Abstract
The aim of this study was to determine the effects of dietary grape seed polyphenols (GSP) supplementation during the late gestation and lactation period on reproductive performance, antioxidative status in serum, nutrient composition, and Ig content in colostrum of multiparous sows. On day 80 of gestation, a total of 64 sows with similar body condition were allocated to a completely randomized block design with 4 dietary treatments (n = 16 sows per treatment): 1) basal diet (CON, control group); 2) basal diet supplemented with 200 IU/kg vitamin E (200VE, positive control group); 3) basal diet supplemented with 200 mg/kg GSP (200GSP); and 4) basal diet supplemented with 300 mg/kg GSP (300GSP). The trial lasted 56 d until the piglets were weaned on day 21 of lactation. Reproductive performance, parameters of antioxidative status, and levels of progesterone (P4) and estradiol (E2) in serum, nutrient composition, and Ig content in colostrum of sows were determined. The number of dead fetuses was reduced, and farrowing survival was significantly improved in the litters from 300GSP-fed (P < 0.05). Preweaning survivability significantly increased in the litters from sows fed 200GSP and 200VE (P < 0.05). The activity of superoxide dismutase and glutathione peroxidase (GSH-Px) in the serum was significantly increased in sows fed 200GSP and 300GSP (P < 0.05). The activity of GSH-Px in the serum also significantly increased in sows fed 200VE (P < 0.05). Sows fed 300GSP had the greatest levels of P4 and E2 in the serum, which was significantly greater than sows fed 200VE and CON (P < 0.05). No significant differences were found among treatments for the content of solids-not-fat, fat, protein, and lactose in colostrum (P > 0.05). However, sows fed GSP had greater IgM and IgG content in colostrum compared with sows fed 200VE and CON (P < 0.05). In conclusion, dietary GSP supplementation during late gestation and lactation improved the farrowing survival and preweaning survivability, enhanced the antioxidant status and hormone levels in serum, and increased the IgM and IgG content in colostrum of sows.
Keywords: antioxidant status, grape seed polyphenols, hormone levels, immunoglobulin, reproductive performance, sow
INTRODUCTION
Gestation is characterized by dynamic changes in several tissues and organs that lead to increased basal oxygen consumption and metabolic status modifications in both the sow and the fetus (Berchieri-ronchi et al., 2015). Gestation is a state of high oxidative stress in animals, which is harmful to embryonic, fetal, and placental development (Al-Gubory et al., 2010; Yin et al., 2014). Reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radical, are some of the most important factors for oxidative stress and lead to significant oxidative damage. An imbalance between ROS and antioxidants is considered responsible for affecting female reproductive processes (Mou et al., 2018). Reactive oxygen species are eliminated or detoxified by different components of enzymatic defense system, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and glutathione reductase, and nonenzymatic antioxidant defense system, such as carotenoids, retinoids, vitamin C, vitamin E, albumin, uric acid, bilirubin, and transferrin (Čolak et al., 2017). Dietary antioxidants have been recognized as important contributors to the total antioxidant capacity of cells and plasma, which have the ability to neutralize ROS and other free radicals produced by oxidative stress (Oliveira et al., 2017). It has been reported that vitamin E supplementation in the sows diet increases milk α-tocopherol levels, prevents vitamin E deficiency in young piglets, improves the weight of piglets at weaning, and enhances the antioxidant activity of sows and piglets (Mahan, 1991, 1994; Shelton et al., 2014; Wang et al., 2017). As a commonly used antioxidant, besides vitamin E, a great number of secondary plant metabolites may be most promising due to their well-established antioxidative and gene regulatory properties (Aguirre et al., 2014; Gessner et al., 2017).
Polyphenols, as well-known natural antioxidants and chemopreventive agents, are one of the most common groups of substances in plants and their derived foods and beverages, as well as in flowers, vegetables, fruits, essential oil, and tea (Zhang and Tsao, 2016). Epidemiological, clinical, and nutritional studies have shown that polyphenols have a wide range of biological activities, include antioxidant (Gessner et al., 2017; Zou et al., 2018), anticancer (Fantini et al., 2015; Niedzwiecki et al., 2016; Colomer et al., 2017), and anti-inflammatory (Joseph et al., 2016; Zhang and Tsao, 2016) properties. Their antioxidant capacity is comparable to vitamin E, which is generally considered to be the main biological antioxidants and minimize the negative consequences of oxidative stress (Iqbal et al., 2015). Grape seed polyphenols (GSP) have been widely used as a human food supplement for health promotion and disease prevention. The polyphenols in grape pomace can increase the total antioxidant status and decrease lipid peroxidation in duodenum and colon, and increase SOD activity in duodenum and CAT and GSH-Px activity in the colon of piglets (Chedea et al., 2018). Feeding polyphenol-rich plant products (grape seed or grape pomace meal extract) has been shown to decrease expression of various proinflammatory genes in duodenum, ileum, and colon of growing pigs (Fiesel et al., 2014). However, the effects of dietary GSP supplementation on reproductive performance and antioxidative status of sows has been less investigated so far. Therefore, our objective is to investigate the reproductive performance, parameters of antioxidative status and hormone levels in serum, and Ig content in colostrum of sows that received GSP during late gestation and lactation.
