Abstract
Activin E, a secreted peptide encoded by the inhibin/activin βE subunit gene, is a member of the transforming growth factor-β superfamily, which is predominantly expressed in the liver. Recent reports have suggested that activin E plays a role in energy homeostasis as a hepatokine. Here, using transgenic mice overexpressing activin E under the control of the β-actin promoter, we demonstrate that activin E controls energy metabolism through brown/beige adipocytes. The glucose tolerance test and insulin tolerance test showed that the insulin sensitivity was improved in the transgenic mice. Furthermore, the mice had a high body temperature compared with wild-type mice. The transgenic brown adipose tissue and mesenteric white adipose tissue showed upregulation of uncoupling protein 1, which enables energy dissipation as heat by uncoupling oxidative phosphorylation from ATP production. Present results indicate that activin E activates energy expenditure through brown/beige adipocyte activation, suggesting that activin E has high potential for obesity therapy.
Keywords: INHBE, browning/beiging, hepatokine, thermogenesis, UCP1
Mammals have two major types of adipose tissues: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT consisting of white adipocytes is involved in the storage of energy in the form of triglycerides. In contrast, BAT consisting of brown adipocytes dissipates stored energy to generate heat in response to cold exposure or excess feeding. Expression of the mitochondrial uncoupling protein 1 (UCP1), which promotes proton leak across the inner mitochondrial membrane, enables brown adipocytes to stimulate energy expenditure [2].
There are two origins of UCP1-positive adipocytes, at least in mice: classical brown adipocytes located at the interscapular BAT (iBAT) and beige adipocytes induced in WAT [5]. Both cell types upregulate UCP1 expression in response to β adrenergic receptor activation [1, 13, 18, 23] and contribute to thermogenesis. However, the commitment/differentiation process is distinct between classical brown adipocytes and beige adipocytes [12, 16]; classical brown adipocytes are derived from skeletal muscle precursors, whereas beige adipocytes arise from smooth muscle-like origins [12, 16].
In mice, increases in the number of beige adipocytes in WAT are associated with the protection against diet-induced obesity and metabolic dysfunction including insulin resistance [10]. In adult humans, the amount of BAT negatively correlates with the body mass index [3]. Furthermore, cold exposure enhances the activity of BAT in humans, which is responsible for the increased energy expenditure [4, 14]. Interestingly, human brown adipocytes possess molecular signatures resembling those of mouse beige adipocytes [19]. These results indicated that brown/beige adipocytes are metabolically important cells for regulating energy metabolism not only in rodents, seasonally hibernating mammals and infants, but also in adult humans [23]. Therefore, thermogenesis in brown/beige adipocytes may have useful therapeutic potential for combating obesity and diabetes. However, less information is available on how the activity of brown/beige adipocytes is regulated.
Hepatokines are secreted from the liver and play important roles in glucose and lipid metabolism [21]. Hepatokines including angiopoietin-related growth factor and fibroblast growth factor 21 are secreted into the circulation and exert their effects in insulin-sensitive tissues. Therefore, dysregulation of these factors is associated with obesity, lipid accumulation in the liver and skeletal muscle, insulin resistance and reduced energy expenditure.
Activin E, a member of the transforming growth factor-β (TGF-β) superfamily, is predominantly expressed in the liver. The mRNA expression of activin E exhibited feeding-related diurnal changes in restricted-feeding rats [15] and upregulation in diet-induced obese mice [7]. Recently, using mice that overexpress activin E in the liver and mice with targeted disruption of the activin E gene, we demonstrated that activin E regulates energy metabolism through activation of brown/beige adipocytes as a hepatokine [8]. To further confirm activin E as a therapeutic target for obesity, in the present study, we investigated the physiological/pharmacological activity of activin E in the regulation of the metabolic pathway using transgenic mice systemically expressing activin E under the β-actin promoter (TgActβE mice) [6].
