Abstract
Purpose
The uterine immunophenotype is relatively poorly understood, with most studies reporting proportions/percentages. A novel technique to calculate local endometrial lymphocyte concentrations is described, and used to compare results between aetiological subgroups such as repeated implantation failure (RIF) and recurrent pregnancy loss (RPL) with male-factor controls.
Methods
455 patients had an endometrial biopsy performed. Background history on initial presentation was used to subdivide the population into RIF (n = 149), RPL (n = 121), primary (n = 76) and secondary infertility (n = 80). A control group was identified comprising male factor infertility aetiology with all female investigations normal (n = 29). Endometrial Tissue was assessed using a comprehensive multi-parameter panel. Lymphocyte subpopulations were calculated using flowcount flurospheres and a mathematical correction applied to determine concentrations per milligram of tissue, based on original biopsy weight and volumetric dilutions.
Results
The flow cytometry technique was successful in determining population centiles for concentrations of endometrial lymphocyte subsets. Distinct differences were noted across the patient groups. Th2 concentrations were significantly higher in the controls (p = 0.0002). All RPL/infertile populations had increased concentrations of peripheral type NK’s (p = 0.016) and B cells (p = 0.045). Relative to male factor controls, CD4+ and CD8+ T lymphocyte populations were increased in RPL patients, and reduced in those with a history of RIF. Th1 concentrations were elevated in the adverse outcome groups (p = 0.032). Concentration centiles alone do not appear to accurately predict outcome with subsequent treatment.
Conclusions
Endometrial biopsy analysis by flow cytometry can provide detailed analysis of constituent lymphocyte subsets by concentration as well as proportion. This novel approach provides additional independent data to further assess the significance of endometrial changes in the setting of reproductive failure.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01427-8) contains supplementary material, which is available to authorized users.
Keywords: Endometrium; ART; Natural killer cells; Lymphocytes; Immunophenotype, cell-count
Introduction
Implantation failure and early pregnancy loss are frequently encountered in gynaecology and reproductive medicine, and despite advances, many cases are still classified as unexplained. Although embryo aneuploidy is typically the primary cause [1, 2], other known influencing factors should be considered, such as infection [3], anatomy [4], endocrine [5], haematological [6, 7], receptivity [8, 9], and immunological [10, 11]. Indeed, preimplantation genetic screening techniques (PGT-A) have shown that transfer of screened euploid blastocysts leads to a live birth in only just over half of all treatment cycles [12], highlighting the multifactorial nature of the embryonic implantation process and the need to further explore alternative hypotheses for failure. Despite these vast gaps in knowledge, the proposition of immune-mediated theories for reproductive failure has, and continues to be, controversial. It is recognized that the maternal immune system, particularly the abundant uterine/decidual natural killer cells (uNK), plays an important role during embryonic implantation [13], but its contribution to abnormal outcomes, particularly with a euploid embryo, is less clearly understood. Currently, a complete description of the normal levels of endometrial lymphocyte subsets is not completely defined. In spite of promising attempts, there is no universally accepted technique to assess the cellular populations present [14], and even less consensus regarding their interpretation or proposed interventions. Peripheral blood immunophenotypes have often been used to test theories of immunological dysfunction in reproductive failure. Interventions based on peripheral blood analysis has many critics and receives only limited acceptance, as admittedly various internal and external factors can have an impact on the findings [15, 16]. There is now also a long history of endometrial tissue analysis by various histological, immunohistochemical, or molecular techniques, which have the potential to contribute to the understanding of “unexplained” implantation failure or pregnancy loss. These tests are, however, still not widely supported for routine use. Despite this, there is still potential that endometrial analysis may yet explain some of the unanswered questions. Variations in endometrial natural killer, T and B lymphocyte populations have all been proposed as contributory factors to adverse reproductive failure outcome [17–20]. An early Canadian publication analysing endometrial biopsies reported that patients with recurrent miscarriage had significant cellular differences, in particular a reduction in CD8+ T lymphocytes and an increase in B cells [21]. Endometrial CD57+ NK cells, identified by immunohistochemistry, representing a mature cytotoxic subset of cells, were also proposed as an early marker for recurrent miscarriage [19]. Interestingly, CD57+ cells are always CD16+ and so are most likely peripheral in origin and do not represent uterine NKs. An important UK study found significantly higher levels of uterine natural killer cells in patients with recurrent miscarriage than in controls, but also demonstrated that corticosteroid therapy could lead to a significant reduction in the amount of CD56+ve endometrial cells [27].
Recent work by Ledee et al. utilising gene expression patterns and cytokine analysis (IL-15/Fn-14 mRNA and IL-I8/TWEAK mRNA) demonstrated dysregulation of endometrial immune profiles in over 80% of cases with RIF [22]. This study also identified that underactivation, as well as overactivation, may have a causative role. A follow-on cohort study also found that adjunctive treatment, tailored to the endometrial status, could significantly improve subsequent live birth rates with ART [23].
There is a lack of study into the actual levels of lymphocytes in the endometrium, and the relative concentrations of each subtype. This directly contrasts to work on blood, where ranges of both proportions and concentrations are widely reported. Development of a technique to measure the concentrations of each subset is needed, and determination of the normal cellular ranges could contribute to the understanding of this advancing field.
Methodology
Analysis was performed on 455 patients, across three university-affiliated ART centres, over a 3-year period. A prospective enrolment design was used. Patients with a history of poor reproductive history, consisting of either pregnancy loss or implantation failure, were offered an endometrial biopsy to assess the local endometrial lymphocyte subsets in advance of their subsequent treatment.
