Abstract
Ocean temperatures are rising; species are shifting poleward, and pH is falling (ocean acidification, OA). We summarise current understanding of OA in the brackish Baltic-Skagerrak System, focussing on the direct, indirect and interactive effects of OA with other anthropogenic drivers on marine biogeochemistry, organisms and ecosystems. Substantial recent advances reveal a pattern of stronger responses (positive or negative) of species than ecosystems, more positive responses at lower trophic levels and strong indirect interactions in food-webs. Common emergent themes were as follows: OA drives planktonic systems toward the microbial loop, reducing energy transfer to zooplankton and fish; and nutrient/food availability ameliorates negative impacts of OA. We identify several key areas for further research, notably the need for OA-relevant biogeochemical and ecosystem models, and understanding the ecological and evolutionary capacity of Baltic-Skagerrak ecosystems to respond to OA and other anthropogenic drivers.
Keywords: Baltic, Ecosystem services, Eutrophication, Indirect effects, Ocean acidification, Warming
Introduction
Globally, increasing emissions of anthropogenic carbon dioxide (CO2) are causing warming of the oceans, melting of sea ice, glaciers and ice sheets, and ocean acidification.1 For the Baltic-Skagerrak System (Fig. 1), these processes are reflected in rising sea level, increased precipitation (leading to locally reduced salinity), increased flooding, coastal erosion, and flow of organic and inorganic matter into coastal waters. All of these add to the direct and indirect effects of ocean acidification in different ways. Anthropogenic ocean acidification arises when anthropogenic emissions of CO2 elevate atmospheric CO2 concentration, resulting in elevated partial pressure of CO2 (pCO2) in the oceans and a corresponding decrease in ocean pH. But pH also varies naturally in the oceans over diurnal and seasonal timescales. This arises due to biogeochemical drivers, such as temperature, salinity, and input of terrestrial organic matter and subsequent decay, as well as through biological processes such as primary production (which increases pH) and respiration (which decreases pH). While the effects of these drivers on marine life are relatively well understood individually, we know less about the effects of combinations of drivers on single species, and little about their effects on entire coastal ecosystems, communities, and society in general—especially for ocean acidification.
Fig. 1.
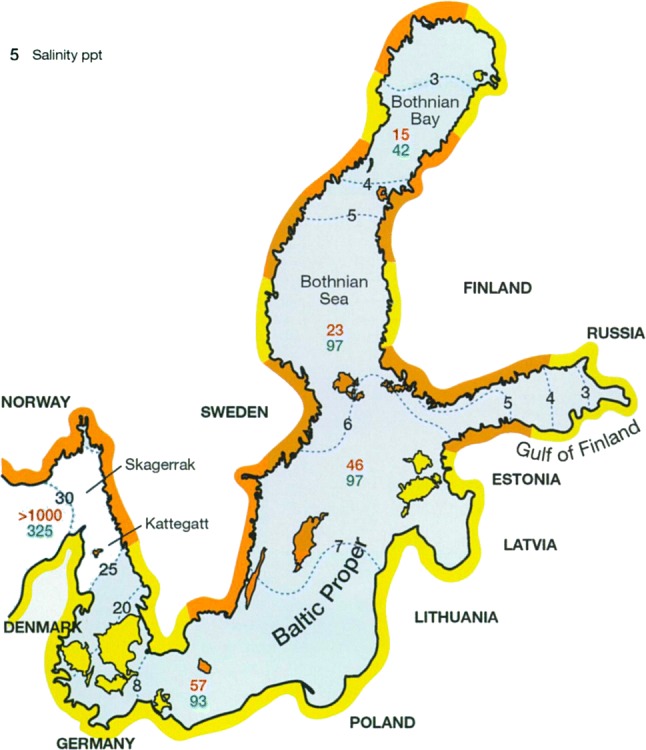
Map of the Baltic-Skagerrak System (Skagerrak, Kattegatt, Baltic Proper, Bothnian Sea, Bothnian Bay, Gulf of Finland). We use the term “Baltic-Skagerrak System” to refer to the entire region from the Skagerrak to the Bothnian Bay.
Modified from Rönnbäck et al. (2007)
Ocean acidification (hereafter, ‘OA’) is recognised in the United Nations’ Sustainable Development Goal 14.3 “Minimize and address the impacts of ocean acidification…”, and has been identified as a cause of substantial impacts on marine ecosystems (Gattuso et al. 2015; Riebesell and Gattuso 2015). Although OA is a global issue caused by rising atmospheric CO2 concentrations (Doney et al. 2009), the degree and effects of OA are geographically heterogeneous due to regional differences in air:sea fluxes and ocean chemistry (Steffen et al. 2015). Multiple recent reviews of OA have concluded that effects on species are generally negative, but that responses vary markedly among species (e.g. Kroeker et al. 2013), notably between field and the laboratory (e.g. Wahl et al. 2018), and that OA effects can be strongly modified by interactions with other drivers (Gunderson et al. 2016) and species (Kroeker et al. 2017). Much of this recent work highlights the importance of non-linear processes, especially when multiple environmental drivers interact (e.g. Albright et al. 2016a; Mostofa et al. 2016; Kroeker et al. 2017). For example, Gao et al. (2018) found that the interactive effects of OA and ocean warming on phytoplankton physiology could be synergistic, neutral, or antagonistic depending on species and prevailing environmental conditions. Equivalent responses have been reported at higher trophic levels (Harvey et al. 2013). At a broader scale, OA impacts on marine ecosystems have the potential to affect a wide range of ecosystem services (ESs). Although a general picture of OA effects on ESs is yet to emerge (Falkenberg and Tubb 2017), some specific OA-related dis-benefits have already been identified (e.g. oysters, Lemasson et al. 2017). Addressing these OA impacts directly may be difficult, however, manipulating non-OA drivers affecting ecosystem resilience or adaptation has been suggested as a viable management policy option to mitigate OA effects (Albright et al. 2016b).
The geological record provides an important environmental archive of past OA events and how past marine ecosystems have responded to changes in pH and ocean biogeochemistry (e.g. Harnik et al. 2012; Hönisch et al. 2012). The most recent global event occurred during the last deglaciation, when atmospheric CO2 increased 30% (from 189 to 265 μatm), leading to a ~ 0.15 unit drop in sea surface pH in the open ocean (e.g. Hönisch and Hemming, 2005). This change corresponded to a decrease of 0.002 units per 100 years, which is much slower than current rates (Zeebe et al. 2016). The coastal seas of the Baltic-Skagerrak System were subjected to considerable environmental changes during the deglaciation and disentangling the potential effect of a regional change in pH (and its magnitude) remains to be done.
The Baltic-Skagerrak System comprises one of the world’s largest permanent salinity gradients (Fig. 1) from the high salinity Skagerrak shores (salinity ~ 30)2 to the shallow, almost freshwater archipelagos of the northern Bothnian Bay (salinity ~ 3). This gradient not only contains multiple different ecosystems, but also creates differences in seawater chemistry that alter the process, and effects, of OA. Hence ocean acidification will impact various parts of the system very differently.
In addition to drivers3 such as OA and warming that are changing marine systems on a global scale, the Baltic-Skagerrak System is also subject to local pressures from eutrophication, freshening, and pollution, resulting from agriculture, tourism, fishing, aquaculture, etc. Local differences in the strength and timing of these drivers, and the natural heterogeneity of coastal seas, create far greater variability and change than that seen in the open ocean. Coastal marine ecosystems of the Baltic have changed markedly during the past 40 years, which is partly attributable to local and regional warming and freshening (Olsson et al. 2012), as well as eutrophication (Olsson et al. 2015). The role of OA in this context is poorly known, not least because most biologists were unaware of its importance until relatively recently (Gattuso and Hansson 2011).
Here we (i) outline the current state of knowledge related to OA in the Baltic-Skagerrak System, as an example of OA-related processes in a brackish coastal sea; (ii) identify knowledge gaps; and (iii) suggest priorities for future research. We focus on the mechanisms that underlie OA, its interactions with other coastal drivers, and its impacts on biogeochemical processes, marine species, and ecosystems. Unless stated otherwise, we consider the biological consequences of “near-future” OA (i.e. pCO2 levels up to ~ 1300 µatm, van Vuuren et al. 2011). Information summarised here was obtained from ISI Web of Science and EconLit databases using the search terms “ocean acidification” or “pCO2”, together with “Baltic, Kattegat, Skagerrak or Finland”. Additional examples, including non-OA examples, were searched for on an ad hoc basis. A companion manuscript (Jagers et al. 2018) addresses the socio-economic background to, and prospective societal solutions for, OA in Swedish coastal seas. Throughout, we use the term “Baltic-Skagerrak System” to refer to all waters from the Skagerrak to the Bothnian Bay, and the relevant regional terms for the different components of the Baltic system (Fig. 1).
Biogeochemical basis of ocean acidification in the Baltic-Skagerrak System
The surface ocean continuously exchanges CO2 with the atmosphere. When atmospheric partial pressure of CO2 (pCO2) is greater than that in the oceans, there is a flux of CO2 from the atmosphere into the oceans (and vice versa). This flux is influenced by primary production—which reduces seawater pCO2 in the summer, and by decomposition and biomineralisation—which cause the release of CO2 in the winter. These processes create a seasonal cycle of pCO2 both in the atmosphere and the ocean. However, as the atmosphere mixes much faster than the ocean, the atmospheric signal is diluted and far less pronounced: for instance the seasonal amplitude in atmospheric pCO2 at the latitude of Sweden is ~ 10 µatm, while that in the coastal ocean can be several hundred µatm (Fig. 2).
Fig. 2.
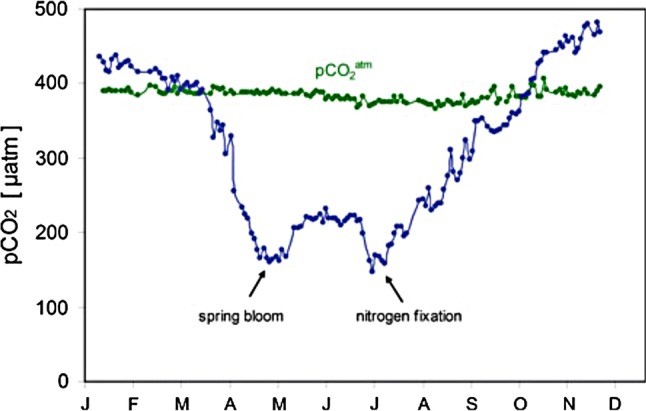
Seasonal changes in phytoplankton productivity absorb dissolved CO2 in the water (blue line) during spring and summer, creating “draw-down” relative to the level of CO2 in the overlying atmosphere (green line). Data from central Baltic Sea, taken from Schneider et al. (2015)
Since the onset of the industrial revolution, seasonally averaged global atmospheric pCO2 has risen from around 280 µatm to > 400 µatm today, and consequently the average pCO2 of the surface ocean has also increased (Bates et al. 2012). Seasonal fluctuations in pCO2 are superimposed on this mean increase, leading to greater extremes of pCO2. Increasing atmospheric pCO2 reduces ocean pH, but the extent of pH reduction depends not only on the pCO2 itself, but also on other properties of the seawater, notably the existing pH, the buffering capacity (the “alkalinity”) of the seawater, and to a small extent the temperature (see Box 1). The chemical buffer capacity, i.e. the change in pH for a given change in pCO2, is determined by the total concentration of all the bases in the seawater, measured as total alkalinity. In most of the world’s coastal oceans, alkalinity is broadly correlated with salinity, and therefore buffer capacity typically decreases with decreasing salinity. However, in the different basins of the Baltic-Skagerrak System, this alkalinity:salinity relationship is complicated by large differences in local geology that influence alkalinity independently (Omstedt et al. 2009; see Box 2). This results in the different low-salinity basins of the Baltic-Skagerrak System having different pHs despite having similar salinities; i.e. low pH in the northern Bothnian Bay and inner Gulf of Finland due to input of low alkalinity river runoff, and high pH in the Gulf of Riga resulting from inflow of high alkalinity runoff (Fig. 3).
| Box 1: Atmospheric CO2 and the acidification of seawater | |
|---|---|
|
Unlike the other atmospheric gases, when CO2 dissolves in seawater it reacts chemically with the water:
The pH of the seawater determines which of these chemical species dominate. Higher pH drives the system farther to the right. At seawater pH’s typical of Swedish coastal waters (7.5–8.5) the carbonate system is dominated by the HCO3– terms, and hence dissolving CO2 in seawater leads to an increase in proton (H+) concentration, and hence increased acidity. Dissolved inorganic carbon (DIC) buffers the dissolution of CO2 in seawater by the CO32– ion reacting with the CO2. Because DIC and alkalinity decline from the Swedish west-coast, through the Baltic Proper and into the Bothnian Bay, the effects of increasing pCO2 are greater in the Baltic than on the west coast (see main text). |
| Box 2: Salinity, alkalinity and pH in the Baltic | |
|---|---|
| In the Baltic, sea surface salinity is determined by the combination of runoff of freshwater together with limited exchange of seawater with the North Sea. In those parts of the Baltic system most distant from the North Sea (Bothnian Bay and eastern Gulf of Finland), the salinity is below 3, rises to around 7 in the Baltic Proper, and then rises rapidly from ~ 8 in the southern Danish straits to ~ 15 in the Kattegat just a hundred km or so to the north. Salinity continues to rise to the north and west, reaching 30 in the western Skagerrak (Fig. 1). The alkalinity of river runoff varies depending on the geology of the drainage basin. The northern Bothnian Bay, which is largely surrounded by granite bedrock, has relatively low alkalinity and pH because the alkalinity of the runoff is low. In contrast, the limestone bedrock that characterises the watersheds flowing into the Gulf of Riga has very high alkalinity, and thus pH in the Gulf of Riga is much higher (Fig. 3). Recent work shows increased weathering of bedrock, increases alkalinity and offsets the effects of ocean acidification (Müller et al. 2016). |
Fig. 3.
