Abstract
Purpose
This research sought to understand IVF-physicians’ knowledge of, experience with, and attitudes toward fertility preservation for cancer patients.
Methods
A 35-question, self-report survey request was emailed to IVF providers who were registered on the IVF-Worldwide.com network (3826 clinics). Physicians submitted responses on the IVF-Worldwide.com website. Survey results were reported as a proportion of the responding clinics.
Results
Survey responses were completed by 321 (8.4%) globally distributed IVF clinics, representing 299,800 IVF cycles. Of these clinics, 86.6% (278) performed fertility preservation, treating approximately 6300 patients annually. However, 18.4% of the centers reported that patients sought advice independently, without an oncologist’s referral. Ovarian tissue cryopreservation was performed by 37.7% of the clinics, yet 52.6% considered the procedure experimental. IVM was performed by 16.5% of responding clinics. A majority (63.6%) of the clinics selected treatment protocols based on each patient’s malignancy. Most respondents (76.3%) disagreed that fertility preservation was not yet successful enough to make it an available option. However, 44.2% believed that pregnancy rates following oocyte cryopreservation could not be determined because not enough oocyte cryopreservation patients had completed embryo transfer.
Conclusions
Most clinics performed fertility preservation, tailoring protocols to each patient’s disease and condition. Almost 20% of patients sought advice independently, indicating that more effort is needed to encourage oncologists to refer patients. Most survey respondents believed that data was not yet available on either live birth outcomes or the best protocol for each disease. Therefore, long-term study must continue, with the establishment of interim milestones and an outcome-tracking registry.
Keywords: Fertility preservation, IVF, IVF-Worldwide, Oncofertility, Survey, Cryopreservation
Introduction
According to the GLOBOCAN project [1], whose results were adopted by the World Health Organization (WHO), the incidence of cancer in women worldwide, excluding non-melanoma skin cancer, is 165.2/100,000. When segmented by age, the cancer incidence for women between ages 15 and 39 is 48.7/100,000, and for women between ages 40 and 44 is 180.1/100,000. Moreover, female cancer patients aged 44 or younger at diagnosis make up about 13% of all newly diagnosed cases worldwide, with breast, cervical, uterine, thyroid, and ovarian cancers being the most prevalent. Because the worldwide mortality rate has declined over the past several years, prevalence and survival have increased [2–5].
Infertility is usually related to cancer treatment side effects rather than the disease itself. Cancer treatment may lead to the loss of reproductive organ function, premature ovarian failure, or the inability to produce mature eggs for ovulation. When presenting disease facts, potential prognoses, and treatment options to the patient, medical professionals should discuss fertility preservation, especially given that women are now choosing to conceive at older ages than they have in the past [6, 7]. This is also important because at the time of diagnosis, 74% of adolescents and young adults indicated a desire to have children in the future [8].
Fertility preservation is an option that has high financial costs, as well as physical effects and emotional repercussions. When presenting fertility preservation considerations to patients, a multi-disciplinary team, including an oncologist, reproductive health specialist, and psychological counselor, is required.
Unfortunately, a standard tool for selecting or recommending the best treatment per patient is not yet available to clinical teams. This lack of a reliable decision-making algorithm that takes into consideration the patient’s treatment options, fertility assessment, and anamnestic information, such as age, past fertility treatments, and comorbidity, emphasizes the need to survey medical professionals for their input.
There are two standard treatment options that reproductive health professionals can offer female patients: oocyte cryopreservation and embryo cryopreservation. There are many ways to perform each procedure, and in selecting the best one, physicians should consider the timing of cancer treatment, treatment regimen, cancer type, patient age, and presence or absence of a partner [9]. Cryopreservation of embryos is the most widely used fertility preservation method. Due to improved vitrification techniques, oocyte survival rates are high, with no significant differences in implantation or pregnancy rates between embryos obtained from cryopreserved mature oocytes and fresh oocytes [10]. However, both methods require approximately 2 weeks to perform, since patients need to undergo controlled ovarian hyperstimulation (COH) prior to the procedure. This time, requirement is not an option for patients encountering aggressive cancers or patients with hormone-sensitive cancers that must be treated immediately. Moreover, these procedures are not options in prepubertal patients.
