Abstract
This study aimed to improve the staining of frozen-thawed Japanese Black bull sperm acrosomes with fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA). Spermatozoa were washed, fixed with 1–3% paraformaldehyde (PFA) in suspension for 10, 20, and 30 min, permeabilized with 0–2% Triton X-100 for 5 min, stained with FITC-PNA, and mounted with different antifade agents (0.22 M 1,4-diazabicyclo [2,2,2] octane (DABCO), SlowFade®, and ProLong®) in suspension (In-suspension) or on a smear (On-smear). The spermatozoa were categorized into seven pattern types either immediately or after storage for 24 hr. Experiment 1 showed that 1) the In-suspension method was better than the On-smear method; 2) if spermatozoa were stained using the In-suspension method and examined immediately, the best antifade agent was SlowFade®; 3) if samples were to be stored after staining using the On-smear method, DABCO should be avoided; 4) if spermatozoa were stained using the In-suspension method, storage of the stained samples was not recommended; and 5) if samples were to be stored after staining using the In-suspension method, ProLong® might be the best antifade agent. The results of experiment 2 showed that the concentration of Triton X-100 could be reduced to 0.1 from 1%. The results of experiment 3 showed that the paraformaldehyde concentration used for a 30 min fixation could be reduced from 3 to 2%. It is expected that the improved staining protocol will be useful to determine bull sperm acrosomal integrity.
Keywords: acrosomal integrity, bull spermatozoa, FITC-PNA staining
Artificial insemination (AI) is an essential technique for producing offspring in the cattle industry, and bull semen used for AI is routinely analyzed to predict fertility. However, subfertile bulls have occasionally been reported to exhibit normal standard semen parameters [7, 14, 15]. Thus, standard semen analyses may fail to accurately estimate fertility, and novel methods to predict bull fertility are required. Among these methods, acrosomal integrity is one of the most powerful estimates of the fertilization ability of spermatozoa, because for successful fertilization by AI, spermatozoa must have intact acrosomes when inseminated into the female reproductive tract and must react in a timely manner when they reach the site of fertilization.
The acrosome can be observed using phase contrast microscopy [1], the triple stain technique [22], or Coomassie blue labeling [8]. More recently, fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA), which specifically binds to the sugar Galactosyl β-1,3 N-acetylgalactosamine in acrosomal membranes [13], has been used as a probe to visualize acrosomal integrity by Kishida et al. [7], Almadaly et al. [1], and Harayama et al. [5]. FITC-conjugated Pisum sativum agglutinin (FITC-PSA) has also been utilized for acrosomal staining [12]. In particular, the report by Harayama et al. [5] showed that categorizing samples according to their FITC-PNA staining pattern could be used to estimate bull fertility; thus, this method can be a useful test in addition to standard semen analyses. This study aimed to improve the staining conditions of the acrosomes categorized according to Harayama et al. [5].
In our previous report on staining with FITC-PNA, spermatozoa were fixed with 4% paraformaldehyde (PFA) for 30 min, permeabilized with 1% Triton X-100 for 5 min, stained with FITC-PNA, and mounted in 1,4-diazabicyclo [2,2,2] octane (DABCO) for analysis according to our own criteria for categorization [1]. When frozen-thawed Japanese Black bull spermatozoa were stained with FITC-PSA, the labeling was not confined to the acrosomal region and there was non-specific labeling of the head and flagellum, whereas FITC-PNA labeling was specific to the acrosomal region [1]. Therefore, FITC-PNA is more suitable for assessing acrosomal status in bull spermatozoa. Moreover, the fluorescent dye FITC is useful as a conjugate to PNA to label sperm acrosomes, but the fluorescence generally fades gradually while the stained samples are being stored or examined under a microscope. Thus, applying an antifade agent is necessary to retard the fading to enable the accurate assessment of acrosomal status.
Since PNA binds to molecules present inside the sperm plasma membrane, it is necessary to permeabilize spermatozoa before they are stained with PNA to reveal the integrity of the acrosome. For frozen-thawed bull spermatozoa, treatment with 1% Triton X-100 for 5 min has been used to permeabilize acrosomes for staining with FITC-PNA [1, 5]. However, because Triton X-100 is also used to extract proteins from cells [3, 6, 17], reducing its concentration might minimize the acrosomal damage caused by excessive treatment.
The aim of this study was to use the categorization criteria developed by Harayama et al. [5], instead of our previous criteria [1], to re-evaluate the protocols for staining frozen-thawed Japanese Black bull spermatozoa with FITC-PNA under gentler conditions to minimize artifactual damage. Therefore, we evaluated the effects of (1) antifades, the sperm environment during staining (on smear or in suspension), and the storage of stained spermatozoa mounted in antifade medium for 24 hr; (2) different concentrations of Triton X-100 (0, 0.1, 0.5, 1, and 2%) used to permeabilize spermatozoa; and (3) different durations of fixation (10, 20, and 30 min) using different concentrations of PFA (1, 2, and 3%) on the staining patterns of the acrosome.
MATERIALS AND METHODS
Reagents and media
All chemicals used in this study were purchased from SigmaAldrich (Sigma-Aldrich, Steinheim, Germany), unless otherwise stated. Saline medium and sucrose medium were used for washing spermatozoa [18, 19].
A solution of 12.5% (w/v) PFA in 0.5 M Tris, adjusted to pH 7.4 at 20°C, was prepared according to Almadaly et al. [1] and kept frozen at −30°C until use. A FITC-PNA stock solution (444 µg/ml of H2O) was prepared and diluted with phosphate buffered saline (PBS (1,060 µl)) to 20 µg/ml according to Almadaly et al. [1].
