Abstract
Pregnancy is a unique immunological situation in which a fetus-bearing paternal histocompatibility antigens can survive in a maternal environment without apparent rejection. To face this challenge, cells of the uterine immune system show characteristic changes in absolute number and composition during pregnancy. Particularly relevant to this process are uterine natural killer (uNK) cells and their cell surface receptors, killer immunoglobulin-like receptors (KIRs). The main purpose of this review is to outline the current body of knowledge on the involvement of KIRs in the complications of pregnancy. Implantation depends on the invasion of embryonic trophoblast cells into maternal uterine tissue and remodeling of the uterine spiral arterioles, which is essential for placental perfusion and successful pregnancy. The proper interaction between maternal KIRs and their ligands human leukocyte antigen (HLA) class I molecules, expressed by the extravillous trophoblast cells, is crucial in this process. KIRs are a complex family that includes both activator and inhibitory receptors. The activation profile is genetically determined in each individual and leads to diverse levels of functionality for NK and T cells on engagement with specific HLA class I molecules. An association between different KIR alleles and HLA molecules has been reported in pregnancy complications, supporting the idea of a relevant role of these receptors in successful pregnancy.
Keywords: KIR, HLA, Recurrent miscarriage, Uterine natural killer, Extravillous trophoblast
Introduction
Pregnancy and its maintenance represents a challenge for the maternal immune system, since it must defend the mother from pathogens while simultaneously tolerating paternal alloantigens from the fetus [1]. Dysregulation of immune responses can lead to reproductive failure. During a normal pregnancy, the maternal immune system recognizes fetal antigens and an equilibrium is established between the defense of the integrity of the uterus (by preventing over-invasion of the endometrium by the trophoblast) and fetal nutrition [2]. In turn, this balance depends on the tolerance of maternal leukocytes to antigens expressed in fetal cells. Conversely, the fetus must develop tolerance to maternal antigens. The immune cell characteristics and composition of the decidua, the maternal portion of the placenta [3], and especially surface receptors on natural killer (NK) cells, one of the most important subpopulations in implantation, are intricately involved in maintaining this homeostasis between uterus integrity and fetal nutrition. Among NK cell receptors, killer immunoglobulin-like receptors (KIRs), which bind to ligands on trophoblast cells, have been associated with reproductive failure [3–5]. The main purpose of this review is to survey the current body of knowledge on the involvement of KIRs in complications of pregnancy.
Leukocyte subpopulations
Several cell types are involved during a successful pregnancy. The process of implantation requires a low degree of inflammation, with the consequent participation of inflammatory cells. Nevertheless, excessive inflammation might cause implantation failure or miscarriage [6]. The control of inflammation depends on the leukocyte populations present in the endometrium and in the mother-fetus interphase [7]. Endometrium leukocyte populations are characteristically different from those typically found in peripheral blood. Indeed, the endometrium is devoid of B cells, has a low amount of neutrophils, and is mainly composed of T cells, macrophages, dendritic cells, and uterine natural killer (uNK) cells [8]. uNK cells are distinct from peripheral NK cells in that they do not express CD16 or CD57 receptors, but instead express CD38 and KIRs, and are characterized as CD56bright. Also, uNK cells exhibit a reduced cytotoxic activity but have a high capacity to produce cytokines and angiogenic factors [9].
The absolute number and proportion of leukocyte subpopulations varies during the menstrual cycle and in early pregnancy. T lymphocytes represent ~ 45% of the total number of leukocytes in the proliferative endometrium. Although this population remains stable during the menstrual cycle or early pregnancy, the T/uNK cell ratio decreases due to an increase in the numbers of uNK cells. Similarly, macrophages represent ~ 15% of the endometrium leukocytes, but this increases to 20% during the secretory phase of the cycle and early pregnancy.
