Abstract
Background
The aim of this study was to identify risk factors for Clostridium difficile infection (CDI) and its attributable mortality and to propose methods to prevent CDI and improve patients’ outcomes.
Methods
CDI was defined as diarrheal patients with stool samples that were positive for C. difficile toxin. Clinical presentations of all patients with CDI and two times as many age- and sex-matched culture-negative controls at the Chang Gung Memorial Hospital in 2014 were identified and compared by multivariate, nonparametric, and Kaplan–Meier survival analysis.
Results
There were no significant differences in ages, sex, or Charlson comorbidity indexes between the CDI group (n = 42) and the control group (n = 86). The multivariate analysis indicated that underlying peptic ulcer disease and previous use of gastric acid inhibitors or third-generation cephalosporins for at least 3 days were significantly more common in patients with CDI than in the controls. Charlson scores were associated with mortality due to CDI. Recommended treatment using oral vancomycin to treat patients with Charlson score ≥ 5 and oral metronidazole or vancomycin to treat those with moderate underlying disease (Charlson score ≥ 2 and ≤ 5) significantly increased survival in these patients (p = 0.001).
Conclusions
Oral vancomycin given to patients with high Charlson scores and oral metronidazole or vancomycin to patients with moderate Charlson scores decreased mortality due to CDI. Restricting the use of third-generation cephalosporins and gastric acid inhibitors is recommended to prevent CDI in hospitalized patients.
Keywords: Risk factor, Clostridium difficile infection, Gastric acid inhibitor, Third-generation cephalosporin, Charlson score
At a glance of commentary
Scientific background on the subject
Patients with C. difficile infection (CDI), one of the leading causes of nosocomial infections globally, ranging from asymptomatic, mild diarrhea to life-threatening pseudomembranous colitis. Rrisk factors for CDI have been found to include previous broad-spectrum antibiotics use, and severe underlying disease, but rare such study has been surveyed in Taiwan.
What this study adds to the field
Restricting the use of third-generation cephalosporins and gastric acid inhibitors is recommended to prevent CDI in hospitalized patients. Oral vancomycin given to patients with high Charlson scores and oral metronidazole or vancomycin to patients with moderate Charlson scores decreased mortality due to CDI.
Clostridium difficile is an anaerobic, Gram-positive, spore-forming bacillus that infects or colonizes the colon [1], [2], [3]. C. difficile infection (CDI) is one of the leading causes of nosocomial infections globally [1], [2], [3]. A recent dramatic increase in the incidence of CDI was found in many countries, including the United States, United Kingdom, and Australia [4], [5], [6], [7].
Some patients with C. difficile-associated disease (CDAD) are asymptomatic after exposure. However, others exhibit disease ranging from mild diarrhea to life-threatening pseudomembranous colitis [8], [9], [10], [11]. In previous studies, risk factors for CDI have been found to include previous broad-spectrum antibiotics use, advanced age, severe underlying disease, and prior hospitalization [9], [10], [11], but rare such study has been surveyed in Taiwan.
Metronidazole is the drug of choice for mild CDI (500 mg by mouth, three times a day for 10–14 days), whereas vancomycin is recommended for more severe CDI cases (125 mg by mouth, four times a day for 10–14 days) [11], [12], [13], [14], [15], [16], [17]. Patients with severe/complicated disease should be treated with vancomycin (125 mg) orally four times daily, with or without metronidazole (500 mg), intravenously every 8 h [11], [12], [13], [14], [15], [16], [17]. Leukocytosis with a white blood cell (WBC) count of ≥ 15,000 cells/mL or a serum creatinine level ≥ 1.5 times the premorbid level has been used as the criterion to define severity of CDI [13]. However, this criterion was only based on expert opinion that leukocytosis seems to reflect the severity of intestinal inflammation [12], and an elevated serum creatinine level may indicate inadequate renal perfusion resulting from severe diarrhea with volume dehydration [12]. These criteria should be examined in additional clinical studies.
By comparing clinical presentations of patients with CDI and those of patients with hospital-acquired diarrhea with an etiology other than C. difficile, our aim was to identify risk factors for acquiring CDI and those associated with attributable mortality to recommend interventions for preventing and treating CDI to improve patient outcomes.
Methods
Study design
This was a retrospective case–control study to survey risk factors associated with CDI and its attributable mortality. For each CDI case, approximately two times as many age- and sex-matched culture-negative controls were selected. Clinical presentations of patients with first episodes of CDI and those of controls were determined at Chang Gung Memorial Hospital (CGMH) between January 2014 and December 2014. This study was approved by the Institutional Review Board (IRB code: 201700587B0), Chang Gung Memorial Hospital, Taoyuan, Taiwan.
