Abstract
Comparative genomics has proven useful in exploring the biodiversity of phages and understanding phage-host interactions. This knowledge is particularly useful for phages infecting Streptococcus thermophilus, as they constitute a constant threat during dairy fermentations. Here, we explore the genetic diversity of S. thermophilus phages to identify genetic determinants with a signature for host specificity, which could be linked to the bacterial receptor genotype. A comparative genomic analysis was performed on 142 S. thermophilus phage genomes, 55 of which were sequenced in this study. Effectively, 94 phages were assigned to the group cos (DT1), 36 to the group pac (O1205), six to the group 5093, and six to the group 987. The core genome-based phylogeny of phages from the two dominating groups and their receptor binding protein (RBP) phylogeny corresponded to the phage host-range. A role of RBP in host recognition was confirmed by constructing a fluorescent derivative of the RBP of phage CHPC951, followed by studying the binding of the protein to the host strain. Furthermore, the RBP phylogeny of the cos group was found to correlate with the host genotype of the exocellular polysaccharide-encoding operon. These findings provide novel insights towards developing strategies to combat phage infections in dairies.
Subject terms: Super-resolution microscopy, Comparative genomics, Bacteriophages
Introduction
Bacteriophages represent a constant threat for the dairy industry worldwide. Infections of the bacterial starters with phages result in acidification failures, frequently leading to a lower quality of dairy products1. Phages infecting Streptococcus thermophilus are important due to the commercial use of thermophilic starter cultures for the production of yoghurt and various types of cheese2–4.
Advances in genome sequencing technologies and bioinformatic tools enable in-depth exploration of dairy phage biodiversity. Genomic studies provide insights into the evolution and relatedness of phages, rendering fast and precise phage taxonomic schemes. These studies are also useful to elucidate mechanisms of phage-host interactions, and this knowledge is essential for the rational design of novel anti-phage strategies1,5. Such efforts include designing PCR methods for phage monitoring6–9, tracking the dynamics of the phage community during dairy fermentations10, identifying groups of genes with host-specificity signatures11, or optimizing starter rotation schemes by selecting phage-unrelated strains12,13.
Genomic studies require access to comprehensive genomics data. As of October 2018, the GenBank database comprised 87 publicly available S. thermophilus phage genomes14–31. Phages infecting S. thermophilus belong to the Siphoviridae family of the Caudovirales order32 and are currently differentiated into four groups: the two dominating groups termed cos and pac33, as well as the 5093 group18,30, and the 987 group17,18. Each group of dairy streptococcal phages displays individual characteristics, which is reflected in their genetic diversity and morphological features, including host-recognition features on the tail-tip9,17,18,30.
Structures on the tail-tip, called antireceptors or receptor binding proteins (RBP), are known to mediate host recognition34–36. Characterization of an RBP gene in cos-group phages revealed three characteristic regions: (i) the conserved region, which corresponds to the amino-terminus of the protein, (ii) the first variable region (VR1), which is only present in some phages, and (iii) the second variable region (VR2), which corresponds to the carboxy-terminus of the protein and was proposed to code for the host recognition domain35. The identity of the RBP gene in pac-group phages was predicted based on its genomic position and homology to the gene in cos-group phages8,35. RBP genes in the 987- and 5093-group phages were established by expressing and purifying phage proteins, followed by studying the inhibitory effect of these proteins on phage adsorption to the host strain9,17.
A phage RBP interacts with a specific component present on the bacterial cell surface. It was speculated that cos and pac phages adsorb to a carbohydrate receptor on the host cell surface35,37,38. In a recent study, we provide genetic and biochemical evidence that specific cell wall glycans, namely exocellular polysaccharides encoded by the eps operon and rhamnose-containing polysaccharides encoded by the rgp operon, can mediate phage adsorption to S. thermophilus39.
Understanding the affinity between a bacterial receptor and a phage RBP is essential for developing knowledge-based tools to counter the threat of phage infections in the dairy industry1. In Lactococcus lactis, a correlation between the bacterial cell wall polysaccharide genotype and the RBP phylogeny of phages from the 936 group was established12. A specific type of operon encoding the biosynthesis of a particular polysaccharide structure, the so-called pellicle, can be recognized by the specific phage RBP domain12,13. For S. thermophilus, the VR2 sequence of cos- and pac-group phages was used to correlate phages with their host-range. Although a relationship between sequence similarity and host specificity of phages was put forward, it cannot be extrapolated to all representatives of the two groups3,8,9,35.
An alternative host-range determinant could be established independent of RBP. Studies on cos- and pac-group phages suggested that two other genes, which code for the tape-measure protein (TMP) and the distal tail protein (Dit), could be related to the host specificity of these phages40. Similarly, the structural module, especially the region surrounding the major capsid protein- and the major tail protein-encoding genes, was observed to be a hotspot for genetic recombination among 936-group of L. lactis phages13,41. Structural proteins belong to a core genome in dairy lactococcal and streptococcal phages9,13. Those phage structures are believed to coevolve with the phage host and therefore, may play a role in phage-host interactions13,41.
The overall objective of this study is to investigate the genetic diversity of a S. thermophilus phage population to identify genetic determinants with a signature for host specificity, which could be linked to the receptor genotype in bacteria. Towards this goal, we expanded the database of S. thermophilus phage genomes by sequencing 55 new phages isolated from dairy fermentations that took place in different years and on different continents. By combining this dataset with publicly available genome sequences, a comparative genomic analysis of 142 phage genomes was performed. Subsequently, the role of a putative RBP of a pac-group phage in host recognition was verified by expressing and purifying a fluorescent derivative of RBP, followed by studying the protein adsorption to the host strain. Finally, the RBP phylogeny was linked to the genotype of the operon encoding biosynthesis of exocellular polysaccharides in S. thermophilus.
Results
General characteristics of the phages and their genomes
To investigate the dairy streptococcal phage population, the genomes of 55 Streptococcus thermophilus phages from the Chr. Hansen Phage Collection (CHPC) were sequenced in this study. The selected samples originated from cheese and yoghurt fermentations performed in various geographic locations, including Europe, North and South America, and they were isolated at various time-points, between 1995 and 2013 (Table 1). These features were expected to provide a broad perspective on genetic diversity and evolution of S. thermophilus phages.
Table 1.
Characteristics of bacteriophage genomes from the Chr. Hansen Phage Collection sequenced in this study.
