Abstract
This study was conducted to evaluate the effects of sequential feeding technique in two genetic lines (GL; Line A [cross having a greater proportion of Pietrain] and Line B [cross having a lower proportion of Pietrain]) of growing-finishing pigs reared under daily cyclic high ambient temperature conditions. Seventy-eight castrated male pigs (22 ± 2.5 kg BW) were housed in a single group and were allocated to one of the three feeding programs: control (CON, 24 h control diet), high-fat/low-crude protein (HF/LP, 24 h high-fat/low-crude protein diet), and sequential feeding (SEQ, control diet from 1800 to 1000 h and HF/LP diet from 1001 to 1759 h). Cyclic high ambient temperature was induced by exposing the pigs to 22ºC ambient temperature from 1800 to 1000 h (time-period 22ºC, TP22) and to 30ºC from 1001 to 1759 h (TP30). The experimental period lasted 84 days and was divided into 3 growth phases, growing 1 (from day 0 to 20), growing 2 (from day 21 to 48) and finishing (from day 49 to 83). Feed intake was recorded in real time using an automatic feeder system. Pigs were weighed at the beginning and end of each experimental phase. Animal body composition was measured through dual-energy X-ray absorptiometry on days 0, 35, and 70. The ambient temperature averaged 22.3 ± 0.4ºC during TP22 and 30.2 ± 0.5ºC during TP30, characterizing the condition of daily ambient temperature variation that which pigs are usually exposed in tropical climate areas. During growing phase 1, the feeding programs had negligible effects on pig performance (P > 0.05), whereas during growing phase 2, ADG was greater in SEQ than in CON pigs (7%; P = 0.04). During the finishing phase, HF/LP pigs had greater ADFI (+ 10%) and ADG (+ 8%) than CON pigs. Lean mass and gain did not differ among feeding programs (P > 0.05). Overall, fat mass and gain were similar between SEQ and HF/LP pigs (P > 0.05), and both were greater than those of CON pigs (P < 0.05). On the basis of pig performance per phase, the supply of high-fat/low-crude protein diets (SEQ and HF/LP feeding) improved the performance of pigs under daily cyclic high ambient temperature. However, the use of these techniques resulted in fatter carcasses and in higher energy cost of gain. Finally, pigs with greater proportion of Pietrain genes had decreased growth performance in our experimental conditions.
Keywords: genotype, nutrition, precision feeding, tropical conditions
INTRODUCTION
Pig production in tropical areas has increased considerably to meet the increased global demand for animal products (Renaudeau et al., 2012). Despite this promising scenario, high ambient temperature has been considered a limiting factor to pig productivity due to its negative effects on metabolism and growth rate (Campos et al., 2017). Nutritional strategies have been evaluated and suggested for pigs exposed to high ambient temperatures. On the basis of the net energy system, heat increment due to metabolic utilization of proteins is greater than for carbohydrates or lipids (Noblet et al., 1994). Therefore, diets with low crude protein and greater energy content have presumably lower heat increment and are expected to be better adapted to high ambient temperature conditions (Renaudeau et al., 2002). However, most studies evaluating such strategies were performed with animals individually housed in experimental cages using a constant high ambient temperature protocol (Renaudeau et al., 2011). These studies do not represent then the practical conditions in which pigs are usually raised in tropical areas, i.e., group-housed in semi-open buildings and exposed to daily variation in ambient temperature. Thus, an important step to more precise and sustainable pig production in hot climate areas might be based on the supply of different diets according to the daily variation in ambient temperature in group-housed pigs. The practice of alternating different diets throughout the day is known as sequential feeding (Bouvarel et al., 2008). In addition, genotypes with different potentials for lean deposition are used in the production systems. Therefore, nutritional strategies for pigs exposed to high ambient temperatures should consider how genotypes could modulate pigs responses. This study was, therefore, performed to evaluate the sequential feeding system for two lines of pigs with different proportion Pietrain genes under daily cyclic high ambient temperature conditions.
MATERIALS AND METHODS
All experimental procedures were reviewed and approved by the Ethical Committee for the Care and Use of Experimental Animals of the School of Agricultural and Veterinarian Sciences of São Paulo State University (protocol No. 18077/16).
Animals and Housing
The study was carried out in the experimental facilities of the São Paulo State University (Unesp), School of Agricultural and Veterinarian Sciences, Jaboticabal, Brazil. A total of 78 barrows (22 ± 2.5 kg) from two different commercial genetic lines (Lines A and B; Agroceres PIC, Rio Claro, Brazil) were used. Both lines consisted of a Large White × Landrace × Duroc × Pietrain multiple cross with Line A pigs having a greater proportion of Pietrain genes than those from Line B. Pigs were housed in a single 95 m2 pen (1.19 m2/animal) with a full concrete floor in a temperature-controlled room. The pen was equipped with five automatic feeders (Automatic and Intelligent Precision Feeder [AIPF]; University of Lleida, Lleida, Spain, Pomar et al., 2011) and 10 beat ball drinkers distributed all over the pen. One transponder (plastic button tag containing passive transponders of radio frequency identification; Allflex, Joinville, SC, Brazil) was inserted in the right ear of each pig using specific tagger pliers, and the animals were introduced to the electronic feeders. The photoperiod was fixed to 12 h of artificial light (0600 to 1800 h).
The temperature-controlled room was equipped with an evaporative pad cooling system (Big Dutchman, Araraquara, SP, Brazil) and electric heaters, automatically controlled to maintain the ambient temperature at 22ºC from 1800 to 1000 h (time-period 22ºC, TP22) and 30ºC from 1001 to 1759 h (TP30). The ambient temperature change from 22 to 30ºC occurred at a rate of 0.3ºC/min, whereas the ambient temperature change from 30 to 22ºC occurred at a rate of 0.5ºC/min. These temperatures and time periods aimed to simulate the cyclic variation in ambient temperature that pigs are usually exposed to in tropical climate areas. During the experiment, the ambient temperature and relative humidity in the room were recorded every 10 min using 2 data loggers (HOBO, Onset Computer Corporation, Bourne, MA) located in the middle of the pen and at half the height of the body of the animals.
Experimental Design
The pigs remained in the experiment for 102 d, which consisted of an 18-d adaptation period and a subsequent 84-d experimental period. The experimental period was divided into 3 phases according to the growing stage of the animals: growing phase 1, from 0 to 20 d; growing phase 2, from 21 to 48 d; and finishing phase, from 49 to 83 d.
At the beginning of the experiment, 39 pigs of each genetic line were randomly assigned to receive one of the feeding programs: control (CON), in which the pigs received a control diet from 0000 to 2359 h; high-fat/low-crude protein (HF/LP), in which the pigs received a high-fat/low-crude protein diet from 0000 to 2359 h; and sequential feeding (SEQ), in which the pigs received the CON diet from 1800 to 1000 h (22ºC) and the HF/LP diet from 1001 to 1759 h (30ºC). Irrespective of the feeding program, feed and water were provided ad libitum during the adaptation and experimental periods. Each feeding station (AIPF) consisted of a single-space feeder in which precision Archimedes screw conveyors deliver volumetric amounts of up to 4 diets contained in independent feed containers located in the upper part of the feeder (Pomar et al., 2011). The AIPF identifies each pig when its head is introduced into the feeder and then delivers feed in response to each animal request according to the assigned experimental feeds (see Experimental Diets section). In this way, any pig in the pen could access any of the feeders and receive the feed prescribed for that animal.
