Abstract
Background:
(R,S)-ketamine has gained attention for its rapid-acting antidepressant actions in patients with treatment-resistant depression. However, widespread use of ketamine is limited by its side effects, abuse potential, and poor oral bioavailability. The ketamine metabolite, (2R,6R)-hydroxynorketamine, exerts rapid antidepressant effects, without ketamine’s adverse effects and abuse potential, in rodents.
Methods:
We evaluated the oral bioavailability of (2R,6R)-hydroxynorketamine in three species (mice, rats, and dogs) and also evaluated five candidate prodrug modifications for their capacity to enhance the oral bioavailability of (2R,6R)-hydroxynorketamine in mice. Oral administration of (2R,6R)-hydroxynorketamine was assessed for adverse behavioral effects and for antidepressant efficacy in the mouse forced-swim and learned helplessness tests.
Results:
(2R,6R)-hydroxynorketamine had absolute bioavailability between 46–52% in mice, 42% in rats, and 58% in dogs. Compared to intraperitoneal injection in mice, the relative oral bioavailability of (2R,6R)-hydroxynorketamine was 62%, which was not improved by any of the candidate prodrugs tested. Following oral administration, (2R,6R)-hydroxynorketamine readily penetrated the brain, with brain to plasma ratios between 0.67–1.2 in mice and rats. Oral administration of (2R,6R)-hydroxynorketamine to mice did not alter locomotor activity or precipitate behaviors associated with discomfort, sickness, or stereotypy up to a dose of 450 mg/kg. Oral (2R,6R)-hydroxynorketamine reduced forced-swim test immobility time (15–150 mg/kg) and reversed learned helplessness (50–150 mg/kg) in mice.
Conclusions:
These results demonstrate that (2R,6R)-hydroxynorketamine has favorable oral bioavailability in three species and exhibits antidepressant efficacy following oral administration in mice.
Keywords: Ketamine, hydroxynorketamine, depression, metabolite, pharmacokinetics, oral bioavailability
(R,S)-ketamine (ketamine) has gained attention for its rapid antidepressant actions in human clinical trials (Berman et al., 2000; DiazGranados et al., 2010; Price et al., 2014; Zarate et al., 2006). Specifically, in treatment-resistant depressed patients, a single infusion of ketamine reverses depressive symptoms within hours, with effects often lasting up to one week. While its antidepressant efficacy appears superior to that of classical antidepressants – which typically require weeks to take effect and are ineffective in a large number of patients (Gaynes et al., 2009; Insel and Wang, 2009) – the widespread use of ketamine as an antidepressant is limited by its dissociative side effects and abuse potential (Krystal et al., 1994; Sassano-Higgins et al., 2016; Zanos et al., 2018). Moreover, due to its poor oral bioavailability (BA; approximately 17–24% in humans) (Chong et al., 2009; Clements et al., 1982; Grant et al., 1981; Yanagihara et al., 2003), ketamine is most frequently administered via intravenous (i.v.) infusion, making outpatient treatment challenging.
Ketamine is rapidly and stereoselectively metabolized into a number of metabolites, including (R,S)-norketamine, (R,S)-dehydronorketamine, hydroxyketamines, and hydroxynorketamines (HNKs) (Adams et al., 1981; Chang and Glazko, 1974; Moaddel et al., 2010; Woolf and Adams, 1987). A number of HNK metabolites have been described, including the (2,4)-, (2,5)-, and (2,6)-HNKs, (Desta et al., 2012; Moaddel et al., 2010; Woolf and Adams, 1987), with (2R,6R;2S,6S)-HNK being the most prevalent in the plasma of humans (Zarate et al., 2012) and in the plasma and brain of rodents (Can et al., 2016; Moaddel et al., 2016; Zanos et al., 2016) following ketamine administration. Although early studies classified (2R,6R;2S,6S)-HNK as an inactive metabolite due to its lack of anesthetic effects (Leung and Baillie, 1986), more recent studies have established the biological activity of this metabolite (Cavalleri et al., 2018; Chou et al., 2018; Faccio et al., 2018; Ho et al., 2018; Kroin et al., 2018; Moaddel et al., 2013; Paul et al., 2014; Pham et al., 2017; Singh et al., 2016; Wray et al., 2018; Yao et al., 2017; Zanos et al., 2016). Notably, it has been demonstrated that, in mice and rats, intraperitoneal (i.p.) administration of the ketamine metabolite (2R,6R)-HNK exerts rapid and sustained antidepressant-relevant behavioral actions (Chou et al., 2018; Pham et al., 2017; Zanos et al., 2016), and lacks the adverse effects and abuse potential associated with ketamine (Zanos et al., 2016).
We evaluated the exposure to (2R,6R)-HNK in the plasma and brains of mice following oral (p.o.) administration and compared it to that of i.p. and i.v. administration, the routes of administration most commonly utilized when studying the antidepressant actions of ketamine in rodents and humans, respectively. We additionally evaluated the exposure to (2R,6R)-HNK in the plasma and brains of rats, and in the plasma of dogs, following p.o. and i.v. administration. To determine if a prodrug strategy could improve its oral BA, we evaluated the exposure to (2R,6R)-HNK in plasma following administration of five prodrug candidates in mice. We confirmed that (2R,6R)-HNK did not exert overt adverse effects when administered orally and evaluated the oral efficacy of (2R,6R)-HNK in two assays of antidepressant efficacy in mice, the forced-swim and learned helplessness tests.
Materials and methods
Animals
Mice.
Male and female CD-1 mice (Charles River, Raleigh, North Carolina, USA), 9–12 weeks old, were used for pharmacokinetic studies of (2R,6R)-HNK and prodrug candidates, and for all behavioral testing. Mice were acclimated to the University of Maryland Baltimore animal facility (Baltimore, MD, USA) for at least one week prior to testing. Mice were group housed in cages of 4–5 per cage with a constant 12-hour light cycle (lights on/off at 07:00/19:00). Food and water were available ad libitum. All mouse pharmacokinetic and behavioral tests were performed during the light phase at the University of Maryland Baltimore, were approved by the University of Maryland Baltimore Animal Care and Use Committee, and were conducted in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). All mouse tests of antidepressant efficacy were performed by a male experimenter.
Rats.
Male Sprague-Dawley rats (Si Bei Fu Laboratory Animal Technology Co.), 6–8 weeks old, were used for the pharmacokinetic study of (2R,6R)-HNK. Rats were housed with a constant 12-hour light cycle, and food and water were available ad libitum, with the following exception: rats were food fasted overnight prior to dosing and fed four hours after administration on the day of testing. All rat pharmacokinetic studies were performed at Pharmaron (Beijing, China) in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Dogs.
Adult male purebred beagle dogs (Covance Laboratories Inc.), 8–13 kg, were used for the pharmacokinetic study of (2R,6R)-HNK. Animals were housed with a constant 12-hour light cycle and food and water were available ad libitum. All canine pharmacokinetic studies were performed at Covance Laboratories Inc. (Madison, Wisconsin, USA) in compliance with the Animal Welfare Act Regulations.
Drugs
(2R,6R)-HNK HCl (1) was synthesized and characterized as described (Morris et al., 2017; Zanos et al., 2016). Prodrug esters (acetate (2a), propionate (2b), pentanoate (2c), octanoate (2d), and benzoate (2e) esters) of (2R,6R)-HNK, substituted at the hydroxyl group, were synthesized and characterized (see Supplementary Material) at the National Center for Advancing Translational Sciences (National Institutes of Health, Rockville, Maryland, USA). Ketamine HCl was purchased from Sigma-Aldrich (USA). All drugs were dissolved in 0.9% saline.
Mouse pharmacokinetic studies
Dosing and sample collection.
(2R,6R)-HNK was administered i.p., p.o., or i.v. (via the tail vein). In one experimental cohort, 50 mg/kg of (2R,6R)-HNK HCl (43.1 mg/kg free base) was administered either p.o. or i.p. to male mice. In separate experiments, the same dose was administered either p.o. or i.v. to male or female mice to determine absolute BA. Prodrug compounds were administered at the dose of 50 mg/kg (HCl salt; free base concentration 44–45 mg/kg), i.p. and p.o., in separate cohorts of male mice. All compounds were administered in a volume of 7.5 mL/kg for i.p. and p.o. administration, and in a volume of 4.0 mL/kg for i.v. injection. Prior to p.o. administration, mice were briefly anesthetized (<2 min) under 3% isoflurane. For i.v. administration, mice were briefly restrained (<2 min) using a ventilated restraint tube and the tail was gently warmed to encourage vasodilation.
