Abstract
Bacterial infection causes nutrient malabsorption in small intestine. KR-32, a kind of synthetic antimicrobial peptide, has the bacteriostatic effect. In the present study, 2 experiments were designed to analyze the effects of KR-32 on fat absorption of piglets with or without Escherichia coli infection. In Exp. 1, 12 weaning piglets (21 d old) were allocated to 2 groups: piglets with an intraperitoneal (i.p.) injection of antimicrobial peptide KR-32 (APK) and piglets with an i.p. injection of an equivalent volume (1 mL) of phosphate-buffered saline (PBS) (CON-1). Results showed that after 7 d of growth, KR-32 did not significantly change growth performance and apparent total tract digestibility (ATTD) of feed nutrients of normal pigs. To confirm whether KR-32 affects those of enterotoxigenic Escherichia coli (ETEC) K88–challenged pigs, we performed Exp. 2, in which 18 piglets (28 d old) were divided into the following 3 groups: 1) piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 (K88 + APK); 2) piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS (K88); and 3) piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an i.p. injection of an equivalent volume of PBS (CON-2). Results showed that ETEC K88 challenge led to poor ADFI, ADG, and G:F in piglets; decreased ATTD of feed nutrients, especially CP and ether extract (EE); and intestinal morphology disorder. After i.p. injection of KR-32, ADG and ATTD of CP and EE were greatly increased, G:F was significantly reduced (P < 0.05), and, especially, ATTD of EE returned to a normal level compared with group CON-2. Fatty acid absorption also highly increased after KR-32 injection. Then we focused on fat digestion and fatty acid uptake. The pH in the intestine and pancreas lipase showed no difference among the 3 treatment groups, whereas fatty acid transporter protein 4 (FATP4) expression was remarkably improved (P < 0.05) and the epithelial barrier was recovered after i.p. injection of KR-32. In conclusion, KR-32, given to ETEC K88–challenged piglets, improved growth performance, ATTD of EE, fatty acid absorption, and intestinal morphology, which indicated that KR-32 was likely to improve the expression of FATP4 and by repairing the epithelial barrier, thereby alleviating fatty acid malabsorption.
Keywords: enterotoxigenic Escherichia coli K88, fatty acid absorption, fatty acid transporter protein 4, KR-32
INTRODUCTION
The small intestinal epithelium is the main site of nutrient absorption. The disruption of the small intestinal epithelial barrier, which mainly means the changes in permeability, is frequently associated with diarrhea, such as steatorrhea (Jonas et al., 1979), which always leads to malabsorption in newborns (Xing et al., 2017). Enterotoxigenic Escherichia coli (ETEC) K88 is one of the main pathogenic bacteria causing intestinal barrier injury, mainly related to tight junction dysfunction (Roselli et al., 2007; Yang et al., 2014; Pan et al., 2017). After colonization in the small intestine and the release of enterotoxins, ETEC K88 induces fluid losses (Fairbrother et al., 2005; Guignot et al., 2007) and disturbs ion transport (Eisenhut, 2006), which causes severe watery diarrhea and subsequent growth retardation in neonatal and postweaning piglets, leading to great economic loss in swine production (Fairbrother et al., 2005).
Many nutrients cannot be directly utilized unless digested and absorbed (Borgstrom, 1968; Gray, 1970; Newcomer, 1973). Digestive enzymes, mainly synthesized and secreted by the pancreas, rely on proper pHs and temperatures to catalyze the degradation of nutrients (Holtzapple et al., 1975). Fats, one of the 3 main nutrients, are hydrolyzed into long-chain fatty acids (LCFAs) and monoacylglycerols by lipases in the small intestine; then, LCFA and monoacylglycerols are transported into enterocytes by fatty acid transport proteins for further assembly (Rossi et al., 2010; Buttet et al., 2014). It is known that LCFAs not only interact with various cell membrane structures, transporters, ion channels, enzymes, and hormone receptors in cells to regulate animals’ physiological process but also play an important role in energy supply, especially in nursery pigs, because fatty acids can provide much more energy than the same mass of proteins and carbohydrates (Fijlstra et al., 2013). The uptake of LCFA from the intestinal lumen into enterocytes is mediated by multiple transporters including fatty acid transporter protein 4 (FATP4), Cluster of Differentiation 36 (CD36, also named fatty acid translocase), and intestinal fatty acid–binding protein (iFABP), which has been demonstrated by gene knockout or overexpression experiments in vitro, as well as in mice (Stahl et al., 1999; Nauli et al., 2006).
Antimicrobial peptides, secreted by various organisms, are small peptides with antibacterial and bacteriostatic functions (Kimbrell, 1991; Javadpour et al., 1996). The concentration of antimicrobial peptides in vivo is much lower than that of antimicrobial peptides for bacteriostasis in vitro (Afacan et al., 2012), which suggests that antimicrobial peptides not only have bacteriostatic and bactericidal effects (Xia et al., 2015) but also take part in regulating immunity (Niyonsaba et al., 2002). Host defense peptide LL-37, an antimicrobial peptide in human, inhibits the migration of p65, a subunit of nuclear factor-κB (NF-κB) (Nijnik et al., 2009), which activates the mitogen-activated protein kinase (MAPK) (Wu et al., 2012) and phosphoinositide 3-kinase (PI3K) (Su et al., 2016) signaling pathways and selectively upregulates the expression of anti-inflammatory factors (Ribon et al., 2019). What is more, LL-37 can directly bind to lipopolysaccharides to inhibit Toll-like receptor 4 (TLR4) and its downstream signaling pathways (Suzuki et al., 2016). Our previous studies reported that antimicrobial peptides could exert immunomodulatory properties and maintain intestinal physical barrier functions in mice and RAW264.7 cells (Zong et al., 2016; Chen et al., 2018). Furthermore, Cathelicidin-WA (CWA, a modified synthetic antimicrobial peptide) could alleviate diarrhea in postweaning piglets and enhance host defense against E. coli O157 infection in mice (Yi et al., 2016, 2017).
We developed a kind of modified synthetic peptide KR-32, according to a natural antimicrobial peptide from snake. Just like CWA, KR-32 exhibits strong antimicrobial activities against Gram-negative enteric bacteria (Wang et al., 2008; Liu et al., 2011) and attenuates intestinal inflammation through immune regulation (Zhang et al., 2015; Yi et al., 2016). The present study explored whether KR-32 could ameliorate fatty acid malabsorption caused by ETEC K88 infection, as fatty acids are necessary nutrients.
The aim of the present study is to investigate the role of KR-32 in digestion or absorption of fats. We confirmed that KR-32 might attenuate fatty acid malabsorption possibly by renewing the protein expression of FATP4 through recovering intestinal integrity.
MATERIALS AND METHODS
Animals, Housing, Experimental Design, and Diets
Procedures used in these experiments were approved by the Zhejiang University (Hangzhou, Zhejiang, P.R. China) Animal Care and Use Committee. The basal diet (Table 1) was formulated as a powder without any in-feed antibiotics, based on equal DM, CP, and ether extract (EE) contents and met the NRC (2012) nutrient requirements.
Table 1.
Ingredient compositions and nutrient concentrations in Exp. 1 and Exp. 2 (as-fed basis)
| Ingredient, % | |
|---|---|
| Corn | 54.90 |
| Extruded soybean | 11.00 |
| Soybean meal | 21.10 |
| Bran | 2.00 |
| Fish meal | 3.00 |
| Soy oil | 2.00 |
| Calcium carbonate | 1.00 |
| Calcium superphosphate | 1.00 |
| Premix1 | 4.00 |
| Total | 100.00 |
| Analyzed composition | |
|---|---|
| DM, % | 88.85 |
| CP, % | 18.58 |
| EE2, % | 6.33 |
| Total AA, % | 14.09 |
| Ca, % | 0.73 |
| Total P, % | 0.59 |
| Glycinin, g/kg | 37.52 |
| β-Conglycinin, % | 40.89 |
1Premix provided per kilogram of complete diet: 6,000 IU vitamin A, 900 IU vitamin D3, 3 mg vitamin E, 30 µg vitamin B12, 0.75 mg vitamin K3, 18 mg d-pantothenic acid, 30 mg niacin, 80 mg iron, 0.45 mg iodine, 20 mg copper, 0.1 mg selenium, and 100 mg zinc.