MATERIALS AND METHODS
Animals
C57BL/6J mice were obtained from Crea Japan (Tokyo, Japan). The transgenic mice overexpressed activin E using the β-actin promoter [TgActβE mice, B6. Cg-Tg (CAG-INHBE) 19Ohm] as described previously [6]. TgActβE19 mice were backcrossed to C57BL/6J mice and analyzed together with control littermates after 10–20 generations of backcrossing. Male mice were used in all experiments. Male mice were housed individually from the age of 6 weeks, maintained in a 12-hr light/dark cycle at 22 ± 4°C, and given standard chow (Labo MR-A1, Nosan Corp., Yokohama, Japan, https://www.nosan.co.jp/business/lifetech/expanim.htm) and water ad libitum. Experimental procedures and the care of animals were performed in accordance with the requirements of the Institutional Animal Care Committee at Kitasato University, in compliance with the National Institutes of Health guidelines.
Physiological measurements
Male TgActβE mice and their wild-type littermates were weighed weekly from 4 through 52 weeks of age. Daily food intake was calculated based on the 5-day intake measurement. Rectal temperature was monitored using a microprobe thermometer system equipped with a rectal probe (Model BAT-12; Muromachi Kikai Co., Tokyo, Japan) at 10:00–11:00 hr during the light cycle.
Blood examination
Intravenous blood was obtained from the tail vein of the mice. Blood glucose levels were measured by a Medisafe FIT® blood glucose meter (Terumo, Tokyo, Japan). The glucose tolerance test (GTT) and the insulin tolerance test (ITT) were performed as described previously [8]. Plasma insulin levels were measured by an immunoassay kit (Mouse Insulin ELISA kit U-type, FUJIFILM Wako Shibayagi Corp., Shibukawa, Japan).
Histological analysis
Tissues were fixed in Bouin’s fluid, embedded in paraffin, and then 4 µm sections were stained with hematoxylin and eosin (HE). Cell size was examined using HE-stained adipose tissues. Four arbitrarily chosen fields of view (0.12 mm2/WAT or 0.03 mm2/BAT per field) were analyzed by the computer program, NIH Image (Image J 1.62, National Institutes of Health, Bethesda, MD, U.S.A.), to estimate the adipocyte size. Subsequently, the density and distribution of adipocytes were calculated.
For immunohistochemistry, a rabbit polyclonal anti-UCP1 antibody (1 µg/ml, Alpha Diagnostic Intl. Inc., San Antonio, CA, U.S.A.) was reacted with deparaffinized sections overnight at 4°C and visualized with 3,3′-diaminobenzidine tetrahydrochloride using a Histofine Simple Stain MAX-PO kit (Nichirei, Tokyo, Japan) as described previously [6]. For estimation of Ucp1-positive area, arbitrary fields of view were analyzed using the NIH Image as described previously [9].
Western blot analysis
Protein extraction from tissues and immunoblotting were performed as described previously [6]. Briefly, proteins were subjected to SDS-polyacrylamide gel electrophoresis using 12% gels, and then transferred onto a polyvinylidene difluoride membranes (Millipore, Billerica, MA, U.S.A.). The membranes were blocked with 5% dry non-fat milk and probed with anti-activin E antibody [6], anti-UCP-1 antibody (Alpha Diagnostic Intl. Inc.) and anti-β-actin antibody (Abcam, Cambridge, U.K.), followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The reaction was detected with a chemiluminescence system (ECL Plus; Amersham Biosciences, Little Chalfont, U.K.). The band intensity was measured by NIH Image (ImageJ 1.37).
RNA isolation and real-time RT-qPCR
Total RNA was isolated from adipose tissues using an Isogen kit (Nippon Gene Co., Ltd., Tokyo, Japan). The cDNA was prepared using ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). cDNA reverse transcribed from 5 ng of total RNA was used as a template for real-time qPCR using Thunderbird SYBR qPCR Mix (Toyobo) as described previously [11]. The oligonucleotide primers for RT-qPCR have been described previously [8]. The Ct values were determined, and the abundance of gene transcripts was analyzed by the ΔΔCt method, using hypoxanthine phosphoribosyltransferase 1 (Hprt1) as the normalization gene.
Statistical analysis
All results are expressed as mean ± SEM. Differences between groups were tested by unpaired t-test (StatVeiw 5.0 software, Abacus Concept, Berkley, CA, U.S.A. or Prism5 software, GraphPad Software Inc., San Diego, CA, U.S.A.); P<0.05 was considered statistically significant.