Following (2R,6R)-HNK administration, samples were collected at 0.167, 0.5, 1, 2, and 4 h after drug administration. For all prodrugs, samples were collected at 0.167, 0.5, and 2 h after administration. Mice were deeply anesthetized under 3–4% isoflurane (placed into induction chamber for approximately two minutes) before being decapitated. Trunk blood and whole brains were immediately collected. Blood was collected into 1.5-mL Eppendorf tubes containing 30 μL disodium EDTA (0.5 M, pH 8.0) and kept on ice until plasma collection (<30 min). Blood was then centrifuged at 8000 × g for six minutes at 4°C to obtain plasma. Plasma was collected into clean Eppendorf tubes and stored at −80°C until analysis. Brains were immediately frozen on dry ice and stored at −80°C until analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of plasma and brain samples.
The concentrations of (2R,6R)-HNK in plasma and brain were determined by achiral LC-MS/MS using a previously described protocol with slight modifications (Moaddel et al., 2010, 2015; Paul et al., 2014). The determination of (2R,6R)-HNK was accomplished using an Eclipse XDB-C18 guard column (4.6 mm × 12.5 mm) and Varian Pursuit XRs 5 C18 analytical column (250 × 4.0 mm ID, 5 um). The mobile phase consisted of ammonium acetate (5 mM, pH 7.6) as Component A and acetonitrile as Component B. A linear gradient was run as follows: 0 min 20% B; 5 min 20% B; 15 min 90% B; 20 min 20% B at a constant flow rate of 0.4 mL/ min. The total run time was 30 min per sample.
For plasma samples, the calibration standard for (2R,6R)-HNK ranged from 39.1 to 20,000 ng/mL for (2R,6R)-HNK and 39.1 ng/mL to 5,000 ng/mL for the prodrugs. The quantification of (2R,6R)-HNK was accomplished by calculating area ratios using d4-ketamine (10 μL of 50 μg/mL solution) as the internal standard. Brains were weighed and suspended in 990 μL of water: methanol (3:2, v/v), d4-ketamine (10 μL of 10 μg/mL) was added and the resulting mixture was homogenized on ice with a polytron homogenizer and centrifuged at 21,000×g for 30 min. The supernatant was collected and processed using 1-mL Oasis HLB solid phase extraction cartridges (Waters Corp., Waltham, Massachusetts, USA). The cartridges were preconditioned with 1 mL of methanol, followed by 1 mL of water and then 1 mL ammonium acetate (10 mM, pH 9.5). The supernatants were added to the cartridges, followed by 1 mL of water and the compounds were eluted with 1 mL of methanol. The eluent was transferred to an autosampler vial for analysis. Quality control standards were prepared at 500 ng/mL, 1,000 ng/ml, 2,000 ng/ml and 10,000 ng/ml for brain and 625 ng/ml, 1,250 ng/ml, 5,000 ng/ml and 10,000 ng/mL for plasma.
MS/MS analysis was performed using a triple quadrupole mass spectrometer model API 4000 system from Applied Biosystems/MDS Sciex equipped with Turbo Ion Spray (TIS) (Applied Biosystems, Foster City, California, USA). The data was acquired and analyzed using Analyst version 1.4.2 (Applied Biosystems). Positive electrospray ionization data were acquired using multiple reaction monitoring (MRM). The TIS instrumental source settings for temperature, curtain gas, ion source gas 1 (nebulizer), ion source gas 2 (turbo ion spray) and ion spray voltage were 600°C, 25 psi, 60 psi, 60 psi and 5500 V, respectively. The TIS compound parameter settings and MRM transitions are provided in Supplementary Material Table S1.
Rat pharmacokinetic studies
Dosing and sample collection.
A single 10 mg/kg dose of (2R,6R)-HNK HCl (10 mg/kg free drug dose; equivalent 11.5 mg/kg HCl salt) was administered p.o. in a volume of 10 mL/kg or i.v. (via the tail vein) in a volume of 5 mL/kg. At 0.083 (i.v. only), 0.25, 0.5, 1, 2, 4, 6 (p.o. only), 8, and 24 h after administration, blood and whole brains were collected. Blood was collected via cardiac puncture into tubes containing potassium EDTA and kept on ice until plasma collection. Plasma was obtained via centrifugation at 4000 × g for five minutes at 4°C. Following exsanguination, animals were transcardially perfused with approximately 20 mL of saline, and brains were collected and immediately frozen. Plasma and brains were stored at −75±15°C until analysis.
LC-MS/MS analysis of plasma and brain samples.
Brains were homogenized in water (1:3 weight: volume ratio). Plasma proteins were precipitated with acetonitrile and centrifugation; the supernatants were diluted 1:3 with water. The concentrations of (2R,6R)-HNK in plasma and brain were determined by achiral LC-MS/MS using a Phenomenex Synergi Polar-RP analytical column (3×50 mm, 2.5 μm). The mobile phase consisted of 98% acetonitrile with 0.1% formic acid as Component A and 95% with 0.1% formic acid as Component B. A linear gradient was run as follows: 0–0.20 min 0% B, 1.90 min 85% B, 2.31 min 0% B with a flow rate of 0.5 mL/min. The quantification of (2R,6R)-HNK was accomplished by calculating area ratios of (2R,6R)-HNK and the internal standard dexamethasone. MS/MS analysis was performed using an API Sciex Qtrap 6500 mass spectrometer (Applied Biosystems). Calibration standards for (2R,6R)-HNK ranged from 0.50–1000 ng/mL. Quality control standards were prepared at 1.00, 2.00, 50.0, and 800 ng/mL. Data were acquired using MRM. The TIS compound parameter settings and MRM transitions for (2R,6R)-HNK and dexamethasone are provided in Supplementary Material Table S2.
Canine pharmacokinetic study
Dosing and sample collection.
A single 10 mg/kg dose of (2R,6R)-HNK HCl (10 mg/kg free drug dose; equivalent 11.5 mg/kg HCl salt) was administered p.o. or i.v. (via a cephalic vein) in a volume of 1.0 mL/kg. For i.v. administration, the dosing apparatus was flushed with approximately 2 mL of saline prior to removing the needle. For p.o. administration, the gavage tube was flushed with approximately 5 mL of water prior to its removal. At 0.083 (i.v. only), 0.167, 0.5, 1, 2, 4, 8, 12 and 24 h after administration, blood (approximately 1 mL) was collected via the jugular vein into tubes containing potassium EDTA. Blood was stored on ice until plasma collection (<1 h). Plasma was obtained via centrifugation (4000×g for five minutes at 4°C) and samples were stored at −70°C until analysis.
LC-MS/MS analysis of plasma.
The concentrations of (2R,6R)-HNK in dog plasma were determined by achiral LC-MS/MS. Plasma samples were extracted and proteins were precipitated in acetonitrile (300 μL of acetonitrile containing 10.0 ng/mL of the internal standard d4-ketamine was added to 50.0 μL of plasma) with centrifugation; the supernatants were evaporated under nitrogen and reconstituted in 5% methanol in water. The determination of (2R,6R)-HNK was accomplished using a Waters Atlantis T3 (50×2.1 mm, 3 μm) column. The mobile phase consisted of 10 mM ammonium acetate with 0.1% formic acid as Component A and methanol with 0.1% formic acid as Component B. A linear gradient was run as follows: 0–0.60 min 0% B, 1.00 min 80% B, 2.10 min 10% B at a constant flow rate of 0.5 mL/min. The autosampler and column temperatures were 5°C and 40°C, respectively. The quantification of (2R,6R)-HNK was accomplished by calculating area ratios using d4-ketamine as the internal standard. MS/MS analysis was performed using a Sciex API-5000 mass spectrometer (Applied Biosystems) with positive electrospray ionization. The TIS settings for temperature, curtain gas, collision gas, and ion spray voltage were 600°C, 30 psi, 10 psi, and 5500 V, respectively. The data were acquired and analyzed using Analyst (version 1.6.2). The calibration standards for (2R,6R)-HNK ranged from 1.00 to 1000 ng/mL for (2R,6R)-HNK. Quality control standards were prepared at 3.00, 40.0, 400, and 800 ng/mL. Data were acquired using MRM. The TIS compound parameter settings and MRM transitions for (2R,6R)-HNK and d4-ketamine are provided in Supplementary Material Table S3.
Bioanalysis of (2R,6R)-HNK
Screening of (2R,6R)-HNK and candidate prodrugs.
The bioanalysis of (2R,6R)-HNK and candidate prodrugs was carried out for three timepoints (0.167, 0.5 and 2.0 hours). The area under the curve (AUC) of plasma concentration versus time was calculated using Phoenix WinNonlin (version 7.0, Certara, St Louis, Missouri, USA). AUClast was calculated as AUC0→t (where t is the time of the last measurable concentration). Relative oral BA was calculated as the ratio of the plasma AUClast of p.o. to that of i.p.