Immature oocyte cryopreservation and in vitro maturation (IVM) is a two-step protocol that can aid patients who are unable to undergo COH, allowing them to begin cancer treatment immediately after oocyte aspiration [11].
Ovarian tissue cryopreservation, an experimental fertility preservation option in most countries of the world, is an invasive procedure performed under general anesthesia to surgically remove ovarian tissue. It is the best option for prepubertal patients, patients who do not have a partner or sperm donor, or patients who choose not to use donated sperm. Since this option depends upon ovarian reserve, the age of the patient should be considered [12].
The aspiration of immature oocytes during ovarian tissue cryopreservation is an emerging experimental option. The oocytes can be cryopreserved, or first undergo in vitro maturation and then be cryopreserved. However, few reproductive medicine centers perform this treatment, and even fewer cases have resulted in live births [13].
Given the basic fertility preservation options available to oncology patients, the aim of this study is to better understand physicians’ knowledge of, experience with, and attitudes toward fertility preservation for cancer patients. The survey reported here collects and summarizes opinions from a large and diverse population of IVF specialists. Published insights may help equip fertility treatment providers and oncologists with better decision-making tools in order to develop optimized fertility preservation approaches and strategies, particularly in the absence of clear clinical trial results.
Materials and methods
The oncofertility survey was structured as a series of 35 multiple-choice questions. In most of the questions, a single answer was required by respondents. A small number of questions allowed multiple answers. The survey was web-based, hosted by IVF-Worldwide.com, which is a comprehensive website for IVF healthcare professionals. The survey link was http://www.ivf-worldwide.com/survey/fertility-preservation.html [14]. Invitations to participate in the survey were emailed on three occasions to 3826 units registered on IVF-Worldwide.com. The survey was administered between December 1, 2016 and January 15, 2017.
Quality assurance
To minimize duplicate clinical unit survey reports and eliminate possible false data, we used a software program (BF Survey, Tamlyn Software, Australia) that compared three parameters from the surveyed clinics’ self-reported data with existing clinic data from the IVF-Worldwide website. Methods used were described in previously reported research from the IVF-Worldwide network [15]. These parameters included the unit name, country, and e-mail address. At least two parameters had to match between the survey and the website for the clinical unit data to be included in the study. If two survey responses shared at least two parameters, the duplicate survey results with the later date were discarded.
Statistical analysis
The analysis was based on simple statistics that gave all IVF centers the same weight, regardless of the number of IVF cycles they performed or the number of fertility preservation patients they treated per year. We excluded the centers that did not perform IVF treatments.
To compare results between the method that assigned equal weights per clinic and the method that weighted clinic responses based on treatment volume, we made two supplemental analyses in which we weighted clinic responses based on the annual number of IVF cycles performed and the fertility preservation patients they treated. In the results that were weighted by number of IVF cycles performed annually, we set the maximum number of IVF cycles to 4500, which was 1.5% of the total of 299,800 annual cycles represented in the survey, in order to limit the influence of large-scale centers. We then compared the results for the survey answers and found a mean error rate of 2% when comparing annual IVF cycle calculations, and a mean error rate of less than 5% when comparing annual fertility preservation patients treated. These results showed that there was no statistically significant difference between weighted and non-weighted clinic responses.
Survey results were calculated by using the formulas described in previously reported research from the IVF-Worldwide network [14].
For example, for a question with four possible answers (a, b, c, d), the following results were calculated:
To estimate the average number of patients seen annually or treated annually using a given procedure (e.g., oocyte cryopreservation), we began with the survey question that segments centers by the average number of patients treated per year. (Possible answers were as follows: None, 1–5, 6–20, 21–50, 51–80, More than 80). A midpoint value was assigned to each of the answers (0, 3, 13, 35, 65, and 100, respectively). The number of respondents in each segment was then multiplied by the midpoint value. For example, if 10 centers selected the answer “1–5,” we multiplied 10 by the midpoint value of “3” to obtain 30 in that segment. The values calculated for each segment were then totaled.
