Abstract
Purpose
The IGF signaling cascade exerts important regulatory functions in human ovarian folliculogenesis. The scope of this study was to evaluate the transcription profile of insulin-like growth factor (IGF) genes during human ovarian follicle development and to analyze follicle fluid levels of key IGF proteins.
Methods
Gene expression profiling was performed with microarray gene analysis. The analysis was assessed from ovarian follicles and granulosa cells (GCs) obtained from isolated stage-specific human ovarian follicles, including preantral follicles, small antral follicles, and preovulatory follicles. Numerous genes involved in the IGF signaling pathway was evaluated and key genes were validated by qPCR from GCs. Protein levels of various IGF components of human follicular fluid (FF) were measured by ELISA and time-resolved immunofluorometric assays (TRIFMA).
Results
The gene expression levels of PAPPA, IGF2, IGF receptors and intracellular IGF-activated genes increased with increasing follicle size. This was especially prominent in the late preovulatory stage where IGF2 expression peaked. Protein levels of intact IGF binding protein-4 decreased significantly in FF from large preovulatory follicles compared with small antral follicles concomitant with higher protein levels of PAPP-A. The IGF modulators IGF-2 receptor, IGFBPs, stanniocalcins, and IGF-2 mRNA binding proteins were all observed to be expressed in the different follicle stages.
Conclusions
This study confirms and highlights the importance of PAPP-A regulating bioactive IGF levels throughout folliculogenesis and especially for the high rate of granulosa cell proliferation and expression of key ovarian hormones important in the last part of the follicular phase of the menstrual cycle.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01432-x) contains supplementary material, which is available to authorized users.
Keywords: IGF system, IGFBPs, PAPP-A, Stanniocalcins, Human ovarian follicles
Introduction
Ligands of the insulin-like growth factor (IGF) system entails two small peptides IGF-1 and IGF-2 structurally related to proinsulin. IGFs are intimately involved in cell growth, differentiation, and metabolism in numerous cell types and tissues [1–3] and initiate signaling through the IGF receptor type 1 (IGF1R) [4]. The IGFs also interact with the insulin receptor (INSR) although with lower affinity than that of insulin [5, 6]. In the circulation, the total level of IGFs is approximately 1000 times higher than insulin, which makes masking of IGF bioactivity extremely important. Regulation of the biological activity of the IGFs is very complex and involves six different IGF-binding proteins (IGFBP-1 to 6) that bind the IGFs with different affinities. Hereby, the IGFBPs prolong the IGFs’ half-lives or block the signaling to a varying extent [6, 7].
Specifically, limited proteolytic cleavage of IGFBPs is believed to be a major mechanism by which bioactive IGF is released from IGFBP/IGF complexes. One key IGFBP protease is pregnancy-associated plasma protein-A (PAPP-A), which cleaves IGFBP-2, IGFBP-4, and IGFBP-5, hereby releasing bioactive IGFs [8, 9]. Cleavage of IGFBP-4 may be limited to PAPP-A only, while other proteinases are capable of cleaving IGFBP-2 and IGFBP-5 [9, 10]. Cleavage of IGFBP-5 is also accomplished by PAPP-A2 (the only global homolog of PAPP-A), HTRA serine peptidase 1 (HTRA1), and the metalloproteinase ADAM9 [11–13]. Cleavage of IGFBP-5 appears to be crucial for proteolysis of IGFBP-4/IGF complexes by PAPP-A since IGFBP-5 sequesters IGFs from IGFBP-4 [14].
When the IGFs bind and activate their corresponding receptors, intracellular signaling proteins termed insulin receptor substrate 1 and 2 (IRS1 and IRS2) become phosphorylated and subsequently activate additional signaling proteins such as AKT that activates intracellular pathways important for cell growth and metabolism [1, 15].
IGF activity is also modulated by the IGF-2 receptor (IGF2R), which is homologous to the cation-independent mannose-6-phosphate receptor. IGF2R internalizes the IGFs upon binding and transports the ligands to lysosomes, thus, downregulating IGF bioavailability [16]. Additionally, the IGF2R exists as a soluble form found in the circulation, where it reduces the biological activity of IGF-2 [17, 18].
Several studies have shown that the IGF system impact on human ovarian follicular development [19–23]. The IGFs’ signal via the IGF1R that is expressed on both granulosa cells (GCs) and theca cells, and has an effect on follicular growth, steroidogenesis, and development important for a successful pregnancy [23–26]. In cultured bovine and human theca cells, androgen production is enhanced when stimulated with IGF-2 in synergy with LH, while androgen production is blocked by adding an IGF1R antibody [27, 28]. Previously, IGF-2 have shown to stimulate estradiol production and follicle diameter in human preantral follicles in culture [29]. This effect was inhibited by IGFBP-4. Further, FSH has been shown to enhance IGF-2 expression in cultured human cumulus cells, which synergistically with FSH increased cell proliferation and expression of CYP19a1 [30]. A recent study in our laboratory supports these earlier studies and proposed that PAPP-A plays an important role in regulating IGF activity [31]. In addition, PAPP-A antigen was found to shift from the theca cells in the small antral follicles to the GCs layer in larger antral follicles with highest staining in GCs from preovulatory follicles, possibly reflecting importance of an augmented IGF activity mediating a high proliferation rate of GCs in growing antral follicles.
Women who become pregnant after undergoing ovarian stimulation, which results in high levels of estradiol, show significantly lower levels of PAPP-A as compared to women who become pregnant naturally [32, 33]. This has been measured at the end of the first trimester in connection with prenatal screening, and the reference interval for normal PAPP-A levels is reduced if the woman underwent ovarian stimulation. In fact, the higher the estradiol concentration during ovarian stimulation, the lower the levels of PAPP-A at prenatal screening [32], which suggests that aberrant IGF signaling is an underlying cause. Fetal growth and development may also be influenced by this abnormal PAPP-A/IGF environment since studies have shown that low levels of PAPP-A associate with high risk of having a child with low birth weight [34, 35].
Previously, two potent inhibitors of PAPP-A, stanniocalcin 1 and 2 (STC1 and STC2), have been characterized [36, 37]. These proteins are known to be expressed and form complexes with PAPP-A in the human ovaries [38]. Additionally, the proteolytic function of PAPP-A is inhibited by the proform of eosinophil major basic protein (proMBP), which forms a heterotetrameric disulfide-bound 2:2 complex with PAPP-A [39, 40].
Post-transcriptional regulation of IGF-2 is an extra layer of control, which occurs via IGF-2 mRNA binding proteins (IMPs). Previous studies have shown antigen levels of IMPs in the adult human ovary [41].
Thus, numerous reports suggest that the IGF system is intimately involved in ovarian follicle development and pregnancy and involves a highly complex regulation of bioactive IGFs.
The aim of the present study was to analyze the transcription profile of IGF genes in isolated stage-specific human follicles or granulosa cells spanning the entire human folliculogenesis and to evaluate follicular fluid (FF) levels of IGF proteins.
Materials and methods
Human ovarian tissue
Granulosa cells and FF from preantral and antral follicles (prior to the preovulatory stage) for mRNA microarray analysis, qPCR, ELISA, and TRIFMA was isolated from patients undergoing fertility preservation by having one ovary excised. This procedure is offered to women diagnosed with cancer or other diseases, where gonadotoxic treatments leave the patients with a high risk of infertility. Only the cortex tissue is cryopreserved, whereas the medulla tissue is normally discarded or used for research purposes [42, 43]. The patients included in this study were diagnosed with mainly breast cancer, Hodgkin’s lymphoma, cervical cancer, and others. None of the patients (N = 48, aged 24–34 years (median = 30)) had any endocrinological and/or ovarian disease. Preantral follicles were isolated as previous described [44] with a diameter ranging from approximately 45 to 200 μm. Isolated preantral follicles from the same patient were pooled and snap-frozen in liquid nitrogen or lysed in RNA lysis buffer and stored at − 80 °C until RNA purification. FF from small antral follicles exposed on the surface of the ovary was collected with a small syringe, and the GCs were isolated from the FF by centrifugation [45]. The small antral follicles ranged in size from 4 to 6 mm in diameter. GCs and FF were snap-frozen in liquid nitrogen and stored at − 80 °C until RNA purification and measurements.
GCs and FF from preovulatory follicles were obtained from women (N = 24, aged 27.9 ± 3.4 years) undergoing IVF treatment. One sample was obtained prior to ovulation induction (pre-OI) and one sample from the same woman was obtained at oocyte aspiration 36 h after ovulation induction (post-OI) as previously described [46, 47]. None of the women had any endocrine abnormalities (e.g., PCOS, endometriosis). Additionally, mural and cumulus cells from preovulatory follicles post-OI were collected from IVF patients (N = 20, aged 24–33 years) as previously described [48]. These patients were referred for IVF due to male factor and/or tubal disease, unexplained infertility, and mild endometriosis.