All samples were taken in the luteal phase of the menstrual cycle, following administration of hormone replacement therapy (HRT) regime, to standardise the environment between cases. Oral estradiol hemihydrate was used for endometrial development; then, an office endometrial biopsy (pipelle™) was procured after five completed days (P+5) of vaginal progesterone therapy (Crinone 8%™, Merck). This timing was chosen, as receptivity studies have shown that P+5/LH+7 coincides with the peak window of implantation. There is potential that differences in cell subtypes could occur at different sites in the endometrium, so a standardised four quadrant biopsy technique was utilised to ensure representative sampling of the entire cavity. Samples were maintained in RPMI 1640 (Sigma–Aldrich UK) at room temperature until ready for analysis. Tissue samples were all analysed less than 24 to 48 h after collection, and carefully temperature controlled throughout, in an attempt to prevent uneven immune cell loss. 7-AAD staining confirmed minimal lymphocyte apoptosis under these conditions unlike the endometrium itself which displays highly individual variations independent of sampling time (Supplementary Fig. 1).
The endometrial preparation and flow cytometric processes were first tested by a pilot study, followed by expansion of the antibody panel for CD markers to provide a comprehensive immunophenotype using proportions as previously described in detail [24]. The addition of 100 μL flowcount™ beads (Beckman Coulter UK Ltd.) were added, of a consistent nature and at a defined concentration, allowed for the addition of volumetric counts of the cellular populations (Fig. 1). Initially, the collected tissue was washed in fresh RPMI to avoid peripheral blood or mucus contamination and ensure only endometrium was assessed. The endometrium was then accurately weighed, mechanically dissociated (MACs™ Milteny Biotech), pelleted at × 500g, and re-suspended to a final volume of 1.5 mL in BD staining buffer (BD Biosciences, UK). Using a non-wash protocol, 200 μL of the re-suspended cell solution was added to each flow tube, already prepared with a standardised 100 μL of a suitable antibody cocktail as described below, incubated at room temperature for 20 min, and 900 μL. VersaLyse™ solution (Beckman Coulter UK Ltd.) was used to eliminate red blood cell contamination.Volumetric dilutions employed are illustrated in Supplementary Figure 2.
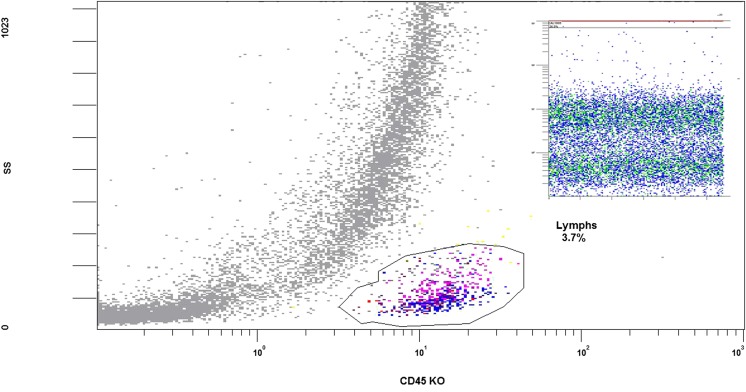
Fig. 1.
Flow cytometer image showing gating of lymphocytes usid CD45 and side scatter and insert, use of flow count flurospheres on FL1 against TIME to illustrate bead capture in a distinct area to all other flourescent cells within the biopsy
Co-localisation of selected antibodies was employed across individual tubes using flow cytometry (Navios™, Beckman Coulter UK LTD) for cellular evaluation, with a 10-colour flow panel and appropriate compensation matrices as previously described [24] and Supplementary Figs. 3, 4 and 5. Cell types were defined according to accepted conventions. Tube 1 assessed uterine/decidual type NK cells (uNK; CD3−, CD16−, CD56bright), peripheral type NKs (pNK; CD16+, CD56dim), natural killer T cells (NKT; CD3+, CD16−, CD56dim), expression of CD57 NK maturity marker (within pNK and NK-T), and B lymphocytes (CD19+). Tube 2 allowed analysis of T lymphocytes (CD4+ and CD8+), and various CD4 subsets including Th1 (CD4+, CD183+, CD196−), Th2 (CD4+, CD183−, CD196−), Th17 (CD4+, CD183−, CD196+), and regulatory T cells (Treg; CD4+, CD127dim, CD25bright). Each tube was made up to a final volume of 100 μL with BD staining buffer.
To allow for patient subgroup analysis, cases with definitive repeated implantation failure (RIF) [n = 149] and recurrent pregnancy loss (RPL) [n = 121] were identified. Although there is no consensus definition, the inclusion criteria for RIF was chosen as > 2 unsuccessful transfers of high morphological grade blastocysts [25]. ASRM and ESHRE guidance was used to define RPL as > 2 clinically detectable concurrent or non-concurrent miscarriages [26, 27]. Patients who presented with miscarriages and/or implantation failure, and were included for analysis, but did not meet the strict definitions for RPL or RIF as described were categorised as either primary (≤ 2 transfers, or questions over embryo quality) or secondary infertility based on their past obstetric history (Table 1). Control samples were taken from cases where the primary aetiology was male factor, with no identifiable female issue. Inclusion criteria for this control group required normal age-related ovarian reserve testing (by AMH and AFC), a normal pelvic ultrasound and saline infusion sonogram (to exclude anatomical causes) and female age ≤ 37 (to limit the influence of oocyte aneuploidy). These cases were chosen as controls because advanced approval to collect and analyse endometrial samples from fertile females in the general population was not obtained with this group therefore representing the most “normal” patients available.
Table 1.
