Abstract
Background
Medullary thyroid carcinoma is a neuroendocrine tumor belonging form a malignant growth of the thyroid parafollicular C-cells, representing from 1 to 10% of all thyroid cancer. The biochemical activity of medullary thyroid carcinoma includes the production of calcitonin and carcinoembryogenic antigen, which are sensitive tumor markers, facilitating the diagnosis, follow-up and prognostication. The diagnosis is reached through the identification of high basal calcitonin serum level or after pentagastrin stimulation test. Medullary thyroid carcinoma is able to produce other relevant biomarkers as procalcitonin, carcinoembryionic antigen and chromogranin A. In Literature are described few cases of medullary thyroid carcinoma without elevation of serum calcitonin, an extremely rare event. The aim of this study was to analyse the presentation, the main features and therapeutic management of medullary thyroid carcinoma associated with negative serum calcitonin levels.
Methods
Using the PubMed database, a systematic review of the current Literature was carried out, up to February 2018. Finally, nineteen articles met our inclusion criteria and were selected according to the modified Newcastle-Ottawa scale.
Results
Fourty-nine patients with definitive pathology confirming medullary thyroid carcinoma and with calcitonin serum level in the normal range were identified (24 female, 24 male and not reported gender in 1 case). Mean age was 51.7 years. Serum calcitonin levels were reported for 20 patients with a mean value of 8.66 pg/mL and a range of 0.8–38 pg/mL. Despite the low or undetectable calcitonin serum level, at immunochemistry in almost the half of the cases reported by the Authors, the tumors presented diffuse or focal positivity for calcitonin and carcinoembryionic antigen, while was reported a chromogranin A positivity in 41 of the 43 tested patients.
Conclusions
Calcitonin negative medullary thyroid carcinoma is an extremely rare pathology. The diagnosis and the surveillance is often challenging and delayed, due to the lack of elevation of serum markers as calcitonin and carcinoembryionic antigen. Further studies are needed, to better define options for management of non secretory medullary thyroid carcinoma and to identify new and reliable biomarkers associated to diagnosis and relapse of this medical dilemma.
Keywords: Medullary thyroid carcinoma, Calcitonin negative, Calcitonin, Carcinoembryonic antigen, Chromogranin a, Procalcitonin, Thyroid nodule
Background
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor (NET) originating from a malignant growth of the thyroid parafollicular C-cells.
At first described by Hazard et al. in 1959, [1] parafollicular C-cells have a neural crest ectoderm and an ultimobranchial body derivation and account for about 1% of all thyroid cells. They have a neuroendocrine role of paramount importance on the calcium homeostasis throughout the production and the secretion of calcitonin (CT) hormone, a 32-aminoacid linear polypeptide.
MTC represent from 1 to 10% of all thyroid cancer with a mean survival of 8.6 years and a 10-years survival rates ranging from 69 to 89%. It is frequently sporadic (75% of cases), otherwise, in case of RET proto-oncogene germline mutation, it has a hereditary pattern (25% of cases). This familial form belongs to multiple endocrine neoplasia type 2 (MEN 2), which present two subtypes MEN 2A – MTC in combination with pheochromocytoma and hyperparathyroidism – and MEN 2B – MTC with an infancy onset, in association to pheochromocytoma, multiple mucosal neuromas, gastrointestinal ganglioneuromatosis and megacolon.
Sporadic MTC has a low growth rate, is well differentiated and generally present a locally aggressiveness. Familial MTC forms, especially in MEN 2B, present a worse prognosis with earlier lymph nodes metastasis and adjacent structures invasion. Central compartment lymph nodes (IV-VI levels) are frequently involved, followed by levels II to V. [2, 3] Metastatic spread to the upper and anterior mediastinum has been described. Haematogenous dissemination involves liver, lungs and bones, even if distant metastases generally occur as a fine miliary pattern, hardly visualized by computed tomography (CT). [2]
Histologically, typical medullary tumor is characterized by round cells producing amyloid substances, separated by fibrous septa and has microcalcification areas. [1]
The biochemical activity of MTC includes the production of CT and carcinoembryogenic antigen (CEA), which are sensitive tumor markers, related to mass size, facilitating the diagnosis, follow-up and prognostication of MTC. In MTC, CT value is high at basal and after pentagastrin stimulation test, resulting in MTC a high sensitivity and specificity indicator of disease. MTC, as other NET, is able to produce many relevant biomarkers as procalcitonin (proCT) the precursor of calcitonin, neuron specific enolase (NSE) and chromogranin A (CgA). [3] Falsely high or low level of CT are associated with several disease such as C-cells hyperplasia, autoimmune thyroiditis, end stage renal disease, lung and prostate cancer and some neuroendocrine tumors. Otherwise, in patients with millimetric MTC, it is possible to identify normal basal level of CT. It is extremely rare to diagnose voluminous and palpable MTC associated with normal CT level, since, in most cases there is a correlation between size and basal CT level. [4] In 1989, Sobol et al. reported the first case of CT negative MTC, and to date only few cases have been occasionally described in Literature. [5]
The aim of his study was to analyse the presentation, the main features and therapeutic management of MTC patients associated with negative serum CT level.
Methods
Using the PubMed database, a systematic review of the current Literature was carried out, up to February 2018. The MeSH (Medical Subject Headings) search terms used were “thyroid”, “medullary”, “carcinoma”, “endocrine” and “neuroendocrine tumors”. The Authors observed that MTC CT-negative was an extremely rare neoplasm. The keywords “calcitonin”, “serum calcitonin”, “calcitonin negative”, “thyroid”, “thyroid gland”, “neuroendocrine”, “neuroendocrine tumor”, “medullary thyroid carcinoma” were used for the research. Several combinations of the keywords and MeSH terms were utilized as showed: “Medullary thyroid carcinoma calcitonin-negative”, “MTC without serum calcitonin”, “Neuroendocrine thyroid tumor lack calcitonin”. The various terms were substituted during the search. References of the more relevant articles were manually searched. The last research was concluded on February 1, 2018.
The search was carried out by two Authors CO, CG and the obtained results were discussed with the senior Author GD. The final article was realised in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. [1] Moreover, the eligible articles were selected according to the modified Newcastle-Ottawa scale in order to satisfy the requirements of the current review. The scale range is from 0 to 9. The studies included were those presenting a score of 6 or higher. [6–8]
The following data were extracted from the included studies: first author, year of data collection, year of publication, country of origin, characteristics of study population, number of patients with MTC CT-negative, clinicopathological characteristics, matching criteria, disease-free survival (DFS) and overall survival (OS).
The inclusion criteria of the study comprised the report of patients with a proven histopathological diagnosis of MTC associated with normal preoperative serum calcitonin, the presence of the evaluation of clinicopathological features and of the analysis of survival. All studies that failed to fulfil the established inclusion criteria and the not English language studies were excluded.