RESULTS
Enhanced insulin sensitivity and body temperature in transgenic mice overexpressing activin E
To further demonstrate activin E as a metabolic regulator, we investigated the phenotype of TgActβE mice, which overexpress activin E under the β-actin promoter.
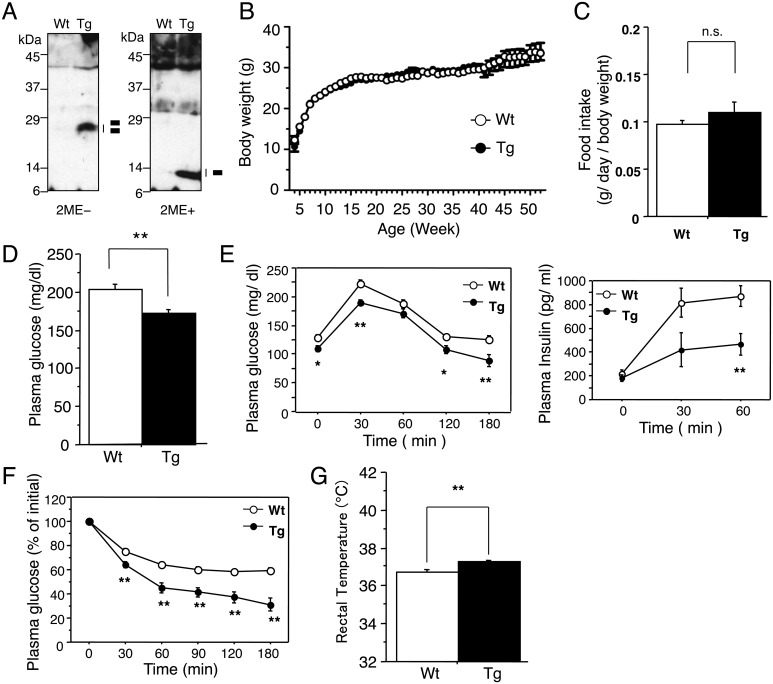
Western blot analysis showed a detectable level of disulfide bond formed activin E in the blood of the transgenic mice, suggesting a possible systemic influence of the mature proteins (Fig. 1A). The body weight and food intake did not show any significant differences between wild-type and TgActβE mice (Fig. 1B, 1C). However, a significant reduction in the blood glucose level was found in TgActβE mice compared with the wild-type mice (Fig. 1D). To investigate insulin sensitivity in TgActβE, GTT and ITT were performed. After the glucose challenge, the blood glucose level was significantly lower in TgActβE mice than in wild-type mice in all the indicated time points in both GTT and ITT (Fig. 1E left panel, 1F). However, the blood insulin level was significantly lower in TgActβE mice compared with the wild-type mice at 30 min after the glucose challenge in GTT (Fig. 1E right panel). The body temperature of TgActβE mice was significantly higher than that of wild-type mice (Fig. 1G). These results indicated increased insulin sensitivity in TgActβE mice, which is possibly linked to the lower blood glucose and higher body temperature in TgActβE mice.
Fig. 1.
Body weight and glucose metabolism of TgActβE mice. (A) Detection of serum activin E in TgActβE mice. The serum was separated on SDS-PAGE under non-reducing (left panel) or reducing (right panel) conditions and probed with anti-activin E antibodies. The migration of activin E is indicated by bars on the right. The bars indicate the activin E mature regions. 2ME, 2-mercaptoethanol. (B) Growth curve. Weekly body weight of TgActβE and wild-type mice was measured from week 4 to week 52. n=11–14 in each group. (C) Food intake. n=6 in each group (18–26 weeks old). (D) Plasma glucose level in mice fasted for 4 h. n=11–12 in each group (18–26 weeks old). (E) Glucose tolerance tests. Plasma glucose (left panel) and insulin (right panel) concentrations were measured at the indicated time points. n=8–11 in each group (9–14 weeks old). (F) Insulin tolerance tests. Plasma glucose concentration was measured at the indicated time points. n=8–10 in each group (8–12 weeks old). (G) Rectal temperature. n=11–14 in each group (11–15 weeks old). Values are mean ± SEM. *P<0.05, **P<0.01.