The use of surplus ovarian tissue, FF, and GCs was approved by the Ethical Committee of the Capital Region (nos. H-4-2011-102 and H-2-2011-044). The use of GCs from follicles pre- and post-OI was approved by the Danish Scientific Ethical committee (SJ-156) and conducted in accordance with Helsinki Declaration II. The use of paired mural and cumulus cells from preovulatory follicles were approved by the Danish Ethical Committee (VN2004/61).
Microarray
Microarray data from four previously published studies were analyzed (Table 1 shows references, number of follicles included in each stage, and the section describing statistical methods shows how normalization was performed) [46–49]. Despite being published in separate studies, all data were generated on the same platform and by the same laboratory: all on the Affymetrix Human Gene ST v1.0 GeneChip array platform (Affymetrix, Santa Clara, California, USA). The amplification and labeling were performed using the Pico amplification kit version 2 from Nugen (Nugen, San Carlos, CA, USA) following the manufacturer’s guidelines. All arrays were stained with phycoerytrin conjugated streptavidin (SAPE) using the Affymetrix Fluidics Station®450 and scanned in the Affymetrix GeneArray® 2500 scanner to obtain fluorescent images as described previously [49]. RNA quality was evaluated with an Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Pico LabChip (Agilent Technologies, Waldbronn, Germany) and all samples showed a high RIN value. These previous studies have not examined the expression of genes related to the IGF system in detail; only a minor analysis has been included in earlier studies showing expression of IGF genes in the preantral stages and IGF2 expression in the preovulatory stage [47, 49]. Microarrays included in this study covered the entire human folliculogenesis from the preantral stage to after induction of ovulation.
Table 1.
Overview of the included ovarian tissue samples for microarray analysis
Validation of microarray results by quantitative reverse transcriptase (RT)-PCR
For the RT-PCR analysis, GCs were isolated from FF from small antral follicles with a diameter of approximately 6 mm during the cryopreservation procedure. In addition, RT-PCR analysis was also performed on paired mural and cumulus cells from preovulatory follicles post-OI aspirated from women undergoing IVF treatment.
RNA isolation for quantitative RT-PCR
RNA for quantitative RT-PCR measures was isolated from the GCs using Trizol reagents (cat. no. 15596026, Ambion; Life Technologies) and 1-bromo-3-chloropropane (cat. no. B9673-200ml; Sigma) and subsequently with an Rneasy minikit 250 (cat. no. 74106; Qiagen) following the manufacturer’s protocol.
Quantitative RT-PCR analysis
For each sample, first-strand cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit from Invitrogen (Invitrogen, 2012; Life Technologies Corporation) following the manufacturer’s protocol. First-strand cDNA was stored at − 80 °C until quantitative RT-PCR analysis.
Quantitative RT-PCR analysis was performed using TaqMan technology (Applied Biosystems), applying TaqMan Gene Expression Master Mix from Invitrogen (Invitrogen, 2012; Life Technologies Corporation) and predesigned Taq-Man Gene Expression Assays for the following genes: IGFBP5 and PAPP-A (ID = Hs00181213_m1 and Hs01032307_m1) (Invitrogen, 2012; Life Technologies Corporation). The cDNA samples were amplified in duplicates using the LightCycler 480 quantitative PCR instrument (Roche). The expression of IGFBP5 and PAPP-A was normalized to GAPDH, and relative quantification according to the comparative cycle threshold method (LightCycler480 Software, Roche) was used to quantify gene expression.
ELISA measurements
Quantification of total and intact IGFBP-4 protein together with total IGFBP-5 protein and detectable free IGF-2 (no pretreatment was performed to separate IGF-2 from its binding proteins) in FF from small antral follicles and in FF from preovulatory follicles pre- and post-OI were performed with ELISA (AL-126, AL-127, AL-128, and AL-131; Ansh Labs, Texas, USA). This was completed according to the manufacturer’s instructions and calibrator A (0 ng/ml antigen) supplied with the ELISA kits was used for FF dilution. FF were diluted 1:2 for IGFBP-4 measurements, 1:5 for IGFBP-5, and 1:3 for IGF-2 measurements.
This study also combined ELISA data from previous studies of PAPP-A protein level in FF from small antral follicles and in FF from preovulatory follicles pre- and post-OI [31, 50].
Time-resolved immunofluorometric assay
Quantification of STC2 protein was measured in FF from small human antral follicles and in FF from preovulatory follicles pre- and post-OI using a time-resolved immunofluorimetric assay (TRIFMA) based on a previously published assay [36]. Samples were diluted 1:10 in 1% (wt/vol) BSA, TBS-Tween 0.05% (pH 7.4), and incubated in monoclonal STC2-coated wells (2 μg/ml, [36]) at 37 °C for 1 h. After washing, 2 μg/ml biotinylated STC2 antibody [36] was diluted in 1% BSA and 0.05% TBS-Tween and incubated for 2 h. After washing, Eu3+-labeled streptavidin was diluted 1:1000 in TBS-T supplemented with 25 μM EDTA and incubated at RT for 1 h. Subsequently, wells were washed and enhancement buffer (Perkin Elmer) was added. The resulting fluorescent was measured using an Enspire Multimode Plate Reader (Perkin Elmer) with excitation 340 nm/emission 615 nm. Blank values were subtracted, and data were analyzed using GraphPad Prism 7.
Statistical analyses
The microarray data analysis was accomplished with R 3.5.1 with the limma and frma packages from Bioconductor (version 3.8). Background subtraction, quantile normalization of probe expression, and summarizing of probe intensities into gene expression were performed using the frozen Robust Multichip Average (fRMA) preprocessing algorithm established by McCall and colleagues [51, 52] and used in several previous studies [46, 53, 54]. The fRMA algorithm allows one to examine microarrays separately and afterwards combine the data for analysis. Information on estimates of probe-specific effects and variances were available from large microarray databases, and these were used on the new arrays to normalize and summarize data. This is important to remove variation from the different arrays and making them comparable [51, 52]. In order to test for statistically significant difference between the expression levels of the selected genes in the different follicular stages, a moderated t test after linear model fit was used.
Analysis of the qPCR, ELISA, and TRIFMA results from the group of small antral follicles and the groups of preovulatory follicles obtained from women undergoing IVF treatment was performed using a t test, since the GCs and FF samples from women undergoing IVF was paired. When analyzing intact IGFBP-4 levels, the Wilcoxon rank sum and Wilcoxon signed-rank test were used since these values did not follow a normal distribution. A p value below 0.05 was considered statistically significant.
Results
Microarray
A total of 85 individual microarrays from individual GC or follicle samples were included in this study (Table 1). In this study, we describe log2 intensity values ≤ 6 as low gene expression, log2 intensity values between 6 and 10 as moderate gene expression, and log2 intensity values ≥ 10 as high gene expression (Fig. 1).
Fig. 1.