MATERIALS AND METHODS
The experimental design and procedures of this study were reviewed and approved by the Institutional Animal Care and Use Committee of Hunan Agricultural University, China.
Animals, Diets, and Experimental Design
Grape seed polyphenols (total polyphenolic ≥ 501.3 mg/g dry weight) were extracted from grape seed and purchased from Hunan PERFLY Biotechnology Co. Ltd (Changsha, China). Sixty-four multiparous crossbred sows (Large White × Landrace, parity 4 and 5) with similar body condition on day 80 of gestation were allocated to 4 dietary treatments (n = 16 sows per treatment) in completely randomized block design and then maintained until the piglets were weaned on day 21 of lactation. The total feeding trial lasted for 56 d. The 4 experimental treatments were defined as follows: 1) basal diet (CON, control group); 2) basal diet supplemented with 200 IU/kg vitamin E (200VE, positive control group); 3) basal diet supplemented with 200 mg/kg GSP (200GSP); and 4) basal diet supplemented with 300 mg/kg GSP (300GSP). The basal diet was formulated to meet or exceed all the nutrient requirements of lactating sows according to National Research Council (NRC, 2012) and contained 50 IU/kg vitamin E. Since an additional 200 IU/kg vitamin E (dl-α-tocopherol acetate, Zhejiang NHU company LTD, Zhejiang, China) was supplemented to 200VE diet, the total vitamin E content of 200VE was 250 IU/kg. The ingredient composition and nutrient levels of the basal diet are shown in Table 1. The levels of vitamin E used in 200VE treatment based on Chen et al. (2016) and Wang et al. (2017). The levels of GSP used based on Meng et al. (2018) and Chedea et al. (2018).
Table 1.
Ingredients composition and nutrient levels of the basal diet1 (air dry basis)
| Ingredients | Composition, % | Items, unit | Nutrient level2 |
|---|---|---|---|
| Corn, 7.8% CP | 65.0 | CP, % | 17.2 |
| Soybean meal, 43% CP | 22.0 | DE, Mcal/kg | 3.30 |
| Wheat bran | 5.0 | DM, % | 87.5 |
| Fish meal, 65% CP | 2.0 | Ether extract, % | 5.27 |
| Soybean oil | 2.5 | Linoleic acid, % | 2.69 |
| Calcium phosphate | 1.1 | Calcium, % | 0.70 |
| Limestone | 0.8 | Total phosphorus, % | 0.61 |
| Salt | 0.3 | Available phosphorus, % | 0.37 |
| l-Lysine, 98% | 0.2 | Lysine, % | 1.10 |
| Threonine, 98% | 0.1 | Threonine, % | 0.68 |
| Vitamin and mineral premix3 | 1.0 | Crude fiber, % | 2.35 |
| Total | 100 |
1The basal diet was used throughout the trial period (from day 80 gestation until the piglets were weaned on day 21 of lactation).
2CP was an analyzed value, whereas the other nutrient levels were calculated.
3The vitamin and mineral premix supplied per kilogram of complete diet: 100 mg Zn (ZnSO4·H2O), 80 mg Fe (FeSO4·H2O), 22 mg Mn (MnSO4·H2O), 25 mg Cu (CuSO4·5H2O), 0.4 mg I (CaI2O6), 0.3 mg Se (Na2SeO3), 14,000 IU vitamin A, 4000 IU vitamin D3, 50 IU vitamin E, 124 mg vitamin K3, 4 mg vitamin B1, 10 mg vitamin B2, 0.04 mg vitamin B12, 4.8 mg vitamin B6, 40 mg niacin, 20 mg d-pantothenate, 2 mg folic acid, and 0.16 mg d-biotin.
The sows in gestation were kept in crates with half-slatted floors until the day 110 of pregnancy, then moved into the farrowing building in farrowing pens with crates. All sows were fed 2.60 kg/d individually, twice daily (at 08:00 and 15:00 h) in 2 equal meals from day 80 of gestation until farrowing, and provided ad libitum after parturition. Water was available ad libitum via nipple drinkers throughout the whole trial period. Cross-fostering was performed between sows in the same treatment and was completed within 24 to 48 h after birth. Litter size was equalized within treatment to achieve 9 to 11 pigs per sow. Relevant parameters of reproductive performance were recorded and calculated as described by Young et al. (2016). The number of total born piglet, born alive, dead fetuses, and mummies per sow were recorded at farrowing. Farrowing survival was calculated as the percent born alive out of the total number farrowed (born alive + dead fetuses + mummies). Preweaning survivability was calculated as the percentage of piglets weaned out of the number of piglets birthed by the sow regardless of whether she nursed them or not. Individual weights were measured at birth for all born alive piglets and at weaning for all weaning piglets. Total live piglet birth weight, average live piglet birth weight, average weight of weaned piglet, and average weight gain of piglet were calculated based on all piglets born to a sow regardless of whether she nursed them or not.