Results
The survey collected responses from 321 IVF centers (8.4% of the 3826 centers) from 65 countries around the world, representing a total of 299,800 annual IVF cycles (Table 1). The majority of the responses came from Europe (44.5%), followed by Asia (19.3%), the USA and Canada (16.5%), South America (12.9%), Australia (4%), and Africa (2.8%). The results were categorized into four topics: oncofertility activity, ovarian tissue cryopreservation and IVM, oocyte cryopreservation protocols, and fertility preservation outcomes.
Table 1.
Geographic distribution of IVF units participating in the fertility preservation survey
| Continent | IVF cycles | % of cycles | IVF units | % of IVF units |
|---|---|---|---|---|
| USA and Canada | 58,300 | 19.5 | 53 | 16.5 |
| South America | 27,700 | 9.2 | 41 | 12.9 |
| Australia and New Zealand | 13,100 | 4.4 | 13 | 4 |
| Asia | 67,100 | 22.4 | 62 | 19.3 |
| Europe | 120,900 | 40.3 | 143 | 44.5 |
| Africa | 12,700 | 4.2 | 9 | 2.8 |
| Total | 299,800 | 100 | 321 | 100 |
Oncofertility activity
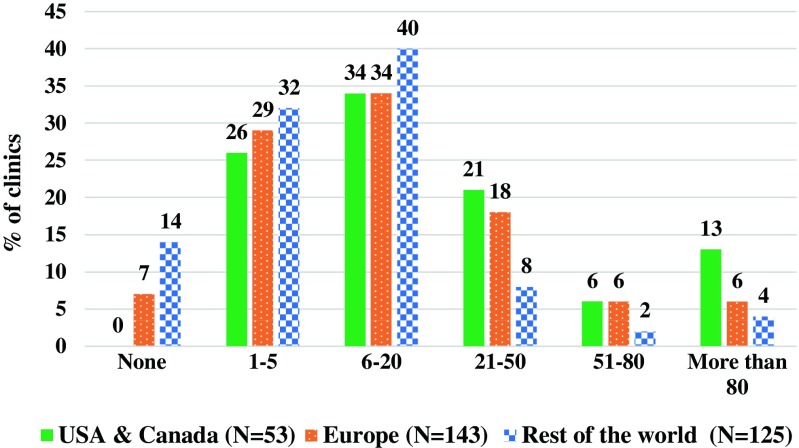
Overall, of these centers, 86.6% (278) offered fertility preservation treatment (Table 2) treating an average of 6300 patients annually (Fig. 1). About 8.4% of the respondents referred patients for treatment and 5.0% of the clinics did not have fertility preservation patients. Most centers (36%) that offered fertility preservation treatment treated between 6 and 20 patients annually. When evaluating the percentage of centers, among the USA/Canada, Europe, and the rest of the world, the distribution and average were similar. Most clinics (74.8%) advised and treated cancer patients that were referred by an oncologist, while in some clinics (18.4%), oncofertility patients sought advice and treatment on their own (Table 3).
Table 2.
Percentage and number of IVF units performing fertility preservation, ovarian tissue cryopreservation, and in vitro maturation (IVM) by region of the 321 total IVF unit respondents
| Fertility preservation | Ovarian tissue cryopreservation | In vitro maturation (IVM) | ||||
|---|---|---|---|---|---|---|
| Region | Number of units | Percentage | Number of units | Percentage | Number of units | Percentage |
| USA and Canada | 53 | 100 | 20 | 37.7 | 7 | 13.2 |
| Europe | 120 | 83.9 | 56 | 39.2 | 26 | 18.2 |
| Rest of the world | 105 | 84 | 45 | 36 | 20 | 16 |
| Total | 278 | 86.6 | 121 | 37.7 | 53 | 16.5 |
Fig. 1.
Percentage of the 321 responding clinics (8.4% of the 3826 centers) treating cancer patients for fertility preservation by region—distributed by number of patients treated per year. The estimated average number of patients treated per year by survey respondents is 6300 (Graph created in Microsoft Excel). “Rest of the world” refers to South America (N = 41), Australia and New Zealand (N = 13), Asia (N = 62), Africa (N = 9)
Table 3.
