Abstract
Background
Platelets play an important role in the pathogenesis of acute coronary syndrome (ACS). Patients with ACS have an increased mean platelet volume (MPV) and immature platelet fraction (IPF) resulting in elevation of thrombotic ability. In this study, we evaluated the diagnostic performance of MPV and IPF in identifying suspected ACS patients at emergency department. Moreover, we investigated the correlation between MPV or IPF with initial troponin I (TnI), one of the current ACS biomarkers.
Methods
This was a single-center study recruiting suspected ACS patients who had acute chest pain at the emergency department. Whole blood samples were obtained from all participants and MPV and IPF were measured by Sysmex XE-5000 hematology analyzer within 20 min of blood sampling. The diagnostic values of MPV and IPF in identifying ACS were analyzed retrospectively.
Result
In this study, 63 in 104 suspected ACS patients were diagnosed as ACS (65.3%). MPV and IPF were higher in ACS patients compared to non-ACS patients (MPV: 10.7 ± 0.80 fL vs 10.0 ± 0.64 fL, p < 0.001; IPF: 3.7 ± 2.64% vs 3.1 ± 2.69%, p = 0.030). MPV and IPF were similar in unstable angina and acute myocardial infarction patients. We showed that elevation of MPV could be an independent predictive factor of ACS (odds ratio: 5.038). At the optimal cut-off value of 10.55 fL (AUC 95% CI: 0.637–0.836), the diagnostic performance of MPV in predicting ACS had an area under a receiver operating characteristic curve (AUC) of 0.736 with sensitivity and specificity of 54.2% and 82.8%, respectively. Patients with both of initial TnI and MPV higher than the established cut-off value had increased incidence (3.792 fold) for ACS development compared to patients with TnI below the cut-off value. Furthermore, diagnosing ACS with both MPV and initial TnI increased the positive predictive value from 84.2% to 86.7%. No correlation was observed between MPV or IPF and the mortality rate of ACS patients (MPV: 3.8% vs 11.1%, p = 0.300; IPF: 12.0% vs 37.5%, p = 0.054).
Conclusion
Here we show that ACS patients have higher MPV and IPF compared to non-ACS patients. We further demonstrate that MPV can be utilized as an independent predictor for early diagnosis of low-risk ACS patients who have acute chest pain.
Keywords: Mean platelet volume, Immature platelet fraction, Acute coronary syndrome, Troponin I
At a glance commentary.
Scientific background on the subject
About 15% of ACS patients are often under-diagnosed. Several Studies have indicated the cause of ACS is associated with atherothrombosis that requires the activation of platelets. In this study, we aimed to investigate the utility of platelet indices, MPV and IPF, as potential biomarkers for early ACS diagnosis.
What this study adds to the field
Results show that MPV can be used as an independent predictive biomarker for the diagnosis of ACS in acute chest pain patients who have low risk at ACS development. We further show that measuring both MPV and initial troponin I enhances the early diagnostic performance for ACS.
Acute coronary syndrome (ACS) includes unstable angina (UA) and acute myocardial infarction (AMI) [1]. Approximately 10–15% of the ACS patients are not accurately diagnosed as they present a normal electrocardiogram (ECG) characteristics and troponin I level during the preliminary checkup at emergency departments [2]. In addition, the level of troponin I is affected by other diseases such as s tachycardia, left side ventricular hypertrophy, coronary artery spasm, direct myocardial injury, heart failure, acute stroke and Kawasaki disease [3], [4], [5], [6], [7], [8], [9]. Due to the lack of accurate inspection and diagnosis, the incidence of ACS is often over estimated. This may lead to inappropriate treatment for non-ACS patients and a waste of medical resources [10].
Current clinical guidelines require that clinicians should provide an accurate diagnosis for ASC patients in 10 min. Clinicians should further classify patients based on their ECG and blood levels of cardiac biomarkers and treat patients within 60 min after diagnosis. Cardiac Troponin I (cTnI) and cardiac troponin T (cTnT) are the current biomarkers for diagnosis of ACS as recommended by the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation [11]. Since the levels of cardiac biomarkers rise only at 3–4 h after the onset of myocardial infarction, these biomarkers have a poor diagnostic value at 2–4 h before the disease onset when the patients already have mild symptoms [12], [13], [14]. Of note, approximately 40–60% of ACS cannot be diagnosed based on the level of initial troponin I (initial TnI) [15]. Thus, in order to provide better diagnosis and treatment for patients with acute chest pain, many studies are exploring a potential new biomarker for ACS diagnosis. For example, ACS is diagnosed by testing the function of endothelial cells [16], measuring the levels of homocysteine [17] or high-sensitive C-reactive protein (hsCRP) [18]. However, these tests are not widely used for clinical decision-making because they are either (i) too expensive for routine clinical analysis, (ii) technically complicated that requires specially trained technicians or (iii) having poor accuracy of the laboratory results due to test variations.
ACS, both UA and AMI, is caused by the sudden disruption of atherosclerotic plaque in coronary artery that leads to partial athrothrombosis. UA is characterized by the partial blockage of coronary artery resulting in reduced blood flow; while AMI is characterized by the total blockage of coronary artery resulting in necrosis of cardiomyocytes [2]. Platelets are playing a crucial role in the formation of atherosclerotic plaque and thrombus. Activation of platelets results in secretion of molecules such as thromboxane A2 and 5-hydroxytryptamine (5HT) that induce vasoconstriction; platelet derived growth factor (PDGF), β-thromboglobulin (β-TG) and platelet factor 4 (PF4) that stimulates arteriosclerosis; leukotrienes that amplify inflammatory responses; and glycoprotein IIb/IIIa receptor that activates haemotatic system for platelet aggregation and thrombus formation [19], [20], [21]. Therefore, anti-platelet drugs are often used to treat patients with cardiovascular disease [22].