Sampling and Assay
On day 110 of pregnancy, 8 sows were randomly chosen from each treatment for blood sampling. Approximately 10 mL of blood was drawn into vacuum tube by puncture of the sow’s auricular vein and directly centrifuged at 2,000 × g for 10 min under 4 °C. Then supernatant serum of each sample was divided into 2 subsamples and stored at −20 °C until analysis. One subsample was used for determining the activities of SOD and GSH-Px, the levels of malondialdehyde (MDA), and total antioxidant capacity (T-AOC) in serum with an ELISA detection kit. The other subsample was used to determine the concentrations of progesterone (P4) and estradiol (E2) in serum by commercial ELISA Kit respectively. SOD kit, GSH-Px kit, MDA kit, and T-AOC kit were all purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). P4 and E2 ELISA Kit were obtained from Wuhan ColorfulGene Biological Technology Co., LTD (Wuhan, China).
Approximately 20 mL of colostrum from 6 sows per treatment was collected from the functional mammary glands after the last piglet was born. The definition of the last piglet born was when the contractions had stopped and after the expulsion of placenta (Kielland et al., 2015). Piglets were not separated from sows before and during colostrum collection. The colostrum sample from each individual was divided into 2 equal portions and stored at −20 °C until further analysis. One colostrum sample was diluted with 3 times its own quantity of purified water and assayed using a fully automated milk analyzer (UL120BC, Hangzhou Ultrasun Technologies Co., Ltd., Hangzhou, China) for nutrient composition (total solids-not-fat, fat, protein, and lactose). The other colostrum sample was used to quantify the content of IgG, IgA, and IgM. Colostral Ig proteins (IgG, IgA, and IgM) were measured with a swine ELISA kit (Wuhan Cusabio Biotech Co., Wuhan, China), and the final values of IgG, IgA, and IgM were expressed in grams per liter.
All of the laboratory analysis procedures followed the manufacturer’s instructions and included 2 duplicates in the same sample. The mean of the duplicates was used for data.
Statistical Analyses
All statistical analysis was performed using the SPSS Statistics 25.0 software (IBM SPSS, Chicago, IL). The differences between treatments were analyzed by one-way analysis of variance, and Duncan’s multiple comparisons tests were used to isolate means that differed. Results are given as mean values and a pooled SEM. A value of P < 0.05 was used to indicate statistical significance.
RESULTS
Reproductive Performance
Sow’s reproductive performance in response to dietary GSP supplementation is described in Table 2. No significant differences in the number of total born piglets, born alive piglets, and mummies were observed among treatments. Compared with the litters from sows fed CON, the number of dead fetuses was reduced (P < 0.05) and farrowing survival was significantly improved (P < 0.05) in the litters from 300GSP-fed. But there was no significant difference between the litters from sows fed 200VE and 200GSP (P > 0.05). Total live piglet birth weight, average live piglet birth weight, average weight of weaned piglet, average weight gain of piglet, and number weaned piglet of sow were not affected by dietary treatment (P > 0.05). However, the preweaning survivability was significantly increased in litters from 200VE- and 200GSP-fed sows compared with litters from CON-fed sows (P < 0.05).
Table 2.
Effects of dietary GSP supplementation on reproductive performance of sows
| Item (n = 16) | CON1 | 200VE1 | 200GSP1 | 300GSP1 | SEM | P-value |
|---|---|---|---|---|---|---|
| Days of pregnancy, d | 116.01 | 115.29 | 114.43 | 115.20 | 0.20 | 0.688 |
| Total born, no. | 12.00 | 11.81 | 11.50 | 11.88 | 0.29 | 0.245 |
| Number of born alive, no. | 9.75 | 10.00 | 10.00 | 10.50 | 0.25 | 0.164 |
| Number of dead fetuses, no. | 1.19a | 1.13ab | 0.75ab | 0.63b | 0.09 | 0.031 |
| Number of mummies, no. | 1.06 | 0.69 | 0.75 | 0.75 | 0.09 | 0.472 |
| Farrowing survival, % | 81.47b | 85.43ab | 87.53ab | 89.32a | 1.06 | 0.009 |
| Total live piglet birth weight, kg | 12.45 | 13.36 | 12.64 | 12.44 | 0.48 | 0.898 |
| Average live piglet birth weight, kg | 1.19 | 1.38 | 1.39 | 1.29 | 0.06 | 0.823 |
| Average weight of weaned piglet, kg | 5.87 | 6.19 | 6.24 | 6.06 | 0.12 | 0.299 |
| Average weight gain of piglet, kg | 4.69 | 4.85 | 4.84 | 4.78 | 0.16 | 0.347 |
| Number weaned piglet by sow, no. | 8.94 | 9.63 | 9.69 | 10.13 | 0.56 | 0.462 |
| Weaning weight by sow, kg | 52.48 | 59.56 | 60.46 | 61.38 | 1.68 | 0.536 |
| Preweaning survivability, % | 91.85b | 96.29a | 96.99a | 95.23ab | 0.76 | 0.016 |
a,bMeans values within a row with different letters superscripts means significantly different (P < 0.05).
1GSP = grape seed polyphenols; CON = basal diet; 200VE = basal diet + 200 IU/kg vitamin E; 200GSP = basal diet + 200 mg/kg GSP; 300GSP = basal diet + 300 mg/kg GSP.