| Phage | Host strain(s) | Group | Year of isolation | Country of origin | Product | Genome size (bp) | #ORFs | GC (%) | Accession # |
|---|---|---|---|---|---|---|---|---|---|
| CHPC1005 | STCH_12 | cos | 2003 | France | cheese | 37,598 | 49 | 38 | MH937483 |
| CHPC1008 | STCH_09 STCH_43 | pac | 2003 | France | cheese | 34,844 | 48 | 40 | MH937484 |
| CHPC1014 | STCH_13 | cos | 2003 | USA | cheese | 35,260 | 49 | 38 | MH937485 |
| CHPC1027 | STCH_12 | cos | 2004 | Italy | cheese | 35,928 | 48 | 38 | MH937486 |
| CHPC1029 | STCH_13 | cos | 2004 | Italy | cheese | 35,920 | 47 | 39 | MH937487 |
| CHPC1033 | STCH_12 | cos | 2004 | Italy | cheese | 36,827 | 49 | 38 | MH937488 |
| CHPC1034 | STCH_13 | cos | 2004 | Italy | cheese | 33,826 | 42 | 39 | MH937489 |
| CHPC1036 | STCH_18 | cos | 2004 | Italy | cheese | 36,333 | 50 | 38 | MH937490 |
| CHPC1037 | STCH_34 | cos | 2005 | USA | cheese | 36,551 | 46 | 38 | MH937491 |
| CHPC1040 | STCH_13 | cos | 2005 | Italy | cheese | 35,851 | 47 | 38 | MH937492 |
| CHPC1041 | STCH_38 | cos | 2005 | Italy | cheese | 38,840 | 55 | 38 | MH937493 |
| CHPC1042 | STCH_07 | pac | 2005 | Italy | cheese | 42,019 | 63 | 39 | MH937494 |
| CHPC1045 | STCH_40 | cos | 2005 | USA | cheese | 34,096 | 46 | 38 | MH937495 |
| CHPC1046 | STCH_14 STCH_39 | cos | 2005 | USA | cheese | 34,790 | 48 | 38 | MH937496 |
| CHPC1048 | STCH_14 STCH_39 | cos | 2005 | USA | cheese | 34,812 | 48 | 38 | MH937497 |
| CHPC1057 | STCH_09 STCH_43 | pac | 2005 | France | yoghurt | 34,845 | 47 | 40 | MH937498 |
| CHPC1062 | STCH_31 | cos | 2005 | France | cheese | 40,037 | 56 | 38 | MH937499 |
| CHPC1067 | STCH_12 | cos | 2005 | France | cheese | 34,355 | 45 | 39 | MH937500 |
| CHPC1073 | STCH_12 | cos | 2006 | France | cheese | 34,017 | 47 | 38 | MH937501 |
| CHPC1083 | STCH_12 | pac | 2006 | USA | cheese | 36,471 | 48 | 39 | MH937502 |
| CHPC1084 | STCH_13 | pac | 2006 | USA | cheese | 36,776 | 49 | 39 | MH937503 |
| CHPC1091 | STCH_04 | cos | 2006 | France | yoghurt | 37,028 | 49 | 38 | MH937504 |
| CHPC1109 | STCH_36 | pac | 2006 | USA | cheese | 33,791 | 47 | 39 | MH937505 |
| CHPC1148 | STCH_41 | cos | 2008 | Germany | cheese | 39,069 | 52 | 38 | MH937506 |
| CHPC1152 | STCH_06 | pac | 2008 | France | yoghurt | 35,353 | 47 | 39 | MH937507 |
| CHPC1156 | STCH_19 | cos | 2009 | USA | cheese | 34,912 | 48 | 38 | MH937508 |
| CHPC1230 | STCH_45 | pac | 2012 | USA | cheese | 39,384 | 57 | 39 | MH937509 |
| CHPC1246 | STCH_07 | pac | 2013 | USA | cheese | 36,876 | 55 | 39 | MH937510 |
| CHPC1247 | STCH_07 | pac | 2013 | France | cheese | 35,543 | 50 | 39 | MH937511 |
| CHPC1248 | STCH_07 | pac | 2013 | France | cheese | 38,383 | 56 | 39 | MH937457 |
| CHPC572 | STCH_24 | cos | N/A | Italy | cheese | 37,005 | 51 | 38 | MH937458 |
| CHPC595 | STCH_25 | cos | N/A | Argentina | cheese | 35,697 | 49 | 39 | MH937459 |
| CHPC640 | STCH_26 STCH_28 | pac | N/A | Italy | cheese | 40,404 | 57 | 38 | MH937460 |
| CHPC642 | STCH_17 | cos | N/A | Italy | cheese | 35,715 | 52 | 38 | MH937461 |
| CHPC663 | STCH_29 | cos | 1995 | Italy | cheese | 38,531 | 54 | 38 | MH937462 |
| CHPC676 | STCH_26 STCH_28 | pac | N/A | Italy | cheese | 40,402 | 55 | 38 | MH937463 |
| CHPC869 | STCH_44 | pac | 1998 | France | yoghurt | 37,080 | 52 | 39 | MH937464 |
| CHPC873 | STCH_42 | cos | 1998 | France | cheese | 38,258 | 53 | 38 | MH937465 |
| CHPC875 | STCH_23 | cos | 1998 | France | cheese | 36,576 | 51 | 39 | MH937466 |
| CHPC877 | STCH_32 STCH_33 | cos | 1998 | France | cheese | 39,965 | 54 | 38 | MH937467 |
| CHPC879 | STCH_20 | cos | 1998 | France | cheese | 36,011 | 48 | 38 | MH937468 |
| CHPC919 | STCH_37 | cos | 2000 | France | cheese | 37,281 | 48 | 38 | MH937469 |
| CHPC925 | STCH_21 | cos | 2000 | France | cheese | 34,759 | 47 | 38 | MH937470 |
| CHPC927 | STCH_32 STCH_33 | cos | 2000 | France | cheese | 37,303 | 50 | 38 | MH937471 |
| CHPC928 | STCH_22 | cos | 2000 | France | cheese | 34,022 | 43 | 38 | MH937472 |
| CHPC929 | STCH_35 | pac | 2000 | France | cheese | 40,874 | 60 | 38 | MH937473 |
| CHPC930 | STCH_30 | cos | 2000 | France | cheese | 36,350 | 49 | 38 | MH937474 |
| CHPC931 | STCH_44 | pac | 2000 | France | yoghurt | 36,490 | 51 | 39 | MH937475 |
| CHPC933 | STCH_02 | pac | 2001 | France | yoghurt | 32,182 | 40 | 40 | MH937476 |
| CHPC950 | STCH_13 | cos | 2002 | UK | cheese | 35,299 | 52 | 39 | MH937477 |
| CHPC951 | STCH_12 | pac | 2002 | USA | cheese | 36,471 | 49 | 39 | MH937478 |
| CHPC952 | STCH_13 | pac | 2002 | USA | cheese | 36,775 | 49 | 39 | MH937479 |
| CHPC954 | STCH_27 | cos | 2002 | USA | cheese | 37,464 | 47 | 38 | MH937480 |
| CHPC979 | STCH_13 | cos | 2002 | Germany | cheese | 34,277 | 50 | 38 | MH937481 |
| CHPC982 | STCH_13 | cos | 2002 | France | cheese | 35,246 | 48 | 38 | MH937482 |
The investigated phages exhibited a narrow host-range. As verified in spot tests with 37 industrial S. thermophilus strains, the studied phages infected their primary host and, in a few cases, only one additional strain (Table S1). Three of the tested strains, STCH_07, STCH_12, and STCH_13, were susceptible to infection by four, seven, and nine specific phages, respectively. These phages were included in the study to examine the genotypic similarity of phages that infect the same host.
The overall genome architecture of the sequenced phages was comparable to the S. thermophilus phage genomes currently available in GenBank. The genome length varied from 32 to 42 kb (average 36.5 kb) and phage genome sequences had a GC content of approximately 38%. Forty to 63 (average 50) coding sequences (CDS) were identified in each genome using RASTtk42. In a previous study, the selected phages were subjected to pac- and cos-grouping using the published multiplex PCR method18. The results of the assay classified 36 phages into the cos group and 19 phages into the pac group. The detailed information on the phages sequenced in this study is presented in Table 1.
Grouping of the S. thermophilus phage population
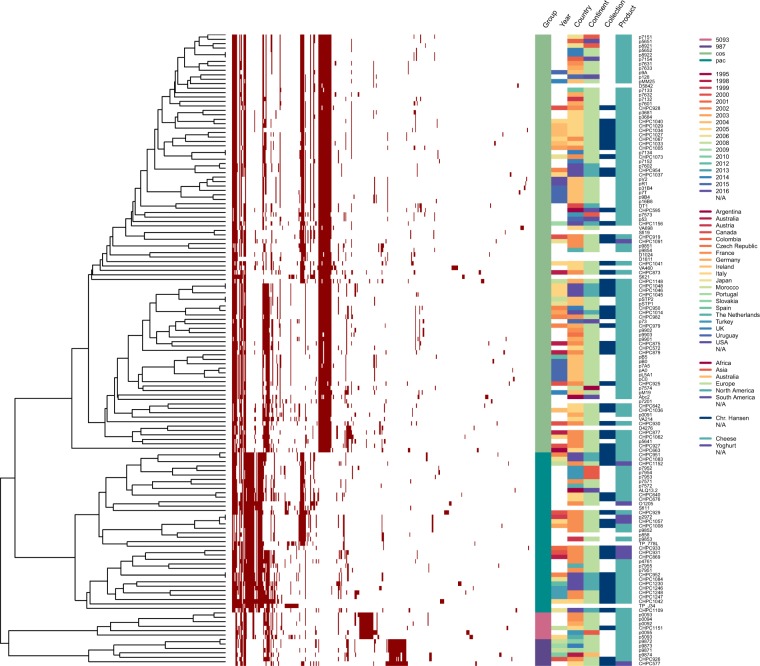
A comparative genomic analysis was performed with 142 S. thermophilus phage genomes: 55 phage sequences obtained in this study and 87 phage genomes available in GenBank (Table S2). This analysis aimed primarily at establishing the genetic relatedness of phages, which should provide a more comprehensive and accurate grouping. The pangenome covering all 142 phages was created through the identification of orthologous gene groups based on sequence similarity (>50% identity) and sequence coverage (>50%). The resulting pangenome information on the absence or presence of orthologous gene groups within the genomes allowed the hierarchical clustering of phages based on their gene content (Fig. 1).