Spermatozoa
Frozen straws of Japanese Black bull semen were generously donated by the Hida Beef Cattle Research Department, Gifu Prefectural Livestock Research Institute, Japan. Cryopreserved spermatozoa from six Japanese Black bulls were used in this study. The use of cryopreserved semen was approved by the Animal Ethics Committee of the Gifu Prefectural Livestock Research Institute, Japan.
Washing and fixing spermatozoa
Spermatozoa were washed as described previously [1] but with a slight modification. Briefly, frozen-thawed spermatozoa were resuspended in saline medium by centrifugation and washed in 4 ml instead of 7.5 ml sucrose medium.
Washed spermatozoa were diluted with PBS to adjust the final sperm concentration to 100 × 106 spermatozoa/ml after fixation. Spermatozoa (230, 210, and 190 µl) were fixed in vials for 10, 20, and 30 min at 20–25°C by adding 20, 40, or 60 µl of 12.5% PFA/0.5 M Tris buffer according to the experimental design to produce final concentrations of 1, 2, or 3%, respectively.
Permeabilization and staining of spermatozoa with FITC-PNA
The On-smear method: The fixed spermatozoa were smeared, permeabilized, and stained as previously described for the smear method by Almadaly et al. [1]. The stained spermatozoa were then covered with 16 µl of 0.22 M DABCO (Sigma-Aldrich) dissolved in a glycerol-PBS mixture (9:1), SlowFade® Diamond Antifade Mountant (Thermo Fisher, West Sacramento, CA, U.S.A.; SlowFade®), or ProLong® Diamond Antifade Mountant (Thermo Fisher; Prolong®) and a cover slip (24 × 50 mm). This procedure was designated the ‘On-smear method’. The storage of stained samples for 24 hr was at 20–25°C in a dark box.
The In-suspension method: Fixed spermatozoa (250 µl, 100 × 106 spermatozoa/ml) were centrifuged at 1,000 × g for 1 min at 20–25°C, and the supernatant was removed. Two hundred microliters of 1% bovine serum albumin (BSA)/glycerol-PBS was added and the spermatozoa were centrifuged again. This rinse was performed twice. The pelleted spermatozoa were permeabilized by adding 200 µl of 0–1% Triton X-100 according to the experimental design for 5 min at 20–25°C and centrifuged at 1,000 × g for 1 min. The supernatant was discarded, 200 µl of 1% BSA/Gly-PBS was added, and the spermatozoa were centrifuged at 1,000 × g for 1 min. This wash was performed twice [21]. The sperm pellets were mixed in a vial with 200 µl of FITC-PNA (20 µg/ml) at 20–25°C for 30 min. Spermatozoa were washed as in the previous step, and the sperm pellets were resuspended in 250 µl PBS. A sample of this (8 µl) was mixed with an equal volume of DABCO, SlowFade®, or ProLong®. Two microliters of the mixture was spotted onto a glass slide, covered with a cover slip (18 × 18 mm), and gently pressed between sheets of tissue paper to remove excess liquid. This procedure was designated the ‘In-suspension method’. The storage of stained samples for 24 hr was at 20–25°C in a dark box.
Examination of sperm acrosomes
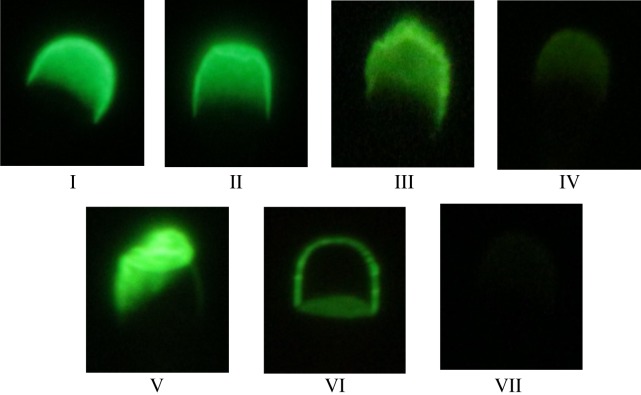
Stained spermatozoa were examined under a phase contrast microscope at a magnification of 1,000 × using fluorescence illumination (mirror unit U-MWB2: dichroic mirror DM500, excitation filter BP460–490, and emission filter BA520IF; Olympus, Tokyo, Japan). The staining patterns of the spermatozoa were first examined using fluorescence optics, then the localization was confirmed using phase contrast optics. Two hundred spermatozoa were examined on each slide, and the staining patterns of the acrosomes were classified into seven categories as described by Harayama et al. [5]: pattern I: normal acrosome; pattern II: slightly disordered acrosome; pattern III: severely disordered acrosome with very bright fluorescence; pattern IV: acrosome with less fluorescence over the entire structure than patterns I and II; pattern V: severely deformed acrosome; pattern VI: acrosome with fluorescence only along its outline; and pattern VII: acrosome with almost no fluorescence. The percentage of spermatozoa showing each pattern was calculated, and patterns I and II were pooled and considered to contain spermatozoa with intact acrosomes. A higher percentage of intact acrosomes (percentage of patterns I + II) and lower percentage of the other patterns (percentage of patterns III to VII) indicated better staining conditions (Fig. 1).
Fig. 1.
Staining patterns of acrosomes stained with FITC-PNA. pattern I: normal acrosome; pattern II: slightly disordered acrosome (patterns I and II are considered as intact); pattern III: severely disordered acrosome with very bright fluorescence; pattern IV: acrosome with less fluorescence over the entire structure than patterns I and II; pattern V: severely deformed acrosome; pattern VI: acrosome with fluorescence only along its outline; and pattern VII: acrosome with almost no fluorescence.