2EE = ether extract.
Experiment 1.
Twelve young female piglets [male Duroc × female (Landrace × Yorkshire), 21 d old, and initial BW of 6.68 ± 0.43 kg] were randomly allocated to 2 treatment groups as follows: 1) piglets with an intraperitoneal (i.p.) injection of an equivalent volume (1 mL) of phosphate-buffered saline (PBS) (CON-1; n = 6) and 2) piglets with an i.p. injection of 0.6 mg/kg antimicrobial peptide KR-32 (APK; n = 6) [the dosage was based on results from CWA (Yi et al., 2016), whose antimicrobial activity is similar with that of KR-32 in vitro pre-experiment, and because the effect of KR-32 is unstable if added into diet or administered orally in piglets, i.p. injection of APK was chosen for piglets]. All piglets were separately fed basal diets for 1 wk, and the experimental group was administered KR-32 once a day for 3 d from day 4.
Experiment 2.
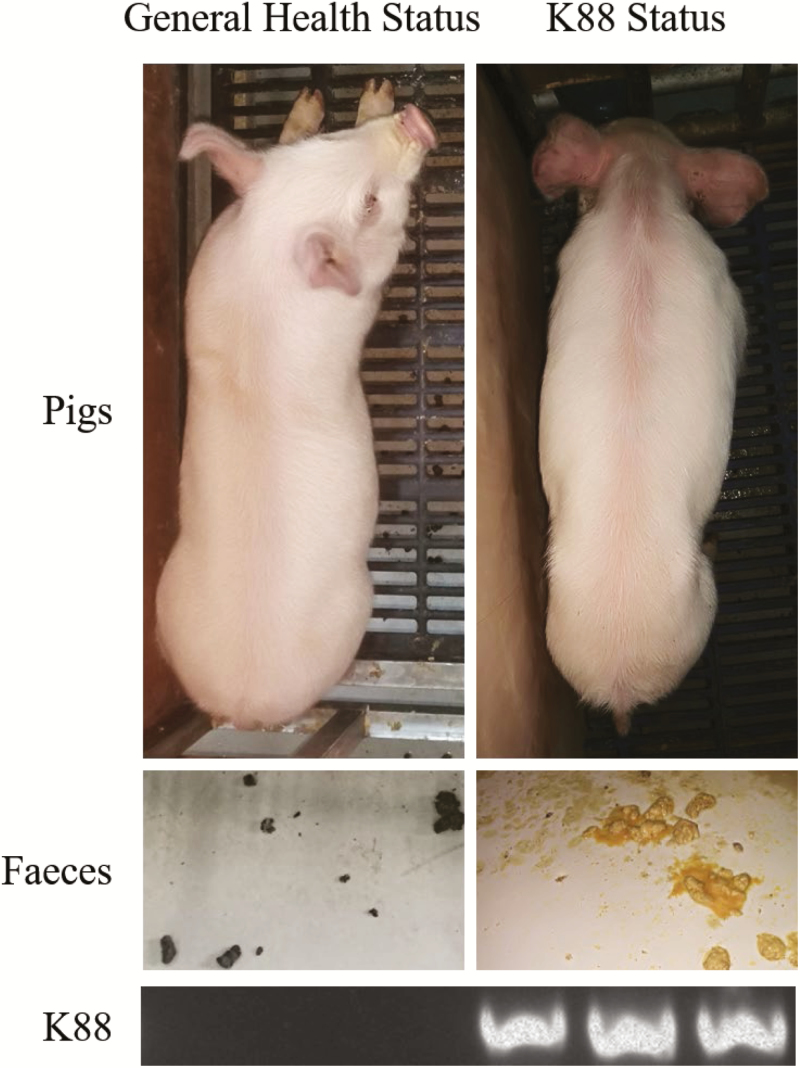
Eighteen young female piglets [male Duroc × female (Landrace × Yorkshire), 28 d old, and initial BW of 7.77 ± 0.35 kg] were randomly allocated to 3 treatment groups as follows: 1) piglets with an oral administration of fresh Luria–Bertani (LB) broth (50 mL) followed by an i.p. injection of PBS, equivalent volume to KR-32 solution (≈1 mL) (CON-2; n = 6); 2) piglets orally challenged with 1 × 1010 cfu ETEC K88 (Che et al., 2017) on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS (K88; n = 6); and 3) piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 (K88 + APK; n = 6). After challenged with ETEC K88, the pigs showed rapid emaciation and the accompanying diarrhea. And ETEC K88 could be detected from fecal in challenged pigs (Fig. 1). All piglets were separately fed basal diets for 1 wk, and the experimental group was administered KR-32 once a day for 3 d from day 4.
Figure 1.
The general health status and the K88 status of the pigs prior to dpi 0. After challenged with enterotoxigenic Escherichia coli (ETEC) K88, physical status, feces, and ETEC K88 in feces of pigs were observed.
The number of weaned piglets with diarrhea and its duration were observed and recorded during the feeding trial. Diarrhea was defined as liquid consistency over a minimum of 2 consecutive days. The incidence of diarrhea (%) was calculated as a percentage of the number of diarrheal piglets during the period divided by the total number of piglets (Qian et al., 2016).
Feces from each pig were collected from day 4 to day 6 between 8:00 and 20:00 each day for apparent total tract digestibility (ATTD) evaluation, and ADG and ADFI were calculated from day 1 to day 6 in Exp. 1 and Exp. 2. On day 7, in Exp. 2, pigs were sacrificed after anesthesia with i.p. injection of 15 mg/kg BW of pentobarbital sodium (Che et al., 2017).
According to Brestensky et al. (2017), coefficients of ATTD of nutrients were calculated using the formula given below:
Nd represents a dietary concentration of the nutrient, Md represents a dietary concentration of marker (acid-insoluble ash as marker in the study), Ni represents a concentration of the nutrient in feces, and Mi represents concentration of marker in feces (all values expressed in grams per kilogram of DM).
BW of piglets was measured on day 1 and day 7 for initial BW and final BW, respectively. Feed intake of piglets was calculated using the following formula:
M (feed intake) represents the feed intake of piglets from day 1 to day 7, M (initial feed) represents the initial feed weight on day 1, and M (remaining feed) represents the left feed weight on day 7 that piglets did not eat yet, and ADFI = M (feed intake)/6.
Antimicrobial Peptide and Bacterial Preparation
The synthetic and purified (>95%) KR-32 peptide (GL Biochem (Shanghai) Ltd., Shanghai, P.R. China) was dissolved in sterile saline before injection. Standard Escherichia strain E. coli O60:K88, producing heat-labile toxins, was purchased from China General Microbiological Culture Collection Center (Beijing, P.R. China). Bacteria were incubated in fresh LB broth at 37 °C, shaken overnight, diluted in fresh LB broth at a ratio of 1:100, and then shaken for 1 h at 37 °C. Bacteria grown to the mid log phase of the growth curve (absorbance under 600 nm = 0.5), which showed high vitality, were used for experiments. Following incubation, the bacteria were concentrated by centrifugation at 3,000 × g for 10 min at 4 °C and resuspended in LB broth. Hundred milliliters of LB broth contain 1 × 1010 cfu ETEC K88 in the end.
Detection of ETEC K88 in feces
Bacterial genomic DNA was extracted from feces of pigs using a TIANamp Stool DNA Kit (TIANGEN, Beijing, P.R. China) according to the manufacturers’ instruction. The PCR reaction was performed in a total volume of 20 μL containing 10 μL of 2× Quick Taq HS DyeMix (TOYOBO, Shanghai, P.R. China), 100 ng of bacterial genomic DNA, and 0.5 pM of each primer. Amplification conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles consisting of 30 s at 94 °C, 30 s at 56 °C, 40 s at 72 °C, and a final extension at 72 °C for 10 min. The PCR products were subjected to electrophoresis and the results were analyzed by gel imaging and analysis system (CliNX, Shanghai, P.R. China). The primers designed for detection of ETEC K88 were as follows: forward: GGTGATTTCAATGGTTCGGTC, reverse: AATGCTACGTTCAGCGGAGCG.