Activation of brown adipocytes and emergence of beige adipocytes in TgActβE mice
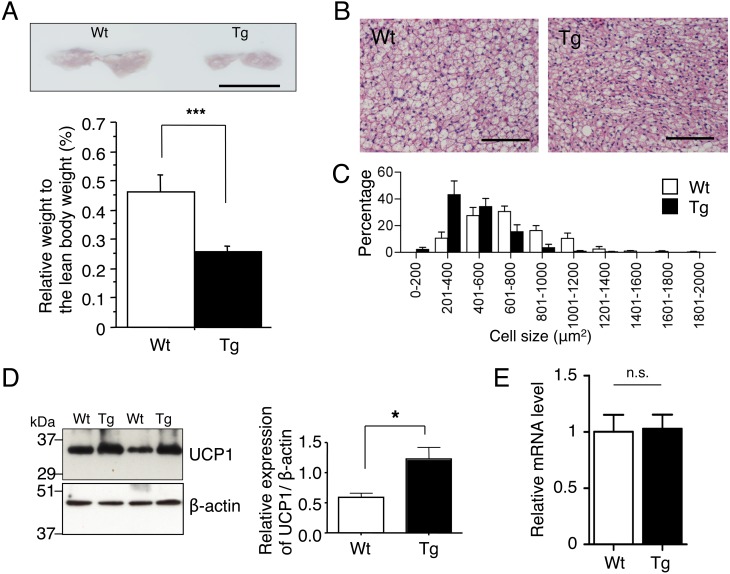
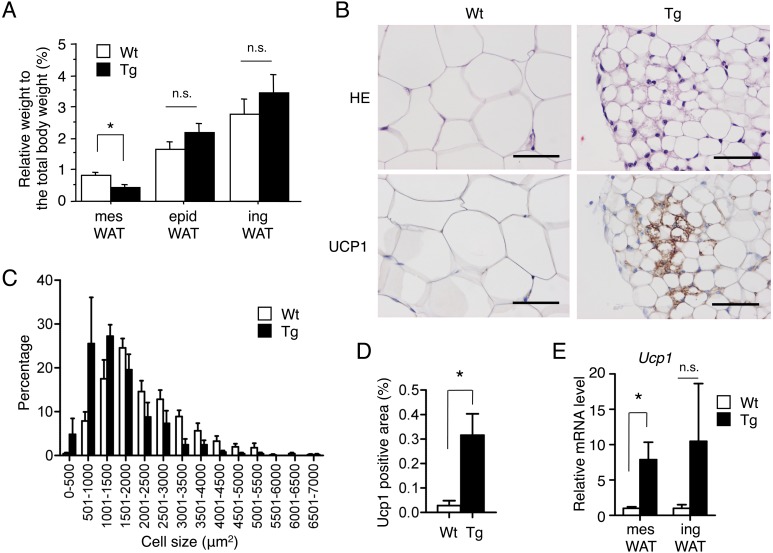
The above results raise the possibility that the increase in insulin sensitivity is due to enhanced heat production by brown adipocytes, beige adipocytes or both. Therefore, we next investigated adipose tissues of TgActβE mice. iBAT of TgActβE mice weighed significantly less than that of wild-type mice (Fig. 2A). Furthermore, histopathological examination revealed it had smaller brown adipocytes compared with the wild-type (Fig. 2B). The histogram showing cell size distribution of transgenic iBAT was shifted to smaller size (Fig. 2C). Moreover, western blot analysis demonstrated that transgenic iBAT had increased expression of UCP1 (Fig. 2D). However, the mRNA expression level of UCP1 in iBAT was comparable between wild-type and TgActβE mice (Fig. 2E). In contrast to iBAT, the weight of inguinal (ing) and epididymal (epid) WAT did not significantly differ between transgenic and wild-type mice (Fig. 3A). However, mesenteric (mes) WAT of TgActβE mice weighed significantly less than that of the wild-type mice (Fig. 3A). Histologically, the size of adipocytes in transgenic mesWAT was small compared with the wild-type (Fig. 3B). The cell size distribution histogram of mesWAT in TgActβE mice was shifted to the left (Fig. 3C). The presence of small adipocytes with multilocular lipid droplets, which were mostly UCP1-positive in mesWAT of TgActβE mice, was detected by immunohistochemistry (Fig. 3B). The Ucp1-positive area was higher in the mesWAT of TgActβE mice than that of wild-type mice (Fig. 3D), suggesting the emergence of beige adipocytes in mesWAT. Additionally, RT-qPCR analysis revealed that the Ucp1 mRNA expression was significantly upregulated in mesWAT of TgActβE mice, but not in ingWAT (Fig. 3E).