Studies have shown that by measuring the platelet activity could identify patients who were at risk of developing cardiovascular diseases. However, measuring the platelet activity for predicting ACS has not been routinely used for decision-making diagnosis and has only been utilized for research proposes. Ex vivo measurement of platelet activity, which only utilizes a few agonists to activate platelet aggregation, often do not reflect the true activity in vivo [23]. Furthermore, anticoagulant in blood collection tube is known to affect the normal function of platelet by chelating calcium in plasma. Until now, the utility of platelet activity in prediction of ACS is still doubtful as there is insufficient data to support the advantage of testing platelet activity and the best cut-off value to identify patients who are at risk of cardiovascular disease is not determined yet. Moreover, many detection methods are costly and time consuming and may require the use of special equipments [24].
During routine blood examination, MPV (mean platelet volume) not only reflects the size of platelet but also positively correlates to the platelet activity. Platelets with increased volume have elevated platelet aggregation ability, formation of thromboxane, release of β-thromboglobulin and expression of adhesion surface molecules [25]. Interestingly, patients with diabetes, hypertension, hypercholesterolemia, smoking habit or obesity, who are also at risk of developing cardiovascular diseases, have higher MPV compared to healthy individuals [26], [27], [28], [29], [30]. This indicates that MPV is an indicator for patients who are at risk of developing cardiovascular diseases and may also be a potential biomarker for ACS diagnosis.
Patients with atherosclerosis have increased utility of platelets [31] that is also presence in patients with other cardiovascular diseases. Peripheral consumption increase counts of immature platelets, which are larger and more reactive than mature platelets. This leads to increase platelet aggregation and reduce treatment response to aspirin and clopidogrel [32]. Because of immature platelets with a large amount of RNA, the ratio of immature to mature platelets (immature platelet fraction, IPF) can be identified by flow cytometry techniques with a nucleic acid-specific dye in reticulocyte/optical platelet channel of the hematology analyzer. Of notice, it has been shown that ACS patients have higher IPF compared to healthy subjects [33].
Although MPV or IPF have thoroughly discussed in ACS, there are still contradictory opinions of MPV or IPF in ACS, which is suggested the diagnostic utilities of MPV and IPF in ACS need to be further addressed. In this study, we collaborated with clinicians at emergency department to recruit patients with acute chest pain. We analyzed the MPV and IPF of the suspected ACS patients and determined the correlations between the incidence of ACS and MPV or IPF. We further inspect the potential use of MPV and/or IPF as ACS prediction biomarkers. In addition, we tested whether MPV and IPF could replace or combined with the current diagnostic marker for ACS (initial TnI), aiming to improve the ACS diagnostic performance.
Material and methods
Patient characteristics
This is a single-center study at the Kaohsiung Chang Gung Memorial Hospital, Taiwan. We recruited 110 Taiwanese adult patients (age >20 years old) who presented with acute chest pain to emergency department. Exclusion criteria include patients with severe hepatic or renal diseases, myeloproliferative disorders, malignancy, idiopathic thrombocytopenia purpura, trauma-related chest pain, taking oral anticoagulant medicine or previously diagnosed as ACS in 8 weeks and were treated accordingly. All participants with ACS were enrolled based upon clinical presentation, physical examination, typical ischemic STT change, and elevated cardiac troponin I that met the criteriae of ACS from guideline recommendation.
Experimental procedures
Venous blood samples were performed EDTA-containing blood collection tubes for CBC assays and heparin-containing blood collection tubes for biochemical assays. Blood samples were analyzed with sysmex XE-5000 automated hematology analyzer (Sysmex, Kobe, Japan) within 20 min from blood collection. Cell blood count (CBC), MPV and IPF were measured. MPV was measured by resistant test [34]. IPF was measured by spectrophotometer detecting the amount of RNA-containing platelets in the total platelet [35]. Measurements were performed according to the manufacturer's protocols. Troponin I was measured by chemiluminescent immunoassay sandwich method using Beckman Unicel DxC 800 (Beckman Coulter Inc., CA, USA).
Disease records
Patients' vital signs, disease histories, laboratory examination results, cardiovascular images and final diagnosis were recorded. All patients filled in a retrospective survey to identify risk factors in development of cardiovascular diseases such as having family history, smoking habit, hypertension or diabetes.
Diagnosis
According to the guidelines agreed by the World Health Organization, the European Society of Cardiology and the American college of Cardiology in August, 2012, ACS is characterized by an increased TnI above the 99th percentile of the upper reference value with laboratory evidence of myocardial ischemia. Furthermore, AMI patients have to present at least one of the following [11]: (i) symptoms of ischemia; (ii) significant EGF findings (new ST-T segment or new left bundle branch block, LBBB); (iii) pathological Q waves in ECG; (iv) cardio imaging showing new loss of viable myocardium or new regional wall motion abnormality; or (v) presence of coronary thrombus by angiography or anatomy. UA patients have to fulfill one of the following: (i) abnormal ST segment (>0.5 mm); (ii) presence of atherosclerosis with narrow degree > 70%; (iii) abnormal regional wall motion. When patients do not achieve these criteria, they are classified as non-ACS.
Statistical analyses
Results were statistically analyzed by using SPSS, version 18.0 (SPSS, Chicago, Illinois, USA). Continuous variables were presented as mean (±standard derivations, SD) and were analyzed using t-test, one-way ANOVA, Mann–Whitney U test or Tukey post hoc test. Categorical variables were presented as ratio and were analyzed with Pearson's Chi–Square test. Multiple logistic regression analysis was applied to determine whether the biomarker(s) could be an independent ACS diagnostic factor. The predictive accuracy of each biomarker was determined by receiver operating characteristic (ROC) curve and the corresponding area under curve (AUC). The optimal cut-off was chosen and the corresponding sensitivity, specificity and likelihood ratios were calculated. p < 0.05 was considered as statistically significant.
Results
A total of 110 patients with acute chest pain at emergency department were enrolled in this study. Six patients who were having hepatic disease (n = 4), renal impairment (n = 1) or hemolymph malignancy (n = 1) were excluded. Thus, a total of 104 patients containing 60 males and 44 females were included in this study (Fig. 1). Laboratory analysis results showed that sixty-three patients were diagnosed as ACS (56.3%) and forty-one patients were diagnosed as non-ACS (Table 1). ACS patients had the increased levels of initial TnI (7.08 ± 15.24 ng/mL vs. 0.88 ± 1.35 ng/mL, p < 0.001), creatine kinase-MB (CK-MB, 37 ± 66.7 ng/mL vs. 15 ± 47.9 ng/mL, p = 0.002), MPV (10.7 ± 0.80 fL vs. 10.0 ± 0.64 fL, p < 0.001) and IPF (3.7 ± 2.64% vs 3.1 ± 2.69%, p = 0.030) compared to non-ACS patients (Table 1).