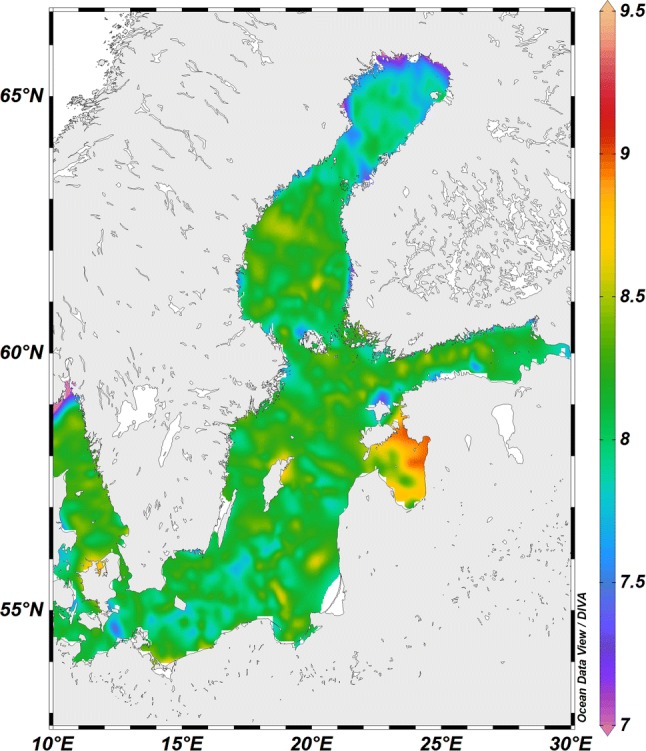
Long-term mean surface water (0–20 m) pH in the Baltic-Skagerrak System, © Adam Ulfsbo. Data are interpolation of historical records collected during all seasons between 1911 and 2003, and therefore only show general patterns in pH distribution. For more information, including station positions, see Hjalmarsson et al. (2008)
Differences in alkalinity combine with differences in the extent of primary production to cause greater seasonal variability in pH in parts of the Baltic compared to the Kattegatt/Skagerrak (Fig. 4). This effect is amplified by the nutrient-rich conditions in the Baltic that cause a stronger “draw-down” of CO2 during the productive season, which results in higher pH during the summer (Fig. 4b). These data are for the open Baltic and Kattegat: in shallow coastal embayments, the processes contributing to this variation generate even stronger pH variation (e.g. Saderne et al. 2013; Wallace et al. 2014; Carstensen et al. 2018).
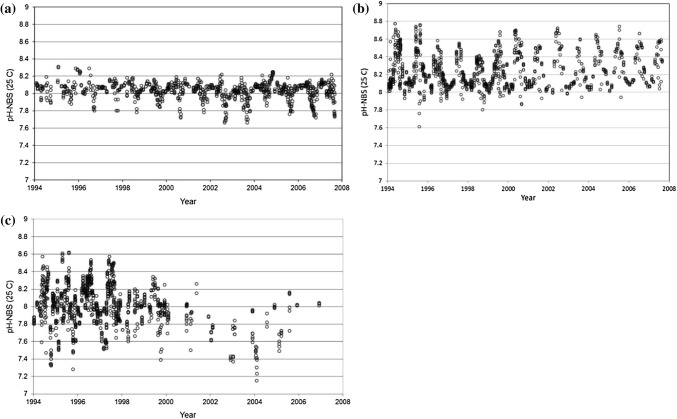
Fig. 4.
Time series of pH in the top 20 m of the water column showing seasonal fluctuations in the open Kattegat (a), Baltic Proper (b) and Bothnian Bay (c). Note that the observational frequency decreased substantially after 2000 in the Bothnian Sea (Data on pHNBS scale, measured at 25 °C from SMHI: http://sharkdata.se/about/)
Seasonal changes in CO2 uptake and release also have implications for other important chemical processes in coastal seas. Photosynthesis by micro- and macroalgae, and marine plants, consumes “acidic” CO2 and hydrogen ions (H+) (see Box 3). This causes pH to increase in summer while the decomposition of organic matter, produces hydrogen ions and lowers pH, reversing this process in winter (Fig. 2). Primary production occurs close to the surface in the surface-lit (photic) zone, but much of the decomposition occurs after this production has died and settled to the seafloor, i.e. deep in the water column or at the sediment surface. In waters with limited exchange, this can completely deplete the available oxygen near the seabed, resulting in bottom waters with very low oxygen (“hypoxic”, i.e. < 30% O2 saturation), or no oxygen (“anoxic”). This influences seawater chemistry resulting in increased solubility of some metals (notably manganese and iron), and in some cases the production of elemental sulphur, all of which influence seawater pH (see Box 3).
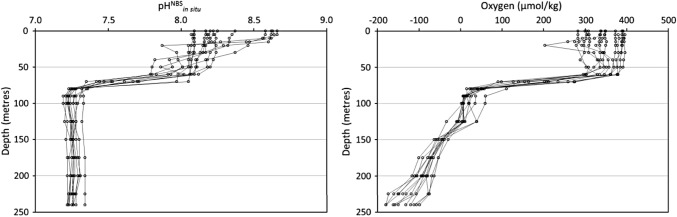
Fig. 5.
Profiles of pH and oxygen in the Gotland Basin for all months during 2008 (after Ulfsbo et al. 2011). Note that negative oxygen concentrations represent sulphide
| Box 3: Chemical consequences of ocean acidification in the Baltic-Skagerrak System | |
|---|---|
|
In the surface mixed layer of the oceans, the photosynthetic capture of light energy to combine CO2, macro-nutrients (such as nitrate and phosphate), and micro-nutrients in the form of trace metals (Me2+, such as iron(II)) to create organic matter and oxygen can be formulated as (1) Decomposition of sedimenting organic matter in deeper water runs in the opposite direction, releasing CO2 and H+. In the following formulation, this CO2 release is illustrated by balancing it to the bicarbonate ion, HCO3−, the dominating form of dissolved inorganic carbon at typical seawater pH (Box 1). (2) Thus decomposition produces hydrogen ions, and hence lowers pH. This reaction normally occurs deep in the water column or at the sediment surface. In waters with limited exchange, the decomposition process (reaction 2) can sometimes completely deplete the available oxygen, resulting in strongly hypoxic or anoxic bottom water. Under these circumstances, other “electron acceptors” are needed to replace oxygen in the decomposition process. The most energetically favourable electron acceptor after oxygen is nitrate, and hence in hypoxic and anoxic areas, decomposition leads to denitrification: (3) When comparing reactions (2) and (3), it can be seen that denitrification generates far fewer hydrogen ions per bicarbonate ion produced. If decomposition proceeds to deplete all the nitrate, then other electron acceptors step in. In seawater, these are (in order) manganese(IV), iron(III) and sulphate. When these are used as electron acceptors the following reactions (4–6) occur (here organic matter is simplified to “carbohydrates”; CH2O(org)): (4) (5) (6) These reactions have very different impacts on pH as both manganese and iron reduction consume H+, whereas sulphate reduction produces H+. An important consequence of this is that the sulphide bottom-water that occurs in the Baltic Proper has close to constant pH, even if the sulphide concentration increases with depth (Fig. 5). Note that temperature affects these biogeochemical reactions as well as the solubility of gases, which results in lower pH (more CO2) in colder waters in equilibrium with the atmosphere. These biochemical processes also occur in the sediment, especially in surface layers where “bioturbation” by the fauna causes mixing of interstitial water with deep waters in the water column. When anoxic water meets oxic water the reduced chemical species are oxidised in reactions that also involve hydrogen ions. For example, when iron(II) is oxidised H+ is produced: (7) But when hydrogen sulphide is oxidised to elemental sulphur H+ is consumed: (8) Or if iron sulphide precipitates H+ is produced: (9) |
In the Baltic-Skagerrak System, nutrient emissions from waste-water treatment and agricultural runoff have caused eutrophication, such that areas of hypoxia/anoxia have increased (Conley et al. 2009, 2011). Anoxia amplifies the effects of eutrophication through chemical reactions between iron (III) and phosphate, which in oxic sediments form precipitations that trap phosphate, but which is dissolved and released when the sediment becomes anoxic and iron(III) is reduced to iron(II). Thus, in anoxic waters phosphate is released back to the water column where, in time, it is mixed up to the surface where it stimulates primary productivity, leading to higher summer pH, as illustrated in the Baltic Proper (Fig. 4).
In addition to influencing alkalinity, river runoff also contains dissolved and particulate organic matter, some of which decays by microbial activity, adding to local deoxygenation and ocean acidification. The dissolved fraction has further impact on pH as these dissolved molecules contain carboxyl groups (organic acids) and thus can reduce pH further. Although uncertainty is large, climate model projections for the coming century show increased precipitation over northern Scandinavia, particularly in the winter months (Christiansen et al. 2015), which implies increased flux of terrestrial organic matter to the coastal seas, potentially adding to OA and deoxygenation.
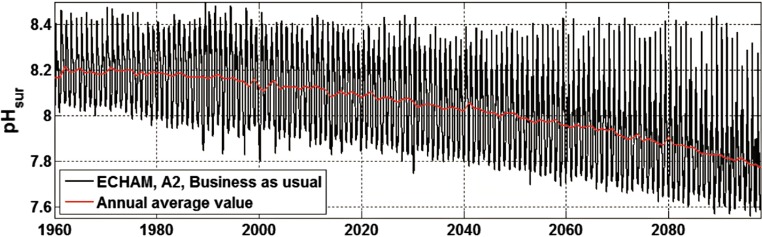
Despite many years of monitoring data, seasonal variation and methodological issues preclude reliable detection of long-term trends in measurements of seawater pH in Baltic-Skagerrak System. Recent modelling, however, projects that the combination of this variation with increasing anthropogenic atmospheric CO2 will lead to greater pH variation and lower pH minima in the surface waters (Fig. 6). Importantly, although projected average pH in the scenario plotted in Fig. 6 reaches 7.8 in the last decade of this century, winter minimum pH already begins to fall below this value in the year 2040. Thus, for species that are sensitive to low pH, the seasonal effects of acidification may be felt far sooner than would be expected from the projected mean annual pH.
Fig. 6.
Daily pH values for surface water in the Eastern Gotland Basin projected from the ECHAM global climate model and the SRES A2 “business as usual” scenario. Note that due to increased nutrient load that stimulates photosynthetic uptake of CO2 from surface waters, summer maximum pH remains mostly constant until ~ 2090, whereas winter minimum pH declines almost linearly throughout the modelled period. (From Omstedt et al. 2012)
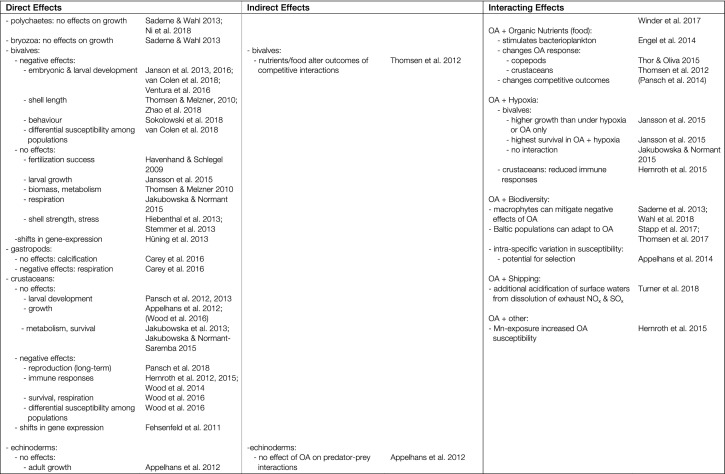
Direct effects of ocean acidification
Ocean acidification impacts the species of the Baltic-Skagerrak System directly, interactively (i.e. in combination with other drivers), and indirectly through competitive and trophic interactions in the ecosystem. In this section, we consider the direct effects of OA on species. The interactive and indirect effects of OA are considered in subsequent sections.
Responses of plankton in the Baltic-Skagerrak System to OA in the form of elevated pCO2 are highly variable, and often context dependent. This applies both among species/OTUs (operational taxonomic units), but also at the community level (Table 1). At the species/OTU level, bacterioplankton abundance typically shows no (or small) responses to OA (e.g. Baltic Proper, Lindh et al. 2013), but at the community level indirect responses can manifest as shifts in community composition, which can be linked to shifts in the phytoplankton community (western Baltic, Bergen et al. 2016; Gulf of Finland, Hornick et al. 2017). Responses of phytoplankton tend to be more variable. Cyanobacterial species in different functional groups display positive, negative, or no, responses to elevated pCO2 (western Baltic, Czerny et al. 2009; Eichner et al. 2014), and responses of spring-bloom diatoms and dinoflagellates also differ. For example, in the Skagerrak, growth rates of diatoms increased under OA (Kremp et al. 2012), but not growth of a toxic dinoflagellate (although toxin production increased, Kremp et al. 2012). Calcification and growth of coccolithophorids under OA varies among clones (multiple locations, Langer et al. 2009) and species (Meyer and Riebesell, 2015), illustrating the difficulties that intra- and interspecific variation create for making generalisations (Eichner et al. 2014; note that coccolithophorids are largely restricted to the higher salinity Kattegat and Skagerrak). At the community level, mesocosm studies show OA can influence phytoplankton community structure, with subtle shifts in dominant taxa such as diatoms, cryptophytes, and cyanobacteria, but stronger shifts in sub-dominant taxa (western Baltic, Sommer et al. 2015; Gulf of Finland, Paul et al. 2016a, b). Overall phytoplankton productivity seems to increase under OA, although there is also a strong seasonal component to this response (Skagerrak, Eberlein et al. 2017).
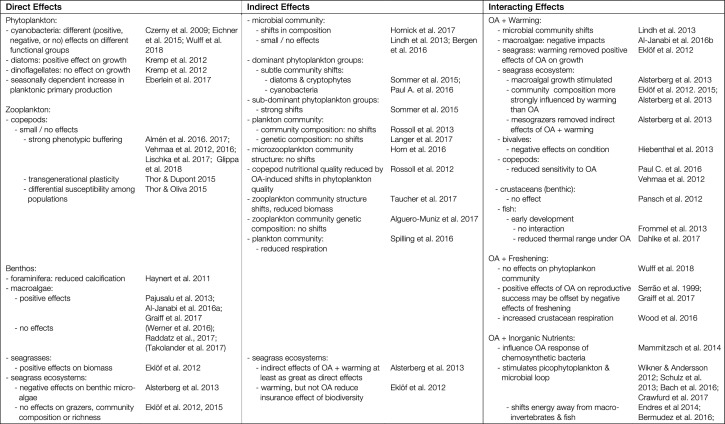
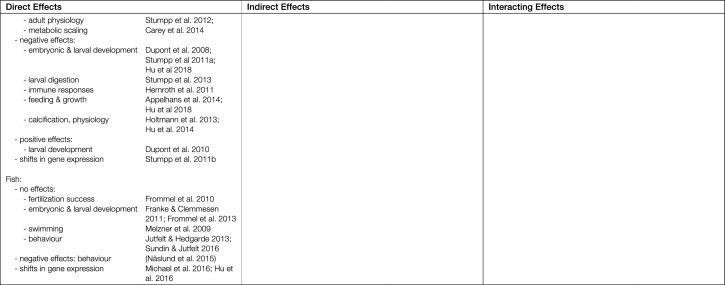
Table 1.
