Abstract
In vitro embryo production (IVP) in cattle has gained worldwide interest in recent years, but the efficiency of using IVP embryos for calf production is far from optimal. This review will examine the pregnancy retention rates of IVP embryos and explore causes for pregnancy failures. Based on work completed over the past 25 yr, only 27% of cattle receiving IVP embryos will produce a live calf. Approximately 60% of these pregnancies fail during the first 6 wk of gestation. When compared with embryos generated by superovulation, pregnancy rates are 10% to 40% lower for cattle carrying IVP embryos, exemplifying that IVP embryos are consistently less competent than in vivo-generated embryos. Several abnormalities have been observed in the morphology of IVP conceptuses. After transfer, IVP embryos are less likely to undergo conceptus elongation, have reduced embryonic disk diameter, and have compromised yolk sac development. Marginal binucleate cell development, cotyledon development, and placental vascularization have also been documented, and these abnormalities are associated with altered fetal growth trajectories. Additionally, in vitro culture conditions increase the risk of large offspring syndrome. Further work is needed to decipher how the embryo culture environment alters post-transfer embryo development and survival. The risk of these neonatal disorders has been reduced by the use of serum-free synthetic oviductal fluid media formations and culture in low oxygen tension. However, alterations are still evident in IVP oocyte and embryo transcript abundances, timing of embryonic cleavage events and blastulation, incidence of aneuploidy, and embryonic methylation status. The inclusion of oviductal and uterine-derived embryokines in culture media is being examined as one way to improve the competency of IVP embryos. To conclude, the evidence presented herein clearly shows that bovine IVP systems still must be refined to make it an economical technology in cattle production systems. However, the current shortcomings do not negate its current value for certain embryo production needs and for investigating early embryonic development in cattle.
Keywords: cattle, conceptus, embryo, in vitro production, pregnancy failure
INTRODUCTION
In vitro production (IVP) of bovine embryos has gained worldwide interest as an assisted reproductive technology (ART) to improve genetic gains in beef and dairy cattle. Combining IVP with ovum pick-up (OPU) from genetically superior females to produce high genetic merit embryos is becoming a popular alternative to artificial insemination (AI) and multiple ovulation and embryo transfer (MOET) in some circumstances (van Wagtendonk-de Leeuw et al., 1998). Also, IVP embryos are useful for impregnating “problem breeders”; cows that may not ovulate or be capable of permitting fertilization (Looney et al., 1994; Block et al., 2010). Additionally, IVP embryos may be used to improve fertility during periods of heat stress, as initial cleavage stage embryos are especially sensitive to environmental stressors (Ealy et al., 1993; Block et al., 2010; Stewart et al., 2011). In 2016, approximately 1 million IVP embryo transfers were completed worldwide, with 400,000 IVP embryos transferred to cattle in North America, 350,000 in South America, and 150,000 in Europe (IETS Data Retrieval Committee Report, December 2017). Thus, IVP has already had an impact on worldwide cattle production, and all indications are that this trend will continue.
In vitro-produced bovine embryos also have gained popularity because they serve as a useful model for studying embryology in cattle. The availability and affordability of slaughterhouse materials provides ample oocyte and embryo numbers for research endeavors involving oocyte maturation, fertilization, and early embryo development. The bovine embryo also has been an excellent model for studying infertility and subfertility in humans. There are many commonalities in technologies used to produce embryos in vitro between cattle and humans. In fact, much of the initial human-based ART work was based on work completed in cattle (Sirard, 2018).
Unfortunately, pregnancy success in cattle after embryo transfer (ET) using IVP embryos is far from ideal. Reviews on this topic from 15+ yr ago indicated that ~50% of pregnancies will be maintained to term in IVP embryo recipients (Farin et al., 2001; Rizos et al., 2002). This percentage has not improved in the past 15 to 20 yr. In fact, pregnancy retention for IVP embryos appears to be worse than originally thought.
This review will provide an update on recent pregnancy retention rates in beef and dairy cattle receiving IVP embryos and describe the predominant developmental abnormalities that exist in IVP embryos after ET. We then will finish this review by providing a brief summary of possible reasons why IVP embryos may be inferior to in vivo-generated embryos at producing viable, healthy offspring.
SEVERITY OF IVP EMBRYO INCOMPETENCY
The studies outlined in Table 1 represent publications from the past 25 yr that investigated pregnancy status after IVP embryo transfer to dairy or beef cattle on at least 2 occasions. Only data from freshly prepared IVP embryos were included to avoid confounding influences that embryo cryopreservation may have on pregnancy outcomes (Agca et al., 1998; Al-Katanani et al., 2002; Block et al., 2010; Stewart et al., 2011). A majority of the studies listed in Table 1 documented pregnancy outcomes from ET studies completed during winter months; however, 4 studies listed in this table were completed in both summer and winter months (Loureiro et al., 2009; Block et al., 2010; Rasmussen et al., 2013; Bonilla et al., 2014). These studies reported no difference in pregnancy outcome, indicating that ET may help overcome environmental stressors caused by seasonality.
Table 1.
Early and late embryonic losses observed after transfer of in vitro-produced bovine embryos to beef and dairy cattle1
| % Pregnant day 18/21 |
% Pregnant day 30/402 |
% Pregnant day 45/902 |
% Calving | Reference |
|---|---|---|---|---|
| 58.0 | 50.0 | 44.0 | 40.5 | Reichenbach et al. (1992) 3 |
| 61.0 | 42.0 | 42.0 | – | McMillan et al. (1997) 4 |
| – | – | 17.7 | 15.2 | Block et al. (2003) 3,5 |
| 64.3 | 32.5 | 24.4 | 22.7 | Block and Hansen (2007) 3,6 |
| – | 37.2 | – | 30.2 | Loureiro et al. (2009) 7 |
| – | 37.4 | 33.5 | – | Pontes et al. (2009) 8 |
| – | 56.3 | 52.6 | – | Block et al. (2010) 7 |
| – | 45.5 | 39.4 | 33.9 | Stewart et al. (2011) 5 |
| – | 24.1 | 20.6 | 17.1 | Rasmussen et al. (2013) 9 |
| – | 29.8 | – | 20.2 | Rasmussen et al. (2013) 10 |
| – | 54.9 | 44.1 | 36.7 | Bonilla et al. (2014) 7 |
| – | 36.4 | 29.2 | 27.2 | Denicol et al. (2014) 5 |
| 58.0 to 64.3 | 24.1 to 56.3 | 17.7 to 52.6 | 15.2 to 40.5 | Range |
| 61.1 | 40.6 | 34.8 | 27.1 | Average |
1Overall means were calculated for pregnancy rates in studies where different embryo or recipient treatments were examined.
2Pregnancy status was completed between day 30 and 40 or between day 45 and 90 in these studies.
3Circulating progesterone concentrations was used to predict pregnancy status at day 21.
4Pregnancy status at day 18 was determined by examining reproductive tracts during necropsy.
5Data represent embryo transfers completed in the summer months in Florida or Texas.
6Data represent embryo transfers completed in the winter months in dairy farms in Florida.
7Data represent work completed both in the summer and winter months in Florida.
8Studies completed on Bos indicus cattle (Nelore).
9Replicate of the study completed in 2 dairies in Colorado in summer and winter months.
10Replicate of the study completed in 3 dairies in Florida in summer and winter months.
As seen in Table 1, the occurrence of pregnancy losses for IVP embryos is alarmingly high. Pregnancies failing to reach term ranged from 59% to 85% in these studies. By contrast, failed pregnancies following AI are estimated at 45% to 65% in lactating dairy cows and 10% to 30% in beef cows (Lucy, 2001; Santos et al., 2004; Perry et al., 2005; Pohler et al., 2015; Wiltbank et al., 2016; Gatea et al., 2018). Further evidence for the increased magnitude of pregnancy losses after IVP embryo transfer is available in studies that compared IVP pregnancies with pregnancies using in vivo-produced embryos generated by superovulation (Table 2). Pregnancy rates were assessed at various times in gestation, but in each study pregnancy rates were lower in IVP-derived pregnancies than in vivo-derived pregnancies (range: 10% to 40% reduction). When averaging outcomes from these studies, pregnancy rates were ~25% lower in IVP embryos than for in vivo-generated embryos.
Table 2.
Difference in pregnancy retention after transfer of in vitro-produced or in vivo-produced bovine embryos1
| Day of pregnancy detection | % Pregnant IVP embryos | % Pregnant in vivo-produced embryos | Reference |
|---|---|---|---|
| 53 | 37.0 | 79.0 | Farin and Farin (1995) |
| 60/70 | 42.9 | 63.5 | Numabe et al. (2000) |
| 43/53 | 52.0 | 70.5 | Farin et al. (1999) 2 |
| 42 | 54.3 | 90.0 | Papadopoulos et al. (2002) |
| Term | 29.5 | 45.5 | McMillan (1998) |
| 60 | 33.5 | 41.5 | Pontes et al. (2009) 3 |
| 30 | 31.0 | 58.8 | Siqueira et al. (2009) |
| Range | 29.5 to 54.3 | 41.5 to 90.0 | |
| Average | 40.1 | 64.1 |
1Treatment means were calculated for pregnancy rates in studies where different embryo or recipient treatments were examined.
2Different embryo grades were examined. Only Grade 1 and 2 embryos were included in these means.
3 Bos indicus cows were used in this study. The study used the same cows for both IVP and in vivo embryo production.
Additional evidence for the incompetency of IVP embryos was observed in studies that compared pregnancy outcomes in cattle receiving either 1 or 2 embryos (McMillan, 1998). Comparing the incidence of open cattle with singleton and twin pregnancies from cattle receiving 1 or 2 embryos has been used in statistical models to assess the relative contributions of the embryo and the recipient for explaining pregnancy failures in cattle (McMillan, 1998). Both recipient and embryo-related problems caused pregnancy losses when using this experimental model in 32 independent studies (1,953 to 2,607 cows/embryo group overall). However, only one-third of the failed pregnancies were attributed to recipient-related problems. The remaining two-thirds of the pregnancy failures were caused by embryo-related problems. Also, when comparing pregnancy outcomes in IVP versus in vivo-generated embryos, embryo survival was consistently 10% to 15% less for IVP embryos than for in vivo-derived embryos in these studies (McMillan, 1998).
