ABSTRACT
This study evaluated the effects of increasing the dose of a 6-phytase from Buttiauxella on phytate degradation, mineral, energy, and AA digestibility in weaned pigs fed complex diets based on wheat, corn, soybean meal, barley, and rapeseed meal. A negative control (NC) diet containing no added inorganic phosphorus (P) and a reduction of 0.1% calcium (Ca) and 36 kcal/kg ME was supplemented with Buttiauxella phytase at 0, 250, 500, 1,000, or 2,000 FTU/kg diet and tested against a nutritionally adequate, positive control (PC) diet. One phytase units (FTU) is the amount of enzyme that liberates 1 micromole of inorganic phosphate per minute from a sodium phytate substrate at pH 5.5 and 37 °C. Barrows (Topigs × Pietrian; initial mean body weight 19.3 kg) were housed individually in metabolic crates and fed the test diets in mash form via 2 equal meals per day for 9 d (fed at 2.5 times the maintenance energy requirement), with 8 replicate pigs per treatment, in 2 experimental runs (total n = 48). After a 3-d adaptation period, urine and feces were collected over 5 d for measurements of apparent total tract digestibility (ATTD) and retention of nutrients. On day 9, pigs were euthanized and ileal digesta collected for measurements of apparent ileal digestibility (AID) of nutrients. Phytase improved (P < 0.05) digestibility of all measured AA except Trp (P < 0.1), and AID P, nitrogen, phytate, ATTD P, Ca versus NC. Increasing phytase dose from 0 (NC) to 2,000 FTU/kg increased AID Lys, Cys, Thr, Val, Ile, Leu, mean AA, P, N, phytate, ATTD P, N, Na, energy, ME, P retention (g/d), and reduced P excretion (g/d) in a linear or exponential manner (P < 0.05). Phytase at 2,000 FTU/kg improved AA digestibility by between +3.1 percentage points (Trp) and +8.8 percentage points (Cys) versus NC (average +6.3 percentage points) (P < 0.05). Phytase inclusion at 2,000 FTU/kg reduced P excretion (g/d) by 57% versus PC (P < 0.05). In conclusion, increasing Buttiauxella phytase in the range of 0 to 2,000 FTU/kg increased phytate degradation, improved AA and P digestibility, and reduced P excretion in weaned pigs fed complex diets.
Keywords: amino acid digestibility, phytase, phytate, weaned pigs
INTRODUCTION
Commercially produced phytases have been available for use in pig and poultry diets since the 1990s. They are now widely accepted as an effective means of improving the availability and utilization of phytate-bound phosphorus (P) from plant-based ingredients, while reducing environmental P excretion and the need to add costly inorganic P ingredients to feed (Selle and Ravindran, 2007). In plant-based ingredients such as cereals and oilseeds that form the basis of pig and poultry diets, up to 80% of total P is in the form of phytate (the salt form of phytic acid, inositol-6-phosphate, IP6) (Selle and Ravindran, 2007, 2008). Phytate is poorly digested by monogastric animals. Phytate also binds readily to proteins and minerals (especially calcium, Ca) at stomach and intestinal pH (Selle et al., 2009a; Selle et al., 2012), forming binary (phytate-protein) or ternary (phytate-mineral-protein) complexes. This not only further reduces the bioavailability of phytate-P, but also that of other minerals, proteins, and nutrients that are bound to the phytate molecule (Selle and Ravindran, 2007; Selle et al., 2009b; Selle et al., 2012). The binding of phytate with proteins can also increase excretion of endogenous AA in high-phytate diets as a result of increased mucin secretion (Selle et al., 2012) and disrupt intestinal sodium balance, which can further affect AA absorption (Cowieson et al., 2004). The extent to which dietary supplementation with phytase can ameliorate these effects and improve the digestibility of nutrients such as AA in addition to P, via so called “extra-phosphoric” effects, is a major current topic of research in pig production.
Phytase activity is expressed as phytase units (FTU), defined as the amount of enzyme that liberates one micromole of inorganic phosphate per minute from a sodium phytate substrate at pH 5.5 and 37 °C (AOAC, 2000a). Studies have shown that a phytase dose of 500 FTU/kg feed typically achieves 40 to 60% phytate degradation by the end of the small intestine, versus 10 to 40% in unsupplemented diets (Dersjant-Li et al., 2015). This highlights the substantial degree of increase that can be achieved, but also that there is room for further improvement. One factor of interest is phytase dose level. A recent review article suggested that phytase dosed at 1,000 FTU/kg typically achieved a further 20% degradation of phytate beyond that achieved by 500 FTU/kg, across pigs and poultry (Dersjant-Li et al., 2015). Greater improvements in the digestibility and retention of P and other nutrients with Buttiauxella phytase dosed at levels above 500 FTU/kg have also been reported, up to a dose of 2,000 FTU/kg (Bento et al., 2012; Adedokun et al., 2015; Dersjant-Li et al. 2017a) or even 20,000 FTU/kg in a recent study (Zeng et al., 2016). It also enhanced growth performance in commercial and research settings (Dersjant-Li et al., 2017a,b; Dersjant-Li et al., 2018). Phytase inclusion at 500 and 1,000 FTU/kg produced increases in ADG (vs. PC) of 4.5 and 5.3% in grower-finisher pigs fed a European-style wheat, corn, barley, and SBM-based diet (Dersjant-Li et al., 2017b).
It is generally recognized that phytase can improve P digestibility, however, the effect of phytase on digestible AA is less consistent (Adeola and Sands, 2003; Adeola and Cowieson, 2011). This may be related to the phytase source and dose level, dietary composition, and phytate source and level in the diet. Adedokun et al. (2015) observed that Buttiauxella phytase at doses between 250 FTU/kg and 2,000 FTU/kg, improved apparent ileal digestibility (AID) of 8 indispensable AA and 6 dispensable AA, in pigs fed diets based on corn, SBM, wheat middlings, and corn DDGS. However, limited data is available on the effects of Buttiauxella phytase on AA digestibility in pigs fed a more complex diet. In addition, evaluating AA digestibility responses alongside effects on phytate degradation and P digestibility may also provide further insight into the modes of action for effects of phytase on these 2 nutrients.