In all the studies, MTC diagnosis was based on the definitive pathology. Microscopically, MTCs features consist of polygonal or fusiform cells, grouped into nests, trabeculae or follicles; in adjacent struma are present amyloid deposits deriving from altered polypeptides of calcitonin. A peculiar characteristic of MTC at an electronic microscope examination, is the presence of electron-bound granules adjacent to the membrane. Histologically, familial MTC can be distinguished from sporadic MTC by the presence of multicentric C-cell hyperplasia in the thyroid parenchyma. The tumor-node-metastasis (TNM) staging system from AJCC was considered for comparison. The clinical characteristics included age, sex, localization of the neoplasm, size, functional hormonal status and presence of symptoms. The OS and DFS of the patients were also analysed.
Results
Twenty-three suitable studies were identified after Literature review. After the removal of a duplicate study, twenty-two articles were selected for the full-text review. A study was excluded because it was in Spanish (Iglesias P et al. Anaplastic variant of thyroid medullar carcinoma. Med Clin (Barc) 1997). An article was excluded because the MTC diagnosis was made post-mortem (Eusebi V et al. Calcitonin free oat-cell carcinoma of the thyroid gland. Virchows Arch A Pathol Anat Histopathol. 1990). Another one was ruled out because was not possible to recover (Diez JJ. et al. Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. Thyroid. 2004) and the last was excluded because it did not meet our inclusion criteria (Mussazhanova Z et al. Radiation-associated small cell neuroendocrine carcinoma of the thyroid: a case report with molecular analyses. Thyroid. 2014). [Fig. 1] Therefore, nineteen responded to our inclusion criteria and were enrolled in the current review. The features of the nineteen selected studies were summarized in Table 1.
Fig. 1.
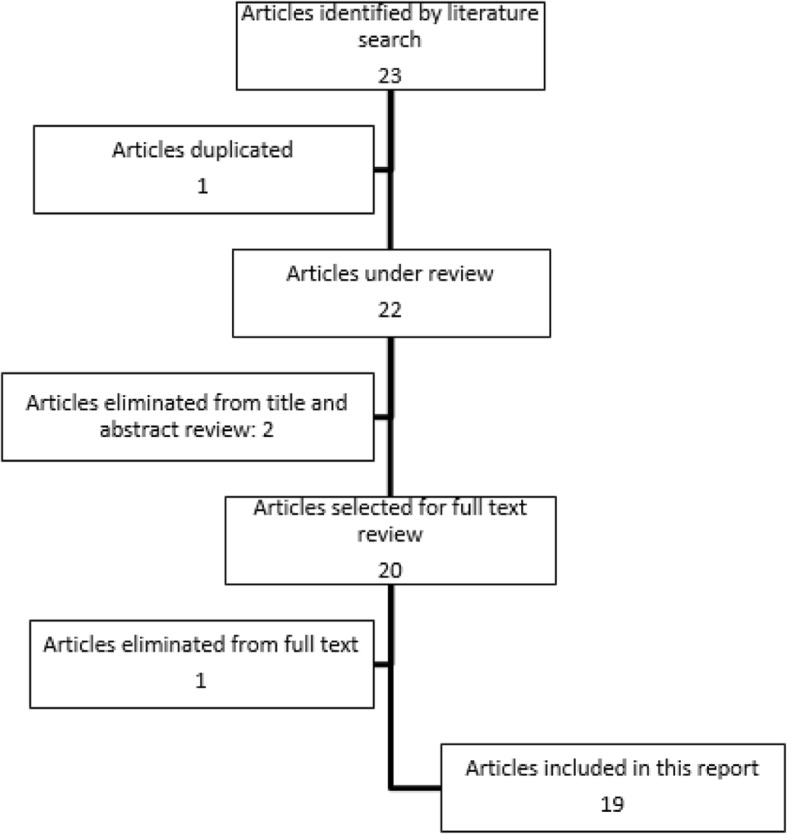
Flow-chart of the papers selection process for CT-negative MTC
Table 1.
Prospectus of the included studies
| Author | Reference | Journal | Year |
|---|---|---|---|
| Sobol RE | Hormone-negative, chromogranin A-positive endocrine tumors. | New England Journal of Medicine | 1989 |
| Schmid KW | “Atypical” medullary thyroid carcinoma with little or no calcitonin expression. | Virchows Archive A Pathological Anatomy and Histopathology | 1998 |
| Redding AH | Normal preoperative calcitonin levels do not always exclude medullary thyroid carcinoma in patients with large palpable thyroid masses. | Thyroid | 2000 |
| Bockhorn M | Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. | Thyroid | 2004 |
| Sand M | Serum calcitonin negative medullary thyroid carcinoma. | World journal of surgical oncology. | 2006 |
| Dora JM | Normal perioperative serum calcitonin levels in patients with advanced medullary thyroid carcinoma: case report and review of the literature. | Thyroid | 2008 |
| Wang TS | Medullary thyroid carcinoma without marked elevation of calcitonin: a diagnostic and surveillance dilemma. | Thyroid | 2008 |
| Giovanella L | Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. | The International Journal of Biological Markers | 2008 |
| Alapat DV | Disparity between tissue and serum calcitonin and CEA in patient with medullary thyroid carcinoma. | Endocrine | 2011 |
| Chernyavsky VS | Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. | Thyroid | 2011 |
| Nakazawa T | C-cell-derived calcitonin-free neuroendocrine carcinoma of the thyroid: the diagnostic importance of CGRP immunoreactivity. | International journal of surgical pathology. | 2011 |
| Frank-Raue K | Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. | Thyroid | 2013 |
| Ismi O | Calcitonin-negative neuroendocrine tumor of thyroid gland mimicking anaplastic carcinoma: an unusual entity. | Gland Surgery | 2014 |
| Brutsaert EF | Medullary thyroid cancer with undetectable serum calcitonin. | The Journal of clinical endocrinology and metabolism. | 2014 |
| Kim JY | A calcitonin-negative neuroendocrine tumor derived from follicular lesions of the thyroid. | Endocrinology and metabolism. | 2015 |
| Kasajima A | A Calcitonin Non-producing Neuroendocrine Tumor of the Thyroid Gland. | Endocrine pathology. | 2016 |
| Samà MT | Clinical challenges with calcitonin-negative medullary thyroid carcinoma. | Journal of cancer research and clinical oncology | 2016 |
| Parmer M | Calcitonin-Negative Neuroendocrine Tumor of the Thyroid. | International journal of surgical pathology. | 2017 |
| Zhou Q | Clinical and pathological analysis of 19 cases of medullary thyroid carcinoma without an increase in calcitonin. | Experimental and toxicologic pathology: official journal of the Gesellschaft für Toxikologische Pathologie. | 2017 |
Demographic and clinicopathological features
From the selected studies, fourty-nine patients with definitive pathology confirming MTC with a CT serum level in the normal range were identified (24 female, 24 male and unknown gender in 1 case). Mean age was 51.7 years, with a median of 53 years and a range of 16–82 years. Mean cancer size was 63 mm (range 10-80 mm), even if in 7 cases the cancer dimension was not reported. Zhou et al. reported 18 cases of monofocal MTC and one patient showed a multifocal tumor, moreover, 14 patients presented a mass larger than 10 mm of volume and 5 smaller than 10 mm of volume. [9] [Table 2] Eight patients (16.6%) presented laterocervical and central compartment lymph nodes metastases on definitive pathology, [4, 9, 10] while 23 patients (47.9%) did not present lymph nodes metastases and 6 (12.5%) had Nx stage at TNM staging. Other reported sites of MTC metastasis were lung, founded in 4 cases (8.3%), [10, 11] brain, in one case (2%) and lymph nodes of other body districts, found in one case (2%). [11, 12]
Table 2.