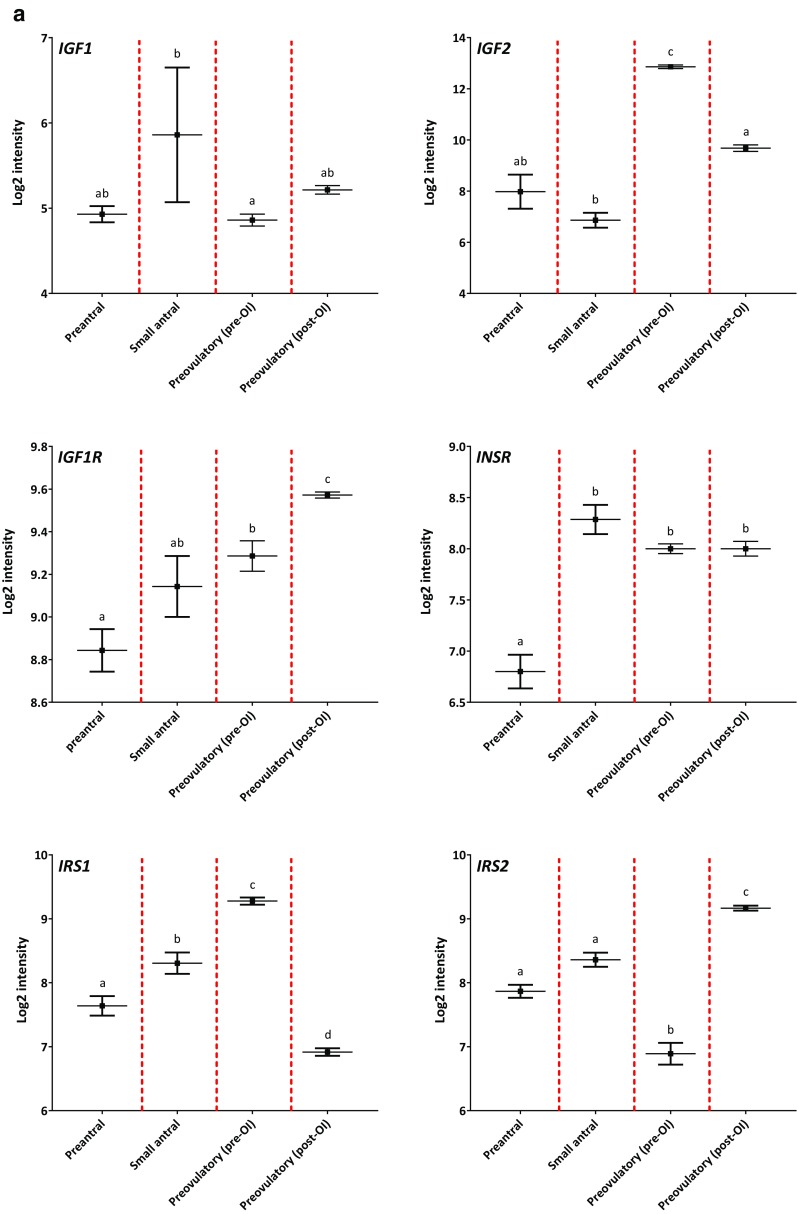
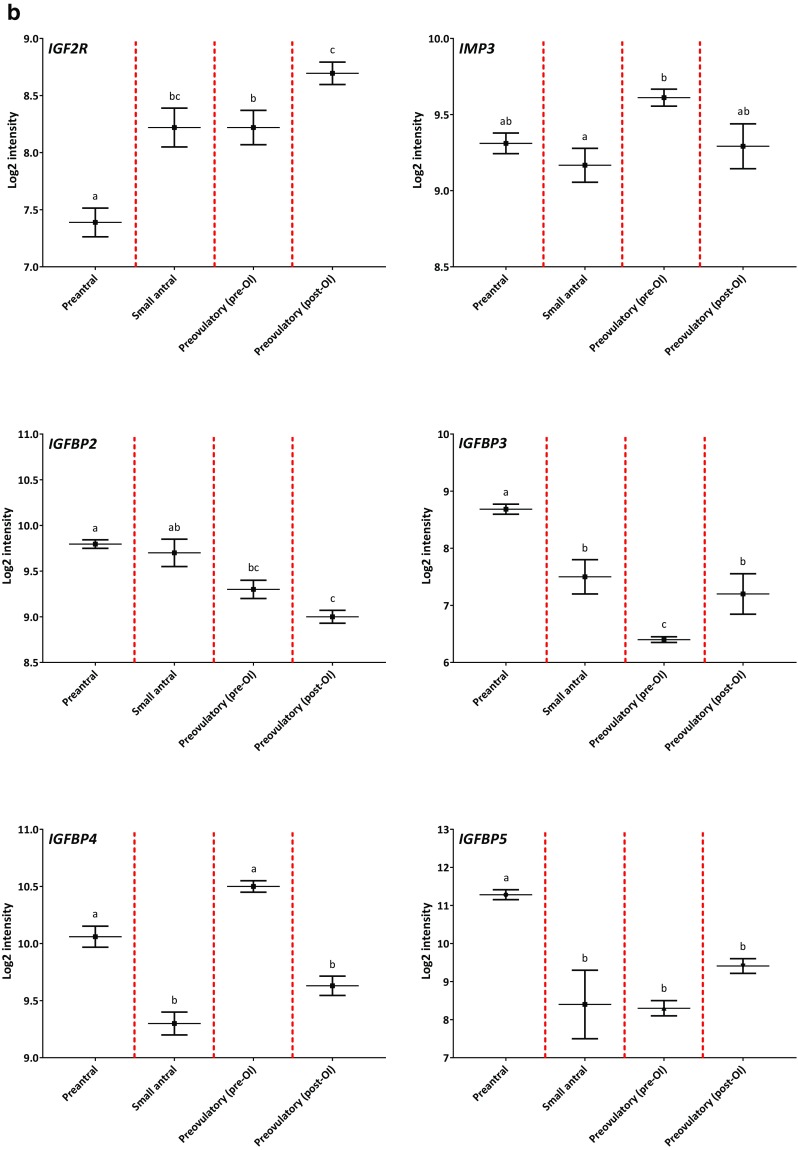
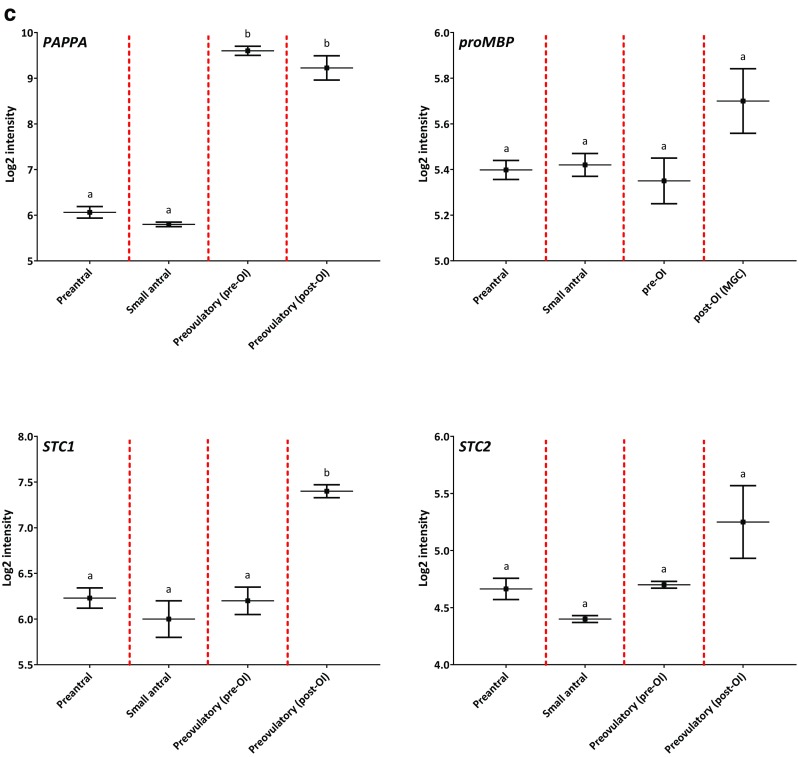
Gene expression profiles of IGF genes during human folliculogenesis. The log2-transformed intensity from the microarray gene expression dataset covering the human folliculogenesis is shown on the y-axis. The following four follicle/granulosa cell (GC) groups are shown on the x-axis: preantral follicles from < 60 μm to > 150 μm in diameter (Preantral), GCs from 4 to 6 mm small antral follicles (Small antral), GCs isolated prior to ovulation induction (Preovulatory (pre-OI)), and GCs isolated after ovulation induction (Preovulatory (post-OI)). The gene name is shown in the upper left corner for each expression profile. Panel (a) shows the profile for IGF1, IGF2, IGF1R, INSR, IRS1, and IRS2. Panel (b) shows the profile for IGF2R and IMP3, IGFBP2, IGFBP3, IGFBP4, and IGFBP5. Panel (c) shows the profile for PAPP-A, ProMBP, STC1, and STC2. Different letters between follicle/GC groups indicate statistical significance. Error bars indicate SEM values
Insulin-like growth factor 1 and 2 (IGF1 and IGF2)
The expression level of IGF1 was overall low in human GCs irrespective of follicular developmental stage (Fig. 1a). In contrast, IGF2 was highly expressed and showed pronounced fluctuations throughout follicular development (Fig. 1a). The expression of IGF2 was moderate in preantral follicles and in small antral follicles, but from this nadir IGF2 expression increased approximately 64-fold in GCs from preovulatory follicles collected pre-OI (p < 0.001) (Fig. 2c). In GCs collected from preovulatory follicles post-OI, the IGF2 expression was reduced 8-fold compared to the peak at pre-OI, but the expression level was significantly higher compared to the level observed in GCs from small antral follicles (p < 0.001).
Fig. 2.