One serving consisted of the amount of feed delivered on each effective serving request (serving size was 25 g). A time lag (18 s) was imposed to ensure that pigs consumed each serving before requesting a new one. The pigs tended to leave the feeder hopper empty or to leave small amounts of feed after at each visit. The feeders were calibrated weekly to convert feed volumes into feed weights. The use of exclusive identification codes per pig allowed recording individual feed intake over the trial. This feature allowed all animals to be housed in the same pen in a single group.
Experimental Diets
For each experimental phase (growing 1, growing 2, and finishing), two corn and soybean meal-based experimental diets were formulated (CON and HF/LP diets; Table 1) to meet the nutritional requirements of the animals with the highest potential for lean deposition (Line A) according to the NRC (2012) recommendations. The CON diets were formulated without the use of oil and crystalline AA, whereas the HF/LP diets were formulated with 4% soybean oil and with supplementation of crystalline AA to obtain a lower CP content than that of the control diets. Within each growth phase, the diets were formulated with similar SID Lys:NE and SID AA (Met, Met + Cys, Thr and Trp):SID Lys ratios.
Table 1.
Ingredients and composition of the experimental diets1
| Items | Growing 1 | Growing 2 | Finishing | |||
|---|---|---|---|---|---|---|
| Control | HF/LP | Control | HF/LP | Control | HF/LP | |
| Ingredient composition, as-fed basis, % | ||||||
| Corn | 63.09 | 67.69 | 66.17 | 68.74 | 71.74 | 76.38 |
| Soybean meal | 31.96 | 22.01 | 28.00 | 19.00 | 22.59 | 13.70 |
| Wheat bran | 2.00 | 2.00 | 2.88 | 4.00 | 3.00 | 2.00 |
| Soy oil | 4.00 | 4.00 | 4.00 | |||
| Dicalcium phosphate | 0.90 | 1.25 | 0.91 | 1.28 | 0.80 | 1.15 |
| Calcium carbonate | 0.90 | 0.74 | 0.91 | 0.73 | 0.82 | 0.66 |
| Salt | 0.40 | 0.41 | 0.38 | 0.38 | 0.35 | 0.36 |
| Dextrin | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Biolys, 56.6% | 0.72 | 0.70 | 0.66 | |||
| dl-Methionine | 0.09 | 0.09 | 0.08 | |||
| l-Threonine | 0.25 | 0.24 | 0.23 | |||
| l-Tryptophan | 0.09 | 0.09 | 0.08 | |||
| Choline chloride | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral and vitamin premix2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.15 | 0.15 |
| Chemical composition3 | ||||||
| ME, MJ/kg | 13.30 | 14.23 | 13.29 | 14.29 | 13.36 | 14.33 |
| NE, MJ/kg | 8.87 | 9.97 | 9.10 | 10.05 | 8.93 | 10.04 |
| DM, % | 88.0 | 88.5 | 88.0 | 88.4 | 87.9 | 84.4 |
| CP, % | 19.8 | 15.7 | 18.4 | 14.6 | 16.4 | 12.7 |
| Ether extract, % | 3.95 | 7.95 | 4.01 | 8.02 | 4.15 | 8.16 |
| Ash, % | 5.39 | 4.92 | 5.27 | 4.78 | 4.78 | 4.29 |
| Crude fiber, % | 3.89 | 3.34 | 3.89 | 3.35 | 3.77 | 3.21 |
| SID4 Lys calculated, % | 0.98 | 1.10 | 0.85 | 0.94 | 0.73 | 0.82 |
| SID Met calculated, % | 0.28 | 0.32 | 0.25 | 0.28 | 0.21 | 0.24 |
| SID Met + Cys calculated, % | 0.55 | 0.62 | 0.48 | 0.53 | 0.42 | 0.48 |
| SID Thr calculated, % | 0.59 | 0.66 | 0.52 | 0.57 | 0.47 | 0.52 |
| SID Trp calculated, % | 0.18 | 0.20 | 0.15 | 0.17 | 0.13 | 0.15 |
| Ca, % | 0.66 | 0.66 | 0.59 | 0.59 | 0.52 | 0.52 |
| Total P, % | 0.43 | 0.44 | 0.38 | 0.38 | 0.33 | 0.34 |
| Digestible P, calculated, % | 0.23 | 0.23 | 0.20 | 0.20 | 0.17 | 0.17 |
1The diets were control and high-fat/low-crude protein (HF/LP).
2Mineral vitamin supplement (per kg of diet): Vit. A (5.250 UI); Vit. D3 (750 UI); Vit. E (11 UI); Vit. K3 (1.5 mg); Vit. B1 (1 mg); Vit. B2 (2,4 mg); Vit. B6 (1 mg); Niacin (30 mg); Pantothenic acid (8.1 mg); Folic acid (0.53 mg); Biotin (0.05 mg); Vit. B12 (16.5 mcg); Copper (13.5 mg); Iodine (0.19 mg); Manganese (37.5 mg); Selenium (0.15 mg); Zinc (72 mg); Iron (72 mg); and Cobalt (0.19 mg).
3All calculated values were obtained using the EvaPig software program (version 1.3.1.4; INRA, Saint-Gilles, France).
4SID = standardized ileal digestible.
The CP, ether extract, starch, crude fiber, and total AA of contents of the ingredients were analyzed by near-infrared reflectance spectroscopy (NIRS) before diet formulation. The coefficient of digestibility of AA was calculated according to Sauvant et al. (2002). The nutritional composition of the raw materials used in the formulation, except for the values obtained by analysis (corn, soybean meal, and wheat bran), was obtained from the Brazilian Poultry and Swine Tables (Rostagno et al., 2011). The feed was steam pelleted at 2.5 mm.
Performance and Body Composition
Pigs were weighed without fasting on the first day of the adaptation period and at the beginning and end of each experimental phase (0, 20, 48, and 84 d). The total body lean and fat mass were measured on day 0, 35, and 70 by dual-energy X-ray absorptiometry (DXA; Hologic Discovery, Hologic Inc., Bedford, MA). For the first DXA scan, 8 pigs per feeding program of each genetic line were randomly selected, and the same animals were used in the subsequent analyses. The animals were fasted for 8 hours before being anesthetized by intramuscular injection of xylazine (1.5 mg/kg) and ketamine (15 mg/kg). The animals were scanned in the prone position, and after each scan, ultrasound images were obtained using ALOKA equipment (500v series with a linear probe of 3.5 MHZ, 13.5 cm) for the analysis of backfat thickness and loin depth. The measurements were taken at the boundary between the thoracic and lumbar vertebrae (P2 point) at 6 cm from the midline (ABCS, 1973).
Analytical Procedures
Representative samples of feed were taken once weekly and pooled per growth phase for dry matter (Method 930.15; AOAC, 2007), ether extract (Method 920.39; AOAC, 2007), crude fiber (Method 978.10; AOAC, 2007), and ash (Method 942.05; AOAC, 2007) analysis according to the procedures of AOAC International (2007). The crude protein content was analyzed using an LECO nitrogen analyzer (Leco Corporation, St. Joseph, MI). Gross energy was measured using an adiabatic bomb calorimeter (Parr Instrument Co., Moline, IL). All the analyses were performed in duplicate at the Animal Nutrition Laboratory (“LANA” São Paulo State University, Department of Animal Science, Brazil). The energy contents of the diets were calculated according to Sauvant et al. (2002).