Fig. 1.
Flowchart of the patient enrollment of the acute chest pain study cohort.
Table 1.
Clinical measurements in ACS and non-ACS patients.
| ACS |
non-ACS |
p-value | |
|---|---|---|---|
| n = 63 | n = 41 | ||
| WBC (1000/μL) | 10.2 ± 4.39 | 11.1 ± 5.94 | 0.629 |
| PLT (1000/μL) | 210 ± 86.5 | 212 ± 96.2 | 0.616 |
| MPV (fL) | 10.7 ± 0.80 | 10.0 ± 0.64 | <0.001*** |
| IPF (%) | 3.7 ± 2.64 | 3.1 ± 2.69 | 0.030* |
| Initial TnI (ng/mL) | 7.08 ± 15.24 | 0.88 ± 1.35 | <0.001*** |
| CK-MB (ng/mL) | 37 ± 66.7 | 15 ± 47.9 | 0.002** |
| hsCRP (mg/L) | 18.22 ± 18.16 | 69.20 ± 96.87 | 0.055 |
| Cholesterol (mg/dL) | 180 ± 44.0 | 165 ± 47.1 | 0.262 |
| LDL (mg/dL) | 102 ± 36.4 | 87 ± 35.2 | 0.067 |
| VLDL (mg/dL) | 24 ± 14.4 | 24 ± 17.3 | 0.687 |
Abbreviations: WBC: white blood cell; PLT: Platelet; hsCRP: high-sensitive C-reactive protein; LDL: Low-density lipoprotein; VLDL: very-low-density lipoprotein.
*, p < 0.05; **, p < 0.01; ***, p < 0.001.
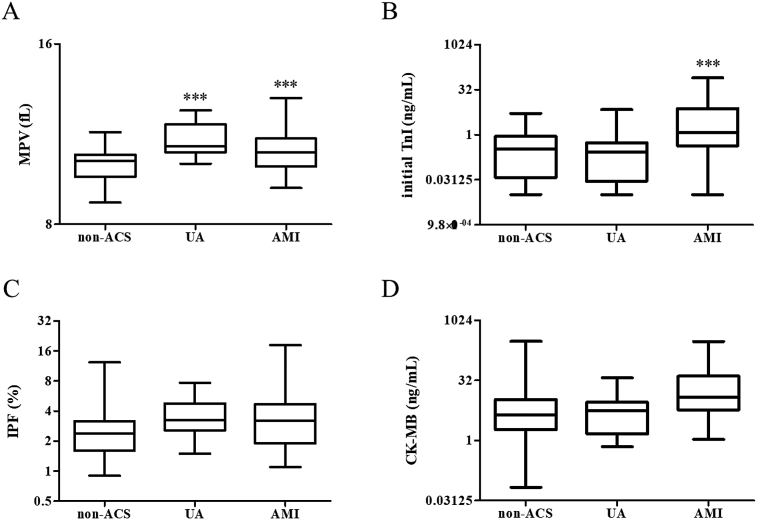
To investigate the differences between AMI and UA patients, we further compared the levels of initial TnI, CK-MB and MPV in AMI, UA and non-ACS patients (Fig. 2). Compared to non-ACS patients, AMI and UA patients had higher MPV (AMI: 10.7 ± 0.79 fL vs 10.0 ± 0.64 fL, p < 0.001UA: 11.1 ± 0.75 fL vs 10.0 ± 0.64 fL, p < 0.001), whereas the level of MPV did not differ between AMI and UA patients (Fig. 2A). Initial TnI was elevated in AMI patients compared to non-ACS patients (8.24 ± 16.36 ng/mL vs 0.88 ± 1.36 ng/mL, p = 0.0081, Fig. 2B). However, other biomarkers, IPF and CK-MB, were not significantly different among the three patient groups. To investigate the predictive value of initial TnI, CK-MB, MPV and IPF for ACS diagnosis, we performed binary logistic regression analyses with each variable. Analysis results suggested that both initial TnI and MPV be utilized as an independent predictor for diagnosing ACS (Table 2).
Fig. 2.
Levels of four biomarkers in UA, AMI and non-ACS patients. (A) MPV; (B) TnI; (C) IPF; (D) CK-MB. ***, p < 0.001.
Table 2.
Correlations between each biomarker and the risk of ACS development.
| Biomarker | OR (95% CI) | p-value |
|---|---|---|
| MPV | 5.083 (1.902–13.586) | <0.001*** |
| IPF | 0.962 (0.644–1.436) | 0.850 |
| Initial TnI | 1.376 (1.041–1.821) | 0.025* |
| CK-MB | 0.992 (0.979–1.005) | 0.241 |
Aberrations: OR: odds ratio.
*, p < 0.05; ***, p < 0.001.
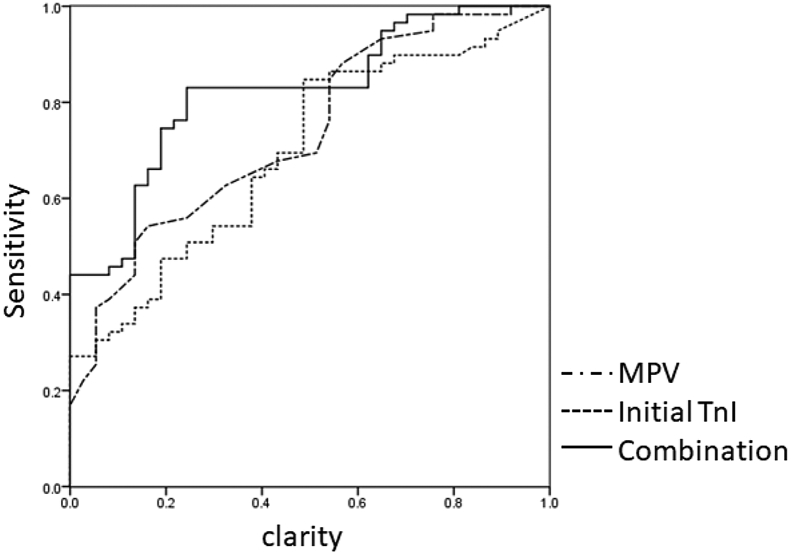
The diagnostic performance of MPV or initial TnI was determined by ROC curve (Fig. 3). Table 3 displayed that the AUC of MPV and initial TnI was 0.736 (95% CI: 0.637–0.836) and 0.696 (95%CI: 0.595–0.798), respectively. In predicting ACS, the optimal cut-off value of MPV and initial TnI was 10.55 fL (sensitivity: 54.2%; specificity: 83.8%) and 0.35 ng/mL (sensitivity: 84.1%; specificity: 51.2%), respectively. Interestingly, the AUC was increased to 0.822 (95%CI: 0.739–0.905) with 83.1% of sensitivity and 75.7% of specificity in the combination of MPV and initial TnI in ACS diagnosis (positive likelihood ratio, LR+: 3.414; negative likelihood ratio, LR-: 0.224, Table 3).
