Abstract
The goal of this study was to test the hypothesis that sodium selenite (ISe), SEL-PLEX (OSe), vs. a 1:1 blend (MIX) of ISe and OSe in a basal vitamin-mineral mix would differentially affect serological and hepatic parameters of growing steers grazing toxic endophyte-infected tall fescue-mixed forage pasture. Predominately Angus steers (BW = 183 ± 34 kg) were randomly selected from herds of fall-calving cows grazing endophyte-infected tall fescue-mixed pasture and consuming vitamin-mineral mixes that contained 35 ppm Se as ISe, OSe, and MIX forms. Steers were weaned, depleted of Se for 98 d, and subjected to summer-long common grazing of an endophyte-infected tall fescue-mixed pasture (0.51 ppm total ergovaline + ergovalinine; 10.1 ha). Steers were assigned (n = 8 per treatment) to the same Se form treatments upon which they were raised. Se treatments were administered by daily top-dressing 85 g of vitamin-mineral mix onto 0.23 kg soyhulls, using in-pasture Calan gates. The PROC MIXED procedure of SAS was used to assess the effect of Se form treatments on serum parameters at day 0, 22, 43, 64, and 86. After slaughter, the effect of Se treatment on hepatic alkaline phosphatase (tissue nonspecific isoform, TNALP) mRNA, protein, and albumin protein content was assessed using the PROC GLM procedure of SAS. Fisher’s protected LSD procedure was used to separate treatment means. Partial correlation analysis was used to evaluate the relationship among whole blood Se concentration and serum parameters, accounting for the effect of time. Across periods, MIX steers had more (P ≤ 0.04) serum albumin than OSe and ISe steers, respectively. However, the relative hepatic bovine serum albumin protein content was not affected (P = 0.28) by Se treatments. Serum alkaline phosphatase activity was greater (P ≤ 0.01) in MIX and OSe steers. Similarly, hepatic TNALP protein content in MIX steers was greater (P = 0.01) than ISe steers. Partial correlation analysis revealed that serum albumin, blood urea nitrogen, and alkaline phosphatase activity were correlated (r ≥ 0.23, P ≤ 0.02) with whole blood Se concentration. In summary, consumption of 3 mg Se/d as OSe or MIX forms of Se in vitamin-mineral mixes increased serum albumin concentration and alkaline phosphatase activity, the reduction of which is associated with fescue toxicosis. We conclude that the organic forms of Se ameliorated the depression of 2 of known serological biomarkers of fescue toxicosis.
Keywords: albumin, alkaline phosphatase, fescue toxicosis, selenium supplementation, steer
Introduction
Ergot alkaloids produced by symbiont endophytes in tall fescue (Lolium arundinaceum) have been identified as causative agents for a variety of negative physiological capacities, collectively known as fescue toxicosis (Strickland et al., 2011). In addition to the classical suppression of serum prolactin, decreased serum alkaline phosphatase activity is another common clinical marker associated with cattle grazing endophyte-infected tall fescue (Boling et al., 1989; Schultze et al., 1999; Brown et al., 2009; Jackson et al., 2015). Other serological markers of fescue toxicosis have been reported, including decreased aspartate aminotransferase activity (Brown et al., 2009), alanine aminotransferase activity (Oliver et al., 2000; Brown et al., 2009), albumin concentration (Jackson et al., 2015), and creatinine kinase activity (Dougherty et al., 1991), but increased creatinine concentration (Schultze et al., 1999). However, the exact mechanisms, as well as the physiological consequence of these serological changes have not been fully delineated.
Besides fescue toxicosis, another challenge faced by many endophyte-infected tall fescue-based beef cattle operations is that the forage often is Se-inadequate (below 0.1 ppm; NASEM, 2016), due to Se-poor soils (Ammerman and Miller, 1975). A common method used to supplement Se to the diet of beef cattle is the inclusion of Se in free-choice vitamin-mineral mixes. Although inorganic forms (sodium selenite, sodium selenite) of Se are most commonly used, the use of organic forms of Se (OSe) in vitamin-mineral mixes typically results in greater blood and tissue Se concentrations (bioavailability) (Nicholson et al., 1991; Gunter et al., 2003; Liao et al., 2011). Interestingly, feeding a 1:1 blend of ISe:OSe (MIX) results in equal amount of Se in whole blood, red blood cells, serum, and liver of heifers as when supplemented with only OSe, both of which are greater than ISe-supplemented heifers (Brennan et al., 2011). Hepatic transcriptome profiles have indicated that, compared to ISe or OSe, MIX uniquely stimulates expression of genes involved in selenoprotein synthesis and glutamate/glutamine metabolism (Matthews et al., 2014). Serendipitously, some of the genes upregulated by MIX were found downregulated in the liver (Liao et al., 2015) and pituitary (Li et al., 2017) of steers grazing high vs. low endophyte-infected forages. Subsequently, a study (Jia et al., 2018) was conducted to evaluate the effect of forms (ISe, OSe, and MIX) of supplemental Se on physiological parameters of growing steers subjected to summer-long grazing of endophyte-infected tall fescue pasture. As a hallmark of fescue toxicosis, the concentration of serum prolactin in this study was decreased over time in all steers. However, across summer-long grazing periods, steers supplemented with MIX or OSe forms of Se had 59% (P < 0.03) and 52% (P < 0.05) more serum prolactin than ISe steers, respectively (Jia et al., 2018).
To elaborate and explore these findings from the initial study (Jia et al., 2018), the first goal of this study was to test the hypotheses that the form (ISe, OSe, and MIX) of supplemental Se in vitamin-mineral mixes consumed by growing beef steers grazing endophyte-infected tall fescue forage would ameliorate the negative serological parameters associated with fescue toxicosis, including depressed serum alkaline phosphatase activity and albumin content. The second goal was to evaluate the potential relationships between whole blood Se concentration and serum clinical parameters.
MATERIALS AND METHODS
All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC protocol #1007A2006).
Animals, Slaughter, and Tissue Collection
Specific descriptions of the animal model, Se treatment administration, and liver sample collection procedures of experimental samples used in the current study have been described (Jia et al., 2018). However, briefly, 24 suckling predominantly Angus beef steers (BW: 183 ± 33.9 kg, age: 166 ± 14.2 d) were randomly selected (n = 8) from herds of fall-calving cows grazing toxic endophyte-infected tall fescue-mixed forage pastures and that had ad libitum access to a common, basal, vitamin-mineral mix that contained either 35 mg/kg Se as ISe (sodium selenite, Prince Se concentrate; Prince Agri Products, Inc., Quincy, IL), OSe (SEL-PLEX, Alltech Inc., Nicholasville, KY), or an 1:1 blend of ISe:OSe (MIX) forms. Over a pre-study, 98-d Se-depletion period (March 11 to June 17, 2015), steers were weaned, trained to consume the basal vitamin-mineral mix (lacking Se) from in-pasture Calan gates and, thereby, commonly depleted of Se. During this 98-d period, the ADG of steers was 0.82 ± 0.18 kg/d.
Steers then began (day 0) an 86-d (June 17 to September 9, 2015) common grazing period in which steers collectively grazed a predominately endophyte-infected tall fescue-mixed pasture (10.1 ha), and individually consumed their respective Se-form-specific vitamin-mineral mix treatments (ISe, OSe, MIX) through the use of in-pasture Calan gates. Steers were assigned to the same Se-form-specific vitamin-mineral mix treatment on which they were raised and which their dams received. The Se-specific mixes contained the basal mix plus 35 mg Se/kg as either ISe, OSe, or MIX. The composition of the basal vitamin-mineral mix was analyzed by Dairy One (Dairy One Cooperative, Inc., Ithaca, NY) and was previously described in the initial study (Jia et al., 2018). To ensure individual consumption of 3 mg Se/d per steer, 85 g of the specific vitamin-mineral mix (35 mg/kg Se) was top-dressed onto 227 g of soyhulls in each individual Calan gate feeder. The consumption of the mixture of soyhulls/vitamin-mineral mix was monitored daily, and all steers consumed all of the mixture every day. Thus, every steer consumed 3 mg of supplemental Se/d. During this 86-d period, 2 ISe steers were removed from the trial due to a bad hoof and failure to consume their mineral treatment. Thus, the final number of experimental observations was as follows: ISe = 6, OSe = 8, and MIX = 8.
Steers were slaughtered over a 26-d period, from day 93 to 119 (September 17 to October 13, 2015) of the study. Specifically, 1 OSe and 1 MIX steer were killed on the first 2 slaughter days. On the remaining 6 slaughter days, 1 steer each from ISe, OSe, and MIX treatment groups was killed. The slaughter process for these steers has been described in detail (Jia et al., 2018). Liver samples were collected from the mid-lower right lobe and placed in foil packs, snap-frozen in liquid nitrogen, and stored at −80 °C for microarray and real-time RT-PCR analyses.
Pasture samples (30 sites per sample day) were collected throughout the common grazing (days 0, 22, 43, 64, 86) and slaughter (day 115) periods and analyzed separately. The mean total concentration of ergovaline and ergovalanine was 0.51 ppm (Jia et al., 2018).