Figure 1.
Hierarchical clustering of a gene content variation among 142 S. thermophilus phages. Pangenome information on the presence or absence of a representative gene of an orthologous group was employed for functional clustering of phages. Gene presence or absence is denoted in the heatmap by red and white colour, respectively. Metadata of the following characteristics is integrated in the dendrogram: the group of a phage (Group), the year and the geographic location of sample isolation (Year, Country, Continent), the type of dairy fermentation the phages were isolated from (Product), the industrial collection of phages (Collection). The varieties within each feature are marked with different colours.
Based on pangenome clustering, S. thermophilus phages were divided into four main clusters, which corresponded to the previously proposed groups: cos, pac, 5093, and 987 (Fig. 1). In addition, two sub-clusters of the cos lineage and three sub-clusters of the pac lineage were observed, which indicated a potential for further division within the two dominating groups. Notably, only a limited number of gene products were complementary within each of the four major clusters, highlighting the mosaic nature of phage genomes.
Factors that shape the genetic relatedness of phages
To identify possible factors that influence the genetic makeup of phages, the visualization of the functional clustering was amended by metadata with the following information: (i) the type of fermentation processes the phages were isolated from, (ii) the industrial collection that phages belong to, (iii) the geographic location, and (iv) the year of sample isolation (Fig. 1). Several relatedness patterns were observed based on the generated network.
Phages with common characteristics, e.g. isolated in the same year or from the same continent, were scattered across the dendrogram. However, samples that originated from one geographic location, but were isolated at various time-points, clustered closely together (Fig. 1, features ‘Year’ and ‘Country’). This observation supports the hypothesis that phage particles with nearly unchanged genotype persist in the processing environment for extended periods of time43,44.
Nine phages from the analysed population were isolated from yoghurt fermentations performed with strains that possess texturizing properties, while the rest of the isolates originated from whey samples of cheese fermentations. Seven of the nine yoghurt-originated phages belonged to the pac group (Fig. 1, feature ‘Product’), which may suggest that pac-group phages have an advantage over cos-group phages for infecting free exopolysaccharide-producing strains.
Phages from CHPC clustered closely together, irrespectively of their geographic origin (Fig. 1, features ‘Continent’ and ‘Collection’). These phages were isolated from the industrial fermentations performed with starter cultures provided by Chr. Hansen. The same bacterial cultures were used in different locations worldwide, which could explain the dissemination of closely related phages across the globe. Thus, the industrialization of the dairy production could be a factor that may shape the diversity of the S. thermophilus phage population.
Core genome as a host-range determinant
To further investigate the genetic similarity of S. thermophilus phages, the core genome was determined from the constructed pangenome of the 142 phage genome sequences. Based on the results, no conserved genes were identified across all members of the examined population. However, one gene coding for a phage protein was present in all members except for phage D1811. This gene had distal genomic location and a representative is ORF43 of CHPC1040. In total 361 genes were covered by the pangenome, and of these 30% were unique genes. These results revealed a significant genetic heterogeneity of S. thermophilus phages.
Even though core genes covering all phage groups were not identified, core genes within each group were recognized. Phages of the two dominating groups cos and pac had 13 core genes each. Phages of the groups 987 and 5093 had 24 and 28 core genes, respectively, which is likely a reflection of the fewer genomes within these groups or that these groups are more genetically related. The genetic elements of the identified core genomes primarily corresponded to the genes coding for structural proteins and genome packaging. Since the cos- and pac-group phages are the most frequently isolated dairy streptococcal phages, we opted to analyse those two groups in detail.
We questioned whether the similarity within the cos and pac core genomes can be attributed to the host-range of the phages. This information could be used to establish starter rotation schemes in dairy plants that could prevent acidification failures due to phage infection. Therefore, phage phylogeny was constructed based on the 13 core genes of the 94 cos-group phages and the 13 core genes of the 36 pac-group phages (Figure S1).
The analyses showed that phages that infected the same host(s) clustered together on well supported branches. Within the cos group, we identified 16 instances of a shared host, i.e. cases where two or more phages infected a single strain, or a pair of phages infected two strains. In 13 of these cases, phages that infected the same strain were phylogenetically related (Figure S1a). Similarly, pac-group phages that infected the same strain were located on the same branches, with only a single exception observed (Figure S1b).
Identifying the antireceptor gene
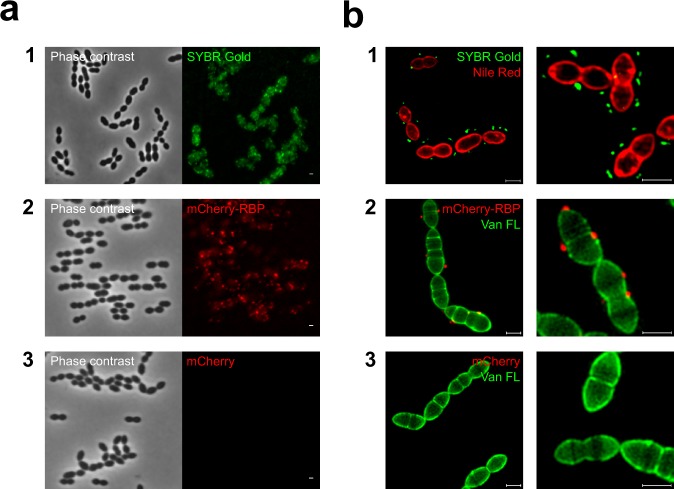
RBP is the factor that mediates host recognition. Thus, a host-related grouping of the cos- and pac-group phages was expected to be generated by comparing the RBP gene sequence. The identity of the RBP in cos-group phages was established previously35. Here, we focused on confirming the function of a putative RBP gene in the pac-group phages, which was predicted based on the genomic position and the similarity to the cos-group phages. Moreover, we wanted to specify whether a single protein encoded by the RBP gene is sufficient to create the irreversible attachment to the phage receptor on the bacterial cell surface. To that end, we constructed and purified a fluorescent variant of a putative RBP from pac-group phage CHPC951 (ORF20) as described in Materials and Methods. Subsequently, we visualized the adsorption of phage CHPC951 and its recombinantly produced RBP to S. thermophilus host strain STCH_12. The images were acquired using both conventional fluorescence microscopy and super-resolution structured illumination microscopy (SIM) (Fig. 2).
Figure 2.
Fluorescence imagining of a phage and a phage antireceptor (RBP) binding to S. thermophilus. Strain STCH_12, phage CHPC951, a fluorescence derivative of the RBP of phage CHPC951, and a purified mCherry protein were used in the experiments. (a) Imagining under a conventional fluorescence microscope: (1) adsorption of SYBR Gold DNA-labelled phages (green) to the host strain; (2) adsorption of mCherry-tagged RBP (red) to the bacterial cells; (3) no adsorption of mCherry proteins to the bacterial cells. (b) Super-resolution structured illumination microscopy (SIM) imagining: (1) bacterial cells were labelled with Nile Red (red) and mixed with SYBR Gold-DNA labelled phages (green); (2) bacterial cells were labelled with Van FL (green) and mixed with mCherry-tagged RBP (red); (3) bacterial cells were labelled with Van FL (green) and mixed with mCherry proteins (red). Scale bars: 1 µm.