Pharmacokinetic analysis.
The pharmacokinetic parameters of (2R,6R)-HNK (five timepoints) were calculated using non-compartmental analysis (Model 200) in the pharmacokinetic software Phoenix WinNonlin (version 7.0, Certara, St Louis, Missouri, USA). The AUC of plasma and tissue concentration versus time was calculated using the linear trapezoidal method. The slope of the apparent terminal phase was estimated by log linear regression using at least three data points and the terminal rate constant (λ) was derived from the slope. AUC0→∞ was calculated as the sum of the AUC0→t (where t is the time of the last measurable concentration) and Ct/λ. Relative BA was calculated as the ratio of the plasma AUC0→∞ of p.o. to that of i.p. administration. Absolute BA was calculated as the ratio of the plasma AUC0→∞ of p.o. to that of i.v. administration. The apparent terminal half-life (t½) was calculated as 0.693/λ.
Behavioral studies
For all behavioral studies, (2R,6R)-HNK was administered i.p. or p.o. (anesthesia was not utilized for behavioral studies). Ketamine was administered i.p.
Open-field test.
Mice were placed into an open field arena (50 cm × 50 cm × 38 cm; length×width×height) for a 60-minute habituation period. Mice then received saline or (2R,6R)-HNK (15, 50, 150, and 450 mg/kg, p.o.) and open-field locomotor activity was assessed for an additional 60 min. Distance travelled and time spent in the central 50% of the arena were analyzed using TopScan software (v2.0; CleverSys, Inc.).
Adverse effects screening.
Mice received a single dose of (2R,6R)-HNK (450 mg/kg, p.o.) and were immediately placed into a clear Plexiglass arena (40 cm × 40 cm × 40 cm) and observed at pre-defined intervals over 60 min. Specifically, each mouse was observed for 30 s every two minutes for the first 20 min following injection, and then 30 s every 10 min from 30–60 min post-injection. The occurrence of the following behaviors, which are associated with discomfort, sickness, and/or stereotypy were scored during each 30-second observation period (adapted from Kelley, 2001): tail flicking (score=0 (absent) or 1 (present)), eye ptosis (i.e. orbital tightening; score=0 (absent) or 1 (present)), piloerection (hair erected; score=0 (absent) or 1 (present)), sniffing (defined as sniffing of the ground of greater intensity than baseline activity; score number bouts), backwards movement (score number bouts), rearing (score number bouts) and grooming (score number bouts). The total score for each behavior was calculated per animal.
Forced-swim test.
Each mouse received an oral gavage (p.o.) immediately followed by a systemic (i.p.) injection. The following treatment groups were included: p.o. saline and i.p. saline; p.o. saline and i.p. ketamine (15 mg/kg); p.o. (2R,6R)-HNK (15, 50, and 150 mg/kg) and i.p. saline. The forced-swim test was performed as previously described (Zanos et al., 2016). Briefly, mice were placed into a clear Plexiglass cylinder (20 cm height × 15 cm diameter) filled to a depth of 15 cm with water (23±1°C) and subjected to a six-minute swim session. Mice were tested in an initial session one hour after treatment and were re-tested 24 h after treatment. Swim sessions were recorded using a digital video camera. The time spent immobile (defined as passive floating with no movements other than those necessary to keep the head above water) was scored during the final four minutes of the session. Sessions were scored by a trained experimenter blind to the treatment groups.
Learned helplessness test.
The learned helplessness test was performed as previously described (Zanos et al., 2016). In brief, mice were placed into one side of a two-chambered shuttle box (Coulbourn instruments) and administered inescapable shocks (day 1; 120 shocks, 0.45 mA). Twenty-four hours later (day 2), mice were screened for “helplessness,” wherein mice were placed into one side of the shuttle box and administered a series of escapable shock trials (i.e. the shuttle door opened with the shock to allow escape to the other side; 30 trials, 0.45 mA). Helpless mice were defined as those with at least 20 total escape failures and at least five escape failures in the last 10 trials. Helpless mice were assigned to treatment groups such that there were no baseline differences in the number of escape failures during screening (p.o. saline and i.p. saline, 25.55±1.02; p.o. saline and i.p. (2R,6R)-HNK, 25.36±0.97; p.o. (2R,6R)-HNK (50 mg/kg) and i.p. saline, 25.91±1.18; p.o. (2R,6R)-HNK (150 mg/kg) and i.p. saline 25.90±1.03; data reported as mean±standard error of the mean (SEM)). On day 3, all helpless mice received an oral gavage (p.o.) immediately followed by an i.p. injection. The following groups were included: p.o. saline and i.p. saline; p.o. saline and i.p. (2R,6R)-HNK (50 mg/kg); and p.o. (2R,6R)-HNK (50 and 150 mg/kg) and i.p. saline. Twenty-four hours after treatment (day 4), mice underwent escapable shock testing (45 trials, 0.45 mA) to assess reversal of helplessness. During the testing phase, the door opened simultaneously with shock onset for the first five trials and with a two-second delay for the remaining 40 trials. The number of escape failures during these last 35 trials and the final 10 trials were used to assess helplessness. Escape failures were recorded using GraphicState software (v3.1; Coulbourn instruments).
Statistical analysis.
All behavioral tests were performed with the experimenter blind to treatment groups and mice were randomly assigned to treatment groups. Statistical analyses were performed using GraphPad Prism software (v6; GraphPad Software, Inc.). All statistical tests were two-tailed, and significance was assigned at p<0.05. When three or more groups were included (open-field test, learned helplessness test, and forced-swim test) one-way analysis of variance (ANOVA) was performed followed by Holm–Šídák post-hoc comparison (with all groups compared to saline control group) when significance was reached. When two groups were included (i.e. adverse effects screening outcomes) an unpaired Student’s t-test was used. Significant results are indicated with asterisks in the figures (*p<0.05, **p<0.01, ***p<0.001).
Results
BA and pharmacokinetic profile of (2R,6R)-HNK in mice
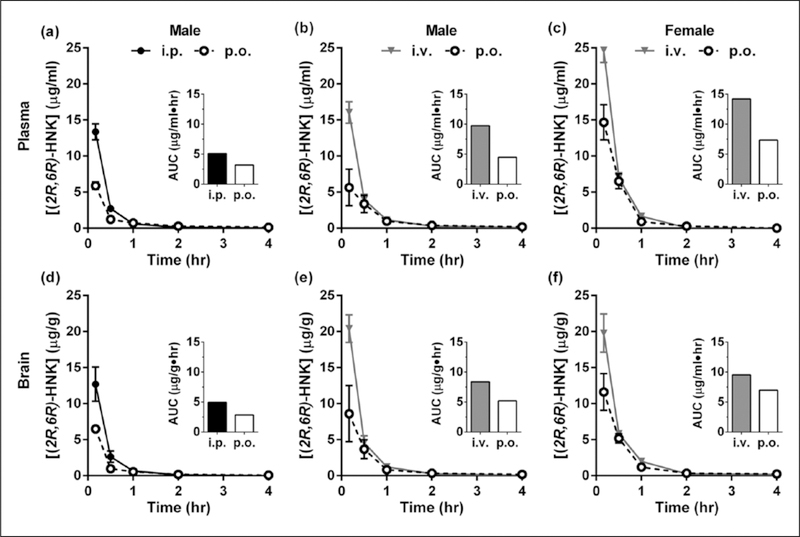
In mice, (2R,6R)-HNK HCl (50 mg/kg) was administered via three different routes (i.p., p.o., and i.v.) to evaluate its BA and pharmacokinetic profile in the plasma and brain. Samples were collected at five time points, ranging from 10 min (0.167 h) to four hours following administration and concentrations of (2R,6R)-HNK were quantitated via LC-MS/MS. For all routes of administration, peak concentrations in the plasma and brain were achieved within 10 min of administration (Tmax=0.167 h; see Figure 1). The half-life (t1/2) was between 0.2–0.8 h in the plasma and 0.5–0.7 h in the brain. Maximum concentrations (Cmax), AUC, BA, and brain penetrance (brain to plasma ratio) are summarized in Tables 1–2.
Figure 1.
Plasma and brain pharmacokinetics of (2R,6R)-hydroxynorketamine (HNK) in mice. (a)–(c) Plasma concentrations of (2R,6R)-HNK following (a) oral (p.o.) or intraperitoneal (i.p.) administration of a single 50 mg/kg dose to male mice, (b) p.o. or intravenous (i.v.) administration of 50 mg/kg to male mice, and (c) p.o. or i.v. administration of 50 mg/kg to female mice. (d)–(f) Brain concentrations corresponding to dosing parameters described in (a)–(c), respectively. Inset: area under the curve (AUC). Data are the mean±standard error of the mean (SEM); n=3–4/group.