The most significant change in leukocyte composition during the menstrual cycle is in the proportion of uNK CD56bright cells. Whereas their total number is similar to that of T cells in the proliferative phase, they represent 70% of all endometrial leukocytes in the secretory phase, and this further increases during decidualization of the endometrium in early pregnancy. Moreover, the size and complexity of uNK cells change after ovulation, from being small and sparse to large with prominent cytoplasmic granules [10]. Indeed, recent studies on the architecture of human decidua have revealed the presence of different subsets of perivascular and stromal cells that are located in distinct decidual layers. Specifically, three major subpopulations of decidual NK cells with distinctive immunomodulatory and chemokine profiles have been described [11]. CD56bright cells normally undergo apoptosis some days before menstruation but are rescued by human chorionic gonadotropin (hCG) if implantation occurs. Indeed, hCG has been shown to act as a homing signal for T regulatory cells to enter the decidua, suggesting a main role for these cells in implantation [12]. Implantation begins with the migration of embryonic trophoblast cells into maternal uterine tissue and the remodeling of the uterine spiral arterioles, with destruction of the media by extravillous trophoblast (EVT) cells. These processes are essential for optimal utero-placental blood perfusion during pregnancy [13]. Remodeling of the uterine spiral arterioles depends on the interaction of the EVT and the decidual immune cells, and failure of this remodeling process is associated with preeclampsia and may be caused by defective EVT endovascular invasion. Indeed, spiral arteriole remodeling is restricted in mice deficient for uNK and T cells, resulting in a preeclampsia-like disorder [14]. Moreover, it has been reported that leukocytes migrate into the arteriolar walls before the arrival of the EVT [15]. That the participation of the EVT is required for spiral arteriole remodeling suggests paracrine regulation [16]. uNK cells play a central role in this complex and dynamic process, particularly the interaction between maternal KIRs expressed by uNK cells and their cognate ligands, human leukocyte antigen (HLA) class I molecules, expressed by the EVT [10]. The presence of fetus-specific anti-HLA antibodies and cytotoxic T lymphocytes (CTLs) has been demonstrated in maternal peripheral blood, but there is no evidence for a response against the fetus at the fetal-maternal interface [17]. A study on the relevance of HLA mismatch between mother and fetus in uncomplicated term pregnancies showed the association of the HLA-C allele mismatch with an abnormal immune response, which led to an increase in the percentage of CD4+ CD25dim-activated T cells in the decidua parietalis together with the presence of functional CD4+ CD25bright regulatory T cells in decidua [17].
The complexity of the killer immunoglobulin-like receptor family
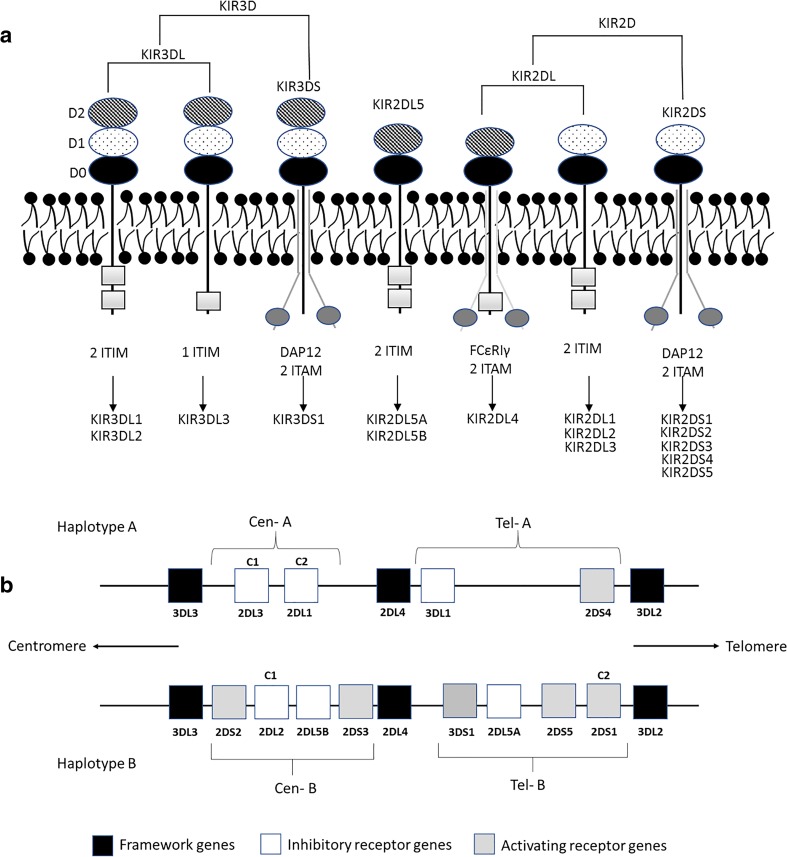
The leukocyte receptor complex (LRC) is formed by a cluster of genes encoding a family of proteins containing immunoglobulin (Ig)-like domains. These include the KIR, “leukocyte Ig-like receptor” (LILR) and “leukocyte-associated Ig-like receptor” (LAIR) families. The “signaling lectins” (SIGLECs) and members of the CD66 family are also found close to the LCR, which is located in human chromosome 19. The KIR gene cluster constitutes a multigene family that currently consists of 15 genes (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5A, KIR2DL5B, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, KIR3DL1, KIR3DL2, KIR3DL3, and KIR3DS1) and two pseudogenes (KIR2DP1 and KIR3DP1). KIR genes encode both activating (KIR2DS and KIR3DS) and inhibitory (KIR2DL and KIR3DL) receptors, with the exception of KIR2DL4, which is unique among the KIR family as its mRNA is detected in most NK populations whereas protein expression is not always evident. Depending on the circumstances, KIR2DL4 can mediate inhibitory or activating signals ([18] see later). The genomic diversity of the KIR family is achieved through differences in gene content, expression, and allelic polymorphism, leading to substantial variation in gene content across individuals and populations [19]. The nomenclature used for the KIR gene family is based on whether they have two (2D) or three (3D) Ig-like domains, and whether they possess a short (S) or a long (L) cytoplasmic tail (Fig. 1). The haplotype variability of the KIR gene family is due to their different number and nature, and KIR alleles can be categorized into two basic groups: haplotypes A and B. Haplotype A contains only one activating KIR gene (KIR2DS4), whereas haplotype B contains various combinations of activating KIR genes: KIR2DS1, -2DS2, -2DS3, -2DS4, -2DS5, and -3DS1. However, the reality is more complex. Linkage disequilibrium studies identified two separate regions in the KIR cluster around the KIR2DL4 gene: a centromeric region, which seems to be driven by the KIR2DL5 and KIR2DL2/2DL3 loci, and a telomeric region driven by the KIR3DL1/3DS1 locus (Fig. 1) [20]. These haplotypes are present in all populations and are thought to be maintained by the balancing selection pressures of infection, immunopathology, and reproduction [21].