Calculations and Statistical Analysis
Feed intake was calculated using the feeding information of each pig (AIPF Software). The days on which pigs were scanned (body composition) were not considered in the analysis. Because TP22 and TP30 had different durations (16 h at 22°C and 8 h at 30°C; on average), feed intake and NE intake per time period was expressed per hour. Lean mass gain and fat mass gain were calculated by the difference between the respective body constituents estimated from the DXA readings at the beginning and end of each period (0 to 35 d; 36 to 70 d). The DXA body lean mass was converted to total body protein (g) according to Pomar and Rivest (1996). The energy retained as protein and fat was calculated assuming that protein gain contained 23.8 MJ/kg (Kleiber, 1961) and fat contained 39.581 MJ/kg (Sainz and Wolff, 1988).
The presence of outliers was evaluated through the residual analysis of data and by daily records of anomalies. The BoxCox and Cramer-von Mises tests were used to verify homogeneity of the variances and normality of the studentized residuals, respectively. For the analysis of variance, the MIXED procedure of SAS (version 9.3; SAS Institute Inc., Cary, NC) was used. For the analysis of feed intake and NE intake per hour, the time period was considered as a fixed effect according to the model:
For the other variables, the model was as follows:
where Yijmk (or Yijk) is observed variable, µ mean, X is covariate (weight of the animal on the first day of the experimental period), and fixed effects of feeding program (FPi; CON, HF/LP, or SEQ), genetic line (GLj; Lines A or B), time period of the day (TPm) and their interactions. The individual pig was considered the experimental unit, and eijmk (or eijk) was considered the random error. The MIXED models included the effect of growth phase as a repeated effect and a compound symmetry covariance structure was used to account for the experimental unit effect over the experimental period; except for growth and body composition averaged variables. The results were considered statistically significant if P < 0.05. When there were differences between feeding programs (P < 0.05), adjusted means were compared using the Tukey–Kramer test. The slope and coefficient of determination of the relationships between NE intake (MJ/d) and energy retained as fat (MJ/d) were estimated using the REG procedure of SAS. In this analysis, it was tested whether there was a linear relationship between the variables at P < 0.05.
RESULTS
Animals and Climatic Conditions
Because of health problems, data from one HF/LP pig of genetic line B were not considered in the performance analysis. Because of adaptation problems to the AIPF, the overall data of two CON pigs (one of each genetic line) and two HF/LP pigs (one of each genetic line) were not included in the performance analysis. During the third body composition evaluation, one HF/LP pig of genetic line B was not scanned due to resistance to anesthesia. Therefore, the body composition data of this animal were not considered in the analysis.
The room ambient temperature averaged 22.3 ± 0.4ºC from 1800 to 1000 h and 30.2 ± 0.5ºC from 1001 to 1759 h. The daily relative humidity averaged 70.3 ± 3.2%. These ambient temperature values were in accordance with the objectives of the experiment.
Performance
In all experimental phases, the effect of BW as a covariate was significant (P < 0.01) for performance and body composition variables. On day 20, 48, and 84 of the experiment, the average BW of the pigs was 45.7 ± 1.1, 74.1 ± 2.3, and 106.9 ± 4.3 kg, respectively. Irrespective of the phase, an interaction between genetic line and time period within the day was observed for feed and NE intake per hour (P < 0.05; Table 2). At TP22, both lines had similar feed and NE intake, while at TP30, line B pigs had greater feed and NE intake than line A.
Table 2.
Effect of the feeding program, genetic line and period of the day on the feed intake and NE intake by hour of growing and finishing pigs1
| Feeding Programs2 | CON | HF/LP | SEQ | Line A | Line B | Statistical Analysis4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period of day | 22 ºC | 30 ºC | 22 ºC | 30 ºC | 22 ºC | 30 ºC | 22 ºC | 30 ºC | 22 ºC | 30 ºC | RSD3 | FP | GL | TP | FP×GL | FP×TP | GL×TP | FP×GL×TP |
| No. of animals | 24 | 24 | 23 | 23 | 26 | 26 | 37 | 37 | 36 | 36 | ||||||||
| Growing phase 1 | ||||||||||||||||||
| Feed intake, g/h | 68.46 | 64.63 | 65.46 | 72.26 | 67.88 | 78.27 | 67.74 | 66.77 | 66.80 | 76.67 | 6.49 | 0.09 | 0.12 | 0.06 | 0.77 | 0.06 | 0.03 | 0.45 |
| NE intake, MJ/h | 0.61 | 0.55 | 0.65 | 0.72 | 0.60 | 0.79 | 0.64 | 0.61 | 0.62 | 0.74 | 3.38 | <0.01 | 0.13 | <0.01 | 0.64 | <0.01 | 0.02 | 0.40 |
| Growing phase 2 | ||||||||||||||||||
| Feed intake, g/h | 77.95 | 79.93 | 81.32 | 91.62 | 89.29 | 83.02 | 81.49 | 66.44 | 84.22 | 103.27 | 3.02 | 0.23 | <0.01 | 0.69 | 0.84 | 0.32 | <0.01 | 0.11 |
| NE intake, MJ/h | 0.71 | 0.74 | 0.82 | 0.93 | 0.81 | 0.88 | 0.77 | 0.64 | 0.79 | 1.00 | 2.78 | 0.01 | <0.01 | 0.29 | 0.74 | 0.66 | <0.01 | 0.42 |
| Finishing phase | ||||||||||||||||||
| Feed intake, g/h | 102.19 | 87.44 | 106.13 | 95.38 | 89.29 | 108.58 | 97.18 | 77.76 | 91.39 | 126.34 | 5.71 | 0.60 | <0.01 | 0.16 | 0.85 | 0.07 | <0.01 | 0.50 |
| NE intake, MJ/h | 0.93 | 0.78 | 1.07 | 0.96 | 0.80 | 1.09 | 0.91 | 0.75 | 0.86 | 1.27 | 6.31 | 0.14 | <0.01 | 0.02 | 0.89 | <0.01 | <0.01 | 0.54 |
| Total experiment | ||||||||||||||||||
| Feed intake, g/h | 82.59 | 78.06 | 84.31 | 86.42 | 82.17 | 89.97 | 82.20 | 70.39 | 80.82 | 102.27 | 5.08 | 0.12 | <0.01 | 0.08 | 0.71 | 0.70 | <0.01 | 0.65 |
| NE intake, MJ/h | 0.77 | 0.70 | 0.84 | 0.87 | 0.74 | 0.90 | 0.77 | 0.68 | 0.76 | 1.00 | 4.98 | <0.01 | <0.01 | <0.01 | 0.75 | 0.09 | <0.01 | 0.32 |
1Initial weight as a covariate was significant for all variables; P < 0.01.
2CON: control diet from 0000 to 2359 h; HF/LP: high-fat/low-crude protein diet from 0000 to 2359 h; sequential feeding (SEQ): 1800 to 1000 h control diet and 1001 to 1759 h high-fat/low-crude protein diet.
3Residual Standard Deviation.
4Data were analyzed using a linear MIXED model including the fixed effects of covariate, feeding program (FP; n=3), genetic line (GL; n=2), time period of the day (TP; n=2), the interaction between FP and GL (FP×GL), FP and PD (FP×TP), GL and PD (GL×TP) and the triple interaction FP×GL×TP.
No interaction between feeding program and genetic line was found for performance traits (P > 0.05; Table 3). In growing phase 1 (0 to 20 d), ADFI, ADG, and feed efficiency were not affected by feeding program (P > 0.05). Net energy intake and energy cost of gain were similar between SEQ and HF/LP pigs (P > 0.05), and both were greater than in CON pigs (15.77 vs. 13.78 MJ/d and 20.9 vs. 18.6 MJ NE/kg of gain, respectively; P < 0.05).
