Abstract
There are minimal data regarding the management of high risk endometrial cancer histologies lacking invasive disease on the final pathology specimen. This study examines a cohort of these patients and assesses outcomes including time to recurrence and risk of death after management with and without adjuvant therapies.
Endometrial cancer patients with minimal or no remaining invasive disease on final pathologic specimen from 1995 to 2010 were included. Surgical procedure was at the discretion of the operating physician. Electronic medical records were used to abstract relevant clinicopathologic data and standard statistical methods were employed.
70 patients met inclusion criteria, of which 26 were high grade histologies. Adjuvant therapies were given in 12 of 26 patients. 6/26 patients recurred, of which 50% were salvaged with therapy at time of recurrence. Overall deaths occurred in 3 of 26 patients in the high risk cohort.
Less than half of the high risk cohort received adjuvant therapies after surgical management. No histologic type was found to increase risk of recurrence, and treatment with initial adjuvant therapy did not significantly reduce recurrence risk. Large scale prospective trials are needed to aid in management of this unique endometrial cancer population.
Keywords: Type II endometrial cancer, Early stage endometrial cancer
Highlights
-
•
This series describes a cohort of high risk with minimal remaining disease on final pathology specimen.
-
•
Patients with clear cell histology who went on to recur did so quickly and were salvageable.
-
•
Treatment with adjuvant therapy after primary surgery did not significantly improve risk of recurrence.
1. Introduction
Endometrial cancer continues as the most commonly diagnosed gynecologic malignancy with 63,000 new cases in 2018 (Cancer Facts, and Figures Atlanta, Georgia, 2018). Endometrial cancer is separated into type 1 carcinomas that includes grade 1 and 2 endometrioid histologies that are less aggressive and often associated with excess circulating estrogen, and type 2, high risk carcinomas, which include all other types (serous, clear cell, carcinosarcoma, mixed, undifferentiated and grade 3 endometrioid histology) and portend a poorer prognosis. In addition, type 2 carcinomas tend to present at advanced stages with extrauterine spread at time of diagnosis (Goff et al., 1994). The NCCN recommendations for stage IA high risk carcinomas include observation or chemotherapy, with or without vaginal brachytherapy (VBT) or external beam radiation, with or without brachytherapy (National Comprehensive Cancer Network, 2018), demonstrating the wide range of adjuvant therapies endorsed by experts. A clinical dilemma then arises when an aggressive histology is found on work-up, but on final pathology there is minimal to no residual disease.
The majority of studies do not specifically focus on stage IA patients in this clinical scenario, which would be useful for guiding adjuvant therapies and counseling patients. We therefore aimed to review our institutional experience managing high risk histologies with minimal residual tumor, as well as report the clinical scenarios of patients who recurred. We hypothesized that in those with high risk histologies, treatment with adjuvant therapy after surgery would decrease recurrence risk.
2. Methods
2.1. Patient population
Women age 18–90 undergoing definitive surgical evaluation for suspected early stage uterine cancers at Washington University in St. Louis after endometrial biopsy or dilation and curettage from 1995 to 2010 were eligible for this IRB approved retrospective cohort study. Patients were included if they had no evidence of residual disease, disease confined to a polyp, or non-invasive disease (endometrial involvement only) on final hysterectomy specimen. High risk, high grade histologies were defined as uterine serous (USC), clear cell (CCC), grade 3 endometrioid (HGE), mixed (M), and carcinosarcoma (CS). Surgical approach was performed at the discretion of the surgeon and during this period, full lymph node dissections were performed. Data were abstracted from electronic medical records, and baseline characteristics and demographic data as well as subsequent treatment, pathology, and clinical outcomes were assessed. Patients without adequate staging, had invasive disease, or did not have available follow up data were excluded. Vital status and duration of follow up was recorded for each patient.
2.2. Adjuvant therapies
Initial therapies included observation, chemotherapy or radiation. Patients who went on to recur were identified and location of recurrence and type of treatment offered was abstracted.
2.3. Statistical analysis
All analyses were performed using SAS Software (v.9.4.; SAS Institute, Cary, NC) and statistical significance was defined as p < .05. Demographic and clinical data were compared among groups using Fisher's exact tests for categorical data and Wilcoxon rank sum tests for non-normal data distribution. Median disease recurrence durations were compared using Wilcoxon rank sum and Kruskal-Wallis tests. Kaplan-Meier curves were produced to visually demonstrate duration to disease recurrence by histology type and treatment type.