CDI cases were defined based on the Society for Healthcare Epidemiology of America (SHEA) and Infectious Diseases Society of America (IDSA) guidelines, using both clinical and laboratory data: (1) the presence of diarrhea, defined as the passage of ≥ 3 unformed stools in ≤ 24 consecutive hours and (2) a stool test positive for the presence of toxigenic C. difficile or its toxins or histopathologic or colonoscopic findings indicating pseudomembranous colitis.12 We excluded patients who were infected by any other pathogenic microbes or who tested positive for norovirus, rotavirus, or Escherichia coli O157.
The control patients were defined as inpatients that presented with diarrhea and were found negative for C. difficile by toxin assay and culture. Cases and controls were matched at a ratio of 1:2 by age (within 10 years) and sex.
Clinical data collection
The following clinical and demographic data were obtained from electronic medical records: age, sex, vital signs, frequency of diarrhea, underlying diseases, exposure to antimicrobials within the 14 days preceding CDI diagnosis, treatment for CDI, length of hospital stay, length of intensive care unit (ICU) stay, surgery related to CDI, and disease outcome.
Definition
Mortality was defined as CDI-attributable death, occurring before signs and symptoms of CDI were resolved [12]. Hypotension, shock, ileus, toxic megacolon, peritonitis, respiratory distress, and hemodynamic instability were considered complications [12], [13], [14], [15], [16], [17]. Based on the time at risk for C. difficile to acquire resistance under antimicrobial selective pressure, prior exposure to antimicrobial agents was defined as ≥ 5 days of therapy during the 14 days prior to isolation of C. difficile [18], [19], [20]. Appropriate antimicrobial therapy was defined as treatment of patients with metronidazole or oral vancomycin within 2 days of CDI onset [18], [19], [20]. Recommended treatment based on Charlson score comprised metronidazole (500 mg) administered orally 3 times per day or oral vancomycin (125 mg) administered orally 4 times per day to patients with moderate underlying diseases (Charlson score ≥ 2 and ≤ 6), and oral vancomycin (125 mg) orally 4 times per day to patients with severe underlying diseases (Charlson score ≥ 6) within 2 days after CDI onset.
Statistical analysis
Data analyses were performed using SPSS software v. 17.0 (SPSS Inc., Chicago, IL, USA). The Chi-square test or Fisher's exact test was used when appropriate to compare proportions. The logistic regression or Wilcoxon rank sum test was used when appropriate to compare continuous variable. Variables with a p value of less than 0.2 in the univariate analysis were added in a forward stepwise manner and selected to create the final model for the multivariate analysis. All statistical analyses were two-sided, and significance was set at p < 0.05.
Results
Clinical characteristics of patients with CDI
Among the 42 patients with CDI, the median age was 67 (interquartile range, 53.3–78.8) years, with the majority of these patients middle-aged or elderly. There were no significant differences in ages, sex, or median Charlson comorbidity indexes between the CDI and control groups (Table 1). The most common clinical manifestation of CDAD was fever (n = 27, 64.3%), followed by vomiting (n = 5, 11.9%), and then abdominal pain (n = 4, 9.5%) (Table 2).
Table 1.
Logistic regression analysis of risk factors for C. difficile infection.
| Characteristics | CDI N = 42 |
Non-CDI N = 86 |
Univariate analysis |
p | Multivariate analysisb |
p |
|---|---|---|---|---|---|---|
| n (%)a | n (%)a | Odds ratio (95% CI) | Multivariate analysisb | |||
| Demographic characteristics | ||||||
| Age, years | 66 (53.3–78.8) | 65 (55.0–80.8) | 1.01 (0.99–1.03) | 0.842 | ||
| Sex, male/female | 20/22 | 52/34 | 0.59 (0.28–1.25) | 0.171 | ||
| Underlying diseases | ||||||
| Charlson score | 3 (2–5) | 3 (1–6) | 1.07 (0.92–1.25) | 0.382 | ||
| Neoplastic diseasec | 16 (38.1) | 29 (33.7) | 1.10 (0.75–1.61) | 0.627 | ||
| Metastatic malignancyc | 5 (14.2) | 15 (17.4) | 0.93 (0.77–1.11) | 0.421 | ||
| Cardiac disease | 7 (16.7) | 16 (18.6) | 0.88 (0.33–2.32) | 0.789 | ||
| Cerebrovascular disease | 14 (33.3) | 19 (22.1) | 1.16 (0.40–1.82) | 0.175 | ||
| Diabetes | 19 (45.2) | 33 (38.4) | 1.15 (0.51–1.48) | 0.598 | ||