Table 3.
Effect of feeding program and genetic line on the performance of growing and finishing pigs1
| Feeding programs2 | Genetic Line | RSD3 | Statistical Analysis (P-value)4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | HF/LP | SEQ | A | B | ||||
| No. of animals | 24 | 23 | 26 | 37 | 36 | - | FP | GL | FP×GL |
| Growing phase 1 (30 to 45 kg BW; 21 d) | |||||||||
| Initial BW, kg | 29.7 | 30.1 | 29.8 | 29.5 | 30.2 | 1.51 | 0.52 | 0.07 | 0.88 |
| Final BW, kg | 45.6 | 45.5 | 46.0 | 44.6 | 46.8 | 3.18 | 0.66 | <0.01 | 0.58 |
| ADFI, kg/d | 1.55 | 1.56 | 1.63 | 1.51 | 1.65 | 0.22 | 0.27 | 0.01 | 0.95 |
| ADG, kg/d | 0.750 | 0.747 | 0.771 | 0.702 | 0.808 | 0.13 | 0.66 | <0.01 | 0.52 |
| Feed efficiency, kg/kg | 0.49 | 0.48 | 0.47 | 0.47 | 0.49 | 0.81 | 0.73 | 0.03 | 0.33 |
| NE intake, MJ/d | 13.78b | 15.50a | 16.04a | 14.49 | 15.73 | 3.04 | <0.01 | 0.01 | 0.86 |
| Energy cost of gain, MJ NE/kg gain | 18.66b | 20.96a | 20.89a | 20.82 | 19.52 | 2.37 | <0.01 | 0.03 | 0.41 |
| Growing phase 2 (45 to 75 kg BW; 28 d) | |||||||||
| Initial BW, kg | 45.6 | 45.5 | 46.0 | 44.6 | 46.8 | 3.91 | 0.66 | <0.01 | 0.58 |
| Final BW, kg | 72.8 | 74.1 | 75.5 | 71.8 | 76.4 | 2.92 | 0.12 | <0.01 | 0.54 |
| ADFI, kg/d | 1.94 | 2.05 | 2.11 | 1.87 | 2.20 | 0.20 | 0.15 | <0.01 | 0.98 |
| ADG, kg/d | 0.974b | 1.016ab | 1.052a | 0.974 | 1.054 | 0.17 | 0.04 | <0.01 | 0.60 |
| Feed efficiency, kg/kg | 0.51 | 0.50 | 0.50 | 0.53 | 0.48 | 0.27 | 0.94 | <0.01 | 0.59 |
| NE intake, MJ/d | 17.69b | 20.58a | 19.94a | 17.76 | 21.05 | 3.14 | <0.01 | <0.01 | 0.93 |
| Energy cost of gain, MJ NE/kg gain | 17.96b | 20.09a | 18.86b | 17.70 | 19.84 | 2.16 | <0.01 | <0.01 | 0.97 |
| Finishing phase (75 to 105 kg BW; 35 d) | |||||||||
| Initial BW, kg | 72.8 | 74.1 | 75.5 | 71.8 | 76.4 | 3.44 | 0.12 | <0.01 | 0.54 |
| Final BW, kg | 104.1 | 108.4 | 108.2 | 102.6 | 111.2 | 8.01 | 0.08 | <0.01 | 0.56 |
| ADFI, kg/d | 2.17b | 2.40a | 2.27ab | 2.12 | 2.44 | 0.38 | 0.02 | <0.01 | 0.91 |
| ADG, kg/d | 0.904b | 0.982a | 0.937ab | 0.885 | 0.997 | 0.17 | 0.04 | <0.01 | 0.63 |
| Feed efficiency, kg/kg | 0.42 | 0.41 | 0.42 | 0.42 | 0.41 | 0.20 | 0.86 | 0.37 | 0.27 |
| NE intake, MJ/d | 19.39c | 24.04a | 21.61b | 20.03 | 23.34 | 2.91 | <0.01 | <0.01 | 0.69 |
| Energy cost of gain, MJ NE/kg gain | 21.64b | 24.52a | 23.07ab | 22.17 | 23.32 | 2.12 | <0.01 | 0.10 | 0.73 |
| Total experiment (84 d) | |||||||||
| Initial BW, kg | 29.7 | 30.1 | 29.8 | 29.5 | 30.2 | 1.51 | 0.52 | 0.07 | 0.88 |
| Final BW, kg | 104.1 | 108.4 | 108.2 | 102.6 | 111.2 | 8.01 | 0.08 | <0.01 | 0.56 |
| ADFI, kg/d | 1.89 | 2.01 | 2.00 | 1.84 | 2.10 | 0.56 | 0.12 | <0.01 | 0.99 |
| ADG, kg/d | 0.874 | 0.918 | 0.920 | 0.854 | 0.943 | 1.12 | 0.16 | <0.01 | 0.54 |
| Feed efficiency, kg/kg | 0.47 | 0.47 | 0.46 | 0.47 | 0.46 | 0.24 | 0.82 | 0.41 | 0.29 |
| NE intake, MJ/d | 16.91b | 20.11a | 19.20a | 17.20 | 20.21 | 3.91 | <0.01 | <0.01 | 0.88 |
| Energy cost of gain, MJ NE/kg gain | 19.15b | 21.85a | 20.94a | 20.22 | 20.96 | 1.02 | <0.01 | <0.01 | 0.22 |
1Initial weight as a covariate was significant for all variables; P < 0.01.
2CON: control diet from 0000 to 2359 h; HF/LP: high-fat/low-crude protein diet from 0000 to 2359 h; sequential feeding (SEQ): 1800 to 1000 h control diet and 1001 to 1759 h high-fat/low-crude protein diet.
3Residual Standard Deviation.
4Data were analyzed using a linear MIXED model including the fixed effects of covariate, feeding program (FP; n=3), genetic line (GL; n=2), and the interaction between FP and GL (FP×GL). The repeated measurements option was used with a compound symmetry covariance structure to account for experimental unit effect over the experimental period.
a, b, c Within a row, means with different superscripts are affected by feeding system (P < 0.05).
In growing phase 2 (21 to 48 d), feeding program did not affect ADFI or feed efficiency (P > 0.05). Pigs in the SEQ program had similar ADG to HF/LP pigs (1.034 kg/d on average; P > 0.05) and greater ADG than CON pigs (1.052 vs. 0.974 kg/d; P = 0.04), whereas ADG did not differ between HF/LP and CON pigs (P > 0.05). The net energy intake was similar between SEQ and HF/LP pigs (P > 0.05), and both were higher than that in CON pigs (20.26 vs. 17.69 MJ/d; P < 0.05). The energy cost of gain was lower in SEQ and CON pigs compared to HF/LP pigs (18.4 vs. 20.1 MJ NE/kg of gain on average; P < 0.05).
In the finishing phase (49 to 83 d), SEQ pigs had similar ADFI, ADG, and energy cost of gain to HF/LP and CON pigs (P > 0.05), whereas HF/LP pigs had greater ADFI (2.40 vs. 2.17 kg/d; P = 0.02), ADG (0.982 vs. 0.904 kg/d; P = 0.04) and energy cost of gain (24.5 vs. 21.6 MJ NE/kg gain, P < 0.01) than CON pigs. The net energy intake was greater in HF/LP pigs compared with the other programs, and greater in SEQ than in CON pigs (24.04 vs. 21.61 vs. 19.39 MJ/d; P < 0.05). When analyzing the entire experimental period (0 to 83 d), ADFI, ADG, and feed efficiency were not affected by feeding program (P > 0.05). Compared with CON pigs, SEQ, and HF/LP pigs had greater NE intake (19.65 vs. 16.91 MJ/d on average; P < 0.05) and energy cost of gain (21.3 vs. 19.1 MJ/kg; P < 0.05).
