Abstract
The use of poison by farmers to control livestock predators is a major threat to vulture populations across Eurasia and Africa. While there is now some understanding of poison use on freehold farmland regions in southern Africa, the prevalence and drivers of this practice are still unknown in communal farmlands. We surveyed 353 communal farmers in Namibia to assess the prevalence of reported poison use and intended poison use and the factors associated with both. We used the Randomised Response Technique, a method deemed to yield more robust estimates of the prevalence of sensitive behaviours compared to direct questioning. We found 1.7% of communal farmers admitted to using poison in the last year. Furthermore, across the study region, predicted poison use was the highest (up to 7%) in areas of the upper north-west. The identified ‘hotspots’ of poison use will assist conservation practitioners to focus their poison-mitigation efforts centred in the areas of the highest need.
Electronic supplementary material
The online version of this article (10.1007/s13280-018-1128-6) contains supplementary material, which is available to authorized users.
Keywords: African vulture crisis, Communal, Conservancy, Farmer, Human–wildlife conflict, Poison use
Introduction
The current biodiversity crisis has recently escalated to a level that scientists now define it as the sixth global mass extinction (Ceballos et al. 2015). As humans are the primary drivers of most threats to the environment, humans will play a key role in trying to tackle these threats and halt biodiversity loss. This mission is challenging and will require a range of approaches and techniques to understand human behaviours, and to trigger behavioural change towards more environmentally sustainable standards.
The majority (73%) of vulture species are under imminent threat of extinction (McClure et al. 2018). Vultures comprise an exclusive functional guild, fundamental for maintaining the balance of the ecosystems they live in (Markandya et al. 2008; Morales-Reyes et al. 2017). Vultures provide important ecosystem services, such as sanitation (i.e. prevention of disease spread), cultural and tourism value (Buechley and Şekercioğlu 2016). These functions are rapidly being lost as vulture populations decline, particularly in the Old World (McClure et al. 2018).
African vulture declines are caused by a multitude of threats, including collisions with, and electrocution by, energy infrastructure, habitat degradation, direct persecution and poisoning (Ogada et al. 2016). Among these, poisoning is considered the primary threat, accounting for 61% of African vulture deaths overall (Ogada et al. 2016).
Namibia was identified as one of Africa’s poisoning hotspots (Santangeli et al. 2016). As the country supports large populations of endangered vultures, there is a need to understand the extent and distribution of important threats, such as poisoning, across the whole territory. A comprehensive survey in 2015 (Santangeli et al. 2016) estimated that on average 20% of Namibia’s freehold farmers use poison to control predators, with hotspots of poisoning reaching 50% prevalence in the southern small stock farming areas. Historically, poisoning was an accepted and widespread method to control predators in Namibia, with strychnine being prescribed by veterinarians (Komen and Brown, pers. comm.). While this became illegal in 2001, using poisons, including pesticides, to kill predators is still relatively common, particularly across the freehold land (Santangeli et al. 2016, 2017). However, to date, little is known about this practice in the communal farmlands of Namibia, as well as in the rest of Africa.
The communal and the freehold farms differ fundamentally in terms of land ownership and land management. It is thus relevant to investigate potential differences in poison use between communal and freehold land. The current land tenure structure in Namibia has been shaped through a long history of colonialism. Much of the central and southern parts of Namibia are divided into freehold farms i.e. fenced land owned by an individual and typically farmed on a commercial scale (Santangeli et al. 2016). In contrast, the north-western, north-central and north-eastern parts of the country are owned by the state and are communally used by local people.
In the mid-1990s, Namibia set up a community based natural resource management scheme to extend land management rights to the people who live on communal land and also to conserve dwindling wildlife populations. 20 years later, large portions of the communal land in Namibia are now under the jurisdiction of conservancies. Many of these conservancies have active ecotourism and hunting trades which support the locals (Naidoo et al. 2016). Namibia’s communal conservancies are typically reported as one of the most successful conservation examples in Africa and worldwide (Jones 2010). While they allowed wildlife populations to recover, growing conflicts have surfaced as wildlife numbers, particularly predators and elephants, increased (Naidoo et al. 2011). Therefore, there could be a risk of escalating poison use, a risk that is unquantified in communal farms.
Here we aim to quantify the prevalence and drivers of poison use by communal farmers in Namibia. We first quantify the prevalence of poison use and intention to use poison by communal farmers. We then identify key socio-ecological factors underlying reported poison use and intention to use poison. We then use these factors to predict where reported poison use and intention to use poison are most prevalent. Next, we compare the current findings with those obtained by Santangeli et al. (2016) in the freehold farmlands of Namibia. This comparison will yield unique insights on how land tenure, culture and farming systems impact land management decisions, mainly the decision to use poison to control predators.