Interactions between the phage and its host strain, as well as between the mCherry-tagged RBP and the bacterial cells were observed in the microscopy assays. A fluorescent signal was detected around the bacterial cells when strain STCH_12 was mixed with SYBR Gold DNA-labelled phage particles (Fig. 2a, panel 1) and with the mCherry-tagged derivative of the phage RBP (Fig. 2a, panel 2). As visualized by SIM, phage particles and mCherry-tagged RBP bound to the host cells (Fig. 2b, panels 1 and 2). For the intact phages, the green fluorescent signal originating from a phage capsid, which contained DNA labelled with SYBR Gold, was localized 0.21 ± 0.07 µm (average ± SD, n = 80 phage capsids) from the bacterial cell surface (Fig. 2b, panel 1). This distance is in accordance to the values determined by electron microscopy for the length of the phage tail39. The red fluorescent signal of mCherry-tagged RBP was localized directly on the cell surface (Fig. 2b, panel 2). The purified mCherry protein, used as a negative control for the assays, did not bind to the bacterial cells (Fig. 2a,b, panel 3). These results highly suggested that the putative RBP gene in the pac-group phage encodes the receptor binding protein and that it is sufficient to attach the mCherry-tagged protein (or a phage) on the bacterial cell surface.
Antireceptor phylogeny correlates with host specificity
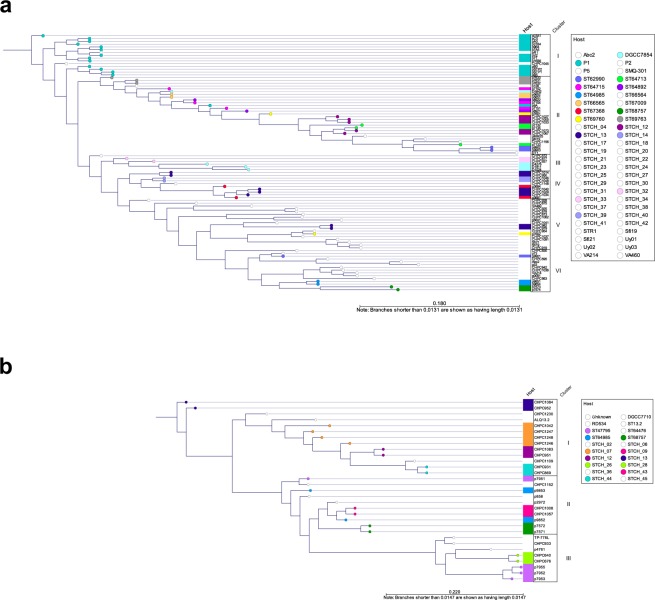
To verify the hypothesis that the RBP can be a gene with a significant signature of host specificity, a phylogenetic analysis of the RBP of the cos- and pac-group phages was performed (Fig. 3, ORFs used in the analysis are listed Table S2). Two cos-group phages (7201, VA698) and five pac-group phages (O1205, Sfi11, CHPC929, 7954, and TP-J34) were excluded from the analysis because the sequence similarity of their RBP was below 30% in comparison to the other RBPs used in the study. Thus, homology with other RBPs, which is a prerequisite for a phylogenetic analysis, could not be established. In 12 out of 16 cases of shared host-ranges within the cos group, phages that infected the same strain(s) clustered together, based on the RBP phylogeny (Fig. 3a). For the pac group, the host specificity correlated well with the RBP phylogeny, with only one exception (Fig. 3b). The correlation between the RBP phylogeny and the host-range of the phages was comparable to the correlations obtained from the core genome analyses.
Figure 3.
Phylogenetic comparison of the antireceptor (RBP) from cos- and pac-group S. thermophilus phages. The phylogenetic tree was inferred from alignment of the RBP protein sequence. (a) The RBP phylogeny of cos-group phages. A colour-coding is used to highlight a host strain that is infected by two or more phages (STCH_12, STCH_13, ST66565, ST67368, ST62990, ST68757, ST64713, ST69760, ST64715, ST69763, ST64892, ST64985, DGCC7854, P1), as well as pairs of strains that are infected by two different phages (STCH_32 and STCH_33, STCH_14 and STCH_39). RBP phylogeny clusters (I to VI) are assigned. (b) The RBP phylogeny of pac-group phages. A colour-coding is used to highlight a host strain that is infected by two or more phages (STCH_07, STCH_12, STCH_13, STCH_44, ST649885, ST47795, ST68757), as well as pairs of strains that are infected by two different phages (STCH_26 and STCH_28, STCH_09 and STCH_43). RBP phylogeny clusters (I to III) are assigned.
Correlation of the antireceptor phylogeny and the eps and rgp operon genotype
Our next goal was to identify the genetic determinants of phages that would correlate with the receptor genotype in bacteria. Therefore, we investigated whether there is a link between the phage RBP phylogeny and the genotype of the eps and rgp operons, presumably encoding phage receptors of S. thermophilus. To address this query, 43 phage genomes from CHPC, 27 cos- and 16 pac-group phages, were selected from the original dataset, and analysed together with their 23 host strains, the genomes of which are part of the internal Chr. Hansen Collection.
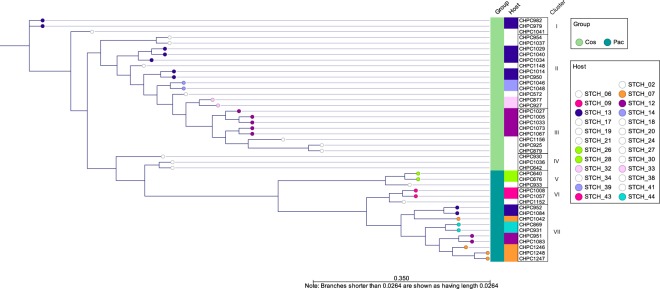
Seven homology clusters were identified based on the RBP phylogeny of the selected phages (Fig. 4). Phages from the cos and pac groups were separated into different clusters. The cos-group phages used in the analysis belonged to the RBP lineages I to IV, while the pac-group phages belonged to the RBP lineages V to VII. Phages that infected the same strain(s) belonged to the same RBP lineage, apart from phages that infected strains STCH_12 and STCH_13. The pac-group phages of these two strains belonged to the RBP lineage VII. The cos-group phages of STCH_12 belonged to the RBP lineage III, while the cos-group phages of STCH_13 belonged to the RBP lineages I and II. The analysis confirmed that RBP phylogeny correlates well with the host association. However, the observed division between cos- and pac-group phages indicated that RBPs of these two phage groups significantly differ, which can result in recognizing different cellular components.
Figure 4.
Phylogenetic comparison of the antireceptor (RBP) from the selected cos- and pac-group S. thermophilus phages. The phylogenetic tree was inferred from alignment of the RBP protein sequence. A colour-coding is used to highlight a host strain that is infected by two or more phages, as well as a pair of strains that are infected by a pair of phages. Metadata on the phage group (cos or pac) is integrated in the dendrogram. RBP phylogeny clusters (I to VII) are assigned.
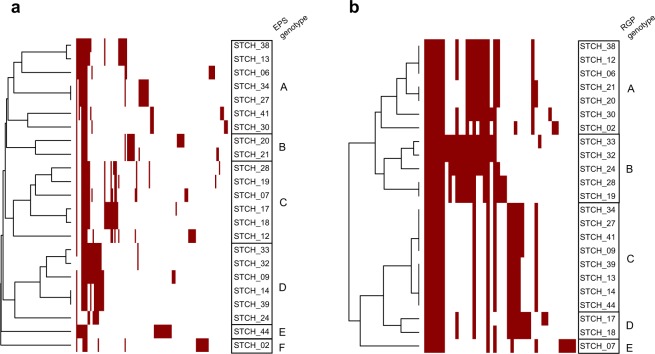
The eps and rgp gene cluster contents of the 23 S. thermophilus strains, which are hosts for the selected phages, were identified as specified in Materials and Methods. The eps clusters contained 17.7 genes on average, of which 13 genes were the minimal number of genes identified, while the rgp clusters contained 16.8 genes on average, with a lowest number of 14. In certain strains, the eps or rgp operon was located in two contigs and thus, genes could be missing in these gene clusters. Hierarchical clustering of the strains based on the presence or absence of orthologous protein groups encoded by the eps and rgp gene clusters was performed (Fig. 5).
Figure 5.
Hierarchical clustering of a gene content variation among eps and rgp gene clusters of 24 S. thermophilus strains. Gene presence or absence is denoted in the heatmap by red and white colour, respectively. (a) EPS genotypes (A to F) and (b) RGP genotypes (A to E) are assigned.