Fig. 1.
Schematic representation of structural characteristics of KIR proteins (a) and typical KIR A and B genotypes (b). ITIM, immunoreceptor tyrosine-based inhibition motifs; DAP12, an activating adaptor protein; ITAM, immunoreceptor tyrosine-based activating motifs; FcεRIγ, an accessory protein; C1/C2, HLA-C alleles bearing C1 or C2 epitopes
It has been described that homozygosity for haplotype A is associated with a more robust response to pathogens such as hepatitis C virus or Ebola virus [22, 23]. Although more studies of susceptibility to infections and KIR haplotypes are warranted, these findings suggest that the KIR A haplotypes can provide more effective immunity against acute viral infections than the KIR B haplotypes, which have an accumulation of attenuated and less polymorphic KIRs. However, haplotype A homozygosity is also associated with preeclampsia. This reflects a compromise of haplotypes A and B between the functions that NK cells exert in immunity and reproduction. The occurrence of an epidemic disease such as Ebola in a population enriches for haplotype A, but the less efficient reproduction of these individuals selects for an increase in haplotype B, restoring the equilibrium between the haplotypes [21].
The different combinations of expressed KIR genes leads to diverse responses in NK and T cells after engagement with their specific HLA class I molecules [24]. Accordingly, KIR-mediated immune responses are uniquely shaped in each individual and are difficult to study collectively. However, in contrast to the villous trophoblast, which is HLA null (to avoid recognition by the immune system of the mother), EVT has a unique HLA profile and does not express the polymorphic T cell ligands HLA-A and HLA-B class I, or any HLA class II molecules, but does express HLA-C allotypes as its only polymorphic HLA class I molecule [25]. The lack of expression of any HLA allele would result in NK cells attacking the EVT since NK cells recognize the absence or the aberrant expression of HLA as indicative of a target cell. In addition, EVT expresses two nonclassical and nonpolymorphic HLA class I molecules: HLA-G and HLA-E. HLA-E binds CD94 (NKG2A) and its activating counterpart CD159c (NKG2C), and HLA-G binds to members of the LILR family and KIR. KIR receptors expressed on NK cells thus play an important role in the induction of NK cell responses, which can be activating or inhibiting depending on the allele presence.
KIR polymorphisms and miscarriage
HLA-G and KIR
HLA-G is a nonclassical HLA class I molecule. It differs from classical HLA class I molecules in expression, genetic diversity, structure, and function. HLA-G is expressed as 7 isoforms: 4 membrane-bound (HLA-G1, -G2, -G3, and -G4) and 3 soluble (HLA-G5, -G6, and -G7) forms generated by alternative splicing. Soluble HLA-G can be also be generated by proteolytic release of the HLA-G membrane-bound forms (shed HLA-G) [26]. HLA-G is stably expressed in the membrane of EVT, and its function seems to be to modulate the secretion of cytokines by decidual lymphocytes for immune tolerance generation and control of EVT invasion, and to contribute to the remodeling of the spiral arteries for implantation and successful pregnancy [27]. HLA-G binds to the inhibitory immunoglobulin-like transcript 2 receptor (ILT2) expressed by all monocytes, B cells, some lineages of T cells, and NK cells. HLA-G also binds to inhibitory ILT4, which is present only on dendritic cells and monocytes, and to KIR2DL4 expressed on all NK cells. As mentioned earlier, KIR2DL4 is unique among KIR receptors, because of its structure, expression, cell localization, and signaling characteristics. Unlike other KIRs that show variegated expression among individual NK cells, KIR2DL4 is present in all haplotypes. KIR2DL4 has a hybrid D0–D2 domain structure and has only one immune receptor tyrosine-based inhibition motif (ITIM) and a positively charged arginine in its transmembrane domain. Therefore, it can both activate and inhibit NK responses to their environment. Its inhibitory potential is very weak, and as an activator, it is involved in chemokine and pro-inflammatory and proangiogenic cytokine secretion rather than cytotoxicity. Unlike the typical cytoplasmic membrane expression of the other KIRs, KIR2DL4 is expressed in endosomes, and its only known ligand is HLA-G. Specifically, KIR2DL4 binds to the soluble form of HLA-G, which accumulates in KIR2DL4+ endosomes and triggers endosomal signaling [28]. HLA-G expression is atypical as it is almost only constitutively expressed in fetal trophoblast cells at the maternal-fetal interface (and in some other tissues and cells, including thymus, cornea, nail matrix, pancreas, and erythroid and endothelial precursors), but it can be upregulated by transformation, neovascularization, inflammation, or infection [29]. Accordingly, soluble HLA-G is secreted by trophoblast cells and might be endocytosed by KIR2DL4 into endosomes, causing a sustained pro-inflammatory and pro-angiogenic secretory response [30].