Demographic and clinicopathological features of CT negative MTC patients
| Author | Gender | Age (years) | Size (mm) | Histology | CT-HIC | CT | Assay | Upper reference limit | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sobol | F | 82 | 20 | WDMTC | Negative | Normal limits | NA | NA |
| 2 | Schmid 1 | M | 28 | NA | WDMTC | Weak | NA | Immunotech | NA |
| 3 | Schmid 2 | M | 46 | NA | WDMTC | Weak | NA | Immunotech | NA |
| 4 | Schmid 3 | F | 45 | NA | WDMTC | Weak | NA | Immunotech | NA |
| 5 | Schmid 4 | M | 37 | NA | WDMTC | Negative | NA | Immunotech | NA |
| 6 | Redding | F | 31 | 45 | WDMTC | Diffuse | 28 | RIA | < 150 |
| 7 | Bockhorn | F | 50 | 20 | PDMTC | Weak | 0.8 | Nichols | < 4.6 |
| 8 | Sand | F | 73 | NA | PDMTC | Weak | 5.3 | Nichols | < 10 |
| 9 | Dora | M | 43 | 20 | PDMTC | Diffuse | 4 | Immunolite | < 12 |
| 10 | Wang | M | 68 | 70 | WDMTC | Weak | 38 | NA | < 10 |
| 11 | Giovanella | F | 43 | 48 | WDMTC | Diffuse | 4.7 | Immunolite | < 10 |
| 12 | Alapat | F | 16 | 30 | WDMTC | Diffuse | 4 | Immunolite | < 4.6 |
| 13 | Chernyavsky | F | 40 | 20 | WDMTC | Negative | 2.1 | Ventana Medical System Inc. | < 5.0 |
| 14 | Nakazawa | M | 76 | 60 | WDMTC | Weak | 22 | NA | < 10 |
| 15 | Frank-Raue 1 | F | 61 | 10 | WDMTC | Weak | 2.9 | Nichols | < 10 |
| 16 | Frank-Raue 2 | M | 70 | 80 | WDMTC | Weak | < 2 | DiaSorin |
< 6.1 male < 3.6 female |
| 17 | Frank-Raue 3 | F | 50 | 20 | WDMTC | Weak | 0.8 | Nichols | < 10 |
| 18 | Frank-Raue 4 | M | 47 | 30 | PDMTC | Focal | 2.6 | Immunolite |
< 8.4 male < 5 female |
| 19 | Frank-Raue 5 | F | 53 | 45 | WDMTC | Diffuse | NA | NA | NA |
| 20 | Frank-Raue 6 | M | 45 | 18 | PDMTC | Weak | 11 | Non commercial | < 18 |
| 21 | Frank-Raue 7 | F | 45 | 55 | PDMTC | Focal | 1.5 | CIS | < 10 |
| 22 | Ismi | NA | 57 | NA | PDMTC | Negative | 5.6 | NA | < 10 |
| 23 | Brutsaert | F | 49 | 26 | WDMTC | Diffuse | < 2 | NA | < 6 |
| 24 | Kim | M | 34 | 10 | PDMTC | Negative | 3.7 | NA | < 10 |
| 25 | Kasajima | F | 48 | 30 | WDMTC | Negative | 29 | NA | NA |
| 26 | Samà 1 | M | 60 | 38 | NA | Focal | 7.8 | NA | NA |
| 27 | Samà 2 | F | 66 | NA | NA | NA | 5 | NA | NA |
| 28 | Samà 3 | M | 53 | 12 | NA | Negative | < 10 | NA | NA |
| 29 | Samà 4 | M | 62 | 45 | NA | Focal | 13 | NA | NA |
| 30 | Parmer | F | 74 | 20 | WDMTC | Negative | Normal limits | NA | NA |
| 31–49 | Zhou |
11 M 8 F |
≥30 3 cases; < 30 16 cases |
≤10mmm 14 cases > 10 mm 5 cases |
NA |
Positive in 8 cases Negative in 11 cases |
NA | NA | NA |
(F female, M male, WDMTC well differentiated MTC, PDMTC poorly differentiated MTC, CT calcitonin, CT-IHC immunohistochemistry for CT, NA Not Avaible)
The clinical presentation, not reported in all cases, were the presence of a palpable mass (11 cases), neck pain (3 cases), an incidentaloma on US (2 cases), shortness of breath (2 cases), cervical lymphadenopathy (1 case), loss of weight (1 case), dysphonia (1 case), paralysis of the ipsilateral vocal cord (1 case), dysphagia (1 case) and diarrhea (1 case).
Only one case presented familiarity with thyroid cancer, [13] even if in 10 cases data on familiarity were not reported. The pathological anamnesis showed 3 patients (6.25%) suffering of thyroid diseases: a case of Hashimoto’s thyroiditis, [14] a case of iatrogenic hyperthyroidism following amiodarone assumption [15] and one of non-toxic multinodular goiter. [12] Furthermore, we found that two patients (4%) were diagnosed with prostate cancer [15] and with breast cancer [16] respectively.
Preoperative evaluation and surgery
Regarding preoperative serum hormones levels, CT levels were reported only for 20 patients with a mean value of 8.66 pg/mL and a range of 0.8–38 pg/mL. (Table 3) In two cases normal CT levels were founded, however, the assay utilized and the considered range values were not specified. [5–10] In one case, it was reported a value of preoperative CT ≤2 pg/mL, [10] in a case a value of < 0.8 pg/mL, [10] in a case a value of < 10 pg/mL [4] and in 5 cases the value of preoperative CT was not reported. In Zhou’s article, preoperative hormones values were not mentioned [9].
Table 3.