Materials and Methods
We conducted surveys with communal farmers during September–November 2016 across central and northern Namibia. Our sampling unit was the household, being represented by one or all members that contributed to the survey. Overall 367 households were surveyed, 14 of which were excluded due to missing or poor-quality data, resulting in 353 households. The majority (n = 255) were surveyed on their farms. Farmers were selected using a systematic approach; a route was chosen and approximately every 10 kilometres we would stop to survey a farmer (Kelley et al. 2003). The remaining surveys (n = 98) took place at agricultural shops, using a convenience sampling technique (Kelley et al. 2003; Santangeli et al. 2016). The latter approach may be affected by non-random sampling biases, but was most logistically efficient for gathering more data. We believe this benefit outweighs possible costs. This approach was also used by Santangeli et al. (2016) who found no indication of any possible biases related to the place of interview (farm vs. shop). Sampling effort was the highest (65% of households surveyed) in the north-west, and lower in eastern (23% of households) and north-central regions (12% of households).
Surveys were conducted in Afrikaans, Damara, Otjiherero, Oshiwambo and English. All surveys were facilitated by the same local field assistant who used the respondents’ preferred language. Sampling in the central north, where people are mainly Oshiwambo speaking, was facilitated by a second, Oshiwambo speaking field assistant. For the most part (95% of surveys), only the local translator and the principal author were present during the survey. All communication during surveys was between the local translator and the farmer, with the principal author being present only for supervision. It is well established that surveys facilitated by a local person and in the preferred language of the respondent/s are important for establishing trust between the two parties (Babbie et al. 2014). This approach improved our chance of eliciting truthful responses from farmers when asking sensitive questions. The local translator would introduce us to the household, reiterating that we were researchers from outside of Namibia. In this way, the households could be assured that we were not from the government and we had no grounds to report them if they admitted to using poison. Each household that participated gave verbal consent, which was translated back to us and we signed consent on their behalf. We guaranteed confidentiality to every household. This study protocol was approved by the University of Cape Town ethics committee (Approval code: FSREC 044–2016). Locations of homesteads are kept confidential to protect farmers’ identity, but an indication of sampling coverage and household density can be found at Fig. 1 in Craig et al. (2018).
Survey design and method
Our survey included a set of 36 structured questions, with a mixture of close- and open-ended questions (Appendix S1). We based the survey upon the Santangeli et al. (2016) survey of freehold farmers to allow comparison between the studies. Our survey was adapted to the communal farming context and simplified to allow ease of translation when surveying households with limited literacy and numeracy. The survey included questions on demographics of the household, livestock ownership and farming challenges, primarily losses to predators. Farmers’ attitudes towards game, vultures, predators and lethal predator control were assessed using statements with a five-point Likert scale (Appendix S1). The attitude statements specifically assessed farmers’ attitudes to the wildlife, vultures and predators living alongside farmers, within the communal farmlands. Lastly, we assessed the sensitive topics of lethal predator control and poison use. The questions were strategically placed at the end of the survey to allow time for the household to become comfortable with us. The survey was co-designed by the authors along with researchers and staff at the University of Cape Town and various organisations in Namibia. The first 14 surveys served as a pilot, after which we reviewed the survey and found that no major changes were necessary.
Sensitive questions
We asked four sensitive questions using the Randomised Response Technique (RRT), with the forced response design (Blair et al. 2015). Two of those questions represent the focus of this study: whether households had used poison to kill predators in the last year and whether they would use poison to kill predators if they lost livestock to predators (Appendix S1). The Randomised Response Technique is a method designed to allow the respondent to answer sensitive questions with a certain degree of anonymity (see below), thereby allowing more freedom to answer honestly compared to direct questioning (Nuno and St John 2015). The use of poison to kill predators is illegal under all circumstances in Namibia, therefore these questions are considered sensitive and farmers would be reluctant to answer. However, in practice, this may not always be the case. For example, during the poison-use survey of commercial freehold farmers in Namibia, Santangeli et al. (2016) noted that farmers were extremely open to admit using poison even though this practice is illegal. With the RRT, a randomising device is used to dictate how the respondent answers a sensitive question. The randomising device introduces a level of chance into the response; in this study, we used a set of coloured balls as a randomiser. One option (white ball) requires the respondent to answer honestly to a yes/no question, a second option (yellow ball) forces to answer yes, irrespective of what the truth is, and a third option (blue ball) forces to answer no. We chose a ratio of 8:1:1 for these options, respectively. The technique gives protection as only the respondent will know what colour ball they picked. The prevalence of the behaviour can then be estimated based on the probability that 10% of the answers were a forced ‘no’ and 10% were a forced ‘yes’ (Blair et al. 2015).
The RRT with forced response design was used by Santangeli et al. (2016) to quantify poison use by freehold farmers. We used the same technique to allow comparison. Furthermore, the RRT has been widely used in the conservation context and has been shown to be effective with people with limited literacy levels (Nuno and St John 2015).
Overall, of the 353 surveys included in the analysis (see “Results”), 61% of the respondents used the technique, but the remaining respondents did not because they preferred to answer directly. We experienced some challenges with the uptake of the RRT, hence, we would give the respondent the opportunity at the end of the survey to tell us the colours of the balls that they drew, if they wished. This allowed us to verify whether the respondent had correctly followed the ‘rules’ of the technique (72% of respondents who used the technique had followed the rules). To maintain consistency, when someone had not used the RRT (39%) or had not followed the rules (17%), we would re-run the randomiser and correct the answer accordingly based on the true answer given by the respondent.