In regard to the genetic line effect on performance, irrespective of the growth phase, pigs from genetic line B had greater ADFI, ADG, and NE intake than pigs from genetic line A (P < 0.05). In growing phase 1, genetic line B pigs were more efficient (0.49 vs. 0.47 kg/kg; P = 0.04) than those from genetic line A. In contrast, animals from genetic line A were more efficient (0.53 vs. 0.48 kg/kg; P < 0.01) than genetic line B pigs in growing phase 2. When analyzing the entire experimental period, pigs from genetic line B had a greater ADFI, ADG, NE intake, and higher energy cost of gain than pigs from genetic line A (P < 0.05).
Body Composition
The results of body composition analysis are presented in Table 4. No interaction between feeding program and genetic line was found for the studied variables (P > 0.05). On day 35, feeding program did not affect lean mass, backfat thickness, or loin depth (P > 0.05). Fat mass was similar between SEQ and HF/LP pigs (P > 0.05), whereas SEQ had greater fat mass than CON pigs (9.58 vs. 8.68 kg; P < 0.01). From 0 to 35 d, feeding programs did not influence (P > 0.05) lean mass gain, energy retained, or energy cost of energy retained (NE intake MJ/energy retained MJ). Pigs in the SEQ and HF/LP programs had similar fat mass gain (P > 0.05), whereas pigs in the SEQ program had greater fat mass gain than CON pigs (17.89 vs 16.27 % BW gain; P = 0.02).
Table 4.
Effect of feeding program and genetic line on the body composition of growing and finishing pigs1
| Feeding programs2 | Genetic Line | RSD3 | Statistical Analysis (P-value)4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | HF/LP | SEQ | A | B | ||||
| No. of animals | 16 | 15 | 16 | 24 | 23 | - | FP | GL | FP×GL |
| Day 0 (initial condition) | |||||||||
| Average BW, kg | 29.98 | 29.57 | 30.30 | 29.53 | 30.20 | 2.14 | 0.74 | 0.08 | 0.95 |
| Lean Mass, kg | 25.58 | 25.35 | 25.43 | 25.30 | 25.66 | 1.38 | 0.23 | 0.11 | 0.44 |
| Fat Mass, kg | 4.09 | 4.23 | 4.17 | 4.02 | 4.25 | 1.28 | 0.33 | 0.18 | 0.50 |
| Backfat thickness, mm | 8.62 | 8.66 | 8.65 | 8.86 | 8.45 | 0.89 | 0.98 | 0.34 | 0.83 |
| Loin depth, mm | 34.86 | 36.43 | 34.52 | 36.25 | 34.27 | 3.62 | 0.26 | 0.21 | 0.33 |
| Day 35 | |||||||||
| Average BW, kg | 57.78 | 58.53 | 59.71 | 57.27 | 60.08 | 4.63 | 0.18 | 0.07 | 0.31 |
| Lean Mass, kg | 48.03 | 48.32 | 49.61 | 47.72 | 49.59 | 3.09 | 0.32 | 0.09 | 0.82 |
| Fat Mass, kg | 8.68b | 9.52a | 9.58a | 8.77 | 9.69 | 3.14 | 0.02 | <0.01 | 0.54 |
| Backfat thickness, mm | 11.19 | 11.92 | 12.31 | 11.75 | 11.87 | 1.29 | 0.39 | 0.82 | 0.96 |
| Loin depth, mm | 47.63 | 51.45 | 51.15 | 50.77 | 49.34 | 3.88 | 0.12 | 0.48 | 0.81 |
| Body tissue gain (0 to 35 d) | |||||||||
| Lean mass gain, % BW gain | 80.76 | 80.10 | 81.31 | 80.28 | 80.99 | 3.72 | 0.14 | 0.12 | 0.56 |
| Fat mass gain, % BW gain | 16.27b | 18.25a | 17.89a | 16.93 | 17.94 | 2.25 | 0.03 | 0.02 | 0.65 |
| Energy retained, MJ/d | 8.29 | 9.06 | 9.31 | 8.33 | 9.65 | 1.76 | 0.17 | <0.01 | 0.53 |
| NE intake MJ / Energy retained MJ | 1.85 | 1.94 | 1.96 | 1.93 | 1.86 | 2.39 | 0.24 | 0.65 | 0.72 |
| Day 70 | |||||||||
| Average BW, kg | 89.71 | 92.08 | 94.01 | 89.20 | 94.66 | 7.23 | 0.06 | <0.01 | 0.07 |
| Lean Mass, kg | 74.08 | 74.67 | 76.28 | 73.32 | 76.33 | 5.14 | 0.18 | 0.14 | 0.33 |
| Fat Mass, kg | 14.22b | 16.67a | 16.36a | 14.57 | 16.93 | 2.12 | <0.01 | <0.01 | 0.81 |
| Backfat thickness, mm | 14.28b | 15.98ab | 16.44a | 14.77 | 16.37 | 3.08 | 0.02 | 0.03 | 0.92 |
| Loin depth, mm | 65.29 | 66.45 | 66.20 | 67.88 | 64.09 | 3.36 | 0.65 | 0.03 | 0.60 |
| Body tissue gain (36 to 70 d) | |||||||||
| Lean mass gain, % BW gain | 79.50 | 79.29 | 78.17 | 78.18 | 79.17 | 3.71 | 0.12 | 0.09 | 0.60 |
| Fat mass gain, % BW gain | 18.00b | 20.52a | 19.21a | 18.10 | 20.92 | 2.69 | 0.04 | 0.01 | 0.48 |
| Energy retained, MJ/d | 10.86 | 11.19 | 11.33 | 10.00 | 11.26 | 1.08 | 0.21 | <0.01 | 0.65 |
| NE intake MJ / Energy retained MJ | 1.70 | 1.82 | 1.91 | 1.70 | 1.92 | 3.01 | 0.31 | <0.01 | 0.46 |
| Total period (0 to 70 d) | |||||||||
| Initial BW, kg | 29.98 | 29.57 | 30.30 | 29.53 | 30.20 | 2.14 | 0.74 | 0.08 | 0.95 |
| Final BW, kg | 89.71 | 92.08 | 94.01 | 89.20 | 94.66 | 7.23 | 0.06 | <0.01 | 0.07 |
| Lean mass gain, % BW gain | 80.86 | 79.14 | 80.06 | 79.88 | 82.04 | 2.03 | 0.10 | 0.04 | 0.39 |
| Fat mass gain, % BW gain | 17.52b | 19.62a | 18.87a | 17.21 | 20.02 | 3.96 | 0.04 | <0.01 | 0.72 |
| Energy retained, MJ/d | 9.57 | 9.93 | 10.12 | 9.09 | 10.22 | 1.06 | 0.18 | 0.03 | 0.95 |
| NE intake MJ / Energy retained MJ | 1.80 | 1.87 | 1.94 | 1.85 | 1.90 | 3.69 | 0.11 | 0.69 | 0.54 |
1Initial weight as a covariate was significant for all variables; P < 0.01.