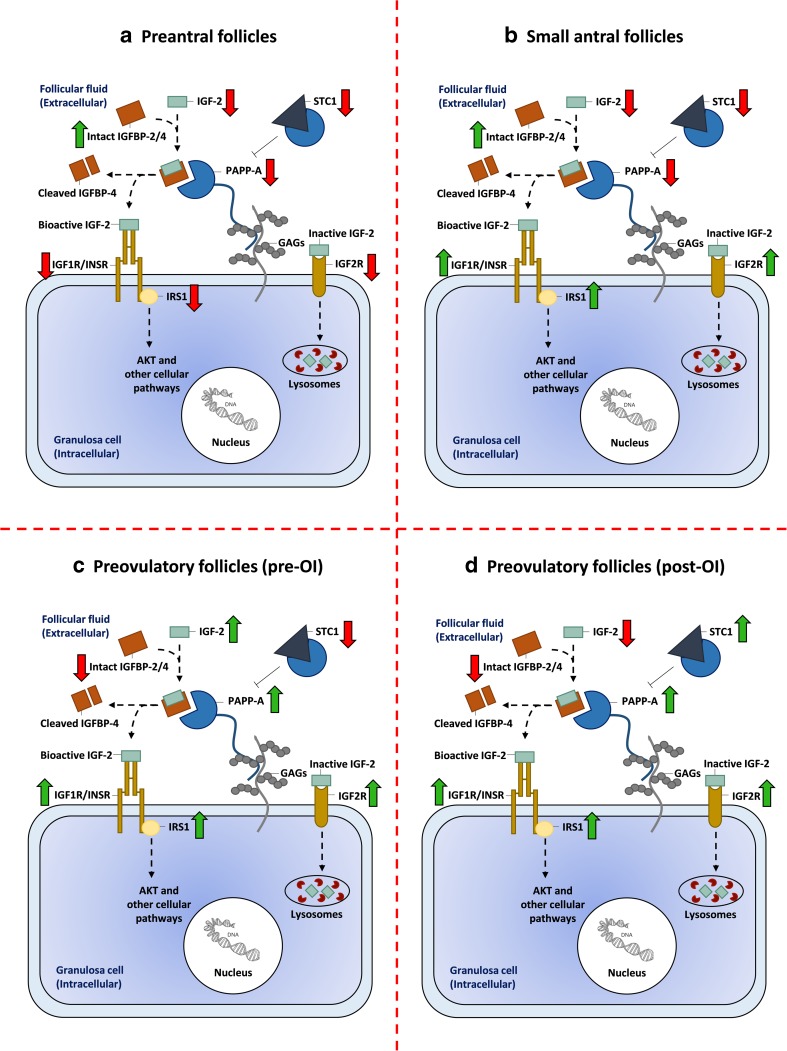
Regulation of IGF-2 bioactivity at the surface of an ovarian granulosa cell. Regulation of IGF bioactivity includes multiple levels of regulatory proteins. This figure depicts some of the most relevant IGF proteins expressed in human ovarian follicles and how they are up- or downregulated in granulosa cells (GCs) from four stages of folliculogenesis: a preantral follicles (< 60 μm to > 150 μm in diameter), b small antral follicles (4–6 mm in diameter), c preovulatory follicles prior to ovulation induction (pre-OI), and d preovulatory follicles after ovulation induction (post-OI). Arrows indicate upregulation (green) or downregulation (red) when comparing the four follicular stages. The following functional statements are based on the observed up- and downregulation of IGF genes in the four follicular stages evaluated in this study: a at the preantral follicle stage, GCs express less IGF-2, IGF1R/INSR, IRS1, PAPP-A, and more IGFBPs that may reflect a limited IGF-2 signaling; b in small antral follicles, GCs continue to express high levels of IGFBPs and less IGF-2 and PAPP-A. In addition, the IGF2R is upregulated in GCs from small antral follicles, which reduces IGF-2 bioactivity. However, small antral follicles also begin to upregulate the IGF1R/INSR, and IRS1 genes important for transducing the signal, indicating a shift to a more active signaling system; c at the preovulatory stage pre-OI, expression of IGF-2, PAPP-A, IGF1R/INSR, and IRS1 increases significantly, whereas IGFBP-2 expression and intact IGFBP-4 protein decreases. This implies an upregulation of IGF bioactivity important for the high rate of GC proliferation and aromatase expression; d at the preovulatory stage post-OI, the GCs downregulate IGF-2 expression and upregulate STC1 that inactivates and modulates PAPP-A activity, which may imply a strict regulation of PAPP-A-mediated IGF signaling after ovulation induction
Insulin-like growth factor receptor type 1 (IGF1R) and insulin receptor (INSR)
The expression level of IGF1R and INSR remained moderate throughout follicular development with an increase as follicle diameter increases (Figs. 1a and 2).
Intracellular signaling proteins (IRS1 and IRS2)
The expression level of IRS1 was moderately expressed in human follicles (Fig. 1a). An increase was observed from preantral follicles to small antral follicles (Figs. 1a and 2). The expression of IRS1 increased significantly in GCs from preovulatory follicles pre-OI compared with small antral follicles (p < 0.001), while the expression level was significantly lower in GCs from preovulatory follicles post-OI compared to earlier stages (p < 0.001).
The expression level of IRS2 was also moderately expressed throughout folliculogenesis (Fig. 1a). The expression level of IRS2 was significantly lower in GCs from preovulatory follicles pre-OI compared to the other follicle groups (p < 0.001); however, the expression level of IRS2 in GCs from preovulatory follicles post-OI was significantly higher compared to the earlier follicle stages (p < 0.001).
Insulin-like growth factor receptor type 2 (IGF2R) and insulin-like growth factor-2 binding protein-3 (IMP3)
The expression level of IGF2R was moderately expressed in human follicles and increased with increasing follicle size (Figs. 1b and 2). The expression level of IMP3 was also moderately expressed in human follicles (Fig. 1b).
Insulin-like growth factor binding proteins (IGFBPs)
The expression level of IGFBP2 was high in the different follicle stages, but a decline was observed in preovulatory follicles (Figs. 1b and 2). A decline in IGFBP3 expression levels was observed in small antral follicles compared with preantral follicles and a further reduction was observed in preovulatory follicles pre-OI compared with small antral follicles (p < 0.001). The expression of IGFBP4 was high in human follicles (Fig. 1b). A significant drop in IGFBP4 expression was observed in small antral follicles compared with preantral follicles. Preovulatory follicles pre-OI expressed higher levels of IGFBP4 compared to small antral follicles (p < 0.001), while the level of IGFBP4 was lower in preovulatory follicles post-OI compared to follicles pre-OI (p < 0.001). The expression level of IGFBP5 was high in preantral follicles but declined significantly in small antral follicles and larger preovulatory follicles (p < 0.001) (Fig. 1b).
Supplementary Fig. 5 displays transcription profiles of IGFBP1 and IGFBP6.
Pregnancy-associated plasma protein-A (PAPPA)
The expression of PAPPA was low to moderate in preantral follicles and in small antral follicles (Fig. 1c). The expression of PAPPA increased markedly in GCs from preovulatory follicles (p < 0.001) (Fig. 2c).
PAPP-A inhibitors: ProMBP, STC1, and STC2
The expression level of proMBP was overall low throughout the different follicle stages (Fig. 1c).
The expression level of STC1 was low to moderate in preantral follicles, small antral follicles, and in GCs from preovulatory follicles pre-OI (Fig. 1c). An increase in the expression level of STC1 was observed in preovulatory follicles post-OI (p < 0.001) (Figs. 1c and 2d). The expression levels of STC2 remained low throughout the different follicle stages (Fig. 1c).
