Abstract
Background
Individuals with cryptococcal antigenemia are at high risk of developing cryptococcal meningitis if untreated. The progression and timing from asymptomatic infection to cryptococcal meningitis is unclear. We describe a subpopulation of individuals with neurologic symptomatic cryptococcal antigenemia but negative cerebral spinal fluid (CSF) studies.
Methods
We evaluated 1201 human immunodeficiency virus–seropositive individuals hospitalized with suspected meningitis in Kampala and Mbarara, Uganda. Baseline characteristics and clinical outcomes of participants with neurologic–symptomatic cryptococcal antigenemia and negative CSF cryptococcal antigen (CrAg) were compared to participants with confirmed CSF CrAg+ cryptococcal meningitis. Additional CSF testing included microscopy, fungal culture, bacterial culture, tuberculosis culture, multiplex FilmArray polymerase chain reaction (PCR; Biofire), and Xpert MTB/Rif.
Results
We found 56% (671/1201) of participants had confirmed CSF CrAg+ cryptococcal meningitis and 4% (54/1201) had neurologic symptomatic cryptococcal antigenemia with negative CSF CrAg. Of those with negative CSF CrAg, 9% (5/54) had Cryptococcus isolated on CSF culture (n = 3) or PCR (n = 2) and 11% (6/54) had confirmed tuberculous meningitis. CSF CrAg-negative patients had lower proportions with CSF pleocytosis (16% vs 26% with ≥5 white cells/μL) and CSF opening pressure >200 mmH2O (16% vs 71%) compared with CSF CrAg-positive patients. No cases of bacterial or viral meningitis were detected by CSF PCR or culture. In-hospital mortality was similar between symptomatic cryptococcal antigenemia (32%) and cryptococcal meningitis (31%; P = .91).
Conclusions
Cryptococcal antigenemia with meningitis symptoms was the third most common meningitis etiology. We postulate this is early cryptococcal meningoencephalitis. Fluconazole monotherapy was suboptimal despite Cryptococcus-negative CSF. Further studies are warranted to understand the clinical course and optimal management of this distinct entity.
Clinical Trials Registration
Keywords: cryptococcal meningitis, HIV, diagnosis, fungal antigen, aseptic meningitis
Blood cryptococcal antigen testing should be considered in all severely immunocompromised human immunodeficiency virus–infected individuals who are hospitalized with suspected meningitis. Fluconazole monotherapy is inadequate for individuals with neurologic symptomatic cryptococcal antigenemia.
Cryptococcal meningitis is the most common cause of meningitis in sub-Saharan Africa and accounts for 15% of human immunodeficiency virus (HIV)/AIDS-related deaths globally [1–3]. Cryptococcal antigenemia, which precedes the development of cryptococcal meningitis, has a prevalence of 8.8% among HIV-infected Ugandan adult outpatients with CD4 T-cell counts <100 cells/μL, and this prevalence is 2-fold greater among those hospitalized [4, 5]. Globally, the cryptococcal antigenemia prevalence is estimated at 6% in HIV-infected individuals with CD4 T-cell counts <100 cells/μL [1].
Current World Health Organization (WHO) guidelines recommend screening all antiretroviral therapy–naive HIV-infected individuals with CD4 T-cell counts <100 cells/μL for cryptococcosis using a serum or plasma cryptococcal antigen (CrAg) test, followed by preemptive antifungal therapy if CrAg positive to diminish the risk of developing cryptococcal meningitis [6, 7]. If untreated, patients with cryptococcal antigenemia progress to develop meningitis symptoms within a median of 22 days (by latex agglutination) [8]. However, the exact timing of developing meningitis symptoms in individuals with a positive serum CrAg vs the ability to detect CrAg in cerebrospinal fluid (CSF) remains unclear. The optimal treatment for this group of patients has not been defined.