Experimental design
Experiment 1: Effect of the staining method (On-smear and In-suspension), different antifades, and 24 hr storage on the acrosomal staining patterns (method × antifade × storage): Spermatozoa that were washed and fixed with 3% PFA for 30 min were divided into two portions and stained by the On-smear method or the In-suspension method, including permeabilization with 1% Triton X-100 for 5 min. Spermatozoa were mounted using the three different antifades, examined for the acrosomal integrity immediately (0 hr) and 24 hr later, and the percentage of spermatozoa showing each staining pattern was determined.
Experiment 2: Effect of different concentrations of Triton X-100 on the staining patterns of acrosomes using the In-suspension method (Triton X-100): Spermatozoa that were washed and fixed with 3% PFA for 30 min were stained using the In-suspension method, including permeabilization with different concentrations of Triton X-100 (0, 0.1, 0.5, 1, and 2%) in 2 ml vials for 5 min at 20–25°C. Stained spermatozoa were mixed with ProLong® and examined for acrosomal integrity immediately, and the percentage of spermatozoa showing each staining pattern was determined.
Experiment 3: Effect of shortened durations of fixation (10, 20, and 30 min) with different concentrations of PFA on the staining patterns of acrosomes using the In-suspension method (shortened fixation): Spermatozoa that were washed and fixed with 1, 2, and 3% PFA for 10, 20, and 30 min were permeabilized with 1% Triton X-100 for 5 min at 20–25°C and were stained using the In-suspension method. Stained spermatozoa were mounted with ProLong® and were examined for acrosomal integrity immediately (0 hr of storage), and the percentage of spermatozoa showing each staining pattern was determined.
Statistical analyses
Results are presented as the mean ± SEM. The data obtained in experiments 1 and 3 were subjected to repeated measures using a two-way ANOVA. When the main effect was significant, the averages of one factor across the other were further compared using a Tukey’s multiple comparison test. When a significant interaction was found, the different treatments were compared using a Bonferroni’s multiple comparison test. A one-way ANOVA was used to analyze the results of experiments 2 and 3, and when the result of the one-way ANOVA was significant, a Tukey’s multiple comparison test was performed to compare the different treatments. Differences with P<0.05 were considered to be statistically significant. All analyses were performed using a statistical software program (GraphPad Prism Version 6.0; GraphPad Software, San Diego, CA, U.S.A.).
RESULTS
Experiment 1: method × antifade × storage
Due to their complexity, the results of experiment 1 were divided into subsets (Tables 1, 2, 3) and analyzed using 2-factor ANOVAs. Therefore, certain parts of Tables 1, 2, 3 represent the same values.
Table 1. Effect of antifade reagents and staining method on the staining patterns of frozen-thawed Japanese Black bull sperm acrosomes stained with FITC-PNA and stored for 0 hr (n=6).
| Pattern | Method | % Patterns with an antifade reagent |
Mean ± SEM | ||
|---|---|---|---|---|---|
| DABCO | SlowFade® | ProLong® | |||
| I+II*br | On-smear | 36.2 ± 5.8 | 37.7 ± 5.5 | 35.7 ± 4.1 | 36.5 ± 0.6 |
| (Intact acrosome) | In-suspension | 41.1 ± 7.3 | 41.4 ± 8.3 | 42.8 ± 7.9 | 41.8 ± 0.5 |
| Mean ± SEM | 38.7 ± 2.5 | 39.5 ± 1.9 | 39.2 ± 3.5 | ||
| IIIa) | On-smear | 40.3 ± 3.5 | 39.2 ± 4.5 | 42.6 ± 3.9 | 40.7 ± 1.0 |
| In-suspension | 37.8 ± 2.2 | 33.7 ± 3.0 | 38.0 ± 2.2 | 36.5 ± 1.4 | |
| Mean ± SEM | 39.0 ± 1.2d) | 36.4 ± 2.7c) | 40.3 ± 2.3d) | ||
| IV | On-smear | 0.6 ± 0.6 | 0.4 ± 0.3 | 0.2 ± 0.2 | 0.4 ± 0.1 |
| In-suspension | 2.1 ± 1.3 | 3.9 ± 2.5 | 1.7 ± 1.4 | 2.6 ± 0.7 | |
| Mean ± SEM | 1.4 ± 0.8 | 2.1 ± 1.7 | 0.9 ± 0.8 | ||
| Vb) | On-smear | 14.2 ± 5.0 | 13.2 ± 5.0 | 12.1 ± 4.5 | 13.1 ± 0.6 |
| In-suspension | 11.1 ± 5.4 | 12.1 ± 5.8 | 9.3 ± 4.7 | 10.8 ± 0.8e) | |
| Mean ± SEM | 12.6 ± 1.6 | 12.6 ± 0.6 | 10.7 ± 1.4 | ||
| VI | On-smear | 8.8 ± 1.3 | 9.6 ± 2.0 | 9.4 ± 1.9 | 9.3 ± 0.3 |
| In-suspension | 7.9 ± 1.7 | 9.0 ± 2.0 | 8.3 ± 1.9 | 8.4 ± 0.3 | |
| Mean ± SEM | 8.3 ± 0.4 | 9.3 ± 0.3 | 8.6 ± 0.5 | ||
| VII | On-smear | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| In-suspension | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Mean ± SEM | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
a) A significant main effect of antifade (P<0.05, two-way ANOVA). b) A significant main effect of method (P<0.05, two-way ANOVA). No significant interaction was found for any of the patterns (P>0.05, two-way ANOVA). c, d) Different superscripts denote a significant difference among the antifades (P<0.05, Tukey’s test). e) Significantly different from the smear (P<0.05, two-way ANOVA).