Chemical Analysis
Feed and feces samples were analyzed for DM, CP, EE, calcium, phosphorus, ash, and AA according to procedures of the AOAC International (2000). β-Conglycinin and glycinin concentrations in feed were analyzed using an indirect ELISA kit (Longzhoufangke Bio Co., Beijing, P.R. China) according to the manufacturer’s instruction.
Collection of Blood and Tissue
After overnight fasting on day 7, all the piglets in Exp. 2 were orally given 10 mL of olive oil. Blood samples were collected in centrifuge tube with heparin before the start of oral fat administration (0 h) and afterwards (at 1, 2, 3, 4, and 6 h after oral gavage) from the anterior vena cava after the pigs were anesthetized with isoflurane (Chi et al., 2017). The collected blood was immediately centrifuged at 2,000 × g for 10 min at 4 °C, and the plasma was stored at −80 °C.
After blood collection, the piglets were sacrificed and the intestines were quickly removed. Small parts of the mid duodenum, jejunum, and ileum were gently washed and then fixed in 4% paraformaldehyde for hematoxylin and eosin (H&E) staining and in 2.5% glutaraldehyde fixative for scanning electron microscopy and transmission electron microscopy. The remaining mid jejunum was opened longitudinally, and the mucosal of mid jejunum was scraped off with slides gently. Then mucosal scrapings and pancreas were collected, quickly frozen in liquid nitrogen, and stored at −80°C for further study.
The Fecal Shedding of Total E. coli
Fresh fecal samples were aseptically obtained from each piglet on day 0, 3, and 6, respectively. Each sample (1 g) was made to 6 serial, 10-fold dilutions according to the fecal E. coli concentrations in the pretest (before the experiment), and 100 μL of each dilution was spread in duplicate onto Eosin Methylene Blue agar plates. The plates were incubated at 37 °C for 24 h. The colonies with metallic color on the agar plates were counted as total E. coli in fecal matter. The numbers of total E. coli present in the undiluted rectal feces were calculated. The results were expressed as log10-transformed data.
Analyses of Total Cholesterol, Triglyceride, and Free Fatty Acid Levels in Plasma and Small Intestine Contents
Plasma total cholesterol (TC), triglyceride (TG) and free fatty acid (FFA) levels were analyzed using an automatic biochemical analyzer (Olympus AU2700) in Affiliated Hospital of Hangzhou Normal University (Hangzhou, China). TG in the small intestine was detected using a TG assay kit (Solarbio, Beijing, P.R. China) and FFAs in the small intestine were detected using FFA assay kits (Solarbio), according to the manufacturers’ instructions.
Detection of Fatty Acids in Plasma
The analysis of plasma fatty acids was performed using gas chromatography–mass spectrometry (GC–MS). GC–MS analysis was used to quantify fatty acid concentrations in piglet serum samples. Serum samples were thawed at room temperature, 10 mL of 1 mol/liter KOH-CH3OH was added into 100 µL of serum samples with sufficient mixing under nitrogen condition, and the samples were distilled for 30 min at 60 °C. After the solution cooled, 4% hydrochloric acid–methanol solutions were added, and the sample was incubated for 10 min under 60 °C water bath. After cooling again, the solution was mixed with n-hexane and saturated sodium chloride solution sufficiently, and then supernatant liquid was injected after centrifugation for GC–MS analysis.
Western Blot Analysis
Scraped intestinal mucosa from the jejunum was homogenized using a Whole Protein Extraction Kit (Nanjing KeyGen Biotech. Co. Ltd., Nanjing, P.R. China), and protein concentrations were quantified using a BCA Protein Quantification Kit (Nanjing KeyGen Biotech. Co. Ltd.). Equivalent proteins were separated using 12% SDS–PAGE and electroblotted onto polyvinylidene fluoride membranes and then blocked with 5% fat-free milk. Then, membranes were incubated overnight at 4 °C with primary antibodies for β-actin (1:500, 60008-1-1g, Proteintech Group, Wuhan, P.R. China), CD36 (1:500, 18836-1-AP, Proteintech Group), iFABP (1:500, 21252-1-AP, Proteintech Group), and FATP4 (1:500, 51035-1-AP, Proteintech Group) and subsequently incubated with goat anti-rabbit IgG secondary horseradish peroxidase-conjugated antibody (1:5000, 10285-1-AP, Proteintech Group) for 1 h at room temperature. The protein blots were photographed using a Tanon 4200SF Chemiluminescent Imaging System (CliNX). Intensity of blots was quantified using ImageJ software.
Intestinal Morphology Analysis
Tissues fixed with paraformaldehyde were embedded in paraffin, and sections (5 µm) were stained with H&E. Images of paraffin section of the jejunum were obtained using a Leica DM3000 Microsystem (Leica Camera AG, Wetzlar, Germany). The villi height and crypt depth were measured using the Leica Application Suite version 3.7.0. Values of villi height or crypt depth were the average of 3 measurements for each pig.
Histologic Scoring
According to image analysis method (Rogers et al., 2016), the severity and extent of inflammation caused by ETEC K88 were scored by a pathologist and 5 scientists with basic histological experience as judges according to the following criteria:
healthy small intestine;
minimal inflammation with minimal to no separation of crypts (generally focal affecting <10% of mucosa);
mild inflammation with mild separation of crypts (generally affecting 11% to 25% of mucosa or mild, diffuse inflammatory infiltrates with minimal separation of crypts);
moderate inflammation with separation of crypts, with or without focal effacement of crypts (generally affecting 26% to 50% of mucosa or moderate, diffuse separation of crypts);
extensive inflammation with marked separation and effacement of crypts (generally affecting 51% to 75% of mucosa); and
diffuse inflammation with marked separation and effacement of crypts (generally affecting >75% of mucosa).
The duodenum, jejunum, and ileum were each scored individually and summed for the total histologic score of each animal. The judges were blinded to the treatment groups at the time of scoring.
Scanning Electron Microscopy and Transmission Electron Microscopy
Jejunum tissue was fixed with 2.5% glutaraldehyde overnight and then with 1% OsO4 for 1 h. The jejunum specimens were then dehydrated in a graded series of ethanol (30%, 50%, 70%, 80%, 90%, 95%, and 100%) for 20 min at each step. Then the samples could be used for scanning electron microscopy and transmission electron microscopy.
The samples for scanning electron microscopy were transferred into an alcohol and isoamyl acetate mixture (1:1, vol: vol) for 30 min followed by isoamyl acetate for 1 h. The specimens were dehydrated with liquid CO2 in a Hitachi model HCP-2 critical point dryer (Hitachi Ltd., Tokyo, Japan). After being coated with gold–palladium, the dehydrated specimens were visualized using a Philips model SU8010 FASEM (Hitachi Ltd.).
The samples for transmission electron microscopy were transferred into pure acetone for 20 min. The specimens were placed in a mixture of pure acetone and Spurr’s resin (1:1 for 1 h and 1:3 for 3 h) and then embedded in Spurr’s resin overnight. Next, the specimens in resin were heated at 70 °C for 9 h to solidify the resin, and then were cut into sections. The sections were stained with uranyl acetate and alkaline lead citrate for 15 min and visualized using transmission electron microscopy (model H-7650; Hitachi Ltd.).
Statistical Analysis
Statistical analysis was performed with SPSS 20.0 software (SAS Inc., Chicago, IL). Student’s t-test or one-way ANOVA followed by LSD multiple comparison tests were used to determine the statistical significance. Differences were considered significant at P <0.05.