Preoperative serum hormones and cytological findings
| Author | CT serum levels | CEA serum | Cytological examination | Immunohistochemistry |
|---|---|---|---|---|
| Sobol | Normal limits | NA | NA | NA |
| Schmid 1 | NA | NA | NA | NA |
| Schmid 2 | NA | NA | NA | NA |
| Schmid 3 | NA | NA | NA | NA |
| Schmid 4 | NA | NA | NA | NA |
| Redding | 8.2 pg/mL | NA | Atypical cells not diagnostic of MTC | Positive for calcitonin |
| Bockhorn | 0.8 pg/mL | Normal limits | Suspicious for MTC or anaplastic cancer | NA |
| Sand | 5.3 pg/mL | NA | NA | NA |
| Dora | 4.0 pg/mL | 0.78 ng/mL | Atypias suggesting for malignancy | NA |
| Wang | 38 pg/mL | 56.7 ng/mL | Discohesive cells with eccentric nuclei, finely granular chromatin and relatively uniform morphology | Negative for calcitonin |
| Giovanella | 4.7 pg/mL | 12.8 ng/mL | Aggregates of elonged cells with finely granular cytoplasm and oval nuclei with coarsely clumped chromatin and nuclear pseudoinclusions | Positive for calcitonin |
| Alapat | 4.0 pg/mL | 1.0 ng/mL | Positive for MTC | Positive for MTC |
| Chernyavsky | 2.1 pg/mL | 0.5 ng/mL | Findings suspicious for a poorly differentiated carcinoma with neuroendocrine differentiation | Negative for calcitonin |
| Nakazawa | 22 pg/mL | NA | Several solid cell clusters | NA |
| Frank-Raue 1 | 2.9 pg/mL | 1.3 ng/mL | Suspected malignancy | NA |
| Frank-Raue 2 | ≤2 pg/mL | 2.1 ng/mL | NA | NA |
| Frank-Raue 3 | < 0.8 pg/mL | 2.8 ng/mL | Positive for MTC | NA |
| Frank-Raue 4 | 2.6 pg/mL | 3.1 ng/mL | NA | NA |
| Frank-Raue 5 | Normal limits | Normal limits | NA | NA |
| Frank-Raue 6 | 11 pg/mL | Normal limits | NA | NA |
| Frank-Raue 7 | 1.5 pg/mL | 1.7 ng/mL | NA | NA |
| Ismi | 5.6 pg/mL | Normal limits | NA | NA |
| Brutsaert | 2.1 pg/mL | 3.1 ng/mL | Positive for malignant cells | Positive for calcitonin in isolated cells |
| Kim | 3.7 pg/mL | NA | Positive for MTC | NA |
| Kasajima | 29 pg/mL | NA | Positive for MTC | Negative for calcitonin |
| Samà 1 | 7.8 pg/mL | NA | NA | NA |
| Samà 2 | 5 pg/mL | NA | NA | NA |
| Samà 3 | < 10 pg/mL | 1.8 ng/mL | NA | NA |
| Samà 4 | 13 pg/mL | 6.3 ng/mL | NA | NA |
| Parmer | NA | NA | Suspected malignancy | NA |
| Zhou | NA | NA |
(CT calcitonin, CEA Carcinoembryonic antigen, NA not avaible)
In 12 patients, a value of CEA was detected with a mean value of 7.22 ng/mL and a range of 0.5–56.7 ng/mL. In 4 cases, a value in the normal range was reported and in 13 cases it was not performed preoperatively. (Table 3).
Twenty-three patients underwent fine-needle cytology (FNC) before surgery: six were positive for MTC, seven were suspicious for MTC, a patient was submitted to lymph nodal biopsy that confirmed diagnosis of MTC. FNC of the remaining 25 patients was not reported. Six FNC were studied with IHC: 3 were calcitonin negative, 2 calcitonin positive and one was confirmed to be MTC [13, 17–22].
Among 49 selected patients, 5 patients underwent total thyroidectomy, [4, 5, 14–16] 7 patients total thyroidectomy with central neck compartment lymphadenectomy or/and lateral compartment lymphadenectomy [11, 13, 19, 21–24] and a patient underwent hemithyroidectomy. [10] As regards the remaining cases, the surgery was not specified in the correspondent articles. (Table 4).
Table 4.
Intraoperative and postoperative findings
| Author | Surgery | Tumor grading | Follow-up | Recurrence | CT-IHC | CGRP-IHC | CgA-IHC | Syn-IHC | TG-IHC | CEA-IHC | RET mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sobol | TT | WDMTC | 6 month | Lymph nodes, liver and bone | – | – | + | NA | NA | + | NA |
| Schmid 1 | NA | WDMTC | NA | NA | NA | + | + | NA | – | – | NA |
| Schmid 2 | NA | WDMTC | 19 month | Lymph nodes | NA | + | + | NA | – | – | NA |
| Schmid 3 | NA | WDMTC | NA | NA | NA | – | + | NA | – | – | NA |
| Schmid 4 | NA | WDMTC | NA | NA | NA | – | + | NA | NA | – | NA |
| Redding | TT + LYA | WDMTC | 43 month | Negative | + | NA | NA | + | NA | + | – |
| Bockhorn | TT + LYA | PDMTC | NA | NA | NA | NA | + | NA | – | + | + |
| Sand | TT + LYA | PDMTC | Deceased 6 weeks | NA | NA | NA | NA | NA | NA | NA | NA |
| Dora | TT | PDMTC | NA | NA | + | NA | + | + | – | NA | – |
| Wang | NA | WDMTC | 12 months | Negative | + | NA | + | NA | – | + | NA |
| Giovanella | TT + LYA | WDMTC | 24 months | NA | + | NA | NA | NA | NA | NA | NA |
| Alapat | TT + LYA | WDMTC | 20 months | Negative | + | NA | + | NA | – | + | NA |
| Chernyavsky | TT + LYA | WDMTC | 12 months | Negative | – | NA | + | + | + | NA | + |
| Nakazawa | TT | WDMTC | 18 months | Negative | NA | NA | + | + | – | NA | NA |
| Frank-Raue 1 | ET | WDMTC | 72 months | Negative | + | NA | + | + | – | + | – |
| Frank-Raue 2 | NA | WDMTC | 25 months | Pulmonary | + | NA | + | + | – | + | – |
| Frank-Raue 3 | NA | WDMTC | 150 months | Lymph modes | + | NA | + | + | – | + | + |
| Frank-Raue 4 | NA | PDMTC | 18 months | Local tumor infiltration | + | NA | + | + | – | + | – |
| Frank-Raue 5 | NA | WDMTC | 21 months | Lymph node, bone, brain | + | NA | + | + | – | + | + |
| Frank-Raue 6 | NA | PDMTC | 21 months | Pulmonary | + | NA | + | + | – | + | + |
| Frank-Raue 7 | NA | PDMTC | 36 months | Dead because of pulmonary failure | + | NA | + | + | – | + | + |
| Ismi | NA | PDMTC | NA | NA | – | NA | + | + | – | NA | NA |
| Brutsaert | TT + LYA | WDMTC | NA | NA | NA | NA | NA | NA | NA | NA | + |
| Kim | ET | PDMTC | 12 months | Negative | – | NA | + | + | + | – | – |
| Kasajima | NA | WDMTC | NA | NA | NA | + | + | + | NA | NA | – |
| Samà 1 | TT | NA | 120 months | Negative | + | NA | + | NA | NA | + | – |
| Samà 2 | NA | NA | 120 months | Negative | NA | NA | NA | NA | NA | NA | NA |
| Samà 3 | NA | NA | 36 months | Negative | – | NA | – | NA | NA | – | + |
| Samà 4 | NA | NA | 36 months | Negative | + | NA | + | NA | NA | – | – |
| Parmer | TT | WDMTC | NA | NA | – | NA | + | + | – | + | NA |
| Zhou | NA | NA | NA | NA |
+ 8 cases - 11 cases |
NA |
+ 18 cases - 1 case |
+ 19 cases |
+ 5 cases - 14 cases |
+ 4 cases - 15 cases |
NA in 15 cases - In 4 cases |
(TT, total thyroidectomy; ET, emithyroidectomy; LYA, lymphadenectomy; WDMTC, well differentiated MTC; PDMTC, poorly differentiated MTC; NA, not avaible; −, negative; +, positive; IHC, immunohistochemistry; CT, calcitonin; CEA, Carcinoembryonic antigen; CGRP, calcitonin gene related peptide; CgA, chromogranin A; Syn, synaptofisine; TG, thyroglobulin)
Definitive pathology examination and immunohistochemistry
Definitive pathology detected 18 cases of well differentiated medullary thyroid carcinoma (WDMTC) and 8 cases of poorly differentiated medullary thyroid carcinoma (PDMTC). In the remaining patients the tumor grading was not evaluated. The following markers were tested at immunohistochemistry (IHC): CT, calcitonin gene related peptide (CGRP), CgA, synaptophysin (Syn), thyroglobulin (TG), CEA and RET oncogene mutations. At IHC, CT resulted positive in 21 of the 38 tested cases, while CgA showed a positive stain in all patients examined. RET oncogene mutation was negative in 4 cases of WDMTC, in 3 cases of PDMTC and in 6 cases of unknown differentiation [4, 9, 10, 14, 16, 19, 22–26]. The complete results were showed in Table 4. Mean follow-up was 41 months, with a range from 6 to 150 months. Recurrence was recorded in 7 patients, 2 of whom had multiple organ recurrence [5, 10]. Two patients died due to complications after surgery [10, 11] and seven patients for the disease progression with metastatic localization in lymph nodes, liver, bones, lungs and brain [5, 10, 18].
