Abstract
Background & Aims:
Use of direct-acting oral anticoagulants (DOACs) is increasing, but little is known about the associated risks in patients undergoing colonoscopy with polypectomy. We aimed to determine the risk of post-polypectomy complications in patients prescribed DOACs.
Methods:
We performed a retrospective analysis using the Clinformatics Data Mart Database (a de-identified administrative database from a large national insurance provider) to identify adults who underwent colonoscopy with polypectomy or endoscopic mucosal resection (EMR) from January 1, 2011, through December 31, 2015. We collected data from 11,504 patients prescribed antithrombotic agents (1590 DOAC, 3471 warfarin, and 6443 clopidogrel) and 599,983 patients not prescribed antithrombotics of interest (controls). We compared 30-day post-polypectomy complications, including gastrointestinal bleeding (GIB), cerebrovascular accident (CVA), myocardial infarction (MI), and hospital admissions, of patients prescribed DOACs, warfarin, or clopidogrel vs controls.
Results:
Post-polypectomy complications were uncommon but occurred in a significantly higher proportion of patients receiving any antithrombotic vs controls (P<0.001). The percentage of patients in the DOAC group with GIB was 0.63% (95% CI, 0.3%–1.2%) vs 0.2% (95% CI, 0.2%–0.3%) in controls. The percentage of patients with CVA in the DOAC group was 0.06% (95% CI, 0.01%–0.35 %) vs 0.04% (95% CI, 0.04%–0.05%) in controls. After we adjusted for bri dge anticoagulation, EMR, Charlson comorbidity index (CCI), and CHADS2 (congestive heart failure, hypertension, age over 75, diabetes, stroke [double weight]) score, patients prescribed DOACs no longer had a statistically significant increase in the odds of GIB (odds ratio [OR], 0.90; 95% CI, 0.44–1.85), CVA (OR, 0.45; 95% CI, 0.06–3.28), MI (OR, 1 .07; 95% CI, 0.14–7.72), or hospital admission (OR, 0.86; 95% CI, 0.64–1.16). C lopidogrel, warfarin, bridge anticoagulation, higher CHADS2, CCI, and EMR were associated with increased odds of complications.
Conclusion:
In our retrospective analysis of a large national dataset, we found that patients prescribed DOACs did not have significantly increased adjusted odds of post-polypectomy GIB, MI, CVA, or hospital admission. Bridge anticoagulation, higher CHADS2 score, CCI, and EMR were risk factors for GIB, MI, CVA, and hospital admissions. Studies are needed to determine the optimal peri-procedural dose for high-risk patients.
Keywords: endoscopy, anticoagulation, colon polyps, outcomes
Introduction
Colonoscopy with polypectomy has been shown to be an effective screening intervention, with associated decreases in colorectal cancer (CRC) incidence by up to 90% and death by up to 50%, in comparison to historical controls.1–3 It is considered a safe, minimally invasive outpatient procedure, with an estimated perforation risk of 0.93%4 and bleeding risk ranging from 0.1% to 10%3. A confounding factor in the risk of post-polypectomy bleeding is the use of antithrombotic agents such as warfarin, clopidogrel and direct-acting oral anticoagulants (DOACs)5.
DOACs, including newer anticoagulation agents such as rivaroxaban, apixaban, dabigatran, and edoxaban, are approved for the prevention of cerebrovascular accidents (CVA) in patients with a history of atrial fibrillation (AF)6 and venous thrombotic embolism (VTE)7. Over the past decade, these agents have been increasingly prescribed, and account for over half of all new anticoagulation prescriptions in patients with AF8. Compared to warfarin, DOACs have been found to reduce the risk of CVA by 19% but increase the risk of all-cause gastrointestinal bleeding (GIB) by 25%9. Individually, apixaban is associated with a lower risk of GIB compared to rivaroxaban or dabigatran10. Current guidelines recommend that DOACs and other antithrombotic medications be held prior to polypectomy, with or without bridge therapy5. However, more recent literature suggests that DOACs may be safer than warfarin in terms for post-endoscopic bleeding11.