Table 2. Effect of antifade reagents and sample storage time on the staining patterns of frozen-thawed Japanese Black bull sperm acrosomes stained with FITC-PNA using the On-smear method (n=6).
| Pattern | Storage time | % Patterns with an antifade reagent |
Mean ± SEM | ||
|---|---|---|---|---|---|
| DABCO | SlowFade® | ProLong® | |||
| I+IIa) | 0 hr | 36.2 ± 5.8 | 37.7 ± 5.5 | 35.7 ± 4.1 | 36.5 ± 0.6 |
| (Intact acrosome) | 24 hr | 24.0 ± 5.6c,e) | 33.5 ± 4.7d) | 35.2 ± 5.4d) | 30.9 ± 3.5 |
| Mean ± SEM | 30.1 ± 6.1 | 35.6 ± 2.1 | 35.5 ± 0.3 | ||
| III | 0 hr | 40.3 ± 3.5 | 39.2 ± 4.5 | 42.6 ± 3.9 | 40.7 ± 1.0 |
| 24 hr | 42.7 ± 5.6 | 41.5 ± 4.5 | 43.4 ± 3.0 | 42.5 ± 0.6 | |
| Mean ± SEM | 41.5 ± 1.2 | 40.3 ± 1.2 | 43.0 ± 0.4 | ||
| IVb) | 0 hr | 0.6 ± 0.6 | 0.4 ± 0.3 | 0.2 ± 0.2 | 0.4 ± 0.1 |
| 24 hr | 10.0 ± 3.3c,e) | 3.2 ± 1.5d) | 0.1 ± 0.1d) | 4.5 ± 2.9 | |
| Mean ± SEM | 5.3 ± 4.7 | 1.8 ± 1.3 | 0.2 ± 0.0 | ||
| V | 0 hr | 14.2 ± 5.0 | 13.2 ± 5.0 | 12.1 ± 4.5 | 13.1 ± 0.6 |
| 24 hr | 13.3 ± 5.2 | 13.0 ± 5.0 | 12.8 ± 5.2 | 13.0 ± 0.1 | |
| Mean ± SEM | 13.7 ± 0.5 | 13.1 ± 0.1 | 12.4 ± 0.3 | ||
| VI | 0 hr | 8.8 ± 1.3 | 9.6 ± 2.0 | 9.4 ± 1.9 | 9.3 ± 0.3 |
| 24 hr | 10.0 ± 1.6 | 8.8 ± 2.2 | 8.5 ± 1.2 | 9.1 ± 0.5 | |
| Mean ± SEM | 9.4 ± 0.6 | 9.2 ± 0.4 | 8.9 ± 0.4 | ||
| VII | 0 hr | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 hr | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Mean ± SEM | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
a, b) Significant interaction between the antifade reagent and the sample storage (P<0.05, two-way ANOVA). c, d) Different superscripts represents significant differences within the same staining pattern (P<0.05, Bonferroni’s test). e) Significantly different from 0 hr (P<0.05, Bonferroni’s test).
Table 3. Effect of antifade reagents and sample storage time on the staining patterns of frozen-thawed Japanese Black bull sperm acrosomes stained with FITC-PNA using the In-suspension method (n=6).
| Pattern | Storage time | % Patterns with an antifade reagent |
Mean ± SEM | ||
|---|---|---|---|---|---|
| DABCO | SlowFade® | ProLong® | |||
| I+IIa) | 0 hr | 41.1 ± 7.3 | 41.4 ± 8.3 | 42.8 ± 7.9 | 42.3 ± 0.6 |
| (Intact acrosome) | 24 hr | 37.6 ± 8.6 | 35.0 ± 7.9 | 40.7 ± 7.4 | 37.8 ± 1.6e) |
| Mean ± SEM | 39.4 ± 1.8 | 39.0 ± 3.9 | 41.7 ± 1.0 | ||
| III | 0 hr | 37.8 ± 2.2 | 33.7 ± 3.0 | 38.0 ± 2.2 | 36.5 ± 1.4 |
| 24 hr | 36.7 ± 5.1 | 37.6 ± 3.0 | 38.6 ± 2.9 | 37.6 ± 0.5 | |
| Mean ± SEM | 37.3 ± 0.6 | 35.7 ± 1.9 | 38.3 ± 0.3 | ||
| IVb) | 0 hr | 2.1 ± 1.3 | 3.9 ± 2.5 | 1.7 ± 1.4 | 2.6 ± 0.7 |
| 24 hr | 4.3 ± 2.3 | 7.4 ± 3.1 | 3.1 ± 1.7 | 4.9 ± 1.3 | |
| Mean ± SEM | 3.2 ± 1.1c,d) | 5.6 ± 1.8c) | 2.4 ± 0.7d) | ||
| V | 0 hr | 11.1 ± 5.4 | 12.1 ± 5.8 | 9.3 ± 4.7 | 10.8 ± 0.8 |
| 24 hr | 12.3 ± 5.7 | 11.3 ± 5.8 | 10.6 ± 5.9 | 11.4 ± 0.5 | |
| Mean ± SEM | 11.7 ± 0.6 | 11.7 ± 0.4 | 9.9 ± 0.6 | ||
| VI | 0 hr | 7.9 ± 1.7 | 9.0 ± 2.0 | 8.3 ± 1.9 | 8.4 ± 0.3 |
| 24 hr | 9.2 ± 2.8 | 8.5 ± 1.1 | 6.9 ± 1.4 | 8.2 ± 0.7 | |
| Mean ± SEM | 8.5 ± 0.6 | 8.8 ± 0.3 | 7.6 ± 0.7 | ||
| VII | 0 hr | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 hr | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.2 ± 0.1 | |
| Mean ± SEM | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | ||
a) A significant main effect of storage (P<0.05, two-way ANOVA). b) A significant main effect of antifade reagent (P<0.05, two-way ANOVA). c, d) Different superscripts differ significantly (P<0.05, Tukey’s test). No significant interaction was found for any of the patterns (P>0.05, two-way ANOVA). e) Significantly different from 0 hr (P<0.05, main effect of time by two-way ANOVA).