Blood Collection and Analyses
Jugular vein blood samples were collected by venipuncture on day 0, 22, 43, 64, and 86. For serum, 16 mL of blood was collected in serum blood collection tubes (Becton Dickinson) without an anticoagulant. Serum was recovered after centrifugation at 3,000 × g for 10 min at 4 °C, and stored at −80 °C. All serum enzymes and analytes were assayed by the American Association for Veterinary Laboratory Diagnosticians approved-University of Kentucky Veterinary Diagnostic Laboratory (Lexington, KY). For serum enzymes, activities of alkaline phosphatase (ALP), E.C. 3.1.3.1; aspartate transaminase (AST/SGOT), E.C. 2.6.1.1; γ-glutamyltransferase (GGT), E.C. 2.3.22; creatine kinase, E.C. 2.7.3.2 were determined as per the manufacturer of the reagent kits (Alfa Wassermann, Diagnostic Technologies, West Caldwell, NJ) using a VET AXCEL Chemical Analyzer (Alfa Wassermann, Diagnostic Technologies, West Caldwell, NJ).
Western Blot Analysis
All western blot and densitometric analyses for the relative expression of targeted proteins were performed using a standard protocol of our lab as previously described (Miles et al., 2015). Approximately 0.2 g of liver was homogenized on ice for 30 s (setting 11, Polytron Model PT10/35, Kinematic Inc., Lucerne, Switzerland) in 7.5 mL of 4 °C sample extraction buffer solution [0.25 mM sucrose, 10 mM HEPES-KOH pH 7.5, 1 mM EDTA, and 50 μL of protease inhibitor (Sigma, St. Louis, MO)]. Protein was quantified by a modified Lowry assay, using bovine serum albumin (BSA) as a standard (Kilberg, 1989). Proteins were separated using 12% SDS-PAGE and electrotransferred onto a 0.45 μm nitrocellulose membrane (Bio-Rad) and then stained with Fast-Green (Fisher, Pittsburgh, PA). The relative amount of stained protein per lane per sample was determined by densitometric analysis and recorded as arbitrary units (Brown et al., 2009).
The use of rabbit IgG anti-BSA polyclonal antibody and rabbit IgG anti-bovine alkaline phosphatase (tissue nonspecific, liver/bone/kidney; TNALP) was validated by pre-adsorption of primary antibodies with their respective antigen polypeptides, using the general procedures of Xue et al. (2011). Immunoreaction products for BSA (Mr = 69 kDa, Supplementary Figure S1) and TNALP (Mr = 21, 37, 48 kDa, Supplementary Figure S2) in liver homogenates (5 μg for BSA, 30 μg for TNALP) were abolished if pre-adsorbed (μg:μg) with increasing amounts of their antigens, thus validating their use. The relative protein content of BSA and TNALP in liver homogenates was detected using procedures as previously described (Miles et al., 2015). Briefly, blots were hybridized with 1 μg of IgG anti-BSA polyclonal antibody (Abcam Inc., Cambridge, MA) per milliliter of blocking solution [3% nonfat dry milk (wt/vol), 30 mM Tris-Cl (pH 7.5), 200 mM NaCl, 0.1% Tween 20 (vol/vol)] for 1.5 h at room temperature with gentle rocking. For TNALP detection, blots were hybridized with 0.6 μg IgG anti-recombinant bovine TNALP polyclonal antibody (MyBioSource, San Diego, CA) per milliliter of blocking solution [5% nonfat dry milk (wt/vol), 30 mM Tris-Cl (pH 7.5), 125 mM NaCl, 0.1% Tween 20 (vol/vol)] for 1.5 h at room temperature with gentle rocking. Protein–primary antibody-binding reactions were visualized with a chemiluminescence kit (Pierce, Rockford, IL) after hybridization of primary antibody with horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham, Arlington Heights, IL; TNALP, 1:5,000).
Densitometric analysis of immunoreactive products was performed as described previously (Miles et al., 2015). After exposure of autoradiographic film (Amersham, Arlington Heights, IL), digital images of all observed immunoreactive species were recorded and quantified as described (Dehnes et al., 1998). Apparent migration weights (Mr) were calculated by regression of the distance migrated against the Mr of a 16 to 185 kDa standard (Gibco BRL, Grand Island, NY) using the Versadoc imaging system (Bio-Rad) and Quantity One software (Version 4.2.3, Bio-Rad). Band intensities of the single immunoreactant (69 kDa) for BSA, and the predominant immunoreactant (37 kDa) for TNALP, were quantified by densitometry (as described above for Fast-Green-stained proteins) and reported as arbitrary units. Densitometric data were corrected for unequal (10%, TNALP; 17%, BSA) loading, transfer, or both, and amount of detected protein normalized to relative amounts of Fast-Green-stained proteins common to all immunoblot lanes/samples (Miles et al., 2015). Digital images were prepared using PowerPoint software (Microsoft, PowerPoint 2013, Bellevue, MA).
Hepatic RNA Extraction and Analysis
For each animal, total RNA was extracted from 300 mg of frozen liver tissue using TRIzol Reagent (Invitrogen Corporation, Carlsbad, CA) following the manufacturer’s instructions. The purity and concentration of total RNA samples was analyzed using a NanoDrop ND-100 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). All samples had an average concentration of 1.05 μg/μL and a high purity with 260/280 absorbance ratios of 1.98 to 2.03 and 260/230 absorbance ratios ranging from 1.89 to 2.18. The integrity of total RNA was examined by gel electrophoresis using an Agilent 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA) at the University of Kentucky Microarray Core Facility. Visualization of gel images and electropherograms showed that all RNA samples were of high quality with 28S/18S rRNA absorbance ratios greater than 2 and RNA integrity numbers (RIN) greater than 8.7.
Real-Time RT-PCR Analysis
The quantification of relative mRNA for genes of interest was performed using standard procedures in our laboratory, as previously described (Cerny et al., 2016). Briefly, 1 µg of each steer’s liver RNA was reversely transcribed to cDNA using the SuperScript III 1st Strand Synthesis System (Invitrogen). Real-time RT-PCR was performed using an Eppendorf Mastercycler ep realplex2 system (Eppendorf, Hamburg, Germany) with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). A total volume of 25 µL was used in each real-time RT-PCR reaction containing 5 µL of cDNA, 1 µL of a 10 µM stock of each primer (forward and reverse), 12.5 µL of 2× SYBR Green PCR Master Mix, and 5.5 µL of nuclease-free water. The relative amount of each transcript was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Primer sets (Supplementary Table S1) for genes of interest were designed using the NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) based on their respective RefSeq sequence (https://www.ncbi.nlm.nih.gov/refseq/, accessed February to April, 2018).
All real-time RT-PCR cDNA products were validated by DNA sequencing and were 99% to 100% identical with their RefSeq (https://www.ncbi.nlm.nih.gov/refseq/) sequences (Supplementary Table S1). Briefly, the PCR-amplified cDNA products were electrophoresed in a 1.2% agarose (ULTRAPURE agarose, Invitrogen, Carlsbad, CA) slab gel. A single cDNA band at the desired size was identified under a UV light, excised from the gel, purified using the PureLink Quick Gel Extraction Kit (Invitrogen, Carlsbad, CA), then sequenced by Eurofins Genomics (Eurofins MWG Operon LLC, Louisville, KY). The resulting sequences (Supplementary Figure S3) were compared to the NCBI RefSeq mRNA sequences used as templates for primer pair set design. From 9 constitutively expressed gene candidates (ACTB, GAPDH, SDHA, PCK2, UBC, YWHAZ, HPRT1, PPIA, TBP) 3 constitutively expressed genes (YWHAZ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta; HPRT1, hypoxanthine phosphoribosyltransferase; PPIA, peptidylprolyl isomerase A) were selected with the lowest average stability value of M = 0.19, calculated using the geNorm software v3.5 (Vandesompele et al., 2002). The relative mRNA expression was normalized to the geometric means of 3 constitutively expressed genes. For the RT-PCR analysis, n = 6, 8, and 8 samples were used for ISe, OSe, and MIX treatments, respectively. RT-PCR reactions were performed in triplicate.
Statistical Analysis
Data are presented as least square means (±SEM). Steers were the experimental units. The effect of Se supplementation on serum analytes was evaluated by ANOVA, using the PROC MIXED procedure of SAS (v 9.4, SAS Inst. Inc., Cary, NC). The statistical model included Se supplementation, time, and their interaction as fixed effects. Class variables were Se supplementation and steer, with steer included in the random statement. The Kenward–Roger adjustment was used to calculate the denominator of df (Kenward and Roger, 1997). Within Se supplement treatments, nonorthogonal polynomial contrasts (linear, quadratic, cubic, and quartic) were used to characterize the effect of Se treatments over time on serum clinical parameters, using the PROC IML procedure of SAS to generate coefficients for unequally spaced contrasts. The highest order contrast that was statistically significant is reported. Partial correlations between whole blood Se concentrations and serum clinical analytes, and controlling the effects of day, were conducted by using the MANOVA/PRINTE statement of PROC GLM procedure of SAS. After slaughter, the effect of Se supplementation on the relative abundance of hepatic mRNA (RT-PCR) and protein (western blotting) was assessed by ANOVA, using the PROC GLM procedure of SAS. For all data, Fisher’s protected LSD procedure was used to separate treatment means.
