Abstract
Background:
Multiple therapies involving ablative and nonablative techniques have been developed for rejuvenation of photodamaged skin. Monopolar radiofrequency (RF) is emerging as a gentler, nonablative skin-tightening device that delivers uniform heat to the dermis at a controlled depth.
Objective:
We evaluated the clinical effects and objectively quantified the histologic changes of the nonablative RF device in the treatment of photoaging.
Methods:
Six individuals of Fitzpatrick skin type III to IV and Glogau class I to II wrinkles were subjected to 3 months of treatment (6 sessions at 2-week intervals). Standard photographs and skin biopsy specimens were obtained at baseline, and at 3 and 6 months after the start of treatment. We performed quantitative evaluation of total elastin, collagen types I and III, and newly synthesized collagen using computerized histometric and immunohistochemical techniques. Blinded photographs were independently scored for wrinkle improvement.
Results:
RF produced noticeable clinical results, with high satisfaction and corresponding facial skin improvement. Compared with the baseline, there was a statistically significant increase in the mean of collagen types I and III, and newly synthesized collagen, while the mean of total elastin was significantly decreased, at the end of treatment and 3 months posttreatment.
Limitations:
A limitation of this study is the small number of patients, yet the results show a significant improvement.
Conclusions:
Although the results may not be as impressive as those obtained by ablative treatments, RF is a promising treatment option for photoaging with fewer side effects and downtime.
Keywords: collagen, elastin, nonablative, radiofrequency, skin aging
There are two clinically and biologically distinct aging processes affecting the skin. The first is intrinsic aging, “the biologic clock,” which affects the skin by slow, irreversible tissue degeneration.1 The second is extrinsic aging, “photoaging,” which was first described in 1986 as the effects of chronic exposure to the elements, primarily ultraviolet radiation on skin.2–4 The histologic and ultrastructural hallmark of photodamaged skin is the accumulation of elastotic material in the papillary and mid dermis, a process known as solar elastosis.5 Collagen, which comprises more than 80% of the total dry weight of the dermis, becomes disorganized with enhanced breakdown and reduced network formation.6 These alterations contribute to the skin sagging and wrinkling.7
For more than half of a decade, many different laser and other light-based systems have been developed and evaluated for their capability to reverse photodamage and age-associated rhytides, a process referred to as photorejuvenation.7,8 Although ablative lasers remain the gold standard for photodamaged skin rejuvenation, their use is associated with significant side effects, and a prolonged and an unpleasant posttreatment downtime.9 Thus, in recent years, interest in ablative treatments has waned and nonablative skin rejuvenation has become an appealing alternative treatment.10 Nonablative laser modalities are designed to produce favorable alterations in the dermis with no epidermal damage. However, laser light can be diffracted, absorbed, or scattered, and only small portions of the emitted energy reach the target of concern. Consequently, the effects are proportionally reduced.11 The monopolar radiofrequency (RF) device is different from cosmetic lasers, as it produces an electric current rather than light. The energy produced is not liable to be diminished by tissue diffraction or absorption by epidermal melanin. As such, RF-based systems are appropriate for any skin type.12 Monopolar RF therapy delivers uniform heat at controlled depth to dermal layers, causing direct collagen contraction and immediate skin tightening.8,13 Subsequent remodeling and reorientation of collagen bundles and the formation of new collagen is achieved over months after treatment.14 The purpose of the current study was to evaluate the effects of, and objectively quantify the histologic facial skin responses to, the monopolar RF device as a nonablative treatment of photoaging, and to assess whether multiple treatments would improve clinical outcome.
METHODS
Study population
This study was conducted on a cohort of 6 female volunteers who desired an improvement in the appearance of facial skin laxity and wrinkles. The individuals, ranging in age from 47 to 62 years with an average of 51.1 ± 5.5 years, were recruited from the dermatology outpatient clinic of Al-Minya University Hospital, Al-Minya, Egypt. Treatment and study details were fully explained to subjects, and all signed an informed consent form. The volunteers were Fitzpatrick skin type III to IV, with class I to II wrinkles based on the Glogau scale.15 Inclusion criteria included bilateral facial changes caused by sun damage. Exclusion criteria were pregnancy or nursing, photosensitivity to sunlight, any sign of infection or inflammatory skin disease, history of hypertrophic scars or keloids, use of oral isotretinoin in the past 12 months, and previous skin rejuvenation procedures in the facial area.