Effects of the staining condition and antifade agent (Table 1)-method × antifade
A two-factor ANOVA of the staining method × antifade agent revealed there was no significant interaction between the two on any of the staining patterns, however, the antifade agent had a significant effect on the percentage of pattern III, and the staining condition affected the percentage of pattern V. The average percentage of intact acrosomes across the antifade agents was higher for the In-suspension method (41.8 ± 0.5%) than the On-smear method (36.5 ± 0.6%), although the difference was not statistically significant (P=0.33). The average percentage of pattern III in the SlowFade® condition was significantly lower than both DABCO and Prolong® (P<0.05 for both, Tukey’s test), and the average percentage of spermatozoa belonging to pattern V across the antifade agents for the In-suspension method (10.8 ± 0.8%) was significantly lower than the On-smear method (13.1 ± 0.6%).
Effects of the antifade agent and sample storage for 24 hr using the On-smear method (Table 2)-antifade × storage (On-smear)
A two-factor ANOVA evaluating the antifade agent × storage for 24 hr using the On-smear method revealed there was a significant interaction between these two factors (P<0.05) on the percentage of intact acrosomes and on the percentage of pattern IV, with a significant decrease in the percentage of intact acrosomes with DABCO after storage for 24 hr compared to that at 0 hr of storage (P<0.05, Bonferroni’s test). The percentage of intact acrosomes after storage for 24 hr was significantly lower for DABCO than for SlowFade® and Prolong® (P<0.05 for each, Bonferroni’s test). The same tendency was seen for the percentage of pattern IV, with a significant increase observed with DABCO after storage (P<0.05, Bonferroni’s test). The percentage of pattern IV after storage for 24 hr was significantly higher for DABCO than for SlowFade® and Prolong® (P<0.05 for each, Bonferroni’s test).
Effects of the antifade agent and sample storage for 24 hr using the In-suspension method (Table 3)-antifade × storage (In-suspension)
A two-factor ANOVA evaluating antifade agent × storage for 24 hr using the In-suspension method revealed there was no significant interaction, however, there was a significant main effect of storage on the percentage of intact acrosomes and of the antifade used on the percentage of pattern IV. The average percentage of intact acrosomes across the antifades significantly decreased after 24 hr (37.8 ± 1.6%) compared to at 0 hr of storage (42.3 ± 0.6%) using the In-suspension method, whereas the average percentage of pattern IV across the storage times was the highest for SlowFade® (5.6 ± 1.8%), intermediate for DABCO (3.2 ± 1.1%), and the lowest for Prolong® (2.4 ± 0.7%; P<0.05 for each, Tukey’s test). Notably, when using Prolong®, the background was clearer during the assessment of the acrosome condition (data not shown).
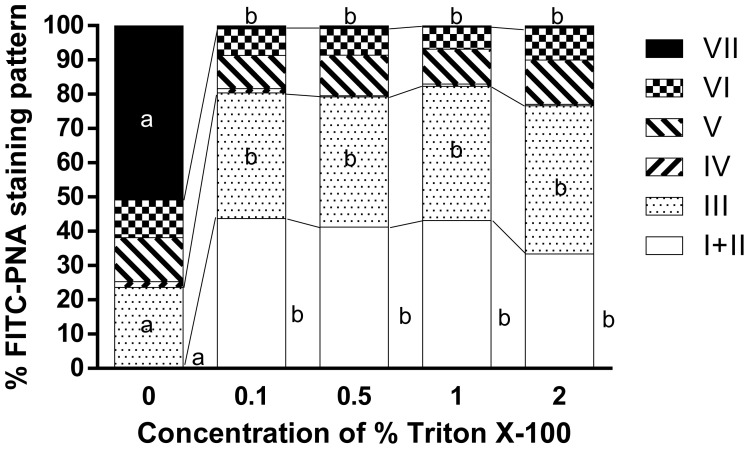
Experiment 2 (Fig. 2)–Triton X-100
When fixed spermatozoa were stained with FITC-PNA without permeabilization (0% Triton X-100 for 5 min), the percentage of intact acrosomes was almost 0% and significantly lower than for 0.1–2% Triton X-100. The percentage of pattern III was significantly higher with 0.1–2% than at 0% (P<0.05), and the percentage of pattern VII was significantly lower with 0.1–2% than at 0% Triton X-100 (P<0.05). There was no significant difference in the percentage of intact acrosomes, pattern III, or pattern VII with 0.1–2% Triton X-100. Although the difference was not significant, the percentage of intact acrosomes was the lowest (33.4 ± 5.5%) with 2% Triton X-100 and the highest (43.7 ± 6.5%) with 0.1% Triton X-100 (Fig. 2 ).
Fig. 2.
Effect of permeabilization using different concentrations of Triton X-100 for 5 min on the staining patterns of frozen-thawed Japanese Black bull sperm acrosomes stained with FITC-PNA using the In-suspension method. Spermatozoa were fixed prior to staining with 3% PFA for 30 min (n=6). a, b: Different superscripts denote a significant difference (P<0.05, one-way ANOVA; P<0.05, Tukey’s test).