RESULTS
Serum Clinical Parameters
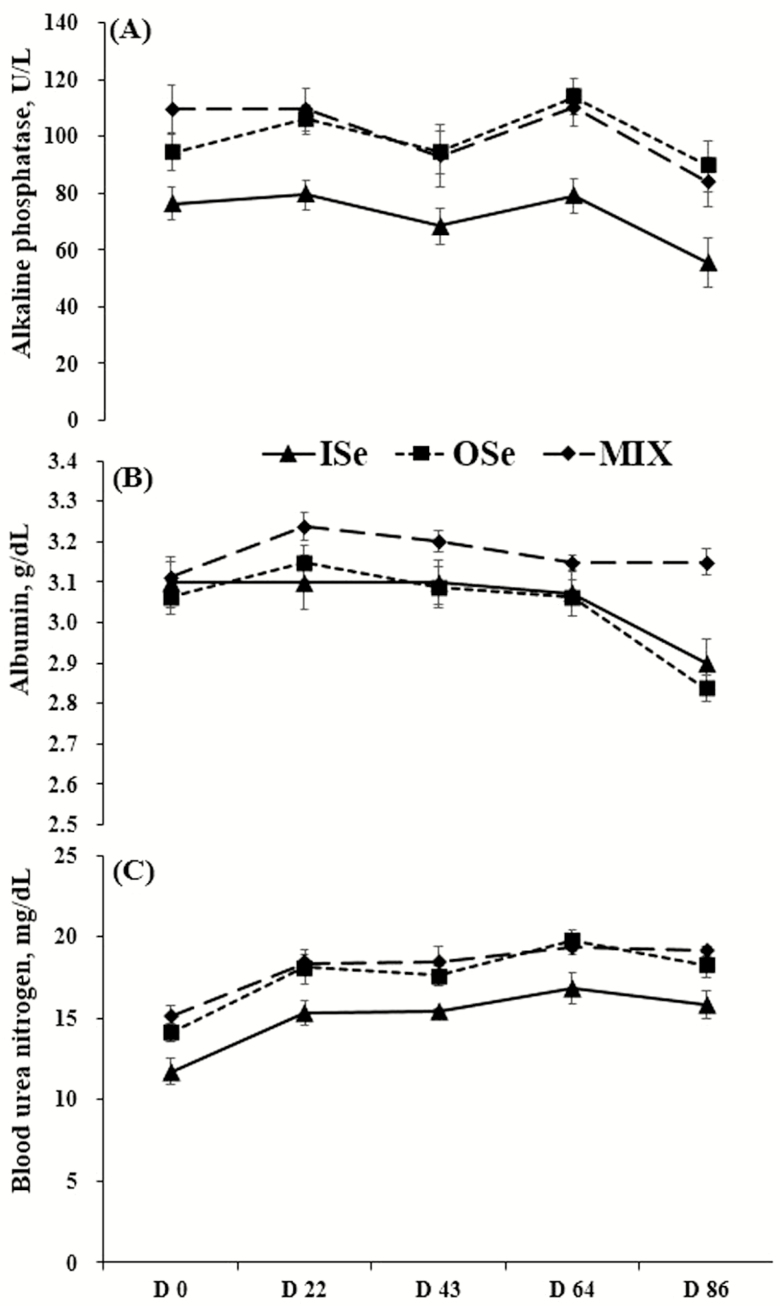
Serum alkaline phosphatase activity was affected (P = 0.01) by Se treatment (Fig. 1A). Across periods, MIX and OSe steers had 42% (P < 0.01) and 40% (P = 0.01) more serum alkaline phosphatase activity than ISe steers, respectively, and did not differ from each other (P = 0.87). A day effect was observed (P < 0.01) in serum alkaline phosphatase activity, but there was no (P = 0.70) Se treatment by day interaction. Across Se treatments, serum alkaline phosphatase activity on day 0, day 22, and day 64 was not different (P ≥ 0.15) within each other, and all greater than that on day 43 (P ≤ 0.07) and day 86 (P ≤ 0.01). Within Se treatments, nonorthogonal polynomial contrast analysis revealed that the serum alkaline phosphatase activity of ISe steers decreased linearly (P = 0.05) in response to time. Similarly, serum alkaline phosphatase activity of MIX steers tended (P = 0.07) to decrease linearly in response to time. In contrast, serum alkaline phosphatase activity of OSe steers did not (P ≥ 0.11) show a significant response to time.
Figure 1.
Serum alkaline phosphatase (A), albumin (B), and blood urea nitrogen (C) concentrations in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe, triangle), SEL-PLEX (OSe, square), or an 1:1 blend of ISe and OSe (MIX, diamond). Data are the least squares means (n = 6 for ISe, n = 8 for OSe and MIX) ± SE. (A) Se form (P = 0.01), time (P < 0.01), and Se form by time interaction (P = 0.70). (B) Se form (P = 0.03), time (P < 0.01), and Se form by time interaction (P < 0.01). (C) Se form (P < 0.01), time (P < 0.01), and Se form by time interaction (P = 0.92). Results for nonorthogonal polynomial contrasts for Se treatments in panels A, B, and C are reported in the Results section of the text. Note that the y-axis for panel B does not begin at 0.
The concentration of serum albumin was affected by Se treatment (P = 0.03), day (P < 0.01), and their interaction (P < 0.01) (Fig. 1B). Specifically, across periods, MIX steers had 4.3% (P = 0.01) and 3.7% (P = 0.04) greater serum albumin concentrations than OSe and ISe steers, respectively, whereas the serum albumin concentration of OSe steers was not significantly different (P = 0.74) from that of ISe steers. Across Se treatments, the concentration of serum albumin was least on day 86 (P < 0.01), whereas day 22 had significantly greater (P < 0.02) serum albumin than day 0 and day 64. The Se treatment by day interaction seems to mostly reflect the differential change of albumin concentration from day 0 to day 22, and day 64 to day 86. Therefore, nonorthogonal polynomial contrast analysis was conducted to characterize the response pattern of serum albumin to time within Se treatments. The concentration of serum albumin essentially decreased in a quadratic manner for both ISe and OSe steers, reflecting a marked drop of 7% (P = 0.10) and 6% (P < 0.01), respectively, from day 64 to day 86. In contrast, the serum albumin concentration of MIX steers did not (P ≥ 0.07) change over time. The differential response in serum albumin concentrations to time between ISe and OSe vs. MIX is reflected by the significant Se treatment by day interaction.
The concentration of blood urea nitrogen was affected (P < 0.01) by Se treatment (Fig. 1C). Specifically, MIX and OSe steers had 21% (P < 0.01) and 17% (P < 0.01) greater blood urea nitrogen than ISe steers, respectively, and did not differ from each other (P = 0.50). A day effect was found (P < 0.01) for blood urea nitrogen, but there was no (P = 0.93) Se treatment by day interaction. Across Se treatments, the concentration of blood urea nitrogen was least on day 0 (P < 0.01), greatest on day 64 (P ≤ 0.05), and not different among day 22, day 43, and day 86 (P ≥ 0.34). Within Se treatments, nonorthogonal polynomial contrast analysis indicated that blood urea nitrogen increased in response to time in a quadratic manner for ISe (P = 0.02), OSe (P = 0.02), and MIX (P = 0.01) steers, apparently reflecting a marked increase from day 0 to day 22 before plateauing.