The risk of procedure-related complications associated with DOACs in patients who undergo colonoscopy with polypectomy remains poorly understood. We hypothesized that patients prescribed DOACs who undergo polypectomy do not have a significantly higher risk of GIB compared to patients on no anticoagulation or patients on other forms of antithrombotics. We aimed to evaluate the risk of complications following polypectomy in patients prescribed DOACs.
Methods:
Data Source:
We queried the Clinformatics Data Mart Database to identify adult patients with an outpatient encounter for colonoscopy with polypectomy or endoscopic mucosal resection (EMR) from January 1, 2011, to December 31, 2015. The Clinformatics Data Mart Database (OptumInsight, Eden Prairie, MN) is a de-identified database that contains inpatient, outpatient, and pharmacy data on 12–14 million individuals annually enrolled in a large commercial insurance plan and Medicare Advantage. We used International Classification of Diseases codes, 9th and 10th revisions (ICD9, ICD10), Current Procedural Terminology (CPT) codes, and pharmacy details.
Cohort Definition:
We identified any polypectomies performed on adult patients using specific CPT codes (44389, 44392, 44394, 44403, 44404, 45380, 45381, 45384, 45385 or 45390) in conjunction with specific ICD9 or ICD10 codes for colon or rectal polyps (Supplemental Table 1). We considered a procedure to be an EMR if the encounter included a CPT code for a snare polypectomy (44394, 45385) along with a CPT code for submucosal injection (44404, 45381) or if the encounter included a CPT code for EMR (45390, 44403). We excluded patients with coagulopathy and renal disease (Supplemental Table 2) as DOAC usage is contraindicated in these patients.
Antithrombotic use:
We compared colonoscopy with polypectomy patients prescribed antithrombotics to a control group of patients who had not been prescribed any antithrombotics of interest. We identified patients with an active prescription for any DOAC, clopidogrel, and warfarin using the corresponding National Drug Code (NDC). We did not identify aspirin or non-steroidal anti-inflammatory drug (NSAID) use, as we did not have access to over-the-counter medication information.
Active prescriptions were defined as those with an initiation date within 90 days prior to the procedure date and continuation after the procedure, indicated by pharmacy refills of the same antithrombotic medication within 90 days after the procedure with less than a one-week gap between the expected refill date and the actual refill date. We assumed that the exact peri-procedural management of the antithrombotic medications was done according to current society guidelines5. We grouped patients according to their antithrombotic prescriptions into DOAC, clopidogrel, or warfarin categories. We identified any patients who were on peri-procedure bridge anticoagulation, defined by prescription of enoxaparin, dalteparin, or fondaparinux within 14 days prior to the colonoscopy. We excluded patients on two or more combinations of DOAC, clopidogrel or warfarin due to the small size of this cohort.
Primary and Secondary Outcomes:
We analyzed clinically significant post-polypectomy GIB as defined by an inpatient encounter for GIB within 30 days of colonoscopy as our primary outcome of interest (Supplemental Table 2b). Our secondary outcomes included inpatient encounters for CVA, VTE, myocardial infarction (MI), and any hospital admission within 30 days of index polypectomy.
Covariates of Interest:
We obtained the demographic information of each patient including age, gender, race, procedure type, and the date of polypectomy. Comorbidities of interest, as defined by specific ICD9 and ICD10 codes prior to polypectomy, included any history of GIB, MI, congestive heart failure, peripheral vascular disease (PAD), CVA, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia, renal disease, malignancy, AIDS/HIV, coagulopathy, AF, VTE, and hypertension (Supplemental Table 2a). We calculated a Charlson comorbidity index (CCI)12 for each patient and a CHADS2 (congestive heart failure, hypertension, age over 75, diabetes, stroke [double weight]) and CHA2D2VASc (congestive heart failure, hypertension, age over 75 [double weight], diabetes, stroke [double weight], age 65–74, sex [female]) score for each patient with AF13
Statistical Analysis:
We summarized the data as means, standard deviations (SD), medians, interquartile ranges (IQR), and proportions. We compared categorical variables using the chi-squared test and continuous variables with the ANOVA test and the Kruskal-Wallis test. We performed multivariate logistic regressions to assess the risk for post-polypectomy GIB, CVA, MI, and admissions for each type of antithrombotic compared to control. We chose the covariates for our multivariate model based on clinically relevant risk factors for post-polypectomy bleeding and thrombotic outcomes. In our multivariate model, we adjusted for CCI, use of bridge anticoagulation, and procedure type (EMR versus polypectomy) based on clinically relevant factors related to post-polypectomy bleeding risk14, 15. We also adjusted for the CHADS2 score, as this is a validated measure of CVA risk in patients with AF. This analysis was performed for each antithrombotic subgroup (DOAC, warfarin, and clopidogrel). A multivariate analysis was not performed for VTE due to the small number of events.