Age profile with deviation, and background obstetric history, of the various patient subgroups investigated. RPL, recurrent pregnancy loss; RIF, recurrent implantation failure; P primary infertility; S secondary infertility; C, control population; MR, miscarriage rate
| Controls | RPL | RIF | P | S | |
|---|---|---|---|---|---|
| Patients (n) | 29 | 121 | 149 | 76 | 80 |
| Mean age | 35.2 | 37.9 | 38.1 | 36.7 | 37.2 |
| SD | 3.1 | 4.0 | 4.4 | 4.5 | 4.1 |
| Mean AMH (pmol/L) | 20.0 | 22.5 | 11.3 | 18.9 | 12.4 |
| Total live births | 10 | 47 | 7 | 0 | 56 |
| Total miscarriages | 4 | 320 | 11 | 0 | 58 |
| MR (%) | 28.6 | 87.2 | 61.1 | – | 50.9 |
The aim of the study was to assess, for the first time, if this flow count technique was valid and reproducible. The primary outcome measure was to determine population percentiles for endometrial lymphocyte subset concentrations (cells/milligram) by flow cytometry. Secondary outcome measures were the comparison of cell counts between patient aetiology subgroups. The tertiary outcome measure was to determine if there was any correlation between lymphocyte subset concentrations and subsequent treatment outcomes following ART cycles from recruited patients over the investigative period. Pregnancy rates, implantation rates and miscarriage rates were calculated. Results were then subdivided based on immunophenotype counts: a high concentration was defined as > 90th percentile, normal between the 25th and 75th percentiles, and low was < 10th percentile.
Advanced approval for the study was obtained from the clinic’s institutional review board, with individual written patient informed consent for the biopsy procedure and subsequent analysis taken, and recorded in the medical chart. The research did not receive any funding or grants from agencies in the public, commercial, or not-for-profit sectors. IBM SPSS v24 was employed to perform appropriate statistical analysis. Using skewness–kurtosis plots, it was determined that the population data was not normally distributed for the majority of parameters, so medians and percentiles were selected as the most representative statistic (Table 3). Non-parametric analysis (Kruskal–Wallis and Mann–Whitney U) was used to look for median differences between groups for scale variables. Results expressed as proportions were compared using chi-square analysis.
Table 3.
Median endometrial concentrations (cells/milligram) of lymphocyte subtypes, across the patient aetiological groups. p values determined using Kruskal Wallis test (SPSS v24). Italics and * indicate statistically significant p values
| Cell type | Controls (cells/milligram) | RPL (cells/milligram) | RIF (cells/milligram) | Primary (cells/milligram) | Secondary (cells/milligram) | p value (overall) |
|---|---|---|---|---|---|---|
| Total lymphocytes | 11,597.3 | 11,469.0 | 9761.3 | 10,193.2 | 11,079.6 | 0.314 |
| pNK | 65.0 | 133.7 | 97.5 | 162.5 | 134.6 | 0.016* |
| NK-T | 278.6 | 305.8 | 231.1 | 312.7 | 311.7 | 0.019* |
| uNK | 5696.1 | 4823.0 | 4917.3 | 5269.6 | 4647.5 | 0.985 |
| CD57 | 32.5 | 55.6 | 33.9 | 63.0 | 51.3 | 0.227 |
| B cells | 48.8 | 79.6 | 70.9 | 80.2 | 80.8 | 0.010* |
| CD8+ | 1462.5 | 1722.8 | 1180.8.7 | 1632.0 | 1933.8 | 0.001* |
| CD4+ | 1007.5 | 1148.4 | 855.6 | 921.9 | 1238.1 | 0.020* |
| T Reg | 97.5 | 78.0 | 54.5 | 63.0 | 65.0 | 0.070 |
| Th1 | 487.5 | 873.4 | 585.0 | 835.7 | 807.7 | 0.032* |
| Th2 | 139.3 | 83.5 | 65.0 | 65.0 | 66.9 | 0.068 |
| Th17 | 31.5 | 19.9 | 22.2 | 24.4 | 32.5 | 0.959 |
Results
Biopsies were analysed for 426 cases and 29 controls to assess endometrial lymphocyte concentrations over the study period. Evaluation of past reproductive history identified 149 patients with clear repeated implantation failure; 121 met the strict criteria for recurrent pregnancy loss; 76 were classified as primary infertility, and 80 had secondary infertility. Demographic and obstetric details across the groups are displayed (Table 1). AMH and age varied across these groups, and ovarian reserve testing results correlated with patient age rather than underlying aetiology as would be expected.
Using this novel flow cytometric technique, the median endometrial lymphocyte count was identified to be 10,562 cells/mg, with an upper limit at the 90th centile of 33,138 cells/mg, suggesting a high degree of variation in this population. Natural killer cells are the major endometrial subset in keeping with published data, and were differentiated into three defined subtypes for further analysis [28]. As has been established, the uterine or decidual type natural killer cell (uNK, CD16−, CD56bright) predominates as the major endometrial lymphocyte (4875 cells/mg). The remaining NK subtypes are all are readily detectable, but present at much lower levels, with median concentrations of 118 cells/mg and 268 cells/mg for peripheral type and NK-T cells, respectively. Interestingly, a proportion of the pNK and NKT populations were identified also co-expressing the maturity marker CD57 in many patients. The potential significance of the presence of this marker for maturity and cytotoxicity needs further study. Regarding endometrial T Lymphocytes, CD8+ cells constitute the majority of this population, at a median concentration of 1532 cells/mg, compared to CD4+ cells at lower count of 1030 cells/mg. Various CD4+ subsets concentrations can also be easily identified by flow cytometry, with Th1 cells as the most prevalent (739 cells/mg). Treg, Th2 and Th17 cells are all present in the endometrium, but at much lower concentrations (Table 2).
Table 2.