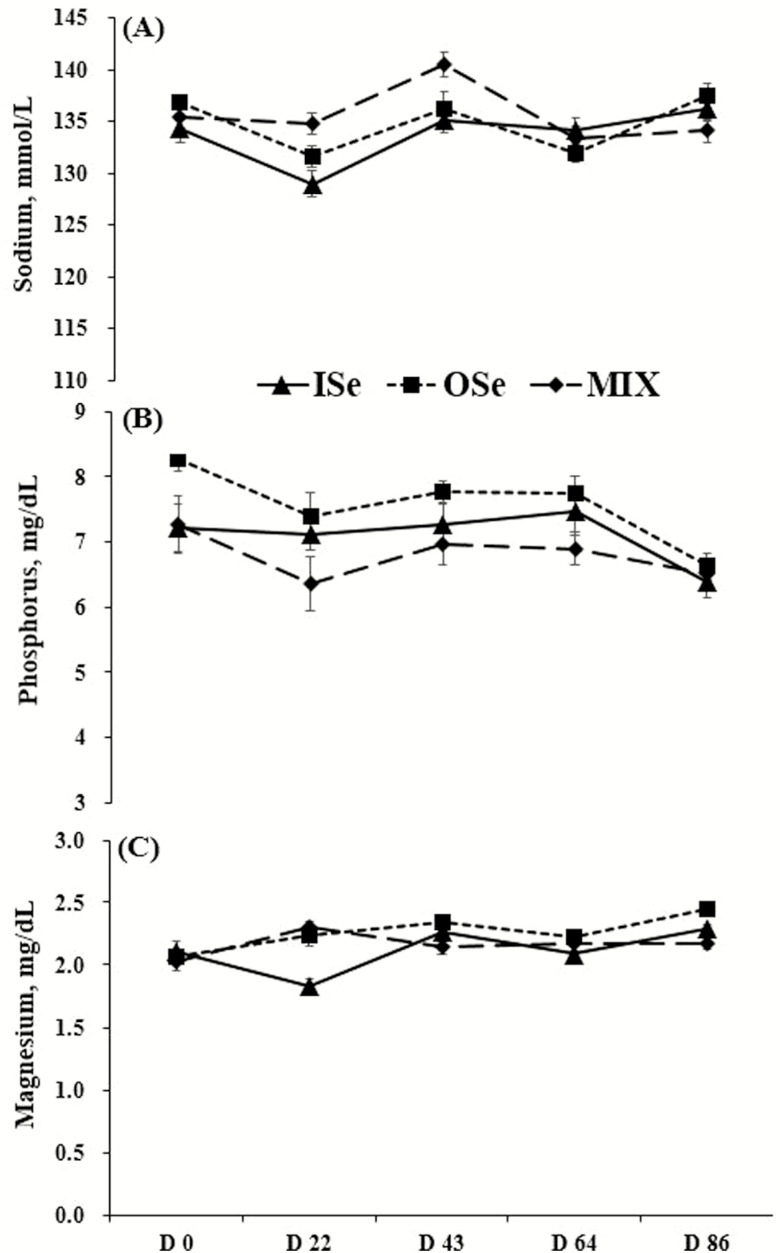
A main effect of Se supplementation treatment (P = 0.03), day (P < 0.01), and their interactions (P < 0.01) were observed for serum sodium concentrations (Fig. 2A). Across periods, MIX steers had 1.4% greater (P < 0.01) serum sodium concentration than ISe steers, but did not differ (P = 0.21) from OSe steers. The concentration of serum sodium between ISe and OSe steers was not different (P = 0.11). Across Se treatments, the concentration of serum sodium among day 0, day 43, and day 86 was not different (P ≥ 0.07), and greater (P ≤ 0.01) than those on day 22 and day 64. Within Se treatments, nonorthogonal polynomial contrast showed that the concentration of serum sodium responded in a quartic manner to time for ISe (P = 0.02), OSe (P < 0.01), and MIX (P = 0.01) steers.
Figure 2.
Serum sodium (A), phosphorus (B), and magnesium (C) concentrations in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe, triangle), SEL-PLEX (OSe, square), or an 1:1 blend of ISe and OSe (MIX, diamond). Data are the least squares means (n = 6 for ISe, n = 8 for OSe and MIX) ± SE. (A) Se form (P = 0.03), time (P < 0.01), and Se form by time interaction (P < 0.01). (B) Se form (P < 0.01), time (P < 0.01), and Se form by time interaction (P = 0.48). (C) Se form (P = 0.02), time (P < 0.01), and Se form by time interaction (P < 0.01). Results for nonorthogonal polynomial contrasts for Se treatments in panels A, B, and C are reported in the Results section of the text. Note that the y-axis for panels A and B does not begin at 0.
The concentration of serum phosphorus was affected (P < 0.01) by Se treatment (Fig. 2B). Specifically, OSe steers had 11% (P < 0.01) and 6.8% (P = 0.04) greater serum phosphorus concentration than MIX and ISe steers, respectively, but ISe and MIX steers did not differ (P = 0.23) from each other. A day effect (P < 0.01) was observed on serum phosphorus concentration, but there was no (P = 0.48) Se treatment by day interaction. Within Se treatments, according to nonorthogonal polynomial contrast analysis, serum phosphorus concentration tended (P = 0.08) to quadratically decrease in response to time in ISe steers. However, the concentration of serum phosphorus in OSe steers decreased in a cubic manner (P < 0.01) over time, whereas it tended (P = 0.07) to cubically decrease in MIX steers.
The concentration of serum magnesium was affected by Se treatment (P = 0.02), day (P < 0.01), and Se treatment by day interaction (P < 0.01) (Fig. 2C). Across periods, OSe steers had 7.2% greater (P < 0.01) serum magnesium concentration than ISe steers, but did not differ (P = 0.06) from MIX steers. The concentration of serum magnesium between ISe and MIX steers did not differ (P = 0.29). Within Se treatments, nonorthogonal polynomial contrast analysis showed an essentially linear (P < 0.01) increase of serum magnesium concentration in response to time for ISe and OSe steers. In contrast, serum magnesium concentrations of MIX steers tended (P = 0.07) to cubically increase in response to time.
The blood urea nitrogen:creatinine ratio was affected by Se treatment (P < 0.01) and day (P < 0.01) (Table 1). However, no Se treatment by day interaction was observed (P = 0.21). More specifically, within the main effect of Se treatments, OSe and MIX steers had 20% (P < 0.01) and 17% (P < 0.01) greater blood urea nitrogen:creatinine ratio than ISe steers. This Se treatment effect might be driven by the combination of elevated blood urea nitrogen concentration (P < 0.01), and unchanged creatinine concentration (P = 0.51) in OSe and MIX steers vs. ISe steers.
Table 1.
Serum clinical parameters in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX)
| Item | Treatment | Day 0 | Day 22 | Day 43 | Day 64 | Day 86 | SEM | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Se form | Day | Se form by Day | ||||||||
| Potassium, mmol/L | ISe | 4.53 | 3.98 | 4.23 | 4.09 | 4.37 | 0.060 | 0.78 | <0.01 | 0.22 |
| OSe | 4.60 | 4.09 | 4.34 | 4.08 | 4.38 | 0.056 | ||||
| MIX | 4.68 | 4.20 | 4.50 | 3.95 | 4.13 | 0.056 | ||||
| Chloride, mmol/L | ISe | 108.4 | 104.3 | 105.7 | 100.4 | 104.7 | 0.53 | 0.40 | <0.01 | <0.01 |
| OSe | 103.3 | 107.0 | 103.1 | 103.6 | 101.9 | 0.49 | ||||
| MIX | 108.5 | 104.5 | 101.4 | 101.1 | 106.4 | 0.49 | ||||
| Calcium, mg/dL | ISe | 10.21 | 10.00 | 9.41 | 9.96 | 9.57 | 0.088 | 0.43 | <0.01 | 0.23 |
| OSe | 9.70 | 10.25 | 9.34 | 9.79 | 9.51 | 0.081 | ||||
| MIX | 9.65 | 10.39 | 9.49 | 9.71 | 10.06 | 0.081 | ||||
| Creatinine, mg/dL | ISe | 1.43 | 1.12 | 1.11 | 1.00 | 1.24 | 0.037 | 0.51 | <0.01 | 0.05 |
| OSe | 1.35 | 1.13 | 1.09 | 1.03 | 1.18 | 0.035 | ||||
| MIX | 1.46 | 1.15 | 1.16 | 1.10 | 1.18 | 0.035 | ||||
| Blood urea nitrogen1:creatinine ratio | ISe | 8.43 | 13.67 | 14.14 | 16.86 | 12.71 | 0.470 | <0.01 | <0.01 | 0.21 |
| OSe | 10.88 | 16.25 | 16.50 | 19.50 | 15.38 | 0.437 | ||||
| MIX | 10.38 | 15.88 | 16.13 | 17.63 | 16.38 | 0.437 | ||||
| Glucose, mg/dL | ISe | 82.9 | 84.3 | 84.7 | 81.6 | 77.7 | 1.48 | 0.79 | <0.01 | 0.35 |
| OSe | 81.4 | 85.1 | 85.6 | 84.8 | 77.8 | 1.38 | ||||
| MIX | 82.9 | 83.5 | 81.8 | 81.6 | 78.1 | 1.38 | ||||
| Creatinine kinase, U/L | ISe | 218.4 | 218.7 | 187.9 | 199.7 | 180.4 | 9.2 | 0.71 | <0.01 | 0.44 |
| OSe | 225.4 | 213.4 | 205.3 | 200.6 | 205.5 | 8.6 | ||||
| MIX | 229.0 | 208.3 | 197.3 | 214.8 | 183.3 | 8.6 | ||||
| Aspartate aminotransferase, U/L | ISe | 63.7 | 55.3 | 56.4 | 58.3 | 54.0 | 2.88 | 0.24 | <0.01 | 0.10 |
| OSe | 62.4 | 60.0 | 60.9 | 61.5 | 58.5 | 2.69 | ||||
| MIX | 70.8 | 60.4 | 60.8 | 63.0 | 65.9 | 2.69 | ||||
| γ-Glutamyltransferase, U/L | ISe | 13.14 | 13.00 | 15.14 | 14.86 | 14.14 | 0.749 | 0.73 | 0.02 | 0.50 |
| OSe | 13.00 | 14.88 | 15.75 | 15.50 | 15.38 | 0.699 | ||||
| MIX | 13.38 | 13.75 | 14.25 | 14.88 | 15.75 | 0.701 | ||||
| Total protein, g/dL | ISe | 6.67 | 6.83 | 7.19 | 6.84 | 7.14 | 0.082 | 0.13 | <0.01 | <0.01 |
| OSe | 6.88 | 7.05 | 7.24 | 7.03 | 6.95 | 0.076 | ||||
| MIX | 7.11 | 7.28 | 7.19 | 7.25 | 7.01 | 0.076 | ||||
| Globulin, g/dL | ISe | 3.57 | 3.73 | 4.09 | 3.77 | 4.24 | 0.094 | 0.61 | <0.01 | <0.01 |
| OSe | 3.81 | 3.90 | 4.15 | 3.96 | 4.11 | 0.088 | ||||
| MIX | 4.00 | 4.04 | 3.99 | 4.10 | 3.86 | 0.088 | ||||
| Albumin1:globulin ratio | ISe | 0.86 | 0.85 | 0.76 | 0.83 | 0.70 | 0.024 | 0.70 | <0.01 | <0.01 |
| OSe | 0.81 | 0.81 | 0.76 | 0.78 | 0.71 | 0.023 | ||||
| MIX | 0.79 | 0.80 | 0.80 | 0.79 | 0.81 | 0.023 |
Data are least squares means (n = 6, ISe; n = 8, OSe; n = 8, MIX).