The majority of studies have described a protective role of HLA-G, including inhibiting allocytotoxic T lymphocyte responses and NK and T cell–mediated cell lysis, inducing a shift from a pro-inflammatory Th1 response to a Th2 response, upregulating inhibitory receptors in NK and T cells, and inducing long-term unresponsiveness of CD4+ T cells (reviewed in [31]). HLA-G inhibits the function of NK cells, T lymphocytes and antigen-presenting cells through direct binding to the inhibitory receptors ILT-2, ILT-4, and KIR2DL4 [32]. Indeed, it has been proposed that this interaction between HLA-G and KIR2DL4 inhibits fetal attack by the maternal immune system and upregulates the secretion of cytokines and angiogenic factors, which favors implantation, placental development, and successful pregnancy [33].
Although HLA-G has comparatively little polymorphism in its coding region, non-coding polymorphisms have been associated with autoimmune disease [29] and, interestingly, with spontaneous abortion [34]. KIR2DL4 also shows low polymorphism, but two common alleles with 9 or 10 consecutive adenines in the transmembrane domain exist. The 10A allele encodes the full-length receptor, whereas the 9A polymorphism has only 9 adenines leading to a frame-shift and stop codon, which generates a truncated receptor lacking the transmembrane region or with a smaller cytoplasmic tail. The function of this truncated receptor is unknown but it is likely involved in miscarriage [35]. KIR2DL4 is expressed in NK CD56bright cells of the decidua and placenta but not in peripheral blood NK CD56bright [36], suggesting a relevant role of this receptor in the maintenance of pregnancy.
HLA-C and KIR
HLA-C alleles present in populations can be divided into two groups. Specifically, Ser77/Asn80 (C1) and Asn77/Lys80 (C2) amino acid positions define serologically distinct allotypes and are found in the region where KIRs bind to HLA-C molecules: KIR2DL2, KIR2DL3, and KIR2DS2 interact with HLA-C1, whereas KIR2DL1 and KIR2DS1 recognize HLA-C2 [10]. HLA-C alleles from the father will be different from the mother, and will be specific for each pregnancy. The combination of the great variety of the polymorphic KIR alleles and the broad diversity of HLA-C alleles generates an extraordinary variability. This is a prolific substrate for natural selection and is one of the mechanisms that lead to immune system adaptation to a continuously changing environment; the functional consequences of which remain largely unexplored.
Maternal-fetal HLA-C mismatch has been associated with increased decidual T cell activation [17]. Interestingly, KIRs are not only expressed in NK cells, but also in CD8+, CD4+, and γδTCR+ T cells [37]. T cells from the decidua (both basalis and parietalis) exhibit different characteristics to the T cells from peripheral blood, with the former showing an increased proportion of atypical T cell populations, such as CD4− CD8− αβTCR+, CD3+ γδTCR+ T, and NK T cells [38]. KIR+ T cells from the decidua are mainly CD4− CD8−, but CD4+ and CD8+ cells are also present. The mechanism of induction of KIR expression on T cells is not yet known, but its function seems to be regulatory [39].
Several studies have examined the relationship between maternal KIR genes and recurrent spontaneous abortion, but the conclusions are conflicting (Table 1). Most studies have involved small patient numbers and, in some cases, different clinical criteria for recruitment. An important factor is the different methodology used for KIR genotyping, especially in view of the complexity of the KIR cluster. As mentioned earlier, two distinct regions in the KIR cluster around the KIR2DL4 gene have been identified [20], although no study has made this distinction, and the majority have examined individual KIR genes. Based on the results, however, what is clear is that the KIR/HLA-C axis provides a mechanism for maternal immune identification of the fetus [10]. In this context, the mechanism of recognition may be modulated by the presence of soluble HLA-G as an inhibitor of the immune response. Indeed, lower levels of soluble HLA-G have been associated with preeclampsia [50] and soluble HLA-G has been postulated as a promoter of tolerance during pregnancy through its engagement with KIR2DL4 or other receptors in uNK cells, such as ILT2 and ILT4 [51].
Table 1.