3. Results
3.1. Demographics
A total of 26 patients with high grade, high risk histology were identified out of 70 patients without residual disease, disease confined to a polyp, or endometrium confined disease within the study period (Table 1). Overall the high risk cohort was comprised of grade 3 endometrioid (23.1%, n = 6), clear cell (19.2%, n = 5), serous (34.6%, n = 9), mixed (19.2%, n = 5), and carcinosarcoma (3.8%, n = 1), respectively. Compared to low grade patients, high risk patients were significantly older (64.3 vs 59.9 years, p = .04), had been followed longer (5.5 vs 2.2 years, p < .01), and had a higher risk of recurrence (6 vs 0, p ≤.01), but otherwise had similar ASA scores, race distribution, and co-morbidities including hypertension, diabetes, and BMI. All patients within the high risk cohort had residual disease on final specimen, versus 33 (75%) of the low grade IA patients. No low grade patients received adjuvant therapies.
Table 1.
Demographics.
| Grade 1 & 2 |
Grade 3 |
P | |
|---|---|---|---|
| (n = 44) | (n = 26) | ||
| Age at diagnosis | 59.5 (54.4–68.3) | 64.3 (58.5–72.9) | 0.04 |
| Race | 0.41 | ||
| Black | 6 (13.6) | 2 (7.7) | |
| Caucasian | 38 (86.4) | 23 (88.5) | |
| Other | 0 (0.0) | 1 (3.9) | |
| Ethnicity | 0.37 | ||
| Hispanic | 0 (0.0) | 1 (3.9) | |
| Comorbidities | |||
| Hypertension | 17 (38.6) | 12 (46.2) | 0.54 |
| Diabetes | 7 (15.9) | 9 (34.6) | 0.07 |
| BMI at Time of Surgery | 34.0 (30.0–42.5) | 31.0 (28.0–38.0) | 0.26 |
| Histology | |||
| Endometrioid | 44 (100.0) | 6 (23.1) | <0.01 |
| Clear cell | 0 (0.0) | 5 (19.2) | <0.01 |
| Papillary serous | 0 (0.0) | 9 (34.6) | <0.01 |
| Carcinosarcoma | 0 (0.0) | 1 (3.8) | 0.37 |
| Mixed | 0 (0.0) | 5 (19.2) | <0.01 |
| Surgical Pathology | <0.01 | ||
| No residual malignancy | 11 (25.0) | 0 (0.0) | |
| Malignancy limited to polyp | 2 (4.6) | 8 (30.8) | |
| Malignancy limited to endometrium | 31 (70.5) | 18 (69.2) | |
| Initial treatment | <0.01 | ||
| Surgery only | 44 (100.0) | 14 (53.8) | |
| Surgery and chemotherapy | 0 (0.0) | 9 (34.6) | |
| Surgery, chemotherapy, and radiation | 0 (0.0) | 3 (11.5) | |
| ASA Score | 0.73 | ||
| 2 | 9 (20.5) | 7 (26.9) | |
| 3 | 34 (77.3) | 19 (73.1) | |
| 4 | 1 (2.3) | 0 (0.0) | |
| Vital statusa | 0.14 | ||
| NED | 36 (81.8) | 16 (61.5) | |
| DOD | 0 (0.0) | 3 (11.5) | |
| LTFU | 8 (18.2) | 7 (26.9) | |
| Recurrence | 0 (0.0) | 6 (23.1) | <0.01 |
| Years from diagnosis to last contact | 2.2 (0.1–5.9) | 5.5 (3.7–7.6) | <0.01 |
NED = No evidence of disease, DOD = dead of disease, LTFU = Lost to follow up.
3.2. Initial treatment
14 of the high risk cohort received no further therapy, while 12 total patients received adjuvant therapies. 9 patients received adjuvant chemotherapy in addition to surgery (34.6%) and 3 received chemotherapy along with radiation (11.5%). No patients were treated with radiation alone. There was no significant difference in the average age of those observed versus those receiving adjuvant therapy (68.8 vs. 64.0 years, p = .16). 10 out of the 12 (83.3%) of the adjuvantly-treated patients had either mixed serous or pure serous histologies. The patient with carcinosarcoma was recommended to receive adjuvant therapy but did not receive it. The mean number of chemotherapy cycles given was 4 ± 1.8, and in all cases a platinum and taxane regimen was given. All patients who received radiation underwent high dose rate VBT to the upper 3–4 cm of the vagina to a total of 21–36 Gy.