Table 1.
Distribution of (2R,6R)-hydroxynorketamine (HNK) in the plasma and brain of mice.
| Sex | Cmax (plasma, μg/mL; brain μg/g) |
AUC0→∞ (plasma, μg/mL×h; brain μg/g×h) |
t1/2 (h) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p.o. | i.p. | i.v. | p.o. | i.p. | i.v. | p.o. | i.p. | i.v. | ||
| Plasma | Male | 5.64−5.90 | 13.4 | 16.0a | 3.18−4.49 | 5.12 | 9.76 | 0.8 | 0.5 | 0.5 |
| Female | 14.7 | ND | 24.7a | 7.33 | ND | 14.2 | 0.3 | ND | 0.2 | |
| Brain | Male | 6.49−8.60 | 12.7 | 20.4a | 2.85−5.19 | 4.94 | 8.38 | 0.7 | 0.4 | 0.5 |
| Female | 11.6 | ND | 19.8a | 6.96 | ND | 9.53 | 0.7 | ND | 0.6 | |
AUC: area under the curve; Cmax: maximum concentration; i.p.: intraperitoneal; i.v.: intravenous; ND: not determined; p.o.: oral; t1/2: half-life.
Parameters calculated from five sampling timepoints following a single dose of 50 mg/kg (43.1 mg/kg free base).
Concentrations measured 10 min after i.v. dosing; values not representative of actual peak.
Table 2.
Bioavailability (BA) and brain penetrance of (2R,6R)-hydroxynorketamine (HNK) in mice.
| Sex | Relative BA (%) | Absolute BA (%) | Brain to plasma ratio |
||
|---|---|---|---|---|---|
| p.o. | i.p. | i.v. | |||
| Male | 62 | 46 | 0.90−1.16 | 0.96 | 0.86 |
| Female | ND | 52 | 0.95 | ND | 0.67 |
i.p.: intraperitoneal; i.v.: intravenous; ND: not determined; p.o.: oral.
Parameters calculated from five sampling timepoints following a single dose of 50 mg/kg (43.1 mg/kg free base).
Following p.o. or i.p. administration of 50 mg/kg of (2R,6R)-HNK to male mice, (Figure 1(a) and (d) and see Tables 1–2), the relative oral BA was 62%. Following p.o. administration, the maximal plasma concentrations (5.90 μg/mL) were 44.1% of that of i.p. administration (13.4 μg/mL). In the brain, the maximal concentrations (6.49 μg/g) were 51% of that of i.p. administration (12.7 μg/g). The ratios of brain to plasma exposure (brain penetrance) were similar for p.o. (0.90) and i.p. (0.96) administration.
In separate experiments, 50 mg/kg of (2R,6R)-HNK was administered either p.o. or i.v. to male (Figure 1(b) and (e) and see Tables 1–2) and female (Figure 1(c) and (f) and see Tables 1–2) mice to assess its absolute oral BA. The absolute oral BA was 46% in males and 52% in females. At the earliest sampling timepoint (10 min after dosing), concentrations following p.o. administration were 35% and 42% of those achieved following i.v. administration, in the plasma (5.64 vs 16.0 μg/mL) and brain (8.60 vs 20.4 μg/g), respectively. Brain penetrance was similar for p.o. (1.16) and i.v. (0.86) administration. Following i.v. administration, the clearance of (2R,6R)-HNK was 74 mL/min/kg in males and 51 mL/min/kg in females (see Table 3). The apparent steady-state volume of distribution was estimated to be 1.7 and 0.80 L/kg in male and female mice, respectively (see Table 3).
Table 3.
Dose-normalized area under the curve (AUC), clearance (Cl), and apparent volume of distribution at steady-state (Vss) following administration (2R,6R)-hydroxynorketamine (HNK) to mice, rats, and dogs.
| Species | Sex | AUC/dose (μg×h×kg/mL/mg) |
Cl (mL/min/kg) |
Vss(L/kg) |
|
|---|---|---|---|---|---|
| i.v. | p.o. | i.v. | i.v. | ||
| Mouse | Male | 0.226 | 0.104 | 74 | 1.7 |
| Female | 0.330 | 0.170 | 51 | 0.80 | |
| Rat | Male | 0.627 | 0.265 | 27 | 7.5 |
| Dog | Male | 0.783 | 0.454 | 21 | 1.2 |
i.v.: intravenous; ND: not determined; p.o.: oral.
BA and pharmacokinetic profile of (2R,6R)-HNK in rats
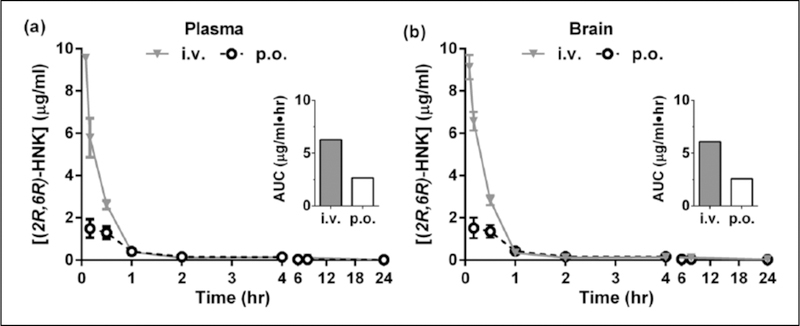
In rats, (2R,6R)-HNK (10 mg/kg free base dose) was administered p.o. and i.v. to evaluate its absolute BA and pharmacokinetic profile in the plasma and brain. The oral BA of (2R,6R)-HNK in rats was 42%. For all routes of administration, peak concentrations in the plasma and brain were measured at the earliest sampling timepoints (i.e. five minutes or 0.083 h for i.v. and 10 min or 0.167 h for p.o. administration; see Figure 2). The half-life (t1/2) was estimated to be 6.9 h in the plasma and 5.7 h in the brain for p.o. administration and was 8.0 h and 8.8 h in the plasma and brain, respectively, for i.v. administration. The brain to plasma ratio was 0.97 for both i.v. and p.o. administration. Cmax and AUC are summarized in Table 4. For i.v. administration, the clearance and volume of distribution were estimated to be 27 mL/min/kg and 7.5 L/kg, respectively (see Table 3).
Figure 2.
Plasma and brain pharmacokinetics of (2R,6R)-hydroxynorketamine (HNK) in rats. Concentration of (2R,6R)-HNK in the (a) plasma and (b) brain of male Sprague-Dawley rats, following a single intravenous (i.v.) or oral (p.o.) dose of 10 mg/kg. Inset: area under the curve (AUC).
Table 4.
Distribution of (2R,6R)-hydroxynorketamine (HNK) in the plasma and brain of rats.
| Cmax (μg/mL) |
AUC0→∞ (μg/mL×h) |
t1/2 (h) |
BA (%) | ||||
|---|---|---|---|---|---|---|---|
| p.o. | i.v. | p.o. | i.v,. | p.o. | i.v. | ||
| Plasma | 1.50 | 9.56a | 2.65 | 6.27 | 6.9 | 8.0 | 42 |
| Brain | 1.52 | 9.13a | 2.58 | 6.09 | 5.7 | 8.8 | ND |
AUC: area under the curve; BA: bioavailability; Cmax: maximum concentration; i.v.: intravenous; ND: not determined; p.o.: oral; t1/2: half-life.
Parameters calculated from eight sampling timepoints following a single dose of 10 mg/kg (free drug dose).
Concentrations measured five minutes after i.v. dosing; values not representative of actual peak.