2CON: control diet from 0000 to 2359 h; HF/LP: high-fat/low-crude protein diet from 0000 to 2359 h; sequential feeding (SEQ): 1800 to 1000 h control diet and 1001 to 1759 h high-fat/low-crude protein diet.
3Residual Standard Deviation.
4Data were analyzed using a linear MIXED model including the fixed effects of covariate, feeding program (FP; n=3), genetic line (GL; n=2), and the interaction between FP and GL (FP×GL). The repeated measurements option was used with a compound symmetry covariance structure to account for experimental unit effect over the experimental period.
a, b, c Within a row, means with different superscripts are affected by feeding system (P < 0.05).
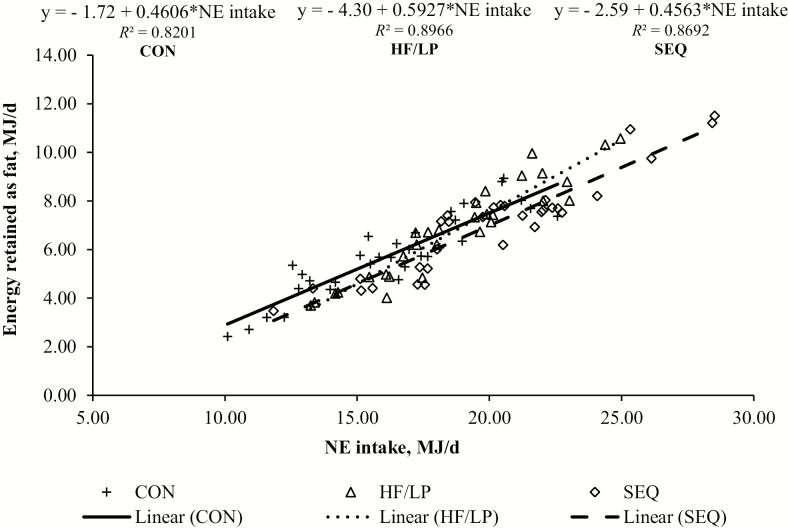
On day 70, feeding program did not affect lean mass or loin depth (P > 0.05). Pigs in the SEQ and HF/LP programs had greater fat mass compared with CON pigs (16.52 vs. 14.22 kg on average; P < 0.05). Backfat thickness was greater in SEQ than in CON pigs (16.4 vs. 14.2 mm; P = 0.02). From 36 to 70 d, fat mass gain was similar between SEQ and HF/LP pigs (P = 0.68), and both were greater than in CON pigs (19.87 vs. 18.00 % BW gain on average; P < 0.05). In regard to the total period (0 to 70 d), feeding program did not affect lean mass gain, energy retained, or energy cost of retained energy (P > 0.05). Pigs in the SEQ and HF/LP feeding programs had greater fat mass gain than CON pigs (19.25 vs. 17.52 % BW gain; P < 0.01). The relationship between NE intake (MJ/d) and energy retained as fat (MJ/d) is presented in Fig. 1. Irrespective of feeding program, energy retained as fat increased linearly in response to increasing NE intake and the slopes of the linear regressions were different from 0 (P < 0.05). In terms of growth performance of the subset of pigs used in the body composition analyses, ADFI, ADG, and feed efficiency were 1.55 kg/d, 0.745 kg/d and 0.48 during the growth phase 1. The respective values for growing phase 2 and finishing were 2.04 kg/d, 1.010 kg/d, and 0.50 kg/kg, and 2.32 kg/d, 0.953 kg/d, and 0.41 kg/kg.
Figure 1.
Relationship between NE intake (MJ/d) and energy retained as fat (MJ/d) for pigs fed CON (control diet from 0000 to 2359 h), HF/LP (high-fat/low-crude protein diet from 0000 to 2359 h) or SEQ (1800 to 1000 h control diet and 1001 to 1759 h high-fat/low-crude protein diet) programs in total period of body composition (0 to 70 d). Data are represented as the linear regression using NE intake as the independent variable.
When analyzing the effects of genetic line, fat mass, fat mass gain, and energy retained were greater (P < 0.05) in pigs from genetic line B than in those from genetic line A in all body composition analyses. Backfat thickness was not influenced by the genetic line on day 35 (P = 0.82); however, on day 70, it was greater in line B than in line A pigs (16.3 vs. 14.4 mm; P = 0.03). Despite of similar lean mass between genetic lines on day 35 and 70 (P > 0.05), greater loin depth was observed in line A pigs on day 70 (67.88 vs. 64.09 mm; P = 0.03). In the total period of evaluation (day 0 to 70), line B pigs had greater lean mass gain than line A pigs (82.04 vs. 79.88 % BW gain; P = 0.04).
DISCUSSION
Overview of the Cyclic Heat Stress and the Sequential Feeding System
This study was conducted to evaluate the sequential feeding technique for two genetic lines of growing-finishing pigs with different proportion of Pietrain genes reared under daily cyclic high ambient temperature conditions. Our first hypothesis was that adjusting diet composition according to daily variation in ambient temperature would benefit the performance and body composition of pigs in relation to those fed a CON diet. Secondly, we hypothesized that pigs with different proportion of Pietrain genes, and presumably with different metabolic heat production and thermoregulatory capacities, would respond differently to feeding programs.
Effect of HF/low-crude protein Sequential Feeding on Pig Performance and Body Composition
According to our results, feeding programs had negligible effects on pig performance during growing phase 1. In contrast, feeding pigs with high-fat/low-CP diets (HF/LP or SEQ programs) during growing 2 and the finishing phase had beneficial effects. For instance, SEQ-fed pigs had greater body weight gain (+7%) during growing phase 2 and HF/LP-fed pigs had greater feed intake (+10%) and body weight gain (+ 8%) during the finishing phase when compared with CON pigs. These results demonstrate the positive effects of the high-fat/low-CP diets as pig body weight increases in association with an increased susceptibility of heavier pigs to the negative effects of high ambient temperatures (Renaudeau et al., 2011). Because feed intake at high ambient temperatures is limited by the capacity of the animal to dissipate heat, the greater feed intake of HF/LP pigs might be explained by the lower thermic effect of the diet. These results are consistent with previous literature (Spencer et al., 2005; Rodrigues et al., 2012) that similarly reported a greater performance of heat-stressed finishing pigs fed high-fat/low-CP diets.
In our study, the difference in NE content between the control and high-fat/low-CP diets was 10% on average, which resulted in greater values of fat mass and fat mass gain in high-fat/low-CP pigs than in CON pigs. These results agree with Le Bellego et al. (2002) that reported greater NE intake and tendency for greater carcass fat in pigs kept at 29°C and fed low-CP diets. In addition, Spencer et al. (2005) observed greater backfat thickness in finishing-pigs fed high-fat/low-CP diet exposed to cyclic ambient temperature of 27°C from 1900 to 100 and 35°C from 1000 to 1900. These results might be explained by an inhibitory effect of high ambient temperature on protein deposition rate that results in greater amount of energy available for lipid deposition. This response can be interpreted as a metabolic adaptation to reduce metabolic heat production in high ambient conditions since the energetic efficiency for lipid deposition is greater than that for protein deposition (van Milgen and Noblet, 2003). In addition, our results demonstrate a linear increase in energy retained as fat as NE intake increased, irrespective of the feeding program.