The aim of this study was thus to determine the effect of increasing the dosing of a Buttiauxella phytase on phytate degradation, mineral, energy, and AA digestibility in weaned pigs fed a complex diet containing wheat, corn, soybean meal, barley, and rapeseed meal, without added inorganic P, when compared with a nutritionally adequate diet.
MATERIALS AND METHODS
Diets and Experimental Design
A 2-round experiment incorporating 6 dietary treatments was conducted to evaluate the effects of a Buttiauxella phytase at 5 dose levels against a nutritionally adequate, positive control (PC) diet (NRC, 2012). Phytase was added to a negative control (NC) diet that was formulated without inorganic phosphate, with a reduction of 0.1% Ca and 36 kcal/kg ME versus PC. The control diets were complex diets based on wheat, corn, barley, soybean meal, rapeseed meal, and contained 0.1% titanium dioxide as an indigestible marker (Table 1). The negative control diet was fed either unsupplemented or supplemented with a microbial 6-phytase from Buttiauxella sp. expressed in Trichoderma reesei (Axtra® PHY, DuPont Animal Nutrition). The phytase was added to the NC at 5 dose levels: 0, 250, 500, 1,000, and 2,000 FTU/kg feed. The diets were fed to pigs in mash form, in 2 equal meals at 0700 h and 1500 h during a 9-d experimental period, providing a feed allowance that was 2.5 times the energy requirement for maintenance.
Table 1.
Composition (%, as-fed), calculated nutrient content (%) of the control diets
| Item | PC | NC |
|---|---|---|
| Ingredient, % | ||
| Wheat | 36.66 | 39.35 |
| Corn | 18.00 | 18.00 |
| Soybean meal (48% CP) | 18.30 | 17.55 |
| Barley | 15.00 | 15.00 |
| Rapeseed meal | 6.00 | 6.00 |
| Vegetable oil | 2.25 | 0.90 |
| L-Lysine HCL | 0.55 | 0.57 |
| Methionine (hydroxyl) | 0.21 | 0.21 |
| L-Threonine | 0.26 | 0.27 |
| L-Tryptophan | 0.08 | 0.08 |
| Salt | 0.44 | 0.44 |
| Limestone | 1.10 | 1.13 |
| Monocalcium-phosphate | 0.65 | - |
| Vitamin-mineral premix1 | 0.50 | 0.50 |
| Calculated nutrients, % | ||
| ME, kcal/kg | 3308 | 3272 |
| NE, kcal/kg | 2457 | 2431 |
| Crude protein | 18.60 | 18.60 |
| dig Lysine | 1.10 | 1.10 |
| dig Methionine + Cysteine | 0.66 | 0.66 |
| dig Threonine | 0.72 | 0.73 |
| dig Tryptophan | 0.24 | 0.24 |
| Calcium | 0.72 | 0.62 |
| total Phosphorus | 0.59 | 0.44 |
| dig Phosphorus | 0.26 | 0.12 |
| Ca:tP | 1.22 | 1.41 |
| Na | 0.2 | 0.2 |
1Supplied per kilogram of diet: 20,000 IU vitamin A; 2,000 IU vitamin D3; 200 mg vitamin E; 2.0 mg vitamin K3; 200 mg vitamin C; 200 mcg biotin; 1.0 mg folic acid; 3.5 mg vitamin B1; 7.0 mg vitamin B2; 6.0 mg vitamin B6; 50 mcg vitamin B12; 35 mg nicotinic acid; 20 mg calpan; 345 mg choline chloride; 300 mg choline; 150 mg Fe (Iron sulfate); 160 mg Cu (copper sulfate); 50 mg Mn (manganese oxide); 120 mg Zn (zinc oxide); 1 mg Ca (calcium iodate); 0.15 mg Co (cobalt carbonate); 0.40 mg Se (sodium selenite).
Pigs and Housing
The experimental procedures adhered to the Animal and Human Welfare Codes/Laboratory practice codes in Germany (LUA Koblenz Animal Care – No. 23 177-07/G12-20–073) and the study protocol was approved by Animal Welfare committee of the University of Applied Science, Bingen, Germany.
Forty-eight healthy Topigs × Pietrian weaned 8-wk-old barrows (mean initial BW of 19.3 kg) were housed individually in piglet metabolic crates and randomly allocated to one of the 6 dietary treatments on the basis of initial BW, with a total of 8 pigs per dietary treatment. The study was performed in 2 experimental runs, with 24 pigs (4 pigs/treatment) in the first run, and 24 in the second. Metabolic crates were housed in a temperature controlled room at 20 °C.
Measurements and Sampling
Following a 3-d adaptation period, total feces and urine were collected 3 times a day for 5 d. Samples were weighed, homogenized, freeze-dried (feces), and stored at −20 °C for determination of total tract digestibility of nutrients. At the end of the sample collection period (day 9), pigs were euthanized by intracardiac injection after sedation with ketamine. Ileal digesta was collected from the last third of the small intestine, weighed, homogenized, freeze-dried, and stored at −20 °C for the determination of nutrient digestibility. Additionally, the femur of the left leg of pigs was extracted surgically and frozen prior to processing for the determination of ash content in fat-free DM.