BA and pharmacokinetic profile of (2R,6R)-HNK in dogs
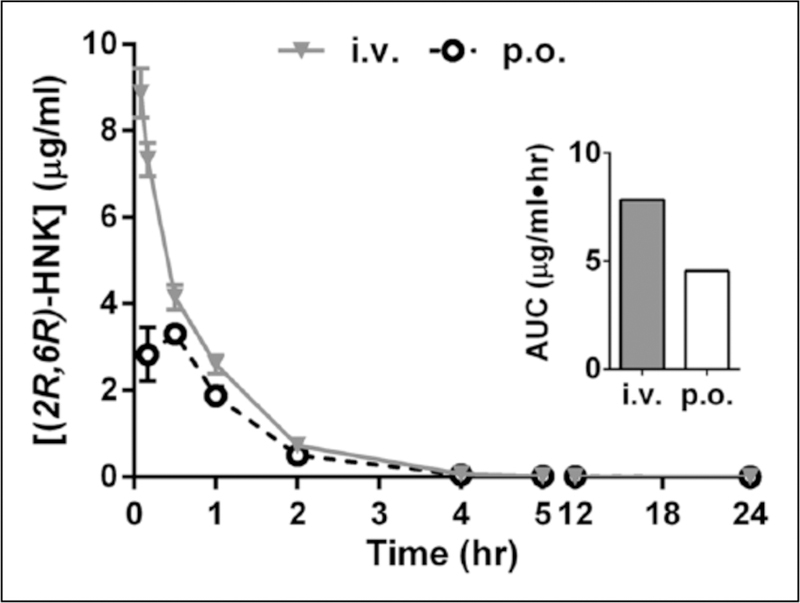
Following administration of a single dose of (2R,6R)-HNK (10 mg/kg free base dose) in beagle dogs (Figure 3), peak plasma concentrations were observed at the first collection time point (five minutes) following i.v. injection and within 30 min following p.o. administration (Tmax=0.28 h). Cmax, AUC, and oral BA are summarized in Table 5. The oral BA was 58%, similar to the values measured in mice. For i.v. and p.o. administration, t1/2 was estimated to be 1.5 and 1.6 h, respectively. Following i.v. administration, the clearance and volume of distribution were estimated to be 21 mL/min/kg and 1.2 L/kg, respectively (see Table 3).
Figure 3.
Oral bioavailability of (2R,6R)-hydroxynorketamine (HNK) in male beagles. Plasma concentrations of (2R,6R)-HNK following oral (p.o.) or intravenous (i.v.) administration of a single 10 mg/kg dose to male beagles. Inset: area under the curve (AUC). Data are the mean±standard error of the mean (SEM); n=3/route.
Table 5.
Distribution of (2R,6R)-hydroxynorketamine (HNK) in the plasma of beagle dogs.
| Cmax (μg/mL) |
AUC0→∞ (μg/mL×h) |
t1/2 (h) |
BA (%) | |||
|---|---|---|---|---|---|---|
| p.o. | i.v. | p.o. | i.v. | p.o. | i.v. | |
| 3.44 | 8.87a | 4.54 | 7.83 | 1.5 | 1.6 | 58 |
AUC: area under the curve; BA: bioavailability; Cmax: maximum concentration; i.v.: intravenous; p.o.: oral; t1/2: half-life;
Parameters calculated from 8–9 sampling timepoints following a single dose of 10 mg/kg (free drug dose).
Concentrations measured five minutes after i.v. dosing; values not representative of actual peak.
Relative oral BA of (2R,6R)-HNK prodrugs candidates in mice
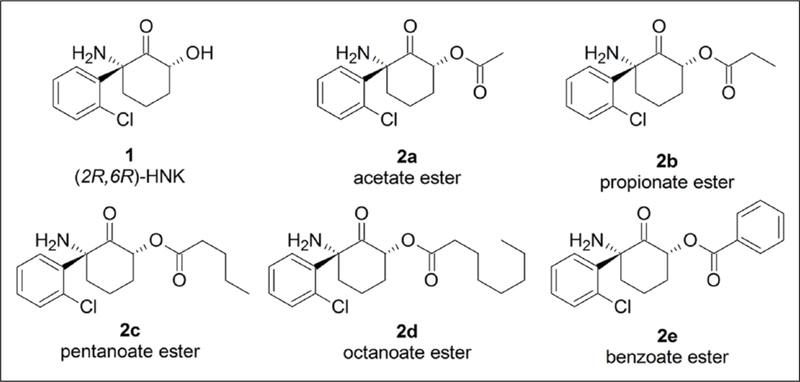
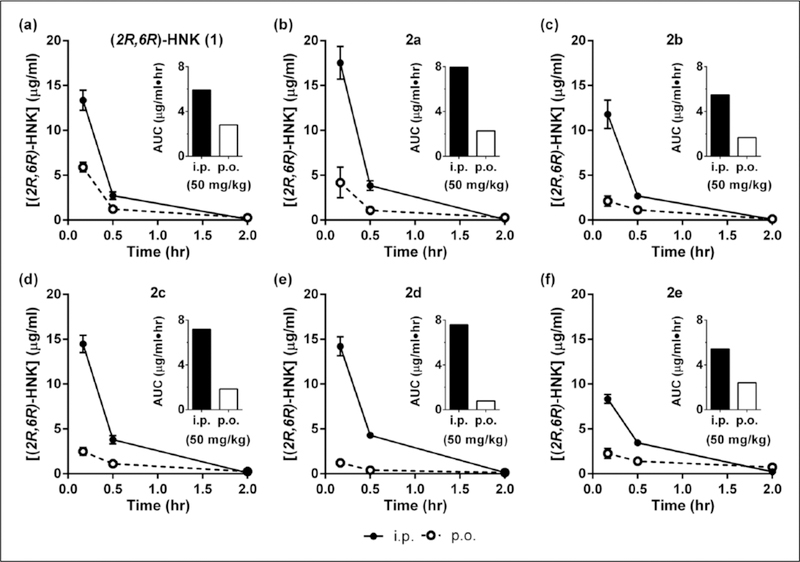
In an effort to improve the oral BA of (2R,6R)-HNK, five prodrug candidates were synthesized by introducing a series of ester modifications onto the free hydroxyl group of (2R,6R)-HNK (see Figure 3). We hypothesized that increasing the lipophilicity of (2R,6R)-HNK via these modifications may increase its oral absorption (see Gao et al., 2017). Once absorbed, the prodrug is converted to the parent drug by circulating esterases (reviewed in Hamada, 2017; Zawilska et al., 2013).
The relative oral BA of five (2R,6R)-HNK prodrug candidates (see Figure 4) was assessed following the administration of a single 50 mg/kg dose (p.o. and i.p.). Plasma was collected at three timepoints (0.167, one, and two hours) and the concentrations of the prodrug and (2R,6R)-HNK were quantitated (Figure 5). To directly compare (2R,6R)-HNK to the prodrugs, the data obtained for (2R,6R)-HNK was reanalyzed with the same three time points included for the prodrug candidates (Figure 5(a)). The choice of three timepoints allowed for a preliminary assessment of whether any prodrug had a robust improvement in the oral BA. The systemic exposure and maximal concentrations of (2R,6R)-HNK are reported in Table 6, with peak concentrations of (2R,6R)-HNK measured at the earliest timepoint (10 min) for all tested compounds. Circulating levels of each prodrug were monitored; however, all were below the limits of quantitation (<10 signal: noise ratio). Following p.o. administration of prodrug candidates, the Cmax of (2R,6R)-HNK varied from 1.23 μg/mL for 2d to 4.18 μg/mL for 2a, whereas administration of (2R,6R)-HNK itself resulted in maximal plasma concentrations of 5.90 μg/mL. Based on the three timepoints, 2d had the lowest systemic exposure following p.o. administration (AUC 0.782 μg/mL/h) and 2a had the highest (AUC 2.26 μg/mL/h), whereas (2R,6R)-HNK itself had an AUC of 2.80 μg/mL/h. We note that the areas calculated from three timepoints in our screening procedure should only be interpreted as relative values and cannot be directly compared with values calculated using the greater number of timepoints. The relative oral BA of the prodrug candidates varied from 10% for 2d to 44% for 2e. When compared at identical timepoints, the relative BA of 2e was similar to, but did not exceed, that of (2R,6R)-HNK itself (47%).
Figure 4.
Candidate prodrug modifications. Chemical structures of the parent compound (2R,6R)-hydroxynorketamine (HNK) (1) parent compound and ester prodrug candidates (2a, 2b, 2c, 2d, and 2e).
Figure 5.
Candidate prodrug modifications do not improve the oral bioavailability of (2R,6R)-HNK in mice. Plasma concentrations of (2R,6R)-HNK following oral (p.o.) (open circles with dashed lines) and systemic (intraperitoneal (i.p.); filled circles with solid lines) administration of a single dose (50 mg/kg) of (a) (2R,6R)-HNK (1) and the (b) acetate (2a), (c) propionate (2b), (d) pentanoate (2c), (e) octanoate (2d), and (f) benzoate (2e) prodrug esters of (2R,6R)-HNK to male mice. Inset: area under the curve (AUC). Data are the mean±standard error of the mean (SEM); n=4/group.
Table 6.