Antioxidant Status and Hormone Levels in Serum
To investigate the effects of dietary supplementation with GSP during late gestation and lactation on antioxidant status, T-AOC, SOD, GSH-Px, and MDA in serum of sows were analyzed, and the results are presented in Table 3. The activity of T-AOC and the content of MDA in the serum of sows were not significantly different among treatments (P > 0.05). There was no significant difference in the activity of SOD in the serum of 200VE-fed sows, compared with the CON-fed sows (P > 0.05), but the activity of SOD in the serum of 200GSP- and 300GSP-fed sows increased significantly (P < 0.05). The activity of GSH-Px in the serum of sows fed 200VE, 200GSP, and 300GSP was significantly increased (P < 0.05). Compared with the positive control (sows fed 200VE), there was no significant difference in the activity of GSH-Px in serum of sows fed 200GSP and 300GSP (P > 0.05).
Table 3.
Effects of dietary GSP supplementation on the antioxidant status in serum of sow
| Items1 (n = 8) | CON2 | 200VE2 | 200GSP2 | 300GSP2 | SEM | P |
|---|---|---|---|---|---|---|
| T-AOC, IU/mL | 29.14 | 26.83 | 32.14 | 41.43 | 4.37 | 0.700 |
| SOD, IU/mL | 37.51b | 42.08b | 61.81a | 66.21a | 3.26 | <0.001 |
| GSH-Px, IU/mL | 417.83b | 586.30a | 562.24a | 620.33a | 23.71 | 0.002 |
| MDA, nmol/mL | 4.13 | 2.94 | 2.56 | 2.33 | 0.37 | 0.339 |
a,bMeans values within a row with different letters superscripts means significantly different (P < 0.05).
1T-AOC = total antioxidant capacity; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; GSH = glutathione; MDA = malonaldehyde.
2GSP = grape seed polyphenols; CON = basal diet; 200VE = basal diet + 200 IU/kg vitamin E; 200GSP = basal diet + 200 mg/kg GSP; 300GSP = basal diet + 300 mg/kg GSP.
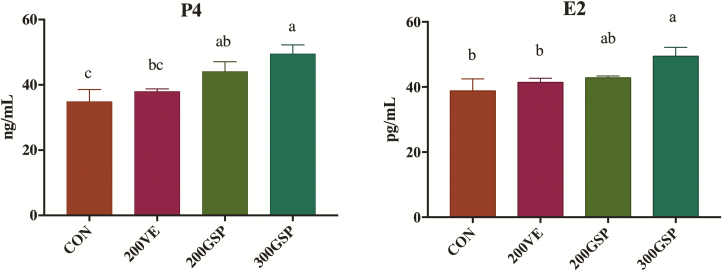
The effects of dietary GSP supplementation during late gestation on the levels of P4 and E2 in the serum of sows are shown in Fig. 1. Sows fed 300GSP had the greatest levels of P4 and E2 in serum, significantly greater than sows fed CON and 200VE, but the difference was not significant compared with sows fed 200GSP (P > 0.05).
Figure 1.
Effect of dietary GSP supplementation on the levels of P4 and E2 in serum of sows. GSP = grape seed polyphenols; CON = basal diet; 200VE = basal diet + 200 IU/kg vitamin E; 200GSP = basal diet + 200 mg/kg GSP; 300GSP = basal diet + 300 mg/kg GSP. P4 = Progesterone; E2 = estradiol. Data are mean ± SEM. a,b,cMeans values within a bar with different letters superscripts means significantly different (P < 0.05).
Nutrient Composition and Ig Content of Colostrum
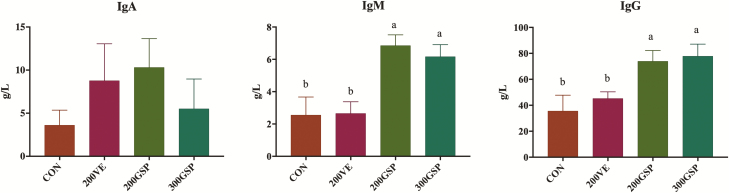
The effects of dietary GSP supplementation during late gestation and lactation on the nutrient composition in colostrum of sows are given in Table 4. No significant differences among treatments were found in colostral nutrient content (solids-not-fat, fat, protein, and lactose; P > 0.05). Figure 2 shows the effects of dietary GSP supplementation during late gestation and lactation on the Ig content in colostrum of sows. There was no significant difference in the concentrations of IgA in colostrum among treatments (P > 0.05). However, sows fed 200GSP and 300GSP had greater content of IgM and IgG in colostrum compared with sows fed 200VE and CON (P < 0.05).
Table 4.
Effect of dietary GSP supplementation on the composition in colostrum of sows
| Item, g/kg | CON1 | 200VE1 | 200GSP1 | 300GSP1 | SEM | P |
|---|---|---|---|---|---|---|
| Solids-not-fat | 215 | 219 | 225 | 218 | 2.43 | 0.248 |
| Fat | 45.5 | 41.0 | 40.1 | 45.8 | 1.64 | 0.285 |
| Protein | 91.8 | 86.5 | 93.0 | 87.0 | 1.18 | 0.059 |
| Lactose | 114 | 115 | 120 | 119 | 1.15 | 0.093 |
1GSP = grape seed polyphenols; CON = basal diet; 200VE = basal diet + 200 IU/kg vitamin E; 200GSP = basal diet + 200 mg/kg GSP; 300GSP = basal diet + 300 mg/kg GSP.