We also asked farmers about poison use in their province. This was done to gain additional information as farmers may be more comfortable discussing their peers’ behaviour than their own. We used province as the focal range for this question because it is a broad enough area that avoids farmers having to report on their immediate neighbours, and thus would be more at ease to reply honestly.
After the first 90 surveys, we realised that reported poison use was lower in the communal areas (see “Results” below) than on freehold farms (Santangeli et al. 2016). Thus, we asked farmers why poison use may be lower in communal than in freehold farmland. This question was asked at the end of each survey so that it did not affect the answers to any of the other questions.
Statistical analysis
We first estimated the prevalence of poison use and intention to use poison from the whole sample (from the responses to the RRT questions; see above) following the equation detailed in Nuno and St John (2015). We then split the sample and looked at the prevalence of poison use among those who used the RRT (using the appropriate formula) and those who did not (direct questioning, thus using simple proportions).
To model poison use, we used poison use (measured by the RRT, question 33 in Appendix S1) as the response variable (1 = household used poison, 0 = household did not use poison). Among all available variables resulting from the questions (see S1), we a priori chose 15 socio-ecological variables-deemed relevant predictors of poison use and with no missing data. We used a variance inflation factor analysis to check for collinearity amongst the chosen variables, with a cut-off generalised variance inflation factor (GVIF) < 2. Two variables were excluded; “ethnicity”, as it was strongly correlated with the spatial variables (as a result of the spatial separation of ethnic groups in Namibia), and ‘number of small livestock lost’ as it was strongly correlated with ‘% livestock lost’. Details, and rationale for inclusion, of the set of 13 uncorrelated variables can be found in Table 1.
Table 1.
The set of uncorrelated predictors used to explain the use of poison, and intention to use poison, by communal farmers in Namibia
| Variable | Type of variable | Rationale for inclusion |
|---|---|---|
| Number of large livestock owned | Continuous, no. cattle, horses, donkeys | Expect those owning more large livestock to experience conflict with predators less often and be less inclined to use poison Santangeli et al. (2016), Schumann (2009) |
| Number of small livestock owned | Continuous, no. goats, sheep, chickens | Expect those owning small livestock to experience more conflict with predators and be more inclined to use poison Santangeli et al. (2016), Schumann (2009) |
| Latitude & longitude of homestead | Continuous, decimal degrees | Account for spatial trends in poison use |
| Attitude to lethal predator control |
Continuous, Likert scale − 2 (against killing predators) + 2 (favour killing predators) |
Expect households in favour of lethal predator control to be more inclined to use poison |
| Number of large livestock lost | Continuous | Large livestock are valuable so expect those losing high numbers of large livestock to be more inclined to use poison |
| Conservancy membership | Categorical (member of conservancy/not member of conservancy) | Expect members of the conservancy to be less inclined to use poison as they are committed to the conservation principles of the conservancy |
| % livestock lost | Continuous | Expect those losing a higher % of livestock to predators to be more inclined to use poison Santangeli et al. (2016) |
| Distance to protected area (km) | Continuous | Expect farmers living closer to protected areas to experience greater conflict with wildlife Newmark et al. (1994), Gillingham and Lee (2003), Brown (2011) and Karanth et al. (2012) |
| Size of household | Continuous | Expect large households to be less tolerant to losses as resources need to be spread among more people |
| Attitude to: vultures/wildlife/predators |
Continuous, Likert scale − 2 (negative attitude) + 2 (positive attitude) |
Expect those with positive attitudes to be less inclined to use poison as these attitudes indicate that they value biodiversity |
The relationship between poison use and the 13 predictors was analysed using Generalized Linear Modelling (GLM). The error structure associated with the model was assumed to be binomial with a link function appropriate for randomised responses. This consists of a modified logit link function that incorporates known probabilities of the forced RRT responses. We ran all model combinations using the 13 predictors. The models were ranked using the Bayesian Information Criterion (BIC). Prevalence of poison use was low, and an initial analysis led to many non-converging models (over 70%). This problem was solved by a sampling without replacement approach, computing all model combinations on 50 resampled datasets. In each resampled dataset, the numbers of zeroes and ones in the response variable were kept equal. Each resampled dataset contained a total of 74 observations. This resulted in a total of 144 495 models reaching convergence across the 50 datasets. Model averaging was performed on each resampled dataset, computed as the average of all the regression coefficients weighted by their BIC weights. A coefficient for each variable was then obtained by taking the mean of the coefficients from the 50 averaged models. For each dataset, a measure of relative importance was calculated using the ratio of absolute values of the t statistics for unstandardised predictors. The model-averaged predicted values from the 95% confidence set were used to map the probability of poison use across the communal regions surveyed. An interpolated map of these fitted probabilities was then created using the inverse distance squared weighting interpolation (IDW) method. In doing so, we first extracted the model-averaged predictions from the 95% confidence set relating poison use as reported by farmers using the Randomised Response Technique to 13 socio-ecological variables. We then used the above-mentioned model-averaged predictions to interpolate the poison prevalence predicted at the survey points across the whole study region using the IDW technique (Neteler and Mitasova 2013).
