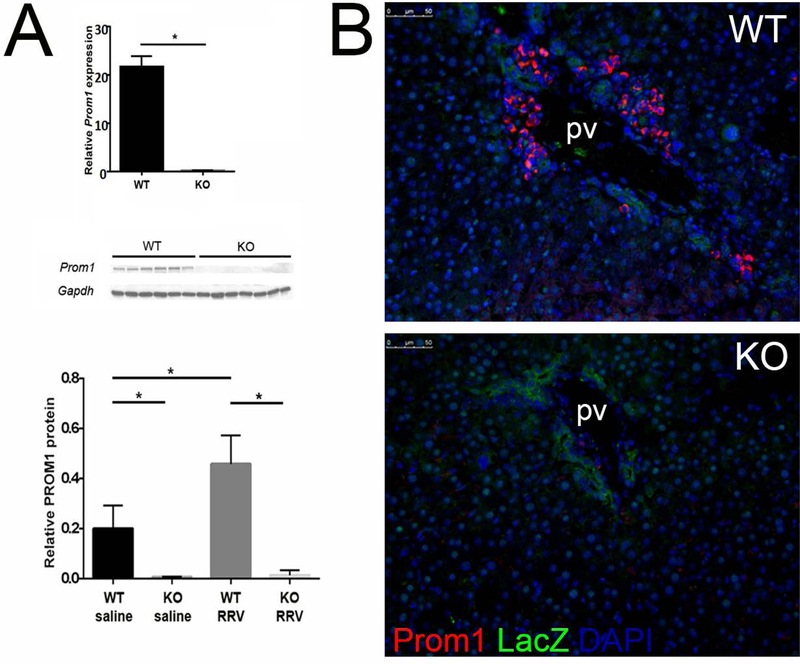
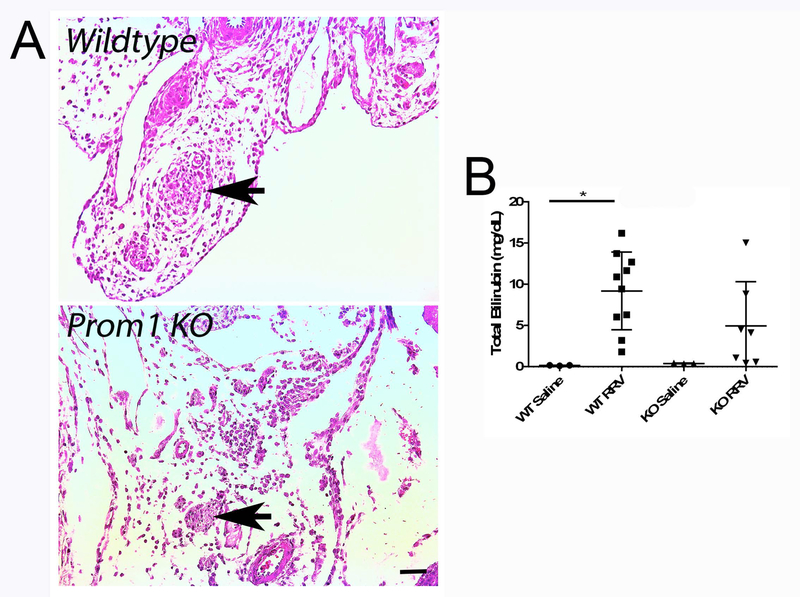
Prom1 expression was significantly decreased in KO livers compared to WT by qPCR (WT=21.59±3.37, KO=0.13±0.25, p<0.0001, n=7), as was PROM1 protein expression by Western blot analysis (WT=3.96±0.24, KO=0.04±0.01, p<0.0001, n=12) (Figure 1A). The robust increase in periportal PROM1pos cells within areas of developing biliary fibrosis, typically observed in Prom1 WT mice by immunofluorescence, was absent in KO mice treated with RRV (Figure 1B), further validating the null mutation phenotype. Importantly, despite Prom1 null mutation, these HPCs are still present and therefore available, as shown by the presence of the LacZ reporter (Figure 1B). Notably, histologic examination revealed equivalent complete extrahepatic biliary obliteration in both WT (7 out of 9) and KO (6 out of 7) mice with RRV treatment (Figure 2A); the remainder of mice manifested near complete luminal obliteration. All RRV-inoculated mice exhibited pale, acholic stool. Survival at 14 days was equivalent between WT and KO (100%). Serum total bilirubin increased significantly in RRV-treated WT mice compared to saline-treated WT controls (9.2±4.7 vs 0.2±0.04 mg/dL, p=0.03, n=23); no significant differences in serum total bilirubin were detected between either WT and KO saline-treated mice (0.2±0.04 mg/dL vs 0.4±0.05 mg/dL, p=0.99) or between WT and KO RRV-treated mice (9.2±4.7 vs 4.9±5.4 mg/dL, p=0.24) (Figure 2B).