Six clusters of the eps operon and five clusters of the rgp operon were identified (Fig. 5). The genotypes were designated as EPS types A to F (for exopolysaccharides biosynthesized via the eps operon) and RGP types A to E (for rhamnose-containing polysaccharides biosynthesized via the rgp operon). Strains that possessed one EPS genotype, possessed separate RGP genotypes (Table 2). An additional hierarchical clustering based on the bacterial pangenome showed that core genome similarity does not reflect the rgp or eps gene content similarity (Figure S2). This observation is in line with the notion that genes of the rgp and eps operons are often acquired by horizonal gene transfer. Therefore, homologs with similar functions can be phylogenetically more distant than other genes within the genome. The established relationships based on the eps and rgp gene cluster contents could reflect the biosynthesis of similar polysaccharide structures by strains that belong to the same EPS or RGP genotype.
Table 2.
List of S. thermophilus phages with the corresponding RBP phylogeny cluster (I to VII), as assigned in Fig. 4, and their host strains with the corresponding EPS genotype (A to F) and RGP genotype (A to E), as assigned in Fig. 5.
| RBP phylogeny cluster | Phage | Host strain | EPS genotype | RGP genotype |
|---|---|---|---|---|
| Ia | CHPC979 | STCH_13 | A | C |
| CHPC982 | ||||
| CHPC1041 | STCH_38 | A | A | |
| IIa | CHPC954 | STCH_27 | A | C |
| CHPC1037 | STCH_34 | A | C | |
| CHPC572 | STCH_24 | D | B | |
| CHPC877 | STCH_32 | D | B | |
| CHPC927 | STCH_33 | D | B | |
| CHPC1014 | STCH_13 | A | C | |
| CHPC1029 | ||||
| CHPC1034 | ||||
| CHPC1040 | ||||
| CHPC950 | ||||
| CHPC1148 | STCH_41 | A | C | |
| CHPC1046 | STCH_39 | D | C | |
| CHPC1048 | STCH_14 | D | C | |
| IIIb | CHPC1005 | STCH_12 | C | A |
| CHPC1027 | ||||
| CHPC1033 | ||||
| CHPC1067 | ||||
| CHPC1073 | ||||
| CHPC1156 | STCH_19 | C | B | |
| CHPC879 | STCH_20 | B | A | |
| CHPC925 | STCH_21 | B | A | |
| IVb | CHPC930 | STCH_30 | A | A |
| CHPC642 | STCH_17 | C | D | |
| CHPC1036 | STCH_18 | C | D | |
| V | CHPC933 | STCH_02 | F | A |
| CHPC640 | STCH_28 | C | B | |
| CHPC676 | ||||
| VI | CHPC1152 | STCH_06 | A | A |
| CHPC1008 | STCH_09 | D | C | |
| CHPC1057 | ||||
| VII | CHPC1083 | STCH_12 | C | A |
| CHPC951 | ||||
| CHPC1084 | STCH_13 | A | C | |
| CHPC952 | ||||
| CHPC869 | STCH_44 | E | C | |
| CHPC931 | ||||
| CHPC1042 | STCH_07 | C | E | |
| CHPC1246 | ||||
| CHPC1247 | ||||
| CHPC1248 |
aProbability of only A and D eps gene clusters for a group size of RBP cluster I and II: < 3E-7. bProbability of only B and C eps gene clusters, except one, for a group size of RBP cluster III and IV: < 2E-4.
Certain correlations between the genotype of the eps operon and the RBP phylogeny of cos-group phages were observed (Table 2). Phages of RBP phylogeny cluster I and II infect strains that possess EPS type A and D, while phages of RBP lineages III and IV preferentially infect strains of EPS type B and C.
Discussion
In this work, the use of comparative genomics enabled accurate grouping of 142 S. thermophilus phages and guided the identification of genetic determinants with a signature of host specificity. Four groups of S. thermophilus phages were defined, and additional subgroups were observed within the two dominating groups, known as cos and pac. The core genome phylogeny and the RBP phylogeny of phages from these two groups could be correlated with the host-range of phages. The role of RBP of pac-group phage CHPC951 in creating the irreversible binding to the host cell surface receptor was confirmed. Finally, a correlation between the eps operon genotype and the RPB phylogeny of phages from the cos group was observed.
The comparative analysis of S. thermophilus phage genomes confirmed that this population can be divided into the previously defined groups cos, pac, 5093, and 9879,18. Considering the growing number of phages of the groups 987 and 5093, which also use pac and cos DNA packaging mechanisms, the conventional classification of S. thermophilus phages based on DNA packaging mechanisms (cos and pac) and structural protein composition should be revised33. Therefore, we propose new names for the two dominating groups: the pac group to be described as group O1205, because phage O1205 was the first pac-group representative defined14, and the cos group to be described as group DT1, because phage DT1 was used as a model of cos-group phages in several studies24,28,35,40. The novel nomenclature will be more accurate in reflecting the current grouping of S. thermophilus phages and allow for further subgrouping within the four major groups.
The results of this study unveiled the mosaic nature of phage genomes and the conservation across the structural genes within the four defined groups, which is in accordance with a previous report9. The relatedness of S. thermophilus phages can be predominantly shaped by industrialization. This observation is not surprising, since the investigated phages originate from industrial fermentations. Indeed, multilocus sequence typing of S. thermophilus strains showed that industrial cultures cluster independently from their geographic origin and fermented product45. The dissemination of phages in industrial fermentations can be due to e.g. the addition of whey protein concentrates to cheese milk. These milk by-products were shown to be a rich source of phages46,47. Thus, global movements of dairy starter cultures and use of products derived from whey likely lead to exchanging phage genomic information and distributing genetically similar phages in different locations across the globe13.
The phylogenetic analyses based on the core genome or the RBP were equally effective in representing the host specificity of phages. Hence, they could both possibly serve to generate predictions on potential phage-bacteria interactions. In this study, we aimed at establishing the relatedness of phages that would correlate with the type of the phage receptor on the bacterial cell surface. The RBP sequence was proven to encode the protein that efficiently adsorbs to a component on the bacterial cell surface. Although some other gene products could additionally assist in host recognition40, the single RBP was sufficient to create the irreversible interaction with the bacterial cell surface receptor. Therefore, the RBP phylogeny was used to link phage relatedness with the receptor genotype of bacteria.
The results of this study indicated the possibility of the unique relationships between the cos-group phage RBP structure and the exocellular polysaccharide structure biosynthesized by the eps operon in S. thermophilus. Indeed, putative receptor mutants of S. thermophilus were shown to acquire mutations in genes belonging the eps operon as a response for the infection by cos-group phages39. The RBP phylogeny of the pac-group phages could not be correlated with specific genotypes of the eps and rgp operons. However, in this study, a limited pool of 16 pac-group phages was used to investigate the association with the receptor genotype. Comparing larger dataset of phage and host genomes would possibly result in a more accurate clustering based on the RBP phylogeny of pac-group phages, and lead to revealing association with the specific RGP or EPS genotypes.
Notably, the clustering based on the RBP phylogeny of the selected phages from the cos and pac groups suggested that phages belonging to different groups recognize diverse receptors on the host cell surface. A single strain likely possesses two types of phage receptors, as a putative receptor mutant of S. thermophilus was reported to acquire resistance towards cos-group phages but remained sensitive towards pac-groups phages48. If the receptors of cos-group phages are polysaccharides biosynthesized via the eps operon, the pac-group phages could recognize RGP biosynthesized via the rgp operon.
In this study, it was assumed that a specific eps genotype is responsible for the biosynthesis of a particular polysaccharide structure. To verify this notion and elucidate genotype-phenotype associations in general, further studies on glycobiology in S. thermophilus are required. The host specificity of phages, which was used for correlating RBP phylogeny with the EPS and RGP genotype, was established based on the spot test, i.e. the ability of phages to form plaques with a bacterial strain. However, successful phage infection is dependent on the receptor recognition as well as additional factors, such as presence of intracellular phage-resistance mechanisms49. Thus, phages with close RBP phylogeny, which did not form plaques with each other’s hosts, could still recognize a specific polysaccharide encoded by a similar eps or rgp operon. To confirm this hypothesis, further studies should be undertaken to examine the adsorption of phages from a given RBP cluster to the strains with the associated EPS genotype.