Basic research studies showing the associations between KIR and HLA and recurrent miscarriage
| Reference | KIR (and HLA) implicated | Type of experiment/objective | Conclusions |
|---|---|---|---|
| [40] | Inhibitory KIRs (2DL1, 2DL2, and 2DL3) | 26 childless couples with ≥ 2 abortions and 26 control couples. KIR genotyping | Some alloimmune abortions may occur when the MHC class I molecules on trophoblasts are recognized by decidual NK cells lacking appropriate inhibitory KIR receptors that would stop activating signals. |
| [41] | No association | 51 women with unexplained recurrent spontaneous abortions consecutively referred/55 controls. KIR genotyping. | The data provide little evidence that KIR polymorphism plays a role in predisposition to recurrent spontaneous abortions. |
| [42] | Inhibitory KIRs (in particular 2DL2) | Cohort of 30 fertile couples (without previous abortions)/139 healthy controls/88 couples with ≥ 3 recurrent spontaneous abortions. KIR genotyping | The balance between inhibitory and activating receptors present in natural killer cells is inclined toward an activating state that may contribute to pregnancy loss. |
| [43] | Activating KIRs (in particular 2DS1). KIR2DS1 in the absence of KIR2DL1/HLA-C2. | 73 pairs of childless couples with ≥ 3 abortions characterized as unexplained and 68 pairs of healthy control couples. KIR genotyping and HLA-C groups C1/C2 identification. | A decrease in the ligands for inhibitory KIRs could potentially lower the threshold for NK cell activation, mediated through activating receptors, thereby contributing to the pathogenesis of recurrent spontaneous abortion. |
| [44] | KIR2DS1 | Male (n = 67) and female (n = 95) partners of couples with ≥ 3 spontaneous miscarriages/269 controls (women primiparae, no miscarriages, or ectopic pregnancies). KIR genotyping and HLA-C groups’ identification. | The findings support the idea that successful placentation depends on the correct balance of uNK cell inhibition and activation in response to trophoblasts. |
| [45] | Activating KIRs | 68 patient couples with recurrent miscarriage and 68 control fertile couples. KIR genotyping | Recurrent miscarriage could be associated with NK cell activation mediated by a profile rich in activating KIR genes. |
| [46] | KIR2DL1/HLA-C2 | 177 couples with recurrent miscarriages (primary aborters, no live births) and 200 healthy couples (at least two live births and with no history of miscarriage, preeclampsia, ectopic pregnancy, or preterm delivery). Maternal KIR gene content and HLA-C genotypes to allele level in couples experiencing recurrent miscarriage and controls. | The activation spectrum of KIR-HLA-C compound genotype for NK cells may contribute to the immunological etiology of recurrent miscarriage. |
| KIR2DS2/HLA-C1 | |||
| [47] | Activating KIRs | 40 women with unexplained recurrent miscarriage and 90 controls. KIR genotyping. | Shifted balance of KIRs toward an activating state in NK cells may contribute to recurrent miscarriage. |
| [48] | Inhibitory KIRs | Retrospective study that included 291 women, with recurrent miscarriages or recurrent implantation failure, who had a total of 1304 assisted reproductive cycles. KIR genotyping. | These new insights could have an impact on the selection of single embryo transfer in patients with miscarriages or recurrent implantation failure, and with a KIR AA haplotype. |
| [49] | KIR2DS1/HLA-C2 | The frequencies of KIR and HLA-C1 and HLA-C2 genes were evaluated in 139 women with ≥ 2 consecutive spontaneous pregnancy losses. | KIR and HLA-C genotyping is important for predicting immune-related problems in women with recurrent pregnancy loss women. |
Expression of HLA class I molecules and successful pregnancy
The expression of HLA molecules is not uniform along normal pregnancy. In the case of HLA-C, immunohistochemistry analysis of placental and decidual tissue samples at different stages of gestation showed a strong staining of EVT membranes at 5 weeks’ gestation, while a decrease in staining intensity was evident from 12 weeks onwards; however, expression was high in fetal amniotic and chorion membranes of 38-week placenta [52]. Expression of HLA-G in pregnancy has been extensively studied. During pregnancy, HLA-G is consistently expressed in EVT of the placenta [51]. HLA-G seems be very relevant for early placentation, as it is expressed by the blastocyst and early embryo and its concentration in blood increases two- to fivefold in pregnant women [53]. Thus, high expression of HLA-G is associated with successful pregnancy, whereas low levels of HLA-G have been associated with pregnancy complications [54, 55]. Indeed, polymorphisms in HLA-G, especially a 14-bp insertion/deletion polymorphism in exon 8 related to low HLA-G expression, have been associated not only with reduced success of in vitro fertilization treatment and pregnancy outcome, but also with lower birth weight and weight of the placenta [31, 52, 56] (Fig. 2).
Fig. 2.