Microscopical examination and anatomopathological features are reported in Table 5. The neuroendocrine component was detected in 23 cases [4, 5, 9, 12, 22, 23, 25, 26]. The amyloid substance was found in 14 patients, [4, 5, 9, 14, 16, 20] lymph nodes metastasis in 7 cases, [9, 11, 13, 14, 20] thyroid capsular invasion in 15 specimens [9, 11, 13, 14, 18, 20, 24] and vascular tumor thrombus in 7 findings. [9, 11, 14, 18, 20]
Table 5.
Definitive pathology examination and immunohistochemistry
| Authors | Cell morphological characteristics | Neuroendocrine tumor structure | Amyloid substance | Lymph node metastasis | Thyroid capsular invasion | Vascular tumor thrombus |
|---|---|---|---|---|---|---|
| Sobol | Ovoid-to-spindle-shaped in groups divided by fibrous septum | Neurosecretory granules | Focal | NA | NA | NA |
| Schmid 1 | Polygonal and spindle cells | NA | NA | NA | Negative | Negative |
| Schmid 2 | Polygonal and spindle cells | NA | NA | NA | Negative | Negative |
| Schmid 3 | Polygonal and spindle cells | NA | NA | NA | Positive | Positive |
| Schmid 4 | Polygonal and spindle cells | NA | NA | NA | Positive | Positive |
| Redding | Nets of fairly uniform cells | NA | NA | NA | Negative | NA |
| Bockhorn | Polyhedral and spindle cells | Positive | NA | NA | Negative | NA |
| Sand | NA | NA | NA | Positive | Positive | Positive |
| Dora | Spindle-shaped celss | NA | Positive | Positive | Positive | Positive |
| Wang | NA | NA | Negative | Positive | Positive | Positive |
| Giovanella | Elongated cells | NA | NA | NA | NA | NA |
| Alapat | Spindle-round-polygonal cells | NA | NA | Positive | Positive | Positive |
| Chernyavsky | Fairly uniform round and polygonal cells | Positive | NA | Negative | Negative | Negative |
| Nakazawa | “Zellballen” pattern | NA | NA | Negative | Negative | Negative |
| Frank-Raue 1 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 2 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 3 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 4 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 5 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 6 | NA | NA | NA | NA | NA | NA |
| Frank-Raue 7 | NA | NA | NA | NA | NA | NA |
| Ismi | Atypical cells | Positive | NA | NA | NA | NA |
| Brutsaert | NA | NA | NA | Negative | Positive | Negative |
| Kim | NA | Positive | NA | Negative | Negative | Negative |
| Kasajima | Polygonal-spindle-shaped cells | Positive | NA | Negative | Negative | Negative |
| Samà 1 | Small-spindle cells | Positive | Positive | NA | NA | NA |
| Samà 2 | NA | NA | NA | NA | NA | NA |
| Samà 3 | NA | NA | NA | NA | NA | NA |
| Samà 4 | NA | NA | NA | NA | NA | NA |
| Parmer | Spindle-round cells | NA | Negative | NA | NA | NA |
| Zhou |
Polygonal cells in 17 cases Spindle cells in 2 cases |
Positive in 16 cases Negative in 3 cases |
Positive in 11 cases Negative in 8 cases |
Positive in 3 cases Negative in 16 cases |
Positive in 8 cases Negative in 11 cases |
Positive in 1 case Negative in 18 cases |
(NA not avaible)
Discussion
MTC is an uncommon and aggressive form of thyroid cancer. Therefore, early identification, surgical resection and careful postoperative surveillance are crucial. The cornerstone in MTC diagnosis and follow-up is the evaluation of CT serum level, which is an index of extreme sensitivity and specificity in case of basal level above of 100 pg/ml. Nevertheless, elevated CT levels may be present in patients affected by autoimmune thyroid disease, in heavy smokers, in end stage renal disease and in patients with pancreas and lung carcinoma [13]. Differential diagnosis is principally formulated on the basis of the pentagastrin stimulating test, which shows an increase in CT above 1000 pg/ml, only in presence of MTC, and throughout the evaluation of CEA and CgA serum levels [1]. New proposed diagnostic tests are the determination of CT on FNC washout fluids, evaluation of serum proCT and calcium stimulation of CT [27–29].
The present review analyze the extremely rare cases of non-secretory MTCs; to date only 49 cases of certified “atypical” MTC have been described in Literature. To the best of our knowledge, this is the first review reporting all cases described in English Literature. Firstly, Sobol et al. reported the case of a 82 years old woman affected by a MTC without CT serum level elevation. The follow up and the relapse identification were achieved through the identification of high CgA serum level, hypothesizing the possibility, in this so-called “chromograninoma”, of an altered co-regulation for genes of CgA and of hormone production [5]. Frank-Raue et al. reported 7 cases of nonsecretory MTC, with a prevalence in his large sporadic MTC population of the 0.83%. Moreover, in Frank-Raue series were reported only a weak or focal immunohistochemical stain in six of the seven cases, even if all cases presented a strong positivity for CgA, suggesting the role of CgA evaluation in addition to CEA in the diagnosis of CT negative MTC [10].