In conclusion, the data generated in this study could be successfully used for accurately grouping S. thermophilus phages and correlating the cos-group phage RBP phylogeny with the genotype of the eps operon. These results should aid in improving starter rotation schemes as well as the selection of strains for culture development.
Materials and Methods
Bacteria, phages, and growth conditions
Streptococcus thermophilus strains and phages used for this study are listed in Table 1. Strains were stored at −40 °C in growth medium supplemented with 15% (wt/vol) glycerol and cultured overnight at 37 °C in LM17 broth (M17 broth [Oxoid, Denmark] with 2% [wt/vol] lactose) or anaerobically at 37 °C on LM17 agar plates (M17 agar [Oxoid] with 2% [wt/vol] lactose). For experiments with phages, the growth medium was additionally supplemented with 10 mM CaCl2 and 10 mM MgCl2 (LM17-Ca/Mg). Phages were propagated on their corresponding host as previously described18 and stored at 4 °C.
Phage titers as well as the host ranges of investigated phages with bacterial strains were determined by using the double agar overlay spot test, as described before50. Following overnight incubation under the appropriate growth conditions, the plaque forming units (PFU) per milliliter were calculated.
Competent cells of Escherichia coli and plasmids used for the cloning procedure were stored at −80 °C. Transformants were selected on LB-Amp agar plates (LB agar [Difco, USA] with 100 µg/ml Ampicillin) and grown in LB-Amp broth (LB broth [Difco] with 100 µg/ml Ampicillin) at 37 °C with aeration at 150 rpm.
Phage genome sequencing
DNA was isolated from the 55 phages listed in Table 1 and whole genome sequencing was performed using the Illumina MiSeq platform with 2 × 250 bp paired end sequencing (Illumina, USA), as described previously18. Sequencing data were processed using CLC Genomics Workbench 8.5 (Invitrogen, Denmark), as described before18. The genome fragments with low coverage (threshold 100 reads) were additionally verified by Sanger sequencing (Macrogen, The Netherlands).
Genomic analysis
Open reading frames (ORFs) in all genomes, including the publicly available ones, were identified and functionally annotated through the RASTtk pipeline42, with default parameters. The translated protein sequences of protein-encoding genes were employed for pangenome construction. Orthologs were identified by blast-based bidirectional best hit (BBH) using Proteinortho51 with 50% identity, 50% coverage and 1.0 similarity cut-offs. The obtained pangenome was visualized by hierarchical clustering with the Jaccard distance metric and UPGMA linkage method, using the hclust and heatmap.2 functions in R.
As no core genes could be identified in the pangenome of the four phage groups, the core genes within each phage group were used to construct phage-group specific phylogeny. For that, the nucleotide sequences of concatenated core genes were first aligned using prank52, with the -F option and otherwise default parameters. Phylogeny was then inferred by the Maximum-Likelihood method using RAxML-NG (https://github.com/amkozlov/raxml-ng), which is based on RAxML53, with the GTRGAMMA nucleotide substitution model and default parameters. For constructing the phylogeny of the RBP protein within the phage genomes, translated protein sequences of the corresponding genes were aligned with prank and phylogeny was inferred using RAxML-NG as described above, but with the WAG substitution model instead of the GTR model. This was performed for the pac and cos phage groups together or individually. Phylogeny clusters were determined by comparing the same phylogenetic tree in phylogram and radial layouts.
The hierarchical clustering of the eps and rgp gene cluster content of selected phage host strains was initiated by identifying the location of the eps and rgp cluster genes within the bacterial genomes. This was performed through a blast search for two flanking genes, i.e. epsA and predicted membrane protein (TMS6) for eps clusters, and radC and bactoprenol glucosyl transferase for rgp clusters, as annotated by RAST54. Ortholog groups of the proteins encoded by the genes within the eps and rgp clusters were determined by blast-based BBH with 40% amino acid sequence identity, 80% coverage and 1.0 similarity cut-offs. A lower percent identity was employed here as the genes within these clusters are often acquired by horizontal gene transfer. Therefore, homologs with similar function are potentially phylogenetically more distant than other genes within the genome. The pangenome of the selected bacterial strains was determined with the same methods and parameters, as described above for the phage pangenome. Subsequent hierarchical clustering of the bacterial pangenome, eps and rgp gene clusters were performed as described for the phage pangenome. Probabilities of selected RBP vs. eps or rgp cluster group distributions were calculated as sampling without replacement.
Fluorescent derivative of a phage antireceptor
A fluorescent derivative of RBP of pac-group phage CHPC951 was cloned and expressed using a commercially available vector pET21a (Novagen, Germany) that was transformed into competent cells of E. coli. The version of mCherry fluorescent protein used for this work was reported before55. PCR amplifications were carried out using Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific, USA). Restriction and ligation enzymes (New England Biolab, USA) were used according to the manufacturer’s protocol. PCR amplicons were purified using Wizard SV Gel and PCR Clean-up System (Promega, USA). Plasmid DNA was isolated using Wizard Plus SV Minipreps (Promega). Sequences of primers used for the cloning procedure are listed in Table 3.
Table 3.
List of primers used in this study.
| Primer name | Sequence, 5′–3′ | Reference |
|---|---|---|
| mCherry_FWD |
GCGGATCCGTGAGCAAGGGCGAGGAGGA TAACATGG |
This study |
| mCherry-His_REV |
CGCGGCCGCAAGCTTTTAGTGATGGTGAT GGTGATGCTTGTACAGCTCGTCC |
|
| mCherry_REV |
GTGAATTCCCTTCCCTCGATCCCGAGATTG TTGTTCTTGTACAGC |
|
| RBP951_FWD |
GGGATCGAGGGAAGGGAATTCACCTTGCT AACAATTCACGACG |
|
| RBP951_REV |
CGCGGCCGCAAGCTTTTAGTGATGGTGATG GTGATGTGCACCTCCTACATATCTTATGACG |
|
| T7 promoter_FWD | TAATACGACTCACTATAGG | Novagen |
| T7 terminator_REV | GCTAGTTATTGCTCAGCGG |
For expression of mCherry not fused to any protein, mCherry sequence with a sequence encoding 6 × His C-terminal-specifying purification tag and an appropriate restriction recognition sequence was amplified using primers mCherry_FWD and mCherry-His_REV. The PCR amplicons were purified, restricted with appropriated enzymes, and ligated into vector pET21a.
For expression of mCherry fused to the phage RBP, mCherry sequence was amplified using primers mCherry_FWD and mCherry_REV. The RBP sequence of phage CHPC951 (ORF20) with a sequence encoding 6 × His C-terminal-specifying purification tag and an appropriate restriction recognition sequence was amplified from phage CHPC951 lysate using primers RBP951_FWD and RBP951_REV. The purified PCR amplicons were joined by an overlap PCR reaction using primers mCherry-FWD and RBP951_REV. The construct encoding mCherry fused to the N-terminal of phage RBP was purified, restricted with appropriate enzymes, and ligated into vector pET21a.
Plasmids were transformed and propagated in E. coli DH5α (Invitrogen). Ampicillin-resistant colonies were screened with primers T7 promoter_FWD and T7 terminator_REV. Constructs were confirmed by Sanger sequencing of the amplified fragments. Plasmids were isolated from positive colonies grown overnight in LB-Amp broth and transformed into competent cells E. coli BL21(DE3) (Novagen). Cells were grown in LB-Amp to OD600 of 0.5. Subsequently, the protein expression was induced by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration 1 mM and incubating overnight under the appropriate growth conditions. Cells were harvested (7,000 rpm for 7 min at 4 °C) and washed twice with equilibration buffer (50 mM Na2PO4, 300 mM NaCl, pH 7.4), followed by lysing at French Press at 1000 psi. The lysate was centrifuged (16,000 rpm for 20 min at 4 °C) to remove cell debris and insoluble components of the sample.
The fluorescent proteins mCherry and mCherry-tagged derivate of the phage RBP were purified from the lysate using cobalt affinity resin (Talon; BD Biosciences, USA), due to the 6-His tag located at the C-terminal end of both proteins, eluted in equilibration buffer with the addition of 5 mM and 10 mM imidazole, and dialyzed overnight against phosphate-buffer saline (PBS) pH 6.0. Proteins were stored at 4 °C in PBS pH 6.0. The samples were separated on SDS-PAGE gels (Bio-Rad, USA) to confirm the presence of induced target proteins. Protein concentration was quantified using Nanodrop (Thermo Fisher Scientific).