Chemical Analysis
Fecal samples were analyzed for DM, GE, phytate, Ca, P, nitrogen, and titanium dioxide marker. Urine samples were analyzed for GE, nitrogen, P, and Ca. Bone samples were analyzed for bone ash, Ca, and P in bone dry matter. Dry matter, Ca, and P were determined according to Naumann and Bassler (2004) VDLUFA methods [DM method: VDLUFA methods Band III (method 3.1); calcium method: VDLUFA methods Band III (method 10.3.2); P method [VDLUFA methods Band III (method 10.6.3)] (VDLUFA, 1976) and phytate by AOAC (1986). Titanium dioxide was determined according to ISO method 17294-2 (ISO, 2016). Nitrogen was analyzed by a nitrogen analyzer (VDLUFA, 2007 Method 4.1.1) and crude protein was then calculated as nitrogen × 6.25. Gross energy was determined by an oxygen bomb calorimeter (IKA-Calorimeter C5000). Ileal digesta samples were ground to pass through a 1.0 mm sieve and analyzed for phytate P, total P, Ca, N, and titanium dioxide, using the above methods. Amino acids in ileal digesta were quantified following oxidation and/or hydrolysis according to ISO method 13903 (2005). Frozen femur samples were thawed, autoclaved, cleaned and dried overnight at 105 °C in a crucible. The clean bones were defatted by soaking in petroleum ether for 24 h and then left to dry overnight in a fume hood before further drying in an oven at 135 °C for 2 h. Sample weight was recorded after 20 min in a desiccator. The defatted, dried bones were then ashed in a muffle furnace at 600 °C for 16 h and reweighed (AOAC, 2000b). Bone ash was ground using a pestle and mortar prior to the determination of Ca and P content using the methods referred to above. Phytase activity in the diets was analyzed by DuPont Research Centre, Brabrand, Denmark, using the methods described by Yu et al. (2012).
Calculations
The apparent total tract digestibility (ATTD) and ileal digestibility (AID) of nutrients and of GE and ME were calculated based on the concentrations of the titanium dioxide marker and nutrient in the feed and total tract/ileal samples, according to the following formula:
where Tid is the titanium concentration in the diet, Tii is the titanium concentration in the ileal digesta or excreta, Ni is the nutrient concentration in the ileal digesta or excreta and Nd is the nutrient concentration in the diet, and;
where GE refers to gross energy (kcal/kg DM), “nutrient” refers to DM, Ca, P, CP, nitrogen, or AA in g/kg DM.
Phosphorus retention was calculated as P intake minus fecal and urinary excretion per pig per day.
Statistical Analysis
For all response criteria, data were analyzed on a per pig basis. Variability in the data was expressed as pooled SEM. Data were analyzed as a randomized complete block design (with experimental run as random effect) using JMP 14.0 (SAS Institute Inc., Cary, NC, 1989–2019). Means separation between PC and NC was achieved using LS means contrast. Phytase effect was tested using a contrast of 0 (NC) versus average of 250, 500, 1,000, and 2,000 FTU/kg. Linear response was tested by fit Y by X to account for uneven phytase dose increase levels. An exponential growth and decay model was applied to test the nonlinear response and the asymptote. Differences were considered significant at P < 0.05 and P < 0.10 was considered a tendency.
RESULTS
Analyzed nutritional contents and phytase activity of the diets are presented in Table 2. In general, phytase activity was somewhat lower than targeted after subtracting the basal phytase level in NC. This may have been due to feed mixing and the acceptable analytical errors; so, it is considered that phytase activity was within an acceptable range.
Table 2.
Analyzed nutritional content (%, DM basis) and phytase activity of the dietary treatments
| NC + phytase levels, FTU/kg | ||||||
|---|---|---|---|---|---|---|
| Item | PC | NC | 250 | 500 | 1,000 | 2,000 |
| DM, % | 90.5 | 90.3 | 90.1 | 89.8 | 89.9 | 89.6 |
| GE, kcal/kg DM | 4321 | 4283 | 4293 | 4281 | 4297 | 4300 |
| Ca, % | 0.73 | 0.67 | 0.70 | 0.64 | 0.66 | 0.60 |
| Phosphorus, % | 0.49 | 0.43 | 0.40 | 0.40 | 0.41 | 0.38 |
| Ca:tP | 1.49 | 1.56 | 1.75 | 1.60 | 1.61 | 1.58 |
| Na, % | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Phytate-P, % | 0.26 | 0.25 | 0.27 | 0.26 | 0.26 | 0.26 |
| Nitrogen, % | 3.54 | 3.48 | 3.47 | 3.56 | 3.65 | 3.50 |
| Lysine, % | 1.47 | 1.48 | 1.40 | 1.40 | 1.45 | 1.46 |
| Methionine, % | 0.28 | 0.28 | 0.28 | 0.27 | 0.27 | 0.29 |
| Cysteine, % | 0.38 | 0.37 | 0.37 | 0.36 | 0.37 | 0.37 |
| Threonine, % | 0.95 | 0.96 | 0.97 | 0.98 | 0.97 | 1.03 |
| Tryptophan, % | 0.23 | 0.23 | 0.23 | 0.25 | 0.25 | 0.22 |
| Valine, % | 0.88 | 0.88 | 0.86 | 0.80 | 0.85 | 0.92 |
| Isoleucine, % | 0.84 | 0.83 | 0.80 | 0.77 | 0.80 | 0.87 |
| Leucine, % | 1.53 | 1.50 | 1.42 | 1.48 | 1.44 | 1.43 |
| Phytase, FTU/kg feed (as is) | 304 | 334 | 442 | 750 | 1020 | 1888 |
NC, negative control; PC, positive control.
Ileal Digestibility, Total Tract Digestibility, and Retention of Nutrients and Energy
The results on apparent ileal and total tract digestibility and retention of nutrients, phytate, and energy are presented in Table 3. Pigs fed NC diets had greater AID and ATTD of Ca, ATTD of energy (P < 0.05), and tendency of greater ATTD of nitrogen (P < 0.10) compared to PC. The addition of phytase to NC improved AID of P, IP6, nitrogen, ATTD of P, Ca, retainable P (g/d), and reduced total P excretion (g/d) (P < 0.05). Increasing phytase dose from 0 (NC) to 2,000 FTU/kg increased AID of P, IP6, nitrogen, ATTD of P, nitrogen, Na, energy, and ME, retainable P (g/d), and reduced P excretion (g/d) (P < 0.05) in a linear or exponential manner. Exponential curve fitting showed that the asymptote (P < 0.05) was reached at 70.3% for AID of P, 80% for AID of nitrogen, 84% for AID of IP6, 71% for ATTD of P, and 0.83 g/d for P excretion. At the top phytase dose, P excretion was reduced by 57% relative to the PC.
