Abstract
This study investigated the direct effects of non-steroidal anti-inflammatory drugs (NSAIDs) and atrial natriuretic peptide (ANP) on canine-derived vascular endothelial cells (VECs). VECs were isolated and cultured from canine arteries and veins. The mRNA expressions of vascular endothelial growth factor receptor 2, cyclooxygenase-2, and natriuretic peptide receptor 1 were detected in the cultured VECs. The viability of the cultured VECs was reduced in a dose-dependent manner by meloxicam, carprofen, and robenacoxib. By contrast, dose escalations of ANP had only marginal influence on the viability of cultured VECs. NSAIDs may potentially serve as not only analgesic agents against cancerous and perioperative pain but also as adjuvant anti-angiogenic drugs in dogs with malignant tumors.
Keywords: anti-angiogenesis, cyclooxygenase-2, dog, non-steroidal anti-inflammatory drugs, vascular endothelial cell
The proliferation of malignant tumors requires neovascularization because tumor cells need oxygen and nutrients delivered in blood [3]. Angiogenesis is involved in tumor growth and metastasis [1]. In angiogenesis, several cytokines and mediators, including vascular endothelial growth factor (VEGF) and fibroblast growth factor, directly affect vascular endothelial cells (VECs) [2, 11].
Anti-angiogenesis therapy is a tumor-dormancy treatment targeting the blood vessels that supply nutrients to malignant tumor tissue. Anti-angiogenesis therapy directed at VEGF is available for the treatment of human colorectal cancer [6]. The inhibition of VECs is a potential adjuvant therapy for the regrowth or metastasis of malignant tumors in dogs, similar to that achieved in humans.
Non-steroidal anti-inflammatory drugs (NSAIDs) are generally applied to treat acute or chronic pain associated with osteoarthritis in dogs [13] and are also prescribed for pain relief from cancer in dogs with malignant tumors [4]. Perioperative administration of NSAIDs is as multimodal analgesia in dogs. In addition, NSAIDs have clinical value for the treatment of canine bladder transitional cell carcinomas [8] and display anti-angiogenesis and anti-inflammatory effects [12]. However, the underlying anti-angiogenic mechanisms still remain unclear.
Atrial natriuretic peptide (ANP) suppresses VEC activity via natriuretic peptide receptor 1 (NPR1) [9]. However, whether ANP directly affects canine VECs is unknown.
The objective of this study was to investigate the direct effects of NSAIDs and ANP on canine-derived VECs. This study was conducted under the Nihon University Animal Care and Use Committee approval (AP15B037). A protocol from a previous study [5, 10] was modified to isolate VECs from the arteries (aorta and bilateral carotid arteries) and veins (caudal vena cava and bilateral jugular veins) of three clinically healthy Beagles. Briefly, after one end of each blood vessel was clamped, 0.25% trypsin solution was injected into the other end using a syringe. Each vessel was filled with trypsin and left for 10 min. The cells in the collected trypsin solution were cultured in an atmosphere containing 5% CO2 at 37°C in MCDB131 medium (Gibco; Thermo Fisher Scientific K.K., Yokohama, Japan) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin (10 units/ml; 10 µg/ml), and 10 mM L-glutamine. The cultured VECs were passaged four times for 3 weeks.
To evaluate the gene expressions of CD31, cyclooxygenase-2 (COX2), VEGF receptor 2 (VEGFR2), and NPR1, the total RNA was extracted from the isolated cells with the NucleoSpin® RNA kit (MACHEREY-NAGEL GmbH & Co., Duren, Germany). RNA integrity was verified using an absorptiometer (NanoDrop 1000; LMS Co., Ltd., Tokyo, Japan). The cDNA was synthesized from 1 µg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific K.K.). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed for each sample for 30 cycles. Specific primers of CD31, COX2, VEGFR2, and NPR1 were designed using the Primer3 Plus software (Table 1). Products amplified by RT-PCR were analyzed by 3% agarose gel electrophoresis.