Summary of observed direct and indirect biological effects of ocean acidification (OA) and interactions with other key anthropogenic drivers in the Baltic-Skagerrak System [Effects and interactions relevant to the Baltic-Skagerrak System, but for which supporting examples come from other regions, are discussed in the main text. References in parentheses show mixed responses]
Among benthic macrophytes, macroalgae show negative, no, or positive, responses to increasing pCO2 (reviewed by Takolander et al. 2017). Elevated pCO2 stimulated growth of filamentous “opportunistic” green- and red-algae (eastern Baltic, Pajusalu et al. 2013), and of the brown alga Fucus, from the western Baltic and Baltic Proper (Al-Janabi et al. 2016a), and had small positive effects on fertilisation success in Fucus (western Baltic, Graiff et al. 2017). Other experiments with Fucus found no (or minor) effects of OA (Pajusalu et al. 2013; western Baltic, Werner et al. 2016; Raddatz et al. 2017). In contrast, seagrasses generally respond more uniformly, and positively, to elevated pCO2, although reported effects are relatively small (Skagerrak, Eklöf et al. 2012; Alsterberg et al. 2013; Portugal, Repolho et al. 2017). In cases where OA has been investigated in combination with warming, responses to OA are frequently overshadowed by the interactive effects of warming (see below).
Studies of zooplankton in the Baltic-Skagerrak System have focused on copepods, which show a high degree of phenotypic buffering to OA in a variety of traits such as body size, reproduction, and behaviour (e.g. Vehmaa et al. 2012, 2016; Almén et al. 2016, 2017; Lischka et al. 2017). Some of these responses have been shown to be enhanced by trans-generational inheritance of plasticity, whereby parental exposure to OA generates more OA-tolerant offspring (Kattegatt, Thor and Dupont, 2015; Baltic Proper, Vehmaa et al. 2016). This plasticity has limits, however, and high pCO2 still had negative effects (Acartia, 1230 µatm CO2, Vehmaa et al. 2016). Importantly, susceptibility of copepods to OA has been shown to vary with location and environmental history (Spitzbergen vs. Skagerrak, Thor and Oliva, 2015). This work also showed that different energetic functions (e.g. ingestion and respiration) can respond differently to OA, yielding non-linear responses at the whole-organism level (Thor and Oliva, 2015). Data for OA responses of gelatinous plankton in the Baltic-Skagerrak System are lacking, however, studies of relevant species from nearby regions indicate they are generally tolerant of OA (Aurelia, North Sea, Algueró-Muñiz et al. 2016), or respond positively (Oikopleura, North Sea, Winder et al. 2017; Bouquet et al. 2018). In summary, these data suggest that the impacts of OA on zooplankton species in the Baltic-Skagerrak System are likely to be small. Interactions among species and effects on the plankton community as a whole are considered in the following section.
In contrast to zooplankton, benthic animals in the Baltic-Skagerrak System generally respond either negatively or neutrally to OA (to date there is one report of a positive response; seastar, Skagerrak, Dupont et al. 2010). Negative responses include reduced embryonic and larval survival (brittle stars, Skagerrak, Dupont et al. 2008; bivalves, Baltic, Jansson et al. 2016; Skagerrak, Ventura et al. 2016; van Colen et al. 2018), delayed larval development (sea urchins, Skagerrak, Stumpp et al. 2011a), and immune suppression (crustaceans, Skagerrak, Wood et al. 2014; Hernroth et al. 2015; see Table 1 for full list). Reports of no, or minor, responses to OA include effects on fertilisation success (bivalves, Skagerrak, Havenhand and Schlegel 2009), adult growth (bivalves, Baltic, Thomsen and Melzner 2010), larval development (crustaceans, Skagerrak, Baltic, Pansch et al. 2012, 2013), and metabolic scaling (echinoderms, Skagerrak, Carey et al. 2014; see Table 1 for full list). Notably, bivalves from parts of the western Baltic that routinely experience strong upwelling/low pH events appear to be tolerant of extreme levels of OA (Thomsen and Melzner 2010; Thomsen et al. 2013), indicating that here too environmental history may be an important determinant of tolerance (Thomsen et al. 2017). As noted earlier for copepods, trans-generational acclimation to OA has also been reported for benthic invertebrates (e.g. Dupont et al. 2013; Hu et al. 2018), and here too, prior environmental history appears to influence tolerance (Hu et al. 2018). Dissecting the effects of trans-generational plasticity from other forms of plasticity is complex and more, targeted experimentation is required before generalisations can be made on the potential for trans-generational plasticity in benthic animals (Torda et al. 2017).
Two bivalve species in particular, Mytilus trossulus/edulis and Macoma baltica, are key components of Baltic-Skagerrak ecosystems, and fundamental to benthic ecosystem functioning in the region (Niiranen et al. 2013). In both cases, these species show few or no responses to OA: Mytilus from the Baltic-Skagerrak System tend to be highly insensitive to OA (Thomsen and Melzner 2010; Thomsen et al. 2010, 2013; Jakubowska and Normant 2015; Ventura et al. 2016), and this response appears to be heritable (Stapp et al. 2017; Thomsen et al. 2017). Although larvae of Macoma baltica from the eastern Baltic are negatively impacted by OA (Jansson et al. 2013, 2016; unlike the adjacent North Sea, van Colen et al. 2018), juvenile survival was positively impacted by OA (in combination with hypoxia, Jansson et al. 2015), and adult Macoma appear to be unaffected, even by very low pH (southern Baltic, Jakubowska and Normant-Saremba 2015). Thus, it seems unlikely that OA will have substantive impacts on these key bivalves—and hence on the primary benthic component of benthic-pelagic coupling—in the Baltic-Skagerrak system.
Commercially and ecologically important fish species in the Baltic-Skagerrak System show generally, but not exclusively, negative direct responses to OA. Growth of larval halibut, herring, and cod were all negatively impacted by OA (Baltic, Franke and Clemmesen 2011; Frommel et al. 2013; Skagerrak, Gräns et al. 2014; Stiasny et al. 2016), but OA had no effects on sperm motility or swimming performance of adult cod (Baltic, Melzner et al. 2009; Frommel et al. 2010). Incorporating the effects of OA in stock recruitment models led to a projected reduction in recruitment of western Baltic cod stocks by 90% (Stiasny et al. 2016). Work on non-commercial species has shown the potential for trans-generational effects that can increase offspring tolerance to OA (sticklebacks, North Sea, Schade et al. 2014), which may ameliorate these effects. The extent to which such effects operate in commercially important species in the Baltic-Skagerrak System is not yet known, however, and currently available data indicate that overall impacts of OA on commercially important fish stocks in the region will likely be negative.
Many of these findings come from coastal habitats, in which diurnal and seasonal pH variations can greatly exceed the shifts expected under OA (western Baltic, Saderne et al. 2013; Kattegat, Carstensen et al. 2018). However, the biological effects of such diurnal pH fluctuations in combination with OA have been investigated rarely (reviewed by Boyd et al. 2016). The only study to test these effects in the Baltic-Skagerrak System investigated responses of the euryhaline barnacle Balanus improvisus (Eriander et al. 2015). This study showed that diurnally fluctuating OA, mimicking future coastal seas, increased variance in growth more than tenfold, revealing strong “winner” and “loser” phenotypes that were not seen in conventional constant OA treatments, and which indicated the potential for adaptation to future OA. Similar differences in responses under constant vs fluctuating OA have been obtained in several other species (Boyd et al. 2016). Collectively, these findings indicate that tolerance to OA in situ may be far more variable than previously thought, and that projecting future impacts of OA from data obtained in experiments using constant OA treatments may be unreliable.
Interactive effects of ocean acidification: Species-level responses
OA does not operate in isolation, and interactive and indirect effects (Box 4) are common. Additional drivers such as warming, freshening, and nutrient input are changing simultaneously in the Baltic-Skagerrak System (Meier 2015), leading to concurrent shifts in eutrophication and hypoxia (Conley et al. 2009, 2011), which interact with the effects of OA. Identifying the consequences of these multiple simultaneous drivers is a complex task (Boyd et al. 2018), yet many investigators have begun to address these in the Baltic-Skagerrak System (Table 1). This section focuses on the species-level effects of these interactions. Indirect effects, which operate via the food-web are considered in the following section.
| Box 4 Direct, indirect, and interactive effects | |
|---|---|
|
Anthropogenic drivers (including OA) rarely operate in isolation. Combinations of driver may have additive, synergistic, or antagonistic effects (Todgham and Stillman 2013). Additive effects are linear, and arise when the effects of multiple combined drivers equals the sum of their effects in isolation. In contrast, synergistic and antagonistic effects are non-linear and arise when the combined effects of multiple drivers is greater than (synergistic) or less than (antagonistic) the sum of those drivers in isolation (Todgham and Stillman 2013; Gunderson et al. 2016). Note that, these non-linear terms relate to the outcome of the interaction, not the effect itself: synergistic negative effects of temperature and OA on growth, for example, will create a greater decrease in growth than the sum of their single effects. Drivers also have direct, and/or indirect, effects on the focal organism or process. Direct effects (of single or multiple drivers) operate directly, such as the impacts of temperature on metabolic rate. Indirect effects operate through chains of interactions, e.g. decreased growth of a filter-feeder due to acidification-induced reduction in nutritional value of its phytoplankton food. Indirect effects typically operate through the food-web, and can be at least as great as direct effects (Alsterberg et al. 2013). |
Over the last 150 years, the Baltic has warmed by 0.4–1.5 °C (Gustafsson et al. 2012). Regional climate models project warming in this region will average + 2–3 °C by the end of this century (Meier 2015). This warming will have substantial direct impacts on marine organisms both independently (e.g. Boyd et al. 2013), and interactively with OA (Doney et al. 2012). Theoretical frameworks for the interaction between warming and acidification are well established (Pörtner and Farrell 2008), and recent work has confirmed this, showing that OA not only reduces the thermal tolerance window, but also can reduce peak performance at optimal temperature (larval cod, Kattegat, Dahlke et al. 2017), and modify intestinal physiology (adult cod, Kattegat, Hu et al. 2016). Other examples show, for example, reduced survival and growth under OA + warming (macroalgae, western Baltic, Al-Janabi et al. 2016b). Surprisingly, however, this pattern is far from uniform, and several investigations have found small, or no, interactive effects of OA + warming (e.g. crustaceans, western Baltic, Pansch et al. 2012; cod, western Baltic, Frommel et al. 2013), or additive effects that averaged the responses seen under each driver independently (e.g. copepods, Gulf of Finland, Vehmaa et al. 2012). Consequently, it is not possible to generalise the biological effects of this interaction (Table 1).
Interactions between OA and salinity are fundamental to the biogeochemistry of OA in the Baltic-Skagerrak System (Box 2, 3). Climate models project freshening by ~ 2 (≈ 10–20%) in the Belt-Seas around Denmark, and by 0.5–1 (≈ 15–30%) in the Bothnian Bay by 2100 (Christiansen et al. 2015; Meier 2015). This will drive species distributions further “out” toward the North Sea (Viitasalo et al. 2015). The likely combined biological effects of this freshening with OA in the Baltic-Skagerrak System have received relatively little attention to date. Available data indicate increased sensitivity to OA in low-salinity populations (crustaceans, Gulf of Bothnia, Wood et al. 2016), potential for freshening to counteract the positive effects of OA on reproductive success (Fucus, Baltic, Serraõ et al. Serrao et al. 1999; Graiff et al. 2017), and no substantive impacts of OA + freshening on microplankton community structure (Baltic Proper, Wulff et al. 2018; Table 1). This important research area requires further experimental investigation before patterns in responses to this interaction can be identified.
The increased precipitation that is projected to lead to freshening will also flush more inorganic and organic nutrients from land into the Baltic-Skagerrak System. The effects of this nutrient enrichment are well understood: it stimulates planktonic primary production, which contributes to increased sedimentation of organic matter to the sea-floor, where it decomposes, reducing O2 concentrations (creating hypoxia) and simultaneously generating CO2 and acidification (Conley et al. 2011; Meier et al. 2011; Meier 2015; Schneider et al. 2015; Box 3). The effects of hypoxia on marine organisms can be strong, and include behavioural changes, reduced performance, and loss of fitness (Gray et al. 2002), as well as reduced benthic productivity and biodiversity (Weigel et al. 2015). The combined effects of OA and hypoxia are now attracting renewed interest from the scientific community, and recent work in the Baltic-Skagerrak System has shown that OA + hypoxia can cause immune suppression in crustaceans (Skagerrak, Hernroth et al. 2015). Other workers, however, have found no effects of OA + hypoxia (Mytilus, southern Baltic, Jakubowska and Normant 2015), and even strong positive responses have been reported (Macoma, Gulf of Finland, Jansson et al. 2015; Table 1). The long-term prevalence of hypoxia in the Baltic-Skagerrak System (Conley et al. 2011) has almost certainly led to acclimation and adaption in many species, however, the extent to which this has also generated tolerance to OA is an exciting prospect that remains to be investigated.