Table 3.
Effects of phytase dose level on apparent ileal digestibility (AID, %) and total tract digestibility (ATTD, %) and retention of nutrients (g/day)
| NC + phytase level, FTU/kg | Probability of contrast | Dose response model1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | PC | NC | 250 | 500 | 1,000 | 2,000 | SEM | PC vs. NC | NC vs. phytase | Linear | Exponential |
| AID P | 48.5 | 50.6 | 56.4 | 63.6 | 68.3 | 69.6 | 5.47 | 0.583 | 0.0002 | <0.001 | 0.016# |
| AID Ca | 52.3 | 66.3 | 66.4 | 63.2 | 69.8 | 66.2 | 4.55 | 0.0013 | 0.7515 | 0.799 | 0.939 |
| AID N | 74.2 | 71.5 | 75.4 | 78.7 | 78.9 | 80.4 | 1.39 | 0.142 | <.0001 | <0.001 | 0.012# |
| AID IP6 | 35.7 | 38.8 | 53.8 | 67.2 | 75.9 | 82.8 | 4.8 | 0.3352 | <.0001 | <0.001 | <0.001# |
| ATTD P | 38.3 | 38.1 | 54.3 | 61.2 | 68.1 | 71.1 | 1.75 | 0.9313 | <.0001 | <0.001 | <0.001# |
| ATTD Ca | 49.7 | 61.9 | 69.9 | 73.5 | 74.1 | 69.9 | 3.74 | 0.0009 | 0.002 | 0.198 | 0.294 |
| ATTD DM | 93.6 | 94.3 | 94.8 | 94.6 | 95 | 95.1 | 0.67 | 0.1485 | 0.1079 | 0.115 | 0.501 |
| ATTD N | 81.6 | 83.2 | 82.1 | 83.8 | 84.6 | 85.1 | 1.11 | 0.0624 | 0.494 | 0.031# | 0.74 |
| ATTD Na | 82.5 | 85.3 | 85.6 | 88.7 | 88.5 | 91.9 | 1.45 | 0.2258 | 0.2465 | <0.001# | 0.997 |
| ATTD energy | 82.7 | 84.6 | 84.1 | 84.3 | 84.7 | 85.9 | 0.58 | 0.0207 | 0.8414 | 0.034# | 0.62 |
| ME, kcal/kg DM | 3516 | 3506 | 3528 | 3530 | 3544 | 3611 | 35.9 | 0.7533 | 0.265 | 0.031# | 0.744 |
| ret P, g/day | 1.31 | 1.05 | 1.42 | 1.55 | 1.65 | 1.63 | 0.23 | 0.1453 | 0.0027 | 0.022 | 0.165# |
| Total P excretion, g/day | 1.89 | 1.61 | 1.16 | 0.93 | 0.88 | 0.82 | 0.01 | 0.123 | <.0001 | <0.001 | 0.004# |
NC, negative control; PC, positive control.
1Linear and exponential regression analysis were performed with increasing phytase dose from 0 (NC) to 2,000 FTU/kg, excluding PC, using JMP fit Y by X for linear response (where P-value is for phytase dose slope) and modeling-nonlinear - exponential growth and decay: fit exponential 3P for exponential response (= a+b * EXP (c * phytase dose)). The P-value in the table is for growth rate. When P-value is below <0.1 for one of the models, a goodness of fit test was done, and the optimal model is the prediction equation with the lowest AIC (measure of fit) and root mean square error (RMSE, measure of precision) and marked with #. An exponential model was used instead of a quadratic response to estimate the asymptote level. The asymptote (P < 0.0001) was reached at 70.3% for AID P, 80% for AID N, 84% for AID IP6, 71% for ATTD P and 0.83 g/d for total P excretion.
Ileal Digestibility of AA
The results on the AID of AA are presented in Table 4. No differences between the PC and NC diets were identified. The addition of phytase improved AID of all AA measured, except Trp (tendency, P = 0.08). Increasing phytase dose from 0 (NC) to 2,000 FTU/kg increased AID of Lys, Cys, Thr, Val, Ile, Leu, and mean AA in a linear or exponential manner, with a tendency of linear increase for AID of Met (P = 0.059). Phytase addition at 2,000 FTU/kg increase AID of Lys by 7 percentage points and mean AA by 6.3 percentage points versus NC.
Table 4.
Effects of phytase dose level on apparent ileal digestibility (AID, %) of AA
| NC+ phytase levels, FTU/kg | Probability of contrast | Dose response model1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | NC | 250 | 500 | 1,000 | 2,000 | SEM | PC vs NC | NC vs phytase | Linear | Exponential | |
| Cysteine | 61.5 | 62.3 | 67.2 | 68.3 | 70.8 | 71.1 | 2.09 | 0.3363 | 0.0001 | 0.015 | 0.163# |
| Isoleucine | 80.1 | 78.5 | 79.8 | 82.3 | 83.2 | 85.3 | 1.15 | 0.4152 | 0.0091 | <0.001 | 0.191# |
| Leucine | 80.6 | 79 | 80.5 | 83 | 84.2 | 85.1 | 1.2 | 0.3966 | 0.0068 | <0.001 | 0.129# |
| Lysine | 84.2 | 81.6 | 84 | 86.7 | 87.3 | 88.6 | 1.49 | 0.1245 | 0.0007 | <0.001 | 0.051# |
| Methionine | 81.7 | 80.5 | 81.6 | 84.5 | 83.7 | 85.7 | 3.26 | 0.3334 | 0.0395 | 0.059# | 0.546 |
| Threonine | 77.2 | 76.7 | 79.4 | 82.3 | 82 | 83.3 | 1.37 | 0.7704 | 0.0005 | 0.001 | 0.056# |
| Tryptophan | 78.9 | 78.9 | 79.5 | 84.1 | 83.4 | 82 | 3.12 | 0.9805 | 0.0836 | 0.228 | 0.375 |
| Valine | 76.6 | 75.3 | 77.2 | 78.6 | 80.8 | 82.4 | 1.98 | 0.5606 | 0.0169 | <0.001 | 0.29# |
| Mean | 77.6 | 76.6 | 78.7 | 81.2 | 81.9 | 82.9 | 1.84 | 0.3414 | 0.0012 | <0.001 | 0.072# |
NC, negative control; PC, positive control.