1Value of blood urea nitrogen and albumin are presented in Fig. 1.
Significant day effects (P ≤ 0.02) were observed for serum potassium, chloride, calcium, creatinine, glucose, creatinine kinase, aspartate aminotransferase, γ-glutamyltransferase, total protein, and globulin analytes, and the albumin:globulin ratio. However, no Se treatment effect (P ≥ 0.13) was observed in these serum parameters. A Se treatment × day interaction was found (P < 0.01) for serum chloride, total protein, and globulin concentrations, and the albumin:globulin ratio.
Hepatic BSA Protein Content, Alkaline Phosphatase mRNA, and Protein Content
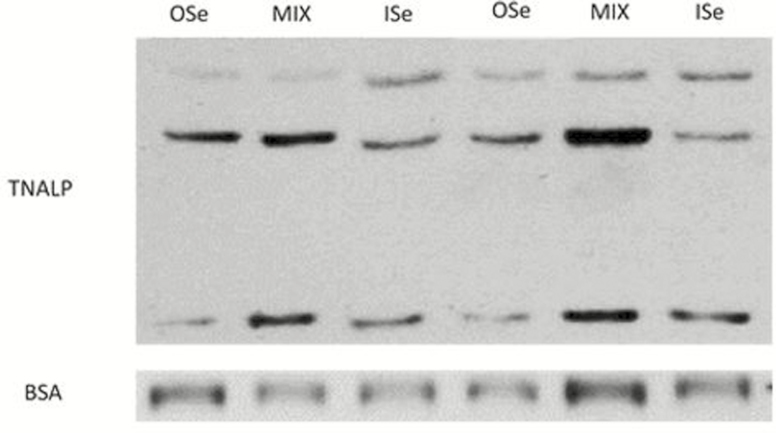
The relative amount of hepatic BSA protein was not affected (P = 0.28) by Se treatment (Table 2; Fig. 3). In contrast, selenium treatment did affect (P = 0.04) the amount of hepatic alkaline phosphatase (tissue nonspecific, liver/bone/kidney, TNALP), with MIX steers having 147% more (P = 0.01) TNALP than ISe steers, and not differing (P = 0.17) from OSe steers (Table 2; Fig. 3). No difference (P = 0.15) was found in TNALP protein content between OSe and ISe steers, despite OSe steers having numerically more (78%) TNALP than ISe steers. In contrast, the relative expression of hepatic TNALP mRNA did not differ (P = 0.37) among Se treatments (Table 2).
Table 2.
Hepatic bovine serum albumin protein content, and alkaline phosphatase (tissue nonspecific, liver/bone/kidney, TNALP) mRNA and protein content, in liver homogenates of slaughtered beef steers grazing endophyte-infected tall fescue that were supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe, n = 6), SEL-PLEX (OSe, n = 8), or an 1:1 blend of ISe and OSe (MIX, n = 8)1
| Item | Treatment2 | ||||
|---|---|---|---|---|---|
| ISe | MIX | OSe | SEM | P-value | |
| Bovine serum albumin, protein3 | 2,329 | 2,579 | 1,825 | 344.3 | 0.28 |
| TNALP, protein3 | 828a | 2,046b | 1,470ab | 298.0 | 0.04 |
| TNALP, mRNA4 | 1.05 | 0.85 | 0.98 | 0.1 | 0.37 |
1Values are least squares means and pooled SEM.
2Means within a row that lack a common letter differ (P < 0.05).
3Values (arbitrary densitometric units) were determined by densitometric evaluation of western blot data (Fig. 3).
4Values are relative level of mRNA expression normalized to the geometric mean of 3 constitutively expressed reference genes: tyrosine 3-monooxygenase/tryptophan 5-monooxygenase (YWHAZ), hypoxanthine phosphoribosyltransferase 1 (HPRT1), and peptidylprolyl isomerase A (PPIA).
Figure 3.
Western blot analysis of alkaline phosphatase (tissue nonspecific, liver/bone/kidney, TNALP), and bovine serum albumin (BSA) content in liver homogenates (45 μg per lane for BSA, 30 μg per lane for TNALP) of slaughtered growing beef steers grazing endophyte-infected tall fescue that were supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe), SEL-PLEX (OSe), or an 1:1 blend of ISe and OSe (MIX). Data are representative of 6 ISe, 8 OSe, and 8 MIX steers (as described in Table 2). The apparent migration weights (kDa) of the lower, middle, and upper immunoreactants for TNALP were 21, 37, and 48, respectively. The apparent migration weight (kDa) of the single immunoreactant for BSA was 69.
Partial Correlation of Whole Blood Se Concentration With Serum Clinical Parameters
For both across and within Se treatments, partial correlation analysis was conducted to determine the relationships between whole blood Se concentrations and serum clinical parameters, while controlling the effect of day (Table 3). Across Se treatments, weak positive correlations (0.244 > r > 0.209) were observed between whole blood Se and serum albumin (P = 0.02), magnesium (P = 0.01), potassium (P = 0.03), glucose (P = 0.03), and total protein (P = 0.01). In contrast, the whole blood Se was weakly and negatively correlated with serum potassium (r = −0.213, P = 0.01).
Table 3.
Partial correlation of serum clinical parameters with whole blood selenium concentration in growing beef steers grazing endophyte-infected tall fescue and supplemented with 3 mg Se/d in vitamin-mineral mixes as sodium selenite (ISe, n = 6), SEL-PLEX (OSe, n = 8), or an 1:1 blend of ISe and OSe (MIX, n = 8)
| Item | Partial correlation1 | |
|---|---|---|
| Coefficient | P-value | |
| Prolactin | −0.046 | 0.64 |
| Blood urea nitrogen | 0.38 | <0.01 |
| Creatinine | −0.443 | <0.01 |
| Blood urea nitrogen/creatinine ratio | 0.527 | <0.01 |
| Glucose | 0.209 | 0.03 |
| Alkaline phosphatase | 0.321 | <0.01 |
| Creatine kinase | −0.13 | 0.18 |
| Aspartate aminotransferase | −0.136 | 0.16 |
| γ-Glutamyltransferase | 0.026 | 0.79 |
| Total protein | 0.244 | 0.01 |
| Albumin | 0.226 | 0.02 |
| Globulin | 0.144 | 0.14 |
| Albumin/globulin ratio | −0.015 | 0.88 |
| Sodium | −0.121 | 0.22 |
| Potassium | −0.213 | 0.03 |
| Chloride | −0.17 | 0.08 |
| Calcium | 0.109 | 0.27 |
| Phosphorus | −0.006 | 0.95 |
| Magnesium | 0.246 | 0.01 |
1Whole blood Se vs. serum analytes, accounting for the effect of time.
Across Se treatments, moderate correlations were found between the whole blood Se and serum alkaline phosphatase (r = 0.321, P < 0.01), blood urea nitrogen (r = 0.38, P < 0.01), creatinine (r = −0.443, P < 0.01), and blood urea nitrogen:creatinine ratios (r = 0.527, P < 0.01).
Discussion
Animal Model
As described previously in the initial report (Jia et al., 2018), the steers of this study had ad libitum access to their respective supplemental Se treatments as suckling calves before their selection into the trial, as did their dams. During this preweaning period, the ad libitum intake of neither dams nor steers was determined. After being weaned, depleted and then repleted with the form of supplemental Se on which they were raised, and adapted to eat from Calan gates, steers then were subjected to summer-long common grazing of tall fescue-mixed pasture, while individually consuming 3 mg/d of their respective Se from initiation of the grazing period until their slaughter (up to 113 d) by use of Calan gates. Although it is likely that the postweaning regimen accounted for the specific Se treatment-specific effects for most measured parameters of this study, the possibility exists that the specific Se treatment-specific effects were influenced by events before imposition of the postweaning regimen. For example, as discussed below for the identified preexisting differences in serum alkaline phosphatase activities and albumin concentrations among ISe, OSe, and MIX steers at day 0, inherent differences in the Se physiology of these steer groups raised on different forms of Se in their vitamin-mineral mixes during suckling may have existed before random selection into the study and imposition of the Se form-specific postweaning regimens. It is for this possibility that steers were assigned to consume the same form of Se in their vitamin-mineral mixes that they and their dams received before their selection into this study.
As described in the Materials and Methods section, ISe, OSe, and MIX steers were randomly selected from 3 cow-calf herds with free access to ISe, OSe, and MIX form of Se in vitamin-mineral mixes, respectively. Because Se was depleted for 98 d after selection, it is reasonable to suggest that the preexisting differences in serum alkaline phosphatase activity and blood urea nitrogen concentration possibly were caused by Se status-induced epigenetic effects.