Next, we used the same set of 13 predictors as above to assess their relationship with intention to use poison (measured by the RRT, question 34 in Appendix S1). In this case, the prevalence of intention to poison was high enough (see “Results”) to allow direct multi-model inference and averaging in the same way as done by Santangeli et al. (2016). Fitted probabilities from this model were then used to map intention of using poison using the same spatial statistics approach as above and as done by Santangeli et al. (2016).
All models were fitted using R 3.3.2 (R Core Team 2016). To fit the poison-use models, the RReg package was used (Heck and Moshagen 2016). Inverse distance squared weighting interpolation was performed using the v.surf.idw GRASS GIS module (Neteler and Mitasova 2013). All maps were created using QGIS (2.18.3) software.
Results
Socio-ecological characteristics of respondents
The majority of respondents were men (78%, n = 353), with mean age 42 (range 18–86). Most respondents identified as Himba (30%), Herero (26.3%), Damara (14.1%) or Ovambo (13.9%). Herero and Himba households owned on average twice as many large livestock (50, n = 199), namely cattle, than Damara (28, n = 50) and Ovambo households (28, n = 48). Most households were registered with a conservancy (59%), with an additional 29% of households living within a conservancy but not yet members. Drought was the main reported cause of livestock loss for 79% of households, followed by predators (11%), disease (4.8%), poisonous plants (3.4%) and theft (1.4%). Moreover, 80% of households had lost livestock to predators in the last year. A total of 47% of households had lost more than a tenth of their livestock. Just six per cent of households who had lost livestock in the last year (17 out of 281) reported that they received compensation from the conservancy for their loss.
Households in the north-west lost the greatest percentage of livestock to predators, compared to the other regions. Most (90%) households had positive attitudes towards game, and 63% held positive attitudes towards vultures (Appendix S1: Q13 a & b, 21, 22; for more information on attitudes to vultures see Craig et al. 2018). Conversely, 73% of households had negative attitudes towards predators and 82% believed that predators that kill livestock should be killed (Appendix S1: Q 13, c-e). There was no relationship between percentage livestock lost and distance to protected area (Pearson’s correlation coefficient = − 0.2, n = 353, p < 0.01). Herding, herding dogs and keeping livestock in kraals (fenced enclosures) were cited by 83% of farmers as best means to protect livestock.
The perceived behaviour of other communal farmers
When asked about how their peers controlled predators, the most commonly mentioned method was snares and traps (32% out of 293 farmers), dogs (26%), firearms (21%) and poison (10%). Reported poison use by peers was the highest in eastern (19%, n = 81) and north-western provinces (13%, n = 229), but was reported to be absent in the north-central provinces (n = 43). Most (93%) farmers did not know or were unwilling to reveal what type of poisons are used. Those who did, reported a wide range of substances, from traditional poisons made from euphorbia plants to chemicals bought from shops.
Sensitive questions on illegal poison use
Overall, the percentage of farmers using poison was 1.7% ± 2.1 (mean ± SE; n = 353). However, 36.0% ± 3.2 of farmers admitted their intention to use poison if they lost livestock to predators. Farmers using the RRT admitted using poison less often (0%) than those who did not use the RRT (6%). RRT uptake differed between ethnic groups, with Damara households using it most often (80%), followed by Herero (69%), Himba (55%) and Ovambo (42%).
The most important predictor of poison use was the number of large livestock owned (Table 2). Notably, households with many large livestock were most inclined to use poison. A similar pattern was seen with intended poison use, with those owning greater numbers of large livestock more likely to admit they would use poison. Probability of poison use also increased northwards and with an increasing number of small livestock owned, although the effect of the latter was weak (Table 2). Attitudes to wildlife, vultures and predators were poor predictors of poison use, but attitudes to lethal predator control was the most important predictor of intended poison use, i.e. a positive attitude towards lethal predator control was associated with intention to use poison (Table 2).
Table 2.