General questions, including oocyte cryopreservation (survey responses per center)
| How would you describe your level of knowledge of oocyte cryopreservation for fertility preservation? | ||||
| Not at all knowledgeable | Aware but do not know much about the topic | Knowledgeable | Very knowledgeable | |
| 6 (1.9%) | 18 (5.6%) | 143 (44.5%) | 154 (48%) | |
| Cancer patients who consult with you for fertility preservation | ||||
| Are referred mostly by oncologists | Are not referred by oncologists: they seek advice on their own | Not applicable: our unit does not receive fertility preservation consultations | ||
| 240 (74.8%) | 59 (18.4%) | 22 (6.9%) | ||
| In your opinion, is there any age limit to the fertility preservation procedure? | ||||
| No | Up to age 35 | Up to age 37 | Up to age 40 | Up to age 42 |
| 49 (15.3%) | 31 (9.7%) | 82 (25.5%) | 100 (31.2%) | 59 (18.4%) |
| For fertility preservation treatment in cancer patients, do you use and prefer | ||||
| Long gonadotropin releasing hormone (GnRH) agonist protocols | Short GnRH agonist protocols | GnRH antagonist protocols | Our unit does not treat patients for fertility preservation | |
| 11 (3.4%) | 35 (10.9%) | 243 (75.7%) | 32 (10%) | |
| In the cases in which you use a GnRH antagonist protocol, do you trigger with an agonist? | ||||
| Yes | No | Our unit does not use GnRH antagonist protocols | Our unit does not treat patients for fertility preservation | |
| 238 (74.1%) | 47 (14.6%) | 7 (2.2%) | 29 (9%) | |
Ovarian tissue cryopreservation and IVM
Ovarian tissue cryopreservation is still experimental in most countries of the world. When asking the respondents how they would describe their level of knowledge of ovarian tissue cryopreservation for fertility preservation, 51.4% replied that they were knowledgeable and 17.4% reported being very knowledgeable (Table 4). Altogether, 39.3% of responding clinics performed ovarian tissue cryopreservation, meaning that an estimated 820 patients underwent this procedure per year. Yet, 52.6% of the respondents believed that ovarian tissue cryopreservation was still considered an experimental procedure (Table 4). A relatively small percentage of respondents (16.5%) performed IVM (Table 2).
Table 4.
Ovarian tissue cryopreservation (survey responses per center)
| How would you describe your level of knowledge of ovarian tissue cryopreservation for fertility preservation? | ||||
| Not at all knowledgeable | Aware but do not know much about the topic | Knowledgeable | Very knowledgeable | |
| 15 (4.7%) | 85 (26.5%) | 165 (51.4%) | 56 (17.4%) | |
| Statement: cryopreservation of ovarian tissue is still an experimental procedure | ||||
| Agree | Neither agree nor disagree | Disagree | ||
| 169 (52.6%) | 67 (20.9%) | 85 (26.5%) | ||
| Please estimate the annual number of patients for whom you perform ovarian tissue cryopreservation*. | ||||
| None | 1–5 | 6–10 | 11–20 | More than 20 |
| 195 (60.7%) | 86 (26.8%) | 17 (5.3%) | 15 (4.7%) | 8 (2.5%) |
*Estimated total number of ovarian tissue cryopreservation procedures performed annually, 820
Oocyte cryopreservation protocols
The core of the survey focused on the topic of oocyte cryopreservation protocols (Table 3). The responses came from experienced clinicians, since 92.5% of the survey respondents described themselves as knowledgeable or very knowledgeable on oocyte cryopreservation procedures.
The upper age limit for fertility preservation was set at 37 by 25.5% of respondents and at 40 by 31.2% of respondents (Table 3). For the vast majority of physicians (75.7%), the GnRH antagonist protocol was the protocol of choice, in which case, 74.1% triggered with a GnRH agonist, while 10.9% preferred a short GnRH agonist protocol and only 3.4% of centers used long GnRH agonist protocols (Table 3).
Most physicians (63.6%) varied their treatment protocols based on the type of patient malignancy (Table 5). Indeed, in patients with hematological diseases, 72% of the respondents replied that they would start treatment immediately (Table 5).
Table 5.