Figure 1.
Knockout of Prom1 (A) Absence of PROM1 expression in Prom1 KO mice by qPCR and Western blot at baseline and follow RRV inoculation by immunofluorescence. (B) PROM1 and LacZ expression 14 days after RRV inoculation. Abbreviations: KO, knockout; PV, portal vein; WT, wildtype. Scale bar = 50 µm. *p<0.05.
Figure 2.
Comparison of Prom1 WT and KO Cholestasis Two Weeks Following RRV Inoculation. (A) Hematoxylin and eosin staining of WT and KO extrahepatic structures (arrows denote obliterated bile duct. Scale bar = 25 µm. (B) Serum total bilirubin levels of WT and KO mice two weeks following saline or RRV injection. Abbreviations: KO, knockout; PV, portal vein; RRV, Rhesus rotavirus; WT, wildtype. * p<0.05.
Null mutation of Prom1 is associated with decreased intrahepatic CK19-positive ductular reactions and collagen deposition.
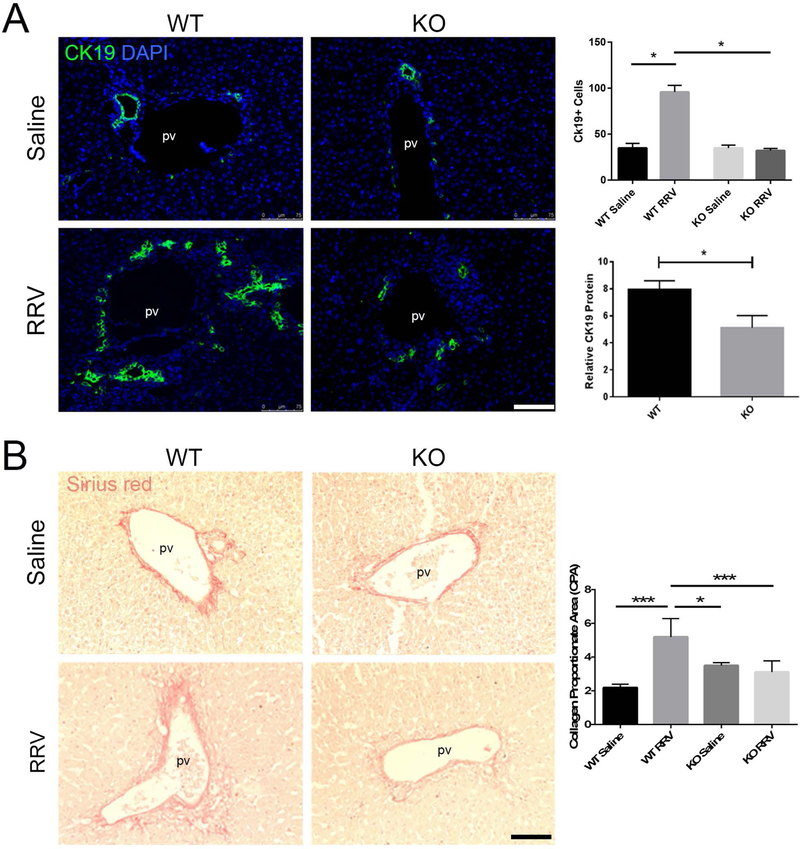
The extent of ductular reactions (DR), typically seen in obstructive cholangiopathies, was assessed. There was no difference in CYTOKERATIN-19 (CK19)-positive cells between saline-injected WT and KO mice (27.4±2.9 vs 32.9±5.5 cells per high power field [hpf], p=0.86, n=6) (Figure 3A). RRV inoculation resulted in a greater ductular reactive response with significantly more CK19-positive DR cells (WT=104.3±14.1, KO=33.6±7.9 cells per hpf, p<0.0001, n=7) and more CK19 protein expression (WT=7.96±1.6, KO=5.12±2.2, p=0.027, n=12) in RRV-treated WT mice compared to KO. Relative Ck19 expression were 0.48±0.2 and 1.2±0.3 in WT and KO RRV treated mice (p=0.0697, n=6).