3.3. Outcomes
Of the high risk cohort, 6 patients recurred (23.1%) with a median follow up time of 5.5 years, and 50% of those recurrences were distant recurrences that were not salvageable (Table 2). 3 out of 6 recurrences were CCC and all 3 were treated with radiation with or without surgery and all were alive without evidence of disease at recent follow up. The other 3 were either pure serous or mixed serous, all of whom are now dead from disease.
Table 2.
Details of Recurrences.
| Histology | Initial Therapy | Recurrence Location | Treatment for Recurrence | Outcome at last follow upa |
|---|---|---|---|---|
| Clear Cell | None | Vaginal cuff | Surgical resection plus radiation | NED |
| Clear Cell | None | Vaginal cuff | Radiation | NED |
| Clear Cell | None | Vaginal cuff | Surgical resection plus radiation | NED |
| Serous | 6 cycles of platinum and taxane | Distant | Surgery plus chemotherapy | DOD |
| Serous | 3 cycles of platinum and taxane | Distant | Chemotherapy | DOD |
| Mixed endometrioid and serous | 1 cycle of platinum and taxane, refused additional cycles | Distant and vaginal cuff | Declined offered chemotherapy | DOD |
NED = No evidence of disease, DOD = Dead of disease.
There was no increased risk of recurrence based on malignancy limited to a polyp versus limited to the endometrium, histology, or treatment with initial adjuvant therapy (Table 3). BMI was significantly higher in those who recurred (43.0 vs 30.5, p = .01), and hypertension (100% vs 30%, p < .01) and diabetes (83% vs 20%, p < .01) were more frequent, as well. 7 of the 26 patients (23.1%) were lost to follow up, defined as no follow up within the prior three years until study end, and were all within the no recurrence group. Overall, 3 of the 26 total patients (11.5%) were dead of disease at study end.
Table 3.
Characteristics of Grade 3 patients by recurrence status.
| Recurred (n = 6) | No recurrence (n = 20) | P | |
|---|---|---|---|
| Age at diagnosis | 69.5 (63.3–72.0) | 64.0 (57.9–73.3) | 0.62 |
| Race | >0.99 | ||
| Black | 0 (0.0) | 2 (10.0) | |
| Caucasian | 6 (100.0) | 17 (85.0) | |
| Other | 0 (0.0) | 1 (5.0) | |
| Ethnicity | >0.99 | ||
| Hispanic | 0 (0.0) | 1 (5.0) | |
| Comorbidities | |||
| Hypertension | 6 (100.0) | 6 (30.0) | <0.01 |
| Diabetes | 5 (83.3) | 4 (20.0) | <0.01 |
| Histology | |||
| Endometrioid | 0 (0.0) | 6 (30.0) | 0.28 |
| Clear cell | 3 (50.0) | 2 (10.0) | 0.06 |
| Serous | 2 (33.3) | 7 (35.0) | >0.99 |
| Carcinosarcoma | 0 (0.0) | 1 (5.0) | >0.99 |
| Mixed | 1 (16.7) | 4 (20.0) | <0.99 |
| Surgical Pathology | 0.33 | ||
| No residual malignancy | 0 (0.0) | 0 (0.0) | |
| Malignancy limited to polyp | 3 (50.0) | 5 (25.0) | |
| Malignancy limited to endometrium | 3 (50.0) | 15 (75.0) | |
| Initial treatment | >0.99 | ||
| Surgery only | 3 (50.0) | 11 (55.0) | |
| Surgery and chemotherapy | 2 (33.3) | 7 (35.0) | |
| Surgery, chemotherapy, and radiation | 1 (16.7) | 2 (10.0) | |
| BMI at time of surgery, Median | 43.0 (32.0–47.0) | 30.5 (27.5–35.5) | 0.01 |
| ASA Score | >0.99 | ||
| 2 | 1 (16.7) | 6 (30.0) | |
| 3 | 5 (83.3) | 14 (70.0) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| Vital statusa | <0.01 | ||
| NED | 3 (50.0) | 13 (65.0) | |
| DOD | 3 (50.0) | 0 (0.0) | |
| LTFU | 0 (0.0) | 7 (35.0) | |
| Years from diagnosis to last contact, Median (IQR) | 7.0 (3.7–9.0) | 5.0 (2.0–7.6) | 0.47 |
NED = No evidence of disease, DOD = dead of disease, LTFU = Lost to follow up.