RESULTS
Growth Performance
The effects of KR-32 on growth performance of piglets challenged with or without ETEC K88 are shown in Table 2. In Exp. 1, there was no significant difference in growth performance between the CON-1 group and the APK group during the experiment period. In Exp. 2, after challenged with ETEC K88, the K88 group showed decreased ADG, ADFI, and G:F compared with CON-2 (P < 0.05). Whereas the K88 + APK group showed small increases in ADG (43 to 116 g) and G:F (0.07 to 0.51) compared with the K88 group (P < 0.05).
Table 2.
Effect of KR-32 on growth performance of piglets challenged with or without enterotoxigenic Escherichia coli (ETEC) K88
| Item | Exp. 1 | Exp. 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group1 | SEM | P-value | Treatment group2 | SEM | P-value | ||||
| CON-1 | APK | CON-2 | K88 | K88 + APK | |||||
| BW, kg | |||||||||
| Initial BW, day 1 | 6.73 | 6.62 | 0.13 | 0.718 | 7.94 | 7.79 | 7.55 | 0.09 | 0.191 |
| Final BW, day 7 | 7.66 | 7.59 | 0.15 | 0.837 | 10.09a | 7.97b | 8.25b | 0.28 | <0.01 |
| ADG, g | 154 | 160 | 15.85 | 0.863 | 358a | 43b | 116c | 37 | <0.01 |
| ADFI, g | 367 | 355 | 0.01 | 0.542 | 518a | 181b | 206b | 41 | <0.01 |
| G:F3 | 0.35 | 0.42 | 0.40 | 0.608 | 0.68a | 0.07b | 0.51a | 2.62 | 0.088 |
a–cMeans within a row with different superscripts differ (P < 0.05).
1CON-1 = piglets with an intraperitoneal (i.p.) injection of an equivalent volume (1 mL) of PBS; APK = piglets with an i.p. injection of antimicrobial peptide KR-32. n = 6 for the CON-1 group and n = 6 for the APK group.
2CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an i.p. injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32. n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group.
3G:F = gain:feed.
Apparent Total Tract Digestibility
The effects of KR-32 on the ATTD of piglets challenged with or without ETEC K88 are shown in Table 3. No difference was found between the CON-1 group and the APK group in Exp. 1, whereas administration of KR-32 alleviated the negative influence of ETEC K88 challenge in pigs in Exp. 2. As shown in Table 3, KR-32 improved (P < 0.05) the ATTD of CP and EE of pigs challenged with ETEC K88 by 17.46% and 32.93%, respectively.
Table 3.
Apparent digestibility of nutrients of weaned pigs treated with KR-32 after challenged with or without enterotoxigenic Escherichia coli (ETEC) K88
| Item | Exp. 1 | Exp. 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group1 | SEM | P-value | Treatment group2 | SEM | P-value | ||||
| CON-1 | APK | CON-2 | K88 | K88+APK | |||||
| OM, % | 77.11 | 76.48 | 0.56 | 0.637 | 75.66 | 69.31 | 74.23 | 1.37 | 0.129 |
| CP, % | 70.33 | 71.33 | 0.68 | 0.539 | 69.66a | 54.06b | 63.50c | 2.39 | <0.01 |
| Crude fat, % | 69.23 | 70.54 | 1.27 | 0.662 | 71.03a | 51.62b | 67.01a | 3.50 | 0.024 |
| Ca, % | 53.10 | 50.13 | 1.75 | 0.459 | 56.92 | 40.24 | 43.58 | 0.04 | 0.141 |
| P, % | 47.10 | 46.61 | 1.31 | 0.874 | 45.33 | 32.33 | 37.67 | 3.04 | 0.228 |
a–cMeans within a row with different superscripts differ (P < 0.05).
1CON-1 = piglets with an intraperitoneal (i.p.) injection of an equivalent volume (1 mL) of PBS; APK = piglets with an i.p. injection of antimicrobial peptide KR-32. n = 6 for the CON-1 group and n = 6 for the APK group.
2CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an i.p. injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32. n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group.
Fecal Shedding of Total E. coli
The result of total E.coli shedding is shown in Table 4. At day 0, the fecal shedding of total E. coli in 3 treatments remained largely consistent before ETEC K88 challenge (P = 0.766). While at day 3, E.coli shedding in ETEC K88–challenged pigs sharply increased compared with that in CON-2 (P < 0.05). After administrated with KR-32 i.p. injection, the fecal shedding of total E.coli returned down significantly at day 6 (P < 0.05).
Table 4.
The fecal shedding of total E. coli of piglets challenged with or without enterotoxigenic Escherichia coli (ETEC) K88
| Shedding data in Exp. 21 | CON-2 | K88 | K88 + APK | SEM | P-value |
|---|---|---|---|---|---|
| Day 0 | 5.17 | 5.18 | 4.76 | 0.25 | 0.766 |
| Day 3 | 5.67a | 9.80b | 9.16b | 0.55 | <0.05 |
| Day 6 | 5.42a | 10.30b | 6.54a | 0.59 | <0.05 |
a–bMeans within a row with different superscripts differ (P < 0.05).
1CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32. n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group.
Fat Absorption
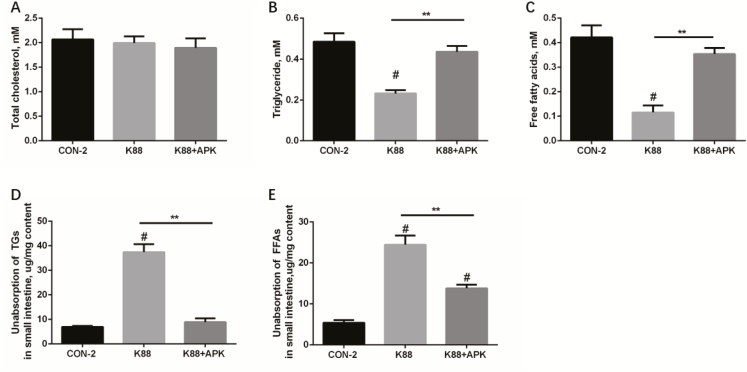
The lipid absorption and metabolic status under different treatments are shown in Fig. 2. TC in plasma displayed similar concentration among 3 groups, but lower concentrations of TGs and FFAs in plasma were observed in K88 group compared with the control (P < 0.05). While in K88 + APK, concentrations of TGs and FFAs in plasma of pigs were at the similar levels with CON-2 (P = 0.05) (Fig. 2A–C). After given olive oil, unabsorbed TG and FFA levels in small intestine of pigs in K88 were more than that in CON-2 (P < 0.05), and i.p. injection of KR-32 reduced unabsorbed TG and FFA levels in small intestine of pigs challenged with ETEC K88 (Fig. 2D–E). KR-32 seems to relieve malabsorption and restore lipid metabolic balance to general status.
Figure 2.
Fat metabolism and absorption in pigs treated by enterotoxigenic Escherichia coli (ETEC) K88 with or without KR-32. (A) Total cholesterol (TC), (B) triglyceride (TG), and (C) FFA concentrations in plasma of fasting pigs that were given olive oil for 2 h were analyzed using an automatic biochemical analyzer. TG (D) and FFA (E) concentrations in small intestine of pigs that were given olive oil were analyzed using assay kits, respectively. **P <0.01 for the K88 group vs. the K88 + APK group; APK = antimicrobial peptide KR-32; #P <0.05 compared with the CON-2 group. CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 in Exp. 2. Results are means and SEM (n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group).