We characterized high-risk patients who were hospitalized with symptoms of meningitis, including headache, neck pain, vomiting, and/or fever, with a positive whole blood CrAg test but with negative CSF evaluation (negative CrAg and no other bacterial, viral, mycobacterial, or fungal etiology identified; hereafter referred to as “symptomatic antigenemia” group). We compared this neurologic symptomatic antigenemia group to patients with confirmed CSF CrAg+ cryptococcal meningitis in terms of clinical presentation and hospital outcomes.
METHODS
Study Participants
As part of screening for enrollment into the Adjunctive Sertraline for Treatment of HIV-Associated Cryptococcal Meningitis (ASTRO-CM) clinical trial [9], we prospectively consented 1201 HIV-infected adults who presented with suspected meningitis to evaluate for the etiology of meningitis at Mulago National Referral Hospital and Mbarara Regional Referral Hospital in Uganda. Study participants were HIV-infected adults (aged ≥18 years) with signs/symptoms of meningitis who provided written informed consent for lumbar puncture, diagnostic testing, and collection of research data. We excluded individuals with past history of cryptococcal meningitis and females who were pregnant. Ethical approvals were obtained from the Uganda National Council of Science and Technology, Mulago Hospital Research and Ethics Committee, and University of Minnesota.
Study Procedures
First, symptomatic hospitalized persons with suspected meningitis underwent finger-stick testing of whole blood with the CrAg lateral flow assay (LFA; Immy, Norman, Oklahoma) [10]. Second, lumbar punctures were performed, with CSF tested using CrAg LFA to establish a diagnosis of cryptococcal meningitis. All CrAg testing was independently repeated in the microbiology laboratory to confirm the point-of-care CrAg result. Tuberculous meningitis (TBM) was diagnosed by Xpert MTB/RIF (Cepheid, Sunnyvale, CA), mycobacterial growth indicator tube culture, or acid-fast bacilli smear [11]. Multiplex polymerase chain reaction (PCR; FilmArray Meningitis/Encephalitis Panel, BioFire Diagnostics, LLC, Salt Lake City, UT) was also performed on a subset of samples to evaluate for potential viral and bacterial etiologies of meningitis [12]. Quantitative fungal cultures were performed on whole CSF, and plates were incubated at 30°C for 10 days on Sabouraud dextrose agar, as previously described [13, 14]. The overall stepwise diagnostic algorithm is presented in Supplementary Figure S1.
Statistical Analyses
We analyzed data based on CSF CrAg test results. Individuals with first-episode cryptococcosis were included in comparative analyses. We compared baseline characteristics and clinical outcomes of individuals in the symptomatic cryptococcal antigenemia group with the cryptococcal meningitis group. We compared continuous variables with Wilcoxon rank sum tests and compared categorical variables with Fisher exact tests. P values <.05 were considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
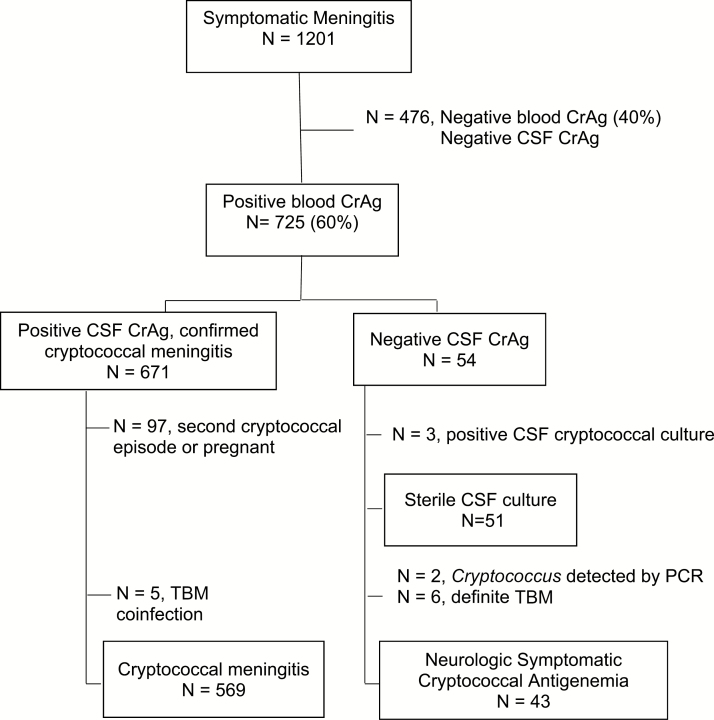
Between August 2013 and May 2017, we consented 1201 HIV-infected participants hospitalized with suspected meningitis for lumbar puncture. All participants first received a CrAg LFA test on finger-stick whole blood. Of these, 60% (725/1201) had a positive blood CrAg test. Of those with a positive blood CrAg, 93% (671/725) had positive CSF CrAg and confirmed cryptococcal meningitis and 7.4% (54/725) had negative CSF CrAg. Of the 574 participants with a first episode of meningitis and a positive CSF CrAg, 0.9% (5/574) had cryptococcal meningitis and microbiologic-confirmed TBM coinfection (Figure 1).