1Linear and exponential regression analysis were performed with increasing phytase dose from 0 (NC) to 2,000 FTU/kg, excluding PC, using JMP fit Y by X (linear response, where P-value is for phytase dose) and modeling-nonlinear - exponential growth and decay: fit exponential 3P (exponential response = a+b * EXP (c * phytase dose)). The P-value in the table is for growth rate. When P-value is below <0.1 for one of the models, a goodness of fit test was done, and the optimal model is the prediction equation with the lowest AIC (measure of fit) and root mean square error (RMSE, measure of precision) and marked with #.
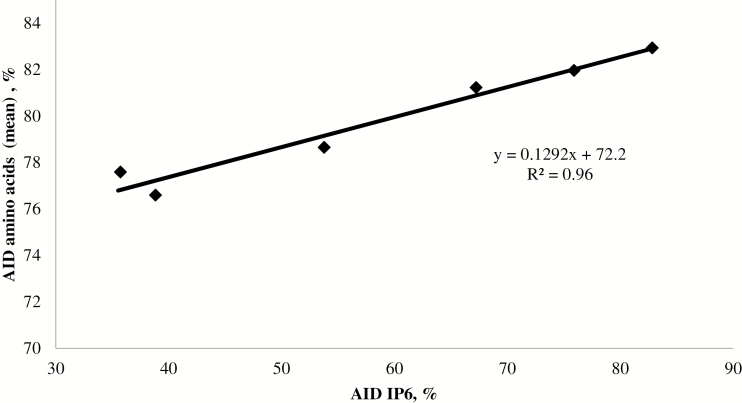
Figure 1 displays the relationship between the AID of AA (averaged, %) and the AID of phytate, calculated using data from all dietary treatments including the NC and PC. There was a significant, positive, linear relationship between these 2 response measures (R2 = 0.959; P < 0.05).
Figure 1.
Relationship between apparent ileal digestibility (AID, %) of phytate (IP6) and AA (average, %) across all dietary treatments in weaned pigs (initial BW 19.3 kg)
Bone Ash and Mineralization
No significant effects of phytase addition on femur ash or bone mineral content were identified (data not shown).
DISCUSSION
Results from the present study have demonstrated a positive dose-dependent effect of Buttiauxella phytase supplementation on digestibility of P and AA. There was evidence of a asymptotic effect of phytase supplementation on P digestibility, P excretion, and P retention. The ATTD of P and P excretion reached an asymptote at a dose level of 2,000 FTU/kg. However, the asymptotic effect of phytase dose was less evident for effects on AA digestibility. With the exception of Trp and tendency for Met, increasing phytase dose from 0 to 2,000 FTU/kg resulted in linear increases in the digestibility of all measured AA, as well as in average AA digestibility. Although the P value for the exponential model was not significant for most of digestible AA, the goodness of fit showed that the exponential model had better measure of fit than the linear response, indicating the digestible AA is approaching to an asymptote. This finding is in contrast to some other studies in the literature, where phytase showed a consistent effect on P digestibility but an inconsistent effect on protein and AA digestibility (Adeola and Sands, 2003; Adeola and Cowieson, 2011). For example, Augspurger and Baker (2004) reported that an Escherichia coli phytase administered at dose levels up to 10,000 FTU/kg improved phytate P utilization but did not affect protein utilization in chicks fed P and AA-deficient diets. The different response on P and AA digestibility may be explained by the 2 different mechanisms of action: 1), P digestibility: phytase increases phosphorus digestibility by the step-wise hydrolysis of phytate, releasing phytate-bound P, and leading to improved digestibility of P. The hydrolysis of phytate could take place along the stomach and small intestine and the P release is a result of the number of phosphate groups released from the inositol ring (Selle and Ravindran, 2007; Cowieson et al., 2009). 2): AA digestibility: phytase increases AA digestibility due to reduction of antinutritional effect of phytate. Phytate can bind to protein directly in the acidic stomach environment, or bind to protein via minerals in the small intestine. Yu et al. (2012) identified that IP6 has a much greater binding capacity to protein compared to lower esters (IP5, IP5, IP3) and thus has a greater negative impact on protein digestion. As a result, phytate (IP6) can increase endogenous AA flow (Cowieson and Ravindran, 2007) and reduce AA absorption due to the reduced function of Na+-dependent transport systems (Selle et al., 2012). To reduce the antinutritional effect of phytate (IP6) on protein digestion, a phytase needs to be able to breakdown phytate quickly in the stomach. The Buttiauxella phytase used in this study had a maximal activity at pH 3 based on an in vitro study (Menezes-Blackburn et al., 2015), which is 235% of its activity at pH 5.5 (the activity of commercial phytase is standardized at pH 5.5). Since the approximate pH of the pig stomach digesta is 3 (Li et al., 2008), it seems likely that the effects on AA digestibility observed in the present study were mediated by the degradation of phytate by Buttiauxella phytase in the stomach, leading to a reduced presence of phytate in the stomach and the small intestine and a reduction in its antinutritional effects. This is in agreement with Adedokun et al. (2015), who suggested that Buttiauxella phytase is active in the acid conditions of the stomach and can rapidly breakdown phytate, reducing the phytate–protein binding, and improving digestibility of AA as a result of an extra-phosphoric effect. In the current study, the increased P level in the PC had no effect on digestibility of AA vs NC, indicating AA digestibility is not directly related to total or available P. The positive linear relationship between ileal AA digestibility and the extent of phytate degradation observed in the present study is suggestive of a direct, causal, link between these 2 variables, that is, that improvements in AA digestibility were due to the increased breakdown of phytate by Buttiauxella phytase.
