Abstract
Background
In Pseudomonas aeruginosa, fluoroquinolone exposure promotes resistance to carbapenems through upregulation of efflux pumps and transcriptional downregulation of the porin OprD. Evidence of this effect among hematologic malignancy (HM) patients or hematopoietic cell transplant (HCT) recipients receiving fluoroquinolone prophylaxis for neutropenia is lacking.
Methods
We retrospectively evaluated episodes of P. aeruginosa bloodstream infections in HM patients or HCT recipients over a 7-year period at our institution. We determined the association of fluoroquinolone prophylaxis at the time of infection with meropenem susceptibility of P. aeruginosa breakthrough isolates and risk factors for meropenem nonsusceptibility. Whole-genome sequencing (WGS) and phenotypic assessments of meropenem efflux pump activity were performed on select isolates to determine the mechanisms of meropenem resistance.
Results
We analyzed 55 episodes of P. aeruginosa bacteremia among 51 patients. Breakthrough bacteremia while on fluoroquinolone prophylaxis was associated with nonsusceptibility to meropenem, but not to antipseudomonal β-lactams or aminoglycosides. The receipt of fluoroquinolone prophylaxis was independently predictive of bacteremia with a meropenem-nonsusceptible isolate. All meropenem-nonsusceptible isolates analyzed by WGS contained oprD inactivating mutations, and all meropenem-nonsusceptible isolates tested demonstrated reductions in the meropenem minimum inhibitory concentration in the presence of an efflux pump inhibitor. A phylogenetic analysis based on WGS revealed several clusters of closely related isolates from different patients.
Conclusions
Fluoroquinolone prophylaxis in HM patients and HCT recipients is associated with breakthrough bacteremia with meropenem-nonsusceptible P. aeruginosa strains, likely due to both mutations increasing efflux pump activity and the epidemiology of P. aeruginosa bloodstream infections in our patient population.
Keywords: Pseudomonas aeruginosa, fluoroquinolone, meropenem, resistance, neutropenia
Fluoroquinolone prophylaxis in neutropenic, hematologic malignancy patients and hematopoietic-transplant recipients is associated with breakthrough bacteremia with meropenem–non-susceptible P. aeruginosa strains. These findings require validation in larger studies and may have implications for the empirical management of febrile neutropenia.
Pseudomonas aeruginosa remains a significant cause of morbidity and mortality among hematologic malignancy (HM) patients and hematopoietic cell transplant (HCT) recipients, particularly during periods of neutropenia [1]. As such, antipseudomonal agents, including carbapenems (meropenem, imipenem-cilastatin), cefepime, and piperacillin-tazobactam, are currently recommended as first-line empirical treatment options for febrile neutropenia in these patients [2].
The widespread use of fluoroquinolone prophylaxis for neutropenic patients has raised concerns about the emergence of resistant organisms, including P. aeruginosa [3]. Specifically, fluoroquinolone exposure promotes P. aeruginosa carbapenem resistance through the upregulation of efflux pumps, such as MexAB-OprM, that export meropenem and through transcriptional downregulation of the carbapenem entry porin OprD [4]. The emergence of carbapenem-resistant P. aeruginosa isolates during fluoroquinolone exposure has been demonstrated both in vitro and in vivo [5–12].
However, evidence of the specific selection of carbapenem-resistant P. aeruginosa by fluoroquinolones in HM patients or HCT recipients receiving fluoroquinolone prophylaxis is lacking. We hypothesized that the prophylactic use of fluoroquinolones among HM patients or HCT recipients would promote carbapenem resistance in P. aeruginosa through upregulation of efflux pump activity and transcriptional downregulation of OprD. In this study, we investigated the impact of fluoroquinolone prophylaxis in these patients on the emergence of P. aeruginosa isolates resistant to meropenem, and we characterized the mechanistic basis of meropenem resistance in selected isolates.
METHODS
Clinical and Microbiological Data
We conducted a retrospective review of P. aeruginosa bloodstream infections in adult (age ≥18 years) HM patients and HCT recipients at Oregon Health and Science University (OHSU) occurring between 1 January 2012 and 31 March 2018. Most HM and HCT patients at OHSU are cared for on a closed, 30-bed ward dedicated to these patients. There are approximately 500 admissions to this ward each year. The study was approved by the OHSU Institutional Review Board.
All cases were identified from the review of blood culture data stored in OHSU’s infection control database. Clinical information, including age, underlying diseases, gender, HCT type, antibiotic exposures within 90 days of bacteremia, date of hospital admission and prior hospitalizations, and absolute neutrophil count (ANC) were obtained from the electronic medical record.