Device and techniques
We used a monopolar RF skin-tightening device (Biorad, Shenzhen GSD Tech Co, Guangdong, China) consisting of RF generator, computerized automatic resistance test technology, a continuous cooling system, and a 3-cm2 tip. The RF generator produces a 6-MHz alternating current that creates an electric field through the skin, and allows for the heating of tissues through their resistance to the flow of electrical current. The physical properties, including frequency generator, frequency of electrical field polarity, and energy output, between the ThermaCool instrument (Solta Medical Inc, Hayward, CA) and our RF instrument are identical. Both instruments use capacitive coupling rather than conductive coupling to deliver the therapeutic energy. Conductive coupling is based on energy concentrated at the tip of an electrode, resulting in accumulation of produced heat at the skin surface in contact with the electrode, which can result in epidermal injury. Capacitive coupling creates a zone of increased temperature through dispersing energy across the skin surface.10,12
Briefly, a topical anesthetic cream (lidocaine 5%) was applied to the treatment area as a thick coating and left for 90 minutes under occlusion, then the cream was gently removed, and the patient was positioned for treatment. A conductive coupling fluid was applied to the treatment site to ensure uniform energy conduction, and enhance the thermal and electrical contact between the treatment tip and the skin. Two initial passes of 150 J each were performed over the entire face to allow uniform contraction of the collagen. We made 3 or more additional passes of 200 J each on the periorbital, nasolabial, and forehead areas. For each session, the total number of passes per treatment area consisted of the two initial passes over the entire face, followed by 3 to 6 passes targeted to treatment regions (total of 5–8 passes/treatment region). These data are described in Table I. Any overlap of pulses was avoided to allow appropriate cooling of the skin for at least 3 minutes between the passes. During each session, we monitored the volunteers for discomfort and intolerable hotness; none of them experienced any signs of edema or heat discomfort.
Table I.
Number of passes for each volunteer per treatment area per session
| Treated area (200 J) |
||||||
|---|---|---|---|---|---|---|
| Periorbital |
Nasolabial |
|||||
| n = 6 | Whole face (150 J) | R | L | R | L | Forehead |
| 5 | 2 | 3 | 3 | 3 | 3 | 3 |
| 1 | 2 | 3 | 3 | 3 | 3 | 6 |
L, Left; R, right.
Treatment regimen and follow-up
Volunteers were subjected to a total of 3 months of treatment (6 sessions at 2-week intervals). They were instructed to avoid the use of ice packs after each session. In addition, sun exposure was avoided using sunscreens to promote the healing response within the dermis and enhance collagen formation. Photographs were taken before and immediately after each session, and at 3 months posttreatment. Punch biopsy specimens (3 mm) were obtained from facial skin at baseline, end of treatment, and 3 months posttreatment. Biopsy specimens after treatment were taken from a site near the pretreatment biopsies.
Histologic staining and measurements
Tissues were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 5 µm-thick sections. All histologic and immunohistochemical staining, evaluation, and studies were carried out in the Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, Philadelphia, PA. Standard hematoxylin-eosin, Verhoeff-van Gieson (elastic fibers), and picrosirius red staining (Direct Red 80, Sigma, St. Louis, MO) (collagen) were performed. The epidermal thickness was measured between the top (from the upper part of granular cell layer) to the bottom (dermoepidermal junction) of the rete ridges. Five measurements were calculated for each tissue using a computerized software analyzer. Picrosirius red was evaluated using a microscope (Nikon, Melville, NY) equipped with filters to provide circularly polarized illumination. Immunohistochemical and picrosirius red staining was quantified using computer-based software (Image-Pro Plus, Media Cybernetics Inc, Silver Spring, MD).