The results of two different models aimed at explaining ‘Reported Poison Use’ and ‘Intended Poison Use’ with 13 socio-ecological uncorrelated factors. Statistics show the coefficient and standard error for each predictor as it relates to the response, as well as the standard error and the relative importance of the predictor. See “Materials and Methods” and Table 1 for more details on the quantitative approach and variable descriptions. In ‘bold’, we highlight strong predictors, namely those where the upper and lower standard errors do not include zero
| Variable | Actual poison use | Intended poison use | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | Rel imp | Coefficient | SE | Rel imp | |
| (Intercept) | − 4.89 | 3.63 | − 1.41 | 0.44 | ||
| Number of large livestock owned | 1.20 | 1.00 | 0.27 | 0.35 | 0.17 | 0.34 |
| Latitude of homestead | 1.19 | 1.03 | 0.21 | − 0.03 | 0.17 | 0.06 |
| No. small livestock owned | 1.09 | 1.63 | 0.18 | 0.09 | 0.16 | 0.06 |
| Attitude to lethal predator control | 0.90 | 1.28 | 0.14 | 0.54 | 0.19 | 0.99 |
| Longitude of homestead | − 0.24 | 1.98 | 0.12 | 0.22 | 0.17 | 0.12 |
| No. large livestock lost | 0.80 | 0.91 | 0.12 | 0.11 | 0.18 | 0.08 |
| Conservancy membership (non member) | − 1.78 | 2.74 | 0.11 | − 0.20 | 0.32 | 0.06 |
| % Livestock lost | − 1.05 | 1.44 | 0.10 | 0.30 | 0.19 | 0.20 |
| Dist. to protected area (km) | − 0.48 | 0.96 | 0.10 | − 0.04 | 0.16 | 0.05 |
| Size of household | − 0.65 | 1.05 | 0.09 | 0.26 | 0.18 | 0.14 |
| Attitude to vultures | − 0.21 | 0.87 | 0.08 | − 0.04 | 0.11 | 0.05 |
| Attitude to game | 0.33 | 1.62 | 0.06 | 0.05 | 0.25 | 0.05 |
| Attitude to predators | 0.14 | 0.49 | 0.03 | 0.05 | 0.12 | 0.06 |
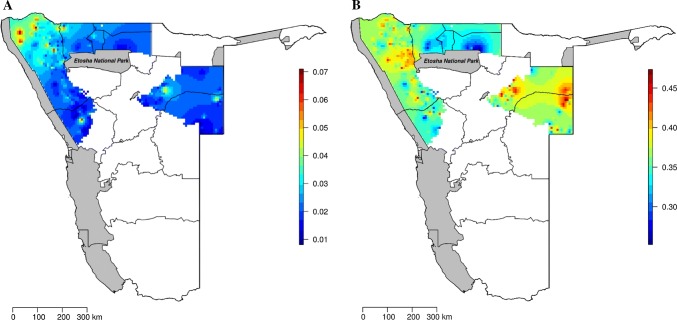
The interpolated map derived from the model predictors further demonstrated that the prevalence of poison use, and intention to use poison are relatively similar in their overall pattern (Fig. 1a, b). Using poison and intending to use poison were not uniform across the communal farmlands. Probability of poison use is the highest in parts of the north-west (up to 7% farmers predicted to be using poison), but very scarce elsewhere, with the exception of few localised areas (Fig. 1a). Similarly, intention to use poison is the highest not only in the north-western areas, but also in the central-east regions (up to 45%; Fig. 1b).
Fig. 1.
The predicted prevalence (on a scale from zero, behaviour is absent, to 1, behaviour present across all farmers) of reported poison use (a) and intention to use poison (b) in the communal farmlands of Namibia. These interpolated maps were derived from the model-averaged predictions based on the 95% confidence set of models on actual and intention to use poison (see “Materials and Methods” and Table 2). Protected areas are highlighted in grey, whereas administrative regions of Namibia are delimited by dark grey continuous lines. Areas in white represent freehold or other areas outside of the study scope
Discussion
In comparison to Namibia’s freehold farmers, poison use in the communal farmland appears to be scarce (Fig. 2). Nevertheless, our spatial model shows certain poison-use hotspot areas in the north-west and central-eastern parts of the country. These areas are largely associated with farmers owning many large livestock, namely cattle. Cattle are not only valuable in monetary terms but are central in the Herero and Himba cultures (Jacobsohn 1995). Since cattle ownership is most common in the Himba and Herero communities, this may indirectly explain higher poison use in those regions. With intention to use poison to control predators, a similar prevalence (over 30% of respondents) was recorded among the communal farmers of this study as for freehold farmers (Santangeli et al. 2016). Our findings highlight how socio-ecological factors such as culture, social norms, type of livestock owned and land tenure influence land management decisions, in this case the decision or intention to use poison to kill predators.
Fig. 2.
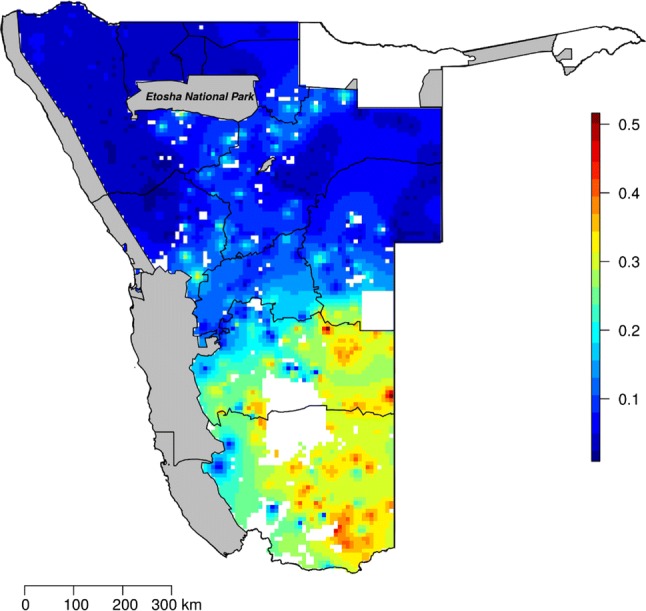
The predicted prevalence of reported poison use in the communal and freehold farmlands of Namibia, using data from (Santangeli et al. 2016). Note the difference in scale compared to Fig. 1a
Poison use in communal versus freehold farmland
While considering the uncertainty in the estimates provided here (see caveats below), the results suggest that poison use on communal farms is lower than on freehold farms in Namibia (Santangeli et al. 2016). However, it is important to note that it is difficult to give an exact figure for the prevalence of poison use, given the challenges we faced in applying the randomised response technique. Nonetheless we are confident that poison use is lower on communal farms, given the households’ reports of poison use in their provinces and discussions with farmers. This was further supported by the very rare occurrence of poisoning events recorded in the field by the conservancy guards who patrol these areas extensively year-round. The explanation communal farmers gave regarding the lower prevalence of poison use there compared to freehold farmland related to land ownership (freehold farmers own their land and can do what they want with it), difficulty in attaining poison and fear of retribution from the conservancy or government on communally used land. Moreover, as communal farmlands are largely unfenced, use of poison needs to be a community decision due to possible indirect risks to other residents, dogs or livestock. The similar prevalence in intention to use poison between freehold and communal farmers suggests that the above-mentioned factors prevent communal farmers from putting their intention to use poison into action (as their actual use of poison is lower than that of freehold farmers). This finding may also represent a potential threat. Should social and logistical limitations in obtaining and using poison drop, poison use in communal areas may reach levels currently seen in the freehold farmland, further increasing the intensity and scale of this threat to vultures.