The results of antimicrobial susceptibility testing, performed as part of routine clinical care by the OHSU microbiology laboratory using VITEK2 (bioMérieux, Durham, NC), were obtained by electronic medical record review. Results for cefepime, ceftazidime, meropenem, ciprofloxacin, piperacillin-tazobactam, gentamicin, and tobramycin were reported as susceptible, resistant, or intermediate, according to Clinical and Laboratory Standards Institute (CLSI) guidelines [13]; breakpoints for these antibiotics did not change during the study period [14]. Additional minimum inhibitory concentration (MIC) determinations for P. aeruginosa isolates submitted for whole-genome sequencing (WGS) were performed by broth microdilution (BMD) in cation-adjusted Mueller Hinton broth, according to CLSI standards [15], using panels prepared at the University of California, Los Angeles, and the University of Pittsburgh.
Neutropenic Prophylaxis
Fluoroquinolone prophylaxis, using levofloxacin at 500 mg orally or intravenously every 24 hours (or adjusted for renal dysfunction), was administered during periods of neutropenia (ANC < 500 cells/mm3), except when contraindicated due to allergy, severe intolerance, or other medical reason(s), at the discretion of the primary team. When a fluoroquinolone could not be used, another agent or no prophylaxis was used, at the discretion of the medical team.
Definitions
Breakthrough bacteremia was defined as the isolation of P. aeruginosa from a blood culture obtained after at least 3 doses of levofloxacin or after 72 hours of another prophylactic agent when levofloxacin was not used. Patients and the respective isolates were included twice in the analysis only if they experienced a second episode of bacteremia at least 90 days after the initial episode. Infections were considered hospital-associated if the bacteremia occurred ≥3 days following admission to OHSU or ≤14 days following discharge from OHSU [11]. Isolates reported as intermediate or resistant were considered nonsusceptible.
Whole-genome Sequencing
Genomic DNA from available clinical isolates was prepared using a DNA Tissue Kit (QIAGEN, Valencia, CA). DNA libraries were created using the Nextera XT DNA kit (Illumina, San Diego, CA) and sequencing was performed on an Illumina MiSeq platform (MiSeq Reagent Kit v3; 600 cycle). Illumina reads underwent adapter and quality trimming on the BaseSpace Onsite Server (Illumina, San Diego, CA) and were assembled de novo using the SPAdes pipeline [16] and PATRIC [17] into larger contigs, which were annotated using RAST [18] and PATRIC. To determine relatedness between isolates, a phylogenetic tree was created using FastTree 2 [19] and visualized using FigTree 1.4.3. The sequence type (ST) of each isolate was determined using a multilocus sequence type 1.8 server and PAst 1.0 [20]. The sequences of clinical isolates were compared to reference strain P. aeruginosa PAO1 (GenBank sequence AE004091.2).
Analysis of Efflux Pump Activity
Representative clinical isolates submitted for WGS were selected for phenotypic analysis of efflux pump activity based on the results of WGS, phylogenetic analysis, and meropenem susceptibility. Fresh clinical isolates and P. aeruginosa American Type Culture Collection (ATCC) 27853 (used as a control strain) were prepared on the day of the experiment for a 0.5 McFarland solution, which was then diluted into Cation-Adjusted Mueller Hinton II Broth (CA-MHB, Remel, Thermo Fisher Scientific, Lenexa, KS) according to CLSI standards. Meropenem (Ark-Pharm, Inc.) was prepared in CA-MHB and the efflux inhibitor phenyl-arginine-β-naphthylamide (PAβN, Sigma-Aldrich; 100 mg/L in CA-MHB) was added. A plate with no PAβN was prepared simultaneously for comparison. The plates were incubated overnight at 35°C and examined the next day for visible cell pellets or turbidity. Meropenem MICs in these experiments were determined by Etest (bioMérieux) using cation-adjusted Mueller-Hinton II agar (Remel).
Statistical Methods
All analyses were performed using Fisher’s exact 2-tailed test or logistic regression analysis (SPSSv24, IBM Corp.).
RESULTS
Patient Characteristics
There were 55 episodes of P. aeruginosa bacteremia among 51 patients included in the analysis. There were 4 patients that each had 2 episodes, occurring 115, 379, 180, and 99 days apart, which were included in the analysis; in each case, the susceptibility pattern of the second isolate differed from that of the first. There were 26 (47.3%) episodes that occurred during fluoroquinolone prophylaxis (25 levofloxacin, 1 moxifloxacin) and 29 (52.7%) in the absence of fluoroquinolone prophylaxis. Among the 29 that occurred in the absence of fluoroquinolone prophylaxis, 21 occurred in the absence of any concurrent antibiotic use and 8 occurred during receipt of another antibiotic (amoxicillin-clavulanate, n = 3; cefpodoxime, n = 2; trimethoprim-sulfamethoxazole, n = 1; cefepime, n = 1; cefazolin, n = 1). The median number of fluoroquinolone doses administered prior to bacteremia was 9 (range, 3–43). Patient characteristics by antibacterial therapy at the time of bacteremia are shown in Table 1. There were no significant differences in patient age, gender, or receipt of HCT between the 2 groups. Neutropenia was more common in the group receiving fluoroquinolone prophylaxis at the time of bacteremia. Of the 3 patients receiving fluoroquinolone prophylaxis at the time of bacteremia who were not neutropenic, 2 were receiving prophylaxis in the setting of acute graft-versus-host disease of the gastrointestinal tract, and 1 had not yet discontinued prophylaxis with ANC recovery following a period of neutropenia.