Immunohistochemical staining
Immunohistochemistry was performed for total elastin and collagen types I and III. Briefly, formalin-fixed, paraffin-embedded tissue slides were heated at 60°C for 30 to 60 minutes. Tissues were then deparaffinized in 100% xylene (5 minutes; 3 times), 100% ethanol (5 minutes; 2 times), 95% ethanol (5 minutes; 2 times), 75% ethanol (2 minutes), 50% ethanol (2 minutes), and distilled water (H2O) (2 minutes). Antigen retrieval was performed by microwave method in 0.1 mol/L sodium citrate (pH 6.0) for 5 minutes. To quench endogenous peroxidase activity, tissues were incubated with 3% hydrogen peroxide in deionized water for 10 minutes at room temperature (RT). Endogenous biotin activity was blocked using an avidin/biotin blocking kit (SP-2001, Vector Laboratories, Burlingame, CA). Sections were then blocked for 60 minutes at RT in blocking buffer (5% normal goat serum, 1% bovine serum albumin [BSA], and 0.02% triton-x-100 [TX-100] in phosphate buffered saline [PBS]). Tissues were incubated with antibodies to elastin (1:300; E4013; Sigma), type I collagen (1:400; sc-59772; Santa Cruz Biotechnology, Santa Cruz, CA), and type III collagen (1:600; ab6310; Abcam, Cambridge, MA) overnight at 4°C. After a 30-minute wash in PBS, tissues were incubated with biotinylated secondary antibody (1:200; PK-6102; Vector Laboratories) for 60 minutes at RT, followed by incubation with ABC reagent (Vectastain Elite ABC peroxidase kits mouse; PK-6102; Vector Laboratories) for 30 minutes at RT. Sections were stained with DAB chromogen substrate kit (K3468; Dako, Real Carpinteria, CA) for 2 to 5 minutes, and then counterstained with hematoxylin (7211; Thermo Fisher Scientific, Waltham, MA). Slides were mounted with Permount (sp15–100; Thermo Fisher Scientific) for viewing using a microscope (Eclipse TE2000-U, Nikon). Digital images were collected using Evolution MP camera (Media Cybernetics Inc).
Statistical analysis
Histologic measurements and quantitative evaluation were analyzed using the software package for statistical science (SPSS for Windows, Version 16, SPSS Inc, Chicago, IL). Statistical analysis was performed using one-way analysis of variance, Wilcoxon-matched pairs signed ranks, and χ2 tests. Data were expressed as mean value ± SD. Statistical significance was defined as P less than or equal to .05.
RESULTS
Clinical evaluation
All 6 volunteers completed the monopolar RF study, and showed clear clinical improvement of skin tightening and rhytides in the periorbital and forehead regions (Fig 1, A). At each end point (before, at the end of, and 3 months after treatment), the volunteers, two doctors, and two independent observers were asked to evaluate the following criteria: improvement of rhytides, skin tightening and texture, and overall volunteer satisfaction. Their evaluations were assessed on a 5-point scale (none = 0%, mild = 1–25%, moderate = 26–50%, good = 51– 75%, and very good = 76–100%). Results obtained were tabulated and compared with baseline for statistical significance with the Pearson χ2 test. The volunteers’ evaluation rates are demonstrated in Fig 1, B. At the end of treatment, subjects showed 35% to 40% improvement in skin tightening (P = .02), 30% to 35% improvement in skin texture (P = .04), 40% to 45% improvement in rhytides (P = .01), and 85% to 90% volunteer satisfaction (P = .001). Three months posttreatment, significant differences were noticed among subjects as they showed 70% to 75% improvements in skin tightening (P = .001), 65% to 70% improvement in skin texture (P = .002), 90% to 95% improvement in rhytides (P = .0001), and volunteer satisfaction increased to 90% to 95% (P = .0001). Regarding doctor and observer assessment rates, data obtained were comparable with volunteers’ evaluation rates. The χ2 test demonstrated statistically significant changes in differences within each criterion compared with baseline. In addition, potential side effects, including erythema, edema, and hypopigmentation or hyperpigmentation were evaluated on a 4-point scale (none, mild, moderate, and severe). Only one volunteer developed slight erythema and mild transient hyperpigmentation 2 days after the fourth session, which subsided 5 days later (Fig 1, C). No scarring was observed.