Fluorescence microscopy
To visualize adsorption of SYBR Gold DNA-labelled phages and the mCherry-tagged proteins to the bacterial cells, fluorescence microscopy experiments were performed. Bacterial cultures at exponential phase (OD600 = 0.5) were used for the assays. Prior to imaging, samples were immobilized on microscope slides covered with a thin layer of 1% agarose in PreC medium56.
Wide-field fluorescence microscopy was performed using Zeiss Axioplan 2 microscope equipped with a Plan-Neofluar objective (100×/1.3 oil Ph3) and a Zeiss Axiocam 503 mono camera (Zeiss, Germany) with 1 s exposure for SYBR Gold and 2 s exposure for mCherry. After acquisition, conventional fluorescence microscopy images were processed using ImageJ software57.
Super-resolution structured illumination microscopy (SIM) was performed in an Elyra PS.1 microscope (Zeiss) and visualized using 561-nm laser with 50 ms exposure for Nile Red and 488-nm laser with 50 ms exposure for SYBR Gold or 561-nm laser with 100 ms exposure for mCherry and 488-nm laser with 100 ms exposure for Van FL. Images were acquired using five grid rotations, followed by reconstruction and processing with ZEN software (black edition, version 14.0.0.201).
To visualize phage adsorption to the host strain, phage lysate was mixed 1000:1 with a 10-fold diluted SYBR Gold stock solution (Invitrogen) and incubated overnight in the dark at 4 °C18,58. For the wide-field fluorescence microscopy, bacterial cultures were mixed with SYBR Gold DNA-labelled phages at a multiplicity of infection approx. 10 (MOI, ratio of PFU to CFU). For SIM, bacterial cells were labelled with Nile Red (Invitrogen) at a final concentration of 2 µg/ml, for 5 min at room temperature with agitation in the dark, washed twice with LM17-CaMg broth, and mixed with SYBR Gold-labelled phages as specified above.
To visualize binding of the phage RBP to the host strain, the mCherry protein and the mCherry-tagged RBP of phage CHPC951 were used. For the wide-field fluorescence microscopy, the bacterial culture was centrifuged at 9,000 × g for 3 min. Cells were resuspended with mCherry and mCherry-tagged RBP at the final concentration 0.4 mg/ml, incubated for 5 min at room temperature and washed once with LM17-CaMg broth. For SIM, bacterial cultures were labelled with Van FL solution (1:1 mixture of vancomycin (Sigma, USA) and the fluorescent BODIPY FL conjugate of vancomycin (Molecular Probes, USA)) at a final concentration of 1 μg/ml, for 5 min at 37 °C with agitation59. Van FL-labelled bacteria were mixed mCherry and mCherry-tagged RBP as specified above.
Accession numbers
Of the phage genomes sequenced in this study: MH937457 to MH937511. Accession numbers of the sequences of eps and rgp gene clusters compared in this study: MK483529 to MK483592.
Supplementary information
Acknowledgements
The authors would like to thank: Mimi Birkelund, Ditte E. Christiansen, Gonçalo Covas, and Denise Coutinho for their help in collecting data; Ana Rute Neves for valuable discussions; Witold Kot and Lars H. Hansen for sequencing of phage genomes at Aarhus University (Roskilde, Denmark). This work is part of an Industrial PhD Project funded by Innovation Fund Denmark and Chr. Hansen A/S, grant no. 4135-00104B.
Author Contributions
P.S. designed and performed the experiments and drafted the manuscript. M.H.R. designed and performed the bioinformatics analyses. J.M.M. and M.G.P. provided SIM analyses. T.J., A.A.Z., S.R.F., F.K.V. participated in the design of the experiments/bioinformatics analyses and the evaluation and interpretation of the results. All authors have reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paula Szymczak and Martin Holm Rau contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44481-z.
References
- 1.Mahony J, van Sinderen D. Novel strategies to prevent or exploit phages in fermentations, insights from phage-host interactions. Curr. Opin. Biotechnol. 2015;32:8–13. doi: 10.1016/j.copbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Hols P, et al. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005;29:435–463. doi: 10.1016/j.femsre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Zinno P, Janzen T, Bennedsen M, Ercolini D, Mauriello G. Characterization of Streptococcus thermophilus lytic bacteriophages from mozzarella cheese plants. Int. J. Food Microbiol. 2010;138:137–144. doi: 10.1016/j.ijfoodmicro.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Reinheimer JA, et al. Natural milk cultures for the production of Argentinian cheeses. J Food Prot. 1997;60:59–63. doi: 10.4315/0362-028X-60.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Mahony J, Bottacini F, van Sinderen D, Fitzgerald GF. Progress in lactic acid bacterial phage research. Microb. Cell Fact. 2014;13(Suppl 1):S1. doi: 10.1186/1475-2859-13-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Rio B, et al. Multiplex PCR for the detection and identification of dairy bacteriophages in milk. Food Microbiol. 2007;24:75–81. doi: 10.1016/j.fm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Dupont K, Vogensen FK, Josephsen J. Detection of lactococcal 936-species bacteriophages in whey by magnetic capture hybridization PCR targeting a variable region of receptor-binding protein genes. J. Appl. Microbiol. 2005;98:1001–1009. doi: 10.1111/j.1365-2672.2005.02548.x. [DOI] [PubMed] [Google Scholar]
- 8.Binetti AG, Del Río B, Martin MC, Álvarez MA. Detection and characterization of Streptococcus thermophilus bacteriophages by use of the antireceptor gene sequence. Appl. Environ. Microbiol. 2005;71:6096–6103. doi: 10.1128/AEM.71.10.6096-6103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonnell B, et al. Global survey and genome exploration of bacteriophages infecting the lactic acid bacterium Streptococcus thermophilus. Front. Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhammed, M. K. et al. Metagenomic analysis of dairy bacteriophages: Extraction method and pilot study on whey samples derived from using undefined and defined mesophilic starter cultures. Appl. Environ. Microbiol. AEM.00888–17, 10.1128/AEM.00888-17 (2017). [DOI] [PMC free article] [PubMed]
- 11.Shapiro JW, Putonti C. Gene co-occurrence networks reflect bacteriphage ecology and evolution. MBio. 2018;9:1–14. doi: 10.3391/mbi.2018.9.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahony J, et al. Investigation of the relationship between lactococcal host cell wall polysaccharide genotype and 936 phage receptor binding protein phylogeny. Appl. Environ. Microbiol. 2013;79:4385–92. doi: 10.1128/AEM.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy J, et al. Comparative genomics and functional analysis of the 936 group of lactococcal Siphoviridae phages. Sci. Rep. 2016;6:21345. doi: 10.1038/srep21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley E, et al. Sequence analysis and characterization of phiO1205, a temperate bacteriophage infecting Streptococcus thermophilus CNRZ1205. Microbiology. 1997;143:3417–3429. doi: 10.1099/00221287-143-11-3417. [DOI] [PubMed] [Google Scholar]
- 15.Desiere F, Lucchini S, Brüssow H. Evolution of Streptococcus thermophilus bacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology. 1998;241:345–56. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 16.Neve H, Freudenberg W, Diestel-Feddersen F, Ehlert R, Heller KJ. Biology of the temperate Streptococcus thermophilus bacteriophage TP-J34 and physical characterization of the phage genome. Virology. 2003;315:184–194. doi: 10.1016/S0042-6822(03)00516-6. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell B, et al. Identification and analysis of a novel group of bacteriophages infecting the lactic acid bacterium Streptococcus thermophilus. Appl. Environ. Microbiol. 2016;82:AEM.00835–16. doi: 10.1128/AEM.00835-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szymczak P, et al. Novel variants of Streptococcus thermophilus bacteriophages are indicative of genetic recombination among phages from different bacterial species. Appl. Environ. Microbiol. 2017;83:1–16. doi: 10.1128/AEM.02748-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achigar R, Magadán AH, Tremblay DM, Julia Pianzzola M, Moineau S. Phage-host interactions in Streptococcus thermophilus: Genome analysis of phages isolated in Uruguay and ectopic spacer acquisition in CRISPR array. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep43438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes AP, et al. An anti-CRISPR from a virulent streptococcal phage inhibits Streptococcus pyogenes Cas9. Nat. Microbiol. 2017;2:1374–1380. doi: 10.1038/s41564-017-0004-7. [DOI] [PubMed] [Google Scholar]