Indirect effects of ocean acidification: Ecosystem responses
Indirect effects of OA—which operate through shifts in competition and trophic interactions—are common in experiments that expose ecological communities to elevated pCO2. The indirect effects of OA on plankton communities vary among systems, but are typically small and subtle. In bacterioplankton, indirect effects were generally small (Baltic Proper, Lindh et al. 2013; western Baltic, Bergen et al. 2016; Table 1), although one study reported substantial shifts in microbial community composition correlated with shifts in phytoplankton community structure (Gulf of Finland, Hornick et al. 2017). Similar, subtle responses were observed in other studies of OA on community composition of phytoplankton (western Baltic, Sommer et al. 2015; Baltic Proper, Paul et al. 2016a, b), although stronger effects were seen in sub-dominant and rare species of phytoplankton (Sommer et al. 2015). These small indirect effects of OA can depend on nutrient availability: when nutrients were not limiting, either through experimental addition or in situ remineralisation, increased pCO2 shifted phytoplankton community composition towards much smaller picophytoplankton (Spitzbergen, Schulz et al. 2013; Kattegat, Bach et al. 2016). This, in turn, indirectly stimulated the microbial loop, which reduced subsequent energy flow to zooplankton and fish (North Sea, Endres et al. 2014; Gulf of Finland, Bermúdez et al. 2016). At an ecosystem level, indirect effects among planktonic species generally lead to small or no effects of OA on community composition (Skagerrak, Horn et al. 2016; Algueró-Muñiz et al. 2017; Langer et al. 2017) and genetic composition (Skagerrak, Langer et al. 2017), although some studies have reported reduced zooplankton biomass (Skagerrak, Taucher et al. 2017), and reduced community respiration (Gulf of Finland, Spilling et al. 2016). Importantly, OA impacts on fatty acid (FA) content of phytoplankton led to radical shifts in FA content, and reduced reproductive output in grazing copepods, indicating the potential for strong OA effects to propagate up the food-web (western Baltic, Rossoll et al. 2012). Field observations of plankton communities also indicate warming-induced shifts toward smaller size composition, with consequent reduction in energy availability to planktivorous fish (Suikkanen et al. 2013). Given that shifts in copepod abundance, size-spectrum, and nutritional quality are likely to cascade to higher trophic levels—and potentially influence regime shifts between cod-dominated and sprat/herring-dominated states in the Baltic (Casini et al. 2009)—there is a clear need to better understand the consequences of indirect and interactive effects of OA in plankton communities of the Baltic-Skagerrak System.
In benthic ecosystems, indirect effects of OA have been reported to be at least as great as direct effects. For example, mesocosm experiments in the Skagerrak showed OA alone had few or no direct or indirect effects, but the combination of OA with warming strongly influenced community composition and productivity (Eklöf et al. 2012, 2015; Alsterberg et al. 2013). These results were similar, but not identical, to those seen in response to warming alone (Alsterberg et al. 2013). Indirect trophic effects were also important: warming together with the loss of a keystone consumer strongly promoted the growth of ephemeral algae (Alsterberg et al. 2013), and had additive positive effects on functional diversity of associated fauna (Eklöf et al. 2015), such that overall indirect effects were at least as strong as direct effects (Alsterberg et al. 2013). Dense stands of benthic macrophytes can also generate substantial diurnal and seasonal pH fluctuations (globally, Hofmann et al. 2011; western Baltic, Saderne et al. 2013), which indirectly impact associated flora and fauna. For example, Baltic macroalgal and seagrass communities generate sufficiently large fluctuations in pH that they can provide temporal refuges from OA stress for calcifying species such as the blue mussel, Mytilus edulis (Wahl et al. 2018). The potential for dense stands of benthic macrophytes to condition the water, and thereby mitigate the impacts of OA throughout the coastal photic zone is clearly considerable (Saderne et al. 2013; Wahl et al. 2018), and warrants further investigation.
Indirect effects of OA also impact benthic-pelagic coupling, which plays a key ecological role throughout the Baltic-Skagerrak System. Sedimentation of planktonic primary production transfers food-energy to the benthos, which—in sufficiently high quantities—can increase food supply to benthic macrofauna, thereby increasing their resilience to OA (western Baltic, Thomsen and Melzner 2010; Melzner et al. 2011; Thomsen et al. 2013; Pansch et al. 2014; Stapp et al. 2017). However, the observation that OA reduces the nutritional value of phytoplankton for copepods (western Baltic, Rossoll et al. 2012), indicates that indirect effects of OA in combination with other drivers are likely to negatively influence the food value of particulates reaching benthic filter-feeders. The extent to which this reduction in food quality is offset by any OA-induced increases in total planktonic primary production (Kremp et al. 2012; Eberlein et al. 2017) remains unclear. Benthic macrofauna in the Baltic-Sakgerrak System are nonetheless projected to be negatively impacted by other, non-OA, drivers such as eutrophication, hypoxia, warming, and freshening (Weigel et al. 2015), which may have cascading effects throughout the ecosystem (Bergström et al. 2015; Viitasalo et al. 2015; Vuorinen et al. 2015), potentially driving regime shifts similar to those seen in the pelagic (Casini et al. 2009).
Modelling shows that controlling eutrophication and fishing pressure is central to managing Baltic ecosystems—not least because responses are non-linear (Niiranen et al. 2013). To date, however, these models have not included direct or indirect effects of OA. In regions where ecosystem management models have included the effects of OA, additive, synergistic, and antagonistic effects of OA with other drivers have been found (e.g. SE Australia, Griffith et al. 2011; US West coast, Kaplan et al. 2010). Including the effects of OA in ecosystem modelling of the Baltic-Skagerrak System is an important, and currently lacking, step that will permit assessment of ecosystem sensitivity to OA. Doing this calls for: (i) improved empirical knowledge/data on the effects of OA on the physiology and demography of key species, and on ecosystem responses; and (ii) modelling that includes the effects of OA at individual, population, and ecosystem levels (e.g. Koenigstein et al. 2016).
In addition to the drivers discussed above, other anthropogenic drivers have specific interactions with OA that influence the chemistry—and potentially biological responses—of the Baltic-Skagerrak System. Among these, shipping contributes directly to OA through the release not only of CO2 but also of the acidic gases SOx and NOx (Omstedt et al. 2015), which dissolve in seawater to create sulphuric and nitric acids. Methods to reduce SOX emissions to the atmosphere include exhaust-gas scrubber systems that absorb the SOx in a counterflow of seawater spray, creating a saline solution of sulphuric acid. Open-loop scrubbers that lack effluent treatment systems release this acid direct to surface waters, causing further acidification. Although the effects of such release are small on a basin scale (Turner et al. 2018), in heavily trafficked areas the effects of releasing acidic scrubber effluent on local seawater pH can be as large as those of anthropogenic ocean acidification (Stips et al. 2016). The biological effects of additional effluent components such as organic microparticles are poorly known.
Warming is also causing marine species distributions to move poleward (so-called “climate tracking”; Pinsky et al. 2013; Hiddink et al. 2015; Molinos et al. 2016). This is projected to increase species turnover (gain of new species, loss of established species) in the coming decades (Molinos et al. 2016). Invasive species are already established throughout the Baltic-Skagerrak System (Hiddink and Coleby 2012; Katsanevakis et al. 2014), with both negative and positive impacts on marine biogeochemistry and ecosystems (reviewed by Katsanevakis et al. 2014) that can interact with OA (Box 3). Although there is strong uncertainty about the timing of further invasions, the establishment of invasive species—especially those tolerant of low pH—seems likely. At the time of writing, no published studies have investigated the effects of OA on warming-driven range shifts and ecosystem structure and function in the Baltic-Skagerrak System.
The role of biodiversity, evolution, and resilience
The impacts of OA and other anthropogenic drivers on marine ecosystems depend not only on the factors and processes considered above. Biodiversity (both genetic variation within species, as well as between species [i.e. richness]), functional diversity (the diversity of ecological functions within an ecosystem), and evolutionary capacity of the component species, are all central to determining the resilience of an ecosystem to disturbance and change (Doney et al. 2012; Loreau and Mazancourt 2013; Gamfeldt et al. 2015; Lefcheck et al. 2015), and hence to OA.
At a within-species-level, anthropogenic pressures are reducing population sizes and genetic diversity (Harley et al. 2006). In smaller populations, stochastic factors such as genetic drift can restrict the ability of populations to adapt to local conditions (Polechová and Barton 2015). Within-species genetic diversity in the Skagerrak and Kattegat is substantially greater than in populations of the same species in the Baltic (Johannesson and André 2006), and consequently we might expect the adaptive capacity of Baltic populations to OA to be less than that in the relatively diverse populations of the Skagerrak and Kattegatt. At present, there are no geographically comprehensive data with which we can test this prediction. Nonetheless, it is clear that some Baltic populations are strongly tolerant to extreme levels of OA (e.g. mussels, western Baltic, Thomsen et al. 2017), and that Baltic populations have adapted rapidly in the past to drivers such as salinity change (Pereyra et al. 2009; and see Johannesson et al. 2011). Experimental laboratory work has also shown that Baltic species can adapt rapidly to high pCO2 conditions (e.g. Lohbeck et al. 2012; Stapp et al. 2017). Although the greater complexity of the natural environment may lead to different outcomes (Bach et al. 2018), available evidence nonetheless suggests there is substantial adaptive capacity in Baltic populations.
At the level of ecological communities, loss of species reduces ecosystem functioning, leading to loss of productivity and greater sensitivity to disturbance (Campbell et al. 2011; Cardinale et al. 2012; Worm et al. 2006; Duffy et al. 2016). Thus, biodiversity is critical to the long-term sustainability of ecosystem functions in the face of environmental change (Loreau and Mazancourt, 2013; Oliver et al. 2015). Examples from marine ecosystems include genetic diversity enhancing the resilience of Baltic seagrass beds (Reusch et al. 2005), and species diversity enhancing the resilience of the California Current pelagic ecosystem (Lindegren et al. 2016). The extent to which resilience to OA is enhanced by biodiversity is less well understood, although work in Swedish seagrass beds indicates that OA + warming can have positive indirect impacts (Alsterberg et al. 2013), and warming (but not OA) reduces the insurance effect of biodiversity (Eklöf et al. 2012). Conversely, investigations of the effects of OA on genetic diversity of plankton communities in large mesocosms found no substantive impacts (Skagerrak, Langer et al. 2017). Unfortunately, comparable data from the Baltic Proper are not available. Nonetheless, it seems likely that the reduced biodiversity observed within and among species in the Baltic Sea will decrease ecosystem resilience to OA and other interacting drivers, rendering the Baltic particularly vulnerable.
Consequences for ecosystem services
The impacts of OA on marine ecosystems are likely to have pervasive consequences. The world’s coastal oceans, including the Baltic-Skagerrak System, generate numerous ecosystem services that are important for human welfare (Millennium Ecosystem Assessment 2005; TEEB 2010; Hattam et al. 2015). Identifying and forecasting the effects of OA on these services in the Baltic-Skagerrak System is, however, a challenging task for several reasons. First, attributing any given ecosystem change to an individual driver, such as OA, is rarely straightforward because multiple interacting drivers simultaneously cause ecosystem change. Shifts in ecosystem services driven by overfishing, eutrophication, habitat destruction, and OA, for example, have their origins in distal phenomena such as urbanisation, global consumption patterns, trade, etc. (Norström et al. 2017; Österblom et al. 2017). This is exemplified in the Baltic-Skagerrak System by eutrophication-driven ecosystem degradation, which has contributed to declines in marine ecosystem goods and services (Rönnbäck et al. 2007; Österblom et al. 2007). Second, these different impacts often have synergistic (reinforcing) or antagonistic (mitigating) effects on each other (Box 4). Ecosystem disturbances operating over long periods can result in small gradual changes in ecosystem structure and functions, yet can sometimes (and especially when disturbances are strong and synergistic) cause rapid shifts that change ecosystem structure, functions, and services (Scheffer et al. 2001; Österblom et al. 2007; Casini et al. 2009). Conversely, negative impacts on extant fisheries may be offset by positive impacts on new, alternate, fishery species (similar to the effects of ocean warming on fisheries catch, Cheung et al. 2010, but see Cheung et al. 2016), although such trade-offs have yet to be demonstrated for OA. Third, the diversity of ecosystem services implies that OA is likely to affect them in different ways. Among the provisioning ecosystem services alone there are multiple examples, such as the production of fish, which can be impacted by the negative effects of OA on coastal fish species (Baltic herring, Franke and Clemmesen 2011; Kattegatt cod, Stiasny et al. 2016); the negative effects of OA on aquaculture (e.g. mussel and oyster growth in the USA, Barton et al. 2012); and the possibility of indirect effects of OA on forage fish in distant waters that that are used for aquaculture feeds. Similar OA-driven shifts can also arise in supporting and cultural ecosystem services through impacts on coastal towns reliant on the fishing industry. Finally, people benefiting from these services may value them in very different ways and will be affected differently depending on their own vulnerability and whether or not replacement services exist.
The potential risks for economic losses can be illustrated by looking at just one key provisioning service, the Swedish fishery sector. In 2016, Swedish fisheries were conservatively estimated to be worth ~ SEK 7.5 billion (recreational = SEK 6.2 billion, SCB 2018; industrial = SEK 1.3 billion, HAV 2014), equivalent to ~ € 730 million. Including Swedish aquaculture (SEK 487 million; SCB 2017), pushes this total to ~ € 780 million. Of course, not all of this value will be lost as a result of OA impacts (and much aquaculture value is in freshwaters), but as outlined earlier, commercially valuable fish species in the Baltic-Skagerrak System respond negatively to OA, and therefore this key provisioning service could incur substantial financial costs. Currently, however, know too little to make valid quantitative assessments of the likely social and economic risks and vulnerabilities arising from biological responses to OA (Falkenberg and Tubb 2017). In order to make these assessments, we need to meet the relevant requirements (Hilmi et al. 2013): (i) accurate determination of the fraction of GNP that depends on provisioning ecosystem services such as fishing and aquaculture, and cultural services such as coastal tourism, that depend on OA sensitive ecosystems; (ii) identifying likely shifts in species composition, and hence food value, of seafood under OA; (iii) projecting changes in human populations dependent on the coastal zone; (iv) determining the vulnerability and sensitivity of these coastal populations to environmental change and assessing their capacity to adapt.
Conclusions
The Baltic-Skagerrak System is characterised by seasonal and diurnal cycles of temperature, primary production, and decomposition, which create strong cycles of pH variation. Interactions with local biogeochemistry and anthropogenic drivers modify these fluctuations, making the detection of decadal pH trends from field observations difficult. However, oceanographic models for the Baltic Proper that incorporate the marine carbonate system project increasing seasonal pH variability and clear long-term (multi-decadal) reductions in mean, and minimum, pH. The results of these models are consistent with projected global trends (Fig. 6; Omstedt et al. 2009, 2012).
The responses of marine organisms in the Baltic-Skagerrak System to levels of ocean acidification (OA) consistent with these trends (pCO2 ≤ 1300 µatm; pH decline ≤ 0.4 units) vary widely from strongly positive, through neutral, to strongly negative. In general, however, we detected a pattern in which species at higher trophic levels tended respond more negatively to OA. Negative responses to OA were more common in macrobenthos and fish than in plankton. Responses to OA were strongly modified by several factors, most prevalent of which was nutritional status (nutrients for primary producers, food for consumers), such that more nutrients/food led to greater resilience to OA. Investigations of the combined effects of OA with warming, freshening, nutrients, or hypoxia frequently showed strong interactions that overwhelmed (warming), or materially changed (hypoxia), responses in comparison to those seen under OA only. In many instances interactive effects of OA + other drivers were small or not detected, and responses to other drivers (notably warming) were greater than those to OA.