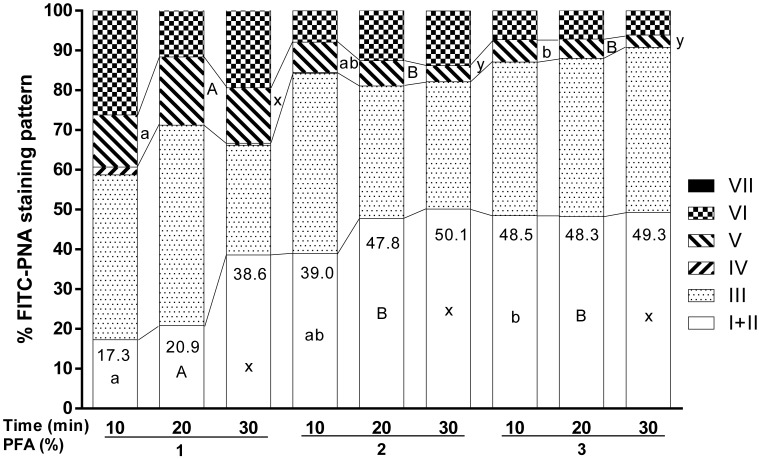
Experiment 3 (Fig. 3)–shortened fixation
A two-way ANOVA evaluating PFA concentration (1, 2, and 3%) × duration (10, 20, and 30 min) of fixation revealed that there was no significant interaction between these two factors on any of the patterns, but there was a significant main effect of the concentration of PFA on the percentage of intact acrosomes and on the percentage of pattern V (P<0.05). With a 10 min duration of fixation, 3% PFA had a significantly higher percentage of intact acrosomes and lower percentage of pattern V than 1% PFA, and 2% PFA produced intermediate results (P<0.05). With 20 min of fixation, 1% PFA had a significantly lower percentage of intact acrosomes and a significantly higher percentage of pattern V than 2 and 3% PFA (P<0.05). With 30 min of fixation, 1% PFA had a higher percentage of pattern V (P<0.05) than the other concentrations, whereas the percentage of intact acrosomes showed no significant difference among the three concentrations. The percentages of intact acrosomes are shown in Fig. 3 , and although it was not statistically significant, the highest percentage of intact acrosomes (50.1%) was observed with 2% PFA fixation for 30 min.
Fig. 3.
Effect of different durations of fixation with different concentrations of PFA on the staining patterns of frozen-thawed Japanese Black bull sperm acrosomes stained with FITC-PNA using the In-suspension method. Fixed spermatozoa were permeabilized prior to staining with 1% Triton X-100 for 5 min (n=3). No significant interaction was found for any of the patterns. A significant main effect of PFA concentration was found for the percentage of intact acrosomes (patterns I + II) and the percentage of pattern V (P<0.05, one-way ANOVA). Different superscripts denote a significant difference among the different concentrations of PFA within the same duration of 10 min (a, b), 20 min (A, B), and 30 min (x, y) (P<0.05 for each, Tukey’s test).
DISCUSSION
The present study evaluated the validity of staining frozen-thawed bull spermatozoa with FITC-PNA in suspension or in a smeared state using the categorization of the acrosomal staining patterns according to Harayama et al. [5], instead of the categorization used in our previous method [1]. Furthermore, we evaluated the possibility of staining acrosomes under gentler conditions to minimize artifactual damage.
The results of experiment 1 (Table 1) showed that the average percentage of intact acrosomes across the antifade agents was not significantly different between the In-suspension method and the On-smear method, but that the percentage of pattern V was significantly lower using the In-suspension method than the On-smear method. These results suggest that if stained samples are examined immediately without storage, as is the usual process, the In-suspension method can stain spermatozoa under gentler conditions, indicating that this method may be better to determine the acrosomal status of the spermatozoa. This finding was contradictory to the results of a previous study which found that on-smear staining was better than staining in a suspension [1]. The reason for this discrepancy might be due to differences in the categorization criteria; in the previous report, slightly damaged acrosomes were categorized as ‘E1’, which was recognized as damaged, whereas in this study, slightly damaged acrosomes were categorized as pattern II, which is recognized as intact according to Harayama et al. [5].
Earlier studies suggested that when mouse spermatozoa were spotted onto a slide then dried, fixed, and stained with FITC-PNA, the acrosomes were better stained than those that were smeared and dried [10]. Similarly, examination by differential interference phase contrast microscopy of a fixed, wet-mounted sample of bull spermatozoa revealed more major abnormalities than a dry-mounted sample [2]. When frozen-thawed bull spermatozoa were smeared without fixation and later stained with Naphthol Yellow S and Erythrosin B, the percentage of spermatozoa showing intact acrosomes was slightly lower than when live, wet spermatozoa or spermatozoa fixed with glutaraldehyde were examined by differential interference contrast microscopy, but this difference was not statistically significant [20]. Similar to these studies, the results of the present study showed that staining spermatozoa in suspension was better than staining a smeared preparation.
In addition, the observation that the percentage of pattern III (severely disordered acrosomes with very bright fluorescence) for SlowFade® was significantly lower than the other two agents at 0 hr of sample storage (Table 1) suggested that if the stained samples are examined immediately without storage, SlowFade® may be the best of the three antifade agents examined. The increase in the percentage of pattern IV after the storage of stained samples either on the smear (Table 2) or in sperm suspension (Table 3) indicated that storage of the stained samples with an antifade agent, regardless of the staining method used, may simply attenuate fluorescence without any damage to sperm acrosomes, and thus storage of stained samples is not recommended.