Fig 1.
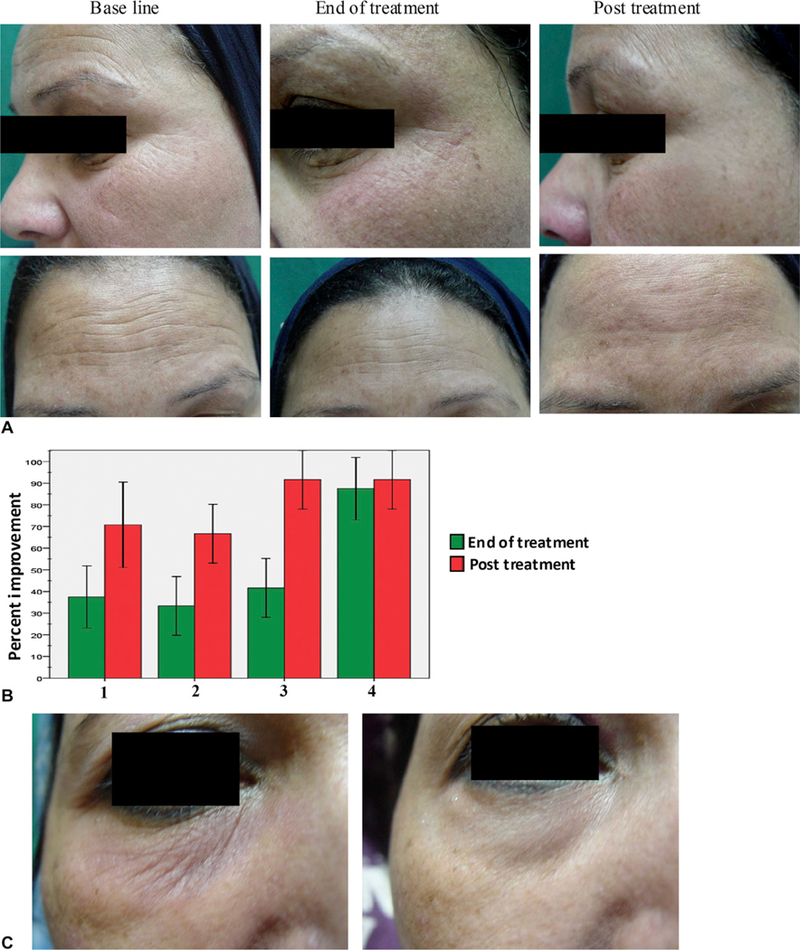
Clinical evaluation of volunteers in response to monopolar radiofrequency treatment. A, Representative photographs of periorbital and forehead areas at baseline, end of treatment, and 3 months posttreatment. B, Volunteers’ evaluation rates showed mean percent improvement of skin tightening (lane 1), skin texture (lane 2), rhytides (lane 3), and overall satisfaction (lane 4) at end of treatment (green) and 3 months posttreatment (red) relative to baseline. C, One volunteer developed slight erythema and mild transient hyperpigmentation 2 days after fourth session (left), which subsided 5 days later (right).
Histologic evaluation showing epidermal changes
Microscopic examination of hematoxylin-eosine stained sections showed epidermal hyperplasia at the end of treatment, which continued to increase 3 months after treatment (Fig 2). The results showed a significant increase in the mean of epidermal thickness from 62.7 ± 2.4 µm before treatment to 67 ± 3.9 µm at the end of treatment (P = .044), followed by a significant increase to 79.5 ± 9.8 µm at 3 months posttreatment (P = .002) (Table II). This was associated with overall morphologic and architectural improvement of the epidermis with development of rete ridges (marked undulations of the dermoepidermal junction). Finally, we observed an increase in granular layer thickness from 6.4 ± 1.1 µm before treatment to 9.9 ± 1.5 µm at the end of treatment and 17.7 ± 3.1 µm at 3 months posttreatment (P = .001 and .0001, respectively) (Table II and Fig 2). This may have resulted from increase in the number and size of the cells in the granular layer.