Responses to OA also vary widely among taxonomic levels: species-level responses were generally stronger (both positive and negative) than responses of communities or ecosystems—indicating the importance of indirect interactions in the ecosystem, which can mediate OA effects on individual taxa. Overall ecosystem (mesocosm) level effects of OA were generally small, although there was evidence for substantial shifts in genetic composition of some plankton communities.
Outlook and research priorities
The findings summarised here represent substantial advances in OA research in the Baltic-Skagerrak System in the last 6 years (cf Havenhand 2012). Nonetheless, our understanding of the interactive and indirect effects of OA remains constrained by our limited knowledge of several key processes, and we know woefully little about how Baltic-Skagerrak species and ecosystems will respond to OA under diurnally, and seasonally, fluctuating cycles (Eriander et al. 2015; Boyd et al. 2016). We echo the sentiments of recent reviews that have emphasised the importance of moving from single drivers, single species, and short timescales, to multiple drivers, ecological communities, and evolutionary responses (e.g. Wernberg et al. 2012; Riebesell and Gattuso 2015; Boyd et al. 2018). In particular, we recommend that future research in the Baltic-Skagerrak System region focus on:
-
(i)
How biogeochemical processes combine to impact the development of OA in brackish coastal seas, and the role of nutrient enrichment and eutrophication; in particular, develop models to project future levels of important drivers under different forcing.
-
(ii)
Quantifying the additive, synergistic (reinforcing), and antagonistic (mediating) responses of keystone, and ecologically dominant, species to OA in combination with other important anthropogenic drivers (notably warming, eutrophication, hypoxia, biodiversity, and fishing).
-
(iii)
Determining the extent of phenotypic plasticity and adaptive capacity of key Baltic-Skagerrak species over multiple generations in response to OA, in combination with other important drivers.
-
(iv)
Quantifying the effects of intra- and interspecific biodiversity on ecosystem responses and resilience to OA in combination with other key drivers; in particular the capacity for OA responses to cascade through the food-web.
-
(v)
Determining the effects of diurnal and seasonal environmental fluctuations, superimposed on OA and other key drivers, on responses of species and ecosystems
-
(vi)
Developing holistic ecosystem modelling frameworks that incorporate the effects of OA with those of other drivers at large spatial scales
-
(vii)
Evaluating the impacts these processes will have on socially and economically important ecosystem services.
Acknowledgements
This work stemmed from a series of workshops on Ocean Acidification sponsored by the Royal Swedish Academy of Sciences. Subsequent workshops, and work by the lead author, was supported by a grant from the Hasselblad Foundation. Earlier versions of the manuscript were improved substantially by comments from Wendy Broadgate, Per Nilsson, and three anonymous referees. We wish to express our gratitude to all. JNH was also supported by a Linnaeus Grant from the Swedish Research Councils VR and FORMAS, to the Centre for Marine Evolutionary Biology (www.cemeb.gu.se). HLF acknowledges funding from the Swedish Research Council FORMAS (Grant No. 210-2012-2140). SN has been supported by the Nordforsk-funded project Green Growth Based on Marine Resources: Ecological and Socio-Economic Constraints (GreenMAR). ASC acknowledges funding from the Ebba and Sven Schwartz foundation.
Biographies
Jonathan N. Havenhand
is a senior researcher in the Department of Marine Sciences at the University of Gothenburg, Sweden. He is based at the Tjärnö Marine Laboratory, where he works on reproductive ecology and adaptations of marine organisms to rapid environmental change. In recent years his research has focussed on the impacts of ocean global change, especially the effects of ocean acidification on marine organisms and ecosystems.
Helena L. Filipsson
is Professor of Quaternary Geology, with a focus on marine geology, at Lund University, Sweden. Her research interests include past and present marine environments and their relation to climate and climate change foremost over the last 130 000 years. She is particularly interested in coastal environments subjected to low oxygen conditions and human impact, and proxy development, i.e. improving the tools used to reconstruct past marine environments.
Susa Niiranen
is a marine ecologist. Her research is focussed on marine food-web dynamics and she uses ecosystem modelling as her main research tool. She is also interested in social-ecological interactions in marine systems.
Max Troell
is an Associate Professor and senior researcher at the Beijer Institute of Ecological Economics where he leads the programme on sustainable seafood. He is also leading research on global food systems and multifunctional land and seascapes at Stockholm Resilience Centre.
Anne-Sophie Crépin
is an Associate Professor and the Deputy Director of the Beijer Institute of Ecological Economics at the Royal Swedish Academy of Sciences. She holds a PhD in economics from Stockholm University and is also a member of the strategic advisory board at the Stockholm Resilience Centre at Stockholm University. Her research interests include the complex links between biosphere and economic dynamics with focus on regime shifts, sustainability and resilience.
Sverker Jagers
is a Professor in Political Science and Director of the Centre for Collective Action Research (CeCAR) at the University of Gothenburg. His research interests include survey and experimental studies of environmental public opinion and drivers of pro-environmental behaviour and country-comparative time-series analyses of environmental performance.
David Langlet
holds the newly established chair in Ocean Governance Law at the School of Business, Economics and Law, University of Gothenburg. His research focuses primarily on the dynamics of multi-level regulation of ocean-related activities.
Simon Matti
is Associate Professor of Political Science at Luleå University of Technology, and a senior researcher at the Centre for Collective Action Research, University of Gothenburg. His main research interests concern the intersect between public policy and public opinion in the environmental and natural resource management field.
David Turner
is Professor Emeritus of Marine Chemistry at the University of Gothenburg. His research interests include the development and application of chemical models for seawater, the development of accurate measurement methods for pH in low-salinity seawater, and understanding the consequences of smokestack emissions from shipping for the marine environment.
Monika Winder
is a Professor in Marine Ecology at Stockhom University with particular interests in the causes and consequences of environmental change for food-web interactions and ecosystem functioning. Her research addresses questions in lakes, estuarine-coastal, and ocean ecosystems with a special emphasis on planktonic organisms.
P. de Wit
is a postdoctoral research fellow in the Department of Marine Sciences at the University of Gothenburg. He has a background in evolutionary biology and molecular ecology, and his current research focuses on the genomics of stress adaptation in marine invertebrates.
Leif G. Anderson
is Professor Emeritus in Marine Chemistry at the University of Gothenburg. His research interest centres on the ocean carbon cycle with a focus on its role in the climate system.
Footnotes
Since the onset of industrialisation, global atmospheric CO2 levels have increased by ~ 45% (from ~ 270 to today’s 400 µatm), causing seawater pH to decrease by about 0.1 units. pH is projected to decrease by another 0.3 units by 2100. Because of the logarithmic nature of the pH scale, these changes equate to increases of ~ 30% and ~ 100% (respectively) in the hydrogen ion content of seawater (Box 1).
The practical salinity scale is dimensionless and therefore we refer to salinity throughout this document simply as a number.
Contributor Information
Jonathan N. Havenhand, Email: jon.havenhand@marine.gu.se
Helena L. Filipsson, Email: helena.filipsson@geol.lu.se
Susa Niiranen, Email: susa.niiranen@su.se.
Max Troell, Email: max@beijer.kva.se.
Anne-Sophie Crépin, Email: annesophie.crepin@beijer.kva.se.
Sverker Jagers, Email: sverker.jagers@pol.gu.se.
David Langlet, Email: david.langlet@law.gu.se.
Simon Matti, Email: simon.matti@ltu.se.
David Turner, Email: david.turner@marine.gu.se.
Monika Winder, Email: monika.winder@su.se.
Pierre de Wit, Email: pierre.de_wit@marine.gu.se.
Leif G. Anderson, Email: leif.anderson@marine.gu.se
References
- Al-Janabi B, Kruse I, Graiff A, Karsten U, Wahl M. Genotypic variation influences tolerance to warming and acidification of early life-stage Fucus vesiculosus L. (Phaeophyceae) in a seasonally fluctuating environment. Marine Biology. 2016;163:15. [Google Scholar]
- Al-Janabi B, Kruse I, Graiff A, Winde V, Lenz M, Wahl M. Buffering and amplifying interactions among OAW (ocean acidification and warming) and nutrient enrichment on early life-stage Fucus vesiculosus L. (Phaeophyceae) and their carry over effects to hypoxia impact. PloS ONE. 2016;11:e0152948. doi: 10.1371/journal.pone.0152948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright R, Caldeira L, Hosfelt J, Kwiatkowski L, Maclaren JK, Mason BM, Nebuchina Y, Ninokawa A, et al. Reversal of ocean acidification enhances net coral reef calcification. Nature. 2016;531:362–365. doi: 10.1038/nature17155. [DOI] [PubMed] [Google Scholar]
- Albright R, Anthony KR, Baird M, Beeden R, Byrne M, Collier C, Dove S, Fabricius K, et al. Ocean acidification: Linking science to management solutions using the Great Barrier Reef as a case study. Journal of Environmental Management. 2016;182:641–650. doi: 10.1016/j.jenvman.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Algueró-Muñiz M, Meunier CL, Holst S, Alvarez-Fernandez S, Boersma M. Withstanding multiple stressors: Ephyrae of the moon jellyfish (Aurelia aurita, Scyphozoa) in a high-temperature, high-CO2 and low-oxygen environment. Marine Biology. 2016;163:185. [Google Scholar]
- Algueró-Muñiz M, Alvarez-Fernandez S, Thor P, Bach LT, Esposito M, Horn HG, Ecker U, Langer JAF, et al. Ocean acidification effects on mesozooplankton community development: Results from a long-term mesocosm experiment. PLoS ONE. 2017;12:21. doi: 10.1371/journal.pone.0175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almén A-K, Vehmaa A, Brutemark A, Bach L, Lischka S, Stuhr A, Furuhagen S, Paul A, et al. Negligible effects of ocean acidification on Eurytemora affinis (Copepoda) offspring production. Biogeosciences. 2016;13:1037–1048. [Google Scholar]
- Almén AK, Brutemark A, Jutfelt F, Riebesell U, Engstrom-Ost J. Ocean acidification causes no detectable effect on swimming activity and body size in a common copepod. Hydrobiologia. 2017;802:235–243. [Google Scholar]
- Alsterberg C, Eklof JS, Gamfeldt L, Havenhand JN, Sundback K. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proceedings of the National Academy of Sciences of the USA. 2013;110:8603–8608. doi: 10.1073/pnas.1303797110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans YS, Thomsen J, Opitz S, Pansch C, Melzner F, Wahl M. Juvenile sea stars exposed to acidification decrease feeding and growth with no acclimation potential. Marine Ecology Progress Series. 2014;509:227–239. [Google Scholar]
- Bach LT, Lohbeck KT, Reusch TB, Riebesell U. Rapid evolution of highly variable competitive abilities in a key phytoplankton species. Nature Ecology and Evolution. 2018;2:611. doi: 10.1038/s41559-018-0474-x. [DOI] [PubMed] [Google Scholar]
- Bach LT, Taucher J, Boxhammer T, Ludwig A, Achterberg EP, Alguero-Muniz M, Anderson LG, Bellworthy J, et al. Influence of ocean acidification on a natural winter-to-summer plankton succession: First insights from a long-term mesocosm study draw attention to periods of low nutrient concentrations. PLoS ONE. 2016;11:e0159068. doi: 10.1371/journal.pone.0159068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton A, Hales B, Waldbusser GG, Langdon C, Feely RA. The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: Implications for near-term ocean acidification effects. Limnology and Oceanography. 2012;57:698–710. [Google Scholar]
- Bates N, Best M, Neely K, Garley R, Dickson A, Johnson R. Detecting anthropogenic carbon dioxide uptake and ocean acidification in the North Atlantic Ocean. Biogeosciences. 2012;9:2509–2522. [Google Scholar]
- Bergen B, Endres S, Engel A, Zark M, Dittmar T, Sommer U, Jurgens K. Acidification and warming affect prominent bacteria in two seasonal phytoplankton bloom mesocosms. Environmental Microbiology. 2016;18:4579–4595. doi: 10.1111/1462-2920.13549. [DOI] [PubMed] [Google Scholar]
- Bergström U, Olsson J, Casini M, Eriksson BK, Fredriksson R, Wennhage H, Appelberg M. Stickleback increase in the Baltic Sea: A thorny issue for coastal predatory fish. Estuarine, Coastal and Shelf Science. 2015;163:134–142. [Google Scholar]
- Bermúdez JR, Riebesell U, Larsen A, Winder M. Ocean acidification reduces transfer of essential biomolecules in a natural plankton community. Scientific Reports. 2016;6:27749. doi: 10.1038/srep27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquet JM, Troedsson C, Novac A, Reeve M, Lechtenborger AK, Massart W, Skaar KS, Aasjord A, et al. Increased fitness of a key appendicularian zooplankton species under warmer, acidified seawater conditions. Plos One. 2018;13:19. doi: 10.1371/journal.pone.0190625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd PW, Collins S, Dupont S, Fabricius K, Gattuso JP, Havenhand J, Hutchins DA, Riebesell U, et al. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—A review. Global Change Biology. 2018;24:2239–2261. doi: 10.1111/gcb.14102. [DOI] [PubMed] [Google Scholar]
- Boyd PW, Cornwall CE, Davison A, Doney SC, Fourquez M, Hurd CL, Lima ID, McMinn A. Biological responses to environmental heterogeneity under future ocean conditions. Global Change Biology. 2016;22:2633–2650. doi: 10.1111/gcb.13287. [DOI] [PubMed] [Google Scholar]
- Boyd PW, Rynearson TA, Armstrong EA, Fu F, Hayashi K, Hu Z, Hutchins DA, Kudela RM, et al. Marine phytoplankton temperature versus growth responses from polar to tropical waters outcome of a scientific community-wide study. PLoS ONE. 2013;8:e63091. doi: 10.1371/journal.pone.0063091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell V, Murphy G, Romanuk TN. Experimental design and the outcome and interpretation of diversity-stability relations. Oikos. 2011;120:399–408. [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Carey N, Dupont S, Lundve B, Sigwart JD. One size fits all: Stability of metabolic scaling under warming and ocean acidification in echinoderms. Marine Biology. 2014;161:2131–2142. [Google Scholar]
- Carey N, Dupont S, Sigwart JD. Sea hare Aplysia punctata (Mollusca: Gastropoda) can maintain shell calcification under extreme ocean acidification. Biological Bulletin. 2016;231:142–151. doi: 10.1086/690094. [DOI] [PubMed] [Google Scholar]
- Carstensen J, Chierici M, Gustafsson BG, Gustafsson E. Long-term and seasonal trends in estuarine and coastal carbonate systems. Global Biogeochemical Cycles. 2018;32:497–513. [Google Scholar]
- Casini M, Hjelm J, Molinero J-C, Lövgren J, Cardinale M, Bartolino V, Belgrano A, Kornilovs G. Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proceedings of the National Academy of Sciences of the USA. 2009;106:197–202. doi: 10.1073/pnas.0806649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WW, Lam VW, Sarmiento JL, Kearney K, Watson R, Zeller D, Pauly D. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology. 2010;16:24–35. [Google Scholar]
- Cheung WW, Reygondeau G, Frölicher TL. Large benefits to marine fisheries of meeting the 1.5 °C global warming target. Science. 2016;354:1591–1594. doi: 10.1126/science.aag2331. [DOI] [PubMed] [Google Scholar]
- Christiansen, O.B., E. Kjellström, and E. Zorita. 2015. Projected Change: Atmosphere. In Second assessment of climate change for the Baltic Sea Basin, ed. BACC II Author Team, 245–261. Heidelberg: Springer.
- Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, et al. Hypoxia-related processes in the Baltic Sea. Environmental Science and Technology. 2009;43:3412–3420. doi: 10.1021/es802762a. [DOI] [PubMed] [Google Scholar]
- Conley DJ, Carstensen J, Aigars J, Axe P, Bonsdorff E, Eremina T, Haahti B-M, Humborg C, et al. Hypoxia is increasing in the coastal zone of the Baltic Sea. Environmental Science and Technology. 2011;45:6777–6783. doi: 10.1021/es201212r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell SD, Doubleday ZA, Foster NR, Hamlyn SB, Harley CD, Helmuth B, Kelaher BP, Nagelkerken I, et al. The duality of ocean acidification as a resource and a stressor. Ecology. 2018;99:1005–1010. doi: 10.1002/ecy.2209. [DOI] [PubMed] [Google Scholar]
- Crawfurd KJ, Alvarez-Fernandez S, Mojica KDA, Riebesell U, Brussaard CPD. Alterations in microbial community composition with increasing fCO2: A mesocosm study in the eastern Baltic Sea. Biogeosciences. 2017;14:3831–3849. [Google Scholar]
- Czerny J, Ramos JBE, Riebesell U. Influence of elevated CO2 concentrations on cell division and nitrogen fixation rates in the bloom-forming cyanobacterium Nodularia spumigena. Biogeosciences. 2009;6:1865–1875. [Google Scholar]
- Dahlke FT, Leo E, Mark FC, Portner HO, Bickmeyer U, Frickenhaus S, Storch D. Effects of ocean acidification increase embryonic sensitivity to thermal extremes in Atlantic cod, Gadus morhua. Global Change Biology. 2017;23:1499–1510. doi: 10.1111/gcb.13527. [DOI] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annual Review of Marine Science. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Doney SC, Ruckelshaus M, Duffy E, Barry J, Chan F, English C, Galindo H, Grebmeier J, et al. Climate change impacts on marine ecosystems. Annual Review of Marine Science. 2012;4:1–27. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- Duffy JE, Lefcheck JS, Stuart-Smith RD, Navarrete SA, Edgar GJ. Biodiversity enhances reef fish biomass and resistance to climate change. Proceedings of the National Academy of Sciences. 2016;113:6230–6235. doi: 10.1073/pnas.1524465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Havenhand J, Thorndyke W, Peck L, Thorndyke M. Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Marine Ecology-Progress Series. 2008;373:285–294. [Google Scholar]
- Dupont S, Lundve B, Thorndyke M. Near future ocean acidification increases growth rate of the lecithotrophic larvae and juveniles of the sea star Crossaster papposus. Journal of Experimental Zoology Part B-Molecular and Developmental Evolution. 2010;314B:382–389. doi: 10.1002/jez.b.21342. [DOI] [PubMed] [Google Scholar]
- Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Marine Biology. 2013;160:1835–1843. [Google Scholar]
- Eberlein T, Wohlrab S, Rost B, John U, Bach LT, Riebesell U, Van de Waal DB. Effects of ocean acidification on primary production in a coastal North Sea phytoplankton community. PLoS ONE. 2017;12:e0172594. doi: 10.1371/journal.pone.0172594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, Rost B, Kranz SA. Diversity of ocean acidification effects on marine N2 fixers. Journal of Experimental Marine Biology and Ecology. 2014;457:199–207. [Google Scholar]
- Eklöf J, Alsterberg C, Havenhand JN, Sundbäck K, Wood H, Gamfeldt L. Experimental climate change weakens the insurance effect of biodiversity. Ecology Letters. 2012;15:864–872. doi: 10.1111/j.1461-0248.2012.01810.x. [DOI] [PubMed] [Google Scholar]
- Eklöf JS, Havenhand JN, Alsterberg C, Gamfeldt L. Community-level effects of rapid experimental warming and consumer loss outweigh effects of rapid ocean acidification. Oikos. 2015;124:1040–1049. [Google Scholar]
- Endres S, Galgani L, Riebesell U, Schulz K-G, Engel A. Stimulated bacterial growth under elevated pCO2: Results from an off-shore mesocosm study. PLoS ONE. 2014;9:e99228. doi: 10.1371/journal.pone.0099228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Piontek J, Grossart HP, Riebesell U, Schulz KG, Sperling M. Impact of CO2 enrichment on organic matter dynamics during nutrient induced coastal phytoplankton blooms. Journal of Plankton Research. 2014;36:641–657. [Google Scholar]
- Eriander L, Wrange A-L, Havenhand JN. Simulated diurnal pH fluctuations radically increase variance in—but not the mean of—growth in the barnacle Balanus improvisus. ICES Journal of Marine Science. 2015;73:596–603. [Google Scholar]
- Falkenberg LJ, Tubb A. Economic effects of ocean acidification: Publication patterns and directions for future research. Ambio. 2017;46:543–553. doi: 10.1007/s13280-017-0895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Clemmesen C. Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.) Biogeosciences. 2011;8:3697–3707. [Google Scholar]
- Fehsenfeld S, Kiko R, Appelhans Y, Towle DW, Zimmer M, Melzner F. Effects of elevated seawater pCO2 on gene expression patterns in the gills of the green crab, Carcinus maenas. BMC Genomics. 2011;12:488. doi: 10.1186/1471-2164-12-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommel AY, Schubert A, Piatkowski U, Clemmesen C. Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Marine Biology. 2013;160:1825–1834. [Google Scholar]
- Frommel AY, Stiebens V, Clemmesen C, Havenhand JN. Effect of ocean acidification on marine fish sperm (Baltic cod: Gadus morhua) Biogeosciences. 2010;7:3915–3919. [Google Scholar]
- Gamfeldt L, Lefcheck JS, Byrnes JE, Cardinale BJ, Duffy JE, Griffin JN. Marine biodiversity and ecosystem functioning: What’s known and what’s next? Oikos. 2015;124:252–265. [Google Scholar]
- Gao K, Zhang Y, Häder D-P. Individual and interactive effects of ocean acidification, global warming, and UV radiation on phytoplankton. Journal of Applied Phycology. 2018;30:743–759. [Google Scholar]
- Gattuso J-P, Hansson L. Ocean acidification: background and history. In: Gattuso J-P, Hansson L, editors. Ocean acidification. Oxford: Oxford University Press; 2011. pp. 1–20. [Google Scholar]
- Gattuso J-P, Magnan A, Billé R, Cheung WW, Howes EL, Joos F, Allemand D, Bopp L, et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science. 2015;349:aac4722. doi: 10.1126/science.aac4722. [DOI] [PubMed] [Google Scholar]
- Glippa O, Engstrom-Ost J, Kanerva M, Rein A, Vuori K. Oxidative stress and antioxidant defense responses in Acartia copepods in relation to environmental factors. PLoS ONE. 2018;13:e195981. doi: 10.1371/journal.pone.0195981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graiff A, Dankworth M, Wahl M, Karsten U, Bartsch I. Seasonal variations of Fucus vesiculosus fertility under ocean acidification and warming in the western Baltic Sea. Botanica Marina. 2017;60:239–255. [Google Scholar]
- Gräns A, Jutfelt F, Sandblom E, Jönsson E, Wiklander K, Seth H, Olsson C, Dupont S, et al. Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. Journal of Experimental Biology. 2014;217:711–717. doi: 10.1242/jeb.096743. [DOI] [PubMed] [Google Scholar]
- Gray JS, Wu RS-S, Or YY. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series. 2002;238:249–279. [Google Scholar]
- Griffith GP, Fulton EA, Richardson AJ. Effects of fishing and acidification-related benthic mortality on the southeast Australian marine ecosystem. Global Change Biology. 2011;17:3058–3074. [Google Scholar]
- Gunderson AR, Armstrong EJ, Stillman JH. Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Annual Review of Marine Science. 2016;8:357–378. doi: 10.1146/annurev-marine-122414-033953. [DOI] [PubMed] [Google Scholar]
- Gustafsson BG, Schenk F, Blenckner T, Eilola K, Meier HM, Müller-Karulis B, Neumann T, Ruoho-Airola T, et al. Reconstructing the development of Baltic Sea eutrophication 1850–2006. Ambio. 2012;41:534–548. doi: 10.1007/s13280-012-0318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, et al. The impacts of climate change in coastal marine systems. Ecology Letters. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Harnik PG, Lotze HK, Anderson SC, Finkel ZV, Finnegan S, Lindberg DR, Liow LH, Lockwood R, et al. Extinctions in ancient and modern seas. Trends in Ecology and Evolution. 2012;27:608–617. doi: 10.1016/j.tree.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Harvey BP, Gwynn-Jones D, Moore PJ. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecology and Evolution. 2013;3:1016–1030. doi: 10.1002/ece3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattam C, Atkins JP, Beaumont N, Börger T, Böhnke-Henrichs A, Burdon D, de Groot R, Hoefnagel E, et al. Marine ecosystem services: linking indicators to their classification. Ecological Indicators. 2015;49:61–75. [Google Scholar]
- HAV. 2014. The balance between the fishing fleet and fishing opportunities. Swedish Agency for Marine and Water Management Report Dnr 1-14 (in Swedish).