THE TIMING OF PREGNANCY LOSSES AFTER TRANSFER OF IVP EMBRYOS
In all the studies listed in Table 1, pregnancy losses were observed throughout gestation, but losses were most prevalent early in gestation. Approximately 40% of cows receiving an IVP embryo at day 7/8 post-estrus were no longer pregnant at day 18 to 21 of gestation, as determined by the absence of a conceptus in excised uteri or low circulating progesterone concentrations reflective of luteal regression (<1 ng/mL). By day 30 to 40 of gestation, ~60% of the recipients were not pregnant when examined via transrectal ultrasonography. Further pregnancy losses were observed thereafter, albeit at a lower rate, with ~5% of pregnancies lost at day 45 and 90 and another ~7% loss thereafter. Only 27.1% of cattle receiving IVP embryos maintained the pregnancy to term.
The timing of IVP embryo pregnancy losses is similar to pregnancy losses observed in dairy and beef cows bred by AI, although the magnitude of these losses is less severe in pregnancies from AI. Fertilization failure occurs in 10% to 20% of inseminated cows, and only 50% to 60% of the fertilized zygotes are viable and have developed to the proper stages of development at day 7 (Pohler et al., 2015; Wiltbank et al., 2016). Another period of pregnancy loss occurs between day 8 and 27 of pregnancy, where losses range from 10% to 20% in beef cattle and 20% to 40% in dairy cattle (Pohler et al., 2015; Wiltbank et al., 2016). Pregnancies surviving to this stage in development experience further losses at a rate of ~15% after day 28 (Perry et al., 2005; Wiltbank et al., 2016).
To summarize up to this point; the various studies presented in Tables 1 and 2 provide compelling evidence supporting the notion that IVP embryos are less competent at maintaining a pregnancy than embryo generated in vivo by using AI or MOET. Although IVP pregnancy failures occur throughout gestation, 80% of all the IVP pregnancy failures will occur by day 40 of gestation (Table 1). The next section will attempt to address the factors involved with the high rate of pregnancy loss in IVP embryos by exploring the differences in embryonic, fetal, and placental development in IVP embryos after ET.
POST-TRANSFER FACTORS THAT INFLUENCE IVP EMBRYO COMPETENCY
Various factors influence pregnancy success after ET in cattle, many of which are not dependent on the source of the embryos. These factors include embryo synchrony with the uterus, early and mid-luteal progesterone concentrations, and embryo grade (Farin et al., 2001; Rizos et al., 2002; Forde and Lonergan, 2017). However, there also are several conceptus, placental and fetal problems associated specifically with the IVP embryo, and all indications are that these problems make IVP-derived pregnancies prone to pregnancy failure. To follow is an overview of developmental abnormalities observed in IVP embryos.
Peri-Attachment Conceptus
Several notable alterations in the embryonic and extraembryonic tissues of the conceptus can be detected in IVP embryos. The term “conceptus” is used to define the embryo and its extraembryonic tissues that develop from the zygote. In cattle, the conceptus will form as a sphere until around day 12 to 14 of gestation, and then it begins to elongate and form a filamentous structure. Conceptuses increase in length and width rapidly thereafter for several days, and by day 17 to 19 each conceptus can span the entire length of a uterine horn (Maddox-Hyttel et al., 2003; Degrelle et al., 2005; Assis Neto et al., 2010). The incidence of elongation is reduced in IVP embryos. At day 16 to 17 of gestation, more degenerated, nonhatched embryos are recovered from recipients with IVP embryos than cows that were bred by AI (Bertolini et al., 2002a; Lonergan et al., 2007; Barnwell et al., 2015). Interestingly, IVP conceptuses were smaller than their in vivo-produced counterparts at day 13 (Lonergan et al., 2007) but were similar in length to in vivo-produced embryos at day 16 and 17 (Bertolini et al., 2002a; Barnwell et al., 2015). This suggests that the initial stages of elongation are compromised in some IVP conceptuses, but those that can undergo elongation may start slowly but eventually will “catch up” to their in vivo-derived counterparts. However, controlled studies are needed to examine multiple stages of conceptus development before such an assertion can be definitely made.
Errors in conceptus elongation in IVP embryos are likely caused by abnormalities in the trophectoderm. The trophectoderm is the outermost layer of the conceptus, and its secretions and direct contact with the endometrium are essential for the maintenance of pregnancy. Recent studies suggest that differences exist in the secretory potential between IVP and in vivo-generated conceptuses. This idea has been best described recently by examining how conceptuses interact with the maternal endometrium. In one recent study, 83 differentially expressed genes were identified in bovine endometrial explants that were cocultured with IVP versus in vivo-generated conceptuses (Mathew et al., 2019). Another study identified 118 differentially expressed genes in the caruncular regions of the endometrium at day 20 of gestation between IVP and in vivo-generated pregnancies (Mansouri-Attia et al., 2009). One conceptus-secreted molecule that may be involved with some of this differential endometrial gene expression is interferon-tau (IFNT), the maternal recognition of pregnancy hormone in cattle (Spencer et al., 2016; Ealy and Wooldridge, 2017; Forde and Lonergan, 2017). Reports are equivocal about whether IFNT mRNA and protein abundance differ between IVP and in vivo-derived blastocysts and elongated conceptuses (Stojkovic et al., 1995; Kubisch et al., 2001; Bertolini et al., 2002a; Arnold et al., 2006). Therefore, the relative importance of IFNT in controlling IVP versus in vivo conceptus changes in endometrial gene expression remains unclear. However, one of the aforementioned studies included an IFNT treatment to endometrial cultures to account for gene expression controlled by IFNT (Mathew et al., 2019). They showed that a majority of the differentially expressed genes identified in that study were not caused by differences in IFNT concentration. Other conceptus-secreted molecules, such as prostaglandins and cortisol, may be involved with the differential expression of endometrial genes between IVP and in vivo-generated conceptuses (Forde et al., 2015), although more work is needed to clarify whether these and other conceptus secretions are altered in IVP conceptuses.
Another component of the peri-attachment conceptus that is altered in IVP embryos is the embryonic disk. This structure develops from the inner cell mass (ICM). It contains epiblast cells, which are the multipotent cells that develop into embryonic mesoderm, endoderm, and ectoderm germ cell lineages (Maddox-Hyttel et al., 2003; Alexopoulos et al., 2008). Reductions in embryonic disk diameter are reported in IVP conceptuses (Bertolini et al., 2002a). This reduction in embryonic disk diameter was more pronounced in female conceptuses than male conceptuses (5-fold vs. 2-fold reduction in diameter, respectively). The reason(s) for this sex-bias is unknown. Also, IVP conceptuses lacking detectable embryonic disks have been reported by several laboratories (Bertolini et al., 2002b; Fischer-Brown et al., 2002, 2004; Block et al., 2007; Loureiro et al., 2011). From 25% to 65% of IVP conceptuses were devoid of an embryonic disk when assessing day 15 to 17 conceptuses by stereomicroscopy. It was not clear whether embryonic disks were too small to detect or if they were indeed absent. However, the lack of detectable embryonic disks compromises pregnancy retention, as pregnancy rates were severely diminished when IVP conceptuses lacking detectable embryonic disks were transferred back into cattle (Fischer-Brown et al., 2004). Therefore, it is likely that poor embryonic disk development is one reason for early pregnancy failures of IVP embryos.
Yolk Sac Development and Function
Yolk sac development and function may be compromised in IVP embryos. The yolk sac develops from the primitive endoderm, which differentiates from a subset of ICM cells at day 8 to 9 of development in cattle by signaling events that rely on fibroblast growth factor (FGF) sensitivity (Yamanaka et al., 2006; Yang et al., 2011; Kuijk et al., 2012). The primitive endoderm cells migrate to the base of the ICM to form the hypoblast, which then proliferates inside the trophectoderm layer to surround the blastocoel cavity. This newly formed yolk sac is indispensable in early pregnancy in mammals because of its role in histotroph digestion during early pregnancy (Shalaby et al., 1995; Arman et al., 1998). Placentomes are not well established in bovine pregnancies until ~ day 40 of pregnancy. Prior to this time the conceptus must rely on histotroph for nourishment, and the yolk sac provides digested histotroph and other nutrients, vitamins, ions, and gases to support embryogenesis (Greenstein et al., 1958; Assis Neto et al., 2010). It also produces several bioactive molecules that facilitate early pregnancy (e.g., transferrin, retinol binding proteins, apolipoproteins, angiogenic factors; Shi et al., 1985; Jollie, 1990; Freyer and Renfree, 2009).
Limited work has been completed on the yolk sac given the difficulty in studying this extraembryonic tissue early in pregnancy and the lack of suitable bovine extraembryonic endoderm cell lines. However, there is some evidence suggesting that yolk sac abnormalities are an underlying cause for at least some pregnancy failures in IVP embryos. Differences in yolk sac structure and gene expression exist between in vivo-generated, IVP and somatic cell nuclear transfer (SCNT) pregnancies at day 20 to 30 of pregnancy. Many SCNT pregnancies contain severe yolk sac deformations (e.g., small, misshapen, necrotic), whereas yolk sac abnormalities in IVP pregnancies are less severe at the macroscopic level. However, microscopic evaluations identified deficiencies in villous formation and vascular development in IVP pregnancies (Alberto et al., 2013; Mess et al., 2017). Also, changes in transcript abundance of vascular endothelial growth factor (VEGF), an important angiogenic factor, and its primary receptor, VEGFR2, were detected in the yolk sac of IVP conceptuses (Mess et al., 2017).