Studies on the effects of Buttiauxella phytase on digestibility of AA in pigs are limited. However, the current study is in agreement with 2 other studies (Adedokun et al., 2015; Velayudhan et al., 2015), both of which have shown that phytase improved digestibility of AA in pigs versus NC. In cannulated grower pigs (average initial BW of 22 and 30 kg in period 1 and 2, respectively) fed corn-SBM-based diets containing wheat middlings and corn DDGs (Adedokun et al., 2015), the overall improvement in AID of AA with the phytase was 2.6 percentage points. In another study in cannulated grower pigs (initial BW 25 kg) fed corn-SBM-based diets without by-products (Velayudhan et al., 2015), mean standard ileal digestibility of indispensable AA and dispensable AA improved by 2.8 and 3.6% with the phytase dosed at 2,000 FTU/kg. The current study showed a greater improvement level of digestibility of dispensable AA, with an increase of 6.3 percentage points at a phytase dose of 2,000 FTU/kg. The different extent of improvement in digestibility of AA observed between these studies may be explained mainly by differences in dietary phytate source and the indigestible protein content of the ingredients used in these studies. The accessibility of phytate by phytase differs from different ingredients (Leske and Coon, 1999). The total AA digestibility in the basal diets was 80.3% in the study by Adedokun et al. (2015), while in the current study, the mean AA digestibility in the NC was 76.6% thus indicating a greater indigestible AA content in the current study. In addition, cannulated pigs were used in the studies of Adedokun et al. (2015) and Velayudhan et al. (2015). However, the current study applied slaughter techniques (all studies applied restrict feeding program). Cannulation has been shown to produce generally lower AA digestibility response levels due to the potential for microbial proliferation and/or inflammation around the cannulation site (Selle and Ravindran, 2008). However, it has also been suggested that flushing the digesta from the distal ileum (used in the current study) may limit the opportunity for AA absorption and this may also reduce AA digestibility estimates (Cowieson et al., 2017a). In broilers, a greater improvement on digestibility of AA (mean AA by 9.2 percentage points across diets with different Ca:P ratios) was seen with the addition of 1,000 FTU/kg Buttiauxella phytase (Amerah et al., 2014). This may partially be explained by the effects of phytate on protein digestibility and that endogenous protein flow may be less consistent and weaker in pigs than poultry (Cowieson and Ravindran, 2007; Woyengo et al., 2009). Recent reviews based on meta-analyses have reported lower effect levels for phytase supplementation on AA digestibility in pigs compared with poultry (Cowieson et al., 2017a, b).
Recent reviews of the effects of microbial phytase on ileal AA digestibility in pigs have reported an average of 2.8% improvement, and increasing phytase beyond 250 FTU/kg in pigs did not deliver any significant further benefit (Cowieson et al., 2017a). The current study showed a greater level of AA digestibility responses to phytase inclusion (on average, + 6.3 percentage points vs. NC) and a linear or exponential increase in AA digestibility with phytase dose continued to be observed up to 2,000 FTU/kg in all but one (Trp) of the measured AA (tendency for Met). These findings suggest that, the greater dose of 2,000 FTU/kg was effective at delivering further increases in AA digestibility. Increasing the phytase dose could be beneficial and lead to improved performance, as several studies have recently indicated a greater benefit of Buttiauxella phytase on ADG when dosed at 2,000 FTU/kg compared with lower doses (Dersjant-Li et al., 2017a,b).
In terms of the individual AA, previous swine studies have generally reported response levels to be greater for Thr, Pro, Gly, and Ser, and lower for Met, Trp, Arg, Glu, and Lys (Adeola and Cowieson, 2011; Cowieson et al., 2017a; Zouaoui et al., 2018). In the present study, effects of phytase were significant across all AA tested, but the overall pattern was similar to the reported trend. The exception was Cys: effects on Cys digestibility were markedly greater in the present study than the average level across phytase studies in pigs reported by Cowieson et al. (2017a) (+8.8 percentage points vs. NC in the present study vs. +2.0% reported by Cowieson et al. 2017a) and were more similar to the average Cys response to phytase reported for broilers (+7.2%; Cowieson et al., 2017b). Interestingly with the same phytase, Adedokun et al. (2015) observed the greatest improvement also with Cys, but the lowest response was Met.
In the current study, phytase dosed at 1,000 FTU/kg and 2,000 FTU/kg increased IP6 digestion from 38.8% in NC to 75.9% and 82.8%, respectively, by the end of the ileum, an increase of 37 and 44 percentage points, respectively. This indicates an effective breakdown of the phytate by a greater dose of the phytase. It is worth noting that this measurement will not have reflected the phytate P made available by phytase but not absorbed in the ileum. The ATTD of P increased from 38% in NC to 68.1% and 71.1% with phytase dosed at 1,000 and 2,000 FTU/kg, respectively, reflecting a 30 and 33 percentage point improvement. This is comparable to the study in grower pigs, where ATTD of P increased from 30% in NC to 66% with the same phytase at 2,000 FTU/kg (36 percentage points increase, Adedokun et al., 2015), and ATTD of P increased from 31% in NC to 73% with the same phytase at 2,000 FTU/kg (39 percentage points increase, Velayudhan et al., 2015). Similarly, in piglets fed wheat based diets, ATTD of P increased from 57.3 in NC to 86.5% (29 percentage points increase) with the same phytase dosed at 2,000 FTU/kg (Dersjant-Li et al., 2017a). Similarly, Bento et al. (2012) reported a linear dose–response effects and improvements in ATTD of P in 2 separate trials involving Buttiauxella phytase at doses of 0 to 2,000 FTU/kg among slightly younger pigs (initial BW 8 to 12 kg).
When expressed as g/day, P excretion was reduced by 57% (vs. PC), or by 1.07 g per pig per day, with phytase inclusion at 2,000 FTU/kg. Dersjant-Li et al. (2017a) reported a similar linear reduction in P excretion with increasing dose of Buttiauxella phytase, by up to 61.5% at 2,000 FTU/kg compared to a PC diet. In practice, a reduction in P excretion of approximately 1 g/animal/day could substantially reduce environmental P excretion during a swine production cycle.