Fig. 2.
Characteristics of iBAT in TgActβE mice. (A) Gross appearance of iBAT of the mice (upper panel). Representative data are shown. Bar, 1 cm. Relative tissue weight expressed as a percentage of total body weight (lower panel). n=11–12 in each group (15–17 weeks old). (B) Histological analysis of iBAT (18 weeks old). Adipose tissue sections were stained with HE. Representative data are shown. Bar, 100 µm. (C) Distribution of the cell size in iBAT. The tissue sections were stained with HE. The adipocyte size in arbitrary fields of view was analyzed by ImageJ (NIH). Results are shown as means ± SEM. n=7 in each group (15–17 weeks old). (D) Western blot analysis of UCP1 expression in iBAT. Representative data are shown (left panel). Densitometric values of UCP1 expression are shown (right panel). n=4 in each group (11–15 weeks old). (E) UCP1 mRNA expression in iBAT. The mRNA was subjected to RT-qPCR. UCP1 expression was normalized to Hprt1 expression. The expression level in the control mice was set to 1. n=4 in each group (24–27 weeks old). Values are mean ± SEM. *P<0.05, ***P<0.005.
Fig. 3.
Characteristics of WAT in TgActβE mice. (A) Adipose tissue weight in TgActβE and wild-type mice. Relative tissue weight expressed as a percentage of total body weight. n=11–16 in each group (14–16 weeks old). (B) HE and immunohistochemical analysis of mesWAT of TgActβE and wild-type mice (18 weeks old) using anti-UCP1 antibodies. Representative data are shown. Bar, 50 µm. (C) Distribution of the cell size in mesWAT. The tissue sections were stained with HE. The adipocyte size in arbitrary fields of view was analyzed by ImageJ (NIH). Results are shown as means ± SEM. n=4 in each group (14–16 weeks old). (D) Ucp1-positive area in mesWAT. The tissue sections were immunostained with Ucp1. The Ucp1-positive area in arbitrary fields of view was analyzed by ImageJ (NIH). Results are shown as means ± SEM. *P<0.05. n=4 in each group (14–16 weeks old). (E) UCP1 expression in WAT. The mRNA of WAT from the mice was subjected to RT-qPCR. UCP1 expression was normalized to Hprt1 expression. The expression level in each WAT of control mice was set to 1. n=4 in each group (24–27 weeks old). Values are mean ± SEM. *P<0.05.
DISCUSSION
Due to their ability to consume energy by heat production, activation of brown adipocytes and induction of beige adipocytes could be a promising approach for fighting obesity. Activation of brown/beige adipocytes is controlled by the activity of the sympathetic nervous system, which is modified by changes in the ambient temperature. Moreover, secreted factors from various organs act as regulators of brown/beige adipocyte activity [5]. Exploration of newly identified activators/inducers of brown/beige adipocytes is important for preventive and curative therapy for obesity. Recently, our data using Tg mice overexpressing activin E in the liver (Alb-ActE mice) indicated that activin E enhances insulin sensitivity through activating brown/beige adipose tissues [8]. In the present study, we showed that systemic overexpression of activin E under the β-actin promoter (TgActβE mice) also enhanced insulin sensitivity and high body temperature. Furthermore, enhanced UCP1 expression in iBAT and mesWAT was observed. These results support the previous results showing that activin E acts as a hepatokine that improves glucose metabolism through activation of brown/beige adipocytes, suggesting that activin E can pharmacologically contribute to obesity therapy.