Chorio-Allantois Development and Function
Various alterations in placentome development and morphology have been noted in IVP pregnancies. The initial trophectoderm adhesion/attachment phases of implantation can be detected at day 16 to 18 of gestation in cattle (Assis Neto et al., 2010). The villous indentations that initiate cotyledon development can be detected around day 32 of gestation, and within the next few weeks numerous placentomes can be detected throughout the placenta (Assis Neto et al., 2010). Several notable changes in placentome development have been observed in IVP pregnancies. Placentomes from IVP embryos are lower in number, smaller in thickness, and larger in size than placentomes from in vivo-generated pregnancies (Farin and Farin, 1995; Bertolini et al., 2002b; Miles et al., 2005). Also, stage-dependent changes in placental development exist in IVP pregnancies. At day 70 of gestation, placentae from IVP embryos have lower fluid volume, reduced blood vessel density, and are less efficient (as determined by comparing fetal weight to placental weight) than placentae from in vivo-generated embryos (Miles et al., 2005). However, at day 180, IVP pregnancies contain heavier placentae that have a greater placental surface area, indicating a potential catch-up mechanism in these pregnancies (Bertolini et al., 2004). At day 222, IVP placentae are still heavier and contain greater fluid volumes than their in vivo-derived counterparts, but overall placentome surface area is reduced when compared with pregnancies from in vivo-generated embryos (Miles et al., 2004). This reduction in surface area is accompanied by an increase in blood vessel density within placentomes. Again, this likely is a compensatory mechanism of IVP pregnancies.
Another notable difference in placentation between IVP and in vivo-derived embryos is in the development and activity of binucleate trophoblast cells. The binucleate cell (also referred to as the trophoblast giant cell) is initially detected at day 16 to 18 of gestation (Wooding, 1982). From day 25 onward binucleate cells will represent approximately 20% of trophectoderm cells within the chorion (Wooding, 1982; Spencer et al., 2008). This cell type differentiates from mononuclear trophectoderm cells and fuses with the endometrial epithelium to form syncytial plaques at the fetal and maternal borders of the placenta. Several hormones and placental factors are produced by binucleate cells, including placental lactogen (PL), prolactin-like proteins, progesterone, and pregnancy-associated glycoproteins (PAGs) (Spencer et al., 2008). Placentae from IVP pregnancies contain reduced binucleate cell numbers (Miles et al., 2004) and reduced PL and PAG concentrations in the maternal circulation (Bertolini et al., 2006). Thus, trophectoderm from IVP embryos appears to be functionally inferior to in vivo-produced embryos.
The mechanisms responsible for these changes in placental development are not well understood. However, not surprisingly, gene imprinting abnormalities are associated with these alterations. Transcript abundance for the paternally imprinted gene, insulin-like growth factor 2 (IGF2), is greater in chorio-allantoic membranes for IVP pregnancies than in vivo-derived placentae at day 33 to 36 of gestation (Perecin et al., 2009). This alteration in IGF2 abundance may be one mechanism responsible for the abnormal placentation observed in IVP pregnancies as IGF2 is associated with the control of embryonic growth.
Allantois development and function may also be compromised in IVP embryos. The allantoic sac is an extraembryonic mesoderm membrane that develops from the embryonic hind gut at day 21 to 25 of gestation. By day 30, vascular networks form within the allantoic mesoderm (Assis Neto et al., 2009, 2010). This vascular network makes the allantois the primary vascular organ of the placenta. The involvement of the allantois in pregnancy losses has not been studied extensively, but the timing of allantoic sac development coincides with a time of pronounced embryonic losses in cattle (Wiltbank et al., 2016). Also, there is one report indicating that ~25% of all IVP pregnancies examined at day 22 to 34 of gestation contain allantoic sacs with reduced development or reduced vascularization (Thompson and Peterson, 2000).
Fetus Development
Several studies have observed reductions in embryo or fetal size at day 37 to 56 of gestation in IVP pregnancies (Bertolini et al., 2002b; Vailes et al., 2019). However, this small IVP fetus phenotype is only temporary. On and after day 180 of gestation, IVP fetuses are larger than their in vivo-derived counterparts (Bertolini et al., 2002b, 2004; Miles et al., 2004). One possible cause for the altered trajectory of fetal growth is that it mirrors the previously described alterations in placental development. Development of both the fetus and placenta appears to be lagging early in gestation, but then can exceed the development of in vivo-generated pregnancies in mid- and late gestation.
Calving Outcomes and Performance
The changes in growth trajectory for the developing IVP fetus described in the above paragraph is a common, core concept that is closely associated with the fetal origins of adult disease (also known as, Developmental origins of health and disease; Fetal programming). One of the first observations made in this field was observed in humans, where fetal underdevelopment during the first trimester due to poor nutrition was followed by compensatory fetal gains in the last 2 trimesters and cardiovascular disorders, among other disorders in adulthood (Fowden et al., 2006). It is now well-documented that alterations in the fetal developmental plan caused by various environmental insults can impact postnatal health and longevity in multiple species (Fowden et al., 2006).
One common outcome of IVP in both humans and cattle is a fetal overgrowth syndrome that produces large for gestational age neonates (Chen et al., 2015). In cattle, this phenomenon is commonly referred to as large offspring syndrome. As in humans and many other mammals, large offspring syndrome is associated with macrosomia (overgrowth), macroglossia (enlarged tongue), abdominal wall defects (e.g., umbilical hernia), and visceromegaly (enlarged visceral organs; Chen et al., 2013). It is still unclear exactly what causes this fetal overgrowth; however, several culture-associated factors are associated with large offspring syndrome. These include the presence of serum in the medium, embryo coculture with feeder cells (e.g., oviductal cells), medium formulation, and oxygen concentration during embryo culture (Yazawa et al., 1997; Farin et al., 2001; Fischer-Brown et al., 2005). These large offspring syndrome pregnancies increase the incidence of dystocia and retained placentae (Bonilla et al., 2014), which may compromise future reproductive capacity and milk yield for the dam (Zaborski et al., 2009). The incidence and severity of large offspring syndrome has been reduced, but has not been totally prevented in the past decade by removing serum from embryo culture media formations, by using media formations that replicate the oviduct environment [e.g., synthetic oviductal fluid (SOF)], and by culturing embryos in an oxygen environment similar to that in the oviduct and uterus (van Wagtendonk-de Leeuw et al., 2000; Fischer-Brown et al., 2005; Bonilla et al., 2014; Siqueira et al., 2017; Tríbulo et al., 2017).
Neonatal health is also compromised in IVP calves, this appears to occur independently of large offspring syndrome. Increases in stillbirths and calf death in the first 20 d of life have been reported in IVP calves (Bonilla et al., 2014). Also, there is a 4% increase in the incidence of congenital abnormalities in IVP calves (van Wagtendonk-de Leeuw et al., 1998). The developmental abnormalities that lead to these neonatal and postnatal problems are unclear. However, alterations in epigenomic profiles likely cause some if not most of these problems. Methylation status, both globally and at specific imprinted regions, is altered in various organs of some IVP fetuses (Suzuki et al., 2009; Hori et al., 2010; Chen et al., 2015). After the neonatal period, IVP-generated calves appear physiological normal to their AI-generated counterparts, at least when examining mortality rates, reproductive function, and lactation performance (Bonilla et al., 2014; Siqueira et al., 2017). However, IVP-generated calves that were conceived using X-sorted semen had an increased risk of mortality and reduced milk yield when compared with calves generated from AI (Siqueira et al., 2017). Thus, the benefits of using sexed semen for genetic improvements may be counterbalanced by adverse embryonic, placental, and/or fetal programming events.
HOW DOES CULTURE INFLUENCE IVP EMBRYO COMPETENCY?
Up to this point in this review we have described the severity and timing of pregnancy losses in IVP embryos and explored the various limitations in embryonic, fetal, and placental development that reduces IVP embryo competency after transfer. So, the question that is assuredly on everyone’s mind is “why are IVP embryos less competent than in vivo-produced embryos?” Unfortunately, a complete picture of issues relating to IVP embryo incompetency is not available. Thus, we are unable to provide a comprehensive story of why IVP embryos are less competent than their in vivo-generated counterparts. Several limitations exist that have hindered the discovery of IVP embryo competency-related factors. One limitation is that few studies have directly compared IVP and in vivo-derived embryos. It is costly to complete both IVP and superovulate cows to recover embryos. Also, even if direct comparisons between embryo types were made, it can be difficult to adequately control for genetic contributions that affect embryo development and gene expression. Many IVP-based studies utilize oocytes from slaughterhouse-derived materials, and the genetics of these cattle can often be different from the genetics of cattle available for superovulation. However, even considering those limitations, IVP embryos clearly deviate from in vivo-produced embryos in several aspects. The upcoming narrative will summarize the major abnormalities in IVP embryo development that have been noted.