Total population endometrial biopsy immunophenotype lymphocyte concentrations (expressed as cells/milligram of endometrial tissue), shown as mean, median and centiles. “Lymphs” is the total concentration of CD45+ lymphocytes in the biopsy cellular population (stromal, endothelial, epithelial, polymorphonuclear etc.)
| Natural killer cells | B cells | T cells | CD4+ subsets | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphs | uNK | NKT | pNK | (CD57+) | CD19+ | CD8+ | CD4+ | T reg | Th1 | Th17 | Th2 | |
| Median | 10,562.5 | 4875.0 | 267.6 | 118.1 | 46.4 | 75.0 | 1532.1 | 1030.2 | 65.0 | 739.4 | 23.5 | 66.9 |
| 10th centile | 3022.5 | 1332.2 | 65.0 | 8.4 | 0.0 | 16.8 | 358.2 | 243.8 | 25.7 | 162.5 | 0.0 | 30.1 |
| 90th centile | 33,138.3 | 15,736.5 | 1183.0 | 748.5 | 317.2 | 422.5 | 6669.0 | 4294.9 | 294.6 | 2720.3 | 162.5 | 312.0 |
Clear and significant differences in lymphocyte subset populations were noted between the different patient aetiology groups (Table 3). Peripheral type NKs were present at higher concentrations in all poor reproductive outcome groups compared to the controls (p = 0.016), potentially identifying this cell type as a useful marker for further research. A similar trend was also observed with CD19+ B lymphocytes (p = 0.01), also suggesting a further marker that could potentially be linked with adverse outcomes. Major differences were identified in the T cell subset concentrations between cases. CD4+ and CD8+ T lymphocytes were increased in RPL patients compared to controls, but when those with a history of implantation failure were assessed, the corresponding values were conversely reduced. Marked differences can be seen when lymphocyte concentrations of miscarriage or implantation failure cases are compared. Pregnancy loss patients have greater concentrations of many lymphocyte subsets, including pNK, NKT, B cells, CD4, CD8, Treg and Th1 (Table 4). The primary and secondary groups had similar concentrations for all parameters, with no significant differences.
Table 4.
Distribution of statistically significant differences (p values) across the patient subpopulations, relative to controls and each other. Significance levels determined with Mann–Whitney U test, using distribution around the median (SPSS v24). Highlighted cells with * indicated statistically significant comparisons
| Cell type | RPL vs control | RIF vs control | RPL vs RIF | Primary vs secondary |
|---|---|---|---|---|
| Total lymphocytes | 0.580 | 0.558 | 0.065 | 0.306 |
| pNK | 0.073 | 0.766 | 0.010* | 0.908 |
| NK-T | 0.437 | 0.299 | 0.004* | 0.786 |
| uNK | 0.863 | 0.802 | 0.972 | 0.501 |
| CD57 | 0.476 | 0.867 | 0.137 | 0.550 |
| B cells | 0.002* | 0.127 | 0.007* | 0.861 |
| CD8+ | 0.888 | 0.047* | 0.001* | 0.287 |
| CD4+ | 0.339 | 0.509 | 0.009* | 0.323 |
| T Reg | 0.832 | 0.071 | 0.008* | 0.516 |
| Th1 | 0.270 | 0.811 | 0.023 * | 0.605 |
| Th2 | 0.179 | 0.020* | 0.131 | 0.864 |
| Th17 | 0.626 | 0.727 | 0.871 | 0.481 |
Recruited patients had 595 embryo transfers performed, 259 from IVF/ICSI cycles and 333 from frozen transfers. Clinical pregnancy success rates from fresh and frozen cycles were equivalent (92/259, 35.5% vs 118/336, 35.1%, p = 0.92) so were grouped together for analysis to improve sample size and statistical power. Implantation rates, pregnancy rates per embryo transfer and miscarriage rates are displayed (Tables 5, 6 and 7). There are observable trends but not statistically significant differences between allocated percentile groups. Continued data collection in appropriately powered studies are required to see if any trends in these areas are potentially significant.
Table 5.
Pregnancy per embryo transfer stratified by lymphocyte subset and associated centile
| Lymphocyte subset | High | Normal | Low | Sig (p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| > 90th C | 25th–75th C | < 10th C | ||||||||
| P | ET | CPR | P | ET | CPR | P | ET | CPR | ||
| uNK | 21 | 58 | 36.2 | 103 | 311 | 33.1 | 16 | 66 | 24.2 | 0.29 |
| pNK | 24 | 70 | 34.3 | 89 | 281 | 31.7 | 21 | 70 | 30.0 | 0.85 |
| CD4 | 24 | 67 | 35.8 | 111 | 298 | 37.2 | 17 | 69 | 24.6 | 0.14 |
| CD8 | 24 | 64 | 37.5 | 107 | 302 | 35.4 | 17 | 65 | 26.2 | 0.30 |
P number of pregnancies, ET embryo transfers, CPR clinical pregnancy rate (%)
Table 6.
Implantation rates stratified by lymphocyte subset and associated centile
| Lymphocyte subset | High | Normal | Low | Sig (p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| > 90th C | 25th–75th C | < 10th C | ||||||||
| EmT | GS | IR | EmT | GS | IR | EmT | GS | IR | ||
| uNK | 91 | 22 | 24.2 | 499 | 91 | 18.2 | 109 | 14 | 12.8 | 0.12 |
| CD4 | 115 | 23 | 20.0 | 479 | 106 | 22.1 | 110 | 15 | 13.6 | 0.33 |
| CD8 | 114 | 21 | 18.4 | 464 | 103 | 22.2 | 107 | 13 | 12.1 | 0.058 |
EmT number of embryos transferred, GS number of gestational sacs seen, IR implantation rate (%)
Table 7.