The effects of phytase on total tract digestibility of Ca are consistent with observations of Adedokun et al. (2015) who demonstrated a 19 percentage points increase in ATTD of Ca (vs. NC) with Buttiauxella phytase dosed at 2,000 FTU/kg. The analyzed Ca to P ratios of the PC and NC diets in the present study were slightly above the optimal ratio of 1.2:1 reported by Qian et al. (1996), which could have had the effect of slightly reducing Ca and P digestibility, since it is well recognized that high Ca levels can reduce bioavailability of both phytate-P and Ca due to binding of phytate, inorganic P, and Ca in the pH conditions of the small intestine (Farkvam et al., 1989; Sandberg et al., 1993; Selle et al., 2009a). No dose–response effect on AID of Ca or ATTD of Ca was seen with increasing phytase dose. However, ATTD of Ca was greater in the phytase treatments versus NC, which may be explained by increased P availability leading to more Ca being absorbed/retained in order to maintain a better Ca: P balance.
No significant effects of phytase addition on femur ash or bone mineral content were identified (data not shown), which is likely because the testing period was not long enough to obtain a significant difference on bone ash.
In conclusion, Buttiauxella phytase added to complex diets based on wheat, corn, soybean meal, barley, and rapeseed meal, deficient in Ca and energy, and without added inorganic P, improved phytate degradation, P digestibility, retention and excretion, and the digestibility of AA in a dose-dependent manner. Increasing Buttiauxella phytase inclusion rate up to 2,000 FTU/kg further increased phytate degradation, improved AA and P digestibility, and reduced P excretion in weaned pigs fed complex diets.
Footnotes
The authors would like to thank Dr Joelle Buck (Reading, UK) for her assistance with the writing of this manuscript.
LITERATURE CITED
- Adedokun S. A., A. Owusu-Asiedu D. Ragland P. Plumstead, and Adeola O.. 2015. The efficacy of a new 6-phytase obtained from Buttiauxella spp. Expressed in Trichoderma reesei on digestibility of amino acids, energy, and nutrients in pigs fed a diet based on corn, soybean meal, wheat middlings, and corn distillers’ dried grains with solubles. J. Anim. Sci. 93:168–175. doi: 10.2527/jas.2014-7912. [DOI] [PubMed] [Google Scholar]
- Adeola O., and Sands J. S.. . 2003. Does supplemental dietary phytase improve amino acid utilization? A perspective that it does not. J. Anim. Sci. 81: E78–E85. [Google Scholar]
- Adeola O., and Cowieson A. J.. . 2011. BOARD-INVITED REVIEW: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Amerah A. M., P. W. Plumstead L. P. Barnard, and Kumar A.. 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 93:906–915. doi: 10.3382/ps.2013-03465. [DOI] [PubMed] [Google Scholar]
- Augspurger N. R., and Baker D. H.. . 2004. High dietary phytase levels maximize phytate-phosphorus utilization but do not affect protein utilization in chicks fed phosphorus- or amino acid-deficient diets. J. Anim. Sci. 82:1100–1107. doi: 10.2527/2004.8241100x. [DOI] [PubMed] [Google Scholar]
- AOAC 1986. Official method 986.11 for phytate in foods. 13th ed. Association of Official Analytical Chemists, Gaithersburg. MD: p. 667–670. [PubMed] [Google Scholar]
- AOAC 2000a. Method 2000.12: Phytase activity in feed: Colorimetric enzymatic method. In Official Methods of Analysis of AOAC International. 17th ed. Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- AOAC 2000b. Official Methods of Analysis of AOAC International. 16th ed. AOAC Int., Association of Official Analytical Chemists, Washington, DC. [Google Scholar]
- Bento M. H., C. Pedersen P. W. Plumstead L. Salmon C. M. Nyachoti, and Bikker P.. 2012. Dose response of a new phytase on dry matter, calcium, and phosphorus digestibility in weaned piglets. J. Anim. Sci. 90(Suppl 4):245–247. doi: 10.2527/jas.53988. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Acamovic T., and Bedford M. R.. . 2004. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from chickens. Br. Poult. Sci. 45: 101–108. doi: 10.1080/00071660410001668923. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., and Ravindran V.. . 2007. Effect of phytic acid and microbial phytase on the flow and amino acid composition of endogenous protein at the terminal ileum of growing broiler chickens. Br. J. Nutr. 98:745–752. doi: 10.1017/S0007114507750894. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Bedford M. R., Selle P. H., and Ravindran V.. . 2009. Phytate and microbial phytase: Implications for endogenous nitrogen losses and nutrient availability. World’s Poult. Sci. J. 65:401–418. doi: 10.1017/S0043933909000294. [DOI] [Google Scholar]
- Cowieson A. J., Ruckenbusch J. P., Sorbara J. O. B., Wilson J. W., Guggenbuhl P., Tanadini L., and Roos F. F.. . 2017a. A systematic view on the effect of microbial phytase on ileal amino acid digestibility in pigs. Anim. Feed Sci. and Tech. 231:138–149. doi: 10.1016/j.anifeedsci.2017.07.007. [DOI] [Google Scholar]
- Cowieson A. J., Ruckenbusch J.-P., Sorbara J. O. B., Wilson J. W., Guggenbuhl P., and Roos F. F.. . 2017b. A systematic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim. Feed Sci. and Tech. 225:182–194. doi: 10.1016/j.anifeedsci.2017.01.008. [DOI] [Google Scholar]
- Dersjant-Li Y., A. Awati H. Schulze, and Partridge G.. 2015. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Wealleans A. L., Barnard L. P., and Lane S.. . 2017a. Effect of increasing Buttiauxella phytase dose on nutrient digestibility and performance in weaned piglets fed corn or wheat-based diets. Anim. Feed Sci. and Tech. 234:101–109. doi: 10.1016/j.anifeedsci.2017.09.008. [DOI] [Google Scholar]
- Dersjant-Li Y., Schuh K., Wealleans A. L., Awati A., and Dusel G.. . 2017b. Effect of a Buttiauxella phytase on production performance in growing/finishing pigs fed a European-style diet without inclusion of inorganic phosphorus. J. Appl. Anim. Nutr. 5:e4:1–7. doi: 10.1017/JAN.2017.3. [DOI] [Google Scholar]
- Dersjant-Li Y., P. Plumstead A. Awati, and Remus J.. 2018. Productive performance of commercial growing and finishing pigs supplemented with a Buttiauxella phytase as a total replacement of inorganic phosphate. Anim. Nutr. 4:351–357. doi: 10.1016/j.aninu.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkvam D. O., Nelson T. S., and Kirby L. K.. . 1989. Calcium and phytic acid in chick nutrition. Nutr. Rep. Int. 40:33–42. [Google Scholar]
- ISO 13903 2005. Animal feeding stuffs - Determination of amino acids content. Available from https://www.iso.org/standard/37258.html. [Google Scholar]
- ISO17294-2 2016. Water quality -- Application of inductively coupled plasma mass spectrometry (ICP-MS) -- Part 2: Determination of selected elements including uranium isotopes. Available from https://www.iso.org/standard/62962.html.