Figure 3.
Prom1 KO Mitigates RRV Mediated Ductular Reactions and Fibrosis (A) Immunofluorescence of CK19 ductular reactions, cell quantification and western blot analysis. (B) Periportal fibrosis (Sirius red staining) two weeks following saline or RRV inoculation. Abbreviations: CPA, collagen proportionate area; KO, knockout; PV, portal vein; RRV, Rhesus rotavirus; WT, wildtype. Scale bar = 75 µm. *p<0.05, ***p<0.001
Given the link between DR and fibrosis, collagen deposition was evaluated histologically as well. RRV-treated WT mice showed significantly increased collagen deposition by Sirius red staining quantified as CPA, compared to saline controls (RRV=5.2±1.1, Saline=2.2±0.2, p=0.0001, n=18) (Figure 3B). RRV-induced fibrosis was not observed between RRV-treated KO vs. saline control (RRV=3.1±0.7, Saline=3.5±0.2, p=0.93, n=9). Most notably, RRV-treated WT mice displayed significantly greater fibrosis than RRV-treated KO mice (WT=5.2±1.1, KO=3.1±0.7, p<0.0005, n=21). Collagen-1α1 expression by qPCR was significantly lower in KO mice (6.8±10.2 vs. 1.7±1.6, p=0.04, n=22). Relative protein levels of portal fibroblastic markers NTPDase II (3.49±0.49 vs 2.33±0.41, p=0.0007, n=13) and MESOTHELIN (7.420±1.1 vs. 2.141±1.1, p<0.0001, n=13), both markers of activated portal fibroblasts, were lower in KO compared to WT). Given the established role of the inflammatory infiltration in the fibrogenesis of the RRV model, inflammatory cytokines were surveyed with qPCR. There were not statistical differences observed between Prom1 WT and KO RRV injured livers (Supplemental Fig. 2).
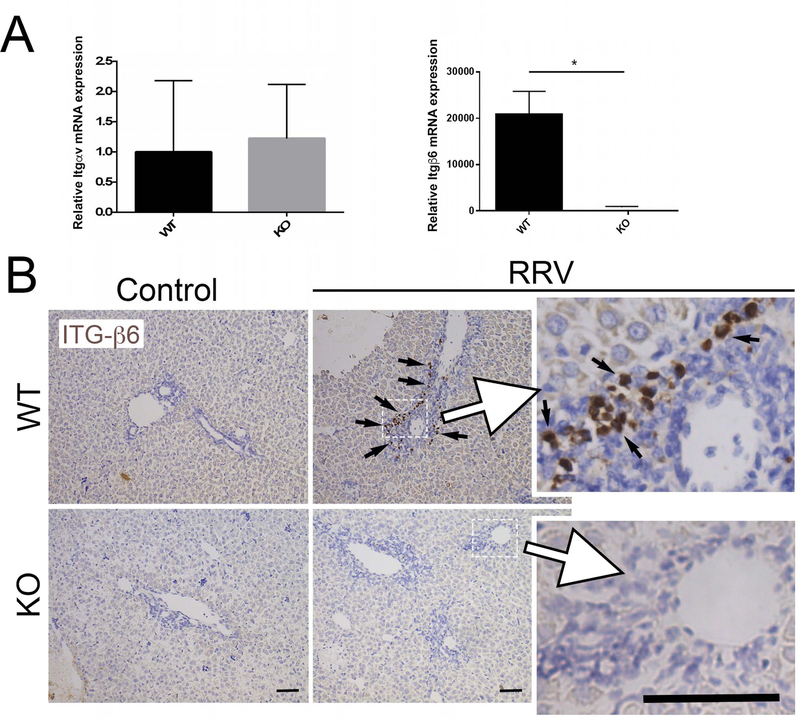
Given that biliary epithelial-specific Integrin-αvβ6 mediates activation of TGFβ, we then sought to determine if null mutation of Prom1 might also decrease expression of genes encoding the Integrin-αv and Integrin-β6 subunits. Integrin-αv expression was not significantly different between WT and KO (1.00±1.18 and 1.22±0.89, p=0.72, n=12, Figure 4A). However, Integrin-β6 expression was significantly lower in the RRV-treated KO mice compared to RRV-treated WT mice (20845±9976 vs. 1.25±0.82, p=0.006, n=8). Immunohistochemistry demonstrated localization of INTEGRIN-β6 within biliary ductular reactions in WT while none was observed in KO (Figure 4B). These data suggest the potential for Prom1 expression to modulate TGFβ-mediated fibrosis through biliary ductular expression of Integrin-β6.