Area under the curve (AUC), maximum concentration (Cmax), and relative bioavailability (BA) of (2R,6R)-hydroxynorketamine (HNK) and prodrug candidates in the plasma of mice.
| Compound | Cmax (μg/mL) |
AUClast (μg/mL×h) |
Relative BA (%) | |||
|---|---|---|---|---|---|---|
| p.o. | i.p. | p.o. | i.p. | |||
| (2R,6R)-HNK (1) | 5.90 | 13.4 | 2.80 | 5.91 | 47 | |
| Prodrug candidates | 2a | 4.18 | 17.5 | 2.26 | 7.96 | 28 |
| 2b | 2.12 | 11.8 | 1.67 | 5.48 | 30 | |
| 2c | 2.49 | 14.5 | 1.84 | 7.18 | 26 | |
| 2d | 1.23 | 14.2 | 0.782 | 7.59 | 10 | |
| 2e | 2.27 | 8.35 | 2.41 | 5.42 | 44 | |
i.p.: intraperitoneal; p.o.: oral.
Parameters calculated from three sampling timepoints following a dose of 50 mg/kg.
Lack of adverse effects of (2R,6R)-HNK in mice
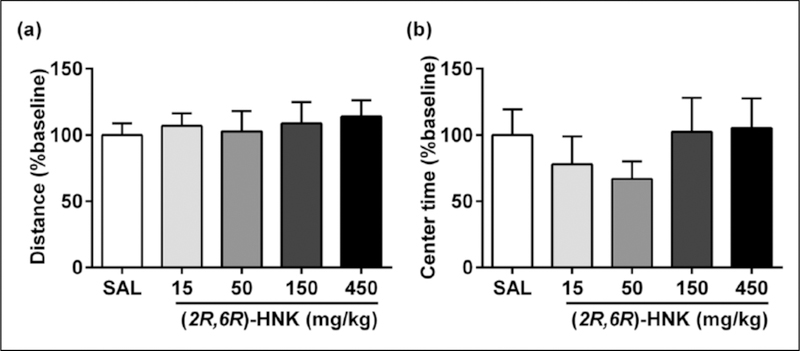
To assess drug effects on locomotion and exploratory behavior, open-field locomotor activity was evaluated following p.o. administration of (2R,6R)-HNK. After a 60-minute habituation period in the open-field arena, mice received (2R,6R)-HNK (15, 50, 150, and 450 mg/kg p.o.) or saline (p.o.). The total distance traveled and time spent in the central 50% of the arena were measured over an additional 60 min after administration. (2R,6R)-HNK did not alter the total distance travelled at any dose tested (p>0.05; Figure 6(a)). Similarly, no significant effect was observed in time spent in the center of the open-field arena (p>0.05; Figure 6(b)).
Figure 6.
(2R,6R)-hydroxynorketamine (HNK) does alter open-field locomotor activity in mice. (a) Oral (p.o.) administration of (2R,6R)-HNK did not alter (a) the total distance travelled (F(4,34)=0.17, p=0.95) or (b) the time spent in the center (central 50%) of the arena (F(4,34)=0.69, p=0.61) at any dose tested (15–450 mg/kg), relative to saline (SAL)-treated controls in the open-field test. Data are the mean±standard error of the mean (SEM); n=7–8/group.
To evaluate additional possible adverse drug effects at a high oral dose, mice were systematically observed for the occurrence of behaviors associated with pain or discomfort, sickness, and stereotypy for one hour following administration of 450 mg/kg of (2R,6R)-HNK or saline (see Table 7). The only behaviors observed consistently were grooming and rearing. Rearing was observed with comparable frequency in both treatment groups, while grooming scores were significantly lower among mice treated with (2R,6R)-HNK (p<0.05). Piloerection was observed in two animals, but occurred equally among saline-(one animal) and (2R,6R)-HNK-treated (one animal) mice, and thus was not attributed to drug effects. Taken together, these observations demonstrate that (2R,6R)-HNK, up to a dose of 450 mg/kg (p.o.), has minimal, if any, adverse effects in mice.
Table 7.
Adverse effects screening outcomes following oral (2R,6R)-hydroxynorketamine (HNK).
| Outcome/Behavior | Mean score±SEM |
|
|---|---|---|
| Saline | (2R,6R)-HNK | |
| Piloerection | 0.25±0.25 | 0.25±0.25 |
| Ptosis | 0 | 0 |
| Tail flicking | 0 | 0 |
| Sniffing | 0 | 0 |
| Backwards movement | 0.75±0.75 | 0 |
| Rearing | 26.50±4.55 | 25.00±6.64 |
| Grooming | 4.25±0.63 | 1.25±0.75a |
SEM: standard error of the mean.
(2R,6R)-HNK administered at 450 mg/kg.
p<0.05; n=4/group.
Oral antidepressant efficacy of (2R,6R)-HNK in mice
The antidepressant efficacy of orally administered (2R,6R)-HNK was evaluated in two established mouse models: the forced-swim test and the learned helplessness test. Injected (i.p.) ketamine and/or (2R,6R)-HNK served as positive controls in both experiments. All mice received an oral gavage immediately followed by an i.p. injection to control for the route of administration.
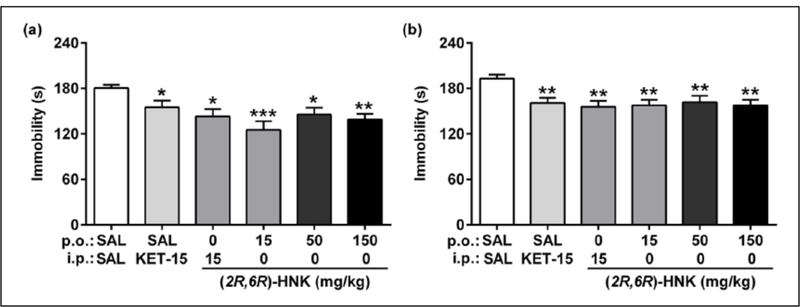
In the forced-swim test, systemic injections of ketamine (15 mg/kg, i.p.) and (2R,6R)-HNK (15 mg/kg, i.p.) reduced immobility time in the forced-swim test both one hour after administration (p<0.05; Figure 7(a)) and in a 24-hour re-test (p<0.01; Figure 7(b)), consistent with the rapid and sustained antidepressant actions of these compounds in this test, as has been previously described (Autry et al., 2011; Li et al., 2010; Pham et al., 2017; Won et al., 2011; Zanos et al., 2016). Orally administered (2R,6R)-HNK also exerted rapid (one hour; Figure 7(a)) and sustained (24 h; Figure 7(b)) reductions in immobility time at doses ranging from 15–150 mg/kg (1-hour test, p<0.05; 24-hour test, p<0.01), establishing the oral efficacy of this compound.
Figure 7.
(2R,6R)-hydroxynorketamine (HNK) reduces immobility time in the mouse forced-swimming test.
Oral (p.o.) (2R,6R)-HNK exerted (a) acute (one hour after treatment; F(5,54)=4.59, p=0.0015) and (b) sustained (24 h after treatment; F(5,54)=3.84, p=0.0048) reductions in immobility time in the forced-swim test, relative to saline (SAL)-treated controls, at all doses tested (15–150 mg/kg). Data are the mean±standard error of the mean (SEM); n=10/group; *p<0.05, **p<0.01, ***p<0.001; i.p.: intraperitoneal.
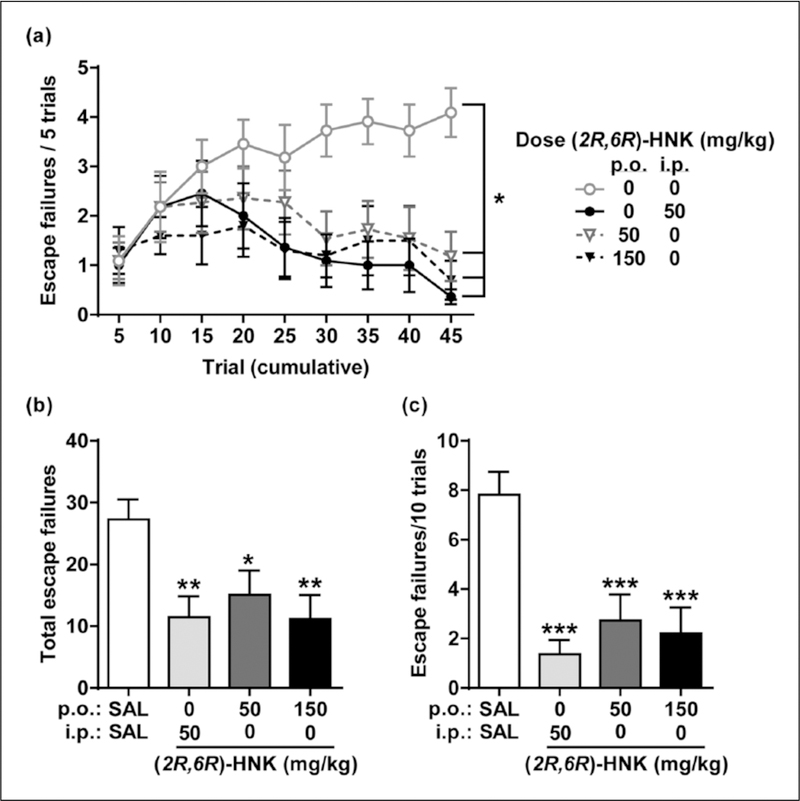
Following inescapable shock induction of learned helplessness, mice received (2R,6R)-HNK, and were tested for reversal of learned helplessness 24 h later. Consistent with a previous report (Zanos et al., 2016) a systemic injection of (2R,6R)-HNK (50 mg/kg, i.p.) reduced the total number of escape failures (p<0.01; Figure 8(a) and (b)) and those in the last 10 trials (p<0.001; Figure 8(c)) relative to saline-treated controls. Orally administered (2R,6R)-HNK (50 and 150 mg/kg, p.o.) was also effective at reversing learned helplessness, as demonstrated by a robust reduction in the number of escape failures (total failures, p<0.05; last 10 trials, p<0.001 Figure 8).