Interestingly, while in freehold farmland the main reported cause of livestock loss was predators, in communal areas it was drought. Communal areas are often overgrazed, and consequently highly vulnerable to droughts. Under projected climate change, deteriorating conditions for livestock farming in communal areas may exacerbate human–wildlife conflicts and trigger an upsurge in poison use.
Factors related to use of poison
We found the most important predictor of poison use for communal farmers to be the number of large livestock owned, namely cattle. Our finding that wealthier (in terms of cattle wealth) individuals are more likely to use poison contrasts with previous work in Tanzania which found a positive link between number of livestock owned and tolerance to predators (Dickman 2005). It seems that particularly in the Namibian context, farmers owning more livestock are less tolerant of predators (Santangeli et al. 2016). Communal farmers with cattle wealth may use poison more frequently because they can afford to purchase it. Wealthier farmers, particularly in the central-east (Otjozondjupa province) tended to have fenced off farms much like the freehold farms allowing them to use poison without indirect risks. Wealthier individuals may also have more power and influence in the community and therefore feel more entitled to use poison.
The low importance of livestock lost in predicting poison use in communal farmland contrasts with the patterns observed in freehold farms (Santangeli et al. 2016). While freehold farmers who use poison are primarily driven by the magnitude of livestock losses (Santangeli et al. 2016), for communal farmers it seems socio-economic and cultural factors drive poison use. Research has shown that anti- and pro- conservation behaviours are strongly influenced by perceived social norms about what is, and is not, an acceptable behaviour (Cialdini et al. 2003). We found further evidence for this, in the regions of high predicted poison use, many farmers indicated that they would use poison and many reported that they knew of others using poison. As such, conservancies and community leaders have an important role to play in challenging the prevailing social norms in ‘hotspot’ areas of poison use.
The role of attitudes in behavioural intention and behaviour
Our study found intention to use poison was the highest among farmers with positive attitudes towards lethal predator control. This was similarly the case with pastoralists’ intention to kill predators in the Maasai Mara (Broekhuis et al. 2018). This is unsurprising given that attitudes and behavioural intentions are often synonymous (Ajzen and Fishbein 2005). However, research shows that attitudes and behavioural intentions fail to consistently translate into behaviour (Ajzen and Fishbein 2005; Waylen et al. 2009). This was clear in our study where we found attitudes to wildlife, including game, predators and vultures and lethal predator control to be poor predictors of poison use. However, it is important to note that we measured broad attitudes towards wildlife and towards killing predators. It is possible that these attitudes are not specific enough to be able to predict poison use. Furthermore, we found proximity to protected area to be a poor predictor of poison use and found that livestock losses near protected areas are not higher than elsewhere. This is somewhat expected given that healthy carnivore populations in Namibia occur within but also outside of protected areas, i.e. in communal conservancies (Naidoo et al. 2016).
Study limitations
Previous research suggested that the RRT can be used successfully in communities with low literacy (Nuno and St John 2015). However, we experienced difficulties with this technique, as many farmers were reluctant to use it or did not understand it. It is therefore advised that researchers test the suitability of this technique in each case, particularly where respondents have low literacy. While our poison-use model was based on few occurrences overall, yielding results associated with high uncertainty, similar results were also found with the model on intended poison use (a less-sensitive question), where occurrence of ‘yes’ answers was much higher. This makes us confident that the findings from our poison-use model conform to what happens in reality, with this being backed by logical interpretation of the findings. A further test of the data collected with the RRT technique would have been to run models separately for the two sets of data, namely those whereby the RRT was used, and those for which it was not used. Unfortunately, given the low incidence of poison use, this was not possible. However, we explored the overall prevalence of poison use among those who used the RRT versus those who did not, and found the latter admitted to using poison more often than the former. This contrasts our expectation that farmers using the technique would answer more truthfully, resulting in higher poison-use prevalence among them.