Sensitivity Analysis:
In order to assess how risk factors for post-polypectomy bleeding complications may differ from post-polypectomy thrombotic complications, we performed additional analyses by including the history of GIB in our multivariate regressions. Considering AF is a common indication for antithrombotic usage, we performed our multivariate regression analysis on an AF-only subgroup. We also assessed the impact of the new risk stratification system, the CHA2DS2VASc score, in our model.
Results:
Patient Characteristics:
We identified 746,492 patients who underwent colonoscopy with polypectomy and excluded 65,568 patients with renal disease and coagulopathy and 37 patients with active prescriptions for two or more anticoagulants of interest (Figure 1). Our final cohort consisted of 1590 patients prescribed any DOAC, 3471 patients prescribed warfarin, and 6443 patients prescribed clopidogrel and 599,983 control patients.
Figure 1.
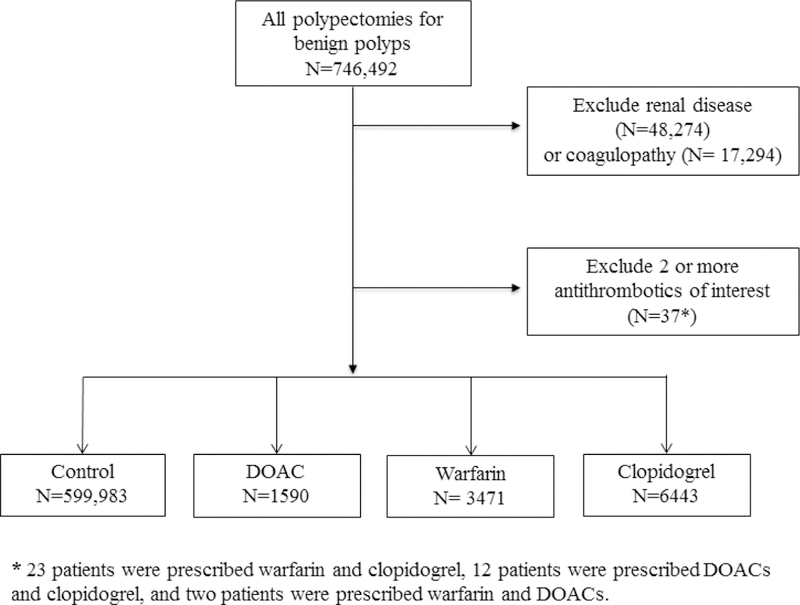
Study flow diagram.
Patient demographics and characteristics are summarized in Tables 1 and 2. Patients prescribed antithrombotics were more often male and older than the control group (P<.001) (Table 1). Patients prescribed antithrombotics had a higher mean CCI (1.7, SD 2.1 [DOACs]; 1.8, SD 1.7 [warfarin]; 1.9, SD 1.7 [clopidogrel]) compared to the control group (0.8, SD 0.5) (P<.001) (Table 2). Patients with a history of AF represented 83.8% of patients in the DOAC group and 64.3% of patients in the warfarin group compared to 1.9% of patients in the control group (P<.001). A higher proportion of patients in the antithrombotic groups also had a history of CVA, VTE, PAD, MI, and GIB compared to the control group (P <.001). Bridge anticoagulation therapy was most commonly used with patients prescribed warfarin (11.4%) compared to patients prescribed DOACs (1.6%), and no patients in the control group (0%) (P<.001).
Table 1.