Also as previously reported in the initial paper of this study (Jia et al., 2018), the mean summer-long concentration of ergot alkaloids in the endophyte-infected tall fescue pasture was 0.51 ppm (ergovaline plus ergovalinine). Importantly, a previous grazing study from our lab showed summer-long grazing of endophyte-infected tall fescue that contained 0.52 ppm ergovaline plus ergovalinine was sufficient to induce fescue toxicosis (Brown et al., 2009). The classic reduction of serum prolactin concentration in steers of the current study confirms the onset of fescue toxicosis. The observed rapid increase and then stabilization in whole blood concentrations of Se in all 3 Se treatment group steers is consistent with our previous Se depletion/repletion regimens with growing beef heifers (Brennan et al., 2011; Liao et al., 2011). Moreover, compared to ISe steers, OSe and MIX steers had greater serum prolactin concentrations across summer-long (86-d) grazing periods than ISe steers (Jia et al., 2018). Taken together, the animal model was successfully established to evaluate the ability of different forms of supplemental Se to ameliorate the negative serological parameters of growing beef steers grazing toxic endophyte-infected tall fescue pasture.
MIX and OSe Forms of Se Supplementation Resulted in Greater Serum Alkaline Phosphatase Activity vs. ISe
Decreased serum total alkaline phosphatase activity has been widely reported in cattle consuming ergot alkaloids from endophyte-infected tall fescue (Boling et al., 1989; Rice et al., 1997; Schultze et al., 1999; Brown et al., 2009). The consistently suppressed serum alkaline phosphatase activity has been regarded as a repeatable clinical measure of fescue toxicosis (Oliver et al., 2000). The exact mechanism of this decrease of serum total alkaline phosphatase activity induced by fescue toxicosis is not understood. However, in vitro studies have shown that cyclic AMP induces de novo synthesis of alkaline phosphatase mRNA and results in an increased alkaline phosphatase protein production in mouse L-cells (Firestone and Heath, 1981). Also, cyclic AMP has been reported to induce the TNALP mRNA expression in L929 fibroblastic cells (Gianni et al., 1993). Due to structural similarities with dopamine and other biogenic amines, ergot alkaloids bind and stimulate dopamine type 2 receptors (Larson et al., 1994; Larson et al., 1999), consequently inhibiting adenylyl cyclase and then cyclic AMP (Fitzgerald and Dinan, 2008). Other researchers (Boling et al., 1989; Rice et al., 1998) have speculated that ergot alkaloids could negatively affect the alkaline phosphatase induction by inhibiting cyclic AMP, thus resulting in a decrease of total alkaline phosphatase or certain alkaline phosphatase isoform activity in the serum.
Throughout the grazing period of the current study, the serum alkaline phosphatase activity was greater in OSe and MIX than ISe steers. A priori, this finding suggests that consumption of organic forms of Se somehow mitigated the negative effects of ergot alkaloid binding of the dopamine type 2 receptor, thus resulting in less repression of cyclic AMP production. However, an alternative or parallel explanation may involve the reported differential effect of Se forms on dopamine-stimulated neuronal signaling (Solovyev, 2015). More specifically, consumption (3 and 9 ppm in drinking water) of ISe (selenite), but not OSe, resulted in increased concentrations of dopamine and its metabolites in murine striatum (Tsunoda et al., 2000). Therefore, compared to ISe, it is reasonable to speculate that OSe-supplemented steers may have had lower dopamine concentrations, and consequently, less activation of dopamine-signaling cascades and less inhibition of cyclic AMP. The inductive effect of cyclic AMP to alkaline phosphatase mRNA and protein, and the greater serum alkaline phosphatase activity found in OSe and MIX than ISe steers in this study appears consistent with the findings in brain tissue (Tsunoda et al., 2000) and cell culture models (Firestone and Heath, 1981; Gianni et al., 1993) discussed above. That is, the consumption of ISe may exacerbate the negative effect of consuming ergot alkaloids has on cyclic AMP-mediated repression of alkaline phosphatase. However, to support this speculation, research is needed to determine the effect of Se form on the expression and function of key enzyme/proteins involved in the dopamine-signaling cascade, and its possible downstream regulatory effects on prolactin and alkaline phosphatase.
Total serum alkaline phosphatase activity is comprised from various soluble alkaline phosphatase released from membranes of different tissues. In cattle, serum alkaline phosphatase mostly originates from liver and bone (Doornenbal et al., 1988), and has been used as a serum biomarker of liver or bone-related diseases. In mammals, including cattle, tissue nonspecific isoenzyme of alkaline phosphatase (TNALP) is expressed in most tissues, including liver, bone, kidney, and placenta (Hank et al., 1993). Therefore, to determine if Se treatments on liver may have contributed to the greater serum alkaline phosphatase activity of OSe and MIX steers, the TNALP mRNA and protein content was measured in homogenates of liver collected at slaughter. In agreement with their study-long increased serum alkaline phosphatase activity, MIX steers had 147% more hepatic TNALP protein content than ISe steers (Table 2). In contrast to the significantly higher serum alkaline phosphatase activity of OSe steers, the hepatic TNALP protein content of OSe steers did not differ, despite being numerically greater (78%). These data indicate that the elevated serum alkaline phosphatase activity in MIX steers was at least partially the result of an increased serum abundance of the liver isoform of alkaline phosphatase. That hepatic mRNA content also did not differ, is consistent with the understanding that hepatic TNALP is commonly regulated by posttranslational modifications (Fernandez and Kidney, 2007).
MIX Form of Se Supplementation Resulted in Greater Serum Albumin Than OSe and ISe
Serum albumin is another parameter previously reported affected by grazing endophyte-infected tall fescue. A summer-long grazing study showed that the serum albumin concentrations was lower (P = 0.05) in steers grazing high endophyte-infected (0.52 ppm of ergovaline and ergovalinine) tall fescue than those grazing low endophyte-infected (0.01 ppm of ergovaline and ergovalinine) tall fescue for 85 d (Brown et al., 2009). Interestingly, this decreased concentration of serum albumin was also observed on days 37 (P = 0.03) and 59 (P = 0.01) of the same steers undergoing summer-long grazing high endophyte-infected tall fescue (Jackson et al., 2015). It is important to note that all steers had ad libitum access (free-choice) to a vitamin-mineral mix that was identical in composition to the ISe treatment in the current study. Although the exact reason behind the serum albumin reduction is not clear, the authors suggested that the hepatic albumin synthetic capacity was reduced in steers grazing endophyte-infected tall fescue (Brown et al., 2009), and possibly as a consequence of reduced intake and malnutrition. In the current study, this reduced serum albumin associated with grazing endophyte-infected tall fescue was alleviated in steers supplemented with the MIX form of Se. However, in contrast to the serum concentrations, the hepatic albumin protein content in MIX steers did not differ from that of OSe and ISe steers (Table 2). Because it is well accepted that serum albumin is synthesized and secreted from hepatocytes into blood circulation (accounting for about 13% of the total protein produced by the liver; Peters, 1977), these data suggest that the increased serum albumin concentration in MIX steers either was not due to an elevated synthetic capacity of hepatic albumin, or that MIX steers had an elevated albumin synthesis capacity that was matched by an increased albumin secretion capacity. To better understand the physiological significance of the greater concentration of serum albumin in MIX steers, research is needed to study the metabolic fate of circulating albumin.
Blood Urea Nitrogen, and Serum Sodium, Phosphorus, and Magnesium Concentration Were Affected by Form of Se
The concentration of blood urea nitrogen is a function of synthesis from hepatic ornithine cycle and renal removal by renal glomerular filtration. In addition, ruminants recycle a considerable amount of blood urea to the rumen by excretion across forestomach epithelia and salivary gland secretion (Archibeque et al., 2001). In the current study, serum creatinine concentrations were not affected by the form of Se supplementation (Table 1). Thus, it is reasonable to suggest that the renal capacity of glomerular filtration did not differ among ISe, OSe, and MIX steers, given that serum creatinine is the most efficient indirect marker of glomerular filtration rate in mammals (Kaneko et al., 2008). Therefore, altered hepatic urea synthesis or gastrointestinal tract urea recycling seems more likely to be responsible for the elevated serum blood urea nitrogen concentration in OSe and MIX steers than that of ISe steers. Interestingly, as reported previously (Jia et al., 2018), the upregulation of glutamine synthetase (critical enzyme in hepatic ammonia fixating) in mRNA, protein content, and enzyme activity in MIX and OSe steers strongly indicates an altered hepatic nitrogen metabolism in MIX- and OSe-treated steers. Taken together, it appears to be reasonable to relate the different blood urea nitrogen profile to the altered hepatic nitrogen metabolism induced by MIX and OSe form of Se.