Protocol used—based on type of cancer (survey responses per center)
| Does your unit have differences in treatment protocols based on the type of the malignancy? | |||
| Yes | No | Our unit does not treat patients for fertility preservation | |
| 204 (63.6%) | 85 (26.5%) | 32 (10%) | |
| In patients with malignant hematological diseases, when do you start fertility preservation treatment? | |||
| Immediately | Wait for the follicular phase to start | Start in the luteal phase | Our unit does not treat patients for fertility preservation |
| 231 (72%) | 45 (14%) | 9 (2.8%) | 36 (11.2%) |
| Do you use controlled ovarian hyperstimulation protocols for fertility preservation in breast cancer patients? | |||
| Yes | No | Our unit does not treat patients for fertility preservation | |
| 269 (83.8%) | 25 (7.8%) | 27 (8.4%) | |
| In breast cancer patients, do you use FSH to stimulate the ovaries? | |||
| Yes | No | Our unit does not treat patients for fertility preservation | |
| 245 (76.3%) | 49 (15.3%) | 27 (8.4%) | |
| In breast cancer patients, do you add aromatase inhibitors to the protocol? | |||
| Yes | No | Our unit does not treat patients for fertility preservation | |
| 237 (73.8%) | 57 (17.8%) | 27 (8.4%) | |
When asked about fertility preservation in breast cancer patients, 83.8% of the respondents used COH protocols, 76.3% used FSH to stimulate the ovaries, and 73.8% added aromatase inhibitors to protocols (Table 5).
Fertility preservation outcomes
One third (33.3%) of respondents believed that 11 to 15 cryopreserved oocytes would be sufficient for fertility preservation, while 37.4% believed that more than 15 oocytes should be cryopreserved (Table 6).
Table 6.
Fertility preservation outcomes (survey responses per center)
| How many cryopreserved patient oocytes would be sufficient for you to recommend that further treatment cycles are not necessary, if the general condition of the patient would allow her to continue cryopreservation therapy? | ||||
| 1–5 | 6–10 | 11–15 | More than 15 | I do not have experience in the field |
| 18 (5.6%) | 51 (15.9%) | 107 (33.3%) | 120 (37.4%) | 25 (7.8%) |
| Statement: the success rate of fertility preservation is not yet good enough to make it an available option. | ||||
| Agree | Neither agree nor disagree | Disagree | ||
| 30 (9.3%) | 46 (14.3%) | 245 (76.3%) | ||
| Were there pregnancies in your center in the following situations? (multiple answers allowed) | ||||
| After cancer treatment from frozen embryos | After cancer treatment from cryopreserved oocytes | After fertility preservation from transplanted ovarian tissue | I am not aware of any pregnancies | There were no pregnancies in women with the situations stated above |
| 182 (56.7%) | 117 (36.4%) | 31 (9.7%) | 57 (17.8%) | 55 (17.1%) |
| The pregnancy rate after oocyte cryopreservation in cancer patients is not yet known because | ||||
| There are not enough patients that benefit from this procedure who have gotten as far as embryo transfer to assess the pregnancy rate | The ability of cryopreserved oocytes to be fertilized is impaired | There is no proper registry | Other reasons | |
| 142 (44.2%) | 15 (4.7%) | 136 (42.4%) | 28 (8.7%) | |
Most respondents (76.3%) disagreed that the success rates of fertility preservation were not yet good enough to make it an available option. However, 44.2% of survey respondents believed that pregnancy rates following oocyte cryopreservation could not be determined because not enough patients who underwent this procedure had progressed as far as embryo transfer. In addition, 42.4% believed that rates could not be determined because there was no proper fertility preservation registry. Over half (56.7%) of the centers reported post-cancer pregnancies following fertility preservation from frozen embryos, 36.4% from cryopreserved oocytes, and 9.7% from ovarian tissue transplantation. (Note that multiple answers to this question were allowed.)
Discussion
This is the first broad-scale international survey of reproductive health professionals on fertility preservation. As such, it has shed light on the prevalence of the practice throughout the world and has provided measures of treatments, patient activity, and opinions. The high percentage of clinics performing fertility preservation and the physicians who believed that fertility preservation success rates were good enough to offer this solution to patients may indicate the degree of confidence that physicians have in the process and the level of patient interest in preserving their fertility.