Changes in oocyte maturation occur when oocytes are removed from the follicle and placed in culture for final maturation. A spontaneous resumption of meiosis is initiated when removing oocytes from follicles, and current IVP protocols promote the final meiotic and ooplasmic maturation by culturing cumulus–oocyte complexes for 21 to 24 h in medium containing glucose, amino acids, and gonadotropins. Selective follicle-derived hormones, such as epidermal growth factor (EGF) and fibroblast growth factor 10 (FGF10) also may be included to improve packaging of ooplasm with maternally derived transcripts (Lonergan et al., 1996; Krisher and Bavister, 1998; Zhang et al., 2010; Caixeta et al., 2013). However, these culture schemes are not identical to oocyte maturation events occurring in the peri-ovulatory follicle. At least 100 genes are differentially expressed between in vitro- and in vivo-matured bovine oocytes (Katz-Jaffe et al., 2009; Adona et al., 2016). Several of these differentially expressed genes represent previously described oocyte competency factors (e.g., aquaporin-3, follistatin, epsin, insulin-like growth factor-binding protein 3, histone deacetylase 2; Meng et al., 2008; Caixeta et al., 2009; Liu and Zheng, 2009; Sawai, 2009; Bonnet et al., 2011). Also, gene ontology profiling found that several genes involved with cellular metabolism were altered by in vitro maturation (Katz-Jaffe et al., 2009; Adona et al., 2016). Consistent with changes in gene expression, DNA methylation patterns are different after in vitro oocyte maturation than following maturation in vivo (Jiang et al., 2018). Other factors that contribute to a bovine oocyte’s competency to generate transferable embryos include methods using to induce follicle development prior to OPU and bovid subspecies (Bos taurus vs. Bos indicus). The involvement of these factors in regulating oocyte competency has been reviewed elsewhere (Baruselli et al., 2012).
In vitro embryo development is distinct from in vivo development in several regards. First, the timing of embryo cleavage and blastulation is delayed during IVP, with IVP embryos reaching the morulae and blastocyst stages 12 to 24 h later than their in vivo-produced counterparts (Holm et al., 2002; Ushijima et al., 2008). Second, blastomere numbers are reduced in IVP embryos (Iwasaki et al., 1990; Knijn et al., 2003; Balasubramanian et al., 2007). Third, differences in metabolic activity are observed between IVP and in vivo-generated embryos (de Souza et al., 2015). Lastly, IVP bovine embryos also have a greater incidence of altered chromosome numbers in their blastomeres. In one study, approximately 20% of blastomeres contained abnormal chromosome counts (Lonergan et al., 2004). Some of the observed aneuploidy and mixoploidy (i.e., mix of normal and aneuploid blastomeres) is caused by fertilization error or polyspermy (Iwasaki et al., 1992; Viuff et al., 1999; Garcia-Herreros et al., 2010). However, aneuploidy also occurs after fertilization, and the incidence of these cleavage stage-dependent errors is greater in IVP embryos than in vivo-produced embryos (Viuff et al., 1999; Lonergan et al., 2004).
Not surprisingly, differential gene expression is observed between IVP and in vivo-generated embryos. In recent studies, between 207 and 793 differentially expressed genes were detected in blastocysts in these 2 groups (Driver et al., 2012; Heras et al., 2016). Embryonic gene expression will vary based on culture media composition (Mohan et al., 2004; Corcoran et al., 2007), so this complicates interpretations. For example, including serum during IVP caused a 5-fold increase in the number of differentially expressed genes observed in IVP embryos when compared with in vivo-generated blastocysts (Heras et al., 2016). Additionally, oxygen tension during IVP can alter gene expression. For example, the abundance of transcripts for glucose transporters and stress-responsive genes are sensitive to oxygen tension, and embryo culture in an oxygen tension observed within the oviduct and uterus (5%) provides a gene expression pattern that is more similar to in vivo-produced embryos than IVP embryos cultured in an atmospheric oxygen condition (21%) (Balasubramanian et al., 2007). Lastly, IVP embryos differ in their epigenetic profiles. Changes in the methylation status of several imprinted genes (H19, IGF2, PEG3, IGFR2, SNRPN) are noted in IVP bovine embryos (Katz-Jaffe et al., 2009; Niemann et al., 2010; Heinzmann et al., 2011).
RECENT ATTEMPTS TO IMPROVE IVP EMBRYO COMPETENCY
All indications are that a subset of IVP embryos suffer from various abnormalities in chromosome number, gene expression, developmental potential, and methylation profiles. Any one of these anomalies could compromise embryo competency. Recent attempts to improve IVP embryo competency have focused on exploring how bioactive oviductal and uterine factors impact IVP embryo development and pregnancy success after ET. These bioactive factors are often referred to as embryotrophic factors or embryokines, given their embryo development-promoting abilities. Embryokines studied recently include colony-stimulating factor 2, EGF, FGF2, and insulin-like growth factor 1 (Block et al., 2003; Loureiro et al., 2009; Arias-Alvarez et al., 2011; Dobbs et al., 2013; Xie et al., 2015). These factors have various activities on embryos (e.g., mitogenic, antiapoptotic, lineage specification), and their inclusion during embryo culture is proposed as a means for refining IVP systems to more closely mimic events occurring in the female reproductive tract during early pregnancy.
One example of using embryokines to improve embryo competency is by including CSF2 in bovine embryo culture media. Supplementing CSF2 to embryo culture medium beginning on day 5 post-IVF increases blastocyst development and post-transfer pregnancy retention in cattle when culture media lacks serum but not when serum is included (Loureiro et al., 2009; Denicol et al., 2014; Tribulo et al., 2017). Also, IVP embryo exposure to CSF2 prior to ET improves postnatal heifer calf growth performance (Kannampuzha-Francis et al., 2015). Potential mechanisms of CSF2 action include promoting early conceptus elongation and survival and altering DNA methylation status (Loureiro et al., 2011; Dobbs et al., 2014).
Another example of improving pregnancy outcomes is by manipulating WNT signaling during in vitro embryo production. The persistence of WNT signaling is associated with embryo incompetency in cattle, pigs, and mice (Xie et al., 2008; Denicol et al., 2013; Lim et al., 2013). Interference in WNT signaling is controlled, at least in part, by endometrial production of Dickkopf-1 (DKK1), an inhibitor of canonical WNT signaling (Cerri et al., 2012). Supplementing DKK1 during IVP embryo culture appears beneficial to calving outcomes. Increases in blastocyst development and pregnancy retention occurred in DKK1 treated IVP embryos in one study lacking serum in culture medium (Denicol et al., 2014), but not in another study that utilized a serum-containing medium ( Tríbulo et al., 2017). In the latter study, a reduction in birth weights was observed in calves from DKK1-exposed IVP embryos (Tríbulo et al., 2017). The mechanism responsible for this outcome is unclear, but this finding may have important implications for further reducing the incidence of large offspring syndrome in IVP pregnancies.
SUMMARY AND CONCLUSIONS
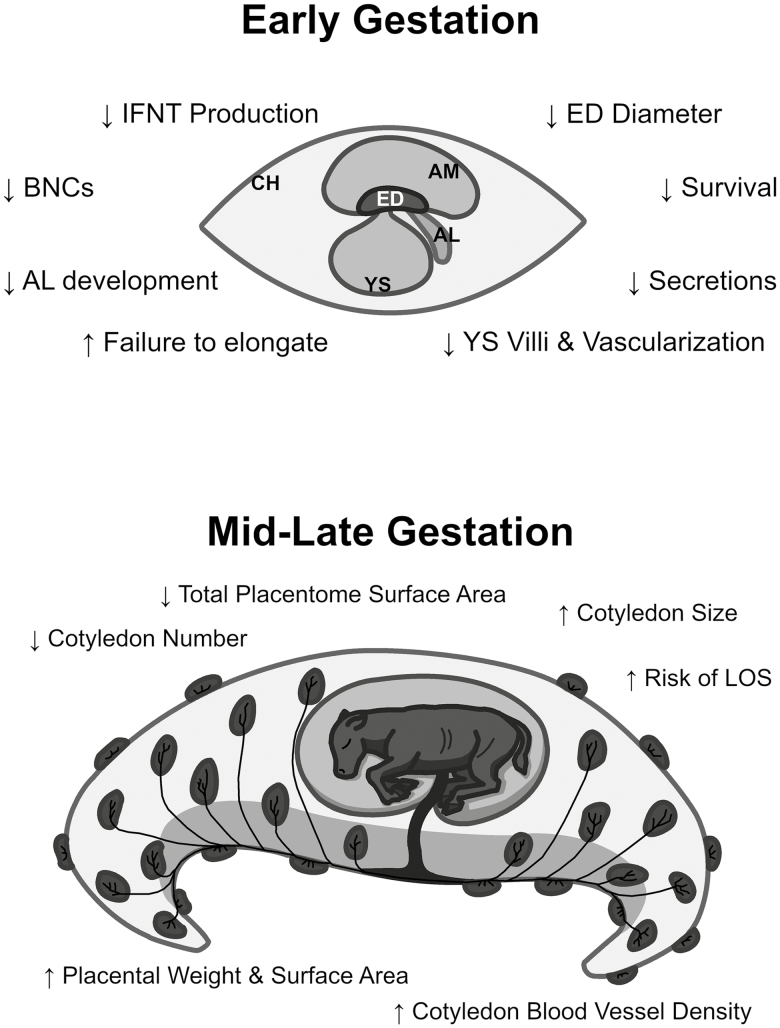
Clearly, post-transfer pregnancy failures are prominent in cattle receiving IVP embryos. There are several proposed mechanisms for why IVP embryos do not survive to term. These are summarized in Fig. 1. A majority of these losses (~80% of all pregnancy failures) occur within the first 6 wk of gestation. Failures occurring during the pre- and peri-implantation period are caused primarily by developmental miscues in embryonic and extraembryonic lineage development. These shortcomings can prevent conceptus elongation or produce elongated conceptuses that contain small or nonexistent embryonic disks or poorly developed yolk sacs. Pregnancy failure is also prominent during the implantation period, where early binucleate cell formation and overall placental and allantoic sac development may be compromised. Lastly, placental development and function during mid- and late gestation and the trajectory of fetal growth are altered in IVP pregnancies, and these can produce large offspring syndrome calves or calves with poor neonatal health. Further work is needed to develop strategies to overcome these post-transfer issues. Medium formulation, oxygen tension, group embryo culture, medium drop size, and other considerations are being used to facilitate embryo competency. Further exploration into how embryokines function during early embryogenesis may hold the key to making future strides in improving post-transfer pregnancy success in IVP embryos.
Figure 1.