Table 1. Primers used for RT-PCR.
| Gene | Accession number | F/R | Sequence (5′−3′) | Tm (°C) | Amplicon Size (bp) |
|---|---|---|---|---|---|
| CD31 | XM_022422841 | F | AATCCCAAATTCCACGTCAG | 63.6 | 346 |
| R | GAATGGAGCACCACAGGTTT | 63.9 | |||
| VEGFR2 | NM_001048024 | F | GACAACCAGACGGACAGTGGTATG | 68.6 | 157 |
| R | ACTGGTAGCCACTCGTTTGGTTAG | 66.1 | |||
| COX2 | NM_001003354 | F | GTTCATTCCTGATCCCCAAG | 63.2 | 186 |
| R | TTGAAAAGGCGCAGTTTATG | 62.6 | |||
| NPR1 | XM_547577 | F | CGCATTGAGCTGACGCGAAA | 71.7 | 249 |
| R | TGAGGTTGCCATGGGAGCAA | 71.1 |
For indirect immunofluorescence analysis of CD31, VECs were seeded onto glass culture slides, fixed with 4% formaldehyde, and permeabilized with 0.1% saponin in phosphate-buffered saline (PBS) for 30 min. The slides were washed with PBS, blocked with PBS containing 1% FBS at room temperature for 20 min, and incubated with primary antibodies against CD31 (1:20 dilution). The slides were then incubated with Alexa488 secondary antibodies (1:500 dilution), followed by nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Images were captured using an upright fluorescence microscope.
Cells were seeded in 96-well plates (1,000 cells/well) in an atmosphere containing 5% CO2 at 37°C in MCDB131 medium for 24 hr. The cells were incubated for 48 hr in MCDB131 supplemented NSAIDs (meloxicam, carprofen, or robenacoxib) or ANP. The control group was cultured in medium alone. To evaluate cell growth inhibition, 10% alamarBlue® Cell Viability Reagent (Invitrogen™; Thermo Fisher Scientific K.K.) was added. Fluorescence was measured using a SpectraMax Gemini EM Microplate Reader (Molecular Devices, LLC., San Jose, CA, U.S.A.) with excitation and emission wavelengths of 570 and 590 nm, respectively. A growth inhibition curve was produced using the average cell viability data for each well.
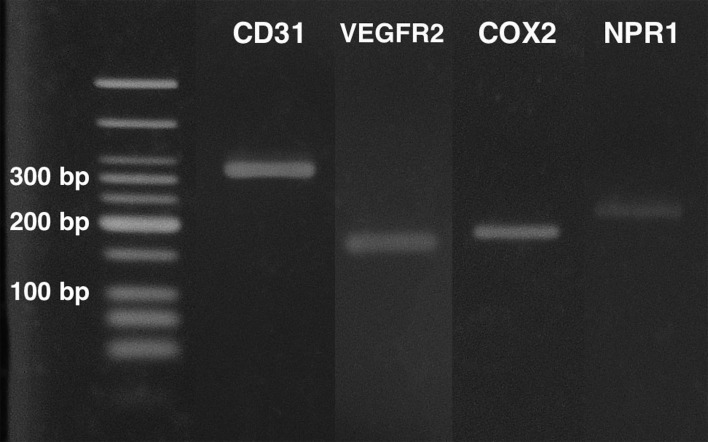
Cells with morphological features of VECs were selected and cultured, and the mRNA expression of CD31, COX2, VEGFR2, and NPR1 was detected in the isolated cells. Electrophoresis revealed one band at the expected molecular weight for each gene (Fig. 1). The indirect immunofluorescence analysis revealed that more than 99% of the cultured cells were CD31-positive (Fig. 2).
Fig. 1.
Agarose gel electrophoresis of RT-PCR amplification of the genes for CD31, COX2, VEGFR2, and NPR1.