Table 1.
Patient Characteristics According to Antibiotic Use at the Time of Bacteremia
| Characteristic | FQ (n = 26) | Non-FQ or None (n = 29) | P Value |
|---|---|---|---|
| Age, y, median (range)a | 61 (25-72) | 60 (21-78) | >.5 |
| Genderb | |||
| Male | 17 (68) | 17 (63) | >.5 |
| Female | 8 (32) | 10 (27) | |
| Underlying diseaseb | |||
| AML | 16 (64) | 11 (40.7) | >.5c |
| MDS | 0 | 5 (18.5) | |
| ALL | 3 (12) | 6 (22.2) | |
| Other | 6d (24) | 5e (18.5) | |
| ANC ≤ 500/mm3a | |||
| Yes | 23 (88.5) | 15 (51.7) | .004 |
| No | 3 (11.5) | 14 (48.3) | |
| HCTa | |||
| Yes | 12f (46.1) | 17g (58.6) | .4 |
| No | 14 (53.9) | 12 (41.4) | |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; ANC, absolute neutrophil count; FQ, fluoroquinolone; HCT, hematopoietic cell transplant; MDS, myelodysplastic syndrome.
aAll bacteremic events.
bUnique patients per category of antibiotic use at time of bacteremia.
cAML + MDS vs others.
dConsisted of 2 lymphoma, 3 mutiple myeloma, and 1 aplastic anemia cases.
eConsisted of 3 lymphoma and 2 chronic lymphocytic leukemia cases.
fConsisted of 9 allogeneic and 3 autologous cases.
gConsisted of 16 allogeneic and 1 autologous cases.
Impact of Fluoroquinolone Prophylaxis on Meropenem Susceptibility Among Breakthrough Isolates
The results of susceptibility testing according to antibiotic use at the time of bacteremia are shown in Table 2. None of the fluoroquinolone-breakthrough P. aeruginosa isolates were susceptible to ciprofloxacin, compared to 21 of 29 (72.4%) of isolates among patients receiving non-fluoroquinolone/no-antibiotic (P < .0001). Only 4 of 26 (15.4%) fluoroquinolone-breakthrough P. aeruginosa isolates were susceptible to meropenem, compared to 21 of 29 (72.4%) among patients receiving non-fluoroquinolone/no- antibiotic (P < .0001). Of the 22 meropenem-nonsusceptible, fluoroquinolone-breakthrough P. aeruginosa isolates, 17 (77.3%) were classified as meropenem-resistant and 5 (22.7%) were meropenem-intermediate. Imipenem susceptibility testing was not routinely performed, although MICs were determined for isolates submitted for WGS (Supplementary Table 1). In contrast to meropenem, there were no significant differences in susceptibilities to other antibiotics tested between the 2 groups. Limiting this analysis to those who were neutropenic at the time of bacteremia did not change these results (data not shown).
Table 2.
Susceptibility of Pseudomonas aeruginosa Isolates According to Antibiotic Use at Time of Bacteremia
| Antibiotic | Antibiotic | P Value | |
|---|---|---|---|
| FQ (n = 26) | Non-FQ or None (n = 29) | ||
| Susceptible isolates, n (%) | Susceptible isolates, n (%) | ||
| Ciprofloxacin | 0 | 21 (72.4) | <.0001 |
| Meropenem | 4 (15.4) | 21 (72.4) | <.0001 |
| Cefepime | 21 (80.8) | 26 (89.6) | .4 |
| Ceftazidime | 19 (73.1) | 25 (86.2) | .3 |
| Gentamicin | 21 (80.8) | 28 (96.5) | .1 |
| P/T | 19 (73.1) | 25 (86.2) | .3 |
| Tobramycin | 25 (96.1) | 29 (100) | .5 |
Abbreviations: FQ, fluoroquinolone; P/T, piperacillin-tazobactam.
Risk Factors for Bacteremia With Meropenem-nonsusceptible P. aeruginosa Isolates
Risk factors for bacteremia with a meropenem-nonsusceptible P. aeruginosa isolate were chosen for analysis based on previously published work [10, 11, 21] and biologic plausibility. On univariate analysis, fluoroquinolone breakthrough and hospital-associated infections were the only factors associated with isolation of a meropenem-nonsusceptible P. aeruginosa bloodstream isolate (Table 3). Notably, only 10 episodes (18.2%) were preceded by meropenem exposure (median doses = 22, range 3–177) within 90 days of bacteremia; no patient received imipenem or doripenem. In a multivariate analysis incorporating fluoroquinolone breakthroughs and hospital-associated infections, only fluoroquinolone breakthroughs (P = .001; odds ratio 11.3, 95% confidence interval 3.1–50.6) were independently predictive of meropenem-nonsusceptibility.
Table 3.