Fig 2.
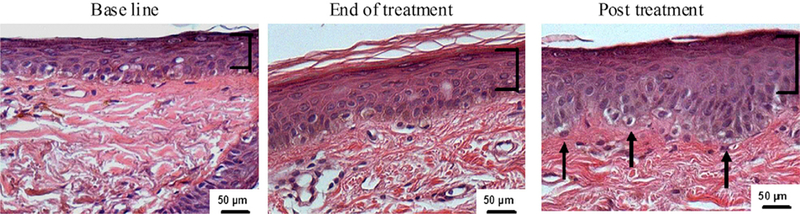
Radiofrequency treatment enhances epidermal hyperplasia. Skin biopsy specimens were formalin fixed and paraffin embedded. Tissue sections were stained with hematoxylineosin, showing increased thickness of epidermis and granular cell layer (brackets) associated with development of rete ridges (arrows) after RF treatment.
Table II.
Histometric analysis of epidermal and granular cell layer thickness
| Thickness, μm* |
Statistical significance |
|||||
|---|---|---|---|---|---|---|
| Baseline | End of treatment | 3 mo Posttreatment | Baseline vs end of treatment | End of treatment vs 3 mo posttreatment | Baseline vs 3 mo posttreatment | |
| Epidermis | 62.7 ± 2.4 | 67 ± 3.9 | 79.5 ± 9.8 | .044† | .016† | .002† |
| Granular cell layer | 6.4 ± 1.1 | 9.9 ± 1.5 | 17.7 ± 3.1 | .001† | .0001† | .0001† |
Mean ± SD; n = 6.
P ≤ .05.
Quantitation of elastin amount in dermis
In photodamaged skin, the level of the connective tissue protein elastin increases, and abnormally accumulates under the epidermis, forming so-called elastotic material. Next, we examined the effects of RF treatment on total dermal elastin by immunohistochemical staining. We observed a slight decrease in elastin level after treatment compared with baseline, which became more pronounced 3 months after treatment (Fig 3, A). This decline in elastin content was associated with translocation of the solar elastotic material away from the epidermis, accompanied by the restoration of normal-appearing elastic fibers within the papillary and upper reticular dermis. These results were confirmed when we assessed the percent area of dermis occupied by elastin using computerized morphometric analysis (Fig 3, B). We detected a slight, but statistically insignificant decrease in elastin staining after RF treatment (49.9 ± 5.3%) compared with baseline (53.7 ± 7.4%) (Fig 3, B). However, a statistically significant decrease in total elastin was observed 3 months after treatment (42.2 ± 3.6%; P = .007).
Fig 3.