Miscarriage rates stratified by lymphocyte subset and associated centile
| Lymphocyte subset | High | Normal | Low | Sig (p) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| > 90th C | 25th–75th C | < 10th C | ||||||||
| CP | OP | MR | CP | OP | MR | CP | OP | MR | ||
| pNK | 24 | 9 | 62.5 | 87 | 42 | 51.7 | 21 | 13 | 38.1 | 0.26 |
| B Cell | 24 | 8 | 66.7 | 84 | 39 | 53.6 | 10 | 5 | 50.0 | 0.48 |
| CD4 | 24 | 12 | 50.0 | 108 | 54 | 50.0 | 17 | 7 | 58.8 | 0.79 |
| CD8 | 24 | 8 | 66.7 | 105 | 52 | 50.4 | 17 | 6 | 64.7 | 0.24 |
CP Total clinical pregnancies, OP ongoing (viable) pregnancies, MR miscarriage rate (%)
Discussion
Endometrial lymphocyte concentrations have been calculated and described in detail, using a novel rapid and objective technique. A unique and reproducible methodology has been developed, allowing the determination of endometrial lymphocyte subset concentrations in cells/milligram, and corresponding percentile-based reference ranges. This newly derived information has the potential to provide a useful basis for further research methodologies in the field of reproductive immunology. Although flow cytometry is a widely used technique for assessing cell populations, it has not typically been employed for endometrial evaluation, where immunohistochemistry has traditionally been utilised. Flow techniques, however, have the potential to provide population and subpopulation analysis in a rapid, detailed, quantitative assessment of now, both cellular concentrations and proportions, with minimal inter-observer subjectivity bias. Actual in situ determination of the location of the examined cells is only lacking. It should be said that consistency with sample timing in menstrual cycle and the tissue preparation techniques are extremely important when collecting and analysing endometrium in order to ensure robust results [29]. Additional potential benefits of flow cytometric evaluation include automation of the analytical phase, further increasing reproducibility, as well as the ability to analyse very small tissue samples, making it a useful tool when assessing endometrium where a typical pipelle biopsy yields approximately 300 mg of tissue.
In this study, several significant differences in individual endometrial lymphocyte subtype concentrations between cases with adverse outcomes and a predefined control population can be seen, as well as between patient populations presenting specifically with miscarriage and implantation failure (Figure 2). The underlying pathology of these latter conditions in particular is still poorly understood but an immunological component has been frequently proposed and may well be relevant in certain cases. Natural killer cells, for example, uniquely exist at the frontier between innate and adaptive immunity and have many characteristics in common with CD8 T cells. They are large granular lymphocytes derived from common lymphoid precursor cells expressing the CD56 surface marker, maturing to produce CD56 bright or dim cells, depending on surface receptor expression. Uterine NKs are said to be intimately involved in trophoblast invasion and spiral artery formation, clear requirements for successful embryo implantation. All NKs, however, have the potential to cause cytotoxic effects by releasing granular components (such as perforin and granzymes) or a variety of potent cytokines (e.g. TNFa, IFNg, GCSF) [30]. The cytotoxicity, gene expression and cytokine profiles of different NK cell subsets vary, possibly influenced by their location. Thus, uterine NKs in general behave quite differently to peripheral type NKs. These latter cells are mainly CD56dim with strong expression of the CD16 transmembrane receptor, while uNK cells are exclusively CD56bright and lack CD16 [31, 32]. CD56dim cells are also more mature with a higher cytotoxic potential than CD56bright [33] The immunoregulatory potential of uterine NK cells is thought to be different to that of peripheral type NKs [34]; however, under the influence of pro-inflammatory cytokines, or other agents, a phenotypic switch to a more aggressive profile is hypothesised [24]. It is likely that pNKs, in particular, are recruited from the peripheral blood as opposed to resident in endometrial tissue. Presence of the CD57 maturity marker could support this theory, as the maturation phases needed to become CD57 positive are unlikely to be completed over the length of one menstrual cycle. A local endometrial origin for uNK cells is, therefore, supported by the consistent observed absence of CD56dim and CD57 markers in this cell type.
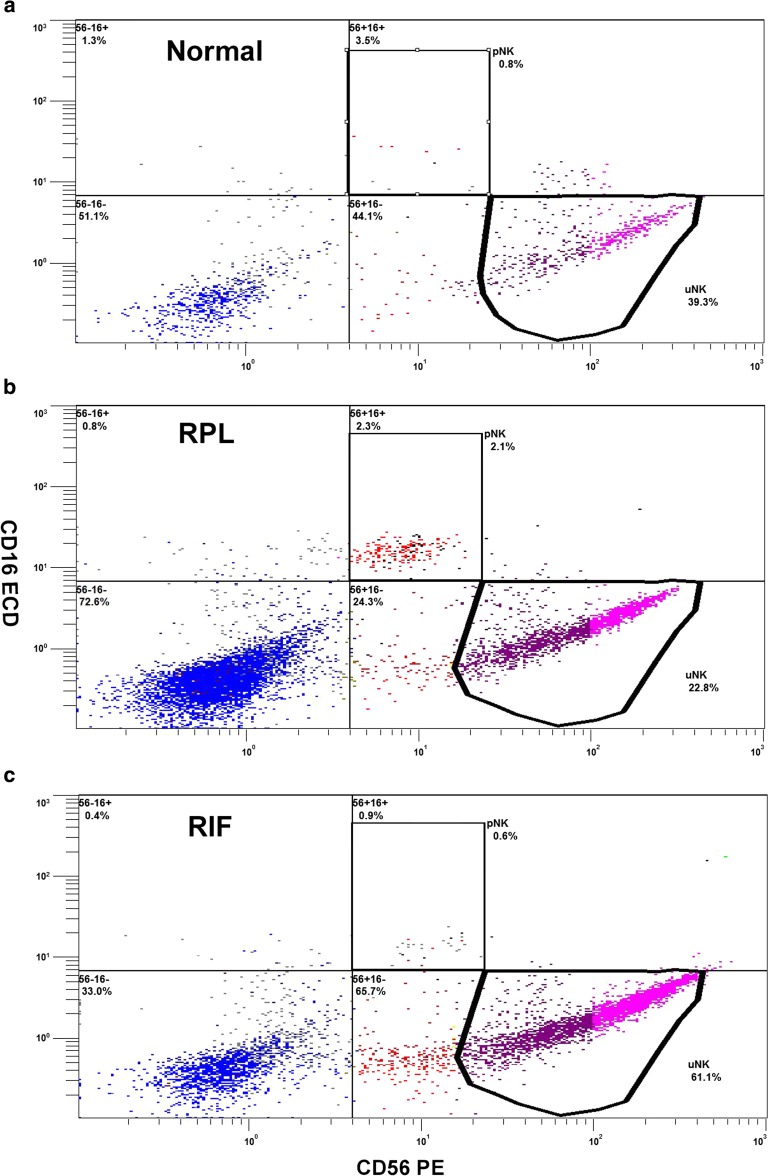
Fig. 2.