| Pulmonary disease | 3 (7.1) | 4 (4.7) | 1.58 (0.34–7.39) | 0.563 | ||
| Hepatic disease | 6 (14.3) | 15 (17.4) | 0.93 (0.53–1.65) | 0.809 | ||
| Renal disease | 16 (39.0) | 23 (26.7) | 1.32 (0.83–1.96) | 0.163 | ||
| Peptic ulcer | 11 (26.2) | 6 (7.0) | 4.73 (1.61–13.90) | 0.005 | 4.77 (1.57–14.45) | 0.006 |
| Dementia | 7 (16.7) | 7 (8.2) | 2.26 (0.74–6.92) | 0.155 | ||
| Previous gastric acid inhibitors for at least 3 days in 14 days before the infection | ||||||
| Esomeprazole | 8 (19.0) | 3 (3.5) | 6.51 (1.63–26.02) | 0.008 | ||
| Pantoprazole | 1 (2.4) | 1 (1.2) | 2.07 (0.13–33.98) | 0.609 | ||
| Lansoprazole | 3 (7.1) | 2 (2.3) | 3.23 (0.52–20.12) | 0.209 | ||
| Proton pump inhibitorsd | 10 (23.8) | 5 (5.8) | 5.06 (1.61–15.97) | 0.006 | ||
| Misoprostol | 1 (2.4) | 1 (1.2) | 2.07 (0.13–33.98) | 0.609 | ||
| Histamine-2 blockers | 1 (2.4) | 1 (1.2) | 12.5 (0.00–115.52) | 0.489 | ||
| Gastric acid inhibitors | 11 (26.2) | 6 (7.0) | 4.73 (1.61–13.90) | 0.005 | 4.77 (1.57–14.45) | 0.006 |
| Previous antibiotics usage for at least 3 days in 14 days before the infection | ||||||
| Cephalosporines | ||||||
| First generation | 3 (7.1) | 2 (2.3) | 3.23 (0.52–20.12) | 0.209 | ||
| Second generation | 1 (2.4) | 4 (4.7) | 0.50 (0.05–4.62) | 0.541 | ||
| Third generation | 21 (50.0) | 23 (26.7) | 2.74 (1.27–5.92) | 0.010 | 2.76 (1.24–6.14) | 0.013 |
| Fourth generation | 4 (9.5) | 9 (10.5) | 0.90 (0.26–3.11) | 0.869 | ||
| All generationse | 26 (61.9) | 34 (39.5) | 2.49 (1.17–5.30) | 0.019 | ||
| Penicillinsf | 6 (14.3) | 5 (5.8) | 2.70 (0.77–9.43) | 0.119 | ||
| Piperacillin/tazobactam | 7 (16.7) | 34 (39.5) | 0.48 (0.19–1.17) | 0.107 | ||
| Glycopeptides | 10 (23.8) | 30 (34.9) | 0.58 (0.25–1.35) | 0.207 | ||
| Imipenem | 15 (35.7) | 23 (26.7) | 1.52 (0.69–3.36) | 0.298 | ||
| Fluoroquinolones | 11 (26.2) | 17 (19.8) | 1.44 (0.60–3.43) | 0.411 | ||
| Cotrimazole | 1 (2.4) | 15 (17.4) | 0.84 (0.47–1.49) | 0.545 | ||
| Aminoglycosides | 7 (16.7) | 5 (5.8) | 1.28 (0.70–2.34) | 0.420 | ||
| Colistin | 1 (2.4) | 5 (5.8) | 0.40 (0.05–3.49) | 0.404 | ||
Abbreviations: CDI: Clostridium difficile infection; ICU: intensive care unit.
Data are presented as median value (interquartile range: Q1-Q3) for continuous variables and number of cases (%) for categorical variables.
All variables with a p value < 0.20 in the univariable analysis were considered for inclusion in the logistic regression model in the multivariable analysis. A forward stepwise selection process was utilized. We found that only underlying peptic ulcer and previous usage of gastric acid inhibitors or third generation cephalosporines for at least 3 days were statistically significant risk factors for acquiring Clostridium difficile infection.
Neoplastic disease included metastatic malignancy and other neoplastic disease.
Because some patients used 2 or more proton pump inhibitors in 14 days before the infection, data here is less than the sum of the case number of using three PPI (esomeprazole, pantoprazole and lansoprazole).
Because some patients used 2 or more generations of cephalosporines in 14 days before the infection, data here is less than the sum of the case number of using 4 generations of cephalosporines (first, second, third and fourth generation).
Data here included all patients using penicillins but excluded those using piperacillin/tazobactam.
Table 2.
Clinical manifestations and outcomes of patients with C. difficile infection compared with those of C. difficile-negative controls.
| Characteristics | CDI N = 42 |
Non-CDI N = 86 |
Odds ratio (95% CI) | p |
|---|---|---|---|---|
| n (%)a | n (%)a | |||
| Clinical manifestations | ||||
| Fever | 27 (64.3) | 33 (38.4) | 2.89 (1.34–6.22) | 0.007 |
| Vomiting | 4 (9.5) | 6 (7.0) | 1.40 (0.37–5.27) | 0.615 |
| Abdominal pain | 5 (11.9) | 12 (14.0) | 0.83 (0.27–2.54) | 0.749 |
| Outcomes | ||||
| Mortality | 8 (19.0) | 15 (17.4) | 1.11 (0.43–2.88) | 0.824 |
| Length of stay in hospital | 43 (24.25–65.25) | 32 (22–67) | 1.00 (0.99–1.01) | 0.544 |
| Length of stay in ICU | 0 (0–3.75) | 0 (0–10.75) | 0.97 (0.94–1.01) | 0.102 |
Data are presented as median value (interquartile range: Q1-Q3) for continuous variables and number of cases (%) for categorical variables. Abbreviations: CDI: Clostridium difficile infection; ICU: intensive care unit.