Risk Factors for Meropenem-nonsusceptibility Among Pseudomonas aeruginosa Bloodstream Isolates
| Risk Factor | MPM NS (n = 30) | MPM S (n = 25) | P Value | OR (95% CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | 21 | 16 | 0.6 | 1.3 (0.4–4.1) |
| Female | 9 | 9 | ||
| Age, median (range) | 61 (22–73) | 63 (21–80) | 0.5 | ND |
| FQ breakthrough | ||||
| Yes | 22 (73.3) | 4 (16) | <.0001 | 14.4 (4.1–62) |
| No | 8 (26.7) | 21 (84) | ||
| HCT recipient | ||||
| Yes | 17 (56.6) | 12 (48) | 0.5 | 1.4 (0.5–4.3) |
| No | 13 (43.4) | 13 (52) | ||
| Neutropenia | ||||
| Yes | 22 (73.3) | 16 (64) | 0.5 | 1.5 (0.5–5) |
| No | 8 (26.7) | 9 (36) | ||
| CP exposurea | ||||
| Yes | 6 (20) | 4 (16) | 0.7 | 1.2 (0.3–5.5) |
| No | 24 (80) | 21 (84) | ||
| Antipseudomonal β-lactam exposurea,b | ||||
| Yes | 15 (50) | 9 (36) | 0.3 | 1.8 (0.6–5.4) |
| No | 15 (50) | 16 (64) | ||
| Hospital-associated infectionc | ||||
| Yes | 27 (90) | 15 (60) | 0.01 | 6 (1.6–30) |
| No | 3 (10) | 10 (40) | ||
| ICU stayd | ||||
| Yes | 3 (10) | 1 (4) | 0.6 | 2.7 (0.3–27.4) |
| No | 27 (90) | 24 (96) | ||
Abbreviations: CI, confidence interval; CP, carbapenem; FQ, fluoroquinolone; HCT, hematopoietic cell transplant; ICU, intensive care unit; MPM, meropenem; ND, not determined; NS, nonsusceptible; OHSU, Oregon Health and Science University; OR, odds ratio; S, susceptible.
aMeasured as ≥1 dose within 90 days of bacteremia.
bIncludes ceftazidime, cefepime, and piperacillin-tazobactam.
cDefined as an infection occuring ≥3 days following OHSU admission or ≤14 days after OHSU discharge.
dAt least 24 hours in a medical or surgical ICU within 90 days prior to bacteremic episode.
Analysis of Genetic Relatedness and Meropenem Resistance Determinants by Whole-genome Sequencing
In order to elucidate the mechanisms of resistance to meropenem among P. aeruginosa isolates in our study population, WGS of 23 isolates obtained between October 2016 and March 2018 was performed. Average WGS coverage was between 16X-25X. MICs of the sequenced isolates to various antibiotics are shown in Supplementary Table 1.
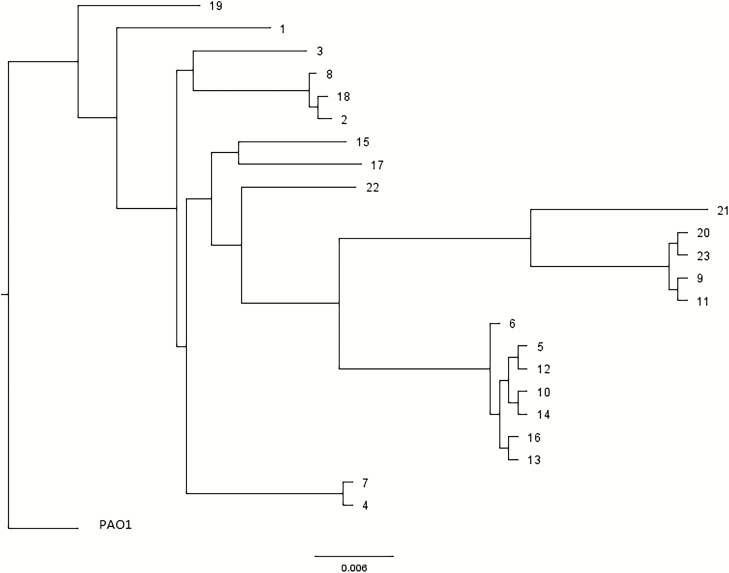
First, a single-nucleotide–variation analysis was performed to determine the degree of genetic relatedness among the sequenced isolates. There were 2 pairs of isolates, obtained from 2 different patients (isolates 4 and 7 from 1 patient obtained 27 days apart, and isolates 10 and 14 from another patient obtained 60 days apart), included in the WGS analysis as controls for assessing genetic relatedness, since the initial and recurrent isolates would be predicted to be closely related [22, 23]. These paired isolates were indeed found to be closely related, and other clusters of closely-related isolates, including ST-111 (n = 7, 2 isolates [10 and 14] from the same patient), ST-446 (n = 4), and ST-281 (n = 3) were also identified (Figure 1 and Table 4). Isolates belonging to the ST-111 and ST-446 clusters comprised 11 of 23 (47.8%) of those analyzed by WGS, but accounted for 76.9% (10 of 13) of the meropenem-nonsusceptible isolates analyzed by WGS.