Proposed causes for pregnancy failures after transfer of in vitro-produced bovine embryos. The various described contributors to these pregnancy losses are indicated relative to the time when they occur. Early gestation is loosely defined as the period between embryo transfer (day 7) and day 30 to 40 of gestation, whereas mid-late gestation is after day 40. Abbreviations: allantois (AL), binucleate cells (BNCs), embryonic disk (ED), interferon-tau (IFNT), large offspring syndrome (LOS), yolk sac (YS).
Regardless of the hurdles that IVP systems still face, the benefits of utilizing this tool cannot be ignored. With this technology, superior females can produce more offspring each year than by MOET alone. Additionally, IVP systems provide researchers with the opportunity to study early bovine embryos so that we may gain a better understanding of what these gametes and embryos need in order to develop successfully in culture.
ACKNOWLEDGMENTS
Funding for this manuscript and work in Dr Ealy’s laboratory was supported by Agriculture and Food Research Initiative Competitive Grant no. 2017-67015-26461 and 2018-67030-28727 from the USDA National Institute of Food and Agriculture. Authors do not have any actual or potential conflicts of interest related to the research presented in this manuscript.
LITERATURE CITED
- Adona P. R., C. L. Leal F. H. Biase T. H. De Bem L. G. Mesquita F. V. Meirelles A. L. Ferraz L. R. Furlan P. S. Monzani, and Guemra S.. 2016. In vitro maturation alters gene expression in bovine oocytes. Zygote 24:624–633. doi: 10.1017/S0967199415000672 [DOI] [PubMed] [Google Scholar]
- Agca Y., Monson R. L., Northey D. L., Mazni O. A., Schaefer D. M., and Rutledge J. J.. . 1998 Transfer of fresh and cryopreserved IVP bovine embryos: normal calving, birth weight and gestation lengths. Theriogenology 50:147–162. doi: 10.1016/S0093-691X(98)00121-6 [DOI] [PubMed] [Google Scholar]
- Alberto M. L., Meirelles F. V., Perecin F., Ambrosio C. E., Favaron P. O., Franciolli A. L., Mess A. M., Dos Santos J. M., Rici R. E., Bertolini M., . et al. 2013. Development of bovine embryos derived from reproductive techniques. Reprod. Fertil. Dev. 25:907–917. doi: 10.1071/RD12092 [DOI] [PubMed] [Google Scholar]
- Alexopoulos N. I., P. Maddox-Hyttel P. Tveden-Nyborg N. T. D’Cruz T. R. Tecirlioglu M. A. Cooney K. Schauser M. K. Holland, and French A. J.. 2008. Developmental disparity between in vitro-produced and somatic cell nuclear transfer bovine days 14 and 21 embryos: implications for embryonic loss. Reproduction 136:433–445. doi: 10.1530/REP-07-0392 [DOI] [PubMed] [Google Scholar]
- Al-Katanani Y. M., Drost M., Monson R. L., Rutledge J. J., Krininger C. E. 3rd, Block J., Thatcher W. W., and Hansen P. J.. . 2002. Pregnancy rates following timed embryo transfer with fresh or vitrified in vitro produced embryos in lactating dairy cows under heat stress conditions. Theriogenology 58:171–182. doi: 10.1016/S0093-691X(02)00916-0 [DOI] [PubMed] [Google Scholar]
- Arias-Alvarez M., P. Bermejo-Alvarez A. Gutierrez-Adan D. Rizos P. L. Lorenzo, and Lonergan P.. 2011. Effect of leptin supplementation during in vitro oocyte maturation and embryo culture on bovine embryo development and gene expression patterns. Theriogenology 75:887–896. doi: 10.1016/j.theriogenology.2010.10.031 [DOI] [PubMed] [Google Scholar]
- Arman E., Haffner-Krausz R., Chen Y., Heath J. K., and Lonai P.. . 1998. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA 95:5082–5087. doi: 10.1073/pnas.95.9.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D. R., Bordignon V., Lefebvre R., Murphy B. D., and Smith L. C.. . 2006. Somatic cell nuclear transfer alters peri-implantation trophoblast differentiation in bovine embryos. Reproduction 132:279–290. [DOI] [PubMed] [Google Scholar]
- Assis Neto A. C., F. T. Pereira T. C. Santos C. E. Ambrosio R. Leiser, and Miglino M. A.. 2010. Morpho-physical recording of bovine conceptus (Bos indicus) and placenta from days 20 to 70 of pregnancy. Reprod. Domest. Anim. 45:760–772. doi: 10.1111/j.1439-0531.2009.01345.x [DOI] [PubMed] [Google Scholar]
- Assis Neto A. C., E. C. Santos F. T. Pereira, and Miglino M. A.. 2009. Initial development of bovine placentation (Bos indicus) from the point of view of the allantois and amnion. Anat. Histol. Embryol. 38:341–347. doi: 10.1111/j.1439-0264.2009.00949.x [DOI] [PubMed] [Google Scholar]
- Balasubramanian S., W. J. Son B. M. Kumar S. A. Ock J. G. Yoo G. S. Im S. Y. Choe, and Rho G. J.. 2007. Expression pattern of oxygen and stress-responsive gene transcripts at various developmental stages of in vitro and in vivo preimplantation bovine embryos. Theriogenology 68:265–275. doi: 10.1016/j.theriogenology.2007.05.044 [DOI] [PubMed] [Google Scholar]
- Barnwell C. V., P. W. Farin C. S. Whisnant J. E. Alexander, and Farin C. E.. 2015. Maternal serum progesterone concentration and early conceptus development of bovine embryos produced in vivo or in vitro. Domest. Anim. Endocrinol. 52:75–81. doi: 10.1016/j.domaniend.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Baruselli P. S., M. F. Sá Filho R. M. Ferreira J. N. Sales L. U. Gimenes L. M. Vieira M. F. Mendanha, and Bó G. A.. 2012. Manipulation of follicle development to ensure optimal oocyte quality and conception rates in cattle. Reprod. Domest. Anim. 47(Suppl. 4):134–141. doi: 10.1111/j.1439-0531.2012.02067.x [DOI] [PubMed] [Google Scholar]
- Bertolini M., S. W. Beam H. Shim L. R. Bertolini A. L. Moyer T. R. Famula, and Anderson G. B.. 2002a. Growth, development, and gene expression by in vivo- and in vitro-produced day 7 and 16 bovine embryos. Mol. Reprod. Dev. 63:318–328. doi: 10.1002/mrd.90015 [DOI] [PubMed] [Google Scholar]
- Bertolini M., Mason J. B., Beam S. W., Carneiro G. F., Sween M. L., Kominek D. J., Moyer A. L., Famula T. R., Sainz R. D., and Anderson G. B.. . 2002b. Morphology and morphometry of in vivo- and in vitro-produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 58:973–994. doi: 10.1002/mrd.90015 [DOI] [PubMed] [Google Scholar]
- Bertolini M., Moyer A. L., Mason J. B., Batchelder C. A., Hoffert K. A., Bertolini L. R., Carneiro G. F., Cargill S. L., Famula T. R., Calvert C. C., . et al. 2004. Evidence of increased substrate availability to in vitro-derived bovine foetuses and association with accelerated conceptus growth. Reproduction 128:341–354. doi: 10.1530/rep.1.00188 [DOI] [PubMed] [Google Scholar]
- Bertolini M., Wallace C. R., and Anderson G. B.. . 2006. Expression profile and protein levels of placental products as indirect measures of placental function in in vitro-derived bovine pregnancies. Reproduction 131:163–173. [DOI] [PubMed] [Google Scholar]
- Block J., L. Bonilla, and Hansen P. J.. 2010. Efficacy of in vitro embryo transfer in lactating dairy cows using fresh or vitrified embryos produced in a novel embryo culture medium. J. Dairy Sci. 93:5234–5242. doi: 10.3168/jds.2010-3443 [DOI] [PubMed] [Google Scholar]
- Block J., M. Drost R. L. Monson J. J. Rutledge R. M. Rivera F. F. Paula-Lopes O. M. Ocon C. E. Krininger J. 3rd Liu, and Hansen P. J.. 2003. Use of insulin-like growth factor-I during embryo culture and treatment of recipients with gonadotropin-releasing hormone to increase pregnancy rates following the transfer of in vitro-produced embryos to heat-stressed, lactating cows. J. Anim. Sci. 81:1590–1602. doi: 10.2527/2003.8161590x [DOI] [PubMed] [Google Scholar]
- Block J., A. E. Fischer-Brown T. M. Rodina A. D. Ealy, and Hansen P. J.. 2007. The effect of in vitro treatment of bovine embryos with IGF-1 on subsequent development in utero to day 14 of gestation. Theriogenology 68:153–161. doi: 10.1016/j.theriogenology.2007.04.045 [DOI] [PubMed] [Google Scholar]
- Block J., and Hansen P. J.. . 2007. Interaction between season and culture with insulin-like growth factor-1 on survival of in vitro produced embryos following transfer to lactating dairy cows. Theriogenology 67:1518–1529. doi:S0093-691X(07)00114-8 [DOI] [PubMed] [Google Scholar]
- Bonilla L., J. Block A. C. Denicol, and Hansen P. J.. 2014. Consequences of transfer of an in vitro-produced embryo for the dam and resultant calf. J. Dairy Sci. 97:229–239. doi:10.3168/jds.2013-6943 [DOI] [PubMed] [Google Scholar]
- Bonnet A., Bevilacqua C., Benne F., Bodin L., Cotinot C., Liaubet L., Sancristobal M., Sarry J., Terenina E., Martin P., . et al. 2011. Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection. BMC Genomics 12:417. doi:10.1186/1471-2164-12-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixeta E. S., M. F. Machado P. Ripamonte C. Price, and Buratini J.. 2013. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod. Fertil. Dev. 25:890–899. doi:10.1071/RD12125 [DOI] [PubMed] [Google Scholar]