Figure 1.
Study population: 1201 individuals with suspected meningitis were screened with a blood cryptococcal antigen (CrAg) test. Overall, 40% (476/1201) were CrAg negative in blood and excluded from this analysis, all of whom were also cerebral spinal fluid (CSF) CrAg negative. Overall, 671 individuals who were blood and CSF CrAg positive were diagnosed with cryptococcal meningitis. Of 574 with first-episode cryptococcal meningitis, 5 had tuberculous meningitis coinfection and were excluded from this analysis. Of the 54 blood CrAg positive and CSF CrAg negative, 3 were later diagnosed by CSF culture and 2 by polymerase chain reaction with cryptococcal meningitis, 6 were diagnosed with microbiologically confirmed tuberculous meningitis, and 43 had neurologic symptomatic cryptococcal antigenemia. Abbreviations: CrAg, cryptococcal antigen; CSF, cerebral spinal fluid; PCR, polymerase chain reaction; TB, tuberculosis; TBM, tuberculous meningitis.
Among the 54 participants with negative CSF CrAg, 3 (6%) subsequently grew Cryptococcus on CSF culture (range, 10–95 colony-forming units/mL) with negative multiplex PCR and 2 patients (13% of 15 tested) had Cryptococcus DNA detected by multiplex PCR with sterile cultures (Table 1). Six participants (11%) were diagnosed with TBM using Xpert MTB/Rif (n = 5), acid-fast bacilli smear microscopy (n = 1), and/or mycobacteria culture (n = 3). There were no cases of viral or bacterial meningitis diagnosed among this group based on CSF profile, multiplex PCR, or bacterial culture. Thus, 43 were included in the final symptomatic antigenemia group for analysis.
Table 1.
Alternative Diagnoses
| Diagnostic Testing | Blood CrAg Positive and CSF CrAg Negative (N = 54) |
Blood and CSF CrAg Positive (N = 574) |
|---|---|---|
| CSF CrAg positive on repeat testing in laboratory | 0 | 573 |
| CSF Cryptococcus culture positive | 3 (6%) | 500 (88%) |
| CSF polymerase chain reaction performed | 15 (28%) | 86 (15%) |
| Positive for Cryptococcus | 2 (13%) | 55 (64%) |
| Positive for herpes human virus 6a | 1 (7%) | … |
| Diagnosed with tuberculous meningitis | 6 (11%) | 5 (1%) |
| Positive CSF acid-fast bacilli smear | 1 of 47 | 0 of 14 |
| Positive CSF Mycobacterium tuberculosis culture | 3 of 33 | 1 of 43 |
| Positive CSF Xpert Mycobacterium tuberculosis/rifampin | 5 of 48 | 4 of 46 |
Abbreviations: CrAg, cryptococcal antigen; CSF, cerebral spinal fluid.
aAdditional polymerase chain reaction testing was negative for Escherichia coli, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae, cytomegalovirus, enterovirus, herpes simplex virus 1 and 2, human parechovirus, and varicella zoster virus.