Figure 2.
Effects of dietary GSP supplementation on Ig in colostrum of sows. GSP = grape seed polyphenols; CON = basal diet; 200VE = basal diet + 200 IU/kg vitamin E; 200GSP = basal diet + 200 mg/kg GSP; 300GSP = basal diet + 300 mg/kg GSP. Data are mean ± SEM. a,bMeans values within a bar with different letters superscripts means significantly different (P < 0.05).
DISCUSSION
Reproductive performance of sows is one of the critical factors influencing the efficiency of swine production (Koketsu et al., 2017; Małopolska et al., 2018). The increase of oxidative stress during gestation, farrowing, and lactation of sows are responsible for the impaired reproductive performance of sows (Kim et al., 2013), which is a difficult problem to be faced in the swine industry. Although in the feed industry, vitamin E is often supplemented to the diet of farm animals to maintain the optimal health, highly productive and reproductive performance (Surai, 2014), previous studies have not been consistent on whether dietary supplementation with a high concentration of vitamin E resulted in a better reproductive performance of sows. Pinelli-Saavedra and Scaife (2005) reported that piglet growth performance and sow reproductive performance were unaffected by the dietary addition of vitamin E (200 and 400 mg/kg). Similarly, Sosnowska et al. (2011) did not observe any significant differences in reproductive performance of sows on the applied addition of 60, 200 mg/kg vitamin E during the last stage of gestation (day 90) and lactation. On the contrary, Wang et al. (2017) demonstrated that the addition of vitamin E to the diet of sows at high concentration (250 IU/kg) improved the weight of piglets at weaning. In the present study, the preweaning survivability was significantly increased in litters from 200VE-fed sows compared with litters from control-fed sows. There are many different reasons that might cause such a phenomenon, and further research is warranted. In addition, we also found that the number of dead fetuses was reduced, farrowing survival was improved with sows fed 300GSP, and the preweaning survivability was significantly increased in litters from 200GSP-fed sows during late gestation and lactation.
Polyphenols are characterized by high antioxidant activity due to their complex chemical structure and minimize the negative consequences of oxidative stress (Lipiński et al., 2017). It has been suggested that polyphenols could be more effective than traditional biological antioxidants, such as vitamin E and C (Surai, 2014). In addition, polyphenols exert nonspecific effects on cell metabolism and deliver health benefits to humans and animals (Petti and Scully, 2009; Kamboh et al., 2015). Garbetta et al. (2018) studied the influences of grape skin polyphenols on the modulation of ROS and GSH levels in basal and in stressed conditions on human intestinal cells (HT-29) and found that grape skin polyphenols exerted antioxidant effects up to 1.3 × 10−6 µg/g and restored the stress-related GSH reduction in stressed conditions. The results are consistent with that of Goutzourelas et al. (2015), who reported that polyphenolic composition of grape stem extracts at low concentrations increased the redox status of endothelial (EA.hy926) and muscle (C2c12) cells. In contrast to the potential antioxidative and anti-inflammatory effects of polyphenols that are well established in model animal and humans, the effects have been less investigated in sows so far (Gessner et al., 2017). Zhao et al. (2018) reported that the activities of T-AOC, glutathione peroxidase 4, and SOD in longissimus dorsi muscle were increased when lambs were fed diet containing 10% wine grape pomace. In addition, several studies developed in pigs have reported a significant increase in the serum antioxidant capacity of weaned piglets or sows that offered diets supplemented with grape seed procyanidins (Hao et al., 2015) or with catechins, which belongs to plant polyphenolic constituents (Fan et al., 2015). Our results demonstrate that dietary GSP supplementation during late gestation exerts a beneficial role in the antioxidant defense capacity of sows, that is achieved by increasing the activity of SOD and GSH-Px in serum in agreement with those in the literature mentioned above. The results of our study also further suggest that the oxidative stress in the process of sow farrowing was reduced by dietary GSP supplementation during late gestation.
Previous studies have shown that P4 plays an important role in establishing and maintaining pregnancy, and E2 is mainly secreted by the fetus, and increases accompanied with the normal development of the fetus, as a communication signal between sow and fetus during gestation (Perry et al., 1973; Wang et al., 2015). In the present study, the levels of P4 in the serum of pregnant sows (110 d) were significantly increased by dietary GSP supplementation during late gestation and the levels of E2 in the serum were also significantly increased in 300GSP-fed sows. According to these results, another hypothesis can be derived, namely, the addition of GSP in the diet of sows in early gestation has a positive impact on the levels of P4 and E2 in serum, thus playing a role in reducing embryo loss and promoting fetal development. In addition, P4 may play an active role in the IgG transfer from plasma to colostrum (Devillers et al., 2004). Jackson et al. (1995) showed that higher colostral IgG content in sows treated with P4 and farrowing at 116 d of gestation instead of 114 d. In this study, sows fed GSP had greater levels of P4 in serum at 110 d of gestation and greater IgG content in colostrum after farrowing. These results are consistent with those of previous studies.