Figure 1.
Single-nucleotide variation analysis of Pseudomonas aeruginosa bloodstream clinical isolates selected for whole-genome sequencing using P. aeruginosa PAO1 strain (GenBank sequence AE004091.2) as the reference. Isolates 4 and 7 were obtained from the same patient, and isolates 10 and 14 were obtained from another patient.
Table 4.
Mutations in Carbapenem Resistance Determinants and Efflux Pump Regulators
| Isolate | ST | MPM MIC | MPM Int | OprDa | MexR | NalC | NalD | MexZ | NfxB |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | ≤0.25 | S | … | … | G71E | … | … | … |
| 2 | 281 | ≤0.25 | S | … | … | G71E, S209R | … | … | … |
| 3 | 17 | 16 | R | G76ARX | … | G71E, S209R | … | … | … |
| 4 | 260 | ≤0.25 | S | - | … | G71E, S209R | … | L138R | … |
| 5 | 111 | 4 | I | S100X | V126E | G71E | … | FSb | … |
| 6 | 111 | ≤0.25 | S | … | V126E | G71E | … | FSb | … |
| 7 | 260 | 1 | S | … | … | G71E | … | L138R | … |
| 8 | 281 | 0.5 | S | … | … | G71E, S209R | … | … | … |
| 9 | 446 | 8 | R | Y328X | V126E | G71E, A145V, S209R | … | … | … |
| 10 | 111 | 4 | I | S100X | V126E | G71E | … | Δ1-28c | … |
| 11 | 446 | 8 | R | Y328X | V126E | G71E, A145V, S209R | … | … | … |
| 12 | 111 | 4 | I | S100X | V126E | G71E | … | Δ1-28c | … |
| 13 | 111 | >16 | R | Y294X | V126E | G71E | … | FSb | … |
| 14 | 111 | 8 | R | S100X | V126E | G71E | … | Δ1-28c | … |
| 15 | 291 | 8 | R | FSd | … | G71E, S209R | … | … | … |
| 16 | 111 | 32 | R | Y294X | V126E | V115X | … | FSb | … |
| 17e | 27 | ND | S | … | V126E,V132A | G71E,S209R | … | L128M | … |
| 18e | 281 | ND | S | … | … | G71E,S209R | … | … | … |
| 19e | Unknown | ND | S | … | … | G71E | … | … | … |
| 20 | 446 | 8 | R | Y328X | V126E | G71E, A145V, S209R | … | … | … |
| 21 | 308 | 32 | R | FSf | V126E | G71E,D79E,S209R | L62P | NS | … |
| 22f | 132 | ND | S | … | … | G71E,S09R | … | … | … |
| 23 | 446 | 4 | I | Y328X | … | G71E, A145V, S209R | … | … | … |
All mutations are at the amino acid level unless otherwise specified.
Abbreviations: ∆, deletion; FS, frameshift; I, intermediate; Int, interpretation; MIC, minimum inhibitory concentration; MPM, meropenem; ND, not determined; NS, not sequenced; nt, nucleotide; R, resistant; S, susceptible; ST, sequence type; X, stop codon.
aOnly inactivating mutations (frameshifts, premature stop codons) are listed.
bDeletion of nt 38-47.
cDeletion of nt 1-84.
dDeletion of nt 62-74.
eMPM MICs not determined. Results shown are as reported by Oregon Health and Science University microbiology using VITEK2.
fInsertion of nt GG at position 484.
Importantly, no isolate was found to harbor a carbapenemase gene. Instead, all meropenem-nonsusceptible isolates contained mutations in oprD that were predicted to result in a loss of expression of functional OprD, including premature stop codons at amino acids 76, 100, 294, and 328, or frameshift mutations (Table 4); no inactivating oprD mutations were found in meropenem-susceptible isolates. No isolate was found to have a functionally significant mutation in ampC, ampR, ampD, or dacB (data not shown).
Genetic Analysis of Efflux Pump Regulators and Phenotypic Assessment of Meropenem Efflux Pump Activity
WGS data for each isolate was assessed for mutations in regulators of efflux pumps MexAB-OprM, MexXY-OprM, and MexCD-OprJ, which have been implicated, to various degrees of evidence, in exporting meropenem [24]. MexAB-OprM expression is negatively regulated by mexR, nalC, and nalD [25]. Functionally-insignificant polymorphisms in mexR (V126E and V132A) and nalC (G71E and S209R) were common (Table 4) [26, 27]. Mutations in nalC that were limited to meropenem-nonsusceptible isolates but of unclear functional significance included A145V (ST-446 isolates 9, 11, 20, and 23) [22, 28, 29], a premature stop codon at amino acid 115 (V115X, isolate 16), and D79E (isolate 21). A previously-undescribed mutation in nalD (L62P) was present in 1 meropenem-nonsusceptible isolate (isolate 21).