In fact, despite the low or undetectable CT serum level, at IHC in almost the half of the cases reported by the Authors, the tumors presented diffuse or focal positivity for CT and CEA, while was reported a CgA positivity in 41 of the 43 tested patients. As the parafollicular C-cells, NET have a neural crest ectoderm derivation and are present in many organs such as pancreas, lung, small bowel and stomach [30]. The differential diagnosis between MTC and NET is often challenging, because morphologically they have both spindle-shaped or round cells in trabecular arrangements with the presence of amyloid. At IHC, both cells stain positive for CgA, NSE and CEA. Therefore, the evaluation of CT serum level and CT at IHC staining are of paramount importance [22]. On these bases, in the reported atypical MTC cases, it is extremely hard to certainly exclude the diagnosis of a primary or secondary thyroidal NET, leading to a possible therapeutic and prognostic misunderstanding.
The differential diagnosis also includes a distinct type of thyroid neoplasm, the hyalinaising trabecular tumors, which share a similar histological pattern and a positive immunostain for CgA, somatostatin and NSE, conversely present as characteristic and distinctive feature the thyroglobulin hyper-expression [31].
The largest clinical series of nonsecretory MTC was reported by Zhou et al., which identified 19 cases of CT negative MTC among their 158 MTC treated patients with a surprising high prevalence of 12,02% [9]. Zhou, in his study, compared MTC patients vs nonsecretory MTC patients, describing usually larger masses in typical MTC group which were also associated with higher rate of lymph nodes metastasis, thus identifying tumor size as an independent survival indicator. Moreover, the study suggested a better oncological outcome for nonsecretory MTC and that the prognosis was related to the CT serum level. [9] Different findings were reported by Frank-Raue et al., which divided nonsecretory MTC patients into two groups, long-term survival (12,5 years) or rapid progression disease (1,75 years), the latter one characterized by over expression of Ki67 and RET gene mutation [10].
The pathophysiology of CT negative MTC is still not clearly understood. Several reasons have been advocated by eminent Authors to explain this medical dilemma. A possible explanation, reported by several papers, is the possibility of calcitonin assay interferences, or hook effect [14, 32]. The hook effect or prozone effect, is observed when a very high amount of an analyte is present in a sample but the observed value is falsely lowered. The mechanism of this significant negative interference is the capability of a high level of an analyte (antigen) to reduce the concentrations of “sandwich” (antibody 1:antigen:antibody 2) complexes that are responsible for generating the signal by forming mostly single antibody:antigen complexes [33]. Dora et al. have obviated to the hook effect bias by performing 1:10 and 1:100 dilutions of the patient serum [14]. Redding et al. have suggested that tumor cells release different types of serum CT, not all recognized by the same antibodies. Therefore, precursor molecules, aberrant CT produced by abnormal secretory mechanism or a high-dose hook effect in the serum immunoassay can be the possible causes of this phenomenon [19]. Nevertheless, several Authors have demonstrated, through the analysis of the immunohistochemical stain using the same antibodies used for serum calcitonin measurement, that parafollicular cells retain the ability to synthesize but not to secrete CT. In this regard, they hypothesized two possible explanations: the parafollicular cells in MTC undergo to a process of dedifferentiation losing the ability to produce CT or the possibility of a preneoplastic impairment in calcitonin secretion [14]. Alapat et al. too hypothesized an alteration of intracellular secretory pathways in tumor cells [13]. Frank-Raue and Brutsaert hypothesized the possibility in neoplastic C-cells, to secrete an altered proportion of N-proCT, mature CT and C-proCT due to an alternative splicing of the CT gene related peptide (CGRP). Moreover, modern monoclonal antibodies measure only monomeric CT, not detecting premature or aberrant form secreted in atypical MTC [10–24]. Similar findings were reported by Bockhorn et al., which suggested that very aggressive and undifferentiated MTC subtypes lose the ability to produce CT. This theory was supported by the recent American Thyroid Association guidelines for the management of MTC [23]. Sand et al. analyzed the nonsecretory MTC DNA with Southern blot hybridizations and identified a mutation of the calcitonin/CGRP gene which might be responsible of the low or undetectable CT serum level [11]. Nakazawa et al. reached the same conclusions, hypothesizing that the loss of calcitonin production reflects a genetic and/or epigenetic interference with CT/CGRP gene [15].
Schmid et al. proposed a different theory based on the thymic origin for the neuroendocrine thyroid tumors without calcitonin expression. In fact morphologically, MTC cells show histological patterns observed in carcinoid tumor of thymus [18, 34].
A rare thyroidal pathology sharing the same peculiar characteristics such as CT negativity stain at IHC and positivity to CgA and NSE have been identified by Chernyavsky et al., who classified this distinct clinical entity as the CT negative NET of the thyroid. However, this challenging diagnosis should be suspected only in case of positivity of thyroglobulin which is the main distinctive feature of this NET [22].
Indications and surgical treatment does not differ from the typical MTC. Total thyroidectomy and appropriate lymphadenectomy is recommended according to the recent guidelines [35, 36]. Due to the unreliability of serum biomarkers, postoperative surveillance in case of CT negative MTC is unclear. In fact the lack of CT increase in case of disease recurrence makes it necessary to perform, in addition to CT, CEA and CgA evaluations, serial and close imaging tests, including neck US and CT, and MRI of liver and chest, even if the identification of small tumor is hard [14–24]. Frank Raue et al., in addition to the conventional imaging techniques identified the 89% of occult persistent MTC with the selective venous catheterization [10]. Fluorine 18-fluorodeoxyglucose (18F-FDG) PET/TC have been proposed in different series as superior than conventional imaging in identifying relapse or disease persistence [35]. Conversely, other Authors considered 18F-FDG as an expensive technique not available in all centers, with a variable sensitivity ranging from 50 to 85% and not able to detect small masses [14].
Therefore, in order to better follow up patients after surgical treatment, new and alternative biomarkers are claimed in nonsecretory MTC. Between them the most reliable and promising indicators are ProCT and CGRP. ProCT is the precursor of CT, with a diagnostic accuracy comparable to CT in terms of identification of primary tumor, extrathyroidal extension and meatastases [37]. It is a very stable protein, easy to manage in pre-analytical level and with an in vivo half-life of 24 h [38]. With these positive features, Pro-CT has a great potential to replace serum CT as a new standard of care in the management of nonsecretory MTC. CGRP is a neuropeptide normally secreted by neurons and expressed both in MTC and in non neoplastic C-Cells. It is generated from the alternative RNA splicing of the CLC-A gene, which encodes for CT and CGRP. Its overexpression is not univocally linked with tumoral growth but is consistent with C-Cell origin, especially in case of co-expression TTF-1 and PAX-8. According to Brutsaert et al., among 18 patients diagnosed of MTC, CGRP was expressed in 66% in primary localization and in 73% of the metastases [24]. Moreover, CGRP is not expressed in follicular lineage and might be used to differentiate thyroidal NET from nonsecretory MTC [16].
Conclusions
CT negative MTC is an extremely rare pathology. The pathophysiology of CT negative MTC is still not clearly understood. Several reasons have been advocated by eminent Authors to explain this medical dilemma, the altered cellular secretion mechanisms, the production of aberrant CT precursors not recognized by the testing antibodies, the hook effect, an ectopic thymic origin, unfortunately without reaching any definitive conclusions. Due to the lack of elevation of serum markers as CT and CEA, atypical MTC is often diagnosed at an advanced stage, leading moreover to a challenging surveillance. The prognosis reported is extremely variable, differing from long term survival and rapid tumor progression in case of poorly differentiated diagnosis, high Ki67 expression and RET mutation. Further studies are needed, to better define options for management of non secretory MTCs and to identify new and reliable biomarkers associated to diagnosis and relapse of this medical dilemma.