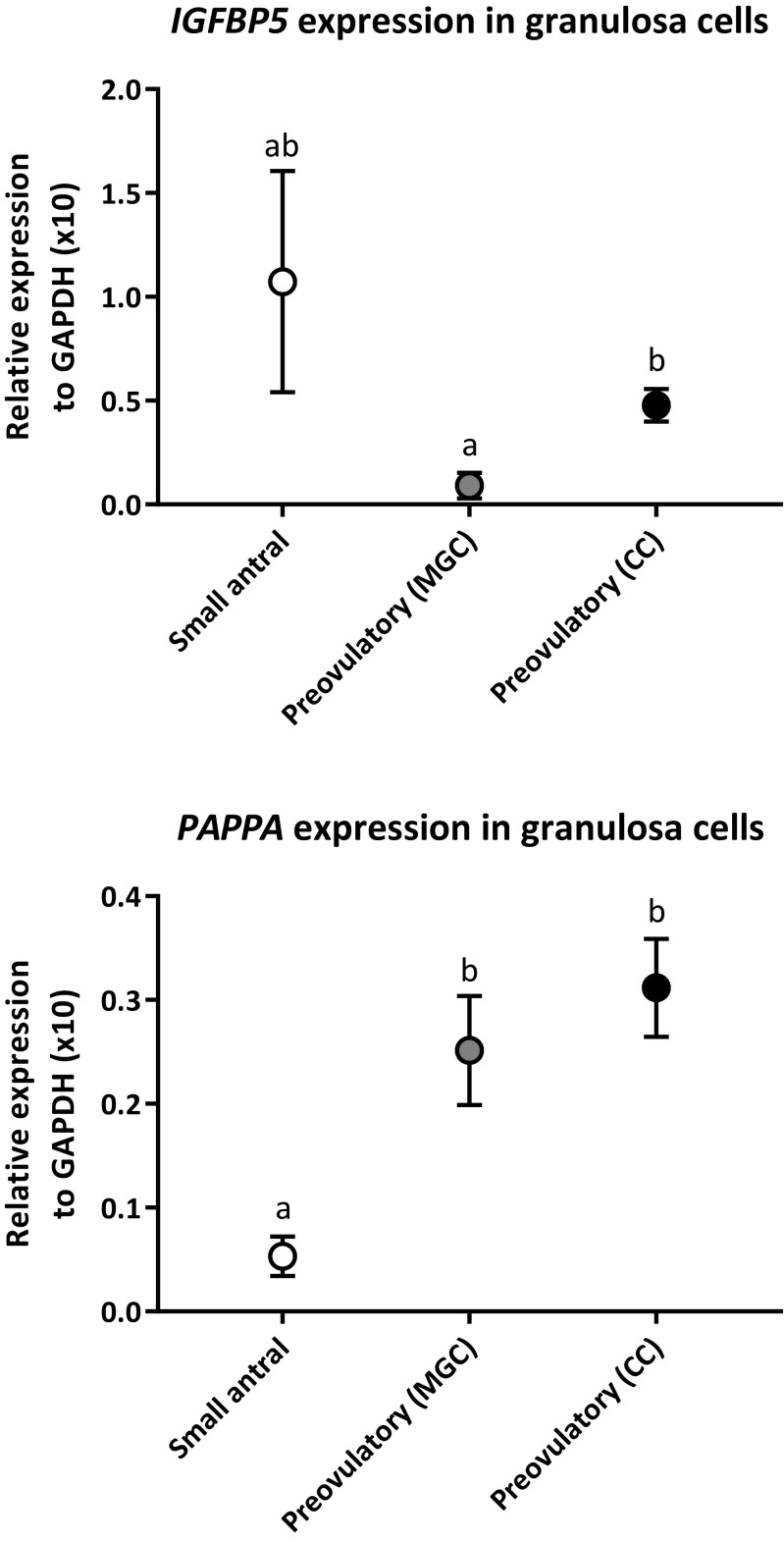
Quantitative RT-PCR of IGFBP5 and PAPPA
To validate the microarray data, mRNA levels of IGFBP5 and PAPPA were measured with qPCR in GCs from small antral follicles together with mural and cumulus cells from preovulatory follicles post-OI (Fig. 3). The gene expression level of IGFBP5 was non-significantly higher in small antral follicles compared to the mural and cumulus cells from preovulatory follicles post-OI. Cumulus cells showed a significantly higher expression of IGFBP5 than mural cells (p = 0.01).
Fig. 3.
RT-qPCR validation of IGFBP5 and PAPPAexpression in GCs from small antral follicles and in mural and cumulus GCs from IVF patients isolated after ovulation induction.IGFBP5 and PAPPA gene expression were normalized to GAPDH expression and the relative expression is displayed. Data are presented as mean values of GC samples measured in duplicates from small antral follicles (Small antral), mural GCs isolated after ovulation induction (Preovulatory (MGC)), and cumulus cells isolated after ovulation induction (Preovulatory (CC)). Error bars indicate SEM values. Different letters between follicle groups indicate statistical significance. Statistical significance for IGFBP5 expression: mural GCs vs. cumulus GCs: p = 0.01. Statistical significance for PAPPA expression: small antral follicles vs. mural GCs: p = 0.006, small antral follicles vs. cumulus GCs: p < 0.001
The expression level of PAPPA was significantly higher in mural and cumulus cells from preovulatory follicles post-OI compared to GCs from small antral follicles (p = 0.006 and p < 0.001, respectively). This confirms the microarray data results.
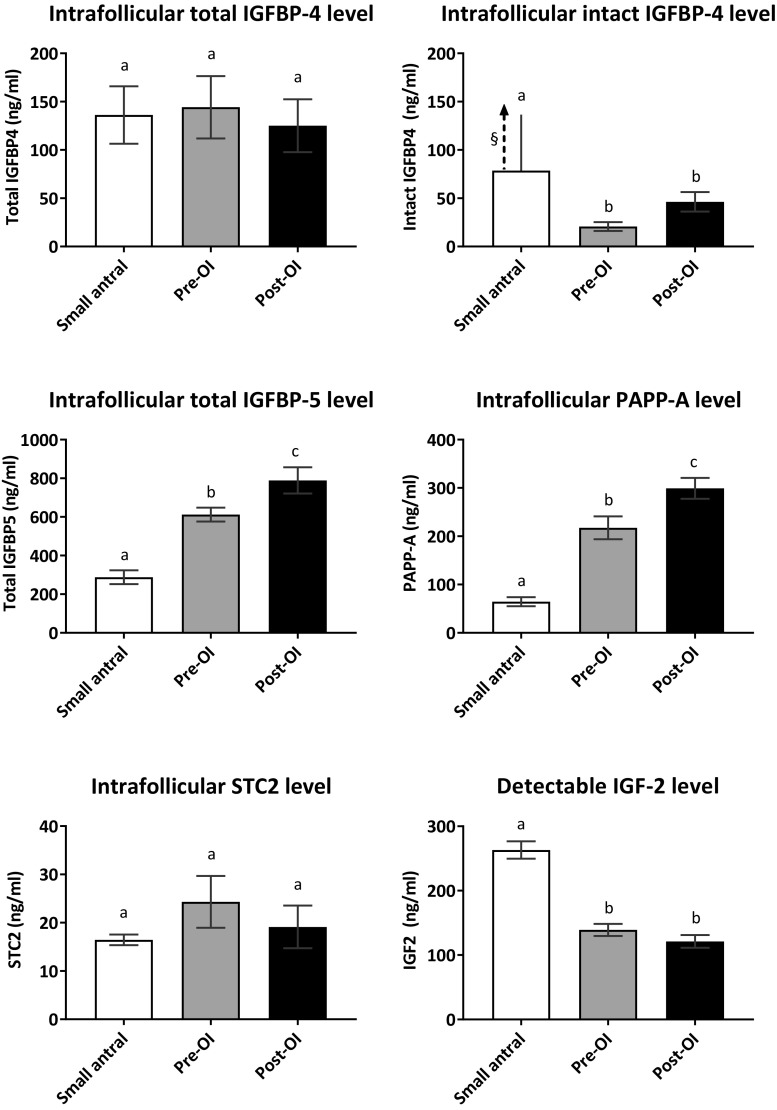
Protein levels of total IGFBP-4, intact IGFBP-4, total IGFBP-5, PAPP-A, STC2, and detectable free IGF-2 in FF from small human antral follicles and preovulatory follicles
The level of total IGFBP-4 protein showed no significant variation between small antral follicles and preovulatory follicles pre-OI and post-OI (Fig. 4). In contrast, intact levels of IGFBP-4 were significantly lower in FF from preovulatory follicles (p < 0.001) compared to FF from small antral follicles (Figs. 2c and 4). The protein levels of total IGFBP-5 were significantly higher in FF from preovulatory follicles compared to small antral follicles (p < 0.001).
Fig. 4.
Antigen levels of total IGFBP-4, intact IGFBP-4, total IGFBP-5, PAPP-A, STC2, and detectable free IGF-2 in FF from small human antral follicles and preovulatory follicles. Data are mean (± SEM) of the intrafollicular levels of total IGFBP-4, intact IGFBP-4, total IGFBP-5, PAPP-A, STC2, and detectable free IGF-2 in FF of small antral follicles (Small antral), preovulatory follicles prior to ovulation induction (Pre-OI), and preovulatory follicles after ovulation induction (Post-OI). §The arrow shows that the mean intact IGFBP-4 protein level in FF from small antral follicles was higher than the upper calibrator of 78 ng/ml supplied with the ELISA kit; thus, an exact mean is not shown. Different letters between follicle groups indicate statistical significance. Statistical significance for FF levels of intact IGFBP-4 protein: small antral follicles vs. follicles pre-OI: p < 0.001, and small antral follicles vs. follicles post-OI: p < 0.001. Statistical significance for FF levels of total IGFBP-5: small antral follicles vs. follicles pre-OI: p < 0.001, small antral follicles vs. follicles post-OI: p < 0.001, and follicles pre-OI vs. follicles post-OI: p = 0.02. Statistical significance for FF levels of PAPP-A: small antral follicles vs. follicles pre-OI: p < 0.001, small antral follicles vs. follicles post-OI: p < 0.001, and follicles pre-OI vs. follicles post-OI: p = 0.02. Statistical significance for FF levels of detectable free IGF-2: small antral follicles vs. follicles pre-OI: p < 0.001 and small antral follicles vs. follicles post-OI: p < 0.001
This study also combined ELISA data from previous studies to highlight that PAPP-A protein level increased significantly in FF from preovulatory follicles compared to small antral follicles (p < 0.001) [31, 50].