Figure 8.
(2R,6R)-hydroxynorketamine (HNK) reverses learned helplessness in mice. Oral (p.o.) (2R,6R)-HNK was effective at reversing helplessness, assessed via a reduction in (a) the number of escape failures over time (effect of treatment, F(3,39)=4.20, p=0.011; effect of time, F(8,312)=3.89, p=0.0002; subjects, F(39,312)=9.88, p<0.001; interaction, F(24,312)=2.51, p=0.0002), (b) the total number of escape failures (F(3,39)=4.20, p=0.011), and (c) the escape failures during the last 10 testing trials (F(3,39)=10.12, p<0.001), relative to saline (SAL)-treated controls. Data are the mean±standard error of the mean (SEM); n=10–11/group; *p<0.05, **p<0.01, ***p<0.001; i.p.: intraperitoneal; KET: ketamine.
Discussion
It has previously been demonstrated that, following i.p. administration, the ketamine metabolite (2R,6R)-HNK exerts rapid and sustained antidepressant-relevant behavioral actions in mice (Chou et al., 2018; Pham et al., 2017; Zanos et al., 2016), without the adverse effects or abuse potential associated with the parent compound (Zanos et al., 2016). Here, we show for the first time that (2R,6R)-HNK has absolute oral BA of between 46–52% in mice, 42% in rats, and 58% in dogs (see Table 2). The oral BA is similar to that of the (2S,6S)-HNK enantiomer previously reported in rats (approximately 46%) (Moaddel et al., 2015). Compared to i.p. administration, (2R,6R)-HNK has a relative oral BA of 62% in male mice. None of the prodrug modifications provided an improvement over the BA for the parent compound. Further, we demonstrate that (2R,6R)-HNK has oral antidepressant efficacy in the mouse forced-swim and learned helplessness tests.
Circulating plasma and brain concentrations of (2R,6R)-HNK were determined in mice following p.o., i.p., or i.v. administration using five sampling timepoints to compare maximal concentrations and exposure between routes of administration and to determine the oral BA. At a dose of 50 mg/kg in male mice (Figures 7 and 8, and see Zanos et al., 2016), the highest measured concentrations following oral administration (plasma, 5.64–5.90 μg/mL; brain, 6.49–8.60 μg/g) were approximately 2–3-fold lower than those measured after i.p. (plasma, 13.4 μg/mL; brain, 12.7 μg/g) and i.v. (plasma, 16.2 μg/mL; brain, 20.4 μg/g) administration. At the same dose in female mice, the highest observed plasma concentrations following p.o. (14.7 μg/mL) and i.v. (24.7 μg/mL) administration were higher (1.5–2.6×) than those measured in males. Comparing within the female cohort, however, similar to the trends observed in males, the maximal levels achieved following p.o. administration were nearly two-fold (1.7×) lower than those following i.v. administration. The absolute oral BA was 46% in males and 52% in females. Because i.p. injection is most commonly used in rodent studies of ketamine and (2R,6R)-HNK, we also calculated the relative oral BA, a comparison of systemic exposure following p.o. to that of i.p. administration. Relative oral BA of (2R,6R)-HNK was 62%. (2R,6R)-HNK readily penetrated the brain, with brain to plasma ratios ranging from 0.67–1.2 for both sexes and all routes of administration.
The oral BA and pharmacokinetic profile of (2R,6R)-HNK was also evaluated in two additional species: rats and dogs. To compare across species and experiments, the total plasma exposure (AUC) was normalized to the free drug dose (see Table 3). The dose-normalized plasma exposure was higher for rats and dogs than mice for both i.v. (mice, 0.226–0.330; rats, 0.627; dogs, 0.783 μg×h×kg/mL/mg) and p.o. (mice, 0.104–0.170; rats, 0.265; dogs, 0.454 μg×h×kg/mL/mg) administration. The clearance of (2R,6R)-HNK was higher in mice (51–74 mL/min/kg) than in rats and dogs (27 and 21 mL/min/kg, respectively), consistent with the higher rates of hepatic blood flow in mice (approximately 2–3-fold higher in mice relative to rats and dogs; Davies and Morris, 1993). However, despite these inter-species differences, oral BA and brain penetrance was similar between all species tested. The absolute oral BA was estimated to be 42% in rats and 58% in dogs (compared to 46–52% in mice, as discussed above). Similar to the findings in mice, (2R,6R)-HNK readily penetrated the brain of rats (brain to plasma ratio of 0.97) following both i.v. and p.o. administration. Overall, our data demonstrate that (2R,6R)-HNK has favorable oral BA (see Cyriac and James, 2014) in three mammalian species, and readily penetrates the brain of rodents.
In an effort to improve the oral BA of (2R,6R)-HNK, five prodrug candidates were synthesized by introducing a series of ester modifications onto the free hydroxyl group of (2R,6R)-HNK (see Figure 3). An analysis of three sampling timepoints for these prodrug candidates was carried out in mice to determine if a meaningful improvement in relative oral BA was observed, compared to (2R,6R)-HNK. Of note, the maximum circulating concentrations of (2R,6R)-HNK were increased following i.p. administration of 2a compared to (2R,6R)-HNK itself (17.5 μg/mL and 13.4 μg/mL, respectively). Similarly, systemic exposure was increased following i.p. administration of 2a (AUC 7.96 μg/mL×h), 2d (AUC 7.59 μg/mL×h), and 2c (AUC 7.18 μg/mL×h), compared to (2R,6R)-HNK itself (AUC 5.91 μg/mL×h). However, for p.o. administration, maximal concentrations and systemic exposures were decreased with increasing acyl chain length for the prodrug candidates relative to (2R,6R)-HNK, with 2a having the highest concentrations and systemic exposure (4.18 μg/mL and 2.26 μg/mL×h, respectively) and 2d having the lowest (1.23 μg/mL and 0.782 μg/mL×h, respectively) (Table 6). Replacing the acyl chain with a benzoyl group (2e) resulted in a slight increase in systemic exposure (2.40 μg/mL×h) following p.o. administration, compared to the other prodrug candidates. As a result, 2e had the highest relative oral BA (44%) of all the prodrugs tested (see Table 6), which was similar to that of the parent drug (2R,6R)-HNK (47% using identical timepoints). Thus, while we hypothesized that increasing the lipophilicity of (2R,6R)-HNK via these modifications would increase its oral absorption (see Gao et al., 2017), and notably ester cleavage in vivo was exceedingly rapid to reveal the parent drug, none of the ester prodrug candidates tested improved the oral BA of (2R,6R)-HNK. Upon retrospective analysis, (2R,6R)-HNK itself exhibited relatively high oral BA in three species (absolute BA 42–52%) and good oral antidepressant efficacy in mice, which may enable the use of the parent drug itself as an oral treatment.
Our data demonstrate that (2R,6R)-HNK has antidepressant efficacy in the mouse FST and LH test following oral administration. Although maximal plasma and brain concentrations and exposure following oral administration of (2R,6R)-HNK were lower than those achieved following i.p. injection, equivalent doses administered via each route produced similar antidepressant effects in the FST (15 mg/kg) and LH test (50 mg/kg). While the minimal effective doses (not determined in the present study) may vary between routes of administration, these data suggest that the oral BA of (2R,6R)-HNK is sufficiently high such that large doses are not required to exert antidepressant effects via the oral route. We note that, similar to published work with the i.p. route (Zanos et al., 2016), orally administered (2R,6R)-HNK appeared to have a broad effective range (Figure 7) in contrast to ketamine which typically shows a U-shaped antidepressant dose-response (Chowdhury et al., 2017; Li et al., 2010; Zanos et al., 2016).