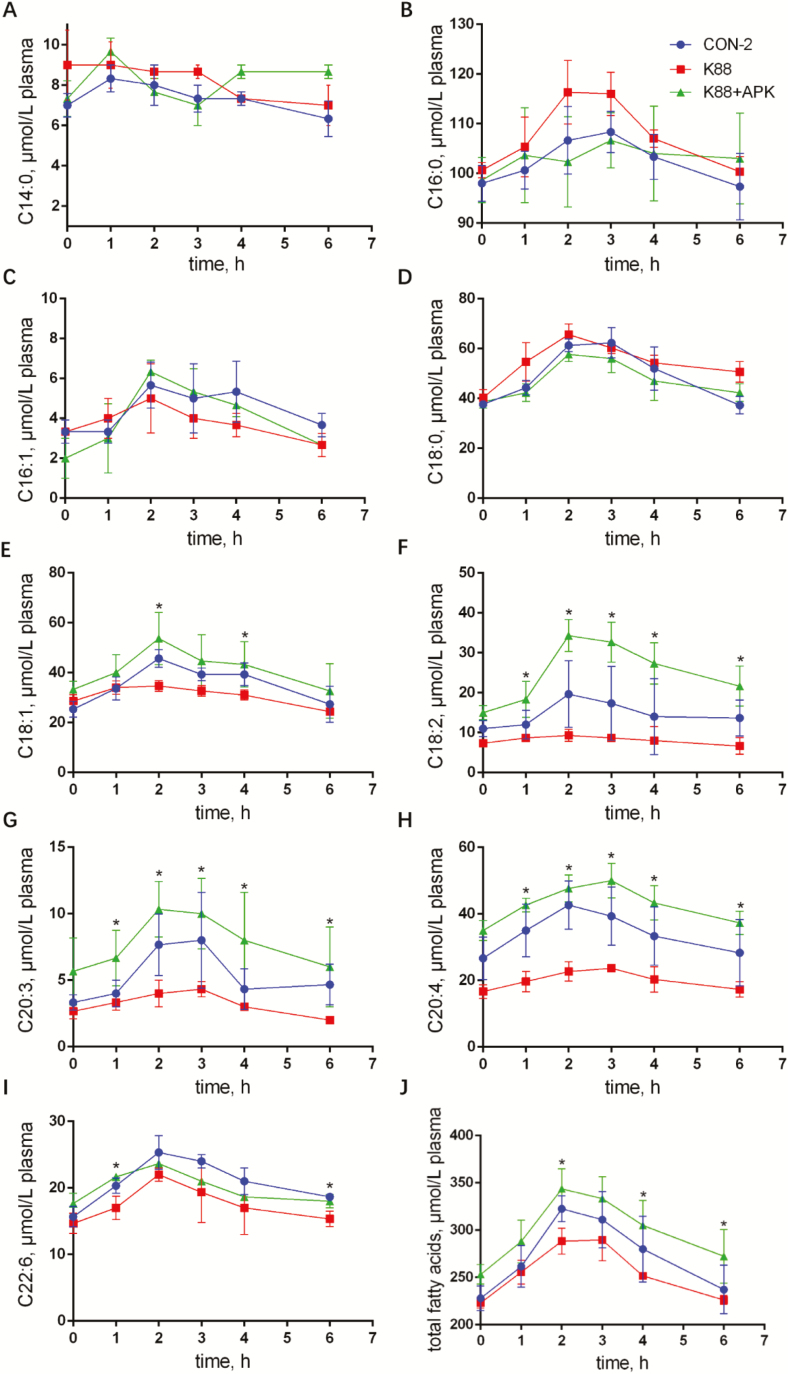
In Exp. 2, 9 fatty acids, C14:0, C16:0, C16:1, C18:0, C18:1, C18:2, C20:3, C20:4, and C22:6, were checked out in serum of piglets. On the whole, the concentrations of fatty acids increased significantly after given olive oil (P < 0.05), and reached the peaks between 2- and 3-h time points, then returned back to normal levels slowly in all the 3 groups. Figure 3 shows that ETEC K88 challenge caused marked reductions in plasma concentrations of C18:1, C18:2, C20:3, C20:4, and C22:6 at all the time points after olive oil infusion compared with the CON-2 group, and there was no lagging but less absorption was observed in K88 group. Whereas fatty acid concentrations in serum of K88 + APK group were significantly improved at most time points, especially C18:2, C20:3, and C20:4 (P < 0.05).
Figure 3.
Concentration–time of fatty acids in the plasma of enterotoxigenic Escherichia coli (ETEC) K88–challenged pigs with or without KR-32. Nine fatty acids C14:0 (A), C16:0 (B), C16:1 (C), C18:0 (D), C18:1 (E), C18:2 (F), C20:3 (G), C20:4 (H), and C22:6 (I) were detected. The sum of these 9 fatty acids was shown as total fatty acids (J). *P <0.05 for the K88 group vs. the K88 + APK group; APK = antimicrobial peptide KR-32. CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 in Exp. 2. Results are means and SEM (n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group).
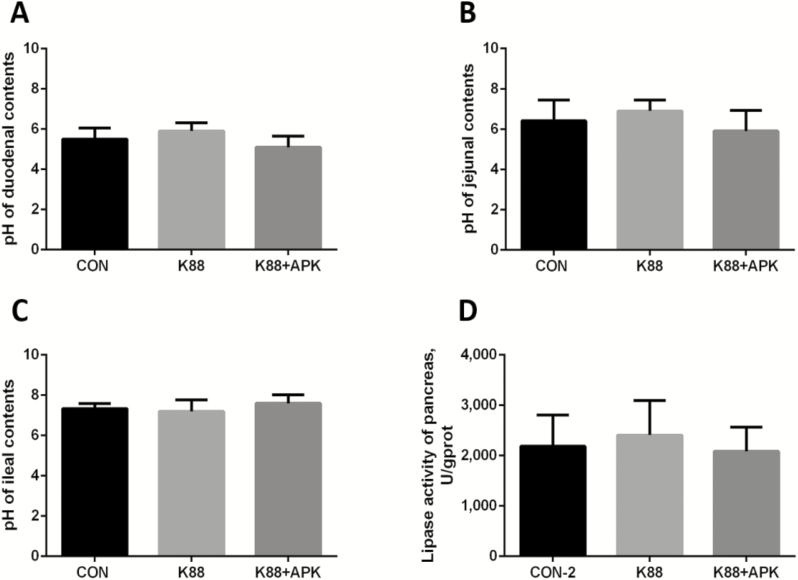
Intestinal pH and Lipase Activity
There was no significant difference in pH values of the duodenum, jejunum, and ileum among all 3 treatment groups in Exp. 2. In the pancreas, there was no significant difference in pH and lipase activity among the 3 groups (CON-2, K88, and K88 + APK; Fig. 4).
Figure 4.
The pH in the intestinal contents and the lipase activities in the pancreas of pigs. The pH values in contents of the duodenum (A), jejunum (B), and ileum (C) and lipase activities in the pancreas (D) of pigs were analyzed. CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu enterotoxigenic Escherichia coli (ETEC) K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 in Exp. 2; APK = antimicrobial peptide KR-32; U = units of lipase activity; gprot = mass of protein (g). Data are means and SD. n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group.
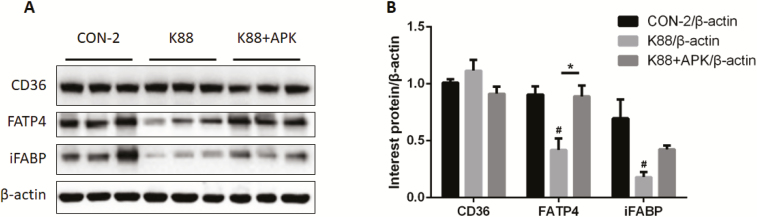
The Expression of FATP4
The expression of proteins involved in the transport of fatty acids in the jejunum mucosa of pigs is shown in Fig. 5. FATP4 and iFABP levels of the jejunum mucosa were significantly decreased in pigs orally treated with ETEC K88 (P < 0.05), whereas i.p. injection of KR-32 prevented FATP4 from decreasing (P < 0.05), but failed to remarkably improve iFABP (P > 0.05). CD36 showed no change among the treatment groups (P > 0.05).
Figure 5.
The expression of proteins involved in the transport of fatty acids in the jejunum mucosa of pigs. (A) Western blot analysis of Cluster of Differentiation 36 (CD36), fatty acid transporter protein 4 (FATP4), and intestinal fatty acid–binding protein (iFABP) expression in jejunum mucosa. (B) Representative histograms of CD36, FATP4, and iFABP. *P <0.05 for the K88 group vs. the K88 + APK group; APK = antimicrobial peptide KR-32; #P <0.05 compared with the CON-2 group. CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu enterotoxigenic Escherichia coli (ETEC) K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 in Exp. 2. Results are means and SEM (n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group).