An allograft of brown adipocytes in mice improved glucose tolerance and increased insulin sensitivity [20]. Therefore, the lower blood glucose level and enhanced insulin sensitivity observed in TgActβE mice are probably due to increased heat production via enhanced UCP1 expression in iBAT and mesWAT. TgActβE mice also showed reduced iBAT and mesWAT tissue weight compared with wild-type mice. This is probably because of enhanced fatty acid oxidation in the transgenic adipose tissues as a result of energy consumption by enhanced UCP1 expression. Although Alb-ActE mice had reduced body weight [8], the body weight did not change in TgActβE mice compared with wild-type mice. This difference is attributed to the lower energy expenditure in TgActβE mice compared with Alb-ActE mice because of the lower browning/beiging of adipose tissues in TgActβE mice compared with Alb-ActE mice [8].
Alb-ActE mice induced browning in ingWAT and in mesWAT [8], whereas the induction of UCP1 expression was limited to mesWAT in TgActβE mice. Although the reason for the different effect of activin E overexpression on fat depots between Alb-ActE mice and TgActβE mice is unclear, a possible explanation is that the TgActβE mice were maintained using a chow diet with high nutritional value compared with the chow diet given to the Alb-ActE mice (CE-2, CLEA Japan Inc., Tokyo, Japan; http://www.clea-japan.com/en/diets/diet_a/a_03.html). Namely, the diet may influence the phenotypic difference between the two transgenic lines. Future studies are needed to clarify the relationship between activin E, the nutritional status and adipocyte browning.
A previous report has demonstrated that reduced serum lipase levels in TgActβE mice attributed to hypoplastic pancreatic acinar cells [6]. It has been shown that a pancreatic lipase inhibitor possessed anti-obesity action through inhibition of absorption of dietary fat [24]. Because we did not observe WAT weight loss in the TgActβE mice, the effect of activin E on pancreatic lipase action may be negligible for energy homeostasis in these mice.
Central treatment with BMP-8B, a member of the TGF-β superfamily, increased sympathetic activation of iBAT and regulated the energy balance in partnership with hypothalamic AMP-activated protein kinase [22]. Previous results have suggested that activin E affects the hypothalamus in TgActβE mice [17]. Therefore, it has been proposed that activin E enhances sympathetic activation of BAT through hypothalamic activation, similarly to BMP-8B. However, in vitro experiments using a brown adipocyte cell line revealed that activin E directly enhanced UCP1 expression [8]. Thus, systemic administration of activin E or drug discovery targeting activin E signal transduction in brown/beige adipocytes could contribute to the therapy for obesity and its related metabolic disorders.
In conclusion, activin E has a distinct pharmacological activity that directly targets brown/beige adipocytes to promote energy expenditure. To explore the possibility of activin E as a preventive or therapeutic target for obesity and its related disorders, the molecular basis of the activin E regulation of UCP1 expression should be explored in the future.
Acknowledgments
The authors thank K. Sanuka and Y. Ichikawa for technical assistance and Dr. R. Nakao for discussion. O.H. was supported by a Grant-in-Aid for Scientific Research from The Japan Society for the Promotion of Science (JSPS KAKENHI Grant JP21580370). M.F. was supported by JSPS KAKENHI Grant JP26450442. A.K. was supported by JSPS KAKENHI JP25640109.