Fig. 2.
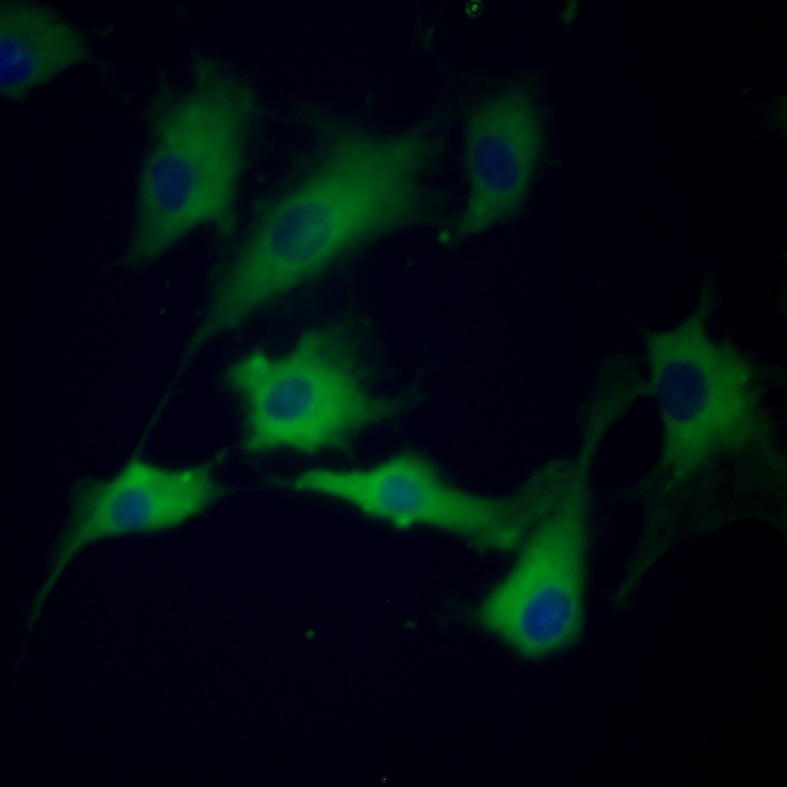
Immunofluorescence analysis of canine-derived VECs. CD31 is stained with Alexa488 (green) and nuclei are stained with DAPI (blue).
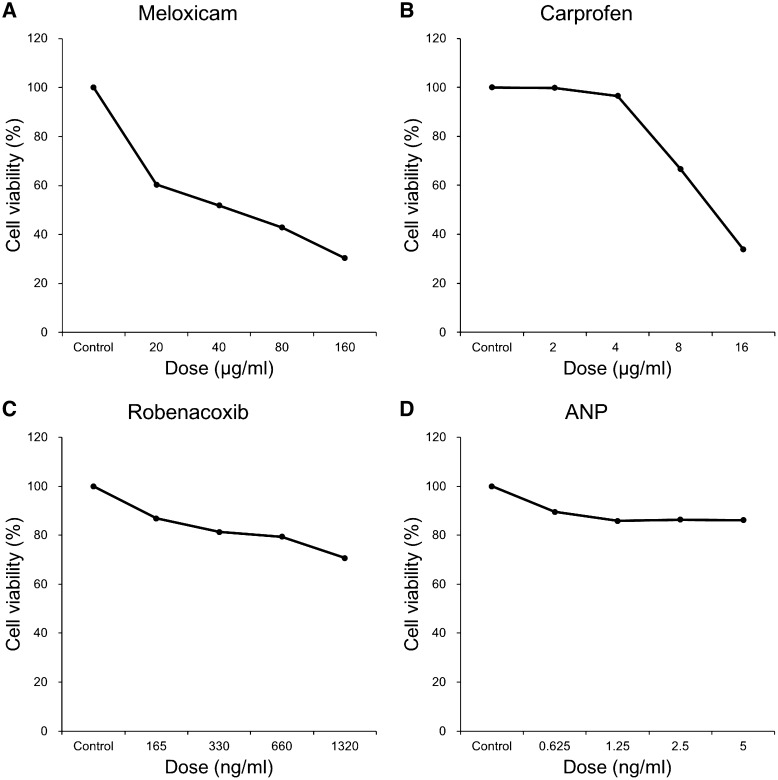
Cell growth inhibition induced by NSAIDs and ANP is illustrated in Fig. 3. At higher concentrations of meloxicam, VEC viability gradually decreased and was reduced to 30% by the addition of meloxicam at 160 µg/ml (Fig. 3A). Dose escalation of carprofen up to 4 µg/ml only marginally influenced VEC viability, but concentrations of ≥8 µg/ml reduced VEC viability in a dose-dependent manner to 34% at 16 µg/ml (Fig. 3B). VEC viability was decreased in a dose-dependent manner by robenacoxib. However, the viability was only reduced to 71% at the maximum robenacoxib concentration of 1,320 ng/ml (Fig. 3C). By contrast, ANP had marginal influence on VEC viability (Fig. 3D).