Kaplan Meier curves were used to compare time to recurrence among the different histological subtypes. Interestingly, while the CCC patients recurred quickly (n = 3, average 14.9 months versus 80.1 months in the serous cohort, p = .08), as mentioned, 100% salvage rate was achieved (Fig. 1); no grade 3 endometrioid histology patients recurred during the study period. Given the small sample size, no multivariable analysis could be performed.
Fig. 1.
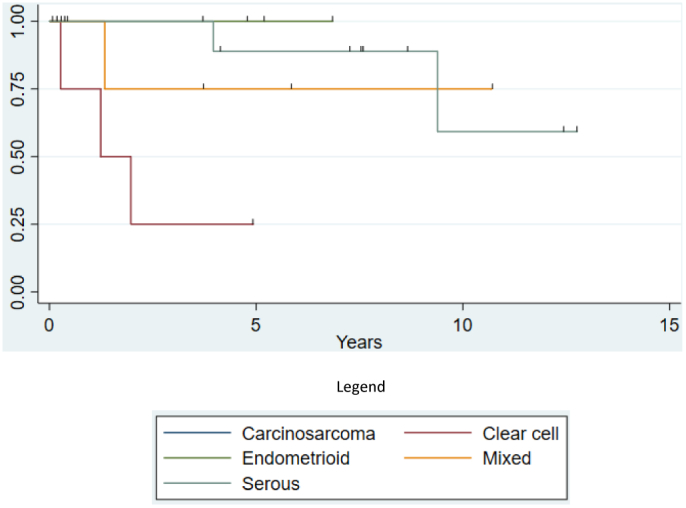
Time to Recurrence Based on Histology.
4. Discussion
Prior studies have examined the outcomes of early stage patients with various high risk histologies both with and without adjuvant therapies. Huh et al. retrospectively evaluated a series of 60 patients at four academic institutions with surgically staged IA-IC uterine serous cancer treated with observation or combinations of chemotherapy and radiation; there was no demonstrated improvement in overall survival in those treated with adjuvant radiation compared to observation, with no recurrences or disease related deaths in the small number of patients treated with at least chemotherapy (n = 8) (Huh et al., 2003). Further studies attempted to clarify outcomes in USC with minimal invasion. Hui et al. evaluated USC patients, surgically staged, with invasion limited to the endometrium or limited to a polyp, and in 22 patients with disease limited to uterus no recurrence was noted during follow up, although the use of adjuvant therapies is not reported (Hui et al., 2004). Kelly et al. evaluated 74 USC patients stage IA-IC, of which 12 had no residual uterine disease on final specimen; Platinum based therapy was associated with a significant improvement in both disease free and overall survival (Kelly et al., 2005). Specifically, those with no residual disease had no recurrences irrespective of adjuvant therapy given. Our own experience found that the patients who recurred with USC after no residual disease, even in the setting of adjuvant therapy, did poorly and succumbed to disease; however out of the included 9 USC patients, only 3 recurred total. Interestingly, all mixed tumors identified in our study included some component of serous tumor in the prior to surgery specimen. It has been suggested that tumor behavior is often driven by serous histology even in a mixed setting, and in our results 80% of mixed tumors received adjuvant therapy up front, with only 1 recurrence.