- Caixeta E. S., P. Ripamonte M. M. Franco J. B. Junior, and Dode M. A.. 2009. Effect of follicle size on mRNA expression in cumulus cells and oocytes of Bos indicus: an approach to identify marker genes for developmental competence. Reprod. Fertil. Dev. 21:655–664. doi:10.1071/RD08201 [DOI] [PubMed] [Google Scholar]
- Cerri R. L., I. M. Thompson I. H. Kim A. D. Ealy P. J. Hansen C. R. Staples J. L. Li J. E. Santos, and Thatcher W. W.. 2012. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J. Dairy Sci. 95:5657–5675. doi:10.3168/jds.2011-5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., D. E. Hagen C. G. Elsik T. Ji C. J. Morris L. E. Moon, and Rivera R. M.. 2015. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 112:4618–4623. doi:10.1073/pnas.1422088112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., K. M. Robbins K. D. Wells, and Rivera R. M.. 2013. Large offspring syndrome: a bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics 8:591–601. doi:10.4161/epi.24655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran D., D. Rizos T. Fair A. C. Evans, and Lonergan P.. 2007. Temporal expression of transcripts related to embryo quality in bovine embryos cultured from the two-cell to blastocyst stage in vitro or in vivo. Mol. Reprod. Dev. 74:972–977. doi:10.1002/mrd.20677 [DOI] [PubMed] [Google Scholar]
- Degrelle S. A., Campion E., Cabau C., Piumi F., Reinaud P., Richard C., Renard J. P., and Hue I.. . 2005. Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev. Biol. 288:448–460. doi:S0012-1606(05)00680-9 [DOI] [PubMed] [Google Scholar]
- Denicol A. C., J. Block D. E. Kelley K. G. Pohler K. B. Dobbs C. J. Mortensen M. S. Ortega, and Hansen P. J.. 2014. The WNT signaling antagonist Dickkopf-1 directs lineage commitment and promotes survival of the preimplantation embryo. FASEB J. 28:3975–3986. doi:10.1096/fj.14-253112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicol A. C., Dobbs K. B., McLean K. M., Carambula S. F., Loureiro B., and Hansen P. J.. . 2013. Canonical WNT signaling regulates development of bovine embryos to the blastocyst stage. Sci. Rep. 3:1266. doi:10.1038/srep01266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs K. B., Gagne D., Fournier E., Dufort I., Robert C., Block J., Sirard M. A., Bonilla L., Ealy A. D., Loureiro B., . et al. 2014. Sexual dimorphism in developmental programming of the bovine preimplantation embryo caused by colony-stimulating factor 2. Biol. Reprod. 91:80. doi:10.1095/biolreprod.114.121087 [DOI] [PubMed] [Google Scholar]
- Dobbs K. B., F. A. Khan M. Sakatani J. I. Moss M. Ozawa A. D. Ealy, and Hansen P. J.. 2013. Regulation of pluripotency of inner cell mass and growth and differentiation of trophectoderm of the bovine embryo by colony stimulating factor 2. Biol. Reprod. 89:141. doi:10.1095/biolreprod.113.113183 [DOI] [PubMed] [Google Scholar]
- Driver A. M., F. Peñagaricano W. Huang K. R. Ahmad K. S. Hackbart M. C. Wiltbank, and Khatib H.. 2012. RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genomics 13:118. doi:10.1186/1471-2164-13-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealy A. D., M. Drost, and Hansen P. J.. 1993. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J. Dairy Sci. 76:2899–2905. doi:10.3168/jds.S0022-0302(93)77629-8 [DOI] [PubMed] [Google Scholar]
- Ealy A. D., and Wooldridge L. K.. . 2017. The evolution of interferon-tau. Reproduction 154:F1–F10. doi:10.1530/REP-17-0292 [DOI] [PubMed] [Google Scholar]
- Farin P. W., Crosier A. E., and Farin C. E.. . 2001. Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology 55:151–170. doi:S0093-691X(00)00452-0 [DOI] [PubMed] [Google Scholar]
- Farin P. W., and Farin C. E.. . 1995. Transfer of bovine embryos produced in vivo or in vitro: survival and fetal development. Biol. Reprod. 52:676–682. doi:10.1095/biolreprod52.3.676 [DOI] [PubMed] [Google Scholar]
- Farin P. W., B. D. Slenning, and Britt J. H.. 1999. Estimates of pregnancy outcomes based on selection of bovine embryos produced in vivo or in vitro. Theriogenology 52:659–670. doi:10.1016/S0093-691X(99)00160-0 [DOI] [PubMed] [Google Scholar]
- Fischer-Brown A., A. Crooks S. Leonard R. Monson D. Northey, and Rutledge J. J.. 2005. Parturition following transfer of embryos produced in two media under two oxygen concentrations. Anim. Reprod. Sci. 87:215–228. doi:10.1016/j.anireprosci.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Fischer-Brown A. E., Lindsey B. R., Ireland F. A., Northey D. L., Monson R. L., Clark S. G., Wheeler M. B., Kesler D. J., Lane S. J., Weigel K. A., . et al. 2004. Embryonic disc development and subsequent viability of cattle embryos following culture in two media under two oxygen concentrations. Reprod. Fertil. Dev. 16:787–793. doi:10.1071/RD04026 [DOI] [PubMed] [Google Scholar]
- Fischer-Brown A., Monson R., Parrish J., and Rutledge J.. . 2002. Cell allocation in bovine embryos cultured in two media under two oxygen concentrations. Zygote 10:341–348. doi:10.1017/S0967199402004082 [DOI] [PubMed] [Google Scholar]
- Forde N., F. W. Bazer T. E. Spencer, and Lonergan P.. 2015. ‘Conceptualizing’ the endometrium: identification of conceptus-derived proteins during early pregnancy in cattle. Biol. Reprod. 92:156. doi:10.1095/biolreprod.115.129296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde N., and Lonergan P.. . 2017. Interferon-tau and fertility in ruminants. Reproduction 154:F33–F43. doi:10.1530/REP-17-0432 [DOI] [PubMed] [Google Scholar]
- Fowden A. L., Giussani D. A., and Forhead A. J.. . 2006. Intrauterine programming of physiological systems: causes and consequences. Physiology 21:29–37. doi:10.1152/physiol.00050.2005 [DOI] [PubMed] [Google Scholar]
- Freyer C., and Renfree M. B.. . 2009. The mammalian yolk sac placenta. J. Exp. Zool. B Mol. Dev. Evol. 312:545–554. doi:10.1002/jez.b.21239 [DOI] [PubMed] [Google Scholar]
- Garcia-Herreros M., T. F. Carter D. A. Villagómez A. D. Macaulay D. Rath W. A. King, and Lonergan P.. 2010. Incidence of chromosomal abnormalities in bovine blastocysts derived from unsorted and sex-sorted spermatozoa. Reprod. Fertil. Dev. 22:1272–1278. doi:10.1071/RD10052 [DOI] [PubMed] [Google Scholar]
- Gatea A. O., M. F. Smith K. G. Pohler T. Egen M. H. C. Pereira J. L. M. Vasconselos J. C. Lawrence, and Green J. A.. 2018. The ability to predict pregnancy loss in cattle with ELISAs that detect pregnancy associated glycoproteins is antibody dependent. Theriogenology 108:269–276. doi:10.1016/j.theriogenology.2017.12.021 [DOI] [PubMed] [Google Scholar]
- Greenstein J. S., Murray R. W., and Foley R. C.. . 1958. Observations on the morphogenesis and histochemistry of the bovine preattachment placenta between 16 and 33 days of gestation. Anat. Rec. 132:321–341. doi:10.1002/ar.1091320308 [DOI] [PubMed] [Google Scholar]
- Heinzmann J., T. Hansmann D. Herrmann C. Wrenzycki U. Zechner T. Haaf, and Niemann H.. 2011. Epigenetic profile of developmentally important genes in bovine oocytes. Mol. Reprod. Dev. 78:188–201. doi:10.1002/mrd.21281 [DOI] [PubMed] [Google Scholar]
- Heras S., De Coninck D. I., Van Poucke M., Goossens K., Bogado Pascottini O., Van Nieuwerburgh F., Deforce D., De Sutter P., Leroy J. L., Gutierrez-Adan A., . et al. 2016. Suboptimal culture conditions induce more deviations in gene expression in male than female bovine blastocysts. BMC Genomics 17:72. doi:10.1186/s12864-016-2393-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm P., Booth P. J., and Callesen H.. . 2002. Kinetics of early in vitro development of bovine in vivo- and in vitro-derived zygotes produced and/or cultured in chemically defined or serum-containing media. Reproduction 123:553–565. doi:10.1530/rep.0.1230553 [PubMed] [Google Scholar]
- Hori N., M. Nagai M. Hirayama T. Hirai K. Matsuda M. Hayashi T. Tanaka T. Ozawa, and Horike S.. 2010. Aberrant CpG methylation of the imprinting control region KvDMR1 detected in assisted reproductive technology-produced calves and pathogenesis of large offspring syndrome. Anim. Reprod. Sci. 122:303–312. doi:10.1016/j.anireprosci.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Iwasaki S., S. Hamano M. Kuwayama M. Yamashita H. Ushijima S. Nagaoka, and Nakahara T.. 1992. Developmental changes in the incidence of chromosome anomalies of bovine embryos fertilized in vitro. J. Exp. Zool. 261:79–85. doi:10.1002/jez.1402610109 [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Yoshiba N., Ushijima H., Watanabe S., and Nakahara T.. . 1990. Morphology and proportion of inner cell mass of bovine blastocysts fertilized in vitro and in vivo. J. Reprod. Fertil. 90:279–284. doi:10.1530/jrf.0.0900279 [DOI] [PubMed] [Google Scholar]