The protein levels of STC2 showed no significant variation in the FF from the three groups of follicles.
Intrafollicular levels of detectable free IGF-2 were significantly higher in small antral follicles compared to preovulatory follicles pre-OI and post-OI (p < 0.001).
Figure 2 is an attempt to provide a schematic display of IGF signaling at four stages of human follicular development.
Discussion
The exceptional complex regulation of the IGF system may reflect its action in many different organs of the body that requires local regulatory mechanisms for controlling bioactivity. Indeed, the human ovarian follicle is no exception, and various regulatory steps interact to control the access of the IGFs to their receptors on the cell surface. Once the receptor is activated, other mechanisms affect and control both intracellular signaling pathways and IGF synthesis.
This study is to our knowledge the first to evaluate the majority of components in the IGF signaling cascade during human folliculogenesis. Despite the complexity, the present study suggests that the IGF system is especially active in the preovulatory phase of follicular development (Fig. 2c). The expression of IGF2 is upregulated approximately 64-fold in the preovulatory stage prior to the midcycle surge of gonadotropins compared to small antral follicles (Fig. 1a), where PAPP-A expression also showed to peak (Fig. 1c). On a functional level, this is backed by the observation showing that PAPP-A protein levels significantly increased while intact IGFBP-4 protein levels significantly decreased in preovulatory follicles compared to small antral follicles (Fig. 4). Since PAPP-A is believed to be the only protease cleaving IGFBP-4 to release IGFs, the reduction of intact IGFBP-4 confirms increased PAPP-A activity and IGF signaling.
The expression of the IGF receptors together with increase of the intracellular signaling molecules, IRS1 and IRS2, with increasing follicle size (Fig. 1a) further supports the hypothesis of a highly active IGF system in late folliculogenesis.
This study confirmed expression of all six IGFBPs as well as the inhibitors of PAPP-A, STC1, and STC2 (Fig. 1b, c). The IMP proteins interfering with translation of IGF-2 were also expressed. The expression levels of most of these genes were relatively constant during follicular development, which suggests that their role in regulating the IGF system is constant and not related to any stage of folliculogenesis.
The present study therefore suggests that the IGF system on a physiological level impacts on the key events taking place in the preovulatory follicle, which is characterized by a very intense proliferation of GCs simultaneous with a substantial synthesis of estradiol and progesterone. Further, the present study enforces that the membrane anchored PAPP-A probably secure IGF signaling by releasing IGF-2 close to the IGF1R present on the cell surface, which is reflected by the significantly reduced levels of intact IGFBP-4.
These data are corroborated by earlier findings in FF, which also suggest a strong involvement of the IGF system in determining steroid output and GC mitosis activity [19, 22, 31, 55–57]. Following the midcycle surge of gonadotropins, the high GC proliferation is terminated, and the steroid output is reorganized to reflect the luteal phase. The expression profiles of the IGF system also reflect this transition with reduced IGF signaling (Fig. 2d). Collectively, the present results suggest that the IGF system is intimately involved in the regulation of follicular growth and development especially in the preovulatory phase of the menstrual cycle.
The importance of the IGF system for fertility has recently been highlighted in a mouse model, where a conditional knockdown of the IGF1R in GCs caused sterility and the ovaries were smaller than controls. Antral follicles did not develop nor were ovulated oocytes observed after ovarian stimulation [24].
Previous studies have suggested that IGF signaling possibly via PAPP-A regulation is important for steroid production in human follicle development [31, 55–58]. In addition, earlier studies showed strong immunoexpression of PAPP-A in GCs from preovulatory follicles together with a strong positive correlation between FF levels of PAPP-A and follicle size [31, 59]. Thus, the presence of increased expression of PAPP-A in large preovulatory follicles confirms and expands on previous observations.
Genes encoding differentiation markers like CYP19a1, LH receptor, and StAR are known to be upregulated by FSH and studies of cultured human cumulus cells have shown that this upregulation is highly dependent on IGF1R activity [60]. This fits with our results showing upregulation of IGF2 expression together with increasing PAPP-A levels at the preovulatory stage underscoring the importance of IGF signaling for differentiation of GCs and the estradiol output, especially when the follicle is approaching ovulation.
Expression of all six IGFBPs, which inhibit the action of IGFs or prolong their half-life, was observed throughout follicle development (Fig. 1b), however with low expression of IGFBP1 and IGFBP6 (Supplementary Fig. 5). Previously, IGFBP-2, IGFBP-3, IGFBP-4, and IGFBP-5 have been observed in human GCs and FF [61–63], which is confirmed and extended by the present results. The present study for the first time demonstrated that the intrafollicular protein level of IGFBP-5 in FF from human preovulatory follicles was significantly higher compared with small antral follicles. As the affinity constant between IGF-2 and IGFBP-5 is around ten times higher than that for IGFBP-4 [14], it is likely that the majority of IGF-2 will be bound by IGFBP-5 rather than IGFBP-4. As PAPP-A cleaves both binding proteins and the levels of intact IGFBP-4 becomes significantly reduced, it is hypothesized that this may reflect a higher conversion of IGFBP-4 by PAPP-A, which only cleaves IGFBP-4 when occupied by IGF-2 thereby leading to higher IGF signaling.
The observed significant rise in IGF2 expression in large preovulatory follicles collected pre-OI in the present study is in line with an earlier study, showing a markedly higher IGF2 expression level in GCs of a 14-mm large follicle compared to smaller follicles [64]. The significant higher expression of IGF2 in GCs from follicles pre-OI compared to follicles post-OI was validated with qPCR by Wissing and colleagues [47]; however, we have not been able to validate the difference in IGF2 expression between GCs isolated from small antral follicles and follicles pre-OI due to lack of material. Previous studies have found significantly higher FF levels of total IGF-2 in human E2-dominant follicles compared to androgen-dominant follicles [19, 55]. The present study found significantly reduced levels of detectable free IGF-2 protein in FF from preovulatory follicles compared to FF from small antral follicles (Fig. 4), which may appear contradictory. However, the assay used to measure detectable free IGF-2 may only partly reflect the true free levels of IGF-2 since this will depend on the affinity constant of the employed antibody, the concentration, cleavage state of the binding proteins, and the soluble IGF2R, which also show high binding affinities toward IGF-2. Thus, it may be difficult to detect the true free biological active IGF-2 concentration in FF, which furthermore is likely to be modulated close to the cell surface. Thus, the biological activity of IGF-2 affecting the receptor is not clarified by the present study, which, however, suggests that at least the cellular synthesis of IGF-2 is highly increased in GCs of preovulatory follicles. Thus, the available assays add another level of complexity analyzing IGF signaling. Assays detecting bioactive IGF-2 in biological fluids are to our knowledge currently not available, and therefore it becomes crucial to address total and intact binding proteins, proteases, and inhibitors of the proteases that may reflect the potential for activation.
Giudice and colleagues showed that FSH increased the expression of IGF2 in human preantral follicles in vitro [29]. In the present study, GCs collected from preovulatory follicles before the midcycle surge are obtained from women who underwent ovarian stimulation with exogenous FSH administration resulting in supra-physiological levels of FSH, while GCs from small antral follicles on the contrary were collected from women in their natural menstrual cycle. Therefore, it cannot be excluded that the 64-fold difference in IGF2 expression is less pronounced when compared to women in their natural menstrual cycle.
The expression levels of the intracellular signaling protein IRS1 was observed to increase significantly in follicles pre-OI compared to small human antral follicles (Figs. 1a and 2c). Previous studies have shown an increase in immunoexpression of IRS1 in human GCs with increasing follicle size, which our findings confirm and extend [65]. The IRS1 pathway has also been shown to be essential for follicle growth and a low expression of IRS1 has been associated with a reduced ovarian activity in mice [66]. The expression level of IRS2 peaked in GCs from preovulatory follicles post-OI, which point toward a possible shift in IGF signaling in the periovulatory events and in the luteal transition being mediated to a higher degree through IRS2 (Fig. 2d).
Another local level of regulation has recently been suggested by the findings of co-localization and formation of inhibitory complexes between STCs and PAPP-A during follicle development [38]. This previous study also found a positive correlation between the total PAPP-A level and the activity in FF from preovulatory follicles pre-OI with markedly lower PAPP-A activity post-OI. This decrease in proteolytic activity was suggested to be modulated by the STCs. In the present study, the expression level of the STC1 was observed to increase in preovulatory follicles post-OI (Figs. 1c and 2d) supporting the previous findings, which suggests a stage-dependent upregulation of STC1 expression important for modulation of PAPP-A mediated IGF signaling after ovulation induction. This may be important for the decline in GC proliferation at the midcycle surge. No significant differences were observed between STC2 protein levels in FF from small antral follicles and preovulatory follicles (Fig. 4).