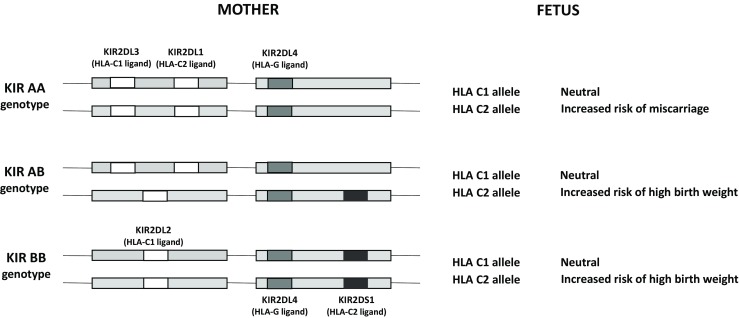
KIR involvement in miscarriage depends on the expression and polymorphism of the classical and nonclassical HLA class I molecules. The HLA-C2 allele is associated with miscarriage when KIR AA genotype is present in uNKs but not when the genotype is AB or BB. KIR2DL4-mediated inhibition of uNKs depends on HLA-G expression in the EVT, described as associated with polymorphisms, especially a 14-bp insertion/deletion polymorphism in exon 8 (adapted from Moffett et al. [57])
HLA-E and HLA-F have not been extensively studied. HLA-E binds to the NK cell receptor CD94/NKG2A promoting immune surveillance, and also to the T cell receptor CD8. The study of its role in successful pregnancy has produced contradictory results, with some associating gene polymorphisms with recurrent spontaneous abortion and others finding no association [55, 58]. HLA-F function remains to be clearly elucidated, but its known receptors are KIR3DL2 and KIR2DS4 [59], and it also interacts with ILT2 and ILT4. During pregnancy, HLA-F is expressed in EVT both on migratory and invasive EVT cells in the first and second trimester, but not in the third trimester [51].
Conclusions
Because of their diversity and capacity to recognize specific HLA class I allotypes, KIRs are good candidates for balancing maternal leukocyte tolerance to the antigens expressed in fetal cells. In turn, the activation profile of KIRs is genetically determined in each individual and leads to diverse levels of functionality in NK and T cells, with several KIR gene alleles (notably, KIR2DL1/L2/L3 and KIR2DL1/S2) potentially associated with pregnancy complications. Overall, these findings support a role for KIRs in successful pregnancy. However, there is still much to be accomplished in this area: to provide alternatives to the traditional methodology of KIR identification, for example through high-resolution genotyping or next-generation sequencing; to understand the biology of EVT and uNK cell interactions; and to improve the number of cases recruited, as well as finding consensus in the clinical inclusion criteria.
Taking into consideration that human blastocysts only express HLA-G, and very small quantities of HLA-C and HLA-E late after implantation, special emphasis should be paid to what type of pregnancy failures may potentially benefit from HLA-C and KIR genotyping as, for example, implantation failures may have a different etiology compared with recurrent miscarriage. Moreover, demographic genetic studies of fertile mothers and their healthy offspring need to be undertaken to better understand the successful KIR/HLA-C allotype combinations of fertility.
If the interaction between HLA-haplotype and KIR holds true, it will be possible to use the identification of KIR and HLA-C alleles to select gametes from donors with specific genotypes, in order to avoid pregnancy complications and improve the probability of a successful pregnancy.
Funding information
This work was supported by a grant from the “Centro Singular de Investigación de Galicia” and the Spanish Ministry of Economy and Competitiveness (Fondo de Investigaciones Sanitarias and Fondos FEDER, grant number PI15/00558).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams Z. Inducing tolerance to pregnancy. N Engl J Med. 2012;367:1159–1161. doi: 10.1056/NEJMcibr1207279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Rango U. Fetal tolerance in human pregnancy--a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115:21–32. doi: 10.1016/j.imlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington MMA. Maternal activating KIRs protect against human reproductive failure mediated by HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy PR, Chazara O, Gardner L, Ivarsson MA, Farrell LE, Xiong S, et al. Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J Immunol. 2016;197:4292–4300. doi: 10.4049/jimmunol.1601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowak I, Wilczyńska K, Wilczyński JR, Malinowski A, Radwan P, Radwan M, et al. KIR, LILRB and their ligands’ genes as potential biomarkers in recurrent implantation failure. Arch Immunol Ther Exp (Warsz) 2017;65:391–399. doi: 10.1007/s00005-017-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35:241–248. doi: 10.1016/j.placenta.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 8.von Rango U, Classen-Linke I, Kertschanska S, Kemp B, Beier HM. Effects of trophoblast invasion on the distribution of leukocytes in uterine and tubal implantation sites. Fertil Steril. 2001;76:116–124. doi: 10.1016/S0015-0282(01)01859-3. [DOI] [PubMed] [Google Scholar]
- 9.Wallace AE, Fraser RCJ. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffett A, Colucci F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev. 2015;267:283–297. doi: 10.1111/imr.12323. [DOI] [PubMed] [Google Scholar]