Risk factors for CDI
To analyze the risk factors for CDI, 42 patients with CDI were matched with 86 controls. Demographic data and clinical characteristics of the patients with CDI and controls are displayed in Table 1. The multivariate analysis indicated that underlying peptic ulcer disease and previous use of gastric acid inhibitors (proton pump inhibitors, histamine 2-blockers, or misoprostol) or third-generation cephalosporins for at least 3 days occurred significantly more frequently in patients with CDI than in the controls (Table 1). Table 2 shows a comparison of clinical manifestations of patients with CDI with those of the controls. The results indicated that patients with CDI were more likely to have fever compared to the controls.
Outcomes of patients with CDI
The outcomes of CDI are presented in Table 2. There were no significant differences in mortality, length of hospital stay, and length of ICU stay when patients with CDI were compared to controls (Table 2).
Risk factors for mortality
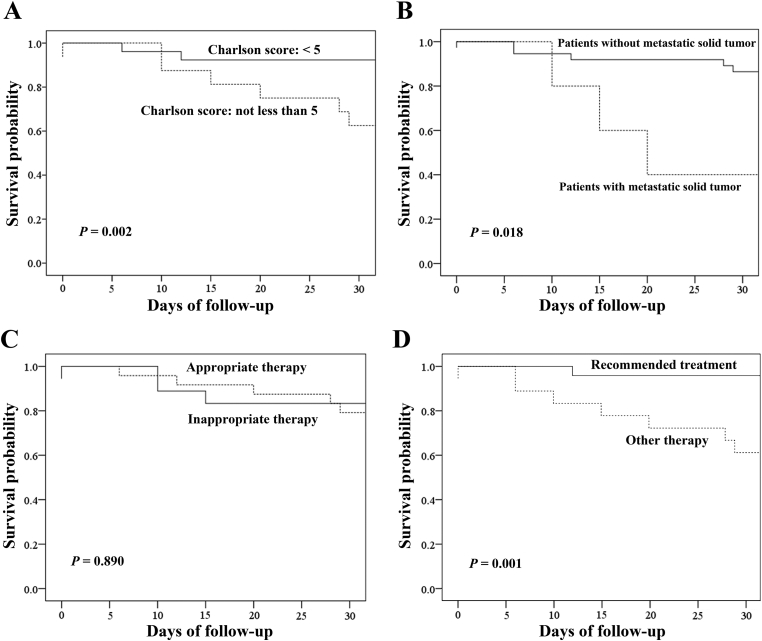
Risk factors for mortality at 30 days were analyzed by comparing the characteristics of survivors and non-survivors (Table 3, Table 4). Based on the multivariate analysis, only Charlson scores were an independent risk factor for mortality (Table 3). Based on the nonparametric analysis, Charlson scores, metastatic malignancy and recommended treatment based on Charlson score were independent risk factors for mortality (Table 4). Nearly 90% of the patients with Charlson scores < 5 survived through day 30, compared to only 60% of patients with Charlson scores ≥ 5 (p = 0.002 by log rank test, Fig. 1A). Nearly 85% of patients without metastatic solid tumors survived through day 30, compared with only 40% of patients with metastatic solid tumors (p = 0.018 by log rank test, Fig. 1B). The Kaplan–Meier survival analysis revealed that appropriate antimicrobial therapy did not affect the time to death in the 30 days of follow-up (p = 0.890 by log rank test, Fig. 1C). Nearly 95% of the patients who received the treatment based on Charlson score survived through day 30, compared with only 60% who did not (p = 0.001 by log rank test, Fig. 1D).
Table 3.
Logistic regression analysis of risk factors for 30-day mortality of patients with C. difficile infection.