- Havenhand JN. How will ocean acidification affect Baltic Sea ecosystems? An assessment of plausible impacts on key functional groups. Ambio. 2012;41:637–644. doi: 10.1007/s13280-012-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenhand JN, Schlegel P. Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences. 2009;6:3009–3015. [Google Scholar]
- Haynert K, Schonfeld J, Riebesell U, Polovodova I. Biometry and dissolution features of the benthic foraminifer Ammonia aomoriensis at high pCO2. Marine Ecology Progress Series. 2011;432:53–67. [Google Scholar]
- Hernroth B, Baden S, Thorndyke M, Dupont S. Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquatic Toxicology. 2011;103:222–224. doi: 10.1016/j.aquatox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Hernroth B, Krång AS, Baden S. Bacteriostatic suppression in Norway lobster (Nephrops norvegicus) exposed to manganese or hypoxia under pressure of ocean acidification. Aquatic Toxicology. 2015;159:217–224. doi: 10.1016/j.aquatox.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Hernroth B, Sköld HN, Wiklander K, Jutfelt F, Baden S. Simulated climate change causes immune suppression and protein damage in the crustacean Nephrops norvegicus. Fish and Shellfish Immunology. 2012;33:1095–1101. doi: 10.1016/j.fsi.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Hiddink JG, Burrows MT, Molinos JG. Temperature tracking by North Sea benthic invertebrates in response to climate change. Global Change Biology. 2015;21:117–129. doi: 10.1111/gcb.12726. [DOI] [PubMed] [Google Scholar]
- Hiddink JG, Coleby C. What is the effect of climate change on marine fish biodiversity in an area of low connectivity, the Baltic Sea? Global Ecology and Biogeography. 2012;21:637–646. [Google Scholar]
- Hiebenthal C, Philipp EER, Eisenhauer A, Wahl M. Effects of seawater pCO2 and temperature on shell growth, shell stability, condition and cellular stress of Western Baltic Sea Mytilus edulis (L.) and Arctica islandica (L.) Marine Biology. 2013;160:2073–2087. [Google Scholar]
- Hilmi N, Allemand D, Dupont S, Safa A, Haraldsson G, Nunes PA, Moore C, Hattam C, et al. Towards improved socio-economic assessments of ocean acidification’s impacts. Marine Biology. 2013;160:1773–1787. doi: 10.1007/s00227-012-2031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson S, Wesslander K, Anderson LG, Omstedt A, Perttilä M, Mintrop L. Distribution, long-term development and mass balance calculation of total alkalinity in the Baltic Sea. Continental Shelf Research. 2008;28:593–601. [Google Scholar]
- Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, Micheli F, Paytan A, Price NN, et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE. 2011;6:e28983. doi: 10.1371/journal.pone.0028983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann WC, Stumpp M, Gutowska MA, Syre S, Himmerkus N, Melzner F, Bleich M. Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: The role of body cavity epithelia and stereom dissolution. Marine Biology. 2013;160:2631–2645. [Google Scholar]
- Horn HG, Sander N, Stuhr A, Alguero-Muniz M, Bach LT, Loder MGJ, Boersma M, Riebesell U, et al. Low CO2 sensitivity of microzooplankton communities in the Gullmar Fjord, Skagerrak: Evidence from a long-term mesocosm study. PLoS ONE. 2016;11:24. doi: 10.1371/journal.pone.0165800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick T, Bach LT, Crawfurd KJ, Spilling K, Achterberg EP, Woodhouse JN, Schulz KG, Brussaard CPD, et al. Ocean acidification impacts bacteria-phytoplankton coupling at low-nutrient conditions. Biogeosciences. 2017;14:1–15. [Google Scholar]
- Hönisch B, Hemming NG. Surface ocean pH response to variations in pCO2 through two full glacial cycles. Earth and Planetary Science Letters. 2005;236:305–314. [Google Scholar]
- Hönisch B, Ridgwell A, Schmidt DN, Thomas E, Gibbs SJ, Sluijs A, Zeebe R, Kump L, et al. The geological record of ocean acidification. Science. 2012;335:1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- Hu MY, Casties I, Stumpp M, Ortega-Martinez O, Dupont S. Energy metabolism and regeneration are impaired by seawater acidification in the infaunal brittlestar Amphiura filiformis. Journal of Experimental Biology. 2014;217:2411–2421. doi: 10.1242/jeb.100024. [DOI] [PubMed] [Google Scholar]
- Hu MY, Michael K, Kreiss CM, Stumpp M, Dupont S, Tseng YC, Lucassen M. Temperature modulates the effects of ocean acidification on intestinal ion transport in Atlantic cod, Gadus morhua. Frontiers in Physiology. 2016;7:18. doi: 10.3389/fphys.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MY, Lein E, Bleich M, Melzner F, Stumpp M. Trans-life cycle acclimation to experimental ocean acidification affects gastric pH homeostasis and larval recruitment in the sea star Asterias rubens. Acta Physiologica. 2018;224:e13075. doi: 10.1111/apha.13075. [DOI] [PubMed] [Google Scholar]
- Hüning A, Melzner F, Thomsen J, Gutowska MA, Kramer L, Frickenhaus S, Rosenstiel P, Portner HO, et al. Impacts of seawater acidification on mantle gene expression patterns of the Baltic Sea blue mussel: implications for shell formation and energy metabolism. Marine Biology. 2013;160:1845–1861. [Google Scholar]
- Jagers SC, Matti S, Crépin A-S, Langlet D, Havenhand JN, Troell M, Filipsson HL, Galaz VR, et al. Societal causes of, and responses to, ocean acidification. Ambio. 2018 doi: 10.1007/s13280-018-1103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowska M, Jerzak M, Normant M, Burska D, Drzazgowski J. Effect of carbon dioxide-induced water acidification on the physiological processes of the baltic isopod Saduria entomon. Journal of Shellfish Research. 2013;32:825–834. [Google Scholar]
- Jakubowska M, Normant M. Metabolic rate and activity of blue mussel Mytilus edulis trossulus under short-term exposure to carbon dioxide-induced water acidification and oxygen deficiency. Marine and Freshwater Behaviour and Physiology. 2015;48:25–39. [Google Scholar]
- Jakubowska M, Normant-Saremba M. The effect of CO2-induced seawater acidification on the behaviour and metabolic rate of the Baltic clam Macoma balthica. Annales Zoologici Fennici. 2015;52:353–367. [Google Scholar]
- Jansson A, Lischka S, Boxhammer T, Schulz KG, Norkko J. Survival and settling of larval Macoma balthica in a large-scale mesocosm experiment at different fCO2 levels. Biogeosciences. 2016;13:3377–3385. [Google Scholar]
- Jansson A, Norkko J, Dupont S, Norkko A. Growth and survival in a changing environment: combined effects of moderate hypoxia and low pH on juvenile bivalve Macoma balthica. Journal of Sea Research. 2015;102:41–47. [Google Scholar]
- Jansson A, Norkko J, Norkko A. Effects of reduced pH on Macoma balthica larvae from a system with naturally fluctuating pH-dynamics. PLoS ONE. 2013;8:e68198. doi: 10.1371/journal.pone.0068198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson K, Andre C. Life on the margin: Genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Molecular Ecology. 2006;15:2013–2029. doi: 10.1111/j.1365-294X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- Johannesson K, Smolarz K, Grahn M, Andre C. The future of Baltic Sea Populations: Local extinction or evolutionary rescue? Ambio. 2011;40:179–190. doi: 10.1007/s13280-010-0129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutfelt F, Hedgarde M. Atlantic cod actively avoid CO2 and predator odour, even after long-term CO2 exposure. Frontiers in Zoology. 2013;10:7. doi: 10.1186/1742-9994-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan IC, Levin PS, Burden M, Fulton EA. Fishing catch shares in the face of global change: A framework for integrating cumulative impacts and single species management. Canadian Journal of Fisheries and Aquatic Sciences. 2010;67:1968–1982. [Google Scholar]
- Katsanevakis S, Wallentinus I, Zenetos A, Leppäkowski E, Oztürk B, Grabowski M, Golani D, Cardoso A. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquatic Invasions. 2014;9:391–423. [Google Scholar]
- Koenigstein S, Mark FC, Gößling-Reisemann S, Reuter H, Poertner HO. Modelling climate change impacts on marine fish populations: Process-based integration of ocean warming, acidification and other environmental drivers. Fish and Fisheries. 2016;17:972–1004. [Google Scholar]
- Kremp A, Godhe A, Egardt J, Dupont S, Suikkanen S, Casabianca S, Penna A. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecology and Evolution. 2012;2:1195–1207. doi: 10.1002/ece3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Global Change Biology. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Harley CD. Embracing interactions in ocean acidification research: Confronting multiple stressor scenarios and context dependence. Biology Letters. 2017;13:20160802. doi: 10.1098/rsbl.2016.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Nehrke G, Probert I, Ly J, Ziveri P. Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences. 2009;6:2637–2646. [Google Scholar]
- Langer JAF, Sharma R, Schmidt SI, Bahrdt S, Horn HG, Alguero-Muniz M, Nam B, Achterberg EP, et al. Community barcoding reveals little effect of ocean acidification on the composition of coastal plankton communities: Evidence from a long-term mesocosm study in the Gullmar Fjord, Skagerrak. Plos One. 2017;12:20. doi: 10.1371/journal.pone.0175808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcheck JS, Byrnes JE, Isbell F, Gamfeldt L, Griffin JN, Eisenhauer N, Hensel MJ, Hector A, et al. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nature Communications. 2015;6:6936. doi: 10.1038/ncomms7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson AJ, Fletcher S, Hall-Spencer JM, Knights AM. Linking the biological impacts of ocean acidification on oysters to changes in ecosystem services: A review. Journal of Experimental Marine Biology and Ecology. 2017;492:49–62. [Google Scholar]
- Lindegren M, Checkley DM, Ohman MD, Koslow JA, Goericke R. Resilience and stability of a pelagic marine ecosystem. Proceedings of the Royal Society B-Biological Sciences. 2016;283:9. doi: 10.1098/rspb.2015.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh MV, Riemann L, Baltar F, Romero-Oliva C, Salomon PS, Graneli E, Pinhassi J. Consequences of increased temperature and acidification on bacterioplankton community composition during a mesocosm spring bloom in the Baltic Sea. Environmental Microbiology Reports. 2013;5:252–262. doi: 10.1111/1758-2229.12009. [DOI] [PubMed] [Google Scholar]
- Lischka S, Bach LT, Schulz K-G, Riebesell U. Ciliate and mesozooplankton community response to increasing CO2 levels in the Baltic Sea: Insights from a large-scale mesocosm experiment. Biogeosciences. 2017;14:447–466. [Google Scholar]
- Lohbeck KT, Riebesell U, Reusch TBH. Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geosciences. 2012;5:346–351. [Google Scholar]
- Loreau M, Mazancourt C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecology Letters. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Mammitzsch K, Jost G, Jurgens K. Impact of dissolved inorganic carbon concentrations and pH on growth of the chemolithoautotrophic epsilonproteobacterium Sulfurimonas gotlandica GD1(T) Microbiology Open. 2014;3:80–88. doi: 10.1002/mbo3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier HEM, Andersson HC, Eilola K, Gustafsson BG, Kuznetsov I, Müller-Karulis B, Neumann T, Savchuk OP. Hypoxia in future climates: A model ensemble study for the Baltic Sea. Geophysical Research Letters. 2011;38:24. [Google Scholar]
- Meier, M. 2015. Projected change: Marine physics. In Second assessment of climate change for the Baltic Sea basin, ed. BACC II Author Team, 243–252. Heidelberg: Springer.
- Melzner F, Gobel S, Langenbuch M, Gutowska MA, Portner HO, Lucassen M. Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater P(CO2) Aquatic Toxicology. 2009;92:30–37. doi: 10.1016/j.aquatox.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Melzner F, Stange P, Trubenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA. Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS ONE. 2011;6:e24223. doi: 10.1371/journal.pone.0024223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Riebesell U. Responses of coccolithophores to ocean acidification: A meta-analysis. Biogeosciences. 2015;12:1671–1682. [Google Scholar]
- Michael K, Kreiss CM, Hu MY, Koschnick N, Bickmeyer U, Dupont S, Portner HO, Lucassen M. Adjustments of molecular key components of branchial ion and pH regulation in Atlantic cod (Gadus morhua) in response to ocean acidification and warming. Comparative Biochemistry and Physiology B-Biochemistry and Molecular Biology. 2016;193:33–46. doi: 10.1016/j.cbpb.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment . Ecosystems and human well-being: Biodiversity synthesis. Washington, DC: Island Press; 2005. [Google Scholar]
- Molinos JG, Halpern BS, Schoeman DS, Brown CJ, Kiessling W, Moore PJ, Pandolfi JM, Poloczanska ES, et al. Climate velocity and the future global redistribution of marine biodiversity. Nature Climate Change. 2016;6:83. [Google Scholar]
- Mostofa KM, Liu C-Q, Zhai W, Minella M, Vione D, Gao K, Minakata D, Arakaki T, et al. Reviews and Syntheses: Ocean acidification and its potential impacts on marine ecosystems. Biogeosciences. 2016;13:1767–1786. [Google Scholar]
- Müller JD, Schneider B, Rehder G. Long-term alkalinity trends in the Baltic Sea and their implications for CO2-induced acidification. Limnology and Oceanography. 2016;61:1984–2002. [Google Scholar]
- Ni S, Taubner I, Bohm F, Winde V, Bottcher ME. Effect of temperature rise and ocean acidification on growth of calcifying tubeworm shells (Spirorbis spirorbis): An in situ benthocosm approach. Biogeosciences. 2018;15:1425–1445. [Google Scholar]
- Niiranen S, Yletyinen J, Tomczak MT, Blenckner T, Hjerne O, MacKenzie BR, Müller-Karulis B, Neumann T, et al. Combined effects of global climate change and regional ecosystem drivers on an exploited marine food web. Global Change Biology. 2013;19:3327–3342. doi: 10.1111/gcb.12309. [DOI] [PubMed] [Google Scholar]
- Näslund J, Lindström E, Lai F, Jutfelt F. Behavioural responses to simulated bird attacks in marine three-spined sticklebacks after exposure to high CO2 levels. Marine and Freshwater Research. 2015;66:877–885. [Google Scholar]
- Norström AV, Balvanera P, Spierenburg MJ, Bouamrane M. Programme on ecosystem change and society: Knowledge for sustainable stewardship of social-ecological systems. Ecology and Society. 2017;22:47. [Google Scholar]
- Oesterwind D, Rau A, Zaiko A. Drivers and pressures—untangling the terms commonly used in marine science and policy. Journal of Environmental Management. 2016;181:8–15. doi: 10.1016/j.jenvman.2016.05.058. [DOI] [PubMed] [Google Scholar]
- Oliver TH, Heard MS, Isaac NJ, Roy DB, Procter D, Eigenbrod F, Freckleton R, Hector A, et al. Biodiversity and resilience of ecosystem functions. Trends in Ecology & Evolution. 2015;30:673–684. doi: 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Olsson J, Bergström L, Gårdmark A. Abiotic drivers of coastal fish community change during four decades in the Baltic Sea. ICES Journal of Marine Science. 2012;69:961–970. [Google Scholar]
- Olsson J, Tomczak MT, Ojaveer H, Gårdmark A, Põllumäe A, Müller-Karulis B, Ustups D, Dinesen GE, et al. Temporal development of coastal ecosystems in the Baltic Sea over the past two decades. ICES Journal of Marine Science. 2015;72:2539–2548. [Google Scholar]
- Omstedt A, Edman M, Claremar B, Frodin P, Gustafsson E, Humborg C, Hägg H, Mörth M, et al. Future changes in the Baltic Sea acid-base (pH) and oxygen balances. Tellus B. 2012;64:19586. [Google Scholar]
- Omstedt A, Edman M, Claremar B, Rutgersson A. Modelling the contributions to marine acidification from deposited SOx, NOx, and NHx in the Baltic Sea: Past and present situations. Continental Shelf Research. 2015;111:234–249. [Google Scholar]
- Omstedt A, Gustafsson E, Wesslander K. Modelling the uptake and release of carbon dioxide in the Baltic Sea surface water. Continental Shelf Research. 2009;29:870–885. [Google Scholar]
- Österblom H, Crona BI, Folke C, Nyström M, Troell M. Marine ecosystem science on an intertwined planet. Ecosystems. 2017;20:54–61. [Google Scholar]
- Österblom H, Hansson S, Larsson U, Hjerne O, Wulff F, Elmgren R, Folke C. Human-induced trophic cascades and ecological regime shifts in the Baltic sea. Ecosystems. 2007;10:877–889. [Google Scholar]
- Pajusalu L, Martin G, Pollumae A. Results of laboratory and field experiments of the direct effect of increasing CO2 on net primary production of macroalgal species in brackish-water ecosystems. Proceedings of the Estonian Academy of Sciences. 2013;62:148–154. [Google Scholar]
- Pansch C, Hattich GSI, Heinrichs ME, Pansch A, Zagrodzka Z, Havenhand JN. Long-term exposure to acidification disrupts reproduction in a marine invertebrate. PLoS ONE. 2018;13:17. doi: 10.1371/journal.pone.0192036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansch C, Nasrolahi A, Appelhans YS, Wahl M. Impacts of ocean warming and acidification on the larval development of the barnacle Amphibalanus improvisus. Journal of Experimental Marine Biology and Ecology. 2012;420:48–55. [Google Scholar]
- Pansch C, Schlegel P, Havenhand J. Larval development of the barnacle Amphibalanus improvisus responds variably but robustly to near-future ocean acidification. ICES Journal of Marine Science. 2013;70:805–811. [Google Scholar]
- Pansch C, Schaub I, Havenhand J, Wahl M. Habitat traits and food availability determine the response of marine invertebrates to ocean acidification. Global Change Biology. 2014;20:765–777. doi: 10.1111/gcb.12478. [DOI] [PubMed] [Google Scholar]
- Paul AJ, Achterberg EP, Bach LT, Boxhammer T, Czerny J, Haunost M, Schulz KG, Stuhr A, et al. No observed effect of ocean acidification on nitrogen biogeochemistry in a summer Baltic Sea plankton community. Biogeosciences. 2016;13:3901–3913. [Google Scholar]
- Paul C, Sommer U, Garzke J, Moustaka-Gouni M, Paul A, Matthiessen B. Effects of increased CO2 concentration on nutrient limited coastal summer plankton depend on temperature. Limnology and Oceanography. 2016;61:853–868. [Google Scholar]
- Pereyra RT, Bergström L, Kautsky L, Johannesson K. Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evolutionary Biology. 2009;9:70. doi: 10.1186/1471-2148-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. Marine taxa track local climate velocities. Science. 2013;341:1239–1242. doi: 10.1126/science.1239352. [DOI] [PubMed] [Google Scholar]
- Polechová J, Barton NH. Limits to adaptation along environmental gradients. Proceedings of the National Academy of Sciences of the USA. 2015;112:6401–6406. doi: 10.1073/pnas.1421515112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP. Physiology and climate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Raddatz S, Guy-Haim T, Rilov G, Wahl M. Future warming and acidification effects on anti-fouling and anti-herbivory traits of the brown alga Fucus vesiculosus (Phaeophyceae) Journal of Phycology. 2017;53:44–58. doi: 10.1111/jpy.12473. [DOI] [PubMed] [Google Scholar]
- Repolho T, Duarte B, Dionisio G, Paula JR, Lopes AR, Rosa IC, Grilo TF, Cacador I, et al. Seagrass ecophysiological performance under ocean warming and acidification. Scientific Reports. 2017;7:41443. doi: 10.1038/srep41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch TBH, Ehlers A, Hammerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences of the USA. 2005;102:2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebesell U, Gattuso J-P. Lessons learned from ocean acidification research. Nature Climate Change. 2015;5:12–14. [Google Scholar]
- Rönnbäck P, Kautsky N, Pihl L, Troell M, Söderqvist T, Wennhage H. Ecosystem goods and services from Swedish coastal habitats: Identification, valuation, and implications of ecosystem shifts. Ambio. 2007;36:534–544. doi: 10.1579/0044-7447(2007)36[534:egasfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rossoll D, Bermúdez R, Hauss H, Schulz KG, Riebesell U, Sommer U, Winder M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE. 2012;7:e34737. doi: 10.1371/journal.pone.0034737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saderne V, Fietzek P, Herman PMJ. Extreme variations of pCO2 and pH in a macrophyte meadow of the Baltic Sea in summer: Evidence of the effect of photosynthesis and local upwelling. PLoS ONE. 2013;8:e62689. doi: 10.1371/journal.pone.0062689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saderne V, Wahl M. Differential responses of calcifying and non-calcifying epibionts of a brown macroalga to present-day and future upwelling pCO2. PLoS ONE. 2013;8:e70455. doi: 10.1371/journal.pone.0070455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCB. 2017. Aquaculture in Sweden in 2016. Swedish Central Bureau of Statistics, Report JO 60 SM 1701, Stockholm, Sweden (in Swedish, English summary).