- Jiang Z., Lin J., Dong H., Zheng X., Marjani S. L., Duan J., Ouyang Z., Chen J., and Tian X. C.. . 2018. DNA methylomes of bovine gametes and in vivo produced preimplantation embryos. Biol. Reprod. 99(5):949–959. doi:10.1093/biolre/ioy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollie W. P. 1990. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology 41:361–381. doi:10.1002/tera.1420410403 [DOI] [PubMed] [Google Scholar]
- Kannampuzha-Francis J., A. C. Denicol B. Loureiro K. Kaniyamattam M. S. Ortega, and Hansen P. J.. 2015. Exposure to colony stimulating factor 2 during preimplantation development increases postnatal growth in cattle. Mol. Reprod. Dev. 82:892–897. doi:10.1002/mrd.22533 [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe M. G., B. R. McCallie K. A. Preis J. Filipovits, and Gardner D. K.. 2009. Transcriptome analysis of in vivo and in vitro matured bovine MII oocytes. Theriogenology 71:939–946. doi:10.1016/j.theriogenology.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Knijn H. M., J. O. Gjørret P. L. Vos P. J. Hendriksen B. C. van der Weijden P. Maddox-Hyttel, and Dieleman S. J.. 2003. Consequences of in vivo development and subsequent culture on apoptosis, cell number, and blastocyst formation in bovine embryos. Biol. Reprod. 69:1371–1378. doi:10.1095/biolreprod.103.017251 [DOI] [PubMed] [Google Scholar]
- Krisher R. L., and Bavister B. D.. . 1998. Responses of oocytes and embryos to the culture environment. Theriogenology 49:103–114. doi:10.1016/S0093-691X(97)00405-6 [DOI] [PubMed] [Google Scholar]
- Kubisch H. M., Larson M. A., Ealy A. D., Murphy C. N., and Roberts R. M.. . 2001. Genetic and environmental determinants of interferon-tau secretion by in vivo- and in vitro-derived bovine blastocysts. Anim. Reprod. Sci. 66:1–13. doi:10.1016/S0378-4320(01)00086-0 [DOI] [PubMed] [Google Scholar]
- Kuijk E. W., L. T. van Tol H. Van de Velde R. Wubbolts M. Welling N. Geijsen, and Roelen B. A.. 2012. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 139:871–882. doi:10.1242/dev.071688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. T., M. K. Gupta S. H. Lee Y. H. Jung D. W. Han, and Lee H. T.. 2013. Possible involvement of Wnt/β-catenin signaling pathway in hatching and trophectoderm differentiation of pig blastocysts. Theriogenology 79:284–290.e281–282. doi:10.1016/j.theriogenology.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Liu Z., and Zheng Y.. . 2009. A requirement for epsin in mitotic membrane and spindle organization. J. Cell Biol. 186:473–480. doi:10.1083/jcb.200902071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P., Carolan C., Van Langendonckt A., Donnay I., Khatir H., and Mermillod P.. . 1996. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol. Reprod. 54:1420–1429. doi:10.1095/biolreprod54.6.1420 [DOI] [PubMed] [Google Scholar]
- Lonergan P., H. G. Pedersen D. Rizos T. Greve P. D. Thomsen T. Fair A. Evans, and Boland M. P.. 2004. Effect of the post-fertilization culture environment on the incidence of chromosome aberrations in bovine blastocysts. Biol. Reprod. 71:1096–1100. doi:10.1095/biolreprod.104.030635 [DOI] [PubMed] [Google Scholar]
- Lonergan P., Woods A., Fair T., Carter F., Rizos D., Ward F., Quinn K., and Evans A.. . 2007. Effect of embryo source and recipient progesterone environment on embryo development in cattle. Reprod. Fertil. Dev. 19:861–868. doi:10.1071/RD07089 [DOI] [PubMed] [Google Scholar]
- Looney C. R., Lindsey B. R., Gonseth C. L., and Johnson D. L.. . 1994. Commercial aspects of oocyte retrieval and in vitro fertilization (IVF) for embryo production in problem cows. Theriogenology 41:67–72. doi:10.1016/S0093-691X(05)80050-0 [Google Scholar]
- Loureiro B., J. Block M. G. Favoreto S. Carambula K. A. Pennington A. D. Ealy, and Hansen P. J.. 2011. Consequences of conceptus exposure to colony-stimulating factor 2 on survival, elongation, interferon-τ secretion, and gene expression. Reproduction 141:617–624. doi:10.1530/REP-10-0511 [DOI] [PubMed] [Google Scholar]
- Loureiro B., L. Bonilla J. Block J. M. Fear A. Q. Bonilla, and Hansen P. J.. 2009. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 150:5046–5054. doi:10.1210/en.2009-0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy M. C. 2001. Reproductive loss in high-producing dairy cattle: where will it end? J. Dairy Sci. 84:1277–1293. doi:10.3168/jds.S0022-0302(01)70158-0 [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel P., Alexopoulos N. I., Vajta G., Lewis I., Rogers P., Cann L., Callesen H., Tveden-Nyborg P., and Trounson A.. . 2003. Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction 125:607–623. doi:10.1530/rep.0.1250607 [PubMed] [Google Scholar]
- Mansouri-Attia N., Sandra O., Aubert J., Degrelle S., Everts R. E., Giraud-Delville C., Heyman Y., Galio L., Hue I., Yang X., . et al. 2009. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc. Natl. Acad. Sci. USA 106:5687–5692. doi:10.1073/pnas.0812722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D. J., Sanchez J. M., Passaro C., Charpigny G., Behura S. K., Spencer T. E., and Lonergan P.. . 2019. Interferon tau-dependent and independent effects of the bovine conceptus on the endometrial transcriptome. Biol. Reprod. 100(2):365–380. doi:10.1093/biolre/ioy199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan W. H. 1998. Statistical models predicting embryo survival to term in cattle after embryo transfer. Theriogenology 50:1053–1070. doi:10.1016/S0093-691X(98)00207-6 [DOI] [PubMed] [Google Scholar]
- McMillan W., Peterson A. J., Hall D., and Donnison M.. . 1997. Embryo and recipient contributions to embryo loss to day 60 in heifers receiving either one or two in vitro-produced embryos. Theriogenology 47:370. (Abstr.). [Google Scholar]
- Meng Q. X., H. J. Gao C. M. Xu M. Y. Dong X. Sheng J. Z. Sheng, and Huang H. F.. 2008. Reduced expression and function of aquaporin-3 in mouse metaphase-II oocytes induced by controlled ovarian hyperstimulation were associated with subsequent low fertilization rate. Cell. Physiol. Biochem. 21:123–128. doi:10.1159/000113754 [DOI] [PubMed] [Google Scholar]
- Mess A. M., A. C. O. Carreira C. Marinovic de Oliveira P. Fratini P. O. Favaron R. D. S. N. Barreto C. Pfarrer F. V. Meirelles, and Miglino M. A.. 2017. Vascularization and VEGF expression altered in bovine yolk sacs from IVF and NT technologies. Theriogenology 87:290–297. doi:10.1016/j.theriogenology.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Miles J. R., C. E. Farin K. F. Rodriguez J. E. Alexander, and Farin P. W.. 2004. Angiogenesis and morphometry of bovine placentas in late gestation from embryos produced in vivo or in vitro. Biol. Reprod. 71:1919–1926. doi:10.1095/biolreprod.104.031427 [DOI] [PubMed] [Google Scholar]
- Miles J. R., C. E. Farin K. F. Rodriguez J. E. Alexander, and Farin P. W.. 2005. Effects of embryo culture on angiogenesis and morphometry of bovine placentas during early gestation. Biol. Reprod. 73:663–671. doi:10.1095/biolreprod.105.040808 [DOI] [PubMed] [Google Scholar]
- Mohan M., A. G. Hurst, and Malayer J. R.. 2004. Global gene expression analysis comparing bovine blastocysts flushed on day 7 or produced in vitro. Mol. Reprod. Dev. 68:288–298. doi:10.1002/mrd.20086 [DOI] [PubMed] [Google Scholar]
- Niemann H., J. W. Carnwath D. Herrmann G. Wieczorek E. Lemme A. Lucas-Hahn, and Olek S.. 2010. DNA methylation patterns reflect epigenetic reprogramming in bovine embryos. Cell. Reprogram. 12:33–42. doi:10.1089/cell.2009.0063 [DOI] [PubMed] [Google Scholar]
- Numabe T., Oikawa T., Kikuchi T., and Horiuchi T.. . 2000. Production efficiency of Japanese black calves by transfer of bovine embryos produced in vitro. Theriogenology 54:1409–1420. doi:10.1016/S0093-691X(00)00463-5 [DOI] [PubMed] [Google Scholar]
- Papadopoulos S., Rizos D., Duffy P., Wade M., Quinn K., Boland M. P., and Lonergan P.. . 2002. Embryo survival and recipient pregnancy rates after transfer of fresh or vitrified, in vivo or in vitro produced ovine blastocysts. Anim. Reprod. Sci. 74:35–44. doi:10.1016/S0378-4320(02)00162-8 [DOI] [PubMed] [Google Scholar]
- Perecin F., S. C. Méo W. Yamazaki C. R. Ferreira G. K. Merighe F. V. Meirelles, and Garcia J. M.. 2009. Imprinted gene expression in in vivo- and in vitro-produced bovine embryos and chorio-allantoic membranes. Genet. Mol. Res. 8:76–85 [DOI] [PubMed] [Google Scholar]
- Perry G. A., M. F. Smith M. C. Lucy J. A. Green T. E. Parks M. D. MacNeil A. J. Roberts, and Geary T. W.. 2005. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 102:5268–5273. doi:10.1073/pnas.0501700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohler K. G., J. A. Green T. W. Geary R. F. Peres M. H. Pereira J. L. Vasconcelos, and Smith M. F.. 2015. Predicting embryo presence and viability. Adv. Anat. Embryol. Cell Biol. 216:253–270. doi:10.1007/978-3-319-15856-3_13 [DOI] [PubMed] [Google Scholar]