Intestinal Integrity
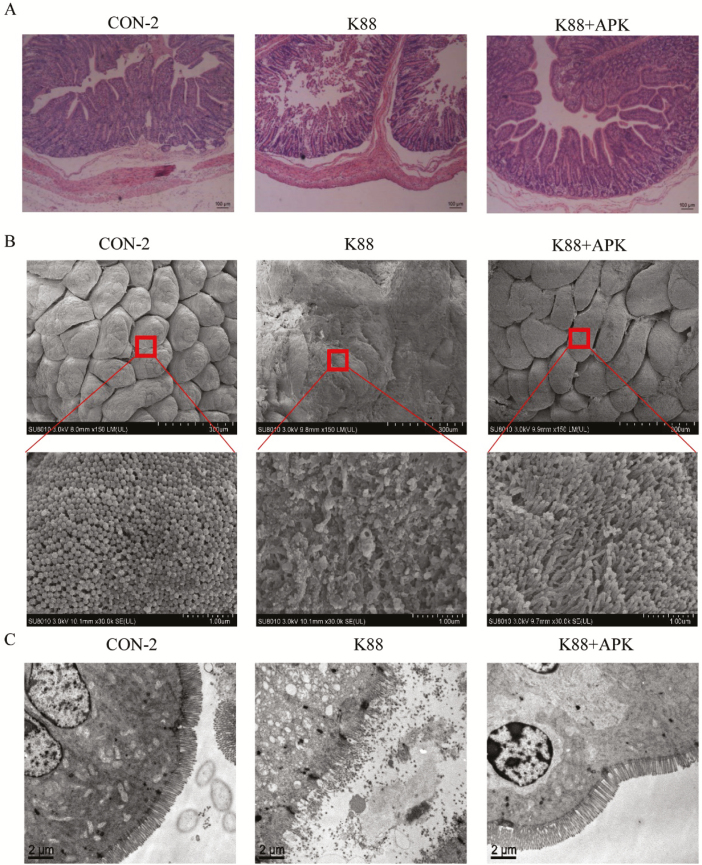
Intestinal epithelia of the jejunum showed serious injury, with discontinuous brush borders and blunt villi, in ETEC K88–challenged pigs, whereas treatment with KR-32 after ETEC challenge resulted in a little improvement (Fig. 6A). Villus height, crypt depth, and the ratio of villus height to crypt depth of different specimens are presented in Table 5. After ETEC K88 infection, villous height (duodenum, jejunum, and ileum) and villous height: crypt depth ratio (duodenum, jejunum and ileum) were decreased (P < 0.05), and crypt depth (jejunum and ileum) was increased dramatically (P < 0.05). While a favorable turn appeared in K88 + APK group, some of the bad conditions were inhibited, villous height of the 3 intestinal segments increased significantly compared with that in K88 group (P < 0.05), and even the villous height and crypt depth of jejunum returned back to control level (P > 0.05). The results suggested KR-32 treatment could ameliorate the villus shortening induced by ETEC K88 infection. Intestinal histologic scores showed remarkable decrease in K88 + APK group compared with ETEC K88–challenged pigs (P < 0.05), and diarrhea rate also deceased but did not show significant difference. Scanning electron microscopy was used to observe the surface of intestinal villi and microvilli in the jejunum (Fig. 6B). The jejunal surface of piglets challenged with ETEC K88 was severely damaged, and KR-32 treatment alleviated the damage caused by ETEC K88. Further observations at 30,000× magnification showed that the microvilli of the jejunal epithelium of ETEC K88–challenged piglets were severely shed but that the KR-32 treatment relieved the disruption of the jejunum with sparsely and irregularly arranged microvilli. The intestinal microvilli in the K88 group appeared to be sparse and different in length at a scale of 2 µm, and the K88 + APK group showed neater intestinal microvilli compared with the K88 group (Fig. 6C).
Figure 6.
Antimicrobial peptide KR-32 improved intestinal morphology. Jejunum sections were used to analyze intestinal morphology. (A) Jejunum tissue stained with hematoxylin and eosin (bars = 100 µm). (B) Scanning electron microscopy images (upper, 150×; lower, 30,000×) of jejunum tissue. (C) Transmission electron microscopy images (bars = 2 µm) of jejunum tissue. CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu enterotoxigenic Escherichia coli (ETEC) K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32 in Exp. 2. APK = antimicrobial peptide KR-32.
Table 5.
Villous height, crypt depth, villous height: crypt depth ratio, histologic score and diarrhea rate [mean (SD)] of the intestine in Exp. 2
| Item | Treatment group1 | ||
|---|---|---|---|
| CON-2 | K88 | K88 + APK | |
| Duodenum | |||
| Villous height, µm | 496 (22)a | 277 (18)b | 416 (30)c |
| Crypt depth, µm | 231 (25) | 286 (8) | 293 (26) |
| Villous height:crypt depth ratio | 2.31 (0.32)a | 0.97 (0.06)b | 1.44 (0.10)b |
| Jejunum | |||
| Villous height, µm | 461 (37)a | 289 (12)b | 427 (23)a |
| Crypt depth, µm | 204 (15)a | 280 (6)b | 210 (18)a |
| Villous height:crypt depth ratio | 2.30 (0.22)a | 1.03 (0.04)b | 2.03 (0.11)a |
| Ileum | |||
| Villous height, µm | 378 (31)a | 265 (15)b | 353 (30)a |
| Crypt depth, µm | 172 (16)a | 269 (9)b | 237 (23)b |
| Villous height: crypt depth ratio | 2.30 (0.27)a | 0.99 (0.09)b | 1.56 (0.19)b |
| Histologic score | 0.22 (0.32)a | 3.50 (0.65)b | 0.97 (0.21)c |
| Diarrhea rate (day 4 to day 6), % | 0 (0)a | 88.9 (17.2)b | 66.6 (29.9)b |
a–cMeans within a row with different superscripts differ (P < 0.05).
1CON-2 = piglets with an oral administration of fresh Luria–Bertani broth (50 mL) followed by an intraperitoneal (i.p.) injection of an equivalent volume of PBS; K88 = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of an equivalent volume (1 mL) of PBS; K88 + APK = piglets orally challenged with 1 × 1010 cfu ETEC K88 on day 1 followed by an i.p. injection of 0.6 mg/kg KR-32. n = 6 for the CON-2 group, n = 5 for the K88 group, and n = 5 for the K88 + APK group.
DISCUSSION
Although antimicrobial peptides were always considered for their bactericidal function, we also wanted to know whether antimicrobial peptides had an effect on nutrient absorption. In the present study, we explored the effect of KR-32 on fat absorption in pigs. KR-32 had no effect on growth performance and ATTD of general healthy pigs, but successfully ameliorated severe nutrient malabsorption caused by ETEC K88 in pigs, maybe through promoting the repair of the intestinal barrier.
According to the results above, we confirmed that ETEC K88 challenge leads to poor growth performance; low ATTD, especially for EE and CP; and fat malabsorption among weaning pigs. Some of these findings are in agreement with previous data showing decreased growth and digestibility in piglets infected with ETEC K88 (Bosi et al., 2004; Che et al., 2017). According to the results of serum fatty acid concentrations changing with time, the peak of fatty acid concentration in each group appeared basically at the same time, about 2 to 3 h after given olive oil. We confirmed that the fatty acid absorption was inhibited rather than delayed in ETEC K88–challenged pigs.
We focused our attention on the progress of fat digestion and fatty acid uptake (Holtzapple et al., 1975) to analyze the reasons for lipid malabsorption caused by ETEC K88. The pH in small intestine contents and lipase secreted by the pancreas (Hartwell, 1938) reflected fat digestibility but shows no remarkable change after ETEC K88 infection, whereas the levels of FATP4 and iFABP, 2 main fatty acid transporters (Nickerson et al., 2009; Venkatachalam et al., 2013), in the K88 group were significantly decreased compared with those in control, which suggested that ETEC K88 mainly inhibited fatty acid uptake, which led to malabsorption of fats.