Sow’s colostrum and milk are very important to the growth and development of piglets during and after lactation (Hurley, 2015). In particular, colostrum has been found to have several advantages over formula. In addition to providing energy and critical nutrients for growth and heat production for newborn piglets, it also has the potential to provide antioxidant protection and maternal immunity to piglets (Berchieri-ronchi et al., 2015), which is a key to the survival of newborn piglets. This study did not find a significant difference in nutrient composition (solids-not-fat, fat, protein, and lactose) in colostrum between sows fed GSP with sows fed CON. These results are consistent with those data obtained in dairy cows by Chedea et al. (2017), using 15% dried grape pomace in the diets, and the milk of cows fed with a grape pomace diet preserves the normal levels of fat, protein, and caseins.
Both IgG and IgM could be absorbed over the gastrointestinal tract during the first 24 to 48 h after birth; therefore, it is considered to be clinically important Ig during the first weeks of life (Sjaastad et al., 2010; Tan et al., 2017). The serum content of IgG in piglet is dependent on several factors, which is strongly associated with the IgG content of colostrum and the amount of colostrum ingested (Rooke and Bland, 2002). In addition, the Igs (IgA, IgG, and IgM) in colostrum are rapidly taken up by nonspecific pinocytosis into the enterocytes of the small intestine of the newborn piglet and localized in vacuoles (Rooke and Bland, 2002). Therefore, the increased IgG content of colostrum will improve the levels of IgG in piglets and potentially improve the survival rate of piglets (Kielland et al., 2015). In the present study, we found that the contents of IgM and IgG in colostrum of sows fed GSP were significantly increased compared with sows fed 200VE or CON. The preweaning survivability of piglets was significantly increased by dietary GSP supplementation of sows during late gestation and lactation. This might be related to the significant increase in IgG and IgM content in colostrum, which may improve the passive immunity and health status of piglets after birth.
CONCLUSIONS
Dietary GSP supplementation of multiparous sows during late gestation and lactation enhanced the antioxidant status and the levels of P4 and E2 in serum. In addition, it increased IgM and IgG content in colostrum of sows, which is potentially beneficial to increase the preweaning survivability of piglets. Overall, our study indicated the potential beneficial effects of dietary GSP supplementation in improving the reproduction performance of sows and suggested that GSP could be useful feed supplements in sow nutrition.
Footnotes
This work was supported by the Natural Science Foundation of Hunan Province, China (No. 2017JJ3135) and the China Scholarship Council (No. 201808430149). The authors declared that they have no conflict of interest.
LITERATURE CITED
- Aguirre L., M. P. Portillo E. Hijona, and Bujanda L.. 2014. Effects of resveratrol and other polyphenols in hepatic steatosis. World J. Gastroenterol. 20:7366–7380. doi: 10.3748/wjg.v20.i23.7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gubory K. H., P. A. Fowler, and Garrel C.. 2010. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 42:1634–1650. doi: 10.1016/j.biocel.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Berchieri-ronchi C. B., Presti P. T., Ferreira A. L. A., Correa C. R., and Salvadori D. M. F.. . 2015. Effects of oxidative stress during human and animal reproductions. Int. J. Nutrol. 8:6–11. [Google Scholar]
- Chedea V. S., Palade L. M., Marin D. E., Pelmus R. S., Habeanu M., Rotar M. C., Gras M. A., Pistol G. C., and Taranu I.. . 2018. Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 10:588. doi: 10.3390/nu10050588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedea V. S., R. S. Pelmus C. Lazar G. C. Pistol L. G. Calin S. M. Toma C. Dragomir, and Taranu I.. 2017. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 97:2516–2523. doi: 10.1002/jsfa.8068 [DOI] [PubMed] [Google Scholar]
- Chen J., Han J. H., Guan W. T., Chen F., Wang C. X., Zhang Y. Z., Lv Y. T., and Lin G.. . 2016. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 221:111–123. doi: 10.1016/j.anifeedsci.2016.08.022 [DOI] [Google Scholar]
- Čolak E., Ignjatović S., Radosavljević A., and Žorić L.. . 2017. The association of enzymatic and non-enzymatic antioxidant defense parameters with inflammatory markers in patients with exudative form of age-related macular degeneration. J. Clin. Biochem. Nutr. 60:100–107. doi: 10.3164/jcbn.16-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer R., A. Sarrats R. Lupu, and Puig T.. 2017. Natural polyphenols and their synthetic analogs as emerging anticancer agents. Curr. Drug Targets 18:147–159. doi: 10.2174/1389450117666160112113930 [DOI] [PubMed] [Google Scholar]