Figure 4.
Impact of Prom1 KO on Integrin Subunit Expression. (A) WT and KO Integrin-αv and Integrin-β6 gene expression two weeks after RRV inoculation. *p<0.05. (B) Immunohistochemical staining for INTEGRIN-β6 in WT and KO mice following saline or RRV inoculation. Black arrows mark positive cells. Abbreviations: ITG-β6, Integrin β6; KO, knockout; RRV, Rhesus rotavirus; WT, wildtype. Scale bar = 25 µm.
Increased PROM1 expression is a feature specific to BA
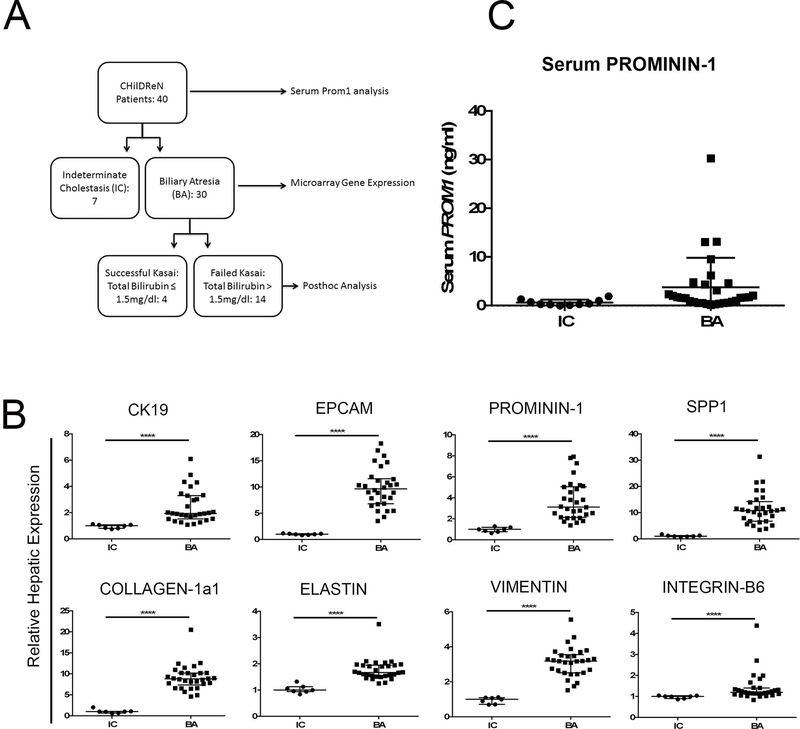
Given the link between Prom1 and ductular proliferation and fibrosis in experimental BA, we sought to determine if BA in human infants might exhibit distinct gene expression patterns from indeterminate cholestasis (IC). Accordingly, liver tissue microarray data from 7 IC and 30 BA patients were obtained through the ChiLDReN network and analyzed for markers of biliary ductular reactions, fibrosis and progenitor cells (Figure 5A). Markers of ductular reactions were upregulated in BA vs IC: CK19 (1.94, 1.99–2.92 vs 1.00, 0.82–1.09, p<0.0001, n=40, Median with 95% C.I.) and EPCAM (9.64, 8.46–11.27 vs 1.00, 0.90–1.14, p<0.0001). Similarly, in BA patients we observed an increase in the fibrosis markers COL-1α1 (8.81, 7.98–10.21 vs 1.00, 0.61–1.49, p<0.0001), ELASTIN (1.67, 1.62–1.92 vs 1.00, 0.89–1.18, p<0.0001), and VIMENTIN (3.19, 2.82–3.45 vs 1.00, 0.78–1.11, p<0.0001). Additionally, there was an increase in progenitor cell marker PROM1 (3.13, 3.03–4.42 vs 1.00, 0.75–1.18, p<0.0001), as well as mediators of fibrosis SPP1 (10.79, 9.30–13.85 vs 1.00, 0.82–1.46, p<0.0001) and INTEGRIN-β6 (1.20, 1.17–1.67 vs 1.00, 0.92–1.04, p<0.0001, Figure 5B)(24). To assess the potential of PROMININ-1 in differentiating IC and BA, we further analyzed serum protein levels from 40 patient (IC=10, BA=30). Although higher serum levels of PROM1 were observed only in BA patients, there was no difference between IC and BA in aggregate (3.74±6.09 vs 0.65±0.61 ng/ml, p=0.12) (Figure 5C).
Figure 5.