The beneficial actions of ketamine and (2R,6R)-HNK, which last for days in mice and up to a week or longer in patients after a single administration, far exceed its time in the body (i.e. Figures 1–3; also see Tables 1, 4, and 5). This suggests that some rapid change is induced while the drug is present, and this change leads to a sustained alteration in brain function that persists long after the drug is cleared (Gould et al., 2018). Thus, it is likely that these rapid-acting antidepressants rapidly engage endogenous processes that promote synaptic growth and strengthening of synapses.
Notably, oral (2R,6R)-HNK did not alter locomotor activity or induce behavioral changes indicative of discomfort, sickness, and/or stereotypy up to a dose of 450 mg/kg, at least nine-fold higher than those doses required for antidepressant effects (15–50 mg/kg) in mice. These results are consistent with the previous finding that (2R,6R)-HNK lacks the hyperlocomotion and dissociative adverse effects associated with ketamine (Zanos et al., 2016) and, taken together, suggest a favorable safety profile, although additional studies are needed to fully investigate potential adverse effects following oral administration.
Although the current study establishes the oral BA in three species and demonstrates oral antidepressant efficacy of (2R,6R)-HNK in mouse behavioral tests, several limitations should be noted. First, a complete dose response relationship of behaviors for oral administration, compared to i.p. injection, has not been established. It is possible that the range of antidepressant doses, including the minimal effective dose needed to exert antidepressant effects, varies between these routes of administration. Thus, in order to further study the antidepressant and/or potential side effects at the most appropriate doses, future studies should establish the full dose response of oral (2R,6R)-HNK. Additionally, while none of the prodrug candidates tested improved the oral BA of (2R,6R)-HNK, these data do not preclude the possibiltiy that alternative prodrug strategies may enhance oral uptake of this compound. For example, amino acid modifications may enable active transport across the intestinal barrier (reviewed in Vig et al., 2013).
While there has been some progress towards the use of intranasal (Lapidus et al., 2014), sublingual (Lara et al., 2013), and subcutaneous (George et al., 2017; Loo et al., 2016) ketamine, most clinical studies utilize i.v. administration. These limitations for its administration, taken together with its side effects and abuse potential, limit the widespread use of ketamine in depression. Here, we show that the ketamine metabolite (2R,6R)-HNK has promising oral BA, and that it exerts antidepressant-like effects via oral administration, without causing overt adverse behavioral effects.
Supplementary Material
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH) grant MH107615 and a Harrington Discovery Institute Scholar-Innovator grant to TDG, NIH/NIGMS T32 GM008181 Training Program in Integrative Membrane Biology (JNH), and the National Institute on Aging (RM), National Institute of Mental Health (CAZ), and National Center for Advancing Ttranslational Sciences (CJT) NIH intramural research programs.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RM and CAZ are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine and other stereoisomeric dehydro- and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. PZ, RM, PJM, CJT, CAZ, and TDG are listed as co-inventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. RM, PJM, CAZ, and CJT have assigned their patent rights to the US government but will share a percentage of any royalties that may be received by the government. PZ and TDG have assigned their patent rights to the University of Maryland Baltimore but will share a percentage of any royalties that may be received by the University of Maryland Baltimore. All other authors declare no competing interests.
References
- Adams JD Jr, Baillie TA, Trevor AJ, et al. (1981) Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom 8: 527–538. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, et al. (2016) Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther 359: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalleri L, Merlo Pich E, Millan MJ, et al. (2018) Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23: 812–823. [DOI] [PubMed] [Google Scholar]
- Chang T and Glazko AJ (1974) Biotransformation and disposition of ketamine. Int Anesthesiol Clin 12: 157–177. [DOI] [PubMed] [Google Scholar]
- Chong C, Schug SA, Page-Sharp M, et al. (2009) Development of a sublingual/oral formulation of ketamine for use in neuropathic pain: Preliminary findings from a three-way randomized, crossover study. Clin Drug Investig 29: 317–324. [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin TB., et al. (2018) (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 139: 1–12. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, et al. (2017) Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS and Grant IS (1982) Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci 71: 539–542. [DOI] [PubMed] [Google Scholar]
- Cyriac JM and James E (2014) Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother 5: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B and Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10: 1093–1095. [DOI] [PubMed] [Google Scholar]
- Desta Z, Moaddel R, Ogburn ET, et al. (2012) Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, et al. (2010) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio AT, Ruperez FJ, Singh NS, et al. (2018) Stereochemical and structural effects of (2R,6R)-hydroxynorketamine on the mitochondrial metabolome in PC-12 cells. Biochim Biophys Acta Gen Subj 1862: 1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Gesenberg C and Zheng W (2017) Oral formulations for preclinical studies: Principle, design and development considerations. In: Yihong Q, Yisheng C, Geoff Z, et al. (eds) Developing Solid Oral Dosage Forms 2 ed. Cambridge, MA: Elsevier Inc, pp. 455–495. [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, et al. (2009) What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60: 1439–1445. [DOI] [PubMed] [Google Scholar]
- George D, Galvez V, Martin D, et al. (2017) Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry 25: 1199–1209. [DOI] [PubMed] [Google Scholar]
- Gould TD, Zarate CA and Thompson SM (2018) Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol 59 Epub ahead of print 8 October 2018 DOI: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant IS, Nimmo WS and Clements JA (1981) Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth 53: 805–810. [DOI] [PubMed] [Google Scholar]
- Hamada Y (2017) Recent progress in prodrug design strategies based on generally applicable modifications. Bioorg Med Chem Lett 27: 1627–1632. [DOI] [PubMed] [Google Scholar]
- Ho MF, Correia C, Ingle JN, et al. (2018) Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol 152: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR and Wang PS (2009) The STAR*D trial: Revealing the need for better treatments. Psychiatr Serv 60: 1466–1467. [DOI] [PubMed] [Google Scholar]
- Kelley AE (2001) Measurement of rodent stereotyped behavior. Curr Protoc Neurosci Chapter 8: Unit 8.8. [DOI] [PubMed]
- Kroin J, Das V, Moric M, et al. (2018) Efficacy of the ketamine metabolite (2R,6R)-hydroxynorketamine in mice models of pain. Reg Anesth Pain Med 42: 507–516. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214. [DOI] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, et al. (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara DR, Bisol LW and Munari LR (2013) Antidepressant, mood stabilizing and precognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol 16: 2111–2117. [DOI] [PubMed] [Google Scholar]
- Leung LY and Baillie TA (1986) Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem 29: 2396–2399. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CK, Galvez V, O’Keefe E, et al. (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134: 48–56. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, et al. (2013) Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol 698: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Dossou KS, et al. (2015) The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect 3: e00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Ramamoorthy A, et al. (2016) Subchronic administration of (R,S)-ketamine induces ketamine ring hydroxylation in Wistar rats. J Pharm Biomed Anal 127: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, et al. (2010) A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 82: 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PJ, Moaddel R, Zanos P, et al. (2017) Synthesis and N-Methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett 19: 4572–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals, 8th edition. Washington (DC): National Academies Press (US). [Google Scholar]
- Paul RK, Singh NS, Khadeer M, et al. (2014) (R,S)-ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 121: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, et al. (2017) Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84: e3–e6. [DOI] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, et al. (2014) Effects of ketamine on explicit and implicit suicidal cognition: A randomized controlled trial in treatment-resistant depression. Depress Anxiety 31: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, et al. (2016) A review of ketamine abuse and diversion. Depress Anxiety 33: 718–727. [DOI] [PubMed] [Google Scholar]
- Singh NS, Rutkowska E, Plazinska A, et al. (2016) Ketamine metabolites enantioselectively decrease intracellular D-serine concentrations in PC-12 cells. PLoS One 11: e0149499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig BS, Huttunen KM, Laine K, et al. (2013) Amino acids as promoieties in prodrug design and development. Adv Drug Deliv Rev 65: 1370–1385. [DOI] [PubMed] [Google Scholar]
- Won J, Marin de Evsikova C, Smith RS, et al. (2011) NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum Mol Genet 20: 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf TF and Adams JD (1987) Biotransformation of ketamine, (Z)-6-hydroxyketamine, and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica 17: 839–847. [DOI] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, et al. (2018) NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry Epub ahead of print 12 June 2018 DOI: 10.1038/s41380-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara Y, Ohtani M, Kariya S, et al. (2003) Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos 24: 37–43. [DOI] [PubMed] [Google Scholar]
- Yao N, Skiteva O, Zhang X, et al. (2017) Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry 23: 2066–2077. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2018) Ketamine and ketamine metabolites pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 70: 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche N, Laje G, et al. (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, et al. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J and Olejniczak AB (2013) Prodrugs: A challenge for the drug development. Pharmacol Rep 65: 1–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.