Flow cytometer montage of the uNK, pNK distribution percentages/counts from three individuals presenting as either “normal”, recurrent implantation failure (RIF) or recurrent pregnancy loss (RPL)
Many studies focusing on these cells in reproductive failure have reported NK cell populations as either numbers or percentages of peripheral blood lymphocytes (flow cytometry), or endometrial stromal cells (immunohistochemistry or flow cytometry), but are often conflicting. A large meta-analysis, for example, found no differences in the percentage of pNK or uNK cells in infertile women compared to controls, no difference in uNK percentage between RPL and controls, but did find a higher pNK percentage in RPL [30]. Lymphocyte concentrations per milligram of tissue presented in this study show similar trends, with no differences in uNK populations between groups, and a trend towards higher pNK concentrations in RPL. Previous work using this same flow technique as described, but for analysing lymphocyte proportions rather than concentrations, identified that the relative total lymphocyte uNK proportion was significantly elevated in RIF, and reduced in RPL [33]. Interestingly, when cellular concentrations are compared, no significant difference in counts is seen between patient aetiologies. Rather than being highest, as was reported with percentages, there are in fact similar uNK concentrations in RIF (4917 vs 5696 cells/mg, p = 0.985). These marked differences between techniques in largely the same patients highlight the challenges encountered when trying to determine if counts or proportions are the more appropriate marker. Previous, studies have also associated lower levels of CD56bright uNK cells with reproductive failure [35]. Although differences are not significant, there could be a possible trend to lower numbers of uNK, Treg, and Th2 concentrations in patients with implantation failure specifically.
Natural killer cell numbers are, of course, only one component of the numerous complex immunological pathways involved in implantation and fetal development. The alloimmune balancing act between T regulatory cells, cytokines, NKTs and Th2 cell bias, for example, in the production of IgG blocking antibodies (to protect the developing embryo and placenta from maternal circulating NKs and T cells) is well established [36], while the third trimester is actually characterised by strong maternal systemic inflammation [37]. T lymphocytes, therefore, are also believed to be involved in the complex interplay that leads to implantation. A higher endometrial CD4/CD8 ratio, elevated CD4 levels and lower CD8 have all been reported in women with implantation failure when compared to controls [38]. Reduced CD8 levels have also been reported in recurrent pregnancy loss [39]. Examining concentrations in this study has shown that CD4 and CD8 counts are significantly lower in implantation failure perhaps supporting a role, either directly or indirectly, for these cells in this complex process. Endometrial B lymphocytes have long been proposed as having an association with recurrent pregnancy loss [21]. Analysing endometrial concentrations confirms that B cells are significantly elevated in all subfertile groups, with high levels seen in cases with RPL, supporting a possible role as a diagnostic marker. Unlike peripheral blood, the uterine environment does not appear to produce B1 cells (CD5+, CD19+) often associated with auto-antibody formation. CD5+ represents a T cell maturity marker and so may indicate a local origin for these lymphocytes. B cell presence itself may not always be deleterious, however, as murine models suggest that the production of protective IL-10 producing B cells (B10 cells) is desirable [40], additionally, blocking antibody production, a cornerstone of materno-fetal tolerance most likely occurs at the synctiotrophoblast level.
Variations in lymphocyte counts and proportions, across differing patient aetiologies, are of course interesting, but there is controversy regarding whether lymphocyte levels themselves have any effect on clinical outcome. A large systematic review has reported that neither uNK or pNK levels had any correlation with either implantation failure or miscarriage [14]. There are however numerous other individual studies suggesting a positive correlation [35, 41, 42]. Potential trends such as the effects of lower uNK/CD4/CD8 concentrations on implantation, or elevated pNK/B/CD8 counts on miscarriage, did not show any statistically significant effect on the subsequent ART outcome in this study population. The reasons for this may be multifactorial; many elements must come together for a successful live birth to be realised in an infertile patient; additionally, the sample size chosen here was not initially determined to evaluate these (tertiary) outcome measures. Nonetheless, the findings can be useful to generate additional hypotheses to guide further trials. Perhaps, the associated cytokine production profiles or identification of inhibitory/activation markers may be a more relevant area for further research. These highly variable findings suggest that rather than immunosuppressive therapeutic agents, which seem to be the “catch all” response in many cases, focusing on stimulatory techniques such as endometrial scratch or G-CSF administration may be more useful in some individuals, particularly in those presenting with implantation failure. Personalised treatment has, indeed, shown success at improving the clinical pregnancy rate in this patient population [22, 43], but it is unclear whether systemic changes are reflected in modifications at the uterine level.
A strength of this study is the fact that the same patient population has previously been examined using immune cell percentages, and now, albeit in a slightly smaller cohort, are being examined using a cell count technique, ostensibly allowing a direct comparison of both methods in the same group of patients. Unfortunately, there are also several limitations to the study. In the absence of preimplantation embryo genetic screening or post-miscarriage cytogenetic analysis, we cannot determine which patients had aneuploid embryos transferred. Limiting the inclusion criteria to proven chromosomally normal embryos would strengthen the data set by excluding the largest cause of implantation failure. Significant numbers of primary and secondary infertility patients were also studied, and these groups have the potential to be more heterogeneous in aetiology than strict RIF or RPL cases. As these groups may have higher rates of embryo aneuploidy, due to less rigid classification criteria, it is possible that many of these cases would have normal endometrial lymphocyte populations. When analysing post-biopsy treatment outcomes as a tertiary measure, the sample size was unfortunately a major limitation. To fully evaluate these findings, larger prospective multicentre data collection is necessary, to allow stratification between fresh or frozen transfers, and female age groups, for example, to confirm if any impact of variations on reproductive outcome is substantive.