Acknowledgements
N/A
Funding
This article did not receive sponsorship for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets used and/or analyzed during the current study are available from the VII Division of General Surgery, Second University of Naples, Via Pansini 5 80131 Naples, on reasonable request.
About the supplement
This article has been published as part of BMC Endocrine Disorders Volume 19 Supplement 1, 2019: Updates and New Technology in Endocrine Surgery. The full contents of the supplement are available online at https://bmcendocrdisord.biomedcentral.com/articles/supplements/volume-19-supplement-1
Abbreviations
- CEA
Carcinoembryonic antigen
- CgA
Chromogranin A
- CGRP
Calcitonin gene related peptide
- CT
Calcitonin
- CT
Computed tomography
- DFS
Disease-free survival
- ET
Emithyroidectomy
- FNC
Fine needle aspiration cytology
- IHC
Immunohistochemistry
- LYA
Lymphadenectomy
- MTC
Medullary thyroid carcinoma
- NET
Neuroendocrine tumor
- NSE
Neuron specific enolase
- OS
Overall survival
- pro-CT
Pro-calcitonin
- Syn
Synaptophysin
- TG
Thyroglobulin
- TT
Total thyroidectomy
- US
Ultrasonography
Authors’ contributions
All authors contributed significantly to the present research and reviewed the entire manuscript. GC: Participated substantially in conception, design and execution of the study and in the analysis and interpretation of the data; also participated substantially in the drafting and editing of the manuscript. OC: Participated substantially in conception and design and of the manuscript. PR, ClG, MC, TE, DCF, DMS, FL, CA, CG, DG: Participated substantially in conception and design of the manuscript and in the analysis and interpretation of the data. All of the authors have read and approved the final manuscript.
Authors’ information
Gambardella Claudio and Mauriello Claudio are PhD student in Medical, Clinical and Sperimental Sciences at Univerity of Campania “Luigi Vanvitelli” – Naples, Italy.
Ethics approval and consent to participate
Not applicable, data retrospectively obtained by clinical records.
Consent for publication
All patients gave written informed consent to publish.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claudio Gambardella, Phone: +390815666648, Email: claudiog86@hotmail.it.
Chiara Offi, Email: chiara.o@live.com.
Renato Patrone, Email: dott.patrone@gmail.com.
Guglielmo Clarizia, Email: clarizia.guglielmo@libero.it.
Claudio Mauriello, Email: claudio.mauriello@live.it.
Ernesto Tartaglia, Email: ernestart@msn.com.
Francesco Di Capua, Email: francesco.dicapua@unicapania.it.
Sergio Di Martino, Email: sergio.dimartino@unicampania.it.
Roberto Maria Romano, Email: robertomaria.romano@gmail.com.
Lorenzo Fiore, Email: lorenzo.fiore@studenti.unicampania.it.
Alessandra Conzo, Email: aleconzo@hotmail.it.
Giovanni Conzo, Email: giovanni.conzo@unicampania.it.
Giovanni Docimo, Email: giovanni.docimo@unicampania.it.
References
- 1.HAZARD JOHN B., HAWK WILLIAM A., CRILE GEORGE. MEDULLARY (SOLID) CARCINOMA OF THE THYROID—A CLINICOPATHOLOGIC ENTITY*. The Journal of Clinical Endocrinology & Metabolism. 1959;19(1):152–161. doi: 10.1210/jcem-19-1-152. [DOI] [PubMed] [Google Scholar]
- 2.Conzo G, Mauriello C, Docimo G, Gambardella C, Thomas G, Cavallo F, Tartaglia E, Napolitano S, Varriale R, Rossetti G, Fei L, Santini L. Clinicopathological pattern of lymph node recurrence of papillary thyroid cancer. Implications for surgery. Int J Surg. 2014;12(Suppl 1):S194–S197. doi: 10.1016/j.ijsu.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Conzo G, Tartaglia E, Avenia N, Calò PG, de Bellis A, Esposito K, Gambardella C, Iorio S, Pasquali D, Santini L, Sinisi MA, Sinisi AA, Testini M, Polistena A, Bellastella G. Role of prophylactic central compartment lymph node dissection in clinically N0 differentiated thyroid cancer patients: analysis of risk factors and review of modern trends. World J Surg Oncol. 2016;14:149. doi: 10.1186/s12957-016-0879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samà MT, Rossetto Giaccherino R, Gallo M, Felicetti F, Maletta F, Bonelli N, Piovesan A, Palestini N, Ghigo E, Arvat E. Clinical challenges with calcitonin-negative medullary thyroid carcinoma. J Cancer Res Clin Oncol. 2016;142(9):2023–2029. doi: 10.1007/s00432-016-2169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobol RE, Memoli V, Deftos LJ. Hormone-negative, chromogranin A-positive endocrine tumors. N Engl J Med. 1989;320(7):444–447. doi: 10.1056/NEJM198902163200707. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa: Ottawa Hospital Research Institute. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp accessed 17 Feb 2014).