There are a lot of studies confirming that nontropic steatorrhea, a kind of fatty diarrhea, is surely related to the intestinal integrity (Schulzke et al., 1998; Montalto et al., 2002; Schumann et al., 2017), and a recent research showed that the loss of claudin-2 and claudin-15 of tight junctions leads to death from serious malnutrition (Wada et al., 2013). We are also aware of epithelial barrier damage after ETEC K88 challenge. Intestinal epithelia of the jejunum showed serious injury, with blunt and short villi, in ETEC K88–challenged pigs, and a reduced ratio of villus height to crypt depth in the K88 group indicated nutrient malabsorption, for ratio of villus height to crypt depth was positive to absorbency to nutrition in intestines (Palander et al., 2013). Scanning electron microscopy and transmission electron microscopy results further showed disordered villi, sparse microvilli, and blurry boundaries between tight junctions, which indicated epithelial barrier damage. It was suggested that ETEC K88 might inhibit fatty acid absorption through destroying intestine integrity. Antimicrobial peptides and host defense peptides could repair the damaged epithelial barrier through sterilization (Jung et al., 2008) or immunity regulation (Niyonsaba et al., 2002; Afacan et al., 2012). KR-32 could relieve epithelial barrier damage caused by ETEC K88, which reduced piglet diarrhea rate and fecal E.coli shedding. What was more, pigs in the K88 + APK treatment group showed improved growth performance and ATTD of EE compared with pigs in the K88 treatment group. Next, we found ETEC K88–challenged pigs absorbed more fatty acids after i.p. injection of KR-32 than pigs did in the CON-2 group, which may be caused by the improved expression of FATP4 through KR-32. KR-32 could effectively restore intestinal integrity in ETEC K88–challenged pigs, which were manifested in decreased level of intestinal damage, increased villus height: crypt depth, improved microvilli form and clear tight junction. It was suggested that KR-32 could relieve fat malabsorption through improving FATP4 expression during the recovery of intestinal integrity.
In conclusion, KR-32 could improve FATP4 expression through epithelial barrier recovery to increase fatty acid uptake and improve growth performance and ATTD of EE in ETEC K88–challenged pigs. In order to understand the molecular mechanism of the relationship between KR-32 and fatty acid transporters in the epithelial barrier, tight junctions will be examined in further research.
Footnotes
This research was supported by the earmarked fund for Key Program of the National Natural Science Foundation of China (3163000269), Modern Agro-industry Technology Research System (CARS-36), and Key Agriculture Program of Zhejiang Major Science and Technology Projects (2015C02022).
LITERATURE CITED
- Afacan N. J., A. T. Yeung O. M. Pena, and Hancock R. E.. 2012. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 18:807–819. doi:10.2174/138161212799277617 [DOI] [PubMed] [Google Scholar]
- AOAC International 2000. Official methods of analysis. 17th ed AOAC Int., Gaithersburg, MD. [Google Scholar]
- Borgstrom B. 1968. Quantitative aspects of intestinal absorption and metabolism of cholesterol and beta-sitosterol in rat. J. Lipid Res. 9:473–481. [PubMed] [Google Scholar]
- Bosi P., L. Casini A. Finamore C. Cremokolini G. Merialdi P. Trevisi F. Nobili, and Mengheri E.. 2004. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82:1764–1772. doi: 10.2527/2004.8261764x [DOI] [PubMed] [Google Scholar]
- Brestensky M., S. Nitrayová J. Heger, and Patráš P.. 2017. Chromic oxide and acid-insoluble ash as markers in digestibility studies with growing pigs and sows. J. Anim. Physiol. Anim. Nutr. (Berl.) 101:46–52. doi: 10.1111/jpn.12503 [DOI] [PubMed] [Google Scholar]
- Buttet M., V. Traynard T. T. Tran P. Besnard H. Poirier, and Niot I.. 2014. From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins. Biochimie 96:37–47. doi: 10.1016/j.biochi.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Che L., Xu Q., Wu C., Luo Y., Huang X., Zhang B., Auclair E., Kiros T., Fang Z., Lin Y., Xu S., Feng B., Li J., and Wu. 2017. Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 118:949–958. doi:10.1017/S0007114517003051 [DOI] [PubMed] [Google Scholar]
- Chen S., Z. Lu F. Wang, and Wang Y.. 2018. Cathelicidin-WA polarizes E. coli K88-induced M1 macrophage to M2-like macrophage in RAW264.7 cells. Int. Immunopharmacol. 54:52–59. doi: 10.1016/j.intimp.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Chi O. Z., Mellender S. J., G. K. Kiss, X. Liu, and H. R. Weiss.. 2017. Blood-brain barrier disruption was less under isoflurane than pentobarbital anesthesia via a PI3K/Akt pathway in early cerebral ischemia. Brain Res. Bull. 131:1–6. doi:10.1016/j.brainresbull.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Eisenhut M. 2006. Changes in ion transport in inflammatory disease. J. Inflammation 3:5. doi:10.1186/1476-9255-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother J. M., E. Nadeau, and Gyles C. L.. 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi:10.1079/ahr2005105 [DOI] [PubMed] [Google Scholar]
- Fijlstra M., W. J. Tissing F. Stellaard H. J. Verkade, and Rings E. H.. 2013. Reduced absorption of long-chain fatty acids during methotrexate-induced gastrointestinal mucositis in the rat. Clin. Nutr. 32:452–459. doi: 10.1016/j.clnu.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Gray G. M. 1970. Carbohydrate digestion and absorption. Gastroenterology 58:96–107. doi:10.1136/jcp.s1-2.1.24 [PubMed] [Google Scholar]
- Guignot J., C. Chaplais M. H. Coconnier-Polter, and Servin A. L.. 2007. The secreted autotransporter toxin, sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 9:204–221. doi: 10.1111/j.1462-5822.2006.00782.x [DOI] [PubMed] [Google Scholar]
- Hartwell G. A. 1938. A note on the digestion of fats by pancreatic lipase. Biochem. J. 32:462–466. doi:10.1136/jcp.s1-2.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzapple P., Koldovsky O., and Smith G.. . 1975. Absorption and digestion of neutral lipids in suckling rats. J. Am. Oil Chem. Soc. 52:A126–A126. [Google Scholar]
- Javadpour M. M., M. M. Juban W. C. Lo S. M. Bishop J. B. Alberty S. M. Cowell C. L. Becker, and McLaughlin M. L.. 1996. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 39:3107–3113. doi: 10.1021/jm9509410 [DOI] [PubMed] [Google Scholar]
- Jonas A., S. Avigad A. Diver-Haber, and Katznelson D.. 1979. Disturbed fat absorption following infectious gastroenteritis in children. J. Pediatr. 95:366–372. [DOI] [PubMed] [Google Scholar]
- Jung W. J., F. Mabood A. Souleimanov X. Zhou S. Jaoua F. Kamoun, and Smith D. L.. 2008. Stability and antibacterial activity of bacteriocins produced by Bacillus thuringiensis and Bacillus thuringiensis ssp. kurstaki. J. Microbiol. Biotechnol. 18:1836–1840. doi:10.4014/jmb.0800.120 [DOI] [PubMed] [Google Scholar]
- Kimbrell D. A. 1991. Insect antibacterial proteins: not just for insects and against bacteria. Bioessays 13:657–663. doi: 10.1002/bies.950131207 [DOI] [PubMed] [Google Scholar]