Clear cell carcinoma is even rarer with large scale prospective trials evaluating early stage management lacking. The Society of Gynecologic Oncology clinical practice committee recommends consideration for adjuvant therapies in early stage disease (Olawaiye and Boruta, 2009). A Taiwanese Gynecologic Oncology Group study sought to retrospectively investigate surgically staged CCC and included 80 stage I patients, of which 37 were observed when tumors were <2 cm and/or stage IA and found an overall survival of 93% (Hsu et al., 2014). Another retrospective investigation of 99 CCC patients, including 16 stage IA patients, found that in the 22 stage I and II patients with thoroughly sampled lymph nodes (>20 pelvic and paraaortic nodes) and varying therapies including observation pursued, only 1 patient recurred (Thomas et al., 2008). CCC is often studied concurrently with USC or in a mixed setting, making conclusions difficult to apply more broadly. Velker et al. reported a single institution experience of CCC and USC with <50% myometrial invasion undergoing adjuvant therapy versus observation, of which only 83% of the cohort had lymph node sampling. Of the 77 patients included, 12 total recurrences were documented and were not significantly different between groups. In a subgroup analysis of the observed patients, patients with myometrial invasion had a non-significant trend towards decreased recurrence free survival (75% versus 93%) (Velker et al., 2016). In our cohort, patients with pure clear cell histologies did well even with recurrence, and were alive and without evidence of disease after appropriate therapies.
Carcinosarcoma (CS), remains an especially difficult disease to manage even at early stages, as greater than half of these patients will have occult metastases (Yamada et al., 2000) and suffer high recurrence rates with a propensity to recur outside the pelvis (Silverberg et al., 1990). A multiinstitutional review of 303 stage I-III patients identified 70 observed stage I patients compared to 29 treated with chemotherapy, 26 radiation therapies, and 30 with combination chemotherapy and radiation. Compared to those stage I and II patients who were treated with chemotherapy, those who were observed had an increased risk of death by greater than four times (Dickson et al., 2015), though authors did not report separate data on stage I patients with minimal or no invasive disease. In our cohort, only one carcinosarcoma without residual disease was evaluable and has unfortunately been lost to follow up for >3 years. More evaluations of patients in this clinical scenario should be considered critical areas of investigation.
High grade endometrioid histology has historically been included within high intermediate risk studies GOG 99 and 249. GOG 99 aimed to outline high intermediate patient subgroups and determine if the addition of adjuvant external beam irradiation lowered the risk of recurrence (Keys et al., 2004), and GOG 249, of which final publication is pending, showed no improvement in outcomes of high risk patients defined by GOG 99 parameters who were then treated with VBT and chemotherapy as compared to standard whole pelvic radiation (Pelvic Radiation Therapy or Vaginal Implant Radiation Therapy, Paclitaxel, and Carboplatin in Treating Patients With High-Risk Stage I or Stage II Endometrial Cancer, 2018). Neither of these trials delineate if preoperative findings of a grade 3 tumor without evidence of residual disease warrants postoperative radiation therapy. In addition, the recently published PORTEC 3 trial did not demonstrate an improvement in survival with the addition of chemotherapy to pelvic radiation even those with invasive stage IA, grade 3 disease (de Boer et al., 2018). In our cohort, no pure grade 3, endometrioid histology tumors recurred.
There are a number of limitations to our study. Given its retrospective nature, it is subject to biases including confounders, chart review errors, and in this case, a >20% loss to follow up rate. In addition, the overall sample size was small and we were unable to create a mutivariable model for risk of recurrence or overall survival.
In our study, less than half of the high risk histology patients received adjuvant therapies and treatment with therapy was not conclusively linked to recurrence risk. Concordant with published data (Calle et al., 2003), obesity and metabolic dysfunction were also more common in the recurrence group. Our experience suggests that in patients with high risk histologies, clear cell carcinomas, while recurring quickly after observation in three patients, were 100% curable with salvage therapy, which suggests upfront observation is reasonable. In addition, while no definitive conclusions can be drawn about the patients who succumbed to recurrence, in all settings pure USC or mixed USC was present on initial biopsy. Additionally, all of these patients received initial adjuvant therapy (ranging from 1 to 6 cycles of carboplatin and taxane). Further studies, including meta-analyses and prospective data if possible, are essential to drawing firm conclusions about management of these patients with high risk but minimal to no residual disease on final pathology.
Author contributions
-
1.
Kathryn A. Mills, M.D.: First author who performed the majority of data collection and quality control, data analysis, and wrote majority of manuscript.
-
2.
Heather Lopez, M.D.: Performed data collection and entry, as well as assisted in manuscript writing and revisions.
-
3.
Lulu Sun, M.D., Ph.D.: Performed data collection and entry, as well as manuscript revision and approval of final submitted version.
-
4.
James C. Cripe, M.D.: Assisted with data analysis and manuscript revisions and approval of final submitted version
-
5.
Taylor Litz MPH: Assisted with statistical analysis and manuscript approval of final submitted version.