Participants in the symptomatic antigenemia group and the cryptococcal meningitis group had similar baseline characteristics (Table 2). The CD4 T-cell counts in the symptomatic antigenemia group (median, 29 cells/µL; interquartile range [IQR], 7–69) were not statistically different from the cryptococcal meningitis group (median, 16 cells/µL; IQR, 6–48; P = .16). Headache was a less common symptom in the symptomatic antigenemia group than the cryptococcal meningitis group (84% vs 98%; P < .01). The proportion of participants on antiretroviral therapy in the symptomatic antigenemia group did not differ from that of the cryptococcal meningitis group (53% vs 52%; P = .84). No participant initiated or changed antiretroviral therapy during hospitalization. A significantly higher proportion of participants in the symptomatic antigenemia group had normal CSF white blood cell counts (<5 cells/µL) compared to the cryptococcal meningitis group (84% vs 64%; P < .01). Similarly, the median CSF opening pressure was 127 mmH2O (IQR 93–157) in the symptomatic antigenemia group compared to 270 mmH2O (IQR, 180–400) in the cryptococcal meningitis group (P < .01). Among those in the symptomatic antigenemia group, 16% (5/32) had elevated CSF opening pressure >200 mmH2O.
Table 2.
Baseline Characteristics of the Study Population
| Characteristic | Neurologic Symptomatic Cryptococcal Antigenemia Group (n = 43) |
Cryptococcal Meningitis Group (n = 569) |
P Value |
|---|---|---|---|
| Men | 44% (29%, 59%) | 59% (55%, 63%) | .06 |
| Age, years | 38 (30, 45) | 35 (29, 40) | .13 |
| Receiving antiretroviral therapy | 53% (39%, 68%) | 52% (48%, 56%) | .84 |
| Fever | 56% (41%, 71%) | 49% (44%, 53%) | .36 |
| Headache | 84% (73%, 95%) | 98% (96%, 99%) | <.01 |
| Photophobia | 12% (2%, 21%) | 27% (23%, 30%) | .03 |
| Seizures | 5% (0%, 11%) | 14% (12%, 17%) | .07 |
| Cough | 21% (9%, 33%) | 20% (17%, 24%) | .91 |
| Abnormal lung exam | 26% (13%, 39%) | 15% (12%, 18%) | .06 |
| Glasgow coma score <15 | 48% (33%, 63%) | 42% (38%, 46%) | .47 |
| Hemoglobin, g/dL | 10.6 (8.2, 11.9) | 11.4 (9.9, 12.9) | <.01 |
| CD4 T cells/μL | 29 (7, 69) | 16 (6, 48) | .16 |
| Creatinine, mg/dL | 0.7 (0.2, 1.1) | 0.7 (0.6, 0.9) | .59 |
| CSF white cells <5 cells/μL | 84% (73%, 95%) | 64% (60%, 68%) | <.01 |
| CSF protein, mg/dL | 31 (22, 67) | 43 (21, 94) | .19 |
| CSF protein <45 mg/dL | 63% (48%, 78%) | 51% (47%, 56%) | .15 |
| Opening pressure, mmH2O | 127 (93, 157) | 270 (180, 400) | <.01 |
| Any abnormal: CSF white cells, protein, or opening pressure | 44% (29%, 59%) | 83% (80%, 86%) | <.01 |
Proportions (95% confidence intervals) displayed are out of N with data in group; median (interquartile range) displayed for continuous variables; P values are from Wilcoxon rank sum tests for continuous variables and Fisher’s exact tests for categorical variables. Persons with tuberculous meningitis coinfection are excluded.
Abbreviation: CSF, cerebral spinal fluid.