Patient demographics. All categories are statistically significant at p<0.001 compared to controls.
| Control N=599,983 |
DOAC N=1590 |
Warfarin N=3471 |
Clopidogrel N=6443 |
|
|---|---|---|---|---|
| Age, Mean (SD) | 60.6 (10.7) | 68.6 (8.6) | 68.9 (9.2) | 67.3 (8.4) |
| Gender, n (%) | ||||
| Male | 305,723 (51.0%) | 1,052 (66.2%) | 2,192 (63.2%) | 4,400 (68.3%) |
| Female | 294,235 (49.0%) | 538 (33.8%) | 1,279 (36.9%) | 2,024 (31.7%) |
| Race, n (%) | ||||
| White | 434,973 (75.9%) | 1,264 (83.4%) | 2,621 (79.6%) | 4,594 (74.9%) |
| Black | 54,527 (9.5%) | 100 (6.6%) | 289 (8.9%) | 666 (10.9%) |
| Asian | 20,033 (3.5%) | 42 (2.8%) | 75 (2.3%) | 263 (4.3%) |
| Hispanic | 43,773 (7.6%) | 60 4.0%) | 183 (5.6%) | 424 (6.9%) |
| Unknown | 19,822 (3.5%) | 50 (3.3%) | 124 (3.8%) | 190 (3.1%) |
SD, Standard Deviation
Table 2.
Patient clinical characteristics. All categories are statistically significant at p<0.001 compared controls.
| Control N=599,983 |
DOAC N=1590 |
Warfarin N=3471 |
Clopidogrel N=6443 |
|
|---|---|---|---|---|
| CCI Score, mean (SD) | 0.8 (0.5) | 1.7 (2.1) | 1.8 (1.7) | 1.9 (1.7) |
| Comorbidities | ||||
| AF, n (%) | 11,322 (1.9%) | 1,332 (83.8%) | 2,231 (64.3%) | 503 (7.8%) |
| CVA, n (%) | 33,094 (5.5%) | 347 (21.8%) | 704 (20.3%) | 2,162 (33.6%) |
| VTE, n (%) | 189 (0.03%) | 19 (1.2%) | 84 (2.4%) | 16 (0.3%) |
| MI, n (%) | 8257 (1.4%) | 137 (8.6%) | 281 (8.1%) | 1504 (23.3%) |
| PAD, n (%) | 37,099 (6.2%) | 1333 (20.9%) | 750 (21.6%) | 2,023 (31.4%) |
| GIB, n (%) | 59,011 (9.8%) | 261 (16.4%) | 497 (14.3%) | 933 (14.5%) |
|
CHADS2 Score, mean (SD) |
0.03 (0.33) | 1.9 (1.4) | 1.6 (1.6) | 0.2 (0.8) |
|
CHA2DS2VASc, mean (SD) |
0.06 (0.5) | 3.0 (2.1) | 2.5 (2.3) | 0.3 (1.2) |
|
Bridge Anticoagulation, n (%) |
0 (0%) | 26 (1.6%) | 394 (11.4%) | 21 (0.3%) |
| EMR, n (%) | 28,323 (4.7%) | 91 (5.7%) | 208 (6.0%) | 6351 (5.5%) |
CCI, Charlson comorbidity index; EMR, endoscopic mucosal resection; SD, standard deviation;
Outcomes Analysis:
Patients prescribed any antithrombotic medications had higher rates of complications compared to controls (P<.001) (Figure 2), though the overall occurrence of complications after polypectomy remained low.
Figure 2.

30-day complications rates after polypectomy. P<0.001 for all outcomes.
Gastrointestinal bleeding
Post-polypectomy GIBs occurred in 10 patients in the DOAC group (rate 0.6%, 95% CI 0.3%−1.2%), 43 patients in the warfarin group (rate 1.2%, 95% CI 0.9%−1.7%) and 59 patients in the clopidogrel group (rate 0.9%, 95% CI 0.7%−1.2%). This compares to 1430 patients with GIBs in the control group (rate 0.2%, 95% CI 0.2% – 0.3%) (Figure 2). There was no significant difference in median days to event (P=.35).
Hospital Admissions
In total, there were 54 admissions in the DOAC group (rate 3.4%, 95% CI 2.6%−4.4%) compared to 143 in the warfarin group, (rate 4.1%, 95% CI 3.7%−5.0%), 217 in the clopidogrel group (rate 3.4%, 95% 3.0% – 3.9%) and 10,782 in the control group (rate 1.8%, 95% CI 1.7%–1.8%) (P<.001) (Figure 2). The median days to admission was 7 days (IQR 3–18) for patients on DOACs, 10 days (IQR 3–21) for patients on warfarin, 13 days (IQR 5–21) for patients on clopidogrel, and 13 days (IQR 4–21) for control patients (P =.05). The most common diagnosis associated with an admission for the entire cohort was post-hemorrhagic anemia (ICD-9 285.1, 14.5%).