Prom1 is increased in Biliary Atresia vs Indeterminate Cholestasis (A) Flow chart of ChiLDReN patients. (B) Hepatic expression of markers of ductular reactions, progenitor cells, and fibrosis in biliary atresia and indeterminate cholestasis patients, by microarray analysis, n=37. (C) Serum level of Prominin-1 in biliary atresia vs indeterminate cholestasis, p=0.12, n=40 Abbreviations: BA, biliary atresia; IC, indeterminate cholestasis. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Successful drainage negatively correlates with PROM1 expression and fibrosis genes at time of Kasai in BA infants.
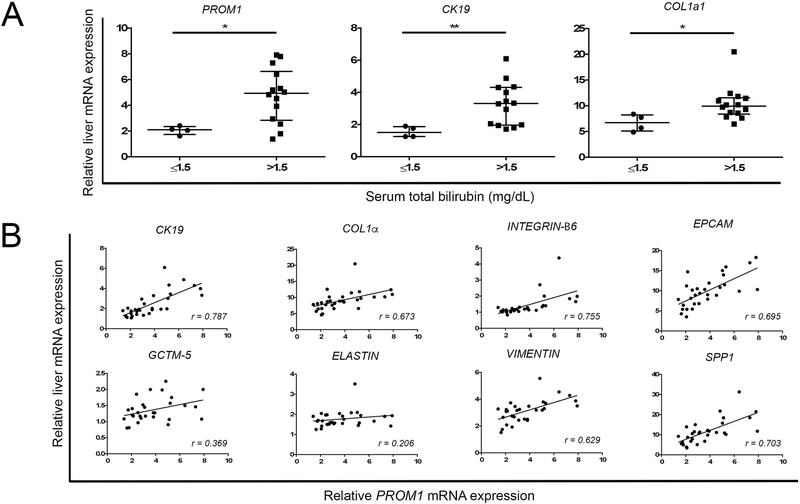
As previously described, infants whose Tbili dropped to ≤ 1.5 mg/dL within 3 months after Kasai portoenterostomy experience much greater survival with native liver. (25) In our cohort of BA patients, age at Kasai (Median 63 vs 66 days, p=0.85, n=18), Ishak fibrosis score (2.0, 2.0–2.0 vs. 2.5, 2.0–3.0, Mann-Whitney test, median and interquartile range, p=0.26, n=18) and preoperative hepatic function panel laboratory tests were not different between those patients who had success drainage (postop TB ≤ 1.5 mg/dL) and those without drainage (>1.5 mg/dL).(Supplemental Fig 3). Post-hoc analysis of post-Kasai BA infants, dichotomized based on successful drainage, had lower levels of PROM1 (2.11, 1.54–2.59 vs 4.93, 3.56–6.00, p=0.024, n=18, Median, 95% C.I.), CK19 (1.52, 1.02–2.09 vs 3.31, 2.53–4.06, p<0.01), and COLLAGEN-1α1 (6.74, 4.08–9.31 vs 9.95, 8.48–12.36, p=0.012) at time of Kasai compared to TB>1.5 (p<0.05). In BA patients, PROM1 expression positively correlated with the biliary epithelial marker, CK19 (r=0.787, p<0.0001), and fibrosis genes, COLLAGEN-1α1 (r=0.673, p<0.0001), INTEGRIN-β6 (r=0.755, p<0.0001), VIMENTIN (r=0.629, p<0.001), and SPP1 (r=0.703, p<0.0001), although not ELASTIN (r=0.206, p=0.27).
Discussion:
In this study, we observed that newborn pups with experimental BA harboring the null mutation of Prom1 exhibit a significant reduction in intrahepatic ductular reactions and periportal fibrosis. This decrease in ductular reactions is also associated with a decrease in Integrin-β6 expression but not Integrin-αv expression. Liver expression of PROM1 is higher in infants with BA compared to those with IC. PROM1 expression strongly correlates with CK19 and COL1α1 expression as well as with other myofibroblastic markers and PROM1 expression is lower in infants post-Kasai with successful drainage. Collectively, these data indicate that Prominin-1 plays an important role in biliary fibrosis associated with BA by promoting the expansion of fibrosis-associated ductular reactions.