Further understanding of the normal endometrial immunophenotype is required to advance this interesting area, and when combined with a more detailed interpretation of various abnormal deviations, it could potentially diagnose a subgroup of infertility patients currently labelled as unexplained. Failure to achieve a live birth despite transferring morphologically high grade or proven euploid blastocysts is a major challenge in reproductive medicine. The development and validation of more detailed endometrial profiling techniques, assessing both receptivity and immunological factors, could help to discover research areas and identify therapeutic interventions for the implantation failure and recurrent miscarriage populations.
Electronic supplementary material
Co-localisation of CD45 lymphocytes with 7-AAD illustrating only live endometrial derived cells are analysed in the lymphocytes gate (JPG 460 kb)
Illustration of mathematical adjustment accounting for biopys weight and dilution in conjunction with flow count fluorospheres to allow the cells to be expressed in a standardised mg format (DOCX 26 kb)
Fluorophores used, detectors and NAVIOS™cytometer compensation settings (DOCX 615 kb)
Flow cytometer images illustrating, in one individual RPL patient, the gating strategy for, in the first instance, (A) dissociated CD45+ lymphocytes separated from the other stromal, epithelial and morphonuclear cells of the endometria. And the cell types investigated, (B) Natural killer Tcells (CD3+ CD56+), (C) peripheral type natural killer cells (CD16+ CD56dim+), uterine natural killer cells (CD16- CD56bright) and (D) CD57+ natural killer cells entirely associated with the CD56dim NK’s (JPG 1485 kb)
broadens the markers into (A) B-cells (CD19+), (B) CD4+ and CD8+ cells, as they appear in the broad lymphocyte gate and (C) as they appear in a CD3+ Tcell gate. Panel D shows the CD4+ subtypes in their various subclasses. Gating here is more difficult and based on individual FMO findings. Panel E illustrates T regulatory cells (CD3+, CD4+ CD25+ CD127dim) (JPG 1457 kb)
Abbreviations
- PGT-A
Preimplantation genetic analysis-aneuploidy
- RPL
Recurrent pregnancy loss
- RM
Recurrent miscarriage
- RIF
Repeat implantation failure
- ART
Assisted reproductive technologies
- AMH
Anti-Mullerian hormone
- AFC
Antral follicle count
- HRT
Hormone replacement therapy
- uNK
Uterine type natural killer cells
- pNK
Peripheral blood type natural killer cells
- CD
Cluster of differentiation
- Th1
T helper type 1 (pro-inflammatory)
- Th2
T helper type 2 (anti-inflammatory)
- TNFa
Tumour necrosis factor alpha
- GCSF
Growth colony stimulating factor
Compliance with ethical standards
Advanced approval for the study was obtained from the clinic’s institutional review board, with individual written patient informed consent for the biopsy procedure and subsequent analysis taken, and recorded in the medical chart.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lathi RB, Westphal LM, Milki AA. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril. 2008;89(2):353–357. doi: 10.1016/j.fertnstert.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 2.El Hachem H, et al. Recurrent pregnancy loss: current perspectives. Int J Women's Health. 2017;9:331–345. doi: 10.2147/IJWH.S100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SEM, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–133. doi: 10.1093/humupd/dmv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozdag G, Aksan G, Esinler I, Yarali H. What is the role of office hysteroscopy in women with failed IVF cycles? Reprod BioMed Online. 2008;17(3):410–415. doi: 10.1016/S1472-6483(10)60226-X. [DOI] [PubMed] [Google Scholar]
- 5.Riccio L, et al. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Stern C, Chamley L. Antiphospholipid antibodies and coagulation defects in women with implantation failure after IVF and recurrent miscarriage. Reprod BioMed Online. 2006;13(1):29–37. doi: 10.1016/S1472-6483(10)62013-5. [DOI] [PubMed] [Google Scholar]
- 7.Di Simone N, et al. Antiphospholipid antibodies affect human endometrial angiogenesis: protective effect of a synthetic peptide (TIFI) mimicking the phospholipid binding site of beta(2) glycoprotein I. Am J Reprod Immunol. 2013;70(4):299–308. doi: 10.1111/aji.12130. [DOI] [PubMed] [Google Scholar]
- 8.Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. 2003;9(6):515–522. doi: 10.1093/humupd/dmg045. [DOI] [PubMed] [Google Scholar]
- 9.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97(5):1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol. 2014;72(2):129–140. doi: 10.1111/aji.12234. [DOI] [PubMed] [Google Scholar]
- 11.Hill JA. Immunological contributions to recurrent pregnancy loss. Baillieres Clin Obstet Gynaecol. 1992;6(3):489–505. doi: 10.1016/S0950-3552(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 12.Franasiak JM, Scott RT. Contribution of immunology to implantation failure of euploid embryos. Fertil Steril. 2017;107(6):1279–1283. doi: 10.1016/j.fertnstert.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 13.van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85(1):4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 14.Tang AW, Alfirevic Z, Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod. 2011;26(8):1971–1980. doi: 10.1093/humrep/der164. [DOI] [PubMed] [Google Scholar]
- 15.Moffett A, Shreeve N. Reply: first do no harm: continuing the uterine NK cell debate. Hum Reprod. 2016;31(1):218–219. doi: 10.1093/humrep/dev290. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124(5):1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quenby S, Farquharson R. Uterine natural killer cells, implantation failure and recurrent miscarriage. Reprod BioMed Online. 2006;13(1):24–28. doi: 10.1016/S1472-6483(10)62012-3. [DOI] [PubMed] [Google Scholar]
- 19.Vassiliadou N, Bulmer JN. Immunohistochemical evidence for increased numbers of ‘classic’ CD57+ natural killer cells in the endometrium of women suffering spontaneous early pregnancy loss. Hum Reprod. 1996;11(7):1569–1574. doi: 10.1093/oxfordjournals.humrep.a019439. [DOI] [PubMed] [Google Scholar]
- 20.Alecsandru D, Garcia-Velasco JA. Why natural killer cells are not enough: a further understanding of killer immunoglobulin-like receptor and human leukocyte antigen. Fertil Steril. 2017;107(6):1273–1278. doi: 10.1016/j.fertnstert.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Lachapelle MH, et al. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996;156(10):4027–4034. [PubMed] [Google Scholar]
- 22.Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, Dubanchet S, Gahéry H, Bensussan A, Chaouat G. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. 2016;75(3):388–401. doi: 10.1111/aji.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledee N, et al. Uterine immune profiling for increasing live birth rate: a one-to-one matched cohort study. J Reprod Immunol. 2017;119:23–30. doi: 10.1016/j.jri.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Marron K, Walsh D, Harrity C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J Assist Reprod Genet. 2018. [DOI] [PMC free article] [PubMed]
- 25.Laufer N, Simon A. Recurrent implantation failure: current update and clinical approach to an ongoing challenge. Fertil Steril. 2012;97(5):1019–1020. doi: 10.1016/j.fertnstert.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Medicine, T.P.C.o.t.A.S.f.R Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 27.ESHRE, <ESHRE RPL Guideline_28112017_FINAL.pdf>. 2017.