| Characteristics | Non-survivors |
Survivors |
Univariate analysis |
p | Multivariate analysisb |
p |
|---|---|---|---|---|---|---|
| N = 8 |
N = 34 |
|||||
| n (%)a | n (%)a | Odds ratio (95% CI) | Odds ratio (95% CI) | |||
| Demographic characteristics | ||||||
| Age, years | 62 (58.5–70) | 69 (53.0–79.5) | 1.01 (0.99–1.03) | 0.916 | ||
| Sex, male/female | 4/4 | 16/18 | 0.60 (0.12–2.92) | 0.527 | ||
| Underlying diseases | ||||||
| Charlson score | 6 (3.5–6.25) | 3 (2–5) | 2.04 (1.17–3.54) | 0.011 | 2.04 (1.17–3.54) | 0.011 |
| Neoplastic diseasec | 3 (37.5) | 13 (38.0) | 0.14 (0.02–0.81) | 0.028 | ||
| Metastatic malignancyc | 3 (37.5) | 2 (6) | 1.46 (1.04–2.04) | 0.028 | ||
| Cardiac disease | 1 (12.5) | 11 (32.4) | 0.52 (0.08–3.33) | 0.487 | ||
| Cerebrovascular disease | 3 (37.5) | 19 (22.1) | 4.33 (0.48–39.36) | 0.193 | ||
| Diabetes | 6 (75.0) | 12 (35.3) | 2.70 (0.08–1.82) | 0.222 | ||
| Pulmonary disease | 0 (0) | 3 (8.8) | 0.44 (0.04–5.53) | 0.523 | ||
| Hepatic disease | 1 (12.5) | 5 (14.7) | 0.05 (0.00–47.20) | 0.390 | ||
| Renal disease | 3 (37.5) | 13 (39.4) | 2.21 (0.39–12.63) | 0.372 | ||
| Peptic ulcer | 2 (25.0) | 9 (26.5) | 0.51 (0.10–2.63) | 0.424 | ||
| Dementia | 2 (25.0) | 5 (14.7) | 1.50 (0.15–14.57) | 0.727 | ||
| CDI Severity | ||||||
| White blood cell count, cells/nL | 10.15 (7.93–13.03) | 10.05 (5.75–11.75) | 1.04 (0.94–1.15) | 0.503 | ||
| White blood cell count > 15 cells/nL | 1 (12.5) | 4 (11.7) | 1.35 (0.14–36.20) | 0.390 | ||
| Creatinine/baseline data | 1.34 (0.96–2.35) | 1.03 (0.85–1.35) | 2.75 (0.85–8.90) | 0.091 | ||
| Creatinine ≥ 1.5 × baseline | 3 (37.5) | 7 (20.5) | 4.21 (0.47–38.36) | 0.195 | ||
| Complication | 1 (12.5) | 1 (2.9) | 4.71 (0.26–84.77) | 0.293 | ||
| Appropriate therapy | 3 (37.5) | 15 (44.1) | 0.76 (0.16–3.70) | 0.734 | ||
| Recommended treatment based on Charlson score | 1 (12.5) | 23 (67.6) | 0.07 (0.01–0.63) | 0.018 | ||
Abbreviations: CDI: Clostridium difficile infection; ICU: intensive care unit.
Data are presented as median value (interquartile range: Q1-Q3) for continuous variables and number of cases (%) for categorical variables.
All variables with a p value < 0.20 in the univariable analysis were considered for inclusion in the logistic regression model in the multivariable analysis. A forward stepwise selection process was utilized. We found that only Charlson score were statistically significant risk factors for 30-day mortality of patients with Clostridium difficile infection.
Neoplastic disease included metastatic malignancy and other neoplastic disease.
Table 4.
Nonparametric analysis of risk factors for 30-day mortality of patients with C. difficile infection.
| Characteristics | Non-survivors |
Survivors |
pb |
|---|---|---|---|
| N = 8 |
N = 34 |
||
| n (%)a | n (%)a | ||
| Demographic characteristics | |||
| Age, years | 62 (58.5–70) | 69 (53.0–79.5) | 0.726 |
| Sex, male/female | 4/4 | 16/18 | 1.000 |
| Underlying diseases | |||
| Charlson score | 6 (3.5–6.25) | 3 (2–5) | 0.014 |
| Neoplastic diseasec | 3 (37.5) | 13 (38.2) | 1.000 |
| Metastatic malignancyc | 3 (37.5) | 2 (5.9) | 0.040 |
| Cardiac disease | 1 (12.5) | 11 (32.4) | 0.402 |
| Cerebrovascular disease | 3 (37.5) | 19 (22.1) | 0.445 |
| Diabetes | 6 (75.0) | 12 (35.3) | 0.056 |
| Pulmonary disease | 0 (0) | 3 (8.8) | 1.000 |
| Hepatic disease | 1 (12.5) | 5 (14.7) | 1.000 |
| Renal disease | 3 (37.5) | 13 (39.4) | 1.000 |
| Peptic ulcer | 2 (25.0) | 9 (26.5) | 1.000 |
| Dementia | 2 (25.0) | 5 (14.7) | 0.601 |
| CDI Severity | |||
| White blood cell count, cells/nL | 10.15 (7.93–13.03) | 10.05 (5.75–11.75) | 0.889 |
| White blood cell count > 15 cells/nL | 1 (12.5) | 4 (11.7) | 1.0 |
| Creatinine/baseline data | 1.34 (0.96–2.35) | 1.03 (0.85–1.35) | 0.161 |
| Creatinine ≥ 1.5 × baseline | 3 (37.5) | 7 (20.5) | 0.369 |
| Complication | 1 (12.5) | 1 (2.9) | 0.348 |
| Appropriate therapy | 3 (37.5) | 15 (44.1) | 1.000 |
| Recommended treatment based on Charlson score | 1 (12.5) | 23 (67.6) | 0.013 |
Abbreviations: CDI: Clostridium difficile infection; ICU: intensive care unit.