It is important to note that the difference in serum alkaline phosphatase activities (Fig. 1A) and blood urea nitrogen concentrations (Fig. 1C) among ISe, OSe, and MIX existed at the beginning of the experiment (day 0). This preexisting difference might be explained by the inherent different Se physiology of these 3 groups of steers raised on different forms of selenium in their vitamin-mineral mixes. As described in the Materials and Methods section, ISe, OSe, and MIX steers were randomly selected from 3 cow-calf herds with free access to ISe, OSe, and MIX form of Se in vitamin-mineral mixes, respectively. Because Se was depleted for 98 d after selection, it is reasonable to suggest that the preexisting differences in serum alkaline phosphatase activity and blood urea nitrogen concentration possibly were caused by Se status-induced epigenetic effects. Importantly, Se status has been shown to affect hepatic DNA methylation in the liver of mice (Speckmann et al., 2017). Moreover, our lab has shown that calf whole blood Se concentrations were correlated to, and affected by, the dam’s sources of Se (Patterson et al., 2013), wherein the whole blood Se concentrations in OSe and MIX calves were higher than for ISe calves. Therefore, it is reasonable to speculate in the current study that a different Se status existed in Se form-specific steers from after birth to weaning (selection), which may have resulted in epigenetic changes that fundamentally affected the metabolism of alkaline phosphatase and blood urea nitrogen.
The literature is limited with regard to the potential interaction of Se supplementation with sodium, phosphorus, and magnesium metabolism. When supplemented with the ISe form of Se, steers grazing high endophyte-infected tall fescue (0.52 ppm total ergot alkaloids: ergovaline plus ergovalinine) had decreased serum sodium concentration on day 36 than that grazing low endophyte-infected tall fescue (Jackson et al., 2015). It was speculated that the reduction of serum prolactin might be responsible for this because prolactin promotes the retention of Na+ and K+ by the kidney (Richardson, 1973) and the absorption of Na+, K+, and Ca2+ by intestinal epithelia (Mainoya, 1975). In the current study, across periods, MIX steers had higher serum sodium concentration than ISe steers (Fig. 2A). Interestingly, the serum prolactin concentration in current study was higher in MIX steers than ISe steers (Jia et al., 2018). Given the various biological effects of prolactin on water and electrolyte balance (Bole-Feysot et al., 1998), the higher serum sodium concentration in MIX steers in the current study could be due to the high serum prolactin concentrations. However, concentrations of serum potassium and calcium were not affected (Table 1) by Se form treatment in this study. Nevertheless, and regardless of their physiological consequences, across periods, the average concentration of serum sodium, phosphorus, and magnesium in ISe, OSe, and MIX steers (Fig. 2) was all within reference range, as serum sodium 132 to 152 mmol/L, serum phosphorus 3.5 to 8 mg/dL, and magnesium 1.7 to 2.9 mg/dL (University of Kentucky Veterinary Diagnostic Lab, http://vdl.uky.edu/).
Whole Blood Se Concentration Is Correlated With Alkaline Phosphatase Activity, and Albumin, Blood Urea Nitrogen, and Magnesium Concentrations
Whole blood Se concentration is one of the parameters commonly determined to indicate Se status, and is well correlated with Se intake in cattle (Patterson et al., 2013). Again as reported (Jia et al., 2018), whole blood Se concentrations were affected by the form of Se supplementation, with OSe and MIX steer concentrations being higher than that of ISe steers. Therefore, the second goal of this study was to evaluate the potential relationships between whole blood Se and serum analytes (Table 3). Due to the significant time effect observed in all serum parameters, the partial correlation was used to account for the time effect. The significant positive correlation between whole blood Se and the serum blood urea nitrogen:creatinine ratio can be mathematically explained by the finding that whole blood Se was positively and negatively correlated with blood urea nitrogen and serum creatinine, respectively. Although the concentrations of serum creatinine and potassium did not differ among Se treatments, significant negative correlations were observed between whole blood Se and serum creatinine and potassium concentrations. The reason and physiological consequences of these negative correlations need to be determined. Nevertheless, significant positive correlations between whole blood Se and serum albumin, alkaline phosphatase activity, magnesium, and blood urea nitrogen, were found, which seems to suggest that the changes of these parameters were associated with the alterations of whole blood Se. In contrast, serum prolactin, sodium, and phosphorus concentrations were affected by the form of supplemental Se, but not correlated with whole blood Se concentration. This inconsistency may be reasonable given the various biological roles of Se and given that selenoproteins perform many of these roles (Labunskyy et al., 2014).
In summary, predominantly Angus steers subjected to summer-long grazing of endophyte-infected pasture and supplemented (3 mg/d) with MIX or OSe forms of Se had higher whole blood Se, serum albumin, and blood urea nitrogen concentrations, and greater serum alkaline phosphatase activity than ISe-supplemented steers. Moreover, these analytes were positively correlated with whole blood Se concentration, which is one of the indicators of whole body Se status. These findings indicate the inclusion of organic forms of Se in vitamin-mineral mixes of steers grazing endophyte-infected tall fescue forages increase the concentration of 2 of known serological biomarkers of fescue toxicosis, that are depressed in cattle suffering from fescue toxicosis. When combined with the understanding that organic forms of Se also elevate depressed serum prolactin concentrations in the same steers (Jia et al., 2018), results of this study indicate that inclusion of 3 mg Se/d in MIX or OSe forms of Se in vitamin-mineral mixes ameliorates the depression of 3 known biomarkers of fescue toxicosis.
Supplementary Material
Acknowledgments
This work is supported by a United States Department of Agriculture-Agricultural Research Service Cooperative Agreement (J.C.M.) and by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch Project No. 1010352.
LITERATURE CITED
- Ammerman C. B., and Miller S. M.. . 1975. Selenium in ruminant nutrition: a review. J. Dairy Sci. 58:1561–1577. doi: 10.3168/jds.S0022-0302(75)84752-7 [DOI] [PubMed] [Google Scholar]
- Archibeque S. L., J. C. Burns, and Huntington G. B.. 2001. Urea flux in beef steers: effects of forage species and nitrogen fertilization. J. Anim. Sci. 79:1937–1943. doi: 10.2527/2001.7971937x [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C., V. Goffin M. Edery N. Binart, and Kelly P. A.. 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19:225–268. doi: 10.1210/edrv.19.3.0334 [DOI] [PubMed] [Google Scholar]
- Boling J. A., L. D. Bunting G. M. Davenport J. L. Van der Veen K. M. Meekins N. W. Bradley, and Kohls R. E.. 1989. Physiological responses of cattle consuming tall fescue to environmental temperature and supplemental phenothiazine. J. Anim. Sci. 67:2377–2385. doi: 10.2527/jas1989.6792377x [DOI] [PubMed] [Google Scholar]
- Brennan K. M., W. R. Burris J. A. Boling, and Matthews J. C.. 2011. Selenium content in blood fractions and liver of beef heifers is greater with a mix of inorganic/organic or organic versus inorganic supplemental selenium but the time required for maximal assimilation is tissue-specific. Biol. Trace Elem. Res. 144:504–516. doi: 10.1007/s12011-011-9069-y [DOI] [PubMed] [Google Scholar]
- Brown K. R., G. A. Anderson K. Son G. Rentfrow L. P. Bush J. L. Klotz J. R. Strickland J. A. Boling, and Matthews J. C.. 2009. Growing steers grazing high versus low endophyte (Neotyphodium coenophialum)-infected tall fescue have reduced serum enzymes, increased hepatic glucogenic enzymes, and reduced liver and carcass mass. J. Anim. Sci. 87:748–760. doi: 10.2527/jas.2008-1108 [DOI] [PubMed] [Google Scholar]
- Cerny K. L., S. Garbacik C. Skees W. R. Burris J. C. Matthews, and Bridges P. J.. 2016. Gestational form of selenium in free-choice mineral mixes affects transcriptome profiles of the neonatal calf testis, including those of steroidogenic and spermatogenic pathways. Biol. Trace Elem. Res. 169:56–68. doi: 10.1007/s12011-015-0386-4 [DOI] [PubMed] [Google Scholar]
- Dehnes Y., F. A. Chaudhry K. Ullensvang K. P. Lehre J. Storm-Mathisen, and Danbolt N. C.. 1998. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18:3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doornenbal H., A. K. Tong, and Murray N. L.. 1988. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Can. J. Vet. Res. 52:99–105. [PMC free article] [PubMed] [Google Scholar]
- Dougherty C. T., L. M. Lauriault N. W. Bradley N. Gay, and Cornelius P. L.. 1991. Induction of tall fescue toxicosis in heat-stressed cattle and its alleviation with thiamin. J. Anim. Sci. 69:1008–1018. doi: 10.2527/1991.6931008x [DOI] [PubMed] [Google Scholar]