Fig. 3.
ROC analyses of MPV, initial TnI and the combination of MPV and initial TnI.
Table 3.
ROC analyses of MPV, initial TnI and the combination of MPV and initial TnI.
| AUC (95% CI) | Optimal cut-off | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|
| Initial TnI | 0.696 (0.595–0.798) | 0.35 ng/mL | 84.1 (0.813–0.869) | 51.2 (0.485–0.539) | 1.725 (1.590–1.860) | 0.31 (0.291–0.329) |
| MPV | 0.736 (0.637–0.836) | 10.55 fL | 54.2 (0.479–0.605) | 83.8 (0.775–0.901) | 3.345 (2.937–3.753) | 0.546 (0.490–0.602) |
| Combined | 0.822 (0.739–0.905) | – | 83.1 (0.802–0.860) | 75.7 (0.726–0.788) | 3.414 (3.159–3.669) | 0.224 (0.198–0.250) |
Patients diagnosed as ACS and non-ACS were grouped into four different classes based on the optimal cut-off values of MPV and initial TnI. High percentage of patients with both MPV and TnI over the optimal cut-off values were diagnosed as ACS (Table 4, p < 0.001). The positive predictive value (PPV) and negative predictive value (NPV) of MPV in diagnosing ACS was 84.2% and 53.4%, respectively. The PPV of initial TnI alone was 73.5% which was then increased to 86.7% when combined with MPV (Table 4). The incidence of developing ACS in patients with both of initial TnI and MPV over the cut-off values is 3.792 fold higher than that in patients who had TnI over the cut-off value only (Table 5). Furthermore, patients were grouped according to the levels of MPV, in which their risk factors for development of ACS including age, gender, BMI, smoking, hypertension, diabetes, hyperlipidemia, and family ACS history were being compared. These data indicated that all the risk factors were not correlated to the risk of developing ACS (Table 6). The mortality rate and each biomarker were compared to understand the correlations of disease prognosis among MPV, IPF, and initial TnI. Patients with ACS or non-ACS in the high IPF levels might tend to have high mortality rate (ACS: 3.8% vs 11.1%, p = 0.300; non-ACS: 12.0% vs 37.5%, p = 0.054), whereas the levels of MPV and initial TnI were not significantly correlated to mortality rate (Table 7).
Table 4.
Comparison of MPV and initial TnI in ACS and non-ACS patients.
| ACS |
non-ACS |
|
|---|---|---|
| n = 59 | n = 37 | |
| MPV (−) Initial TnI (−) | 3 (5.1%) | 17 (45.9%) |
| MPV (−) Initial TnI (+) | 24 (40.7%) | 14 (37.8%) |
| MPV (+) Initial TnI (−) | 6 (10.2%) | 2 (5.4%) |
| MPV (+) Initial TnI (+) | 26 (44.1%) | 4 (10.8%) |
Patients were grouped according to their levels of MPV and initial TnI. The cut-off values of MPV and TnI were 10.55 fL and 0.35 ng/mL, respectively.
Positive predict value of high MPV = 84.2%.
Negative predict value of low MPV = 53.4%.
Positive predict value of high initial TnI = 73.5%.
Negative predict value of low initial TnI = 67.9%.
Positive predict value of high MPV and initial TnI = 86.7%.
Table 5.
Associations of MPV and initial TnI with the risk of developing ACS.
| Laboratory results | OR (95% CI) | p-value |
|---|---|---|
| MPV (−) Initial TnI (+) | 1 [reference value] | |
| MPV (+) Initial TnI (+) | 3.792 (1.095–13.129) | 0.035* |
*, p < 0.05.
Table 6.
Patient characteristic.
| MPV < 10.55 fL |
MPV ≧ 10.55 fL |
p-value | |
|---|---|---|---|
| n = 46 | n = 58 | ||
| Age | 61 ± 9.1 | 62 ± 18.3 | 0.543 |
| Gender (male/female) | 30/16 | 30/28 | 0.123 |
| BMI | 24.8 ± 4.7 | 24.4 ± 4.2 | 0.829 |
| Cardiovascular risk factors, n (%) | |||
| Smoking | 19 (41.3) | 17 (29.3) | 0.205 |
| Hypertension | 30 (62.5) | 37 (63.8) | 0.884 |
| Diabetes | 22 (47.8) | 23 (39.7) | 0.408 |
| Hypercholesterolemia | 12 (26.1) | 16 (27.6) | 0.868 |
| Family history | 8 (14.7) | 10 (17.2) | 0.988 |
Patients with the higher or lower cut-off values of MPV levels were grouped according to age, gender, BMI, and cardiovascular risk.
Table 7.
Associations between each biomarker with the mortality rate of ACS.
| MPV |
p-value | IPF |
p-value | Initial TnI |
p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10.4 fL | ≧10.4 fL | <2.80% | ≧2.80% | <0.571 ng/mL | ≧0.571 ng/mL | |||||
| ACS | Patients (n) | 22 | 37 | 0.272 | 26 | 36 | 0.300 | 26 | 37 | 0.952 |
| Deaths (n) | 3 | 2 | 1 | 4 | 2 | 3 | ||||
| Mortality rate | 13.6% | 5.4% | 3.8% | 11.1% | 7.7% | 8.1% | ||||
| non-ACS | Patients (n) | 25 | 12 | 0.513 | 25 | 16 | 0.054 | 26 | 15 | 0.819 |
| Deaths (n) | 4 | 3 | 3 | 6 | 6 | 3 | ||||
| Mortality rate | 16.0% | 25.0% | 12.0% | 37.5% | 23.1% | 20.0% | ||||
Data are given as median.