A significant interaction between the antifade agent and the storage of samples for 24 hr on the percentage of intact acrosomes and the percentage of pattern IV using the On-smear method (Table 2) suggests that depending on the antifade agent used, the storage of stained samples on slides with an antifade may in fact attenuate fluorescence more severely. A significantly lower percentage of intact acrosomes and higher percentage of pattern IV was observed with DABCO than with the other two agents (Table 2), suggesting that DABCO should be avoided if the stained sample are to be stored on slides with an antifade agent for up to 24 hr.
The results of the In-suspension method (Table 3) suggest that when spermatozoa are stained using the In-suspension method, storage of the stained samples in suspension with an antifade agent is not recommended, as this resulted in a decrease in the percentage of intact acrosomes. Nevertheless, the lower percentage of pattern IV with the use of Prolong® suggests that this antifade agent is the best to use if the stained sample is to be stored in suspension with an antifade agent for up to 24 hr.
SlowFade® is designed to help maintain the fluorescence of a histological section or a cell preparation stained with a fluorescent probe for immediate examination or for examination after storage for a relatively short period of time (2–3 weeks, according to the manufacturer’s instructions). It has been shown that SlowFade® has a quenching effect on the initial fluorescence but also slows down the rate of fading [9]. For the longer storage of fluorescent samples, Prolong® is suitable to keep fluorescent preparations for up to several months (according to the manufacturer’s instructions), but this antifade is designed to seal a histological section on the slide since this agent solidifies after being applied to the preparation (according to the manufacturer’s instructions). In agreement with the manufacturer’s instructions, SlowFade® was the best when stained spermatozoa were examined immediately after staining (Table 1), whereas Prolong® was the best when the samples stained using the In-suspension method were kept for 24 hr (Table 3).
The results of experiment 2 (Fig. 2) indicate that a concentration of at least 0.1% Triton X-100 might damage spermatozoa to some extent. A significantly higher percentage of pattern VII (acrosomes with almost no fluorescence) was observed with 0% Triton X-100 than the other concentrations, indicating that without permeabilization, FITC-PNA does not enter the acrosome and cannot bind to the molecules present inside, but that it can enter and stain damaged acrosomes. This result is similar to reports that stained un-permeabilized spermatozoa with FITC-PNA and identified damaged acrosomes by the fluorescence-positive spermatozoa [4, 16]. Conversely, the presence of all patterns when using between 0.1 and 2% Triton X-100 suggests that the concentration of Triton X-100 may be suitable within that range for spermatozoa permeabilization. Treatment with 1% Triton X-100 for 5 min has been used to permeabilize frozen-thawed bull spermatozoa [1, 5] and treatment with 1% for 10 min has also been successfully employed in human spermatozoa [11]. The present study clearly shows that the Triton X-100 concentration can be reduced to 0.1%, which minimizes the damage to bull spermatozoa.
Since the effect of fixation duration was not significant in experiment 3 (Fig. 3), we decided to compare the effects of PFA concentration within the same duration of fixation (10, 20, or 30 min). The results showed that with 10 min of fixation, 3% PFA was the best, 2% PFA was intermediate, and 1% was worse than the other treatments according to the percentage of intact acrosomes and pattern V (Fig. 3). This suggests that the fixation time can be shortened from 30 min to 10 min if 3% PFA is used. With 20 min of fixation, 2 and 3% PFA resulted in similar percentages of intact acrosomes and pattern IV, which were both significantly different from 1%, suggesting that the fixation time can be shortened to 20 min if 2 or 3% PFA is used. Since there was no significant difference in the percentage of intact acrosomes among 1, 2, and 3% PFA treatments for 30 min, but a significant increase in the percentage of pattern V at 1% PFA, this suggests that if spermatozoa are fixed for 30 min, the PFA concentration can be reduced from 3 to 2%. Altogether, the improved fixation conditions were 10 min with 3% PFA, 20 min with 2 or 3% PFA, or 30 min with 2% PFA. Since the highest percentage of intact acrosomes was achieved with 30 min of 2% PFA treatment (Fig. 3), this condition is proposed as the best one.
According to the results of this study, the following new protocol is proposed: spermatozoa should be fixed with 2% PFA for 30 min, permeabilized with 0.1% Triton for 5 min, stained with FITC-PNA, mounted with the antifade agent SlowFade®, and immediately evaluated using the categorization described by Harayama et al. [5]. In all steps, spermatozoa should be handled in suspension. Since it has been shown that the FITC-PNA staining patterns of the frozen-thawed bull spermatozoa used in this study are related to bull fertility [5], it is expected that the modified staining protocol described here will be a useful tool to routinely examine bull spermatozoa to estimate a bull’s fertility.
Acknowledgments
Reza Rajabi-Toustani was awarded the Japanese government (MONBUKAGAKUSHO: MEXT) scholarship by the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was supported in part by a JSPS KAKENHI Grant-in Aid for Scientific Research (C) No. 26450427.