Cerebrovascular accidents
There was only one admission for CVA in the DOAC group (0.06%, 95% CI 0.01% – 0.35%). There were 13 admissions for CVA in the warfarin group (0.4%, 95% CI 0.2% – 0.6%), 18 admissions in the clopidogrel group (0.3%, 95% CI 0.2% – 0.5%), and 238 patients admitted for CVA (rate 0.04%, 95% CI 0.04% – 0.05%) in the control group (Figure 2). The median days to CVA was 10 days in the DOAC group, 22 days (IQR 6–22) in the clopidogrel group, 13 days (IQR 5–18) in the warfarin group, and 13 days (IQR 5 – 21) for the control group (P=.05).
Other complications
There were two admissions for MI in the DOAC group (rate 0.13%, 95% CI 0.04% – 0.46%), 5 in the warfarin group (rate 0.1%, 95% CI 0.1% – 0.3%) and 20 in the clopidogrel group (rate 0.3%, 95% CI 0.2% – 0.5%) (Figure 2). In contrast, there were 179 patients admitted for MI in the control group (rate 0.03%, 95% CI 0.03% – 0.03%). There was no significant difference in the days to event between the groups, P=.33. There were no admissions for VTE in the DOAC or warfarin cohort, only one in the clopidogrel cohort, and four in the control group.
Multivariate Analysis
After adjusting for bridge anticoagulation, procedure type, CCI, and CHADS2 score, we found that patients with an active DOAC prescription did not have a statistically significant increase in the odds of GIB (OR 0.90, 95% CI 0.44–1.85), CVA (OR 0.45, 95% CI 0.06–3.38), MI (OR 1.07, 95% CI 0.14–7.72), or admissions (OR 0.86, 95% CI 0.64 to 1.16) compared to control (Figure 3, Table 3). Odds of GIB, CVA, and readmission remained elevated with both warfarin and clopidogrel prescriptions. Warfarin was associated with an OR of 1.90 for GIB (95% CI 1.28–2.83), an OR of 2.57 for CVA (95% CI 1.28–5.17), an OR of 1.07 for MI (95% CI 0.63–5.80), and an OR of 1.07 for admissions (95% CI 0.87–1.31). Clopidogrel was associated with the highest odds of GIB (OR 2.84, 95% CI 2.16–3.73), CVA (OR 4.04, 95% CI 2.42–6.74), MI (OR 5.79, 95% CI 3.49–9.62), and admissions (OR 1.33, 95% CI 1.15–1.54) compared to the other antithrombotics.
Figure 3.
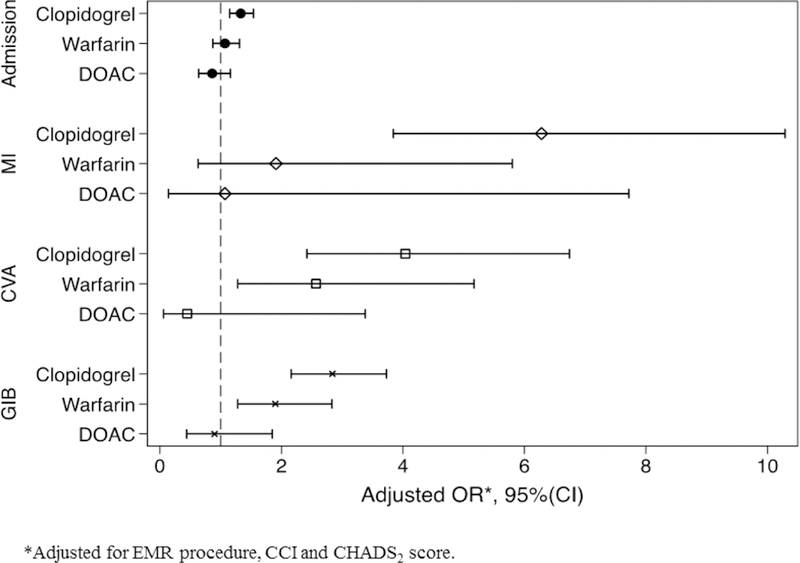
The adjusted odds ratio of 30-day complications after polypectomy by antithrombotic type with no antithrombotic or aspirin as reference. The multivariate model is adjusted for EMR procedure, CCI and CHADS2 score. The results of the full multivariate model are summarized in Table 3.