Discussion
To achieve early diagnosis of ACS and provide appropriate therapy, many research teams are engaged in identifying new ACS diagnostic markers. Although biomarkers such as CK-MB, myoglobin or brain natriuretic peptide (BNP) are currently utilized, the sensitivities and specificities of these biomarkers for the detection of early ACS are moderate [36]. It has been shown recently that activation and aggregation of platelets plays an essential role in formation of coronary thrombus and induction of ACS. ACS is a presentation of atherosclerosis that is caused by accumulation of lipid and cholesterol in coronary arteries resulting in formation atherosclerotic plaques. The rupture of atherosclerotic plaques is associated with a series of thrombosis reactions that leads to AMI or UA. Thus, platelets and their activation factors, which participate in thrombosis, are potential biomarkers for diagnosis of ACS [37].
Here we showed that ACS patients had higher MPV compared to non-ACS patients, suggesting that MPV be utilized as an independent biomarker for ACS diagnosis (Table 2). The AUC of the MPV in ACS diagnosis was 0.736, and the optimal cut-off was set as 10.55 fL with the 54.2% of sensitivity, the 83.3% of specificity, the 3.345 of LR+, and the 0.546 of LR—. When MPV was combined to initial TnI for ACS diagnosis, the ACU was increased to 0.822 in 83.1% of sensitivity, 75.7% of specificity, 3.414 of LR+, and 0.224 of LR— (Table 3). In standard diagnosis of TnI, TnI should be examined and monitored in multiple time course to diagnose as ACS. In fact, initial TnI showed 84.1% sensitivity but 51.2% specificity. If the combination of initial TnI and MPV were performed, the specificity of combination could increase to 75.7% and more than specificity of TnI alone. The positive predictive value (PPV) of initial TnI alone was 73.5% which was then increased to 86.7% when combined with MPV in ACS (Table 4). The probability of patients with ACS was significantly increased, while abnormal levels of both MPV and initial TnI were detected in patients (Table 5). These results were consistent with previous studies [38], [39], [40] and indicated that MPV is a suitable biomarker for the detection of suspected ACS patients of different races in different countries. We further demonstrated that the detection of ACS by MPV set at the cut-off of 10.55 fL is not affected by other known ACS risk factors (Table 6). Thus, we suggest that MPV be served as a valuable biomarker for the diagnosis of ACS. That is to say, when patients with acute chest pain in emergency department, measuring both initial TnI and MPV may help clinical physicians rule out non-ACS, improve accuracy for diagnosing ACS, not wait for multiple TnI detection, shorten the time of diagnosis and Door-to-Balloon Time to achieve early diagnosis of ACS.
Initial TnI is a sensitive biomarker for detecting myocardial injuries but it is not solely associated with atherosclerosis. For instance, we observe that 49% of non-ACS patients had an initial TnI over the clinical cut-off value. In contrast, 15% of ACS patients had an initial TnI below the cut-off value. Thus, initial TnI is good enough for ruling out non-ACS patients but not for ruling in ACS patients. On the other hand, MPV could be calculated and provided from standard CBC instruction. It is the cheap and fast diagnosis to assist diagnosis in ACS and easily apply to combination of initial TnI. We demonstrated that measuring both initial TnI and MPV increased the specificity in ACS diagnosis (Table 4, Table 5). This means that patients with normal levels of TnI and MPV have very low incidence of developing ACS. Thus, the combination of initial TnI and MPV may provide the decrease of bias jugged by first line physicians and shorten time of therapeutic intervention. Though our study, we verified the diagnostic utilities of combination with initial TnI and MPV in ACS as well as provided new application for fast ACS diagnosis for Asian population.
We showed the ACS patients with the high levels of MPV. Increased MPV is positively associated with the probability of thrombosis. Platelets with increased volume have elevated enzymatic activities and increased membrane receptors GPIIb/IIIa expression which will accelerate the adhesion of platelet to intracellular collagen and will ultimately lead to arterial thrombosis [41]. The use of MPV for differentiation of UA and AMI patients is still controversial as some studies showed that MPV could be a differentiation biomarker [39], [40], but some systemic studies showed that MPV could not differentiate between UA and AMI [42], [43]. We detected an increased MPV in UA and AMI patients compared to that in non-ACS patients, but the levels of MPV between UA and AMI patients were not significantly different (Fig. 2). Therefore, MPV could not differentiate UA and AMI patients based on our current study.
Besides from elevation of MPV, IPF is also increased in ACS patients. When patients have an increased platelet aggregation and formation of thrombus, mature platelets are being consumed. To compensate for the loss of platelets, a positive feedback mechanism is turned on to produce more platelets which are immature with increased volume. Thus, the platelet count was similar in ACS and non-ACS patients, but the levels of MPV and IPF were elevated in ACS patients (Table 1). It has been shown that IPF is positively correlated to MPV [44]. Yet, we showed that IPF could not be an independent predictor for ACS diagnosis. Platelet activity is known to associate with platelet volume and the presence of immature platelets. Of notice, not all platelets with increased volume are immature platelets [45]. High IPF is generally caused by the fast-response of megakaryocytes, which produce activated immature platelets to compensate for rapid platelet loss. In ACS patients, there is a steady platelet consumption which is due to endothelial cells injury and formation of atherosclerotic plaque during the disease progression. To compensate for the long-term loss of platelets, megakaryocytes are stimulated by thrombopoietin and transformed into polyploidy megakaryocytes which produce mature platelet with increased volume by slow-response [37]. Thus, IPF alone may not be a good biomarker for identifying ACS patients.