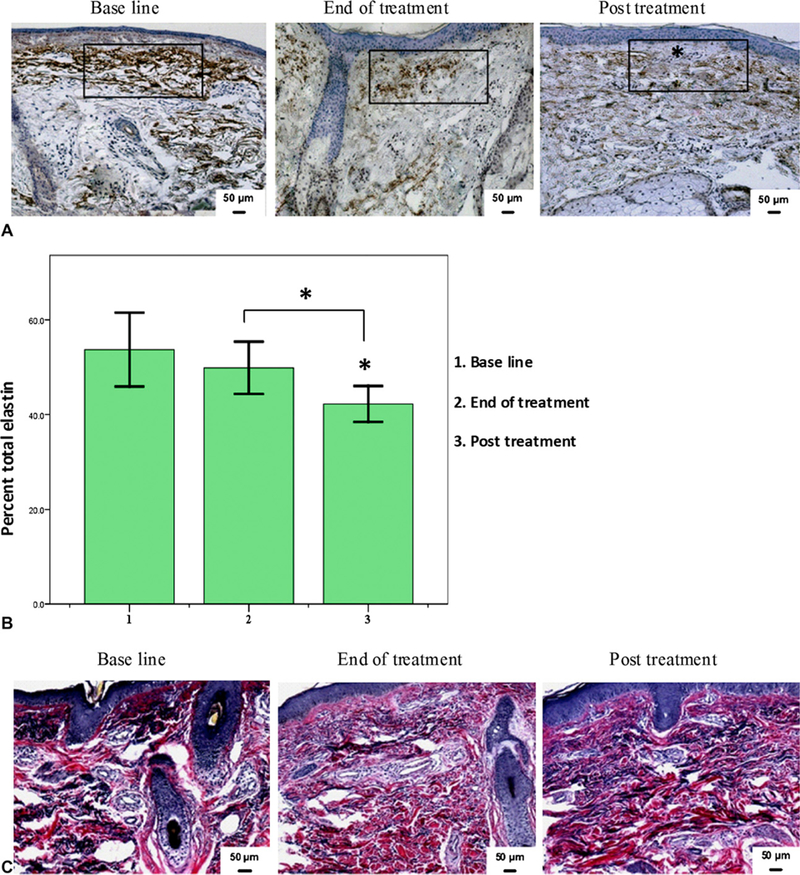
Volunteers treated with monopolar radiofrequency showed decrease in total dermal elastin. A, Skin tissues at baseline, end of treatment, and after RF treatment were immunostained for total elastin. Representative samples show decrease in elastin level. Area (rectangle) was used to assess elastin staining level. *Grenz zone. B, Percent of dermis occupied by elastin showing significant decrease in total elastotic material after treatment. *P ≤ .05. C, Verhoeff-van Gieson special stain showing similar decrease in elastic fibers after RF treatment.
These changes in elastin content were confirmed by Verhoeff-van Gieson special stain (Fig 3, C). This stain is useful in differentiating elastic tissue (blue black to black) from collagen (red). The elastic fibers (shown in black) are objectively decreased in content after treatment with restoration of normal-appearing elastic fibers within the papillary and reticular dermis.
Evaluation of collagen changes in dermis
Evaluation of immunohistochemical staining for total collagen (Fig 4, A, top row) revealed a narrow collagen band (grenz zone, 9.8 ± 3 µm) at the dermoepidermal junction in volunteers before treatment. This band of collagen increased slightly to 11 ± 3.6 µm at the end of treatment (P = .573). Three months posttreatment, staining of skin biopsy specimens revealed a significant increase in the thickness of the collagen band to 15.6 ± 2.3 µm (P = .004). Quantitative assessment of the percentage of dermis-positive collagen showed significant increase in content of type I collagen (Fig 4, A, middle row) from 65.8 ± 4.7% before treatment to 72.2 ± 4.3% at the end of treatment (P = .034) and 81.2 ± 4.5% at 3 months posttreatment (P = .0001). Finally, assessment of collagen type III revealed a significant increase from 60.9 ± 2.5% at the baseline to 66.5 ± 4.4% at the end of treatment and to 73.6 ± 4.8% at 3 months posttreatment (P = .028 and .0001, respectively) (Table III and Fig 4, B). In summary, our data show that enhancement of collagen expression continued to increase 3 months after RF treatment.
Fig 4.