- Devillers N., C. Farmer A. M. Mounier J. Le Dividich, and Prunier A.. 2004. Hormones, igg and lactose changes around parturition in plasma, and colostrum or saliva of multiparous sows. Reprod. Nutr. Dev. 44:381–396. doi:10.1051/rnd:2004043 [DOI] [PubMed] [Google Scholar]
- Fan Z., Y. Xiao Y. Chen X. Wu G. Zhang Q. Wang, and Xie C.. 2015. Effects of catechins on litter size, reproductive performance and antioxidative status in gestating sows. Anim. Nutr. 1:271–275. doi: 10.1016/j.aninu.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M., M. Benvenuto L. Masuelli G. V. Frajese I. Tresoldi A. Modesti, and Bei R.. 2015. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 16:9236–9282. doi: 10.3390/ijms16059236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesel A., D. K. Gessner E. Most, and Eder K.. 2014. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 10:196. doi: 10.1186/s12917-014-0196-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbetta A., L. Nicassio I. D’Antuono A. Cardinali V. Linsalata G. Attolico, and Minervini F.. 2018. Influence of in vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food Res. Int. 106:878–884. doi: 10.1016/j.foodres.2018.01.072 [DOI] [PubMed] [Google Scholar]
- Gessner D. K., R. Ringseis, and Eder K.. 2017. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 101:605–628. doi: 10.1111/jpn.12579 [DOI] [PubMed] [Google Scholar]
- Goutzourelas N., D. Stagos Y. Spanidis M. Liosi A. Apostolou A. Priftis S. Haroutounian D. A. Spandidos A. M. Tsatsakis, and Kouretas D.. 2015. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 12:5846–5856. doi: 10.3892/mmr.2015.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R., Li Q., Zhao J., Li H., Wang W., and Gao J.. . 2015. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 178:237–242. doi: 10.1016/j.livsci.2015.06.004 [DOI] [Google Scholar]
- Hurley W. L. 2015. Composition of sow colostrum and milk. In: The gestating and lactating sow. Wageningen Academic Publishers, Wageningen, The Netherlands: p. 193–230. [Google Scholar]
- Iqbal Z., Kamran Z., Sultan J. I., Ali A., Ahmad S., Shahzad M. I., Ahsan U., Ashraf S., and Sohail M. U.. . 2015. Replacement effect of vitamin E with grape polyphenols on antioxidant status, immune, and organs histopathological responses in broilers from 1- to 35-d age. J. Appl. Poult. Res. 24:127–134. doi: 10.3382/japr/pfv009 [DOI] [Google Scholar]
- Jackson J. R., W. L. Hurley R. A. Easter A. H. Jensen, and Odle J.. 1995. Effects of induced or delayed parturition and supplemental dietary fat on colostrum and milk composition in sows. J. Anim. Sci. 73:1906–1913. doi: 10.2527/1995.7371906x [DOI] [PubMed] [Google Scholar]
- Joseph S. V., I. Edirisinghe, and Burton-Freeman B. M.. 2016. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 56:419–444. doi: 10.1080/10408398.2013.767221 [DOI] [PubMed] [Google Scholar]
- A. A. Kamboh. 2015. Flavonoids: health promoting phytochemicals for animal production-a review. J. Anim. Heal. Prod. 3:6–13. doi:10.14737/journal.jahp/2015/3.1.6.13 [Google Scholar]
- Kielland C., V. Rootwelt O. Reksen, and Framstad T.. 2015. The association between immunoglobulin G in sow colostrum and piglet plasma. J. Anim. Sci. 93:4453–4462. doi: 10.2527/jas.2014-8713 [DOI] [PubMed] [Google Scholar]
- Kim S. W., A. C. Weaver Y. B. Shen, and Zhao Y.. 2013. Improving efficiency of sow productivity: Nutrition and health. J. Anim. Sci. Biotechnol. 4:26. doi: 10.1186/2049-1891-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu Y., S. Tani, and Iida R.. 2017. Factors for improving reproductive performance of sows and herd productivity in commercial breeding herds. Porcine Health Manag. 3:1. doi: 10.1186/s40813-016-0049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipiński K., Mazur M., Antoszkiewicz Z., and Purwin C.. . 2017. Polyphenols in monogastric nutrition – A review. Ann. Anim. Sci. 17:41–58. doi: 10.1515/aoas-2016-0042 [DOI] [Google Scholar]
- Mahan D. C. 1991. Assessment of the influence of dietary vitamin E on sows and offspring in three parities: Reproductive performance, tissue tocopherol, and effects on progeny. J. Anim. Sci. 69:2904–2917. doi: 10.2527/1991.6972904x [DOI] [PubMed] [Google Scholar]
- Mahan D. C. 1994. Effects of dietary vitamin E on sow reproductive performance over a five-parity period. J. Anim. Sci. 72:2870–2879. doi: 10.2527/1994.72112870x [DOI] [PubMed] [Google Scholar]
- Małopolska M. M., Tuz R., Lambert B. D., Nowicki J., and Schwarz T.. . 2018. The replacement gilt: Current strategies for improvement of the breeding herd. J. Swine Heal. Prod. 26:208–214. [Google Scholar]