- 11.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 13.Burton GJ, Woods AW, Jauniaux EKJ. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster MFS. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI200422991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lash GE, Pitman H, Morgan HL, Innes BA, Agwu CNBJ. Decidual macrophages: key regulators of vascular remodeling in human pregnancy. J Leukoc Biol. 2016;100:315–325. doi: 10.1189/jlb.1A0815-351R. [DOI] [PubMed] [Google Scholar]
- 16.Choudhury RH, Dunk CE, Lye SJ, Aplin JD, Harris LK., Jr Extravillous trophoblast and endothelial cell crosstalk mediates leukocyte infiltration to the early remodeling decidual spiral Arteriole Wall. J Immunol. 2017;198:4115–4128. doi: 10.4049/jimmunol.1601175. [DOI] [PubMed] [Google Scholar]
- 17.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg-v.d.Voort-Maarschalk MM, Roelen DL, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:147–156. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting Edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor gamma protein. J Immunol. 2005;174:3859–63. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15778339. [DOI] [PubMed]
- 19.Hsu KC, Chida S, Geraghty DE, Dupont B. The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev. 2002;190:40–52. doi: 10.1034/j.1600-065X.2002.19004.x. [DOI] [PubMed] [Google Scholar]
- 20.Pyo CW, Guethlein LA, Vu Q, Wang R, Abi-Rached L, Norman PJ, et al. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS One. 2010;5(12):e15115. [DOI] [PMC free article] [PubMed]
- 21.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wauquier N, Padilla C, Becquart P, Leroy E, Vieillard V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics. 2010;62:767–771. doi: 10.1007/s00251-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut. 2011;60:268–278. doi: 10.1136/gut.2010.212555. [DOI] [PubMed] [Google Scholar]
- 24.Olcese L, Cambiaggi ASG, et al. Human diversity of killer cell immunoglobulin-like receptors and disease. J Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 25.King A, Burrows TD, Hiby SE, Bowen JM, Joseph S, Verma S, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–387. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 26.Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O, Mittal P, Gotsch F, Chaiworapongsa T, Edwin SS, Pacora P, Hassan SS. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22:1151–1166. doi: 10.3109/14767050903019684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak I, Malinowski A, Barcz E, Wilczyński JR, Wagner M, Majorczyk E, Motak-Pochrzęst H, Banasik MKP. Possible role of HLA-G, LILRB1 and KIR2DL4 gene polymorphisms in spontaneous miscarriage. Arch Immunol Ther Exp. 2016;64:505–514. doi: 10.1007/s00005-016-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park GM, Lee S, Park B, Kim E, Shin J, Cho KAK. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem Biophys Res Commun. 2004;313:606–611. doi: 10.1016/j.bbrc.2003.11.153. [DOI] [PubMed] [Google Scholar]
- 29.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125–132. doi: 10.1016/j.it.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan SLE. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol. 2012;3:258. doi: 10.3389/fimmu.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persson G, Melsted WN, Nilsson LL, Hviid TVF. HLA class Ib in pregnancy and pregnancy-related disorders. Immunogenetics. 2017;69:581–595. doi: 10.1007/s00251-017-0988-4. [DOI] [PubMed] [Google Scholar]
- 32.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/S0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue S, Yang J, Yao F, Xu L, Fan L. Recurrent spontaneous abortions patients have more −14 bp/+14 bp heterozygotes in the 3’UT region of the HLA-G gene in a Chinese Han population. Tissue Antigens. 2007;69(Suppl 1):153–155. doi: 10.1111/j.1399-0039.2006.763_7.x. [DOI] [PubMed] [Google Scholar]
- 35.Goodridge JP, Witt CS, Christiansen FTWH. KIR2DL4 (CD158d) genotype influences expression and function in NK cells. J Immunol. 2003;171:1768–1774. doi: 10.4049/jimmunol.171.4.1768. [DOI] [PubMed] [Google Scholar]
- 36.Makrigiannakis A, Petsas G, Toth B, Relakis KJU. Recent advances in understanding immunology of reproductive failure. J Reprod Immunol. 2011;90:96–104. doi: 10.1016/j.jri.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 37.van Bergen J, Thompson A, van der Slik A, Ottenhoff TH, Gussekloo JKF. Phenotypic and functional characterization of CD4 T cells expressing killer Ig-like receptors. J Immunol. 2004;173:6719–6726. doi: 10.4049/jimmunol.173.11.6719. [DOI] [PubMed] [Google Scholar]
- 38.Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SACF. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol. 2009;80:22–32. doi: 10.1016/j.jri.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Guerra N, Guillard M, Angevin E, Echchakir H, Escudier B, Moretta A, Chouaib SCA. Killer inhibitory receptor (CD158b) modulates the lytic activity of tumor-specific T lymphocytes infiltrating renal cell carcinomas. Blood. 2000;95:2883–2889. [PubMed] [Google Scholar]