Previous studies observed a significant increase in STC1 mRNA levels in mice ovaries after treatment with hCG [67]. This might explain the upregulation of STC1 in preovulatory follicles after ovulation induction as shown in this study. However, only little is known on the specific regulation of STCs synthesis in the human ovary, and this needs to be studied in more detail in coming studies.
The present study is mainly based on gene expression data and may not reflect actual functional protein levels. In this context, it is also important to notice that the present study found expression of the IMPs that regulates translation of IGF-2. Our data confirm previous studies [41] and highlight that gene expression does not necessary reflect translation especially in connection with IGF-system. In our study, however, gene expression levels of PAPPA, IGFBP4, IGFBP5, and STC2 were confirmed by qPCR and protein measures and are in line with previous studies from our laboratory [31, 59].
The patients from whom one ovary was excised for fertility preservation had different diagnosis, which could affect the results in this study since different types of cancers have been shown to affect levels of proteases like PAPP-A and levels of IGFs [68, 69]. However, all patients were early-stage disease patients, and further, this study included samples from patients with different types of diagnoses, thus, not reflecting one specific disease. None of the patients had any ovarian disease, and the change in expression associated with different cancer types may be tissue specific and affects organs with tumor cells.
Conclusion
In conclusion, the expression of PAPP-A with its specific proteolytic activity toward IGFBP-4/IGF-2 complexes increased significantly in preovulatory follicles coinciding with a reduction of intact levels of IGFBP-4, which potentially reflects an increased IGF signaling (Fig. 2c). The increased IGF-2 is likely to augment steroid production and rapid GC proliferation as seen in final stages of follicle development, prior to the mid-cycle surge. Transcription levels of intracellular signaling proteins increase with increasing follicle size, supporting the hypothesis that IGF signaling is essential in late folliculogenesis. The moderate expression of IGF components in the early stages of follicular development implies a role for IGF activity regarding GC differentiation and growth in preantral follicles; however, the high expression of IGFBPs and IMP3 may also be important to maintain the follicles in their arrested state. An increase in STC1 expression and FF-levels of IGFBP-5 protein seems to be important to sustain a strict regulation of the IGF signaling in the periovulatory phase.
These data show how the expression of IGF components fluctuate during human ovarian folliculogenesis, and a better understanding of essential pathways like this may provide markers of follicular health or targets for induction of follicle activation and hereby help in improving treatments at the IVF clinics.
Electronic supplementary material
Additional gene expression profiles of IGF genes during human folliculogenesis (TIF 1.83 MB)
Acknowledgements
We acknowledge the Core Facility for helping with fine microarray analysis. Furthermore, we are thankful for the work performed at the fertility clinics in regard to collecting the granulosa cells and follicular fluids from IVF patients. Finally, we thank Pernille Rimmer Noer from the Department of Molecular Biology and Genetics at University of Aarhus for technical assistance.
Funding
The financial support from The Novo Nordisk Foundation, the Lundbeck Foundation, and Gangstedfonden is gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference list
- 1.Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48:1–9. doi: 10.1007/s00592-010-0227-z. [DOI] [PubMed] [Google Scholar]
- 2.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. doi: 10.1016/S0092-8674(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 3.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 4.Le Roith D. The insulin-like growth factor system. Exp Diabesity Res. 2003;4:205–212. doi: 10.1155/EDR.2003.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform a, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan S, Baylink DJ, Pettis JL. Insulin-like growth factor (IGF)-binding proteins in serum—do they have additional roles besides modulating the endocrine IGF actions? J Clin Endocrinol Metab. 1996;81:3817–3820. doi: 10.1210/jcem.81.11.8923818. [DOI] [PubMed] [Google Scholar]
- 7.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 8.Boldt HB, Conover CA. Pregnancy-associated plasma protein-a (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Hormon IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal. 2015;9:177–187. doi: 10.1007/s12079-015-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laursen LS, Overgaard MT, Söe R, Boldt HB, Sottrup-jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-a (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/S0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 11.Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, Junker U, Jones SA, Clausen T, Ehrmann M. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 12.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276:21849–21853. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- 14.Laursen LS, Sorensen KK, Andersen MH, Oxvig C. Regulation of insulin-like growth factor bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol. 2007;21:1246–1257. doi: 10.1210/me.2006-0522. [DOI] [PubMed] [Google Scholar]
- 15.Law NC, White MF, Hunzicker-Dunn ME. G protein-coupled receptors (GPCRs) that signal via protein kinase a (PKA) cross-talk at insulin receptor substrate 1 (IRS1) to activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. J Biol Chem. 2016;291:27160–27169. doi: 10.1074/jbc.M116.763235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res. 2010;16:2512–2517. doi: 10.1158/1078-0432.CCR-09-2232. [DOI] [PubMed] [Google Scholar]
- 17.Scott CD, Kiess W. Soluble M6P/IGFIIR in the circulation. Best Pract Res Clin Endocrinol Metab. 2015;29:723–733. doi: 10.1016/j.beem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Zaina S, Squire S. The soluble type 2 insulin-like growth factor (IGF-II) receptor reduces organ size by IGF-II-mediated and IGF-II-independent mechanisms. J Biol Chem. 1998;273:28610–28616. doi: 10.1074/jbc.273.44.28610. [DOI] [PubMed] [Google Scholar]
- 19.Dessel THJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, Braat DD, Fauser BC, Giudice LC. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81:1224–1231. doi: 10.1210/jcem.81.3.8772603. [DOI] [PubMed] [Google Scholar]
- 20.Geisthovel F, Moretti-Rojas I, Asch RH, Rojas FJ. Expression of insulin-like growth factor-II (IGF-II) messenger ribonucleic acid (mRNA), but not IGF-I mRNA, in human preovulatory granulosa cells. Hum Reprod. 1989;4:899–902. doi: 10.1093/oxfordjournals.humrep.a137007. [DOI] [PubMed] [Google Scholar]
- 21.Nyegaard M, Overgaard MT, Su YQ, Hamilton AE, Kwintkiewicz J, Hsieh M, Nayak NR, Conti M, Conover CA, Giudice LC. (2010). Lack of functional pregnancy-associated plasma protein-A (PAPP-A) compromises mouse ovarian steroidogenesis and female fertility. Biol Reprod. 2010;82:112–138. doi: 10.1095/biolreprod.109.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spicer LJ, Aad PY. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 2007;77:18–27. doi: 10.1095/biolreprod.106.058230. [DOI] [PubMed] [Google Scholar]
- 23.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27:511–513. doi: 10.1210/me.2012-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology. 2017;158:2309–2318. doi: 10.1210/en.2017-00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjö T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–331. doi: 10.1016/S0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs SA, Webber LJ, Stark J, Rice S, Margara R, Lavery S, Trew GH, Hardy K, Franks S. Role of insulin-like growth factors in initiation of follicle growth in normal and polycystic human ovaries. J Clin Endocrinol Metab. 2013;98:3298–3305. doi: 10.1210/jc.2013-1378. [DOI] [PubMed] [Google Scholar]
- 27.Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod. 1995;10:75–81. doi: 10.1093/humrep/10.1.75. [DOI] [PubMed] [Google Scholar]
- 28.Spicer LJ, Voge JL, Allen DT. Insulin-like growth factor-II stimulates steroidogenesis in cultured bovine thecal cells. Mol Cell Endocrinol. 2004;227:1–7. doi: 10.1016/j.mce.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Yuan W, Giudice LC. Insulin-like growth factor-II mediates the steroidogenic and growth promoting actions of follicle stimulating hormone on human ovarian pre-antral follicles cultured in vitro. J Clin Endocrinol Metab. 1999;84:1479–1482. doi: 10.1210/jcem.84.4.5727. [DOI] [PubMed] [Google Scholar]
- 30.Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, Stocco C. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab. 2015;100:E1046–E1055. doi: 10.1210/jc.2015-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bøtkjær JA, Jeppesen JV, Wissing ML, Kløverpris S, Oxvig C, Mason JI, Borgbo T, Andersen CY. Pregnancy-associated plasma protein a in human ovarian follicles and its association with intrafollicular hormone levels. Fertil Steril. 2015;104:1294–1301. doi: 10.1016/j.fertnstert.2015.07.1152. [DOI] [PubMed] [Google Scholar]