The lack of difference in extrahepatic biliary obstruction suggest that RRV tropism for biliary epithelia as previously described (26, 27), remains unaffected by null mutation of Prom1. Our data indicate that the phenotypic impact arises predominantly downstream of the biliary injury in this model. Ductular reactions, the hallmark pathologic finding typical of cholestatic liver injury, arise from Prominin-1 expressing hepatic progenitor cells within the portal triads of the hepatic lobule. (13, 14) Using a thioacetamide-induced chronic liver injury, Kamimoto et al demonstrated that while the majority of intrahepatic biliary epithelial cells undergo a limited number of cell divisions during biliary tree remodeling, a small subset of Prom1-expressing progenitor cells maintain proliferative capacity and continually generate new biliary epithelial cells (13). Similarly, Nguyen et al similarly showed an increasing fraction of biliary epithelial cells comprising ductular reactions arise from Prom1-expressing progenitors following bile duct ligation. Interestingly, Prom1 expression did not increase during hepatotoxic carbon tetrachloride model suggesting that this response may be cholestatic injury-specific (14). Zhu et al demonstrated outward radial expansion of Prom1-expressing progenitor cell lineage following liver injury with DDC into both ductular reactions, as well as hepatocytes, from portal triads (12). We previously demonstrated an association between the expansion of a population PROM1-expressing HPC during RRV-induced experimental BA (9). As Prom1 upregulation is a feature both of adult bile duct ligation as well as neonatal experimental biliary atresia, this may be a common reactive pathway of biliary obstruction. In this study, we show both decreased ductular reactions as well as periportal fibrosis observed in experimental BA with null mutation of Prom1. Notably, the presence of LacZ expression in the Prom1 KO mouse (Figure 1B) indicates that null mutation of Prom1 does not lead to the loss of HPC. These data suggest a potential block in the differentiation pathway from HPC to biliary ductular epithelium with knockout of Prom1.