Of 6 symptomatic antigenemia participants with TBM coinfection, CSF was more inflammatory compared to symptomatic antigenemia participants without TBM. Participants with TBM coinfection presented more frequently with abnormal CSF pleocytosis ≥5 white cells/µL (67% vs 16%; P = .02); however, the proportions presenting with elevated protein >45 mg/dL (60% vs 37%; P = .37) and opening pressure >200 mmH2O (50% vs 16%; P = .33) were similar when compared to those with symptomatic antigenemia.
Among the 96% (585/612) with known in-hospital outcomes, early mortality did not differ in the symptomatic antigenemia group: 32% (11/34) who were treated with fluconazole monotherapy (initially 800 mg/day) vs 31% (173/551) in the cryptococcal meningitis group who were treated with combination amphotericin B and fluconazole (P = .91). Among patients in the symptomatic antigenemia group, the median time to in-hospital death was 7 days (IQR, 4–18 days; maximum, 31 days), and a further 21% had unknown outcome (eg, fled from the hospital/lost to follow-up). Thus, outcomes in this symptomatic antigenemia group, who generally appeared less ill at baseline, were suboptimal when receiving fluconazole monotherapy.
DISCUSSION
We identified a group of HIV-infected individuals with cryptococcal antigenemia and symptomatic meningitis but without microbiologic evidence of CSF infection. We propose that these persons most likely have early cryptococcal meningitis. In the absence of testing blood for CrAg and only testing CSF, we would have missed their diagnosis. It is likely that these missed diagnoses commonly occur in immunocompromised populations throughout the world. These neurologic symptomatic antigenemia patients had relatively milder symptoms at baseline, normal CSF opening pressures, and slightly higher CD4 T-cell counts, yet they still had 32% known early in-hospital mortality with the WHO and US recommended fluconazole monotherapy for CrAg antigenemia. While anecdotally recognized over the past approximately 4 decades, this is the first cohort to describe this population and the prevalence (1 in 25 adults presenting with meningitis symptoms in Uganda).
There are 2 important lessons from our findings. First, diagnostic testing of immunocompromised individuals with suspected central nervous system (CNS) infection should always include blood testing of cryptococcal antigen, particularly in the absence of an identified CSF pathogen. Second, CNS cryptococcosis is a meningoencephalitis where early in infection, yeasts can be present in the brain parenchyma without CSF involvement. Untreated, eventually CSF involvement will occur based on the natural history of Cryptococcus neoformans. [8]. A South African autopsy study reported intraparenchymal Cryptococcus in the brains of patients with cryptococcal antigenemia who received preemptive fluconazole therapy and later died [15].
Second, the current guidelines recommending fluconazole therapy for isolated cryptococcal antigenemia are inadequate [7, 16, 17]. While these guidelines may be adequate for asymptomatic antigenemia, for persons with symptomatic CNS disease and no other identifiable cause of symptoms, patients should be approached similarly to CNS infection, with consideration of more aggressive management with amphotericin-based therapy or combination fluconazole and flucytosine. Of notable concern is the similar in-hospital mortality among those with symptomatic antigenemia compared with those with symptomatic cryptococcal meningitis. Despite the fact that these symptomatic antigenemia patients were identified early during the continuum of cryptococcal disease while progressing to overt cryptococcal meningitis, we observed unacceptably high early mortality (32%). The proximal cause of death was unclear, based on the available data. Even with preemptive fluconazole monotherapy in asymptomatic cryptococcal antigenemia persons screened in a multisite cryptococcal screening and treatment program in Kampala, Uganda, 28% were symptomatic at the time of clinic return [18]. Of these symptomatic cryptococcal antigenemia persons identified in outpatient clinics, 54% were dead or lost at 6 months [18]. Fluconazole monotherapy, even at 1200 mg/day for CNS disease, has been associated with a 68% mortality in a recent Ethiopian study [19].