Data are presented as median value (interquartile range: Q1-Q3) for continuous variables and number of cases (%) for categorical variables.
Fisher's exact test or Chi-square test (Fisher's exact test was used if more than 20% of cell expectations are less than 5; otherwise, Chi-square test was used) was used to calculate the p values for classification variables; Wilcoxon rank sum test was used to calculate the p values for continuous variables.
Neoplastic disease included metastatic malignancy and other neoplastic disease.
Fig. 1.
Kaplan–Meier survival curves of patients with Clostridium difficile infection (CDI). (A) Kaplan–Meier survival curve of patients with Charlson scores < 5 (solid line) compared with those with Charlson scores ≥ 5 (dotted line) (p = 0.002 by log-rank test). (B) Kaplan–Meier survival curve of patients without metastatic solid tumors (solid line) compared with those with metastatic solid tumors (dotted line) (p = 0.018 by log-rank test). (C) Kaplan–Meier survival curve of patients who received appropriate therapy (dotted line) compared with those who did not receive appropriate therapy (solid line) (p = 0.890 by log-rank test). (D) Kaplan–Meier survival curve of patients who received recommended treatment based on Charlson score (solid line) compared with those who did not receive such treatment (dotted line) (p = 0.001 by log-rank test).
Discussion
The most important finding in this study was that patients treated with the treatment based on Charlson score had a greater probability of survival than those without such therapy. This recommended therapy was according to Charlson scores (≥ 5), rather than based on leukocytosis (> 1.5 cells/mL) or elevated creatinine levels (≥ 1.5 × baseline) used in most studies [12], [13], [14], [15], [16], [17]. In this study, patients with higher Charlson scores who were treated with oral metronidazole were more likely to die than those with severe comorbidities that were treated with oral vancomycin. Therefore, oral vancomycin given for 2 days is recommended for patients with higher Charlson scores, even if they did not have leukocytosis, elevated creatinine levels, or any complications. All patients with mild underlying disease (Charlson scores < 2) survived, even without treatment with oral metronidazole or vancomycin. Close outpatient monitoring without the administration of antibiotics has been recommended for outpatients with CDI [11]. A larger study to determine the value of oral antibiotics for the treatment of hospitalized patients with CDI and mild underlying diseases should be conducted.
The efficacy and safety of antibiotic therapy for CDI were reviewed in 2017, but the efficacy of antibiotic treatment for severe CDI is uncertain, as most studies have excluded patients with severe disease [15]. Whether antibiotics are necessary to treat mild CDI remains unknown because of the lack of “no treatment” control studies 15 Many studies have suggested that vancomycin is superior to metronidazole, especially for the treatment of patients with severe CDI [12], [13], [14], [15], [16], [17]; this was supported by the results of our study. Oral metronidazole was used to treat most of the patients with CDI in this study, because the relative effectiveness of these antibiotics was unknown and metronidazole is far less costly than vancomycin. In our study, the treatment based on Charlson score using oral vancomycin to treat patients with CDI with Charlson scores ≥ 5 significantly increased the probability of their survival compared to treatment with oral metronidazole. This is because patients with severe underlying diseases, but without leukocytosis or increased creatinine levels, died if they were treated with oral metronidazole according to the current guidelines.
Guidelines for CDI treatment are often stratified by the presence of leukocytosis or elevated serum creatinine levels [12], [13], [14], [15], [16], [17]. However, in addition to elevated WBC counts of > 20 × 109/L [21], WBC count >15 × 109/L [22], and creatinine concentrations > 133 μmol/L [23], there are many additional risk factors for CDI-associated mortality, such as malignancy [22], [24], low albumin levels [22], [23], ICU admission [22], [24], response failure [22], ischemic heart disease [23], acute renal failure [24], liver disease [24], inflammatory bowel disease [24], [25], cardiopulmonary disease [24], diabetes mellitus [26] and shock [26]. Most of these reported independent risk factors, including malignancy [22], [24], ischemic heart disease [23], acute renal failure [24], liver disease [24], inflammatory bowel disease [24], cardiopulmonary disease [24], and diabetes mellitus [26] are comorbidities that can affect Charlson scores, an independent risk factor identified in our study. Results of previous studies indicate that underlying diseases are as important as leukocytosis or elevated creatinine levels in determining the outcomes of patients with CDI [21], [22], [23], [24], [25], [26]. Therefore, it is reasonable to give oral vancomycin, which has been found to be superior for the treatment of patients with severe CDI [16], to patients with severe underlying diseases, which have been associated with higher mortality in our previous study and many others [21], [22], [23], [24], [25], [26].