Table 3.
Results of the complete multivariate regression model.
| OR (95% CI) | GIB | CVA | MI | Any Admission |
|---|---|---|---|---|
| Antithrombotic | ||||
| DOAC | 0.90 (0.44–1.85) | 0.45 (0.06–3.38) | 1.07 (0.14–7.72) | 0.86 (0.64–1.16) |
| Warfarin | 1.90 (1.28–2.83) | 2.57 (1.28–5.17) | 1.91 (0.63–5.80) | 1.07 (0.87–1.31) |
| Clopidogrel | 2.84 (2.16–3.73) | 4.04 (2.42–6.74) | 6.28 (3.84–10.29) | 1.33 (1.15–1.54) |
| CCI | 1.16 (1.12–1.20) | 1.30 (1.23–1.37) | 1.27 (1.87–4.30) | 1.22 (1.21–1.24) |
| CHADS2 Score | 1.34 (1.25–1.43) | 1.49 (1.32–1.67) | 1.16 (0.97–1.39) | 1.26 (1.22–1.30) |
| EMR | 4.96 (4.36–5.64) | 2.00 (1.32–3.03) | 2.84 (1.87–4.30) | 5.18 (4.93–5.44) |
| Bridge anticoagulation | 3.29 (1.68–6.44) | 2.64 (0.74–9.44) | 4.36 (0.85–22.43) | 1.67 (1.06–2.65) |
CCI, Charlson comorbidity index; CI, confidence interval; CVA, cerebrovascular accident; DOAC, direct-acting oral anticoagulant; EMR, endoscopic mucosal resection; GIB, gastrointestinal bleeding; OR, odds ratio;
EMR procedure type, bridge anticoagulation, higher CCI, and CHADS2 score were also found to be independent risk factors for GIB, CVA, MI, and admissions (Table 3). EMR was associated with the highest odds of GIB (OR 4.96, 95% CI 4.36–5.64), and admissions (OR 5.18, 95% CI 4.93–5.44). Bridge anticoagulation use was also an independent risk factor for GIB (OR 3.29, 95% CI 1.68–6.44) and admissions (OR 1.67, 95% CI 1.06–2.65)
Sensitivity Analysis:
Active DOAC prescriptions were not associated with a significant increase in the odds of GIB, CVA, MI, or admissions (Supplemental Table 3) when the model was adjusted for a history of GIB, though a history of GIB was associated with an increase in the odds of post-polypectomy GIB (OR 1.42, 95% CI 1.23–1.64) and admissions (OR 1.46, 95% CI 1.38–1.54). We did not find the use of CHA2DS2VASc score to affect the findings of the multivariate model (Supplemental Table 4A). In the AF subgroup, no antithrombotics were associated with statistically significant increased odds of GIB, CVA, MI, or admissions (Supplemental Table 4B).
Discussion:
Our findings provide necessary data for the understanding of adverse events in patients prescribed DOACs who undergo colonoscopy with polypectomy. The overall rates of complications in polypectomy or EMR patients were low, but higher in patients prescribed any antithrombotics compared to patients in the control group (P< 0.001). After adjusting for bridge anticoagulation use, patient comorbidities, and procedure type, patients prescribed DOACs did not have a statistically significant increase in the odds of GIB, CVA, MI, or admissions as compared to the control group. Clopidogrel and warfarin remained associated with an increased risk of post-polypectomy bleeding, CVA, MI, and admissions compared to the control group.