Popov et al showed that Integrin-αvβ6 is overexpressed de novo by biliary epithelial cells in cirrhotic patients and mice with experimental biliary fibrosis (17). Furthermore, Integrin-αvβ6 is essential for progression of biliary fibrosis and can be targeted therapeutically using selective blocking antibodies or pharmacologic inhibitors (28). Importantly, Peng et al demonstrated that actively proliferating hepatic progenitor cells express Integrin-αvβ6 and that blockage of either Integrin-αv or Integrin-β6 results in arrest of hepatic progenitor cell proliferation and ductular reaction formation, as well as their consequent biliary fibrosis and tumorigenesis (29). Here, we demonstrate a significant reduction in ductular reactions and Integrin-β6 expression in the Prom1 KO mice with RRV-induced BA. Our findings here are consistent with published observations and suggest a similar mechanism in fibrogenesis associated with biliary atresia.
We previously reported a nonsignificant negative correlation between hepatic PROM1 expression at the time of Kasai operation and likelihood of achieving successful biliary drainage, as defined as reaching a serum total bilirubin of ≤1.5 mg/dL within 3 months after Kasai operation, albeit with the highest levels of expression being in the two patients with the highest serum bilirubin levels.(9) In the current study, utilizing microarray analysis data of liver samples from 30 infants with BA enrolled in ChiLDReN, we demonstrate significantly higher levels of PROM1, COL1α1, CK19 expression within failed Kasai cohort. We also show strong positive correlations between PROM1 expression and numerous a set of genes associated with fibrosis, which further supports a potential physiologic link between Prom1 and liver fibrosis. PROM1 levels were significantly higher in the livers of BA infants compared to a cohort of non-BA indeterminate cholestasis livers, indicating a potential BA disease-specific upregulation of expression. Interestingly, while a serum level of PROM1 alone is insufficient to distinguish BA from non-BA diagnostically, the highest serum levels documented were associated with BA, again indicating some degree of disease specificity.
The mechanism by which Prom1 impacts fibrosis associated with cholestasis is unclear. Moreover, little is known regarding the function of Prom1 aside of its presences as a marker of progenitor and stem cells in a variety of organs. In our study, loss of Integrin-αvβ6 associated with KO genotype likely contributes although the link between Prom1 and Integrin-αvβ6 is less clear. We posit that null mutation of Prom1 negatively impacts the cholangiocyte differentiation pathway of HPC within the portal trial. Our data demonstrating decreased CK19 positivity is consistent with this. However, we cannot exclude the possibility that Prom1 directly regulates Integrin-αvβ6.
In summary, we conclude that Prom1 is functionally involved in fibrogenesis associated with BA via transdifferentiation of HPC and expansion of ductular reactions which express profibrogenic Integrin subunits. While further studies are needed, these observations suggest that Prominin-1 may be a reasonable target for anti-fibrogenic strategies in the treatment of biliary atresia.
Supplementary Material
Figure 6.
Prom1 Expression Correlated with Increased Fibrosis in Biliary Atresia (A) Microarray analysis of relative PROM1, CK19, and COL1α expression at time of Kasai portoenterostomy in patients who subsequently had successful drainage (total serum bilirubin ≤1.5 mg/dL within 3 months post-Kasai) compared to those with unsuccessful drainage (total serum bilirubin > 1.5 mg/dL 3 months post-Kasai). (B) Linear correlation of PROM1 expression with various fibrosis-relevant genes. Each point represents a single patient. * denotes p<0.05. ** denotes p<0.01.
Abbreviations:
- BA
Biliary atresia
- ChiLDReN
Childhood Liver Disease Research Network
- CK19
Cytokeratin-19
- COL1α1
Collagen-1α1
- CPA
Collagen proportionate area
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- DR
Ductular reaction
- HPC
Hepatic progenitor cell
- IC
Indeterminate cholestasis
- KO
Knockout
- LacZ
β-galactosidase
- PROM1
Prominin-1
- RRV
Rhesus rotavirus
- TGFβ
Transforming growth factor β
- WT
Wildtype
Footnotes
University of Southern California/Children’s Hospital Los Angeles Institutional Review Board/IACUC
None of the authors have any conflicts of interest to disclose.