Therefore, given the implementation of national CrAg screening programs where patients with symptomatic cryptococcal antigenemia will increasingly be identified, we posit that treating individuals with symptomatic cryptococcal antigenemia with a short course of amphotericin-based therapy could potentially improve their clinical outcomes. However, the dose and duration should be the subject of future research. We would also recommend an enhanced diagnostic approach, especially considering that 11% of those with symptomatic antigenemia had confirmed TBM coinfection. A further proportion may have had TBM missed on CSF testing, thus cryptococcal antigenemia alone still requires comprehensive diagnostic evaluation. CSF culture detected Cryptococcus in 3 false-negative CSF CrAg tests among 1201 (0.25%) CSF processed; all of whom had their cryptococcal infection detected by blood CrAg testing. In comparison, in the absence of blood CrAg testing, 4.2% (51/1201) would have not had their cryptococcal infection detected by standard CSF culture, CrAg testing, and microscopy. Thus, we strongly recommend an enhanced diagnostic work up for all immunocompromised patients with suspected CNS infection to include blood CrAg testing.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
ASTRO-CM team members. Henry W. Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Jayne Ellis, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Emily E. Evans, Sruti S. Velamakanni, Bilal Jawed, Katelyn A. Pastick, Matthew Merry, Anna Stadelman, Andrew G. Flynn, A. Wendy Fujita, Liliane Mukaremera, Bozena M. Morawski, Kabanda Taseera, Kirsten Nielsen, Paul R. Bohjanen, and Andrew Kambugu.
Financial support. This research was supported by the National Institute of Neurological Disorders and Stroke (R01NS086312), Fogarty International Center (K01TW010268, R25TW009345), National Institute of Allergy and Infectious Diseases (T32AI055433), United Kingdom Medical Research Council/DfID/Wellcome Trust Global Clinical Trials (M007413/1), and Grand Challenges Canada (S4-0296-01). D. B. M. and R. K. are currently supported by the DELTAS Africa Initiative grant (DEL-15-011) to THRiVE-2. The DELTAS Africa Initiative is a funding scheme of the Accelerating Excellence in Science in Africa with funding from the Wellcome Trust (grant 107742/Z/15/Z) and the UK government.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Adjunctive Sertraline for Treatment of HIV-associated Cryptococcal Meningitis Team:
Henry W Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Jayne Ellis, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Emily E Evans, Sruti S Velamakanni, Bilal Jawed, Katelyn A Pastick, Matthew Merry, Anna Stadelman, Andrew G Flynn, A Wendy Fujita, Liliane Mukaremera, Bozena M Morawski, Kabanda Taseera, Kirsten Nielsen, Paul R Bohjanen, and Andrew Kambugu
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oyella J, Meya D, Bajunirwe F, Kamya MR. Response to comment on “prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda (Oyella et al. 2012).” J Int AIDS Soc 2012; 15:18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization; Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 7. World Health Organization; Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children, March 2018 Available at: http://www.who.int/iris/handle/10665/260399. Accessed 10 May 2018. [PubMed] [Google Scholar]
- 8. French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002; 16:1031–8. [DOI] [PubMed] [Google Scholar]
- 9. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams DA, Kiiza T, Kwizera R, et al. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 2015; 61:464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahr NC, Tugume L, Rajasingham R, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF. Int J Tuberc Lung Dis 2015; 19:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhein J, Bahr NC, Hemmert AC, et al. ; ASTRO-CM Team. Diagnostic performance of a multiplex PCR assay for meningitis in an HIV-infected population in Uganda. Diagn Microbiol Infect Dis 2016; 84:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45:76–80. [DOI] [PubMed] [Google Scholar]
- 14. Dyal J, Akampurira A, Rhein J, et al. ; ASTRO-CM Trial Team. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wake RM, Omar T, Karat AS, et al. Post-mortem cryptococcal meningitis following treatment for cryptococcal antigenaemia. In: 25th Conference on retroviruses and opportunistic infections (CROI 2018) Boston, Massachusetts, 2018:Abstract 788, session P-O4. [Google Scholar]
- 16. Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV clinics in Uganda. J Acquir Immune Defic Syndr 2018; 78:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beyene T, Zewde AG, Balcha A, et al. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis 2017; 65:2126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.