Our study highlights the relative safety of DOACs compared to warfarin and clopidogrel. We found that patients prescribed warfarin and clopidogrel had an increased risk for GIB, CVA, MI, and admissions, although the increase in the odds of admissions and MI in the warfarin group was not statistically significant. These findings were seen throughout most of our sensitivity analyses with the exception of the AF-only sub-group; however, we note that the AF-only cohort consisted of 11,322 patients and thus is likely underpowered. A 2012 study, which compared patients on dabigatran to patients on warfarin, found similar risks of peri-procedural bleeding and thromboembolic events between the two medications16. More recent studies have suggested DOACs may have a lower risk of post-polypectomy bleeding compared to warfarin11, 17, 18 in line with our study findings. Similarly, clopidogrel use has also been associated with an increased risk of post-polypectomy bleeding19, 20.
Our analyzed cohort is one of the largest studied antithrombotic groups who underwent colonoscopy with polypectomy and includes both men and women with a mean age of 61.2 years (SD 10.7) and a mean CCI of 0.87 (SD 1.34). Such a robust dataset allowed us to perform risk stratification to facilitate guidance on the highest risk polypectomy group. Providers should still use caution in managing antithrombotics in older patients with more comorbid conditions, as we found increasing CCI and CHADS2 score to be additional risk factors for complications in our multivariate regression model (Table 3).
The use of bridge anticoagulation has been linked to a higher risk of GIB21. We also found bridge anticoagulation to be an independent risk factor for GIB and 30-day admissions. Other risk factors for post-polypectomy bleeding that had been previously identified include larger, complex polyps15, and a right-sided location22. While we could not identify the exact location or characteristic of the polyp, we found EMR is an independent risk factor for all complications with a five-fold increase in the odds of GIB and admissions and a two-fold increase in the odds of CVA and MI.
Our study is not without limitations. We recognize that since this study is a retrospective analysis of administrative data there may have been information bias present. We minimized misclassification errors by including only polypectomies for benign polyps by associating the appropriate CPT codes for the polypectomy procedure with the ICD9 or ICD10 codes for benign polyps. We applied strict active prescription criteria by requiring both an active prescription at the time of procedure as well as a medication refill within 90 days after the procedure and no more than a 7-day gap between refills. However, it is possible that patients could have been instructed to continue or stop anticoagulation in accordance with current guidelines. Furthermore, peri-procedural medication management may have differed between the antithrombotic groups. In addition, as only prescription medication information was available, we could not reliably identify patients on over-the-counter aspirin and NSAIDs. Patients may have been on aspirin or NSAIDs in combination with an antithrombotic of interest. Importantly, aspirin and NSAID use have been shown to be safe in polypectomy23 and are not routinely held prior to procedures. Therefore, we do not believe the inability to identify usage significantly impacts our findings.
In our analysis, we adjusted for multiple patient and procedure-related factors, however, there may be unmeasured confounders contributing to our findings. We also note that there is a difference in cohort size between the 3 antithrombotic groups of interest. We performed post-hoc power analyses to assess the strength of our findings, as we recognize that patients on warfarin and clopidogrel were more represented in the antithrombotic cohort (i.e. 1590 patients prescribed DOACs compared to 3471 prescribed warfarin and 6443 prescribed clopidogrel). Despite the smaller DOAC cohort, post-hoc power analyses reveal that we achieved a power of 82% for GIB and 98.1% for admissions outcomes using the effect sizes observed and a significance level of 0.05. The power was less than 80% for CVA and MI. Larger studies on post-polypectomy CVA and MI complications in patients on DOACS may provide stronger conclusions.
Conclusion:
Our study found that active DOAC prescriptions were not associated with significantly increased adjusted odds of GI bleeding, CVA, MI or 30-day admissions after polypectomy compared to patients in the control group after adjusting for procedure complexity, bridge anticoagulation use, CHADS2 score, and CCI. Patients with a higher CHADS2 score, comorbidity index, bridge anticoagulation use, or undergoing EMR may be at higher risk. Further studies on high-risk patients, optimal peri-procedural dosing, and bleeding prophylaxis are needed.
Supplementary Material
What You Need to Know.
Background:
The risk of post-polypectomy complications in patients on DOACs is poorly understood. We compared post-polypectomy outcomes of patients prescribed DOACs, warfarin, clopidogrel to control patients with no antithrombotic prescriptions.
Findings:
Patients prescribed DOACs had low but increased rates of complications compared to controls, but this was non-significant on multivariable regression. Other risk factors include patient comorbidities, bridge anticoagulation, and EMRs.