Many observational studies have shown that prior treatment with antimicrobials is the main risk factor for CDI [11], [12], [13], [27], [28]. CDI is known to be the cause of up to 25% of antibiotic-associated diarrheal cases [8]. Our study revealed that using a third-generation cephalosporin for ≥ 3 days (odds ratio: 2.74, 95% confidence interval, 1.27–5.92) was associated with an increased risk of CDI, whereas use for < 3 days did not significantly increase the risk of CDI. This result might imply that using broad-spectrum antibiotics for an extended period has negative effects on the normal flora, thus increasing the potential for CDI [29]. Previous studies have shown that the occurrence of CDI is associated with the administration of clindamycin [30], [31], extended-spectrum penicillins [30], [31], and cephalosporins [30], [31]. Furthermore, three recent meta-analyses found that cephalosporins and clindamycin were the most strongly associated with hospital-associated CDI [32], whereas community-associated CDI was associated with use of clindamycin, cephalosporins, or quinolones [33], [34]. A recent review indicated that prior use of almost all antibiotics is associated with CDI, with cephalosporins, ampicillin, amoxicillin, clindamycin, and fluoroquinolones being the antibiotics most commonly associated with these infections [9].
In some studies, the use of a proton pump inhibitor was found to be a risk factor for CDI, because gastric acid suppression elevates the gastrointestinal pH, allowing more vegetative C. difficile organisms to reach the colon [35], [36]. However, other studies have shown that proton pump inhibitor therapy is not associated with CDI and have attributed CDI risk to C. difficile spores, the acid-resistant vectors for infection that remain viable in the low pH of the stomach [37], [38], In this study, we found that patients with underlying peptic ulcer disease were at greater risk for CDI. All patients with peptic ulcers in our study were given gastric acid inhibitors, which are also associated with risk of CDI.
In our study, outcomes, including mortality, length of hospital stay, and length of ICU stay, were not significantly different between patients with CDI and controls. In contrast, Vonberg et al. found that both increased length of hospital stay and length of ICU stay were associated with CDI [39]. The discrepancy might result from the relatively limited sample size in this study.
Conclusions
Patients with diarrhea, fever, peptic ulcers, and previous use of gastric acid inhibitors or third-generation cephalosporins were more likely to be diagnosed with CDI than patients without these conditions. High Charlson scores were associated with mortality due to CDI. Recommended treatment based on Charlson score using oral vancomycin to treat patients with Charlson scores ≥ 5 and oral metronidazole or vancomycin to treat those with moderate underlying disease (Charlson scores ≥ and ≤ 5) within 2 days of CDI onset significantly increased their survival times.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank professor Tzu-Lan Wu for providing bacterial isolates for this study. This study was supported by grants from National Science Council, Executive Yuan, Taiwan (100-2314-B-182A-049 and 102-2314-B-182A-023) and Chang Gung Memorial Hospital (CMRPG490052, CMRPG3E0482 and CMRPG4C0032), Taiwan.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2018.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chitnis A.S., Holzbauer S.M., Belflower R.M., Winston L.G., Bamberg W.M., Lyons C. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 2.Peery A.F., Dellon E.S., Lund J., Crockett S.D., McGowan C.E., Bulsiewicz W.J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czepiel J., Dróżdż M., Pituch H., Kuijper E.J., Perucki W., Mielimonka A. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019 doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessa F.C., Mu Y., Bamberg W.M., Beldavs Z.G., Dumyati G.K., Dunn J.R. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox M.H., Shetty N., Fawley W.N., Shemko M., Coen P., Birtles A. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55:1056–1063. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 6.Banaei N., Anikst V., Schroeder L.F. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2368–2369. doi: 10.1056/NEJMc1505190. [DOI] [PubMed] [Google Scholar]
- 7.Planche T.D., Davies K.A., Coen P.G., Finney J.M., Monahan I.M., Morris K.A. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect Dis. 2013;13:936–945. doi: 10.1016/S1473-3099(13)70200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett J.G., Gerding D.N. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46:S12–S18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 9.Leffler Daniel A., Thomas Lamont J. Review article: Clostridium difficile infection. N Engl J Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 10.Kee V.R. Clostridium difficile infection in older adults: a review and update on its management. Am J Geriatr Pharmacother. 2012;10:14–24. doi: 10.1016/j.amjopharm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Anjewierden S., Han Z., Foster C.B., Pant C., Deshpande A. Risk factors for Clostridium difficile infection in pediatric inpatients: A meta-analysis and systematic review. Infect Control Hosp Epidemiol. 2019;40:420–426. doi: 10.1017/ice.2019.23. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S.H., Gerding D.N., Johnson S., Kelly C.P., Loo V.G., McDonald L.C. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 13.Ong G.K., Reidy T.J., Huk M.D., Lane F.R. Clostridium difficile colitis: a clinical review. Am J Surg. 2017;213:565–571. doi: 10.1016/j.amjsurg.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano L.M., Edmiston C.E., Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery. 2017;162:325–348. doi: 10.1016/j.surg.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Nelson R.L., Suda K.J., Evans C.T. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017;3:CD004610. doi: 10.1002/14651858.CD004610.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zar F.A., Bakkanagari S.R., Moorthi K.M.L.S.T., Davis M.B. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 17.Rajasingham R., Enns E.A., Khoruts A., Vaughn B.P. Cost-effectiveness of treatment regimens for Clostridioides difficile infection―an evaluation of the 2018 Infectious Diseases Society of America guidelines. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz318. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H.Y., Chen C.L., Wu S.R., Huang C.W., Chiu C.H. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. 2014;42:1081–1088. doi: 10.1097/CCM.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.Y., Chen C.L., Liu S.Y., Yan Y.S., Chang C.J., Chiu C.H. Impact of molecular epidemiology and reduced susceptibility to glycopeptides and daptomycin on outcomes of patients with methicillin-resistant Staphylococcus aureus Bacteremia. PLoS One. 2015;10:e0136171. doi: 10.1371/journal.pone.0136171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H.Y., Huang C.W., Chen C.L., Wang Y.H., Chang C.J., Chiu C.H. Emergence in Taiwan of novel imipenem-resistant Acinetobacter baumannii ST455 causing bloodstream infection in critical patients. J Microbiol Immunol Infect. 2015;48:588–596. doi: 10.1016/j.jmii.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Solomon K., Martin A.J., O'Donoghue C., Chen X., Fenelon L., Fanning S. Mortality in patients with Clostridium difficile infection correlates with host proinflammatory and humoral immune responses. J Med Microbiol. 2013;62:1453–1460. doi: 10.1099/jmm.0.058479-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim E.S., Kim Y.J., Park C.W., Cho K.B., Jang B.K., Chung W.J. Response failure to the treatment of Clostridium difficile infection and its impact on 30-day mortality. Hepatogastroenterology. 2013;60:543–548. doi: 10.5754/hge12730. [DOI] [PubMed] [Google Scholar]
- 23.Wilson V., Cheek L., Satta G., Walker-Bone K., Cubbon M., Citron D. Predictors of death after Clostridium difficile infection: a report on 128 strain-typed cases from a teaching hospital in the United Kingdom. Clin Infect Dis. 2010;50:e77–e81. doi: 10.1086/653012. [DOI] [PubMed] [Google Scholar]
- 24.Kassam Z., Cribb Fabersunne C., Smith M.B., Alm E.J., Kaplan G.G., Nguyen G.C. Clostridium difficile associated risk of death score (CARDS): a novel severity score to predict mortality among hospitalised patients with C. difficile infection. Aliment Pharmacol Ther. 2016;43:725–733. doi: 10.1111/apt.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananthakrishnan A.N., McGinley E.L., Binion D.G. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 26.Honda H., Yamazaki A., Sato Y., Dubberke E.R. Incidence and mortality associated with Clostridium difficile infection at a Japanese tertiary care center. Anaerobe. 2014;25:5–10. doi: 10.1016/j.anaerobe.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas C., Stevenson M., Riley T.V. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 28.Hensgens M.P., Goorhuis A., van Kinschot C.M., Crobach M.J., Harmanus C., Kuijper E.J. Clostridium difficile infection in an endemic setting in The Netherlands. Eur J Clin Microbiol Infect Dis. 2011;30:587–593. doi: 10.1007/s10096-010-1127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borriello S.P. The influence of the normal flora on Clostridium difficile colonisation of the gut. Ann Med. 1990;22:61–67. doi: 10.3109/07853899009147244. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett J.G. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15:573–581. doi: 10.1093/clind/15.4.573. [DOI] [PubMed] [Google Scholar]
- 31.Barbut F. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect. 2001;7:405–411. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 32.Slimings C., Riley T.V. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 33.Deshpande A., Pasupuleti V., Thota P., Pant C., Rolston D.D., Sferra T.J. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68:1951–1961. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 34.Brown K.A., Khanafer N., Daneman N., Fisman D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57:2326–2332. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dial S., Delaney J.A., Barkun A.N., Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 36.Howell M.D., Novack V., Grgurich P., Soulliard D., Novack L., Pencina M. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 37.Henrich T.J., Krakower D., Bitton A., Yokoe D.S. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novack L., Kogan S., Gimpelevich L., Howell M., Borer A., Kelly C.P. Acid suppression theory does not predispose to Clostridium difficile infection: the case of the potential bias. PLoS One. 2014;9:e110790. doi: 10.1371/journal.pone.0110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vonberg R.P., Kuijper E.J., Wilcox M.H., Barbut F., Tüll P., Gastmeier P. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008;14(Suppl 5):2–20. doi: 10.1111/j.1469-0691.2008.01992.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.