Many clinics are choosing to tailor protocols based on patient disease and condition [16]. This was also demonstrated by the majority of responders in the survey. However, data is not yet available to confirm the best protocol for all the complex patient factors that can affect outcomes, including disease type, stage, condition, age, and fertility goals [17].
The finding that 18.4% of clinics had patients who sought fertility preservation on their own without an oncologist referral should help drive increased oncologist awareness on raising the topic of fertility preservation with their patients. This data is supported by a study published by Shnorhavorian et al. [18], which found that factors such as gender, education, insurance status, medical factors, patient socioeconomic status, and child-rearing status may affect whether patients and their physicians have discussions and take actions to preserve fertility during cancer treatment [18]. The study by Shnorhavorian et al. was based on a survey among 459 adolescents and young adults who were diagnosed with cancer in 2007 or 2008.
In a Hong Kong-based survey among 457 clinicians in clinical oncology, hematology, obstetrics, gynecology, pediatrics, and surgery departments in various public hospitals, only 45.6% were familiar with fertility preservation [19]. The factors considered most important for referral were patient prognosis, patient’s desire to have children, time available before commencing gonadotoxic treatment, type of cancer, and type of gonadotoxic treatment. The majority of clinicians did not refer their patients for fertility preservation due to a lack of available time before treatment, considerable risk of recurrence, poor prognosis, financial constraints, need for cancer treatment as the top priority at the time, and a lack of awareness of fertility preservation services. Almost all clinicians agreed that a dedicated center should be set up for fertility preservation, and 76.5% agreed that fertility preservation should be provided as a public service.
Types of tissues to cryopreserve
In the current survey, oocytes were the most common tissue to cryopreserve (92.5%). High oocyte preservation prevalence implies that oocyte vitrification techniques are used regularly. Oocyte cryopreservation is also recommended by the Fertility Preservation-ESHRE-ASRM 2015 Expert Group [17].
As reported above, 52.6% of the units surveyed responded that ovarian tissue cryopreservation was still considered an experimental procedure. The procedure is becoming a valuable established approach to the preservation of fertility, especially in prepubertal girls, since it is the only possible solution for this patient group. Yet, more accurate data is needed both on the likelihood of successful childbirth after this procedure and on the factors that underpin the successful application of this approach, to help promote effective use. The procedure is available today in many countries and so far, experts in the field estimate that more than 130 healthy children have been born worldwide through its application (unpublished data). Recently, a study was published showing that it may be possible to test cryopreserved tissue for remaining cancer cells before reimplantation [20].
Time in the cycle to start ovarian stimulation
It is important to note that 72% of survey respondents advocated starting ovarian stimulation immediately upon a hematological malignancy diagnosis even if it was during the luteal phase of a patient’s menstrual cycle. A recent study by Ubaldi et al. showed no statistically significant differences in metaphase II (MII) oocytes from the follicular phase versus from the stimulated luteal phase (3.4 ± 1.9 vs. 4.1 ± 2.5). No differences were observed in the euploid blastocyst formation rate calculated either per biopsied blastocyst (46.9% vs. 44.8%) or per injected MII oocyte (16.2% vs. 15.0%) [21]. This study and other observations in the field clearly support that ovarian stimulation for oocyte/embryo cryopreservation can start any time during the menstrual cycle.
Number of oocytes retrieved
The highest percentage of the respondents (37.4%) recommended that the optimal number of oocytes for cryopreservation was 15 or more. We need to take into consideration that in most cryopreservation cases, patients have only one cycle for egg collection prior to cancer treatment, and in most cases, patients’ physical conditions likely affect ovarian response. This aligns with the findings by Goldman et al. [22] that nearly 20 eggs are needed to achieve a live birth [22]. Infertile women or women with cancer may not have good-quality eggs, and therefore, may have inferior outcomes from oocyte cryopreservation. This potential limitation has to be presented during fertility preservation counseling and decision-making. It is important to the practice of fertility preservation to collect data and analyze outcomes on mature oocyte cryopreservation among cancer therapy survivors who subsequently undergo assisted reproductive technology (ART) using their previously cryopreserved oocytes.