Irrespective of growth phase, our study demonstrated negligible effects of feeding program on feed efficiency. Accordingly, Kerr et al. (2003) did not observe effects of diets varying in CP and EM content (16% CP, 12% CP + AA, and 12% CP) on feed efficiency in pigs kept at a constant ambient temperature of 33°C. In contrast, a high-fat diet (4.5% of soybean oil addition) improved the feed efficiency in growing pigs reared at a constant ambient temperature of 32ºC (Wolp et al., 2012). In fact, because of the large environmental and experimental variability between studies (duration of high ambient temperature exposure, feed composition, management and sanitary status, BW, and sex) it is still difficult to predict the direct effects of low heat increment diets on feed efficiency in pig reared under high ambient temperature conditions.
During the finishing phase, a worse energy cost of gain was observed in high-fat/low-CP than in CON pigs in association with greater fat mass. This result contrasts those of Le Bellego et al. (2002) that reported similar values of energy cost of gain between control and high-fat/low-CP diets in pigs individually housed and at a constant temperature of 29°C. It should be noted that pigs housed individually have different performance than group-housed pigs (Bornett et al., 2000), and this difference may become more evident in limiting conditions (temperature, nutrition, health challenge, etc.). Therefore, the higher ADFI in pigs receiving high-fat/low-CP diet can be understood as an attempt of the animals to adapt to our experimental conditions (housing and diurnal thermal challenge).
Overall, the performance results were similar between pigs in the SEQ and HF/LP programs. These findings suggest that high-fat/low-protein and SEQ programs are potential strategies to improve the performance (ADFI, ADG) of pigs under cyclic high ambient temperature condition when compared with standard feeding programs. As feed represents a large part of the cost of pig production and the energy component represents the greatest proportion of feed cost (Noblet, 2007; Velayudhan et al., 2015), the SEQ feeding program may be economically advantageous than the HF/LP program since SEQ pigs had the best values for energy cost of gain and NE intake in the growing 2 and finishing phases, respectively. Accordingly, sequential feeding with low and high-protein diets improved the weight gain (10%) and feed efficiency (8%) in growing geese exposed to ambient temperature variations between 22°C and 29°C (Ho et al., 2015). In contrast, no effect of sequential feeding with diets varying in CP and energy content was observed in male broilers reared at a constant ambient temperature of 37°C (Bouvarel et al., 2004).
The similar results for lean mass and gain between feeding programs suggest that a reduction of up to 4% CP with correct AA supplementation does not affect the lean body mass of pigs under cyclic high ambient temperature. These results agree with those of Le Bellego et al. (2002) and Kerr et al. (2003), who reported no differences in lean mass in pigs receiving decreased CP levels (−4%) and exposed to high ambient temperature conditions (29ºC and 33ºC, respectively). However, there is a limit to CP reduction even with AA supplementation. Some authors suggest that a CP reduction is considered beneficial up to 4% below the NRC (2012) recommendations (Gloaguen et al., 2014; Peng et al., 2016; Fan et al., 2017; Ma et al., 2018). It should be noted that these latter studies did not consider the effects of high ambient temperature. As metabolic and physiological processes change or are activated in response to high temperatures (Campos et al., 2017), additional studies may be required to evaluate the effects of different CP levels with AA supplementation in pigs exposed to hot conditions.
Effect of Genetic Line on the Performance and Body Composition of Pigs Exposed to Daily Cyclic High Ambient Temperature Conditions
According to our results, although the genetic lines showed similar responses to the feeding programs, they responded differently to cyclic high ambient temperature exposure. In fact, animal responses to high ambient temperatures are highly variable within a population, and part of this variability has a genetic basis (Renaudeau et al., 2012; Lan et al., 2016). Overall, line B pigs had greater growth performance (ADFI, NE intake, and ADG) than line A pigs. Likewise, greater lean and fat mass during the total period (+4 and +14%; respectively) and greater body weight at the end of the experiment (+8.6 kg/animal) was observed in line B pigs. In addition, while line A pigs reduced their feed and NE intake during the period of high ambient temperature, this response was not observed in line B pigs. Pietrain pigs are commonly used in breeding programs due to their greater potential and efficiency of protein deposition (Jiang et al., 2012; Ropka-Molik et al., 2018). However, increased lean tissue deposition and growth rate are associated with increased metabolic heat production (Brown-Brandl et al., 2004) and therefore, with high susceptibility to heat stress (Renaudeau, 2005; Rauw et al., 2017). Part of this greater metabolic heat production is explained by the higher energetic cost of protein deposition relative to lipid (Renaudeau et al., 2012). The synthesis of a peptide bond from amino acids requires at least 5 ATP, while lipid deposition has an energy cost of 2 ATP (van Milgen et al., 2001; van Milgen and Noblet, 2003). Therefore, heat production associated with proteogenesis is greater compared to that of lipogenesis (Rauw et al., 2017). Line A pigs had a higher proportion of Pietrain genes which might explain their decreased growth performance in our experimental conditions when compared to pigs from genetic line B. Interestingly, genetic line feed efficiency varied according to the experimental phases. Unlike growing phase 1, in growing phase 2, line A pigs were more efficient and had lower energy cost of gain than the line B pigs, but both lines had the same results for these variables during the finishing phase.
The results of this study suggest that the supply of high-fat/low-CP diets (SEQ and HF/LP feeding) improve growth performance of growing-finishing pigs under daily cyclic high ambient temperatures. However, the use of these techniques resulted in fatter carcasses and in higher energy cost of gain. Our results also suggest that genetic selection for lean growth negatively affects growth performance of pigs reared under high ambient temperature conditions.
Conflict of interest statement. None declared.
Footnotes
The authors thank (grant no. 2012/03781-0 and Fellowship no. 2016/08682-1) the São Paulo Research Foundation (FAPESP) (Brazil) for their financial support of this project.