Fig. 3.
Growth inhibitory curves of canine-derived VECs treated with meloxicam (A), carprofen (B), robenacoxib (C), and ANP (D). VEC viability decreased in a dose-dependent manner with NSAID treatment but was only marginally influenced by ANP.
Previous studies have described the isolation and culture of canine VECs derived from arteries and veins using collagenase [5, 10]. In our study, trypsin was used for the intraluminal exfoliation of VECs from canine vessels because of the mRNA expression of CD31 and the positive detection of surface CD31 antigens. The canine-derived VECs obtained by this method should be available for in vitro studies on the mechanisms and roles of VECs on the angiogenesis. In addition, the mRNA expressions of COX2, VEGFR2, and NPR1 were detected in canine-derived VECs. These molecules are thought to be associated with angiogenesis, so canine-derived VECs should prove useful for investigations of potential therapeutic anti-angiogenesis candidates for the treatment of canine malignant tumors.
Presently, meloxicam, carprofen, and robenacoxib directly inhibited the proliferation of VECs. These NSAIDs are COX2 inhibitors and have demonstrated anti-angiogenic activity in mice [7]. The mechanisms of action are thought to be associated with prostaglandin-E2 produced by COX2 [7]. Prostaglandin-E2 increases the synthesis of VEGF, which promotes the VEC proliferation. VEGF synthesis is reduced by NSAIDs in mouse endovascular cells [14]. In clinical settings, however, the dose of NSAIDs influences the intended therapeutic effects but has undesirable side effects. In our study, the four concentrations of each drug were allocated to be one quarter, one half, exactly, and double that of the maximum blood concentration after the subcutaneous administration of the usual dose for analgesia. The concentrations of meloxicam, carprofen, and robenacoxib equivalent to the maximum blood concentration reduced cell viability to 43, 67, and 79%, respectively. Therefore, meloxicam, carprofen, and robenacoxib have potential for adjuvant anti-angiogenesis therapy for canine malignant tumors.
Perioperative administration of ANP decreases the postoperative metastasis in human patients with lung cancer [9]. ANP is thought to inhibit the adhesion of cancer cells to VECs because of the suppression of E-selectin synthesis promoted by cancer-related inflammation. In our study, canine-derived VECs expressed mRNA of NPR1, a receptor of ANP. However, its direct influences on the proliferation of VECs remain unclear.
One of major limitations in our study was that only the direct effects of NSAIDs but not the inhibitory mechanisms of canine-derived VECs on cell growth were demonstrated. In humans, several studies have reported the anti-angiogenic mechanisms of NSAIDs [7, 14]. Further investigations are required to clarify the mechanisms of cell growth inhibition in canine-derived VECs.