- 8.Cohen EG, Shaha AR, Rinaldo A, Devaney KO, Ferlito A. Medullary thyroid carcinoma. Acta Otolaryngol. 2004;124(5):544–557. doi: 10.1080/00016480310015704. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Yue S, Cheng Y, Jin J, Xu H. Clinical and pathological analysis of 19 cases of medullary thyroid carcinoma without an increase in calcitonin. Exp Toxicol Pathol. 2017;69(8):575–579. doi: 10.1016/j.etp.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Frank-Raue K, Machens A, Leidig-Bruckner G, Rondot S, Haag C, Schulze E, Lorenz A, Kreissl MC, Dralle H, Raue F, Schmid KW. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid. 2013;23(3):294–300. doi: 10.1089/thy.2012.0236. [DOI] [PubMed] [Google Scholar]
- 11.Sand M, Gelos M, Sand D, Bechara FG, Bonhag G, Welsing E, Mann B. Serum calcitonin negative medullary thyroid carcinoma. World J Surg Oncol. 2006;4:97. doi: 10.1186/1477-7819-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismi O, Arpaci RB, Berkesoglu M, Dag A, Sezer E, Bal KK, Vayısoğlu Y. Calcitonin-negative neuroendocrine tumor of thyroid gland mimicking anaplastic carcinoma: an unusual entity. Gland Surg. 2015;4(4):344–349. doi: 10.3978/j.issn.2227-684X.2015.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alapat DV, Ain KB, Sloan DA, Monaghan KG, Karabakhtsian RG. Disparity between tissue and serum calcitonin and carcinoembryonic antigen in a patient with medullary thyroid carcinoma. Endocrine. 2011;39(2):148–152. doi: 10.1007/s12020-010-9433-2. [DOI] [PubMed] [Google Scholar]
- 14.Dora JM, Canalli MH, Capp C, Puñales MK, Vieira JG, Maia AL. Normal perioperative serum calcitonin levels in patients with advanced medullary thyroid carcinoma: case report and review of the literature. Thyroid. 2008;18(8):895–899. doi: 10.1089/thy.2007.0231. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa T, Cameselle-Teijeiro J, Vinagre J, Soares P, Rousseau E, Eloy C, Sobrinho-Simões M. C-cell-derived calcitonin-free neuroendocrine carcinoma of the thyroid: the diagnostic importance of CGRP immunoreactivity. Int J Surg Pathol. 2014;22(6):530–535. doi: 10.1177/1066896914525228. [DOI] [PubMed] [Google Scholar]
- 16.Parmer M, Milan S, Torabi A. Calcitonin-negative neuroendocrine tumor of the thyroid. Int J Surg Pathol. 2017;25(2):191–194. doi: 10.1177/1066896916670989. [DOI] [PubMed] [Google Scholar]
- 17.Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ, 3rd, Ganly I, Hodak SP, Kebebew E, Patel KN, Shaha A, Steward DL, Tufano RP, Wiseman SM, Carty SE. American Thyroid Association surgical affairs committee. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25(7):760–768. doi: 10.1089/thy.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid KW, Ensinger C. "atypical" medullary thyroid carcinoma with little or no calcitonin expression. Virchows Arch. 1998;433(3):209–215. doi: 10.1007/s004280050238. [DOI] [PubMed] [Google Scholar]
- 19.Redding AH, Levine SN, Fowler MR. Normal preoperative calcitonin levels do not always exclude medullary thyroid carcinoma in patients with large palpable thyroid masses. Thyroid. 2000;10(10):919–922. doi: 10.1089/thy.2000.10.919. [DOI] [PubMed] [Google Scholar]
- 20.Wang TS, Ocal IT, Sosa JA, Cox H, Roman S. Medullary thyroid carcinoma without marked elevation of calcitonin: a diagnostic and surveillance dilemma. Thyroid. 2008;18(8):889–894. doi: 10.1089/thy.2007.0413. [DOI] [PubMed] [Google Scholar]
- 21.Giovanella L, Crippa S, Cariani L. Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. Int J Biol Markers. 2008;23(2):129–131. doi: 10.1177/172460080802300212. [DOI] [PubMed] [Google Scholar]
- 22.Chernyavsky VS, Farghani S, Davidov T, Ma L, Barnard N, Amorosa LF, Trooskin SZ. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid. 2011;21(2):193–196. doi: 10.1089/thy.2010.0299. [DOI] [PubMed] [Google Scholar]
- 23.Bockhorn M, Frilling A, Rewerk S, Liedke M, Dirsch O, Schmid KW, Broelsch CE. Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. Thyroid. 2004;14(6):468–470. doi: 10.1089/105072504323150813. [DOI] [PubMed] [Google Scholar]
- 24.Brutsaert EF, Gersten AJ, Tassler AB, Surks MI. Medullary thyroid cancer with undetectable serum calcitonin. J Clin Endocrinol Metab. 2015;100(2):337–341. doi: 10.1210/jc.2014-3095. [DOI] [PubMed] [Google Scholar]
- 25.Kim GY, Park CY, Cho CH, Park JS, Jung ED, Jeon EJA. Calcitonin-negative neuroendocrine tumor derived from follicular lesions of the thyroid. Endocrinol Metab (Seoul) 2015;30(2):221–225. doi: 10.3803/EnM.2015.30.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasajima A, Cameselle-Teijeiro J, Loidi L, Takahashi Y, Nakashima N, Sato S, Fujishima F, Watanabe M, Nakazawa T, Naganuma H, Kondo T, Kato R, Sasano H. A calcitonin non-producing neuroendocrine tumor of the thyroid gland. Endocr Pathol. 2016;27(4):325–331. doi: 10.1007/s12022-016-9416-9. [DOI] [PubMed] [Google Scholar]
- 27.Trimboli P, Cremonini N, Ceriani L, Saggiorato E, Guidobaldi L, Romanelli F, Ventura C, Laurenti O, Messuti I, Solaroli E, Madaio R, Bongiovanni M, Orlandi F, Crescenzi A, Valabrega S, Giovanella L. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol. 2014;80(1):135–140. doi: 10.1111/cen.12234. [DOI] [PubMed] [Google Scholar]
- 28.Mian C, Perrino M, Colombo C, Cavedon E, Pennelli G, Ferrero S, De Leo S, Sarais C, Cacciatore C, Manfredi GI, Verga U, Iacobone M, De Pasquale L, Pelizzo MR, Vicentini L, Persani L, Fugazzola L. Refining calcium test for the diagnosis of medullary thyroid cancer: cutoffs, procedures, and safety. J Clin Endocrinol Metab. 2014;99(5):1656–1664. doi: 10.1210/jc.2013-4088. [DOI] [PubMed] [Google Scholar]
- 29.Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99(8):2986–2994. doi: 10.1210/jc.2014-1278. [DOI] [PubMed] [Google Scholar]
- 30.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88(5):1139–1148. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1139::AID-CNCR26>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Schmid KW, Mesewinkel F, Böcker W. Hyalinizing trabecular adenoma of the thyroid--morphology and differential diagnosis. Acta Med Austriaca. 1996;23(1–2):65–68. [PubMed] [Google Scholar]
- 32.Leboeuf R, Langlois MF, Martin M, Ahnadi CE, Fink GD. "hook effect" in calcitonin immunoradiometric assay in patients with metastatic medullary thyroid carcinoma: case report and review of the literature. J Clin Endocrinol Metab. 2006;91(2):361–364. doi: 10.1210/jc.2005-1429. [DOI] [PubMed] [Google Scholar]
- 33.Schiettecatte J, Anckaert E, Smitz J. Interferences in immunoassays. In: Chiu NHL, editor. Advances in immunoassay technology. InTech. 2012. pp. 45–62. [Google Scholar]
- 34.Schmid KW. Calcitonin-free medullary thyroid carcinoma: evidence for a neuroendocrine thyroid carcinoma of thymic origin? J Pathol (Lond) 1997;181:32A. [Google Scholar]
- 35.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG. American Thyroid Association guidelines task force on medullary thyroid carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conzo G, Docimo G, Pasquali D, Mauriello C, Gambardella C, Esposito D, Tartaglia E, Della Pietra C, Napolitano S, Rizzuto A, Santini L. Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg. 2013;13(S2):1–6. doi: 10.1186/1471-2482-13-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, Crocetti U, Attard M, Maranghi M, Torlontano M, Filetti S. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92(2):450–455. doi: 10.1210/jc.2006-1590. [DOI] [PubMed] [Google Scholar]
- 38.Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clin Chem Lab Med. 2015;53(10):1507–1514. doi: 10.1515/cclm-2015-0058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The datasets used and/or analyzed during the current study are available from the VII Division of General Surgery, Second University of Naples, Via Pansini 5 80131 Naples, on reasonable request.