- 21.Hynes AP, et al. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05092-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte VS, et al. Draft genome sequences of three virulent Streptococcus thermophilus bacteriophages isolated from the dairy environment in the Veneto region of Italy. Genome Announc. 2018;6:1–2. doi: 10.1128/genomeA.00045-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavelle K, et al. A decade of Streptococcus thermophilus phage evolution in an Irish dairy plant. Appl. Environ. Microbiol. 2018;84:1–17. doi: 10.1128/AEM.02855-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremblay DM, Moineau S. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology. 1999;255:63–76. doi: 10.1006/viro.1998.9525. [DOI] [PubMed] [Google Scholar]
- 25.Lucchini S, Desiere F, Brüssow H. The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: Whole genome comparison of cos-site phages Sfi19 and Sfi21. Virology. 1999;260:232–243. doi: 10.1006/viro.1999.9814. [DOI] [PubMed] [Google Scholar]
- 26.Stanley E, Walsh L, van der Zwet A, Fitzgerald GF, van Sinderen D. Identification of four loci isolated from two Streptococcus thermophilus phage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol. Lett. 2000;182:271–277. doi: 10.1111/j.1574-6968.2000.tb08907.x. [DOI] [PubMed] [Google Scholar]
- 27.Lévesque C, et al. Genomic organization and molecular analysis of virulent bacteriophage 2972 infecting an exopolysaccharide-producing Streptococcus thermophilus Strain. Appl. Environ. Microbiol. 2005;71:4057–4068. doi: 10.1128/AEM.71.7.4057-4068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deveau H, et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guglielmotti DM, et al. Genome analysis of two virulent Streptococcus thermophilus phages isolated in Argentina. Int. J. Food Microbiol. 2009;136:101–109. doi: 10.1016/j.ijfoodmicro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Mills S, et al. A new phage on the ‘Mozzarella’ block: Bacteriophage 5093 shares a low level of homology with other Streptococcus thermophilus phages. Int. Dairy J. 2011;21:963–969. doi: 10.1016/j.idairyj.2011.06.003. [DOI] [Google Scholar]
- 31.Ali Y, et al. Temperate Streptococcus thermophilus phages expressing superinfection exclusion proteins of the Ltp type. Front. Microbiol. 2014;5:1–23. doi: 10.3389/fmicb.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veesler D, Cambillau C. A Common evolutionary origin for tailed-bacteriophage functional modules and bacterial machineries. Microbiol. Mol. Biol. Rev. 2011;75:423–433. doi: 10.1128/MMBR.00014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Marrec C, et al. Two groups of bacteriophages infecting Streptococcus thermophilus can be distinguished on the basis of mode of packaging and genetic determinants for major structural proteins. Appl. Environ. Microbiol. 1997;63:3246–53. doi: 10.1128/aem.63.8.3246-3253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins B, et al. Structure and functional analysis of the host recognition device of lactococcal phage Tuc2009. J. Virol. 2013;87:8429–8440. doi: 10.1128/JVI.00907-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duplessis M, Moineau S. Identification of a genetic determinant responsible for host specificity in Streptococcus thermophilus bacteriophages. Mol. Microbiol. 2001;41:325–336. doi: 10.1046/j.1365-2958.2001.02521.x. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay, D. M. et al. Receptor-binding protein of Lactococcus lactis phages: Identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 7 (2006). [DOI] [PMC free article] [PubMed]
- 37.Quiberoni A, Stiefel JI, Reinheimer JA. Characterization of phage receptors in Streptococcus thermophilus using purified cell walls obtained by a simple protocol. J. Appl. Microbiol. 2000;89:1059–1065. doi: 10.1046/j.1365-2672.2000.01214.x. [DOI] [PubMed] [Google Scholar]
- 38.Binetti AG, Quiberoni A, Reinheimer JA. Phage adsorption to Streptococcus thermophilus. Influence of environmental factors and characterization of cell-receptors. Food Res. Int. 2002;35:73–83. doi: 10.1016/S0963-9969(01)00121-1. [DOI] [Google Scholar]
- 39.Szymczak, P. et al. Cell wall glycans mediate recognition of the dairy bacterium Streptococcus themophilus by bacteriophages. Appl. Environ. Microbiol. 10.1128/AEM.01847-18 (2018). [DOI] [PMC free article] [PubMed]
- 40.Duplessis M, Lévesque CM, Moineau S. Characterization of Streptococcus thermophilus host range phage mutants. Appl. Environ. Microbiol. 2006;72:3036–3041. doi: 10.1128/AEM.72.4.3036-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro-Nallar E, et al. Population genomics and phylogeography of an australian dairy factory derived lytic bacteriophage. Genome Biol. Evol. 2012;4:382–393. doi: 10.1093/gbe/evs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brettin T, et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015;5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahony J, van Sinderen D. Current taxonomy of phages infecting lactic acid bacteria. Front. Microbiol. 2014;5:7. doi: 10.3389/fmicb.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavelle, K. et al. A decade of Streptococcus thermophilus phage evolution in an Irish dairy plant. Appl. Environ. Microbiol. AEM. 02855–17, 10.1128/AEM.02855-17 (2018). [DOI] [PMC free article] [PubMed]
- 45.Delorme C, et al. Study of Streptococcus thermophilus population on a world-wide and historical collection by a new MLST scheme. Int. J. Food Microbiol. 2017;242:70–81. doi: 10.1016/j.ijfoodmicro.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau GM, Moineau S. Evolution of Lactococcus lactis phages within a cheese factory. Appl. Environ. Microbiol. 2009;75:5336–44. doi: 10.1128/AEM.00761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner N, et al. Whey powders are a rich source and excellent storage matrix for dairy bacteriophages. Int. J. Food Microbiol. 2017;241:308–317. doi: 10.1016/j.ijfoodmicro.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 48.McDonnell, B., Mahony, J., Hanemaaijer, L., Kouwen, T. R. H. M. & van Sinderen, D. Generation of bacteriophage-insensitive mutants of Streptococcus thermophilus via an antisense RNA CRISPR-Cas silencing approach. Appl. Environ. Microbiol. 1–14, 10.1128/AEM.01733-17 (2018). [DOI] [PMC free article] [PubMed]
- 49.Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8:317–27. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 50.Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009;501:287–292. doi: 10.1007/978-1-60327-164-6_24. [DOI] [PubMed] [Google Scholar]
- 51.Lechner M, et al. Proteinortho: Detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Löytynoja A, Goldman N. From The Cover: An algorithm for progressive multiple alignment of sequences with insertions. Proc. Natl. Acad. Sci. 2005;102:10557–10562. doi: 10.1073/pnas.0409137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Overbeek R, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42:206–214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atilano, M. L., Yates, J., Glittenberg, M., Filipe, S. R. & Ligoxygakis, P. Wall teichoic acids of Staphylococcus aureus limit recognition by the Drosophila peptidoglycan recognition protein-SA to promote pathogenicity. PLoS Pathog. 7 (2011). [DOI] [PMC free article] [PubMed]
- 56.Henriques, M. X., Catalão, M. J., Figueiredo, J., Gomes, J. P. & Filipe, S. R. Construction of improved tools for protein localization studies in Streptococcus pneumoniae. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 57.Abràmoff, M. D., Hospitals, I., Magalhães, P. J. & Abràmoff, M. Image processing with ImageJ. Biophotonics Int (2004).
- 58.Dupont K, Vogensen FK, Neve H, Bresciani J, Josephsen J. Identification of the receptor-binding protein in 936-species lactococcal bacteriophages. Appl. Environ. Microbiol. 2004;70:5818–5824. doi: 10.1128/AEM.70.10.5818-5824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereira PM, Filipe SR, Tomasz A, Pinho MG. Fluorescence ratio imaging microscopy shows decreased access of vancomycin to cell wall synthetic sites in vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2007;51:3627–3633. doi: 10.1128/AAC.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.