- Leske K. L., and Coon C. N.. . 1999. A bioassay to determine the effect of phytase on phytate phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poult. Sci. 78:1151–1157. doi: 10.1093/ps/78.8.1151. [DOI] [PubMed] [Google Scholar]
- Li Z., Yi G., Yin J., Sun P., Li D. F., and Knigh C.. . 2008. Effects of organic acids on growth performance, gastrointestinal pH, intestinal microbial populations and immune responses of weaned pigs. Asian-Austr. J. Anim. Sci. 21: 252–261. doi: 10.5713/ajas.2008.70089. [DOI] [Google Scholar]
- Menezes-Blackburn D., S. Gabler, and Greiner R.. 2015. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agric. Food Chem. 63:6142–6149. doi: 10.1021/acs.jafc.5b01996. [DOI] [PubMed] [Google Scholar]
- Naumann C., and Bassler R.. . 2004. Die chemische Untersuchung von Futtermittel. VDLUFA-Verlag, Darmstadt, Germany. [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Qian H., E. T. Kornegay, and Conner D. E. Jr. 1996. Adverse effects of wide calcium:phosphorus ratios on supplemental phytase efficacy for weanling pigs fed two dietary phosphorus levels. J. Anim. Sci. 74:1288–1297. [DOI] [PubMed] [Google Scholar]
- Sandberg A. S., T. Larsen, and Sandström B.. 1993. High dietary calcium level decreases colonic phytate degradation in pigs fed a rapeseed diet. J. Nutr. 123:559–566. doi: 10.1093/jn/123.3.559. [DOI] [PubMed] [Google Scholar]
- Selle P. H., and Ravindran V.. . 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135:1–41. doi: 10.1016/j.anifeedsci.2006.06.010. [DOI] [Google Scholar]
- Selle P. H., and Ravindran V.. . 2008. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 113:99–122. doi: 10.1016/j.livsci.2007.05.014. [DOI] [Google Scholar]
- Selle P. H., Cowieson A. J., and Ravindran V.. . 2009a. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 124:126–141. doi: 10.1016/j.livsci.2009.01.006. [DOI] [Google Scholar]
- Selle P. H., Ravindran V., and Partridge G.. . 2009b. Beneficial effects of xylanase and/or phytase inclusions on ileal amino acid digestibility, energy utilization, mineral retention and growth performance in wheat-based broiler diets. Anim. Feed Sci. Tech. 153: 303–313. doi: 10.1016/j.anifeedsci.2009.06.011. [DOI] [Google Scholar]
- Selle P. H., A. J. Cowieson N. P. Cowieson, and Ravindran V.. 2012. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 25:1–17. doi: 10.1017/S0954422411000151. [DOI] [PubMed] [Google Scholar]
- VDLUFA 1976: Methodenbuch III, Die chemische Untersuchung von Futtermitteln, 6. Erg. 2006, VDLUFA-Verlag, Darmstadt, ISBN 978-3-941273-14-6. [Google Scholar]
- Velayudhan D. E., Heo J. M., Dersjant-Li Y., Owusu-Asiedu A. and Nyachoti C. M.. . 2015. Efficacy of novel 6-phytase from Buttiauxella sp. on ileal and total tract nutrient digestibility in growing pigs fed a corn-soy based diet. Anim. Feed Sci. Technol. 210:217–224. doi: 10.1016/j.anifeedsci.2015.10.005. [DOI] [Google Scholar]
- Woyengo T. A., A. J. Cowieson O. Adeola, and Nyachoti C. M.. 2009. Ileal digestibility and endogenous flow of minerals and amino acids: Responses to dietary phytic acid in piglets. Br. J. Nutr. 102:428–433. doi: 10.1017/S0007114508184719. [DOI] [PubMed] [Google Scholar]
- Yu S., A. Cowieson C. Gilbert P. Plumstead, and Dalsgaard S.. 2012. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 90:1824–1832. doi: 10.2527/jas.2011-3866. [DOI] [PubMed] [Google Scholar]
- Zeng Z. K., Q. Y. Li P. F. Zhao X. Xu Q. Y. Tian H. L. Wang L. Pan S. Yu, and Piao X. S.. 2016. A new phytase continuously hydrolyzes phytate and improves amino acid digestibility and mineral balance in growing pigs fed phosphorous-deficient diet. J. Anim. Sci. 94:629–638. doi: 10.2527/jas.2015-9143. [DOI] [PubMed] [Google Scholar]
- Zouaoui M., Létourneau-Montminy M. P., and Guay F.. . 2018. Effect of phytase on amino acid digestibility in pig: A meta-analysis. Anim. Feed Sci. and Tech. 238:18–28. doi: 10.1016/j.anifeedsci.2018.01.019. [DOI] [Google Scholar]