REFERENCES
- 1.Almadaly E., El-Kon I., Heleil B., Fattouh S., Mukoujima K., Ueda T., Hoshino Y., Takasu M., Murase T.2012. Methodological factors affecting the results of staining frozen-thawed fertile and subfertile Japanese Black bull spermatozoa for acrosomal status. Anim. Reprod. Sci. 136: 23–32. doi: 10.1016/j.anireprosci.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 2.Freneau G. E., Chenoweth P. J., Ellis R., Rupp G.2010. Sperm morphology of beef bulls evaluated by two different methods. Anim. Reprod. Sci. 118: 176–181. doi: 10.1016/j.anireprosci.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 3.Gennuso F., Fernetti C., Tirolo C., Testa N., L’Episcopo F., Caniglia S., Morale M. C., Ostrow J. D., Pascolo L., Tiribelli C., Marchetti B.2004. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1). Proc. Natl. Acad. Sci. U.S.A. 101: 2470–2475. doi: 10.1073/pnas.0308452100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gürler H., Calisici O., Bollwein H.2015. Inter- and intra-individual variability of total antioxidant capacity of bovine seminal plasma and relationships with sperm quality before and after cryopreservation. Anim. Reprod. Sci. 155: 99–105. doi: 10.1016/j.anireprosci.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Harayama H., Nishijima K., Murase T., Sakase M., Fukushima M.2010. Relationship of protein tyrosine phosphorylation state with tolerance to frozen storage and the potential to undergo cyclic AMP-dependent hyperactivation in the spermatozoa of Japanese Black bulls. Mol. Reprod. Dev. 77: 910–921. doi: 10.1002/mrd.21233 [DOI] [PubMed] [Google Scholar]
- 6.Hipfner D. R., Gauldie S. D., Deeley R. G., Cole S. P. C.1994. Detection of the Mr 190,000 multidrug resistance protein, MRP, with monoclonal antibodies. Cancer Res. 54: 5788–5792. [PubMed] [Google Scholar]
- 7.Kishida K., Sakase M., Minami K., Arai M. M., Syoji R., Kohama N., Akiyama T., Oka A., Harayama H., Fukushima M.2015. Effects of acrosomal conditions of frozen-thawed spermatozoa on the results of artificial insemination in Japanese Black cattle. J. Reprod. Dev. 61: 519–524. doi: 10.1262/jrd.2015-073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson J. L., Miller D. J.1999. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 52: 445–449. doi: [DOI] [PubMed] [Google Scholar]
- 9.Longin A., Souchier C., Ffrench M., Bryon P. A.1993. Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J. Histochem. Cytochem. 41: 1833–1840. doi: 10.1177/41.12.8245431 [DOI] [PubMed] [Google Scholar]
- 10.Lybaert P., Danguy A., Leleux F., Meuris S., Lebrun P.2009. Improved methodology for the detection and quantification of the acrosome reaction in mouse spermatozoa. Histol. Histopathol. 24: 999–1007. [DOI] [PubMed] [Google Scholar]
- 11.Mahony M. C., Clark G. F., Oehninger S., Acosta A. A., Hodgen G. D.1993. Fucoidin binding activity and its localization on human spermatozoa. Contraception 48: 277–289. doi: 10.1016/0010-7824(93)90146-X [DOI] [PubMed] [Google Scholar]
- 12.Mendoza C., Carreras A., Moos J., Tesarik J.1992. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J. Reprod. Fertil. 95: 755–763. doi: 10.1530/jrf.0.0950755 [DOI] [PubMed] [Google Scholar]
- 13.Mortimer D., Curtis E. F., Miller R. G.1987. Specific labelling by peanut agglutinin of the outer acrosomal membrane of the human spermatozoon. J. Reprod. Fertil. 81: 127–135. doi: 10.1530/jrf.0.0810127 [DOI] [PubMed] [Google Scholar]
- 14.Murase T., El-Kon I., Harayama H., Mukoujima K., Takasu M., Sakai K.2010. Hyperactivated motility of frozen-thawed spermatozoa from fertile and subfertile Japanese black bulls induced by cyclic adenosine 3′,5′-monophosphate analogue, cBiMPS. J. Reprod. Dev. 56: 36–40. doi: 10.1262/jrd.09-082N [DOI] [PubMed] [Google Scholar]
- 15.Murase T., Mukohjima K., Sakaguchi S., Ohtani T., Tsubota T., Kita I.2001. Characterization of frozen-thawed Japanese Black bull spermatozoa by standard semen analysis, mucus penetration test and the ability to undergo the acrosome reaction in response to calcium and the calcium ionophore A23187. J. Reprod. Dev. 47: 237–243. doi: 10.1262/jrd.47.237 [DOI] [Google Scholar]
- 16.Prathalingam N. S., Holt W. V., Revell S. G., Jones S., Watson P. F.2006. Dilution of spermatozoa results in improved viability following a 24 h storage period but decreased acrosome integrity following cryopreservation. Anim. Reprod. Sci. 91: 11–22. doi: 10.1016/j.anireprosci.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 17.Rajagopal A., Pant A. C., Simon S. M., Chen Y.2002. In vivo analysis of human multidrug resistance protein 1 (MRP1) activity using transient expression of fluorescently tagged MRP1. Cancer Res. 62: 391–396. [PubMed] [Google Scholar]
- 18.Roldan E. R., Murase T., Shi Q. X.1994. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science 266: 1578–1581. doi: 10.1126/science.7985030 [DOI] [PubMed] [Google Scholar]
- 19.Roldan E. R. S., Harrison R. A. P.1989. Polyphosphoinositide breakdown and subsequent exocytosis in the Ca2+/ionophore-induced acrosome reaction of mammalian spermatozoa. Biochem. J. 259: 397–406. doi: 10.1042/bj2590397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinholt H. C., Chandler J. E., Tirado V.1991. Evaluating acrosome reaction steps with brightfield and differential interference contrast microscopy techniques. J. Dairy Sci. 74: 3822–3826. doi: 10.3168/jds.S0022-0302(91)78574-3 [DOI] [PubMed] [Google Scholar]
- 21.Tabuchi T., Shidara O., Harayama H.2008. A 32-kDa tyrosine-phosphorylated protein shows a protease-dependent increase in dead boar spermatozoa. J. Reprod. Dev. 54: 502–507. doi: 10.1262/jrd.20021 [DOI] [PubMed] [Google Scholar]
- 22.Talbot P., Chacon R. S.1981. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J. Exp. Zool. 215: 201–208. doi: 10.1002/jez.1402150210 [DOI] [PubMed] [Google Scholar]