REFERENCES
- 1.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., Cinti S.2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298: E1244–E1253. doi: 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- 2.Cannon B., Nedergaard J.2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277–359. doi: 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 3.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R.2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. doi: 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess A. M., Chen Y. C., Sze C., Wang K., English J., Chan O., Holman A. R., Tal I., Palmer M. R., Kolodny G. M., Kahn C. R.2012. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. U.S.A. 109: 10001–10005. doi: 10.1073/pnas.1207911109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harms M., Seale P.2013. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19: 1252–1263. doi: 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto O., Ushiro Y., Sekiyama K., Yamaguchi O., Yoshioka K., Mutoh K., Hasegawa Y.2006. Impaired growth of pancreatic exocrine cells in transgenic mice expressing human activin betaE subunit. Biochem. Biophys. Res. Commun. 341: 416–424. doi: 10.1016/j.bbrc.2005.12.205 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto O., Sekiyama K., Matsuo T., Hasegawa Y.2009. Implication of activin E in glucose metabolism: transcriptional regulation of the inhibin/activin betaE subunit gene in the liver. Life Sci. 85: 534–540. doi: 10.1016/j.lfs.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto O., Funaba M., Sekiyama K., Doi S., Shindo D., Satoh R., Itoi H., Oiwa H., Morita M., Suzuki C., Sugiyama M., Yamakawa N., Takada H., Matsumura S., Inoue K., Oyadomari S., Sugino H., Kurisaki A.2018. Activin E controls energy homeostasis in both brown and white adipose tissues as a hepatokine. Cell Reports 25: 1193–1203. doi: 10.1016/j.celrep.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto O., Noda T., Morita A., Morita M., Ohtsuki H., Sugiyama M., Funaba M.2016. Castration induced browning in subcutaneous white adipose tissue in male mice. Biochem. Biophys. Res. Commun. 478: 1746–1750. doi: 10.1016/j.bbrc.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 10.Kajimura S., Saito M.2014. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu. Rev. Physiol. 76: 225–249. doi: 10.1146/annurev-physiol-021113-170252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida R., Yoshida H., Murakami M., Shirai M., Hashimoto O., Kawada T., Matsui T., Funaba M.2016. Direct action of capsaicin in brown adipogenesis and activation of brown adipocytes. Cell Biochem. Funct. 34: 34–41. doi: 10.1002/cbf.3162 [DOI] [PubMed] [Google Scholar]
- 12.Long J. Z., Svensson K. J., Tsai L., Zeng X., Roh H. C., Kong X., Rao R. R., Lou J., Lokurkar I., Baur W., Castellot J. J., Jr., Rosen E. D., Spiegelman B. M.2014. A smooth muscle-like origin for beige adipocytes. Cell Metab. 19: 810–820. doi: 10.1016/j.cmet.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno H., Shinoda K., Spiegelman B. M., Kajimura S.2012. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15: 395–404. doi: 10.1016/j.cmet.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., Carpentier A. C.2012. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122: 545–552. doi: 10.1172/JCI60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgarkia-Dara C., Vejda S., Erlach N., Losert A., Bursch W., Berger W., Schulte-Hermann R., Grusch M.2006. The activin axis in liver biology and disease. Mutat. Res. 613: 123–137. doi: 10.1016/j.mrrev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M.2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967. doi: 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiyama K., Hashimoto O., Ushiro Y., Adachi C., Kikusui T., Tanemura K., Hasegawa Y.2009. Abnormalities in aggression and anxiety in transgenic mice overexpressing activin E. Biochem. Biophys. Res. Commun. 385: 319–323. doi: 10.1016/j.bbrc.2009.05.054 [DOI] [PubMed] [Google Scholar]
- 18.Sharp L. Z., Shinoda K., Ohno H., Scheel D. W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V., Kajimura S.2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452. doi: 10.1371/journal.pone.0049452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinoda K., Luijten I. H., Hasegawa Y., Hong H., Sonne S. B., Kim M., Xue R., Chondronikola M., Cypess A. M., Tseng Y. H., Nedergaard J., Sidossis L. S., Kajimura S.2015. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 21: 389–394. doi: 10.1038/nm.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanford K. I., Middelbeek R. J. W., Townsend K. L., An D., Nygaard E. B., Hitchcox K. M., Markan K. R., Nakano K., Hirshman M. F., Tseng Y. H., Goodyear L. J.2013. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123: 215–223. doi: 10.1172/JCI62308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefan N., Häring H. U.2013. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 9: 144–152. doi: 10.1038/nrendo.2012.258 [DOI] [PubMed] [Google Scholar]
- 22.Whittle A. J., Carobbio S., Martins L., Slawik M., Hondares E., Vázquez M. J., Morgan D., Csikasz R. I., Gallego R., Rodriguez-Cuenca S., Dale M., Virtue S., Villarroya F., Cannon B., Rahmouni K., López M., Vidal-Puig A.2012. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149: 871–885. doi: 10.1016/j.cell.2012.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., Schrauwen P., Spiegelman B. M.2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376. doi: 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada Y., Kato T., Ogino H., Ashina S., Kato K.2008. Cetilistat (ATL-962), a novel pancreatic lipase inhibitor, ameliorates body weight gain and improves lipid profiles in rats. Horm. Metab. Res. 40: 539–543. doi: 10.1055/s-2008-1076699 [DOI] [PubMed] [Google Scholar]