Implications for patient care:
Patients prescribed DOACs do not have increased adjusted odds of post-polypectomy complications. Providers should use caution in managing patients with bridge anticoagulation, higher comorbidities, and undergoing EMRs.
Acknowledgments
Grant Support:
JXY: NIH TL1 TR 001084, NIH T32 DK 007056
JL: NIH KL2 TR 001083, NIH UL1 TR 001085
Abbreviations:
- AF
atrial fibrillation
- CCI
Charlson comorbidity index
- CHADS2
congestive heart failure, hypertension, age over 75, diabetes, stroke (double weight)
- CHA2DS2VASc
congestive heart failure, hypertension, age over 75 (double weight), diabetes, stroke (double weight), age 65–74, sex (female)
- CI
confidence interval
- CPT
current procedural terminology
- CRC
colorectal cancer
- CVA
cerebrovascular accident
- DOAC
direct-acting oral anticoagulant
- EMR
endoscopic mucosal resection
- GIB
gastrointestinal bleeding
- ICD9
international classification of diseases codes, 9th revision
- ICD10
international classification of diseases codes, 10th revision
- IQR
interquartile range
- MI
myocardial infarction
- NDC
national drug code
- OR
odds ratio
- PAD
peripheral vascular disease
- SD
standard deviation
- VTE
venous thromboembolism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Citations:
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 2.Shaukat A, Church TR. Colorectal-cancer incidence and mortality after screening. N Engl J Med 2013;369:2355. [DOI] [PubMed] [Google Scholar]
- 3.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–14. [DOI] [PubMed] [Google Scholar]
- 4.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–57, W152. [DOI] [PubMed] [Google Scholar]
- 5.Committee ASoP, Acosta RD, Abraham NS, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3–16. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, et al. 2014. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 8.Barnes GD, Lucas E, Alexander GC, et al. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med 2015;128:1300–5 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 10.Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology 2017;152:1014–1022 e1. [DOI] [PubMed] [Google Scholar]
- 11.Nagata N, Yasunaga H, Matsui H, et al. Therapeutic endoscopy-related GI bleeding and thromboembolic events in patients using warfarin or direct oral anticoagulants: results from a large nationwide database analysis. Gut 2017. [DOI] [PMC free article] [PubMed]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 13.Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed]
- 14.Kim HS, Kim TI, Kim WH, et al. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am J Gastroenterol 2006;101:1333–41. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Lee HJ, Ahn JW, et al. Risk factors for delayed post-polypectomy hemorrhage: a case-control study. J Gastroenterol Hepatol 2013;28:645–9. [DOI] [PubMed] [Google Scholar]
- 16.Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa N, Nagata N, Watanabe K, et al. Post-polypectomy bleeding and thromboembolism risks associated with warfarin vs direct oral anticoagulants. World J Gastroenterol 2018;24:1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fast Stats: An interactive tool for access to SEER cancer statistics Volume 2018: Surveillance Research Program, National Cancer Institute. [Google Scholar]
- 19.Feagins LA, Iqbal R, Harford WV, et al. Low rate of postpolypectomy bleeding among patients who continue thienopyridine therapy during colonoscopy. Clin Gastroenterol Hepatol 2013;11:1325–32. [DOI] [PubMed] [Google Scholar]
- 20.Lin D, Soetikno RM, McQuaid K, et al. Risk factors for postpolypectomy bleeding in patients receiving anticoagulation or antiplatelet medications. Gastrointest Endosc 2018;87:1106–1113. [DOI] [PubMed] [Google Scholar]
- 21.Ishigami H, Arai M, Matsumura T, et al. Heparin-bridging therapy is associated with a high risk of post-polypectomy bleeding regardless of polyp size. Dig Endosc 2017;29:65–72. [DOI] [PubMed] [Google Scholar]
- 22.Buddingh KT, Herngreen T, Haringsma J, et al. Location in the right hemi-colon is an independent risk factor for delayed post-polypectomy hemorrhage: a multi-center case-control study. Am J Gastroenterol 2011;106:1119–24. [DOI] [PubMed] [Google Scholar]
- 23.Yousfi M, Gostout CJ, Baron TH, et al. Postpolypectomy lower gastrointestinal bleeding: potential role of aspirin. Am J Gastroenterol 2004;99:1785–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