- Pontes J. H., Nonato-Junior I., Sanches B. V., Ereno-Junior J. C., Uvo S., Barreiros T. R., Oliveira J. A., Hasler J. F., and Seneda M. M.. . 2009. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 71:690–697. doi:S0093-691X(08)00692-4 [DOI] [PubMed] [Google Scholar]
- Rasmussen S., J. Block G. E. Seidel Z. Jr. Brink K. McSweeney P. W. Farin L. Bonilla, and Hansen P. J.. 2013. Pregnancy rates of lactating cows after transfer of in vitro produced embryos using X-sorted sperm. Theriogenology 79:453–461. doi:10.1016/j.theriogenology.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Reichenbach H. D., Liebrich J., Berg U., and Brem G.. . 1992. Pregnancy rates and births after unilateral or bilateral transfer of bovine embryos produced in vitro. J. Reprod. Fertil. 95:363–370. doi:10.1530/jrf.0.0950363 [DOI] [PubMed] [Google Scholar]
- Rizos D., F. Ward P. Duffy M. P. Boland, and Lonergan P.. 2002. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 61:234–248. doi:10.1002/mrd.1153 [DOI] [PubMed] [Google Scholar]
- Santos J. E., Thatcher W. W., Chebel R. C., Cerri R. L., and Galvao K. N.. . 2004. The effect of embryonic death rates in cattle on the efficacy of estrus synchronization programs. Anim. Reprod. Sci. 82–83:513–535. doi:10.1016/j.anireprosci.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Sawai K. 2009. Studies on gene expression in bovine embryos derived from somatic cell nuclear transfer. J. Reprod. Dev. 55:11–16. doi:10.1262/jrd.20131 [DOI] [PubMed] [Google Scholar]
- Shalaby F., J. Rossant T. P. Yamaguchi M. Gertsenstein X. F. Wu M. L. Breitman, and Schuh A. C.. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62–66. doi:10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- Shi W. K., B. Hopkins S. Thompson J. K. Heath B. M. Luke, and Graham C. F.. 1985. Synthesis of apolipoproteins, alphafoetoprotein, albumin, and transferrin by the human foetal yolk sack and other foetal organs. J. Embryol. Exp. Morphol. 85:191–206. [PubMed] [Google Scholar]
- Siqueira L. G. B., S. Dikmen M. S. Ortega, and Hansen P. J.. 2017. Postnatal phenotype of dairy cows is altered by in vitro embryo production using reverse X-sorted semen. J. Dairy Sci. 100:5899–5908. doi:10.3168/jds.2016-12539 [DOI] [PubMed] [Google Scholar]
- Siqueira L. G., C. A. Torres E. D. Souza P. L. Monteiro E. K. Jr. Arashiro L. S. Camargo C. A. Fernandes, and Viana J. H.. 2009. Pregnancy rates and corpus luteum-related factors affecting pregnancy establishment in bovine recipients synchronized for fixed-time embryo transfer. Theriogenology 72:949–958. doi:10.1016/j.theriogenology.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Sirard M. A. 2018. 40 years of bovine IVF in the new genomic selection context. Reproduction 156:R1–R7. doi:10.1530/REP-18-0008 [DOI] [PubMed] [Google Scholar]
- de Souza D. K., L. P. Salles, and Rosa e Silva A. A.. 2015. Aspects of energetic substrate metabolism of in vitro and in vivo bovine embryos. Braz. J. Med. Biol. Res. 48:191–197. doi:10.1590/1414-431X20143744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. E., N. Forde, and Lonergan P.. 2016. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod. Fertil. Dev. 29:84–100. doi:10.1071/RD16359 [DOI] [PubMed] [Google Scholar]
- Spencer T. E., O. Sandra, and Wolf E.. 2008. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction 135:165–179. doi:10.1530/REP-07-0327 [DOI] [PubMed] [Google Scholar]
- Stewart B. M., J. Block P. Morelli A. E. Navarette M. Amstalden L. Bonilla P. J. Hansen, and Bilby T. R.. 2011. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J. Dairy Sci. 94:3437–3445. doi:10.3168/jds.2010-4008 [DOI] [PubMed] [Google Scholar]
- Stojkovic M., Wolf E., Buttner M., Berg U., Charpigny G., Schmitt A., and Brem G.. . 1995. Secretion of biologically active interferon tau by in vitro-derived bovine trophoblastic tissue. Biol. Reprod. 53:1500–1507. doi:10.1095/biolreprod53.6.1500 [DOI] [PubMed] [Google Scholar]
- Suzuki J., Jr., Therrien J., Filion F., Lefebvre R., Goff A. K., and Smith L. C.. . 2009. In vitro culture and somatic cell nuclear transfer affect imprinting of SNRPN gene in pre- and post-implantation stages of development in cattle. BMC Dev. Biol. 9:9. doi:10.1186/1471-213X-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. G., and Peterson A. J.. . 2000. Bovine embryo culture in vitro: new developments and post-transfer consequences. Hum. Reprod. 15(Suppl. 5):59–67. doi:10.1093/humrep/15.suppl_5.59 [DOI] [PubMed] [Google Scholar]
- Tríbulo P., B. H. Bernal Ballesteros A. Ruiz A. Tríbulo R. J. Tríbulo H. E. Tríbulo G. A. Bo, and Hansen P. J.. 2017. Consequences of exposure of embryos produced in vitro in a serum-containing medium to Dickkopf-related protein 1 and colony stimulating factor 2 on blastocyst yield, pregnancy rate, and birth weight. J. Anim. Sci. 95:4407–4412. doi:10.2527/jas2017.1927 [DOI] [PubMed] [Google Scholar]
- Ushijima H., Akiyama K., and Tajima T.. . 2008. Transition of cell numbers in bovine preimplantation embryos: in vivo collected and in vitro produced embryos. J. Reprod. Dev. 54:239–243. doi:10.1262/jrd.19128 [DOI] [PubMed] [Google Scholar]
- Vailes M. T., S. R. McCoski L. K. Wooldridge S. T. Reese K. G. Pohler D. A. Roper V. R. Mercadante, and Ealy A. D.. 2019. Post-transfer outcomes in cultured bovine embryos supplemented with epidermal growth factor, fibroblast growth factor 2, and insulin-like growth factor 1. Theriogenology 124:1–8. doi:10.1016/j.theriogenology.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Viuff D., Rickords L., Offenberg H., Hyttel P., Avery B., Greve T., Olsaker I., Williams J. L., Callesen H., and Thomsen P. D.. . 1999. A high proportion of bovine blastocysts produced in vitro are mixoploid. Biol. Reprod. 60:1273–1278. doi:10.1095/biolreprod60.6.1273 [DOI] [PubMed] [Google Scholar]
- van Wagtendonk-de Leeuw A. M., Aerts B. J., and den Daas J. H.. . 1998. Abnormal offspring following in vitro production of bovine preimplantation embryos: a field study. Theriogenology 49:883–894. doi:S0093-691X(98)00038-7 [DOI] [PubMed] [Google Scholar]
- van Wagtendonk-de Leeuw A. M., Mullaart E., de Roos A. P., Merton J. S., den Daas J. H., Kemp B., and de Ruigh L.. . 2000. Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring. Theriogenology 53:575–597. doi:10.1016/S0093-691X(99)00259-9 [DOI] [PubMed] [Google Scholar]
- Wiltbank M. C., G. M. Baez A. Garcia-Guerra M. Z. Toledo P. L. Monteiro L. F. Melo J. C. Ochoa J. E. Santos, and Sartori R.. 2016. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 86:239–253. doi:10.1016/j.theriogenology.2016.04.037 [DOI] [PubMed] [Google Scholar]
- Wooding F. B. 1982. The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31–39 [PubMed] [Google Scholar]
- Xie M., McCoski S. R., Johnson S. E., Rhoads M. L., and Ealy A. D.. . 2015. Combinatorial effects of epidermal growth factor, fibroblast growth factor 2 and insulin-like growth factor 1 on trophoblast cell proliferation and embryogenesis in cattle. Reprod. Fertil. Dev. 29(2):419–430. doi:10.1071/RD15226 [DOI] [PubMed] [Google Scholar]
- Xie H., S. Tranguch X. Jia H. Zhang S. K. Das S. K. Dey C. J. Kuo, and Wang H.. 2008. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development 135:717–727. doi:10.1242/dev.015339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y., A. Ralston R. O. Stephenson, and Rossant J.. 2006. Cell and molecular regulation of the mouse blastocyst. Dev. Dyn. 235:2301–2314. doi:10.1002/dvdy.20844 [DOI] [PubMed] [Google Scholar]
- Yang Q. E., S. D. Fields K. Zhang M. Ozawa S. E. Johnson, and Ealy A. D.. 2011. Fibroblast growth factor 2 promotes primitive endoderm development in bovine blastocyst outgrowths. Biol. Reprod. 85:946–953. doi:10.1095/biolreprod.111.093203 [DOI] [PubMed] [Google Scholar]
- Yazawa S., Aoyagi Y., Konishi M., and Takedomi T.. . 1997. Characterization and cytogenetic analysis of Japanese Black calves produced by nuclear transfer. Theriogenology 48:641–650. doi:10.1016/S0093-691X(97)00280-X [DOI] [PubMed] [Google Scholar]
- Zaborski D., W. Grzesiak I. Szatkowska A. Dybus M. Muszynska, and Jedrzejczak M.. 2009. Factors affecting dystocia in cattle. Reprod. Domest. Anim. 44:540–551. doi:10.1111/j.1439-0531.2008.01123.x [DOI] [PubMed] [Google Scholar]
- Zhang K., P. J. Hansen, and Ealy A. D.. 2010. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction 140:815–826. doi:10.1530/REP-10-0190 [DOI] [PubMed] [Google Scholar]