Recommendations and conclusions
To tackle poisoning, the human–wildlife conflict must be alleviated. Recent evidence suggests that lethal predator control is often ineffective (van Eeden et al. 2018), although this method, used cautiously, can be effective in ameliorating community anger after losses. But navigating the conflict and finding ways for farmers to co-exist with carnivores is the most sustainable, albeit challenging, way forward. This can be achieved by different means and should be always based on the best available evidence (van Eeden et al. 2018). Communal conservancies have an important role to play in this regard, particularly in the north-west and eastern communal areas, where risk of poison use is the highest. A first step would be improving the equality in sharing conservancy benefits and the costs among conservancy members. Our discussions with communal farmers revealed that social norms represent a major deterrent for poison use. In order to strengthen this, community leaders in high-risk areas should become involved and support education campaigns against the use of poison and the side effects this entails.
While poison use appears relatively scarce in communal areas, even a single poisoning event could devastate vulture populations. Thus, it is important to carefully address this threat, particularly in light of the widespread intention of interviewed farmers to possibly use poison in the future. Unfortunately, this issue is not unique to Namibia and in many parts of Africa poison use is widespread. When addressing this threat, it is vital to consider the social dimension underlying its emergence and spread. Ultimately, we believe that our results will be key in informing conservation practitioners and conservancy managers on the extent and drivers of poison use, and in effectively addressing this threat, starting from the hotspots of poison use identified with this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the time given by every farmer who participated in this research. We thank Ndapanda Kasaona and Ndina Hapinge for their translation services in the field. We are grateful to Liz Komen, Chris Brown, Holger Kolberg, the MET, Annatjie du Preez, NACSO, Basilia Shivute, Teo Ntinda, Andrew Malherbe, Vince and Edgar Naude, Timm Hoffmann and Jane Turpie for their advice and support while planning and implementing this research. We thank the British Ecological Society, Mohamed bin Zayed Species Conservation fund (Project Number: 142510056) and the National Research Foundation for funding the field work for this research. We also thank two anonymous reviewers for their constructive comments. Lastly, we thank the Agra stores in Opuwo, Omaruru, Khorixas and Outjo for allowing us to survey in their stores.
Biographies
Christie A. Craig
received her MSc (Conservation Biology) degree from the University of Cape Town. Her research interests include community conservation, human–wildlife conflicts, sustainability and conservation biology.
Robert L. Thomson
is a Senior Lecturer at the FitzPatrick Institute of African Ornithology. His main research falls within behavioural and community ecology but he is expanding into more applied themes in conservation biology.
Marco Girardello
is based at the Azorean Biodiversity Group, Portugal. His research interests include macroecology, biogeography and conservation biology.
Andrea Santangeli
is a postdoctoral fellow at the Finnish Museum of Natural History. His research interests include applied ecology and conservation science with particular focus on bottom-up approaches to solve conservation issues.
Contributor Information
Christie A. Craig, Email: christiea250@gmail.com
Robert L. Thomson, Email: robert.thomson@uct.ac.za
Marco Girardello, Email: marco.girardello@gmail.com.
Andrea Santangeli, Email: andrea.santangeli@helsinki.fi.
References
- Ajzen I, Fishbein M. The influence of attitudes on behavior. In: Albarracin D, Johnston B, Zanna M, editors. Handbook of attitudes and attitude change: Basic principles. New York: Psychology Press; 2005. pp. 173–221. [Google Scholar]
- Babbie E, Mouton J, Vorster P, Prozesky B. The practice of social research (South African edition) Cape Town: Oxford University Press; 2014. [Google Scholar]
- Blair G, Imai K, Zhou Y-Y. Design and analysis of the randomized response technique. Journal of the American Statistical Association. 2015;110:1304–1319. doi: 10.1080/01621459.2015.1050028. [DOI] [Google Scholar]
- Broekhuis F, Kaelo M, Sakat DK, Elliot NB. Human–wildlife coexistence: Attitudes and behavioural intentions towards predators in the Maasai Mara, Kenya. Oryx. 2018 [Google Scholar]
- Brown, C. 2011. Analysis of human-wildlife conflict in the MCA-supported conservancies for the five-year period of 2006–2010. Namibia Nature Foundation.
- Buechley ER, Şekercioğlu ÇH. The avian scavenger crisis: Looming extinctions, trophic cascades, and loss of critical ecosystem functions. Biological Conservation. 2016;198:220–228. doi: 10.1016/j.biocon.2016.04.001. [DOI] [Google Scholar]
- Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sciences Advances. 2015;1:1–5. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini S, Crafting A, Plous S, Cialdini RB. Crafting normative messages to protect the environment. Current Directions in Psychological Science. 2003;12:105–109. doi: 10.1111/1467-8721.01242. [DOI] [Google Scholar]
- Craig CA, Thomson RL, Santangeli A. Communal farmers of Namibia appreciate vultures and the ecosystem services they provide. Ostrich. 2018 [Google Scholar]
- Dickman, A. J. 2005. An assessment of pastoralist attitudes and wildlife conflict in the Rungwa-Ruaha region, Tanzania, with particular reference to large carnivores. MSc. Dissertation: University of Oxford.