The results of present study showed significant elevation of MPV and IPF in the patients with ACS compared to those without ACS. The diagnosis performance of MPV in predicting ACS had an AUC of 0.736 and specificity of 82.8%. As a result, MPV could be considered as an add-on biomarker to exclude ACS if patients presenting with chest pain at ER had a slightly elevated troponin-I level. It is a common medical practice that serially negative cardiac troponin-I could help to exclude ACS at ER. For those patients with slightly elevated troponin-I, cardiac cath is a gold standard diagnostic tool to evaluate coronary anatomy and survey culprit lesions. However, not every patient with suspicious ACS had a desire to undergo cardiac cath, e.g., those with elderly, high-risk bleeding, advanced renal insufficiency and multiple comorbidities. In this circumstance, MPV and IPF helped to further increase in the ACS diagnosis. Furthermore, those patients with negative MPV expression had a low probability of ACS. In the future, to combine ECG with MPV, IPF and troponin-I to evaluate ACS accuracy deserves further investigation.
Studies on the predictive value of MPV or IPF for the prognosis of ACS patients are still contradictory [46], [47]. We observed an increased mortality rate in ACS patients with high IPF (11.1%), compared to patients with high MPV (5.4%) or patients with increased initial TnI (8.1%). We also observed a trend of high mortality rate in all patients with increased IPF. Correlation between mortality rate and the level of IPF was also observed in other studies. For instance, patients with major adverse cardiac events (MACEs) patients had higher IPF that was positively correlated to the mortality rate. Increased IPF is caused by an elevated platelet turnover rate that may lead to poor treatment response and disease prognosis [32], [45]. Positive correlation between mortality and IPF was also observed in non-cardiovascular diseases such as sepsis and disseminated intravascular coagulation [48], [49]. The absence of significant correlation between mortality rate and IPF may due to the low number of participants and the short follow-up period in this cohort study.
Despite the fact that both MPV and IPF are platelet-associated measurements, these values may represent different clinical meanings as observed in this study. MPV represents the volume of platelet and remains constant within 4 month after the measurement. Increased MPV indicates increased aggregation activity that may induce thrombosis. IPF is the ratio of immature platelet to total platelet in blood and represents the platelet-producing activity of megakaryocytes in bone marrow. As MPV remains constant for at 4 months, MPV reflects the previous status of patients and may be clinically useful in predicting the onset of ACS. Since peripheral immature platelets are degraded within 24 h, a sudden increased of IPF indicates an increased platelet consumption, increased platelet turnover and activation of megakaryocyte in stable atherosclerosis. Thus, IPF may predict ACS prognosis. However, MPV and IPF have not yet clinically used as MPV increases when the storage time of the blood sample is prolonged. Therefore, it is crucial to measure MPV within 30 min of blood sampling [50]. This limitation can be easily resolved as these measurements are performed in an automatic analyzer. In this study, in order to reduce technical variations, blood samples were collected into K2 EDTA blood tube and were measured within 20 min of sampling. Blood samples were transported to the central laboratory through pneumatic tube system and analyzed by the sysmex XE-5000 automated hematology analyzer. The development of automatic analyzer allows a speedy measurement of MPV and IPF at a reasonable cost. However, MPV may vary according to the detection methods [34] and IPF may differ in between races [35], future research to determine the optimal MPV and IPF cut-off values for Taiwanese patients is warranted.
Conclusions
Hereby, we analyzed the predictive performances of MPV and IPF on ACS diagnosis and prognosis, respectively. MPV can be utilized as an independent predictor for ACS diagnosis and it can further enhance the predictive value of TnI in ACS diagnosis by increasing the sensitivity, specificity, PPV and NPV. When a patient, who has low risk of developing ACS, presenting with an acute chest pain at emergency department, measuring both MPV and initial TnI will be useful in ACS diagnosis. IPF is associated with the disease prognosis and it should be monitored after even after treatment. Moreover, MPV and IPF are speedy measured by automatic analyzer with easy protocol and low cost. We recommend including both MPV and IPF measurements for suspected ACS patients in emergency departments.
Study limitation
There are still some limitations in this study. First, the automated and standardized instruments were performed to detect MPV and IPF, which could be provided test results with faster diagnosis, better precision and reproducibility. However, the normal range of MPV may be variant in different instrument or race, so each lab should be established the evaluable cut-off value. Second, to be properly measured MPV, the timing of test must be strictly controlled. Because the MPV increases with anticoagulant placement time and decreases due to the dilution of cytoplasmic content [51], all blood samples in this study were measured within 20 min after blood draw to reduce these effects. Since MPV is calculated by the following formula MPV (fl) = [(platelet (%)/Platelet count (X109/l)], MPV will be hard to calculated when the number of platelets in patient is very low [52]. Third, some reports indicate that MPV and IPF are related to cardiovascular mortality. However, since the sample size in this study was limited and lack of long term following-up, we did not observe the correlation between mortality and these biomarkers. Therefore, the relevance of these biomarkers to patient outcomes may be further addressed by large sample size, long term following-up and multi-center studies. Fourth, the diagnosis of coronary spasm-related type II MI is diagnosed at cath lab where angiographic study showed no culprit lesion on ST elevation territory and dynamic lesion stenosis after intracoronary injection of provocative methylergonovine and subsequent relieving of nitroglycerin. Therefore, we did not evaluate the ACS patient with coronary spasm in this study. However, we overcame the issue by confirming diagnosis of ACS with angiographic results and serial follow-up of cardiac troponin-I. Fifth, medications prescribed for ACS including antiplatelet or antithrombotic agents, statin, and cardo-selective beta blocker were not contraindicated for coronary spasm. Based upon majority of ACS patients should be treated with aforementioned cardiac protective agents in the beginning, even though we did not further diagnose possible ACS resulted from coronary spasm, the treatment for ACS did not influence our study results. Also, those with coronary spasm usually presented with dynamic ST segment depression on ECG rather than ST elevation, so only minority of spasm associated ACS would be enrolled in the present study. Thus, MPV could be used as a predictor of ACS for the relatively low-risk patients presenting with acute ischemic chest pain and was not influenced by initial treatment for ACS.
Ethical approval
This study was approved by the ethical committee of the Kaohsiung Chang Gung Memorial Hospital, Taiwan (IRB no. 105-0947C). Written informed consent was obtained from all participants after all aspects of this study were explained.