- Liu Y. F., Luan C., Xia X., An S., and Wang Y. Z.. . 2011. Antibacterial activity, cytotoxicity and mechanisms of action of cathelicidin peptides against enteric pathogens in weaning piglets. Int. J. Pept. Res. Ther. 17:175–184. doi:10.1007/s10989-011-9255-y [Google Scholar]
- Montalto M., L. Cuoco R. Ricci N. Maggiano F. M. Vecchio, and Gasbarrini G.. 2002. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion 65:227–233. doi: 10.1159/000063817 [DOI] [PubMed] [Google Scholar]
- Nauli A. M., F. Nassir S. Zheng Q. Yang C. M. Lo S. B. Vonlehmden D. Lee R. J. Jandacek N. A. Abumrad, and Tso P.. 2006. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131:1197–1207. doi: 10.1053/j.gastro.2006.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer A. D. 1973. Digestion and absorption of protein. Mayo Clin. Proc. 48:624–629. doi:10.1146/annurev.me.29.020178.000531 [PubMed] [Google Scholar]
- Nickerson J. G., Alkhateeb H., Benton C. R., Lally J., Nickerson J., Han X., Wilson M. H., Jain S. S., Snook L. A., Glatz J. F. C., A. Chabowski, J. J. F. P. Luiken, and A. Bonen. 2009. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J. Biol. Chem. 284:16522–16530. doi:10.1074/jbc.M109.004788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A., J. Pistolic A. Wyatt S. Tam, and Hancock R. E.. 2009. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J. Immunol. 183:5788–5798. doi: 10.4049/jimmunol.0901491 [DOI] [PubMed] [Google Scholar]
- Niyonsaba F., K. Iwabuchi A. Someya M. Hirata H. Matsuda H. Ogawa, and Nagaoka I.. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20–26. doi:10.1046/j.1365-2567.2002.01398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Palander P. A., Heinonen M., I. Simpura, S. A. Edwards, and A. E. Valros. 2013. Jejunal morphology and blood metabolites in tail biting, victim and control pigs. Animal 7:1523–1531. doi:10.1017/s1751731113000669 [DOI] [PubMed] [Google Scholar]
- Pan L., P. F. Zhao X. K. Ma Q. H. Shang Y. T. Xu S. F. Long Y. Wu F. M. Yuan, and Piao X. S.. 2017. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 95:2627–2639. doi: 10.2527/jas.2016.1243 [DOI] [PubMed] [Google Scholar]
- Qian L., X. Yue L. Hu Y. Ma, and Han X.. 2016. Changes in diarrhea, nutrients apparent digestibility, digestive enzyme activities of weaned piglets in response to chitosan-zinc chelate. Anim. Sci. J. 87:564–569. doi: 10.1111/asj.12460 [DOI] [PubMed] [Google Scholar]
- Ribon M., S. Seninet J. Mussard M. Sebbag C. Clavel G. Serre M. C. Boissier L. Semerano, and Decker P.. 2019. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1Q and LL-37. J. Autoimmun. 98:122–131. doi: 10.1016/j.jaut.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Rogers R., J. Eastham-Anderson J. DeVoss J. Lesch D. Yan M. Xu M. Solon K. Hotzel L. Diehl, and Webster J. D.. 2016. Image analysis-based approaches for scoring mouse models of colitis. Vet. Pathol. 53:200–210. doi: 10.1177/0300985815579998 [DOI] [PubMed] [Google Scholar]
- Roselli M., A. Finamore M. S. Britti S. R. Konstantinov H. Smidt W. M. de Vos, and Mengheri E.. 2007. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 137:2709–2716. doi: 10.1093/jn/137.12.2709 [DOI] [PubMed] [Google Scholar]
- Rossi R., Pastorelli G., Cannata S., and Corino C.. . 2010. Recent advances in the use of fatty acids as supplements in pig diets: a review. Anim. Feed Sci. Technol. 162:1–11. doi:10.1016/j.anifeedsci.2010.08.013 [Google Scholar]
- Schulzke J. D., C. J. Bentzel I. Schulzke E. O. Riecken, and Fromm M.. 1998. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr. Res. 43(4 Pt 1):435–441. doi: 10.1203/00006450-199804000-00001 [DOI] [PubMed] [Google Scholar]
- Schumann M., B. Siegmund J. D. Schulzke, and Fromm M.. 2017. Celiac disease: role of the epithelial barrier. Cell. Mol. Gastroenterol. Hepatol. 3:150–162. doi: 10.1016/j.jcmgh.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A., Hirsch D. J., Gimeno R. E., Punreddy S., Ge P., Watson N., Patel S., Kotler M., Raimondi A., Tartaglia L. A., and Lodish H. F.. . 1999. Identification of the major intestinal fatty acid transport protein. Mol. Cell 4:299–308. doi:10.1016/S1097-2765(00)80332-9 [DOI] [PubMed] [Google Scholar]
- Su W., Y. Chen C. Wang X. Ding G. Rwibasira, and Kong Y.. 2016. Human cathelicidin LL-37 inhibits platelet aggregation and thrombosis via Src/PI3K/Akt signaling. Biochem. Biophys. Res. Commun. 473:283–289. doi: 10.1016/j.bbrc.2016.03.095 [DOI] [PubMed] [Google Scholar]
- Suzuki K., T. Murakami Z. Hu H. Tamura K. Kuwahara-Arai T. Iba, and Nagaoka I.. 2016. Human host defense cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake by liver sinusoidal endothelial cells without cell activation. J. Immunol. 196:1338–1347. doi: 10.4049/jimmunol.1403203 [DOI] [PubMed] [Google Scholar]
- Venkatachalam A. B., D. L. Sawler, and Wright J. M.. 2013. Tissue-specific transcriptional modulation of fatty acid-binding protein genes, fabp2, fabp3 and fabp6, by fatty acids and the peroxisome proliferator, clofibrate, in zebrafish (Danio rerio). Gene 520:14–21. doi: 10.1016/j.gene.2013.02.034 [DOI] [PubMed] [Google Scholar]
- Wada M., A. Tamura N. Takahashi, and Tsukita S.. 2013. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 144:369–380. doi: 10.1053/j.gastro.2012.10.035 [DOI] [PubMed] [Google Scholar]
- Wang Y., J. Hong X. Liu H. Yang R. Liu J. Wu A. Wang D. Lin, and Lai R.. 2008. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS ONE 3:e3217. doi: 10.1371/journal.pone.0003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., C. H. Kim R. Liu M. Kucia W. Marlicz N. Greco J. Ratajczak M. J. Laughlin, and Ratajczak M. Z.. 2012. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia 26:736–745. doi: 10.1038/leu.2011.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., L. Zhang, and Wang Y.. 2015. The antimicrobial peptide cathelicidin-BF could be a potential therapeutic for Salmonella typhimurium infection. Microbiol. Res. 171:45–51. doi: 10.1016/j.micres.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Xing T., R. Camacho Salazar, and Chen Y. H.. 2017. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers 5:e1356901. doi: 10.1080/21688370.2017.1356901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. M., Z. Y. Jiang C. T. Zheng L. Wang, and Yang X. F.. 2014. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 92:1496–1503. doi: 10.2527/jas.2013-6619 [DOI] [PubMed] [Google Scholar]
- Yi H., W. Hu S. Chen Z. Lu, and Wang Y.. 2017. Cathelicidin-WA improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic Escherichia coli O157:H7 infection. J. Immunol. 198:1696–1705. doi: 10.4049/jimmunol.1601221 [DOI] [PubMed] [Google Scholar]
- Yi H., L. Zhang Z. Gan H. Xiong C. Yu H. Du, and Wang Y.. 2016. High therapeutic efficacy of cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6:25679. doi: 10.1038/srep25679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., X. Xia F. Han Q. Jiang Y. Rong D. Song, and Wang Y.. 2015. Cathelicidin-BF, a novel antimicrobial peptide from Bungarus fasciatus, attenuates disease in a dextran sulfate sodium model of colitis. Mol. Pharm. 12:1648–1661. doi: 10.1021/acs.molpharmaceut.5b00069 [DOI] [PubMed] [Google Scholar]
- Zong X., W. Hu D. Song Z. Li H. Du Z. Lu, and Wang Y.. 2016. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 104:74–82. doi: 10.1016/j.bcp.2016.01.009 [DOI] [PubMed] [Google Scholar]