-
6.
Premal H. Thaker, M.D., M.S.: Assisted with manuscript revisions and approval of final submitted version.
-
7.
Matthew A. Powell, M.D.: Assisted with manuscript revisions and approval of final submitted version.
-
8.
David G. Mutch, M.D.: Assisted with manuscript revisions and approval of final submitted version.
-
9.
Katherine C. Fuh, M.D., Ph.D. Senior author who helped with initial design, IRB submission, data entry, and manuscript writing and approval of final submitted version.
Conflict of interest statement
The authors declare no competing or conflicts of interest regarding this work.
References
- Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from Cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Cancer Facts & Figures Atlanta, Georgia. American Cancer Society; 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf Available from: [Google Scholar]
- de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E.L., Vogel R.I., Gehrig P.A., Pierce S., Havrilesky L., Secord A.A. A multi-institutional study of outcomes in stage I–III uterine carcinosarcoma. Gynecol. Oncol. 2015;139(2):275–282. doi: 10.1016/j.ygyno.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B.A., Kato D., Schmidt R.A., Ek M., Ferry J.A., Muntz H.G. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol. Oncol. 1994;54(3):264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- Hsu K.-F., Chou H.-H., Huang C.-Y., Fu H.-C., Chiang A.-J., Tsai H.-W. Prognostic factors and treatment outcomes for patients with surgically staged uterine clear cell carcinoma focusing on the early stage: a Taiwanese Gynecologic Oncology Group study. Gynecol. Oncol. 2014;134(3):516–522. doi: 10.1016/j.ygyno.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Huh W.K., Powell M., Leath C.A., Straughn J.M., Cohn D.E., Gold M.A. Uterine papillary serous carcinoma: comparisons of outcomes in surgical Stage I patients with and without adjuvant therapy. Gynecol. Oncol. 2003;91(3):470–475. doi: 10.1016/j.ygyno.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Hui P., Kelly M., O'Malley D.M., Tavassoli F., Schwartz P.E. Minimal uterine serous carcinoma: a clinicopathological study of 40 cases. Mod. Pathol. 2004;18:75. doi: 10.1038/modpathol.3800271. [DOI] [PubMed] [Google Scholar]
- Kelly M.G., O'Malley D.M., Hui P., McAlpine J., Yu H., Rutherford T.J. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol. Oncol. 2005;98(3):353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Keys H.M., Roberts J.A., Brunetto V.L., Zaino R.J., Spirtos N.M., Bloss J.D. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2004;92(3):744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network Uterine Neoplasms 2018 [Updated 5/25/2018. 2] 2018. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf Available from:
- Olawaiye A.B., Boruta D.M. Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol. Oncol. 2009;113(2):277–283. doi: 10.1016/j.ygyno.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Pelvic Radiation Therapy or Vaginal Implant Radiation Therapy, Paclitaxel, and Carboplatin in Treating Patients With High-Risk Stage I or Stage II Endometrial Cancer. 2018. https://clinicaltrials.gov/ct2/show/NCT00807768 Available from.
- Silverberg S.G., Major F.J., Blessing J.A., Fetter B., Askin F.B., Liao S.-Y. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: a gynecologic oncology group pathologic study of 203 cases. Int. J. Gynecol. Pathol. 1990;9(1):1–19. doi: 10.1097/00004347-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Thomas M., Mariani A., Wright J.D., Madarek E.O.S., Powell M.A., Mutch D.G. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma: a multi-institutional review. Gynecol. Oncol. 2008;108(2):293–297. doi: 10.1016/j.ygyno.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Velker V., D'Souza D., Prefontaine M., McGee J., Leung E. Role of adjuvant therapy for stage IA serous and clear cell uterine cancer: is observation a valid strategy? Int. J. Gynecol. Cancer. 2016;26(3):491–496. doi: 10.1097/IGC.0000000000000643. [DOI] [PubMed] [Google Scholar]
- Yamada S.D., Burger R.A., Brewster W.R., Anton D., Kohler M.F., Monk B.J. Pathologic variables and adjuvant therapy as predictors of recurrence and survival for patients with surgically evaluated carcinosarcoma of the uterus. Cancer. 2000;88(12):2782–2786. doi: 10.1002/1097-0142(20000615)88:12<2782::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]