Conflicts of interest
We declare that no conflict of interest.
Acknowledgments
This study was financially supported by the Kaohsiung Chang Gung Memorial Hospital, Taiwan (grant no. CMRPG8E0241). We appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, Taiwan, for statistics works and suggestion.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Achar S.A., Kundu S., Norcross W.A. Diagnosis of acute coronary syndrome. Am Fam Physician. 2005;72:119–126. [PubMed] [Google Scholar]
- 2.Hamm C.W., Bertrand M., Braunwald E. Acute coronary syndrome without ST elevation: implementation of new guidelines. Lancet (London, England) 2001;358:1533–1538. doi: 10.1016/S0140-6736(01)06585-0. [DOI] [PubMed] [Google Scholar]
- 3.Bakshi T.K., Choo M.K., Edwards C.C., Scott A.G., Hart H.H., Armstrong G.P. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J. 2002;32:520–525. doi: 10.1046/j.1445-5994.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Hamwi S.M., Sharma A.K., Weissman N.J., Goldstein S.A., Apple S., Canos D.A. Troponin-I elevation in patients with increased left ventricular mass. Am J Cardiol. 2003;92:88–90. doi: 10.1016/s0002-9149(03)00477-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang C.H., Hsieh C.L., Dai M.H., Chen C.H., Lai Y.F. Inter-rater reliability and validity of the stroke rehabilitation assessment of movement (stream) instrument. J Rehabil Med. 2002;34:20–24. doi: 10.1080/165019702317242668. [DOI] [PubMed] [Google Scholar]
- 6.Hijazi Z., Siegbahn A., Andersson U., Granger C.B., Alexander J.H., Atar D. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. doi: 10.1161/CIRCULATIONAHA.113.006286. [DOI] [PubMed] [Google Scholar]
- 7.Velmahos G.C., Karaiskakis M., Salim A., Toutouzas K.G., Murray J., Asensio J. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma. 2003;54:45–50. doi: 10.1097/00005373-200301000-00006. discussion -1. [DOI] [PubMed] [Google Scholar]
- 8.Horwich T.B., Patel J., MacLellan W.R., Fonarow G.C. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation. 2003;108:833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 9.Kim M., Kim K. Elevation of cardiac troponin I in the acute stage of Kawasaki disease. Pediatr Cardiol. 1999;20:184–188. doi: 10.1007/s002469900437. [DOI] [PubMed] [Google Scholar]
- 10.Solinas L., Raucci R., Terrazzino S., Moscariello F., Pertoldi F., Vajto S. Prevalence, clinical characteristics, resource utilization and outcome of patients with acute chest pain in the emergency department. A multicenter, prospective, observational study in north-eastern Italy. Ital Heart J. 2003;4:318–324. [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., White H.D. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 12.Apple F.S., Wu A.H. Myocardial infarction redefined: role of cardiac troponin testing. Clin Chem. 2001;47:377–379. [PubMed] [Google Scholar]
- 13.Panteghini M. IFCC committee on standardization of markers of cardiac damage: premises and project presentation. International federation of clinical chemistry and laboratory medicine. Clin Chem Lab Med. 1998;36:887–893. doi: 10.1515/CCLM.1998.155. [DOI] [PubMed] [Google Scholar]
- 14.Penttila I., Penttila K., Rantanen T. Laboratory diagnosis of patients with acute chest pain. Clin Chem Lab Med. 2000;38:187–197. doi: 10.1515/CCLM.2000.027. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G., Montagnana M., Salvagno G.L., Guidi G.C. Potential value for new diagnostic markers in the early recognition of acute coronary syndromes. CJEM. 2006;8:27–31. doi: 10.1017/s148180350001335x. [DOI] [PubMed] [Google Scholar]
- 16.Toutouzas P., Richter D. Carotid intima-media thickness (cIMT): a useful clinic tool or research luxury? Another view of the ENHANCE trial. Angiology. 2008;59 doi: 10.1177/0003319708321748. 77s–9s. [DOI] [PubMed] [Google Scholar]
- 17.Oudi M.E., Aouni Z., Mazigh C., Khochkar R., Gazoueni E., Haouela H. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol. 2010;15:e25–e28. [PMC free article] [PubMed] [Google Scholar]
- 18.Hemingway H., Philipson P., Chen R., Fitzpatrick N.K., Damant J., Shipley M. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppinger J.A., Cagney G., Toomey S., Kislinger T., Belton O., McRedmond J.P. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 20.Gawaz M., Langer H., May A.E. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willoughby S., Holmes A., Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs: J Work Group Cardiovasc Nurs Eur Soc Cardiol. 2002;1:273–288. doi: 10.1016/s1474-5151(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 22.Clappers N., Brouwer M.A., Verheugt F.W.A. Antiplatelet treatment for coronary heart disease. Heart. 2007;93:258–265. doi: 10.1136/hrt.2005.071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorog D.A., Sweeny J.M., Fuster V. Antiplatelet drug ‘resistance’. Part 2: laboratory resistance to antiplatelet drugs-fact or artifact? Nat Rev Cardiol. 2009;6:365–373. doi: 10.1038/nrcardio.2009.13. [DOI] [PubMed] [Google Scholar]
- 24.Karon B.S., Tolan N.V., Koch C.D., Wockenfus A.M., Miller R.S., Lingineni R.K. Precision and reliability of 5 platelet function tests in healthy volunteers and donors on daily antiplatelet agent therapy. Clin Chem. 2014;60:1524–1531. doi: 10.1373/clinchem.2014.226332. [DOI] [PubMed] [Google Scholar]
- 25.Bath P.M., Butterworth R.J. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis: Int J Haemost Thromb. 1996;7:157–161. [PubMed] [Google Scholar]
- 26.Coban E., Ozdogan M., Yazicioglu G., Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–982. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 27.Kario K., Matsuo T., Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14:281–287. doi: 10.1111/j.1365-2257.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 28.Nadar S., Blann A.D., Lip G.Y. Platelet morphology and plasma indices of platelet activation in essential hypertension: effects of amlodipine-based antihypertensive therapy. Ann Med. 2004;36:552–557. doi: 10.1080/07853890410017386. [DOI] [PubMed] [Google Scholar]