- 28.Harrity, C., Bereir M.M., Walsh D.J., Marron K.D., Moving from peripheral blood to local uterine immunophenotype analysis in patients with poor reproductive history: pilot study of a novel technique. Ir J Med Sci, 2018. [DOI] [PubMed]
- 29.Laird, L., Li, B, <RCOG 2016 guidelines.pdf>. 2016.
- 30.Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(3):429–438. doi: 10.1093/humupd/dmt056. [DOI] [PubMed] [Google Scholar]
- 31.Kwak-Kim J, Gilman-Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol. 2008;59(5):388–400. doi: 10.1111/j.1600-0897.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 32.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42(4):511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Thiruchelvam U, Wingfield M, O'Farrelly C. Natural killer cells: key players in endometriosis. Am J Reprod Immunol. 2015;74(4):291–301. doi: 10.1111/aji.12408. [DOI] [PubMed] [Google Scholar]
- 34.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198(8):1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, Kimura H, Mizunuma H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90(1):105–110. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Hyde KJ, Schust DJ. Immunologic challenges of human reproduction: an evolving story. Fertil Steril. 2016;106(3):499–510. doi: 10.1016/j.fertnstert.2016.07.1073. [DOI] [PubMed] [Google Scholar]
- 37.Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24:S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 38.Y., E.-S., <Endometrial CD4+ And CD8+ in Women with Failed Implantation Following Embryo Transfer.pdf>. International Journal of Obstetrics and Gynaecology Research (IJOGR), 2016. Vol. 3 (1): p. 209–220.
- 39.Mamedaliyeva NM, et al. Clinical and immunological parallels in pregnancy loss. Gynecol Endocrinol. 2017;33(sup1):5–7. doi: 10.1080/09513590.2017.1404238. [DOI] [PubMed] [Google Scholar]
- 40.Fettke F, et al. B cells: the old new players in reproductive immunology. Front Immunol. 2014;5:285–5. [DOI] [PMC free article] [PubMed]
- 41.Chen X, Mariee N, Jiang L, Liu Y, Wang CC, Li TC, Laird S. Measurement of uterine natural killer (uNK) cell percentage in the peri-implantation endometrium from fertile women and women with recurrent reproductive failure: establishment of a reference range. Am J Obstet Gynecol. 2017;217:680.e1–680.e6. doi: 10.1016/j.ajog.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Coulam CB, Acacio B. Does immunotherapy for treatment of reproductive failure enhance live births? Am J Reprod Immunol. 2012;67(4):296–304. doi: 10.1111/j.1600-0897.2012.01111.x. [DOI] [PubMed] [Google Scholar]
- 43.Harrity C, Shkrobot L, Walsh D, Marron K. ART implantation failure and miscarriage in patients with elevated intracellular cytokine ratios: response to immune support therapy. Fertil Res Pract. 2018;4:7. doi: 10.1186/s40738-018-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-localisation of CD45 lymphocytes with 7-AAD illustrating only live endometrial derived cells are analysed in the lymphocytes gate (JPG 460 kb)
Illustration of mathematical adjustment accounting for biopys weight and dilution in conjunction with flow count fluorospheres to allow the cells to be expressed in a standardised mg format (DOCX 26 kb)
Fluorophores used, detectors and NAVIOS™cytometer compensation settings (DOCX 615 kb)
Flow cytometer images illustrating, in one individual RPL patient, the gating strategy for, in the first instance, (A) dissociated CD45+ lymphocytes separated from the other stromal, epithelial and morphonuclear cells of the endometria. And the cell types investigated, (B) Natural killer Tcells (CD3+ CD56+), (C) peripheral type natural killer cells (CD16+ CD56dim+), uterine natural killer cells (CD16- CD56bright) and (D) CD57+ natural killer cells entirely associated with the CD56dim NK’s (JPG 1485 kb)
broadens the markers into (A) B-cells (CD19+), (B) CD4+ and CD8+ cells, as they appear in the broad lymphocyte gate and (C) as they appear in a CD3+ Tcell gate. Panel D shows the CD4+ subtypes in their various subclasses. Gating here is more difficult and based on individual FMO findings. Panel E illustrates T regulatory cells (CD3+, CD4+ CD25+ CD127dim) (JPG 1457 kb)