- Gillingham S, Lee PC. People and protected areas: A study of local perceptions of wildlife crop-damage conflict in an area bordering the Selous Game Reserve, Tanzania. Oryx. 2003;37:316–325. doi: 10.1017/S0030605303000577. [DOI] [Google Scholar]
- Heck, D.W., and M. Moshagen. 2016. RRreg: Correlation and regression analyses for randomized response data. R package version 0.6.1.
- Jacobsohn, M. 1995. Negatiating meaning and change in space and material culture: An ethno-archaeological study among semi-nomadic himba and herero herders in north-western Namibia. PhD. Thesis: University of Cape Town.
- Jones B. The evolution of Namibia’s communal conservancies: The politics of natural resource governance in Africa. In: Nelson F, editor. Community rights, conservation & contested land. Abingdon: Earthscan; 2010. pp. 106–120. [Google Scholar]
- Karanth KK, Gopalaswamy AM, Defries R, Ballal N. Assessing patterns of human-wildlife conflicts and compensation around a central Indian protected area. PLoS One. 2012;7:1–13. doi: 10.1371/journal.pone.0050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. International Journal for Quality in Health Care. 2003;15:261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- Markandya A, Taylor T, Longo A, Murty MN, Murty S, Dhavala K. Counting the cost of vulture decline: An appraisal of the human health and other benefits of vultures in India. Ecological Economics. 2008;67:194–204. doi: 10.1016/j.ecolecon.2008.04.020. [DOI] [Google Scholar]
- McClure CJW, Westrip JRS, Johnson JA, Schulwitz SE, Virani MZ, Davies R, Symes A, Wheatley H, et al. State of the world’s raptors: Distributions, threats, and conservation recommendations. Biological Conservation Online. 2018 [Google Scholar]
- Morales-Reyes Z, Perez-Garcia JM, Moleon M, Botella F, Carrete M, Donzar JA, Ainara C-A, Eneko A, et al. Evaluation of the network of protection areas for the feeding of scavengers in Spain: From biodiversity conservation to greenhouse gas emission savings. Journal of Applied Ecology. 2017;54:1120–1129. doi: 10.1111/1365-2664.12833. [DOI] [Google Scholar]
- Naidoo R, Weaver LC, de Longcamp M, du Plessis P. Namibia’s community-based natural resource management programme: An unrecognized payments for ecosystem services scheme. Environmental Conservation. 2011;38:445–453. doi: 10.1017/S0376892911000476. [DOI] [Google Scholar]
- Naidoo R, Weaver LC, Diggle RW, Matongo G, Stuart-Hill G, Thouless C. Complementary benefits of tourism and hunting to communal conservancies in Namibia. Conservation Biology. 2016;30:628–638. doi: 10.1111/cobi.12643. [DOI] [PubMed] [Google Scholar]
- Neteler M, Mitasova H. Open source GIS: A GRASS GIS approach. New York: Springer Science & Business Media; 2013. [Google Scholar]
- Newmark WD, Manyanza DN, Gamassa DM, Sariko HI. The conflict between wildlife and local people living adjacent to protected areas in Tanzania: Human density as a predictor. Conservation Biology. 1994;8:249–255. doi: 10.1046/j.1523-1739.1994.08010249.x. [DOI] [Google Scholar]
- Nuno A, St John FAV. How to ask sensitive questions in conservation: A review of specialized questioning techniques. Biological Conservation. 2015;189:5–15. doi: 10.1016/j.biocon.2014.09.047. [DOI] [Google Scholar]
- Ogada D, Shaw P, Beyers RL, Buij R, Murn C, Thiollay JM, Beale CM, Holdo RM, et al. Another continental vulture crisis: Africa’s vultures collapsing toward extinction. Conservation Letters. 2016;9:89–97. doi: 10.1111/conl.12182. [DOI] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- Santangeli A, Arkumarev V, Rust NA, Girardello M. Understanding, quantifying and mapping the use of poison by commercial farmers in Namibia–Implications for scavengers’ conservation and ecosystem health. Biological Conservation. 2016;204:205–211. doi: 10.1016/j.biocon.2016.10.018. [DOI] [Google Scholar]
- Santangeli A, Arkumarev V, Komen L, Bridgeford P, Kolberg H. Unearthing poison use and consequent anecdotal vulture mortalities in Namibia’s commercial farmland–Implications for conservation. Ostrich. 2017;88:147–154. doi: 10.2989/00306525.2017.1321051. [DOI] [Google Scholar]
- Schumann, B. 2009. The needs of emerging commercial farmers in Namibia in relation to human-carnivore conflict. MSc Dissertation: Cape Peninsula University of Technology.
- van Eeden LM, Eklund A, Miller JRB, López-Bao JV, Chapron G, et al. Carnivore conservation needs evidence-based livestock protection. PLoS Biology. 2018;16:e2005577. doi: 10.1371/journal.pbio.2005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waylen K, McGowan PJK, Milner-Gulland EJ. Ecotourism positively affects awareness and attitudes but not conservation behaviours: A case study at Grande Riviere, Trinidad. Oryx. 2009;43:343. doi: 10.1017/S0030605309000064. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.