- 29.Papanas N., Symeonidis G., Maltezos E., Mavridis G., Karavageli E., Vosnakidis T. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–478. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 30.Pathansali R., Smith N., Bath P. Altered megakaryocyte-platelet haemostatic axis in hypercholesterolaemia. Platelets. 2001;12:292–297. doi: 10.1080/09537100120058810. [DOI] [PubMed] [Google Scholar]
- 31.Lordkipanidze M. Platelet turnover in atherothrombotic disease. Curr Pharmaceut Des. 2012;18:5328–5343. doi: 10.2174/138161212803251952. [DOI] [PubMed] [Google Scholar]
- 32.Grove E.L., Hvas A.M., Mortensen S.B., Larsen S.B., Kristensen S.D. Effect of platelet turnover on whole blood platelet aggregation in patients with coronary artery disease. J Thromb Haemost: JTH. 2011;9:185–191. doi: 10.1111/j.1538-7836.2010.04115.x. [DOI] [PubMed] [Google Scholar]
- 33.Grove E.L., Hvas A.M., Kristensen S.D. Immature platelets in patients with acute coronary syndromes. Thromb Haemost. 2009;101:151–156. [PubMed] [Google Scholar]
- 34.Latger-Cannard V., Hoarau M., Salignac S., Baumgart D., Nurden P., Lecompte T. Mean platelet volume: comparison of three analysers towards standardization of platelet morphological phenotype. Int J Lab Hematol. 2012;34:300–310. doi: 10.1111/j.1751-553X.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 35.Jung H., Jeon H.K., Kim H.J., Kim S.H. Immature platelet fraction: establishment of a reference interval and diagnostic measure for thrombocytopenia. Korean J Lab Med. 2010;30:451–459. doi: 10.3343/kjlm.2010.30.5.451. [DOI] [PubMed] [Google Scholar]
- 36.Moe K.T., Wong P. Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singap. 2010;39:210–215. [PubMed] [Google Scholar]
- 37.Martin J.F., Kristensen S.D., Mathur A., Grove E.L., Choudry F.A. The causal role of megakaryocyte-platelet hyperactivity in acute coronary syndromes. Nat Rev Cardiol. 2012;9:658–670. doi: 10.1038/nrcardio.2012.131. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz M.B., Cihan G., Guray Y., Guray U., Kisacik H.L., Sasmaz H. Role of mean platelet volume in triagging acute coronary syndromes. J Thromb Thrombolysis. 2008;26:49–54. doi: 10.1007/s11239-007-0078-9. [DOI] [PubMed] [Google Scholar]
- 39.Chu H., Chen W.L., Huang C.C., Chang H.Y., Kuo H.Y., Gau C.M. Diagnostic performance of mean platelet volume for patients with acute coronary syndrome visiting an emergency department with acute chest pain: the Chinese scenario. Emerg Med J. 2011;28:569–574. doi: 10.1136/emj.2010.093096. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Xu X.L., Li X.M., Zhao R., Yang X., Cong H.L. Diagnostic value of mean platelet volume combined with troponin I for acute coronary syndrome. Am J Med Sci. 2016;352:159–165. doi: 10.1016/j.amjms.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Giles H., Smith R.E., Martin J.F. Platelet glycoprotein IIb–IIIa and size are increased in acute myocardial infarction. Eur J Clin Investig. 1994;24:69–72. doi: 10.1111/j.1365-2362.1994.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 42.Chu S.G., Becker R.C., Berger P.B., Bhatt D.L., Eikelboom J.W., Konkle B. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansanayudh N., Anothaisintawee T., Muntham D., McEvoy M., Attia J., Thakkinstian A. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2014;175:433–440. doi: 10.1016/j.ijcard.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Ingram M., Coopersmith A. Reticulated platelets following acute blood loss. Br J Haematol. 1969;17:225–229. doi: 10.1111/j.1365-2141.1969.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 45.Cesari F., Marcucci R., Gori A.M., Caporale R., Fanelli A., Casola G. Reticulated platelets predict cardiovascular death in acute coronary syndrome patients. Insights from the AMI-florence 2 study. Thromb Haemost. 2013;109:846–853. doi: 10.1160/TH12-09-0709. [DOI] [PubMed] [Google Scholar]
- 46.Berny-Lang M.A., Darling C.E., Frelinger A.L., Barnard M.R., Smith C.S., Michelson A.D. Do immature platelet levels in chest pain patients presenting to the emergency department aid in the diagnosis of acute coronary syndrome? Int J Lab Hematol. 2015;37:112–119. doi: 10.1111/ijlh.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taskesen T., Sekhon H., Wroblewski I., Goldfarb M., Ahmad M.B., Nguyen Q.T. Usefulness of mean platelet volume to predict significant coronary artery disease in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol. 2017;119:192–196. doi: 10.1016/j.amjcard.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 48.Hong K.H., Kim H.K., Kim J.E., Jung J.S., Han K.S., Cho H.I. Prognostic value of immature platelet fraction and plasma thrombopoietin in disseminated intravascular coagulation. Blood Coagul Fibrinolysis: Int J Haemost Thromb. 2009;20:409–414. doi: 10.1097/MBC.0b013e32832b1866. [DOI] [PubMed] [Google Scholar]
- 49.Muronoi T., Koyama K., Nunomiya S., Lefor A.K., Wada M., Koinuma T. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb Res. 2016;144:169–175. doi: 10.1016/j.thromres.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Joergensen M.K., Bathum L. Reference intervals for mean platelet volume and immature platelet fraction determined on a sysmex XE5000 hematology analyzer. Scand J Clin Lab Investig. 2016;76:172–176. doi: 10.3109/00365513.2015.1124448. [DOI] [PubMed] [Google Scholar]
- 51.Machin S., Briggs C. Mean platelet volume: a quick, easy determinant of thrombotic risk? J Thromb Haemost. 2010;8:146–147. doi: 10.1111/j.1538-7836.2009.03673.x. [DOI] [PubMed] [Google Scholar]
- 52.Bhat R., Pai S. Immature platelet fraction: a platelet parameter with significant clinical utility. Am J Clin Pathol. 2015;144 A142. [Google Scholar]