Limitations and reasons for caution
The large and geographically diverse clinic sample in this survey reflects a wide range of opinions; however, the source of data in this survey came mainly from centers that were likely to be deeply involved in fertility preservation. This implies that it is possible that the majority of responses received were from units that were involved in fertility preservation. Therefore, the results may be skewed or biased toward fertility preservation experts versus mainstream or novice practitioners, and clinics that did not practice or refer patients for fertility preservation may have been underrepresented.
The largest percentage (44.5%) of units that responded to the survey came from Europe, where free healthcare or subsidized universal healthcare is widely available, indicating that European physicians may have different perspectives toward the treatment than their peers from other parts of the world. The survey results should be used with great caution; they by no means should replace evidence-based medicine and do not suggest that clinicians follow the practices from the survey. They simply reflect the opinions and experience of medical directors from hundreds of IVF units worldwide at a specific point in time. Because only the first survey response per clinic was accepted in order to prevent skewed results, this study did not assess whether there were different opinions within each clinic.
The survey did not ask respondents to specify each type of cancer treated. This could be a subject to be covered in a future survey.
In the questionnaire question, “The pregnancy rate after oocyte cryopreservation in cancer patients is not yet known because,” the respondent was not given a choice of disagreeing with the statement. Therefore, the respondents who believed that the pregnancy rate is known may have selected ‘Other Reasons’ or another option. This may have slightly skewed the results of this question.
Fertility preservation outcomes and data availability
Data collection on successful pregnancy outcomes is still in its infancy. To determine recommended protocols, the outcomes of many more cancer patients who choose to undergo fertility preservation need to be evaluated. Due to the nature of cancer, patients typically pursue pregnancy once they have gone into remission or recover from the disease, a process that could take years. A certain percentage of cancer patients die before IVF can be performed. For those who survive, personal considerations, such as age and partner status, could also factor into the equation. Therefore, fertility preservation success may be determined differently depending upon each patient factor. Long-term studies must continue, with protocols being updated periodically and clear milestones defined along this path toward success. The results point to a clear need for a fertility preservation registry, one that would provide a real-world view of clinical practice, patient outcomes, and comparative effectiveness in order to help determine best practices and to support physicians and patients in decision-making.
In conclusion, this survey has revealed that a vast majority of IVF centers that responded to the survey performed fertility preservation procedures. In the current absence of clinical trial results that indicate clear treatment protocol recommendations, reliance on the “wisdom of the crowd” through insightful IVF-practitioner survey input is critical to decision-making as oncofertility, oncology, and technology continue to evolve. This first-of-its-kind worldwide survey is an important starting point from which to measure progress.
Funding sources
Technical aspects of the IVF-Worldwide survey were supported by an unrestricted grant from Teva Pharmaceutical Industries Ltd. (grant number: TST_TPU_SRVS_2017_21763).
Compliance with ethical standards
Conflict of interest
In terms of disclosures, Gon Shoham has nothing to disclose; Rachel Levy-Toledano was a consultant for Teva Europe Medical Affairs; Milton Leong is an executive at IVF-Worldwide; Ariel Weissman has nothing to disclose; Yuval Yaron has nothing to disclose and Zeev Shoham is an executive at IVF-Worldwide.
Research involving human participants and/or animals
This IVF-Worldwide survey consists of physician self-reports on activity volumes and opinions on medical practices. The survey does not involve research on human or animal subjects and this article does not study human or animal subjects. Therefore, formal institutional review board approval was not necessary. The survey was conducted as an open-access questionnaire to IVF-Worldwide.com members who voluntarily answered the study questions. Patient data for this research remained anonymous since no patient data was collected. Therefore, no informed consent documentation was required. The study did not involve the use of laboratory animals.
Footnotes
This article has not been published elsewhere and has not been submitted simultaneously for publication elsewhere. The co-authors have not submitted similar journal articles or conference papers to this publication submission. The authors are not aware of similar or significantly overlapping contributions in other journal articles or conference papers published.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide. 2012. http://gco.iarc.fr/.