- Meng Q., Guo T., Li G., Sun S., He S., Cheng B., Shi B., and Shan A.. . 2018. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 9:34. doi: 10.1186/s40104-018-0248-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou D., J., Wang H., Liu Y., Chen L., Che Z., Fang S., Xu Y., Lin B., Feng J., Li, et al. 2018. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition 45:76–84. doi: 10.1016/j.nut.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Niedzwiecki A., Roomi M. W., Kalinovsky T., and Rath M.. . 2016. Anticancer efficacy of polyphenols and their combinations. Nutrients 8:552. doi: 10.1016/j.ymssp.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. rev National Academic Press, Washington, DC. [Google Scholar]
- Oliveira A. B., de Almeida Lopes M. M., Moura C. F. H., de Siqueira Oliveira L., de Souza K. O., Filho E. G., Urban L., and de Miranda M. R. A.. . 2017. Effects of organic vs. conventional farming systems on quality and antioxidant metabolism of passion fruit during maturation. Sci. Hortic. 222:84–89. doi: 10.1016/j.scienta.2017.05.021 [DOI] [Google Scholar]
- Perry J. S., R. B. Heap, and Amoroso E. C.. 1973. Steroid hormone production by pig blastocysts. Nature 245:45–47. doi: 10.1038/245045a0 [DOI] [PubMed] [Google Scholar]
- S. Petti, andScully C. . . . 2009. Polyphenols, oral health and disease: a review. J. Dent. 37:413–423. doi:10.1016/j.jdent.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Pinelli-Saavedra A., and Scaife J. R.. . 2005. Pre- and postnatal transfer of vitamins E and C to piglets in sows supplemented with vitamin E and vitamin C. Livest. Prod. Sci. 97:231–240. doi: 10.1016/j.livprodsci.2005.05.001 [DOI] [Google Scholar]
- Rooke J. A., and Bland I. M.. . 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78: 13–23. doi:10.1016/S0301-6226(02)00182-3 [Google Scholar]
- Shelton N. W., S. S., Dritz J. L., Nelssen M. D., Tokach R. D., Goodband J. M., DeRouchey H., Yang D. A., Hill D., Holzgraefe D. H., Hall, et al. 2014. Effects of dietary vitamin E concentration and source on sow, milk, and pig concentrations of α-tocopherol. J. Anim. Sci. 92:4547–4556. doi: 10.2527/jas.2014-7311 [DOI] [PubMed] [Google Scholar]
- Sjaastad Ø. V., Sand O., and Hove K.. . 2010. Physiology of domestic animals. 2nd ed. Scandinavian Veterinary Press, ; Oslo, Norway. [Google Scholar]
- Sosnowska A., Kawecka M., Jacyno E., Kołodziej-Skalska A., Kamyczek M., and Matysiak B.. . 2011. Effect of dietary vitamins E and C supplementation on performance of sows and piglets. Acta Agric. Scand. A Anim. Sci. 61:196–203. doi: 10.1080/09064702.2012.666560 [DOI] [Google Scholar]
- Surai P. F. 2014. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 98:19–31. doi: 10.1111/jpn.12070 [DOI] [PubMed] [Google Scholar]
- Tan L., T. Wei A. Yuan J. He J. Liu D. Xu, and Yang Q.. 2017. Dietary supplementation of astragalus polysaccharides enhanced immune components and growth factors EGF and IGF-1 in sow colostrum. J. Immunol. Res. 2017:9253208. doi: 10.1155/2017/9253208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., X. Xu G. Su B. Shi, and Shan A.. 2017. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 84:8–13. doi: 10.1017/S0022029916000650 [DOI] [PubMed] [Google Scholar]
- Wang X., H. Qiu M. Zhang X. Cai Y. Qu D. Hu X. Zhao E. Zhou S. Liu, and Xiao Y.. 2015. Distribution of highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) in different stages of gestation sows: HP-PRRSV distribution in gestation sows. Vet. Immunol. Immunopathol. 166:88–94. doi: 10.1016/j.vetimm.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Yin J., M. M. Wu H. Xiao W. K. Ren J. L. Duan G. Yang T. J. Li, and Yin Y. L.. 2014. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92:612–619. doi: 10.2527/jas.2013-6986 [DOI] [PubMed] [Google Scholar]
- Young J. M., R. Bergsma E. F. Knol J. F. Patience, and Dekkers J. C.. 2016. Effect of selection for residual feed intake during the grow/finish phase of production on sow reproductive performance and lactation efficiency. J. Anim. Sci. 94:4120–4132. doi: 10.2527/jas.2015-0130 [DOI] [PubMed] [Google Scholar]
- Zhang H., and Tsao R.. . 2016. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 8:33–42. doi: 10.1016/j.cofs.2016.02.002 [DOI] [Google Scholar]
- Zhao J. X., Li Q., Zhang R. X., Liu W. Z., Ren Y. S., Zhang C. X., and Zhang J. X.. . 2018. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed Sci. Technol. 236:76–85. doi: 10.1016/j.anifeedsci.2017.12.004 [DOI] [Google Scholar]
- Zou X., Xiao R., Li H., Liu T., Liao Y., Wang Y., Wu S., and Li Z.. . 2018. Effect of a novel strain of Lactobacillus brevis M8 and tea polyphenol diets on performance, meat quality and intestinal microbiota in broilers. Ital. J. Anim. Sci. 17:396–407. doi: 10.1080/1828051X.2017.1365260 [DOI] [Google Scholar]