In conclusion, our study demonstrates that NSAIDs directly inhibited canine-derived VECs. NSAIDs may have potential value as analgesics against cancerous and perioperative pain and also as adjuvant anti-angiogenic drugs in dogs with malignant tumors.
REFERENCES
- 1.Argyle D. J., Khanna C.2012. Tumor Biology and Metastasis. pp. 30–50. In: Withrow and McEwen’s Small Animal Clinical Oncology, 5th ed. (Withrow, S. J., Vail, D. M. and Page, R. L. eds.), Elsevier Saunders, St. Louis. [Google Scholar]
- 2.Ferrara N., Henzel W. J.1989. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161: 851–858. doi: 10.1016/0006-291X(89)92678-8 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J.1971. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285: 1182–1186. doi: 10.1056/NEJM197111182852108 [DOI] [PubMed] [Google Scholar]
- 4.Gaynor J. S.2008. Control of cancer pain in veterinary patients. Vet. Clin. North Am. Small Anim. Pract. 38: 1429–1448, viii. doi: 10.1016/j.cvsm.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 5.Hu Q., Chai J., Liu L., Hou Y., Wang Y., Li B., Yang H.2013. [Isolation, culture, and identification of canine umbilical vein vascular endothelial cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 27: 460–463. [PubMed] [Google Scholar]
- 6.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., Ferrara N., Fyfe G., Rogers B., Ross R., Kabbinavar F.2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350: 2335–2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 7.Leahy K. M., Ornberg R. L., Wang Y., Zweifel B. S., Koki A. T., Masferrer J. L.2002. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 62: 625–631. [PubMed] [Google Scholar]
- 8.McMillan S. K., Boria P., Moore G. E., Widmer W. R., Bonney P. L., Knapp D. W.2011. Antitumor effects of deracoxib treatment in 26 dogs with transitional cell carcinoma of the urinary bladder. J. Am. Vet. Med. Assoc. 239: 1084–1089. doi: 10.2460/javma.239.8.1084 [DOI] [PubMed] [Google Scholar]
- 9.Nojiri T., Hosoda H., Tokudome T., Miura K., Ishikane S., Otani K., Kishimoto I., Shintani Y., Inoue M., Kimura T., Sawabata N., Minami M., Nakagiri T., Funaki S., Takeuchi Y., Maeda H., Kidoya H., Kiyonari H., Shioi G., Arai Y., Hasegawa T., Takakura N., Hori M., Ohno Y., Miyazato M., Mochizuki N., Okumura M., Kangawa K.2015. Atrial natriuretic peptide prevents cancer metastasis through vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 112: 4086–4091. doi: 10.1073/pnas.1417273112 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Oosterhoff L. A., Kruitwagen H. S., Spee B., van Steenbeek F. G.2016. Isolation and culture of primary endothelial cells from canine arteries and veins. J. Vis. Exp. 18: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seghezzi G., Patel S., Ren C. J., Gualandris A., Pintucci G., Robbins E. S., Shapiro R. L., Galloway A. C., Rifkin D. B., Mignatti P.1998. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 141: 1659–1673. doi: 10.1083/jcb.141.7.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujii M., Kawano S., Tsuji S., Sawaoka H., Hori M., DuBois R. N.1998. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716. doi: 10.1016/S0092-8674(00)81433-6 [DOI] [PubMed] [Google Scholar]
- 13.Vasseur P. B., Johnson A. L., Budsberg S. C., Lincoln J. D., Toombs J. P., Whitehair J. G., Lentz E. L.1995. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 206: 807–811. [PubMed] [Google Scholar]
- 14.Yoshida S., Amano H., Hayashi I., Kitasato H., Kamata M., Inukai M., Yoshimura H., Majima M.2003. COX-2/VEGF-dependent facilitation of tumor-associated angiogenesis and tumor growth in vivo. Lab. Invest. 83: 1385–1394. doi: 10.1097/01.LAB.0000090159.53224.B9 [DOI] [PubMed] [Google Scholar]