LITERATURE CITED
- ABCS, Associação Brasileira dos Criadores de Suínos 1973. Método Brasileiro de classificação de carcaças, pub. tec. n.2. Estrela, Rio Grande do Sul. [Google Scholar]
- AOAC 2007. Official methods of analysis. 18th ed Assoc. Off. Anal. Chem., Gaithersburg, MD. [Google Scholar]
- Bornett H. L., Morgan C. A., Lawrence A. B., and Mann J.. . 2000. The effect of group housing on feeding patterns and social behaviour of previously individually housed growing pigs. Appl. Anim. Behav. Sci. 70:127–141. doi: 10.1016/S0168-1591(00)00146-5 [DOI] [PubMed] [Google Scholar]
- Bouvarel I., Barrier-Guillot B., Larroude P., Boutten B., Leterrier C., Merlet F., Vilariño M., Roffidal L., Tesseraud S., Castaing J., . et al. 2004. Sequential feeding programs for broiler chickens: twenty-four- and forty-eight-hour cycles. Poult. Sci. 83:49–60. doi: 10.1093/ps/83.1.49 [DOI] [PubMed] [Google Scholar]
- Bouvarel I., Chagneau A. M., Lescoat P., Tesseraud S., and Leterrier C.. . 2008. Forty-eight-hour cycle sequential feeding with diets varying in protein and energy contents: adaptation in broilers at different ages. Poult. Sci. 87:196–203. doi: 10.3382/ps.2007-00205 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Nienaber J. A., Xin H., and Gates R. S.. . 2004. A literature review of swine heat production. Trans. ASAE. 47:259–270. doi:10.13031/2013.15867 [Google Scholar]
- Campos P. H. R. F., Le Floc’h N., Noblet J., and Renaudeau D.. . 2017. Physiological responses of growing pigs to high ambient temperature and/or inflammatory challenges. R. Bras. Zootec. 46:537–544. doi: 10.1590/s1806-92902017000600009 [DOI] [Google Scholar]
- Fan P., Liu P., Song P., Chen X., and Ma X.. . 2017. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 7:43412. doi: 10.1038/srep43412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloaguen M., Le Floc’h N., Corrent E., Primot Y., and van Milgen J.. . 2014. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim. Sci. 92:637–644. doi: 10.2527/jas.2013-6514 [DOI] [PubMed] [Google Scholar]
- Ho S. Y., Chen Y. H., and Yang S. K.. . 2015. Effects of sequential feeding with low- and high-protein diets on growth performances and plasma metabolite levels in geese. Animal 9:952–957. doi: 10.1017/S1751731114003267. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Z., Zhu L., Tang G. Q., Li M. Z., Jiang A. A., Cen W. M., Xing S. H., Chen J. N., Wen A. X., He T., . et al. 2012. Carcass and meat quality traits of four commercial pig crossbreeds in China. Genet. Mol. Res. 11:4447–4455. doi: 10.4238/2012.September.19.6 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Yen J. T., Nienaber J. A., and Easter R. A.. . 2003. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 81:1998–2007. doi: 10.2527/2003.8181998x [DOI] [PubMed] [Google Scholar]
- Kleiber M. 1961. The fire of life: an introduction to animal energetics. John Wiley and Sons, New York, USA. [Google Scholar]
- Lan X., Hsieh J. C. F., Schmidt C. J., Zhu Q., and Lamont S. J.. . 2016. Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genomics. 17:955. doi: 10.1186/s12864-016-3291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bellego L., van Milgen J., and Noblet J.. . 2002. Effect of high temperature and low-protein diets on the performance of growing-finishing pigs. J. Anim. Sci. 80:691–701. doi:10.2527/2002.803691x [DOI] [PubMed] [Google Scholar]
- Ma X., Tian Z., Deng D., Cui Y., and Qiu Y.. . 2018. Effect of dietary protein level on the expression of proteins in the gastrointestinal tract of young pigs. J. Agric. Food Chem. 66:4364–4372. doi: 10.1021/acs.jafc.7b05655 [DOI] [PubMed] [Google Scholar]
- van Milgen J., and Noblet J.. . 2003. Partitioning of energy intake to heat, protein, and fat in growing pigs. J. Anim. Sci. 81 (Suppl.):E86–E93. doi:10.2527/2003.8114_suppl_2E86x [Google Scholar]
- van Milgen J., Noblet J., and Dubois S.. . 2001. Energetic efficiency of starch, protein and lipid utilization in growing pigs. J. Nutr. 131:1309–1318. doi: 10.1093/jn/131.4.1309 [DOI] [PubMed] [Google Scholar]
- Noblet J. 2007. Net energy evaluation of feeds and determination of net energy requirements for pigs. R. Bras. Zootec. 36:277–284. doi: 10.1590/S1516-35982007001000025 [DOI] [Google Scholar]
- Noblet J., Fortune H., Shi X. S., and Dubois S.. . 1994. Prediction of net energy value of feeds for growing pigs. J. Anim. Sci. 72:344–354. doi:10.2527/1994.722344x [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed National Academies Press, Washington, DC. [Google Scholar]
- Peng X., Hu L., Liu Y., Yan C., Fang Z. F., Lin Y., Xu S. Y., Li J., Wu C. M., Chen D. W., . et al. 2016. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal 10:1812–1820. doi: 10.1017/S1751731116000999 [DOI] [PubMed] [Google Scholar]
- Pomar J., López V., and Pomar C.. . 2011. Agent-based simulation framework for virtual prototyping of advanced livestock precision feeding systems. Comput. Electron. Agric. 78:88–97. doi: 10.1016/j.compag.2011.06.004 [DOI] [Google Scholar]
- Pomar C., and Rivest J.. . 1996. The effect of body position and data analysis on the estimation of body composition of pigs by dual energy xray absorptiometry (DEXA). In: Proc. 46th Annual Conference Canadian Society of Animal Science; Lethbridge, AB, Canada p. 26. (Abstr.) [Google Scholar]
- Rauw W. M., Mayorga E. J., Lei S. M., Dekkers J. C. M., Patience J. F., Gabler N. K., Lonergan S. M., and Baumgard L. H.. . 2017. Effects of diet and genetics on growth performance of pigs in response to repeated exposure to heat stress. Front. Genet. 8:155. doi: 10.3389/fgene.2017.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D. 2005. Effects of short-term exposure to high ambient temperature and relative humidity on thermoregulatory responses of European (Large White) and Caribbean (Creole) restrictively-fed growing pigs. Anim. Res. 54:81–93. doi:10.1051/animres:2005005 [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. . 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Gourdine J. L., and St-Pierre N. R.. . 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Quiniou N., Dubois S., and Noblet J.. . 2002. Effect of high ambient temperature and dietary protein level on feeding behaviour of multiparous lactating sows. Anim. Res. 51:227–243. doi:10.1051/animres:2002020 [DOI] [PubMed] [Google Scholar]
- Rodrigues N. E. B., Tadeu Filho E., Zangeronimo M. G., Cantarelli V. S., Rodrigues P. B., Rodrigues Filho M., Gomide E. M., and Betarelli R. P.. . 2012. Reduction in the protein level and addition of oil in diets for finishing pugs under different temperatures. R. Bras. Zootec. 41:1878–1883. doi: 10.1590/S1516-35982012000800011 [DOI] [Google Scholar]
- Ropka-Molik K., Pawlina-Tyszko K., Żukowski K., Piórkowska K., Grzegorz Z., Gurgul A., Derebecka N., and Wesoly J.. . 2018. Examining the genetic background of porcine muscle growth and development based on transcriptome and miRNAome data. J. Mol. Sci. 19:1208. doi: 10.3390/ijms19041208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H. S., Abino L. F. T., Donzele J. L., Gomes P. C., Oliveira R. F., Lopes D. C., Ferreira A. S., Barreto S. L. T., and Euclides R. F.. . 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 3rd ed UFV, Viçosa. [Google Scholar]
- Sainz R. D., and Wolff J. E.. . 1988. Effects of the beta-agonist, cimaterol, on growth, body composition and energy expenditure in rats. Br. J. Nutr. 60:85–90. [DOI] [PubMed] [Google Scholar]
- Sauvant D., Perez J. M., and Tran G.. . 2002. Tables de composition et de valeur nutritive des matières premières destinées aux animaux d’élevage. INRA, Versailles. [Google Scholar]
- Spencer J. D., Gaines A. M., Berg E. P., and Allee G. L.. . 2005. Diet modifications to improve finishing pig growth performance and pork quality attributes during periods of heat stress. J. Anim. Sci. 83:243–254. doi:10.2527/2005.831243x [DOI] [PubMed] [Google Scholar]
- Velayudhan D. E., Kim I. H., and Nyachoti C. M.. . 2015. Characterization of dietary energy in swine feed and feed ingredients: a review of recent research results. Asian-Australas. J. Anim. Sci. 28:1–13. doi: 10.5713/ajas.14.0001R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolp R., Rodrigues N., Zangeronimo M., Cantarelli V., Fialho E., Philomeno R., Alvarenga R., and Rocha L.. . 2012. Soybean oil and crude protein levels for growing pigs kept under heat stress conditions. Livest. Sci. 147:148–153. doi: 10.1016/j.livsci.2012.04.01 [DOI] [Google Scholar]