Amino-terminal frameshift (isolates 5, 6, 13, and 16) or deletion (isolates 10, 12, and 14) mutations in the MexXY-OprM–negative regulator mexZ were present in all ST-111 isolates. Additional mutations in mexZ, previously described and of unclear significance, included L128M and L138R [29, 30]. No mutations in NfxB, the regulator of MexCD-OprJ, were found.
A phenotypic assessment of meropenem efflux pump activity was performed on a subset of the sequenced clinical isolates using the efflux pump inhibitor PAβN (100 mg/L) [31, 32]. Importantly, no decrease in the meropenem MIC was observed for the meropenem-susceptible isolates 1 and 2 or the ATCC control strain, indicating the lack of an efflux pump–independent effect of PAβN on meropenem activity (Table 5). In contrast, all meropenem-nonsusceptible isolates demonstrated 2- to 8-fold reductions in meropenem MICs with the addition of PAβN. Notably, isolates 3 and 11 contained no functionally-significant mutations in any efflux pump regulator analyzed, indicating that efflux pump activity may not be accurately predicted by the genotypic analysis performed.
Table 5.
Effect of Efflux Pump Inhibition on Meropenem Minimum Inhibitory Concentrations for Select Pseudomonas aeruginosa Isolates
| Isolate | MIC (mg/L) | |
|---|---|---|
| PAβN- | PAβN+ | |
| ATCC | 0.5 | 1 |
| 1 | 1 | 2 |
| 2 | 0.25 | 2 |
| 3 | 32 | 8 |
| 5 | 8 | 4 |
| 9 | 4 | 2 |
| 10 | 4 | 2 |
| 11 | 32 | 4 |
| 12 | 4 | 2 |
| 13 | 32 | 16 |
| 16 | 32 | 4 |
Abbreviations: ATCC, American Type Culture Collection; MIC, minimum inhibitory concentration; PAβN, phenyl-arginine-β-naphthylamide.
DISCUSSION
We performed this study to test the hypothesis that fluoroquinolone prophylaxis in HM patients and HCT recipients promotes the emergence of meropenem resistance among P. aeruginosa by upregulation of efflux pumps, which export meropenem, and transcriptional downregulation of OprD, which limits meropenem entry. We found that fluoroquinolone prophylaxis was, indeed, strongly associated with breakthrough bacteremia with P. aeruginosa strains nonsusceptible to meropenem but not consistently nonsusceptible to other antipseudomonal antibiotics. However, our findings suggest that the selection of meropenem-nonsusceptible P. aeruginosa isolates during fluoroquinolone prophylaxis may be due not only to the induction of cross-resistance mechanisms, but may be further influenced by the epidemiology of P. aeruginosa bloodstream infections at our center.
We found evidence of increased efflux activity in all of the meropenem-nonsusceptible isolates tested. The specific pump(s) that is (are) upregulated to promote meropenem efflux remains to be determined. MexAB-OprM has been most consistently associated with meropenem export [25], and it is upregulation of MexAB-OprM that has been demonstrated to promote meropenem resistance as a result of fluoroquinolone exposure in vitro [7]. Although only 1 isolate sequenced by WGS (isolate 16) possessed an inactivating mutation in 1 of the MexAB-OprM negative regulators (nalC V115X), it is not clear that increased MexAB-OprM expression and activity can be accurately predicted from our genotypic analysis, since isolates 3 and 11 demonstrated consistent reductions in meropenem MICs in the presence of PAβN, despite having no known relevant mutations in any efflux pump regulator analyzed. Inactivating frameshift and amino-terminal deletion mutations in the MexXY-OprM–negative regulator mexZ among ST-111 cluster isolates suggest that this efflux pump may be upregulated, although its activity on meropenem has not been as conclusively documented as that of MexAB-OprM [33]. Direct quantitative analysis of MexAB-OprM expression and other candidate efflux pumps will be required to determine their contributory role in resistance to meropenem in these isolates.
We also found that all meropenem-nonsusceptible isolates analyzed by WGS contained inactivating mutations in oprD, rendering any fluoroquinolone-induced transcriptional downregulation of oprD irrelevant in terms of meropenem activity. This was a surprising finding, since oprD mutations generally arise under carbapenem–selected pressure rather than fluoroquinolone–selective pressure [4]. Only 1 of 13 (7.7%) meropenem-nonsusceptible isolates found to contain an oprD inactivating mutation by WGS was obtained following meropenem exposure within 90 days, and overall only 6 of 30 (20%) meropenem-nonsusceptible isolates in the entire study were obtained following meropenem exposure within 90 days.