- 40.Varla-Leftherioti M, Spyropoulou-Vlachou M, Niokou D, Keramitsoglou T, Darlamitsou A, Tsekoura C, et al. Natural killer (NK) cell receptors’ repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol. 2003;49:183–191. doi: 10.1034/j.1600-0897.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 41.Witt CS, Goodridge J, Gerbase-DeLima MG, Daher S, Christiansen FT. Maternal KIR repertoire is not associated with recurrent spontaneous abortion. Hum Reprod. 2004;19:2653–2657. doi: 10.1093/humrep/deh483. [DOI] [PubMed] [Google Scholar]
- 42.Flores AC, Marcos CY, Paladino N, Arruvito L, Williams F, Middleton D, et al. KIR receptors and HLA-C in the maintenance of pregnancy. Tissue Antigens. 2007;69(Suppl 1):112–3. [DOI] [PubMed]
- 43.Wang S, Zhao YR, Jiao YL, Wang LC, Li JF, Cui B, et al. Increased activating killer immunoglobulin-like receptor genes and decreased specific HLA-C alleles in couples with recurrent spontaneous abortion. Biochem Biophys Res Commun. 2007;360:696–701. doi: 10.1016/j.bbrc.2007.06.125. [DOI] [PubMed] [Google Scholar]
- 44.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 45.Vargas RG, Bompeixe EP, França PP, Marques de Moraes M, da Graça Bicalho M. Activating killer cell immunoglobulin-like receptor genes’ association with recurrent miscarriage. Am J Reprod Immunol. 2009;62:34–43. doi: 10.1111/j.1600-0897.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 46.Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum Reprod. 2011;26:491–497. doi: 10.1093/humrep/deq341. [DOI] [PubMed] [Google Scholar]
- 47.Ozturk OG, Sahin G, Karacor EDZ, Kucukgoz U. Evaluation of KIR genes in recurrent miscarriage. J Assist Reprod Genet. 2012;29:933–938. doi: 10.1007/s10815-012-9811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, et al. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum Reprod. 2014;29:2637–2643. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
- 49.Dambaeva SV, Lee DH, Sung N, Chen CY, Bao S, Gilman-Sachs A, et al. Recurrent pregnancy loss in women with killer cell immunoglobulin-like receptor KIR2DS1 is associated with an increased HLA-C2 allelic frequency. Am J Reprod Immunol. 2016;75:94–103. doi: 10.1111/aji.12453. [DOI] [PubMed] [Google Scholar]
- 50.Hackmon R, Koifman A, Hyodo H, Hyobo H, Glickman H, Sheiner E, et al. Reduced third-trimester levels of soluble human leukocyte antigen G protein in severe preeclampsia. Am J Obstet Gynecol. 2007;197:255.e1–255.e5. doi: 10.1016/j.ajog.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 51.Hackmon R, Pinnaduwage L, Zhang J, Lye SJ, Geraghty DE, Dunk CE. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am J Reprod Immunol. 2017;77:e12643. doi: 10.1111/aji.12643. [DOI] [PubMed] [Google Scholar]
- 52.García-Láez V, Serra V, Bellver J, Ferro J, Vidal C, De los Santos JM, et al. Gene polymorphisms and HLA-G expression in spontaneous abortions. Med Reprod Embriol Clin. 2015;2:82–92. [Google Scholar]
- 53.Klitkou L, Dahl M, Hviid TVF, Djurisic S, Piosik ZM, Skovbo P, et al. Human leukocyte antigen (HLA)-G during pregnancy part I: correlations between maternal soluble HLA-G at midterm, at term, and umbilical cord blood soluble HLA-G at term. Hum Immunol. 2015;76:254–259. doi: 10.1016/j.humimm.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Kofod L, Lindhard A, Bzorek M, Eriksen JO, Larsen LG, Hviid TVF. Endometrial immune markers are potential predictors of normal fertility and pregnancy after in vitro fertilization. Am J Reprod Immunol. 2017;78(3):e12684. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28440588. [DOI] [PubMed]
- 55.Pfeiffer KA, Fimmers R, Engels G, van der Ven H, van der Ven K. The HLA-G genotype is potentially associated with idiopathic recurrent spontaneous abortion. Mol Hum Reprod. 2001;7:373–378. doi: 10.1093/molehr/7.4.373. [DOI] [PubMed] [Google Scholar]
- 56.Emmery J, Christiansen OB, Nilsson LL, Dahl M, Skovbo P, Møller AM, et al. Associations between fetal HLA-G genotype and birth weight and placental weight in a large cohort of pregnant women - possible implications for HLA diversity. J Reprod Immunol. 2017;120:8–14. doi: 10.1016/j.jri.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Moffett A, Chazara O, Colucci F, Johnson MH. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: too early for clinical intervention. Reprod Biomed Online. 2016;33:763–769. doi: 10.1016/j.rbmo.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Tripathi P, Naik S, Agrawal S. HLA-E and immunobiology of pregnancy. Tissue Antigens. 2006;67:207–213. doi: 10.1111/j.1399-0039.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Beltran WF, Hölzemer A, Martrus G, Chung AW, Pacheco Y, Simoneau CR, et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat Immunol. 2016;17:1067–1074. doi: 10.1038/ni.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]