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2012;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int J Cancer. 2012;2014:e359–e386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, Ferlay J. Incidence/mortality data. [Online]. http://globocan.iarc.fr. [DOI] [PubMed]
- 5.de Roo SF, Rashedi AS, Beerendonk CCM, Anazodo A, de Man AM, Nelen WLDM, Woodruff TK. Global oncofertility index-data gap slows progress. Biol Reprod. 2017;96(6):1124–1128. doi: 10.1093/biolre/iox051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. National Vital Statistics Reports: Births: Final Data for 2015. National Center for Health Statistics 2017;66(1). [PubMed]
- 7.Rashedi AS, de Roo SF, Ataman LM, Edmonds ME, Silva AA, Scarella A, Horbaczewska A, Anazodo A, Arvas A, Ramalho de Carvalho B, Sartorio C, Beerendonk CCM, Diaz-Garcia C, Suh CS, Melo C, Andersen CY, Motta E, Greenblatt EM, van Moer E, Zand E, Reis FM, Sánchez F, Terrado G, Rodrigues JK, Marcos de Meneses e Silva J, Smitz J, Medrano J, Lee JR, Winkler-Crepaz K, Smith K, Ferreira Melo e Silva LH, Wildt L, Salama M, del Mar Andrés M, Bourlon MT, Vega M, Chehin MB, de Vos M, Khrouf M, Suzuki N, Azmy O, Fontoura P, Campos-Junior PHA, Mallmann P, Azambuja R, Marinho RM, Anderson RA, Jach R, Antunes RA, Mitchell R, Fathi R, Adiga SK, Takae S, Kim SH, Romero S, Grieco SC, Shaulov T, Furui T, Almeida-Santos T, Nelen W, Jayasinghe Y, Sugishita Y, Woodruff TK. Survey of third-party parenting options associated with fertility preservation available to patients with cancer around the globe. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.18.99800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geue K, et al. The desire for children and fertility issues among young German cancer survivors. J Adolesc Health. 2013;54(5):27–35. doi: 10.1016/j.jadohealth.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Kim SK, Lee JR, Woodruff TK. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J Gynecol Oncol. 2015;27(2):e22. [DOI] [PMC free article] [PubMed]
- 10.Goldman KN, Kramer Y, Hodes-Wertz B, Noyes N, McCaffrey C, Grifo JA. Long-term cryopreservation of human oocytes does not increase embryonic aneuploidy. Fertil Steril. 2015;103(3):662–668. doi: 10.1016/j.fertnstert.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Oktay K, Buyuk E, Rodriguez-Wallberg KA, Sahin G. In vitro maturation improves oocyte or embryo cryopreservation outcome in breast cancer patients undergoing ovarian stimulation for fertility preservation. Reprod BioMed Online. 2010;20(5):634–638. doi: 10.1016/j.rbmo.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 13.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, Loh SF, Chia YN. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 14.IVF-Worldwide. Fertility preservation survey, 2016. [Online]. http://www.ivf-worldwide.com/survey/fertility-preservation.html.
- 15.Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod BioMed Online. 2012;25(2):139–145. doi: 10.1016/j.rbmo.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Loren AW, Mangu PB, Nohr Beck L, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. Fertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez F, International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108(3):407–415. doi: 10.1016/j.fertnstert.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Shnorhavorian M, Harlan LC, Smith AW, Keegan TH, Lynch CF, Prasad PK, Cress RD, Wu XC, Hamilton AS, Parsons HM, Keel G, Charlesworth SE, Schwartz SM. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: a population-based study. AYA HOPE Study Collaborative Group. Cancer. 2015;121:3499–3506. doi: 10.1002/cncr.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung JP, Lao TT, Li TC. Evaluation of the awareness of, attitude to, and knowledge about fertility preservation in cancer patients among clinical practitioners in Hong Kong. Hong Kong Med J. 2017;23:556–561. doi: 10.12809/hkmj176840. [DOI] [PubMed] [Google Scholar]
- 20.Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, Schiff E, Orvieto R, Meirow D. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril. 2018;109:48–53. doi: 10.1016/j.fertnstert.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, Trabucco E, Venturella R, Vajta G, Rienzi L. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–1495. doi: 10.1016/j.fertnstert.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril. 2013;100:712–717. doi: 10.1016/j.fertnstert.2013.04.040. [DOI] [PubMed] [Google Scholar]