Reference:
- 1.Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med 2011;62:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman AG, Mack CL. Biliary atresia: cellular dynamics and immune dysregulation. Semin Pediatr Surg 2012;21:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo A, Esquivel CO. Current options for management of biliary atresia. Pediatr Transplant 2013;17:95–98. [DOI] [PubMed] [Google Scholar]
- 4.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet 2009;374:1704–1713. [DOI] [PubMed] [Google Scholar]
- 5.Zagory JA, Nguyen MV, Wang KS. Recent advances in the pathogenesis and management of biliary atresia. Curr Opin Pediatr 2015;27:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis 2007;27:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007;46:566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Superina R, Magee JC, Brandt ML, Healey PJ, Tiao G, Ryckman F, Karrer FM, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg 2011;254:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavila N, James D, Shivakumar P, Nguyen MV, Utley S, Mak K, Wu A, et al. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 2014;60:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavila N, James D, Utley S, Cu N, Coblens O, Mak K, Rountree CB, et al. Fibroblast growth factor receptor-mediated activation of AKT-beta-catenin-CBP pathway regulates survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells. PLoS One 2012;7:e50401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009;457:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Finkelstein D, Gao C, Shi L, Wang Y, Lopez-Terrada D, Wang K, et al. Multi-organ Mapping of Cancer Risk. Cell 2016;166:1132–1146 e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamimoto K, Kaneko K, Kok CY, Okada H, Miyajima A, Itoh T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen MV, Zagory JA, Dietz WH, Park A, Fenlon M, Zhao M, Xu J, et al. Hepatic Prominin-1 expression is associated with biliary fibrosis. Surgery 2017. [DOI] [PMC free article] [PubMed]
- 15.Patsenker E, Stickel F. Role of integrins in fibrosing liver diseases. Am J Physiol Gastrointest Liver Physiol 2011;301:G425–434. [DOI] [PubMed] [Google Scholar]
- 16.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology 2008;135:660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol 2008;48:453–464. [DOI] [PubMed] [Google Scholar]
- 18.Nadler EP, Patterson D, Violette S, Weinreb P, Lewis M, Magid MS, Greco MA. Integrin alphavbeta6 and mediators of extracellular matrix deposition are up-regulated in experimental biliary atresia. J Surg Res 2009;154:21–29. [DOI] [PubMed] [Google Scholar]
- 19.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004;114:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utley S, James D, Mavila N, Nguyen MV, Vendryes C, Salisbury SM, Phan J, et al. Fibroblast Growth Factor signaling regulates the expansion of A6-expressing hepatocytes in association with AKT-dependent β-catenin activation. . Journal of Hepatology 2014;60:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981;1:431–435]. J Hepatol 2003;38:382–386. [DOI] [PubMed] [Google Scholar]
- 22.Lee WS, Looi LM. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J Gastroenterol 2009;15:5326–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyer K, Kaimal V, Pacheco C, Mourya R, Xu H, Shivakumar P, Chakraborty R, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med 2010;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R, Leung TM, et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 2014;63:1805–1818. [DOI] [PubMed] [Google Scholar]
- 25.Shneider BL, Magee JC, Karpen SJ, Rand EB, Narkewicz MR, Bass LM, Schwarz K, et al. Total Serum Bilirubin within 3 Months of Hepatoportoenterostomy Predicts Short-Term Outcomes in Biliary Atresia. J Pediatr 2016;170:211–217 e211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coots A, Donnelly B, Mohanty SK, McNeal M, Sestak K, Tiao G. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. J Surg Res 2012;177:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty SK, Donnelly B, Bondoc A, Jafri M, Walther A, Coots A, McNeal M, et al. Rotavirus replication in the cholangiocyte mediates the temporal dependence of murine biliary atresia. PLoS One 2013;8:e69069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 2007;46:1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng ZW, Ikenaga N, Liu SB, Sverdlov DY, Vaid KA, Dixit R, Weinreb PH, et al. Integrin alphavbeta6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology 2016;63:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.