The finding of infections with oprD-mutant isolates in patients not exposed to carbapenems raises the possibility that these infections may result, in part, from cross-colonization following admission to our HCT/HM unit. Indeed, the phylogenetic analysis of isolates sequenced by WGS revealed several clusters of closely-related isolates, with ST-111 and ST-446 cluster isolates representing 76.9% of meropenem-nonsusceptible isolates sequenced by WGS. Additionally, all infections caused by ST-111 and ST-446 isolates were hospital-associated, reflecting an overall association between hospital-associated infections and meropenem non-susceptibility (Table 3). Whether bacteremia with clonal P. aeruginosa strains, indicative of cross-colonization, is common in HCT/HM wards is unclear, since the molecular epidemiology of non-outbreak, invasive P. aeruginosa in these patients, and in hospitalized patients in general [34], is poorly understood. Notably, meropenem-nonsusceptible P. aeruginosa isolates consistently accounted for approximately 30% of all P. aeruginosa bloodstream isolates during each year of this study among the study population, indicating that our results are unlikely to be due to an outbreak of meropenem-nonsusceptible strains in our HCT/HM unit, but probably reflect the long-term persistence of such strains.
Taken together, our findings suggest that both cross-colonization with OprD-deficient P. aeruginosa strains and the selection of efflux pump, overexpressing strains during fluoroquinolone prophylaxis ultimately lead to breakthrough infections with isolates resistant to meropenem. Indeed, both the loss of OprD and upregulation of efflux pump activity concurrently are required for meropenem-resistant categorization (MIC ≥ 8 µg/mL) [4], which represented the majority (77.3%) of meropenem-nonsusceptible, fluoroquinolone-breakthrough isolates in this study. A sequential analysis of patients’ colonizing P. aeruginosa strains that were present at the time of admission and over the course of hospitalization prior to and during fluoroquinolone exposure will be informative.
This work represents, to the best of our knowledge, the first evidence that fluoroquinolone prophylaxis in HM patients or HCT recipients is independently predictive of meropenem non-susceptibility among breakthrough P. aeruginosa bloodstream isolates. Due in part to antipseudomonal activity, meropenem is currently recommended as a first-line option for the empirical management of febrile neutropenia, based on studies that were mostly performed prior to the routine, widespread use of levofloxacin prophylaxis [2]. Confirmation of our results in larger studies should prompt a reassessment of this recommendation in patients receiving fluoroquinolone prophylaxis.
Our study has important limitations. The single-center nature of this study limits generalizability of our findings to similar patient populations at other centers, especially if our results are, in part, reflective of dominant P. aeruginosa strains unique to our HM/HCT ward. The relatively small number of isolates that were available for WGS analysis limits the ability to make firm conclusions pertaining to genetic mechanisms of resistance and the epidemiology of the P. aeruginosa strains causing invasive infections in our patient population. Only 13 meropenem-nonsusceptible isolates were available for confirmatory MIC testing by BMD. However, among those 13, there were no categorical discordant results, consistent with findings demonstrating no major errors in meropenem testing between VITEK2 and BMD [35]. Conclusions pertaining to the cause-and-effect relationship between fluoroquinolone exposure and efflux pump activity affecting meropenem susceptibility cannot be made at this time.
In conclusion, we found that fluoroquinolone prophylaxis in HM patients and HCT recipients selects for meropenem-nonsusceptible P. aeruginosa isolates during episodes of breakthrough bacteremia. Further genetic and phenotypic studies may provide novel information pertaining to the fundamental mechanisms by which fluoroquinolones promote meropenem resistance and the epidemiology of invasive P. aeruginosa infections in these patients, with potential implications for the empirical management of patients with febrile neutropenia.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Bruce Dougan (Kaiser Microbiology Laboratory) for providing bacteria isolates, Mike Lasarev (Oregon Institute of Occupational Health Sciences) for statistical advice, and Lynne Strasfeld (Oregon Health and Science University Division of Infectious Diseases) for critical review of the manuscript.
Financial support. This work was supported by an Oregon Health and Science University Core Pilot Fund Award (to M. H.). Y. D. received support from the National Institutes of Health (grant numbers R01AI104895, R21AI123747 and R21AI135522. R. K. S. received support from the National Institutes of Health (grant number K08AI114883).