- SCB. 2018. Recreational fishing in Sweden 2016. Swedish Central Bureau of Statistics, Report JO 57 SM 1801, Stockholm, Sweden (in Swedish, English summary).
- Schade FM, Clemmesen C, Wegner KM. Within-and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus) Marine Biology. 2014;161:1667–1676. [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- Schneider, B., K. Eilola, K. Lukkari, B. Müller-Karulis. and T. Neumann. 2015. Environmental impacts— marine biogeochemistry. In Second assessment of climate change for the Baltic Sea basin, ed. BACC II Author Team, 337–362. Heidelberg: Springer.
- Schulz KG, Bellerby R, Brussaard CP, Büdenbender J, Czerny J, Engel A, Fischer M, Koch-Klavsen S, et al. Temporal biomass dynamics of an Arctic plankton bloom in response to increasing levels of atmospheric carbon dioxide. Biogeosciences. 2013;10:161–180. [Google Scholar]
- Serrao EA, Brawley SH, Hedman J, Kautsky L, Samuelson G. Reproductive success of Fucus vesiculosus (Phaeophyceae) in the Baltic Sea. Journal of Phycology. 1999;35:254–269. [Google Scholar]
- Sokolowski A, Brulinska D, Mirny Z, Burska D, Pryputniewicz-Flis D. Differing responses of the estuarine bivalve Limecola balthica to lowered water pH caused by potential CO2 leaks from a sub-seabed storage site in the Baltic Sea: An experimental study. Marine Pollution Bulletin. 2018;127:761–773. doi: 10.1016/j.marpolbul.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Sommer U, Paul C, Moustaka-Gouni M. Warming and ocean acidification effects on phytoplankton -from species shifts to size shifts within species in a mesocosm experiment. PLoS ONE. 2015;10:e125239. doi: 10.1371/journal.pone.0125239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilling K, Paul AJ, Virkkala N, Hastings T, Lischka S, Stuhr A, Bermúdez R, Czerny J, et al. Ocean acidification decreases plankton respiration: Evidence from a mesocosm experiment. Biogeosciences. 2016;13:4707–4719. [Google Scholar]
- Stapp LS, Thomsen J, Schade H, Bock C, Melzner F, Portner HO, Lannig G. Intra-population variability of ocean acidification impacts on the physiology of Baltic blue mussels (Mytilus edulis): Integrating tissue and organism response. Journal of Comparative Physiology B. 2017;187:529–543. doi: 10.1007/s00360-016-1053-6. [DOI] [PubMed] [Google Scholar]
- Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, et al. Planetary boundaries: Guiding human development on a changing planet. Science. 2015;347:1259855. doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- Stemmer K, Nehrke G, Brey T. Elevated CO2 levels do not affect the shell structure of the bivalve Arctica islandica from the western Baltic. PLoS ONE. 2013;8:e70106. doi: 10.1371/journal.pone.0070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny MH, Mittermayer FH, Sswat M, Voss R, Jutfelt F, Chierici M, Puvanendran V, Mortensen A, et al. Ocean acidification effects on Atlantic cod larval survival and recruitment to the fished population. PLoS ONE. 2016;11:e0155448. doi: 10.1371/journal.pone.0155448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stips, A., K. Bolding, D. Macias, J. Bruggeman, and C. Coughlan. 2016. Scoping report on the potential impact of on-board desulphurisation on water quality in SOx emission control areas. EUR 27886. 10.2788/336630.
- Stumpp M, Dupont S, Thorndyke MC, Melzner F. CO2 induced seawater acidification impacts sea urchin larval development II: Gene expression patterns in pluteus larvae. Comparative Biochemistry and Physiology A-Molecular and Integrative Physiology. 2011;160:320–330. doi: 10.1016/j.cbpa.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont ST. CO2 induced seawater acidification impacts sea urchin larval development I: Elevated metabolic rates decrease scope for growth and induce developmental delay. Comparative Biochemistry and Physiology A-Molecular and Integrative Physiology. 2011;160:331–340. doi: 10.1016/j.cbpa.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Stumpp M, Hu M, Casties I, Saborowski R, Bleich M, Melzner F, Dupont S. Digestion in sea urchin larvae impaired under ocean acidification. Nature Climate Change. 2013;3:1044–1049. [Google Scholar]
- Stumpp M, Trubenbach K, Brennecke D, Hu MY, Melzner F. Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquatic Toxicology. 2012;110:194–207. doi: 10.1016/j.aquatox.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Suikkanen S, Pulina S, Engström-Öst J, Lehtiniemi M, Lehtinen S, Brutemark A. Climate change and eutrophication induced shifts in northern summer plankton communities. PLoS ONE. 2013;8:e66475. doi: 10.1371/journal.pone.0066475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin J, Jutfelt F. 9-28 d of exposure to elevated pCO2 reduces avoidance of predator odour but had no effect on behavioural lateralization or swimming activity in a temperate wrasse (Ctenolabrus rupestris) ICES Journal of Marine Science. 2016;73:620–632. [Google Scholar]
- Takolander A, Cabeza M, Leskinen E. Climate change can cause complex responses in Baltic Sea macroalgae: A systematic review. Journal of Sea Research. 2017;123:16–29. [Google Scholar]
- Taucher J, Haunost M, Boxhammer T, Bach LT, Alguero-Muniz M, Riebesell U. Influence of ocean acidification on plankton community structure during a winter-to-summer succession: An imaging approach indicates that copepods can benefit from elevated CO2 via indirect food web effects. PLoS ONE. 2017;12:23. doi: 10.1371/journal.pone.0169737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEEB . The economics of ecosystems and biodiversity: Mainstreaming the economics of nature: A synthesis of the approach, conclusions and recommendations of TEEB. Brussels: UNEP-EU; 2010. [Google Scholar]
- Thomsen J, Casties I, Pansch C, Kortzinger A, Melzner F. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Global Change Biology. 2013;19:1017–1027. doi: 10.1111/gcb.12109. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Melzner F. Moderate seawater acidification does not elicit long-term metabolic depression in the blue mussel Mytilus edulis. Marine Biology. 2010;157:2667–2676. [Google Scholar]
- Thomsen J, Stapp LS, Haynert K, Schade H, Danelli M, Lannig G, Wegner KM, Melzner F. Naturally acidified habitat selects for ocean acidification-tolerant mussels. Science Advances. 2017;3:e1602411. doi: 10.1126/sciadv.1602411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor P, Dupont S. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Global Change Biology. 2015;21:2261–2271. doi: 10.1111/gcb.12815. [DOI] [PubMed] [Google Scholar]
- Thor P, Oliva EO. Ocean acidification elicits different energetic responses in an Arctic and a boreal population of the copepod Pseudocalanus acuspes. Marine Biology. 2015;162:799–807. [Google Scholar]
- Todgham AE, Stillman JH. Physiological responses to shifts in multiple environmental stressors: Relevance in a changing world. Integrative and Comparative Biology. 2013;53:539–544. doi: 10.1093/icb/ict086. [DOI] [PubMed] [Google Scholar]
- Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, et al. Rapid adaptive responses to climate change in corals. Nature Climate Change. 2017;7:627. [Google Scholar]
- Turner DR, Edman M, Gallego-Urrea JA, Claremar B, Hassellov IM, Omstedt A, Rutgersson A. The potential future contribution of shipping to acidification of the Baltic Sea. Ambio. 2018;47:368–378. doi: 10.1007/s13280-017-0950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfsbo A, Hulth S, Anderson LG. pH and biogeochemical processes in the Gotland Basin of the Baltic Sea. Marine Chemistry. 2011;127:20–30. [Google Scholar]
- van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, et al. The representative concentration pathways: An overview. Climatic Change. 2011;109:5. [Google Scholar]
- Van Colen C, Jansson A, Saunier A, Lacoue-Labathe T, Vincx M. Biogeographic vulnerability to ocean acidification and warming in a marine bivalve. Marine Pollution Bulletin. 2018;126:308–311. doi: 10.1016/j.marpolbul.2017.10.092. [DOI] [PubMed] [Google Scholar]
- Vehmaa A, Almén A-K, Brutemark A, Paul AJ, Riebesell U, Furuhagen S, Engström-Öst J. Ocean acidification challenges copepod reproductive plasticity. Biogeosciences. 2016;13:6171–6182. [Google Scholar]
- Vehmaa A, Brutemark A, Engstrom-Ost J. Maternal effects may act as an adaptation mechanism for copepods facing pH and temperature changes. PLoS ONE. 2012;7:8. doi: 10.1371/journal.pone.0048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Schulz S, Dupont S. Maintained larval growth in mussel larvae exposed to acidified under-saturated seawater. Scientific Reports. 2016;6:23728. doi: 10.1038/srep23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitasalo, M., T. Blenckner, A. Gårdmark, H. Kaartokallio, L. Kautsky, H. Kuosa, M. Lindegren, A. Norkko, et al. 2015. Environmental impacts—marine ecosystems. In Second assessment of climate change for the Baltic Sea basin, ed. BACC II Author Team, 363–380. Heidelberg: Springer.
- Vuorinen I, Hänninen J, Rajasilta M, Laine P, Eklund J, Montesino-Pouzols F, Corona F, Junker K, et al. Scenario simulations of future salinity and ecological consequences in the Baltic Sea and adjacent North Sea areas–implications for environmental monitoring. Ecological Indicators. 2015;50:196–205. doi: 10.1016/j.ecolind.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller J, Pansch C, Sawall Y. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnology and Oceanography. 2018;63:3–21. [Google Scholar]
- Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ. Coastal ocean acidification: The other eutrophication problem. Estuarine, Coastal and Shelf Science. 2014;148:1–13. [Google Scholar]
- Weigel B, Andersson HC, Meier HEM, Blenckner T, Snickars M, Bonsdorff E. Long-term progression and drivers of coastal zoobenthos in a changing system. Marine Ecology Progress Series. 2015;528:141–159. [Google Scholar]
- Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Global Change Biology. 2012;18:1491–1498. [Google Scholar]
- Werner FJ, Graiff A, Matthiessen B. Temperature effects on seaweed-sustaining top-down control vary with season. Oecologia. 2016;180:889–901. doi: 10.1007/s00442-015-3489-x. [DOI] [PubMed] [Google Scholar]
- Wikner J, Andersson A. Increased freshwater discharge shifts the trophic balance in the coastal zone of the northern Baltic Sea. Global Change Biology. 2012;18:2509–2519. [Google Scholar]
- Winder M, Bouquet JM, Rafael Bermúdez J, Berger SA, Hansen T, Brandes J, Sazhin AF, Nejstgaard JC, et al. Increased appendicularian zooplankton alter carbon cycling under warmer more acidified ocean conditions. Limnology and Oceanography. 2017;62:1541–1551. [Google Scholar]
- Wood HL, Skold HN, Eriksson SP. Health and population-dependent effects of ocean acidification on the marine isopod Idotea balthica. Marine Biology. 2014;161:2423–2431. [Google Scholar]
- Wood HL, Sundell K, Almroth BC, Skold HN, Eriksson SP. Population-dependent effects of ocean acidification. Proceedings of the Royal Society B-Biological Sciences. 2016;283:7. doi: 10.1098/rspb.2016.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- Wulff A, Karlberg M, Olofsson M, Torstensson A, Riemann L, Steinhoff FS, Mohlin M, Ekstrand N, et al. Ocean acidification and desalination: Climate-driven change in a Baltic Sea summer microplanktonic community. Marine Biology. 2018;165:63. doi: 10.1007/s00227-018-3321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeebe RE, Ridgwell A, Zachos JC. Anthropogenic carbon release rate unprecedented during the past 66 million years. Nature Geosciences. 2016;9:325–329. [Google Scholar]
- Zhao L, Milano S, Walliser EO, Schone BR. Bivalve shell formation in a naturally CO2-enriched habitat: Unraveling the resilience mechanisms from elemental signatures. Chemosphere. 2018;203:132–138. doi: 10.1016/j.chemosphere.2018.03.180. [DOI] [PubMed] [Google Scholar]