- 32.Giorgetti C, Vanden Meerschaut F, De Roo C, Saunier O, Quarello E, Hairion D, Penaranda G, Chabert-Orsini V, De Sutter P. Multivariate analysis identifies the estradiol level at ovulation triggering as an independent predictor of the first trimester pregnancy-associated plasma protein-A level in IVF/ICSI pregnancies. Hum Reprod. 2013;28:2636–2642. doi: 10.1093/humrep/det295. [DOI] [PubMed] [Google Scholar]
- 33.Hunt LP, McInerney-Leo AM, Sinnott S, Sutton B, Cincotta R, Duncombe G, Chua J, Peterson M. Low first-trimester PAPP-A in IVF (fresh and frozen-thawed) pregnancies, likely due to a biological cause. J Assist Reprod Genet. 2017;34:1367–1375. doi: 10.1007/s10815-017-0996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, Hankins G, Berkowitz RL, Merkatz I, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Vidaver J, D'Alton ME. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER trial) Am J Obstet Gynecol. 2004;191:1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Kirkegaard I, Henriksen TB, Uldbjerg N. Early fetal growth, PAPP-A and free β-hCG in relation to risk of delivering a small-for-gestational age infant. Ultrasound Obstet Gynecol. 2011;37:341–347. doi: 10.1002/uog.8808. [DOI] [PubMed] [Google Scholar]
- 36.Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Fuchtbauer EM, Laursen LS, Oxvig C. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem. 2015;290:3430–3439. doi: 10.1074/jbc.M114.611665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kløverpris S, Mikkelsen JH, Pedersen JH, Jepsen MR, Laursen LS, Petersen SV, Oxvig C. Stanniocalcin-1 potently inhibits the proteolytic activity of the metalloproteinase pregnancy-associated plasma protein-A. J Biol Chem. 2015;290:21915–21924. doi: 10.1074/jbc.M115.650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development. Hum Reprod. 2016;31:866–874. doi: 10.1093/humrep/dew013. [DOI] [PubMed] [Google Scholar]
- 39.Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem. 2000;275:31128–31133. doi: 10.1074/jbc.M001384200. [DOI] [PubMed] [Google Scholar]
- 40.Oxvig C, Sand O, Kristensen T, Gleich GJ, Sottrup-Jensen L. Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein. J Biol Chem. 1993;268:12243–12246. [PubMed] [Google Scholar]
- 41.Hammer NA, Hansen TV, Byskov AG, Rajpert-De Meyts E, Grøndahl ML, Bredkjaer HE, Wewer UM, Christiansen J, Nielsen FC. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction. 2005;130:203–212. doi: 10.1530/rep.1.00664. [DOI] [PubMed] [Google Scholar]
- 42.Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595–608. doi: 10.2217/fon.12.47. [DOI] [PubMed] [Google Scholar]
- 43.Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, Andersen AN. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod. 2008;23:2475–2483. doi: 10.1093/humrep/den248. [DOI] [PubMed] [Google Scholar]
- 44.Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–166. doi: 10.1093/humrep/deq318. [DOI] [PubMed] [Google Scholar]
- 45.Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 46.Petersen TS, Kristensen SG, Jeppesen JV, Grøndahl ML, Wissing ML, Macklon KT, Andersen CY. Distribution and function of 3′,5′-cyclic-AMP phosphodiesterases in the human ovary. Mol Cell Endocrinol. 2015;403:10–20. doi: 10.1016/j.mce.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Høst T, Borup R, Grøndahl ML. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29:997–1010. doi: 10.1093/humrep/deu008. [DOI] [PubMed] [Google Scholar]
- 48.Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grøndahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2013;100:994–1001. doi: 10.1016/j.fertnstert.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol. 2015;401:189–201. doi: 10.1016/j.mce.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Bøtkjær JA, Borgbo T, Kløverpris S, Noer PR, Oxvig C, Andersen CY. Effect of pregnancy-associated plasma protein-A (PAPP-A) single-nucleotide polymorphisms on the level and activity of PAPP-A and the hormone profile in fluid from normal human small antral follicles. Fertil Steril. 2016;106:1778–1786.e8. doi: 10.1016/j.fertnstert.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 51.McCall MN, Bolstad BM, Irizarry RA. Frozen robust multiarray analysis (fRMA) Biostatistics. 2010;11:242–253. doi: 10.1093/biostatistics/kxp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCall MN, Jaffee HA, Irizarry RA. fRMA ST: frozen robust multiarray analysis for Affymetrix exon and gene ST arrays. Bioinformatics. 2012;28:3153–3154. doi: 10.1093/bioinformatics/bts588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sánchez-Valle J, Tejero H, Ibáñez K, Portero JL, Krallinger M, Al-Shahrour F, Tabarés-Seisdedos R, Baudot A, Valencia A. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s disease, glioblastoma and lung cancer. Sci Rep. 2017;7:4474. doi: 10.1038/s41598-017-04400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamatas GN, Wu J, PAPP-As A, Mirmirani P, McCormick TS, Cooper KD, Consolo M, Schastnaya J, Ozerov IV, Aliper A, Zhavoronkov A. An analysis of gene expression data involving examination of signaling pathways activation reveals new insights into the mechanism of action of minoxidil topical foam in men with androgenetic alopecia. Cell Cycle. 2017;16:1578–1584. doi: 10.1080/15384101.2017.1327492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandrasekher YA, Van Dessel HJ, Fauser BC, Giudice LC. Estrogen- but not androgen-dominant human ovarian follicular fluid contains an insulin-like growth factor binding protein-4 protease. J Clin Endocrinol Metab. 1995;80:2734–2739. doi: 10.1210/jcem.80.9.7545699. [DOI] [PubMed] [Google Scholar]
- 56.Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, Oxvig C, Giudice LC. Pregnancy-associated plasma protein-A is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology. 2001;142:2155. doi: 10.1210/endo.142.5.8286. [DOI] [PubMed] [Google Scholar]
- 57.Yong EL, Baird DT, Yates R, Reichert LE, Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–849. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 58.Stocco C, Baumgarten SC, Armouti M, Fierro MA, Winston NJ, Scoccia B, Zamah AM. Genome-wide interactions between FSH and insulin-like growth factors in the regulation of human granulosa cell differentiation. Hum Reprod. 2017;32:905–914. doi: 10.1093/humrep/dex002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, Ernst E, Macklon KT, Andersen CY. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol (Lausanne) 2018;8:376. doi: 10.3389/fendo.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995–3004. doi: 10.1210/jc.2014-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cataldo NA, Giudice LC. Insulin-like growth factor binding protein profiles in human ovarian follicular fluid correlate with follicular functional status. J Clin Endocrinol Metab. 1992;74:821–829. doi: 10.1210/jcem.74.4.1372322. [DOI] [PubMed] [Google Scholar]
- 62.El-Roeiy A, Chen X, Roberts VJ, Shimasakai S, Ling N, LeRoith D, Roberts CT, Jr, Yen SS. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1–6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab. 1994;78:1488–1496. doi: 10.1210/jcem.78.6.7515389. [DOI] [PubMed] [Google Scholar]
- 63.Zhou J, Bondy C. Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod. 1993;48:467–482. doi: 10.1095/biolreprod48.3.467. [DOI] [PubMed] [Google Scholar]
- 64.Voutilainen R, Franks S, Mason HD, Martikainen H. Expression of insulin-like growth factor (IGF), IGF-binding protein, and IGF receptor messenger ribonucleic acids in normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:1003–1008. doi: 10.1210/jcem.81.3.8772565. [DOI] [PubMed] [Google Scholar]
- 65.Wu XK, Sallinen K, Anttila L, Mäkinen M, Luo C, Pöllänen P, Erkkola R. Expression of insulin-receptor substrate-1 and -2 in ovaries from women with insulin resistance and from controls. Fertil Steril. 2000;74:564–572. doi: 10.1016/S0015-0282(00)00688-9. [DOI] [PubMed] [Google Scholar]
- 66.Schneider A, Zhi X, Moreira F, Lucia T, Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120. doi: 10.1186/s13048-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deol HK, Varghese R, Wagner GF, Dimattia GE. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology. 2000;141:3412–3421. doi: 10.1210/endo.141.9.7658. [DOI] [PubMed] [Google Scholar]
- 68.Loddo M, Andryszkiewicz J, Acebes SR, Stoeber K, Jones A, Dafou D, Apostolidou S, Wollenschlaeger A, Widschwendter M, Sainsbury R, Tudzarova S, Williams GH. Pregnancy-associated plasma protein a regulates mitosis and is epigenetically silenced in breast cancer. J Pathol. 2014;233:344–356. doi: 10.1002/path.4393. [DOI] [PubMed] [Google Scholar]
- 69.Mathur SP, Mathur RS, Young RC. Cervical epidermal growth factor-receptor (EGF-R) and serum insulin-like growth factor II (IGF-II) levels are potential markers for cervical cancer. Am J Reprod Immunol. 2000;44:222–230. doi: 10.1111/j.8755-8920.2000.440406.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional gene expression profiles of IGF genes during human folliculogenesis (TIF 1.83 MB)