Potential conflicts of interest. Y. D. received personal fees from Allergan, The Medicines Company, Roche, Tetraphase, Achaogen, Merck, Meiji, Shionogi, and Curetis; grants from The Medicines Company and Accelerate Diagnostics; and personal fees from Gladius, outside the submitted work. J. S. L. has served as a consultant for Merck, Achaogen, Accelerate Diagnostics, The Medicines Company, Shionogi, and Tetraphase. R. K. S. has served as a consultant or on advisory boards for Accelerate Diagnostics, Allergan, The Medicines Company, Achaogen, and Pfizer; and has received investigator-initiated grant funding from Merck, Accelerate Diagnostics, Allergan, Melinta, Shionogi, Achoegen, Venatorx, and Roche. J. S. R. M. H. is employed by Accelerate Diagnostics and owns stock in Accelerate Diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis 2017; 19:e12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:427–31. [DOI] [PubMed] [Google Scholar]
- 3. Bow EJ. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr Opin Infect Dis 2011; 24:545–53. [DOI] [PubMed] [Google Scholar]
- 4. Livermore DM. Of Pseudomonas, porins, pumps and carbapenems. J Antimicrob Chemother 2001; 47:247–50. [DOI] [PubMed] [Google Scholar]
- 5. Rådberg G, Nilsson LE, Svensson S. Development of quinolone-imipenem cross resistance in Pseudomonas aeruginosa during exposure to ciprofloxacin. Antimicrob Agents Chemother 1990; 34:2142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother 1992; 36:1847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanimoto K, Tomita H, Fujimoto S, Okuzumi K, Ike Y. Fluoroquinolone enhances the mutation frequency for meropenem-selected carbapenem resistance in Pseudomonas aeruginosa, but use of the high-potency drug doripenem inhibits mutant formation. Antimicrob Agents Chemother 2008; 52:3795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aubert G, Pozzetto B, Dorche G. Emergence of quinolone-imipenem cross-resistance in Pseudomonas aeruginosa after fluoroquinolone therapy. J Antimicrob Chemother 1992; 29:307–12. [DOI] [PubMed] [Google Scholar]
- 9. Michéa-Hamzehpour M, Auckenthaler R, Regamey P, Pechère JC. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother 1987; 31:1803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol 2006; 27:893–900. [DOI] [PubMed] [Google Scholar]
- 11. López-Dupla M, Martínez JA, Vidal F, et al. Previous ciprofloxacin exposure is associated with resistance to beta-lactam antibiotics in subsequent Pseudomonas aeruginosa bacteremic isolates. Am J Infect Control 2009; 37:753–8. [DOI] [PubMed] [Google Scholar]
- 12. Wolter DJ, Acquazzino D, Goering RV, Sammut P, Khalaf N, Hanson ND. Emergence of carbapenem resistance in Pseudomonas aeruginosa isolates from a patient with cystic fibrosis in the absence of carbapenem therapy. Clin Infect Dis 2008; 46:e137–41. [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement M100-S22. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2012. [Google Scholar]
- 14. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing M100-S28. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2018. [Google Scholar]
- 15. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M07-11. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2018. [Google Scholar]
- 16. Nurk S, Bankevich A, Antipov D, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol 2013; 20:714–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wattam AR, Abraham D, Dalay O, et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 2014; 42:D581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Overbeek R, Olson R, Pusch GD, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 2014; 42:D206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 2010; 5:e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012; 50:1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Averbuch D, Tridello G, Hoek J, et al. Antimicrobial resistance in gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: intercontinental prospective study of the infectious diseases working party of the European bone marrow transplantation group. Clin Infect Dis 2017; 65:1819–28. [DOI] [PubMed] [Google Scholar]
- 22. Domitrovic TN, Hujer AM, Perez F, et al. Multidrug resistant Pseudomonas aeruginosa causing prosthetic valve endocarditis: a genetic-based chronicle of evolving antibiotic resistance. Open Forum Infect Dis 2016; 3:ofw188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCARTHY KL, Kidd TJ, Paterson DL. Pseudomonas aeruginosa blood stream infection isolates from patients with recurrent blood stream infection: Is it the same genotype?Epidemiol Infect 2017; 145:3040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare?Clin Infect Dis 2002; 34:634–40. [DOI] [PubMed] [Google Scholar]
- 25. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cabot G, Ocampo-Sosa AA, Domínguez MA, et al. ; Spanish Network for Research in Infectious Diseases (REIPI). Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob Agents Chemother 2012; 56:6349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Llanes C, Hocquet D, Vogne C, Benali-Baitich D, Neuwirth C, Plésiat P. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob Agents Chemother 2004; 48:1797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campo Esquisabel AB, Rodríguez MC, Campo-Sosa AO, Rodríguez C, Martínez-Martínez L. Mechanisms of resistance in clinical isolates of Pseudomonas aeruginosa less susceptible to cefepime than to ceftazidime. Clin Microbiol Infect 2011; 17:1817–22. [DOI] [PubMed] [Google Scholar]
- 29. Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and OprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 2006; 50:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hocquet D, Nordmann P, El Garch F, Cabanne L, Plésiat P. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006; 50:1347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J Antimicrob Chemother 2014; 69:1804–14. [DOI] [PubMed] [Google Scholar]
- 32. Kern WV, Steinke P, Schumacher A, Schuster S, von Baum H, Bohnert JA. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J Antimicrob Chemother 2006; 57:339–43. [DOI] [PubMed] [Google Scholar]
- 33. Morita Y, Tomida J, Kawamura Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front Microbiol 2012; 3:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCarthy KL, Kidd TJ, Paterson DL. Molecular epidemiology of Pseudomonas aeruginosa bloodstream infection isolates in a non-outbreak setting. J Med Microbiol 2017; 66:154–9. [DOI] [PubMed] [Google Scholar]
- 35. Bobenchik AM, Deak E, Hindler JA, Charlton CL, Humphries RM. Performance of Vitek 2 for antimicrobial susceptibility testing of Acinetobacter baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia with Vitek 2 (2009 FDA) and CLSI M100S 26th Edition breakpoints. J Clin Microbiol 2017; 55:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.