- Fernandez N. J., and Kidney B. A.. . 2007. Alkaline phosphatase: beyond the liver. Vet. Clin. Pathol. 36:223–233. doi: 10.1111/j.1939-165X.2007.tb00216.x [DOI] [PubMed] [Google Scholar]
- Firestone G. L., and Heath E. C.. . 1981. The cyclic AMP-mediated induction of alkaline phosphatase in mouse L-cells. J. Biol. Chem. 256:1396–1403. [PubMed] [Google Scholar]
- Fitzgerald P., and Dinan T. G.. . 2008. Prolactin and dopamine: what is the connection? A review article. J. Psychopharmacol. 22:12–19. doi: 10.1177/0269216307087148 [DOI] [PubMed] [Google Scholar]
- Giannì M., M. Terao S. Sozzani, and Garattini E.. 1993. Retinoic acid and cyclic AMP synergistically induce the expression of liver/bone/kidney-type alkaline phosphatase gene in L929 fibroblastic cells. Biochem. J. 296(Pt 1):67–77. doi: 10.1042/bj2960067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter S. A., P. A. Beck, and Phillips J. K.. 2003. Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J. Anim. Sci. 81:856–864. doi: 10.2527/2003.814856x [DOI] [PubMed] [Google Scholar]
- Hank A. M., W. E. Hoffmann R. K. Sanecki D. J. Schaeffer, and Dorner J. L.. 1993. Quantitative determination of equine alkaline phosphatase isoenzymes in foal and adult serum. J. Vet. Intern. Med. 7:20–24. doi: 10.1111/j.1939-1676.1933.tb03164.x [DOI] [PubMed] [Google Scholar]
- Jackson J. J., M. D. Lindemann J. A. Boling, and Matthews J. C.. 2015. Summer-long grazing of high vs. low endophyte (Neotyphodium coenophialum)-infected tall fescue by growing beef steers results in distinct temporal blood analyte response patterns, with poor correlation to serum prolactin levels. Front. Vet. Sci. 2:77. doi: 10.3389/fvets.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Q. Li W. R. Burris G. E. Aiken P. J. Bridges, and Matthews J. C.. 2018. Forms of selenium in vitamin-mineral mixes differentially affect serum prolactin concentration and hepatic glutamine synthetase activity of steers grazing endophyte-infected tall fescue. J. Anim. Sci. 96:715–727. doi: 10.1093/jas/skx068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko J. J., Harvey J. W., and Bruss M. L.. . 2008. Clinical biochemistry of domestic animals. 6th ed. Academic Press, Burlington, MA. [Google Scholar]
- Kenward M. G., and Roger J. H.. . 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi: 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. 1989. Measurement of amino acid transport by hepatocytes in suspension or monolayer culture. Methods Enzymol. 173:564–575. doi: 10.1016/S0076-6879(89)73039-1 [DOI] [PubMed] [Google Scholar]
- Labunskyy V. M., D. L. Hatfield, and Gladyshev V. N.. 2014. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 94:739–777. doi: 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B. T., D. L. Harmon E. L. Piper L. M. Griffis, and Bush L. P.. 1999. Alkaloid binding and activation of D2 dopamine receptors in cell culture. J. Anim. Sci. 77:942–947. doi: 10.2527/1999.774942x [DOI] [PubMed] [Google Scholar]
- Larson B. T., D. M. Sullivan M. D. Samford M. S. Kerley J. A. Paterson, and Turner J. T.. 1994. D2 dopamine receptor response to endophyte-infected tall fescue and an antagonist in the rat. J. Anim. Sci. 72:2905–2910. doi: 10.2527/1994.72112905x [DOI] [PubMed] [Google Scholar]
- Li Q., R. Hegge P. J. Bridges, and Matthews J. C.. 2017. Pituitary genomic expression profiles of steers are altered by grazing of high vs. low endophyte-infected tall fescue forages. PLoS One 12:e0184612. doi: 10.1371/journal.pone.0184612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S. F., J. A. Boling, and Matthews J. C.. 2015. Gene expression profiling indicates an increased capacity for proline, serine, and ATP synthesis and mitochondrial mass by the liver of steers grazing high vs. low endophyte-infected tall fescue. J. Anim. Sci. 93:5659–5671. doi: 10.2527/jas.2015-9193 [DOI] [PubMed] [Google Scholar]
- Liao S. F., K. R. Brown A. J. Stromberg W. R. Burris J. A. Boling, and Matthews J. C.. 2011. Dietary supplementation of selenium in inorganic and organic forms differentially and commonly alters blood and liver selenium concentrations and liver gene expression profiles of growing beef heifers. Biol. Trace Elem. Res. 140:151–169. doi: 10.1007/s12011-010-8685-2 [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mainoya J. R. 1975. Effects of bovine growth hormone, human placental lactogen and ovine prolactin on intestinal fluid and ion transport in the rat. Endocrinology 96:1165–1170. doi: 10.1210/endo-96-5-1165 [DOI] [PubMed] [Google Scholar]
- Matthews J. C., Z. Zhang J. D. Patterson P. J. Bridges A. J. Stromberg, and Boling J. A.. 2014. Hepatic transcriptome profiles differ among maturing beef heifers supplemented with inorganic, organic, or mixed (50% inorganic:50% organic) forms of dietary selenium. Biol. Trace Elem. Res. 160:321–339. doi: 10.1007/s12011-014-0050-4 [DOI] [PubMed] [Google Scholar]
- Miles E. D., B. W. McBride Y. Jia S. F. Liao J. A. Boling P. J. Bridges, and Matthews J. C.. 2015. Glutamine synthetase and alanine transaminase expression are decreased in livers of aged vs. young beef cows and GS can be upregulated by 17β-estradiol implants. J. Anim. Sci. 93:4500–4509. doi: 10.2527/jas.2015-9294 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM) 2016. Nutrient Requirements of Beef Cattle: Eighth Revised Edition. The National Academies Press, Washington, DC: p. 127–128. doi: 10.17226/19014 [DOI] [Google Scholar]
- Nicholson J. W. G., McQueen R. E., and Bush R. S.. . 1991. Response of growing cattle to supplementation with organically bound or inorganic sources of selenium or yeast cultures. Can. J. Anim. Sci. 71:803–811. doi: 10.4141/cjas91-095 [DOI] [Google Scholar]
- Oliver J. W., A. E. Schultze B. W. Rohrbach H. A. Fribourg T. Ingle, and Waller J. C.. 2000. Alterations in hemograms and serum biochemical analytes of steers after prolonged consumption of endophyte-infected tall fescue. J. Anim. Sci. 78:1029–1035. doi:10.17266119014 [DOI] [PubMed] [Google Scholar]
- Patterson J. D., W. R. Burris J. A. Boling, and Matthews J. C.. 2013. Individual intake of free-choice mineral mix by grazing beef cows may be less than typical formulation assumptions and form of selenium in mineral mix affects blood se concentrations of cows and their suckling calves. Biol. Trace Elem. Res. 155:38–48. doi: 10.1007/s12011-013-9768-7 [DOI] [PubMed] [Google Scholar]
- Peters T.,, Jr. 1977. Serum albumin: recent progress in the understanding of its structure and biosynthesis. Clin. Chem. 23:5–12. [PubMed] [Google Scholar]
- Rice R. L., D. J. Blodgett G. G. Schurig W. S. Swecker J. P. Fontenot V. G. Allen, and Akers R. M.. 1997. Evaluation of humoral immune responses in cattle grazing endophyte-infected or endophyte-free fescue. Vet. Immunol. Immunopathol. 59:285–291. doi: 10.1016/S0165-2427(97)00079-2 [DOI] [PubMed] [Google Scholar]
- Rice R. L., G. G. Schurig, and Blodgett D. J.. 1998. Evaluation of physiologic indices in mice vaccinated with protein-ergotamine conjugates and fed an endophyte-infected fescue diet. Am. J. Vet. Res. 59:1258–1262. [PubMed] [Google Scholar]
- Richardson B. P. 1973. Evidence for a physiological role of prolactin in osmoregulation in the rat after its inhibition by 2-bromo- -ergokryptine. Br. J. Pharmacol. 47:623P–624P. [PMC free article] [PubMed] [Google Scholar]
- Schultze A. E., B. W. Rohrbach H. A. Fribourg J. C. Waller, and Oliver J. W.. 1999. Alterations in bovine serum biochemistry profiles associated with prolonged consumption of endophyte-infected tall fescue. Vet. Hum. Toxicol. 41:133–139. [PubMed] [Google Scholar]
- Solovyev N. D. 2015. Importance of selenium and selenoprotein for brain function: from antioxidant protection to neuronal signalling. J. Inorg. Biochem. 153:1–12. doi: 10.1016/j.jinorgbio.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Speckmann B., S. Schulz F. Hiller D. Hesse F. Schumacher B. Kleuser J. Geisel R. Obeid T. Grune, and Kipp A. P.. 2017. Selenium increases hepatic DNA methylation and modulates one-carbon metabolism in the liver of mice. J. Nutr. Biochem. 48:112–119. doi: 10.1016/j.jnutbio.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Strickland J. R., M. L. Looper J. C. Matthews C. F. Rosenkrans M. D. Jr Flythe, and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi: 10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Tsunoda M., V. J. Johnson, and Sharma R. P.. 2000. Increase in dopamine metabolites in murine striatum after oral exposure to inorganic but not organic form of selenium. Arch. Environ. Contam. Toxicol. 39:32–37. doi: 10.1007/s002440010076 [DOI] [PubMed] [Google Scholar]
- Vandesompele J., K. De Preter F. Pattyn B. Poppe N. Van Roy A. De Paepe, and Speleman F.. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., S. F. Liao J. R. Strickland J. A. Boling, and Matthews J. C.. 2011. Bovine neuronal vesicular glutamate transporter activity is inhibited by ergovaline and other ergopeptines. J. Dairy Sci. 94:3331–3341. doi: 10.3168/jds.2010-3612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.