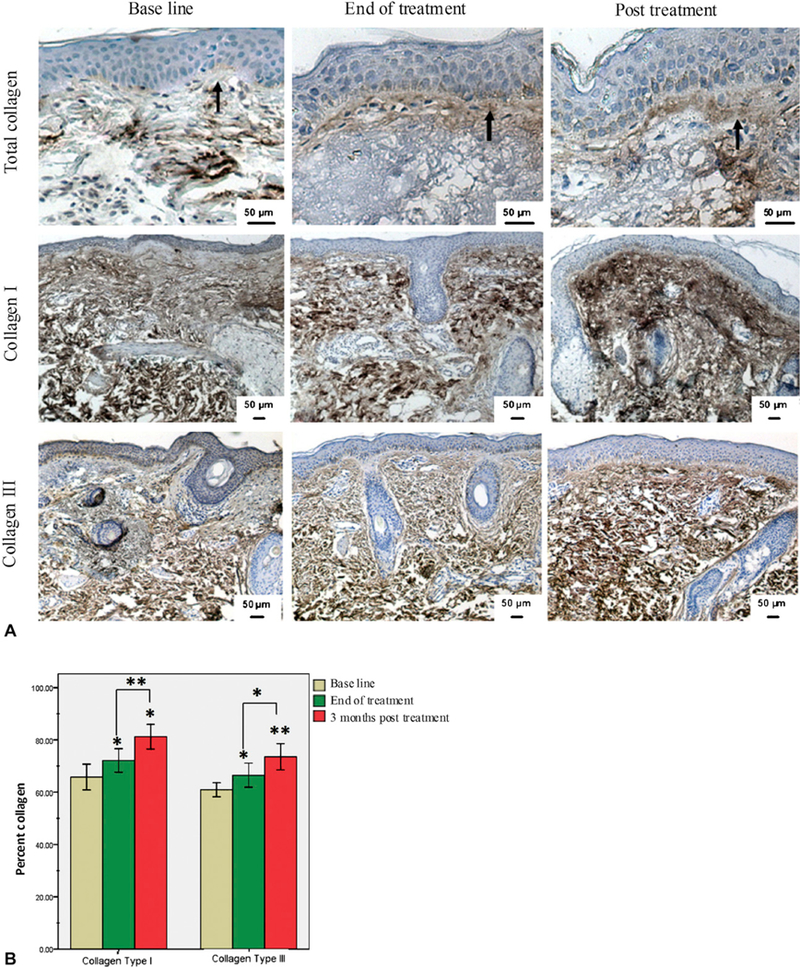
Increase in dermal collagen content in response to radiofrequency. A, Immunohisto-chemical staining of skin tissues at baseline (left), end of treatment (middle), and after RF treatment (right) for total collagen (top) and collagen types I (middle) and III (bottom). Increase in collagen band thickness at dermoepidermal junction was observed after RF treatment compared with baseline (arrows). B, Collagen level was measured and values presented as percentage of dermis-positive collagen. Data showed statistically significant increase in both collagen I and III in response to RF. *P ≤ .05; **P ≤ .001.
Table III.
Quantitative analysis of total elastin and collagen (newly synthesized and types I and III) at the 3 time points
| Relative content, %* |
Statistical significance |
|||||
|---|---|---|---|---|---|---|
| Baseline | End of treatment | 3 mo Posttreatment | Baseline vs end of treatment | End of treatment vs 3 mo posttreatment | Baseline vs 3 mo posttreatment | |
| Total elastin | 53.7 ± 7.4 | 49.9 ± 5.3 | 42.2 ± 3.6 | .324 | .015† | .007† |
| Newly synthesized collagen | 15.3 ± 4.3 | 21.7 ± 3.1 | 26.9 ± 3.7 | .014† | .024† | .001† |
| Total collagen type I | 65.8 ± 4.7 | 72.2 ± 4.3 | 81.2 ± 4.5 | .034† | .005† | .0001† |
| Total collagen type III | 60.9 ± 2.5 | 66.5 ± 4.4 | 73.6 ± 4.8 | .028† | .023† | .0001† |
Mean 6 SD; n = 6.
P ≤ .05.
As collagen matures, the optical properties of the fibers show signs of an increase in birefringence (the ability to change color under polarized light) with consequent decrease in light penetration. When tissues are stained with picrosirius red and viewed under polarized microscope, large collagen fibers stain red while the thinner ones, which represent the newly synthesized fibers, are stained yellow to orange.16,17 To assess whether the increase in collagen level observed by immunohistochemistry was a result of increase in newly synthesized collagen formation, we stained the tissues with picrosirius red. The results showed an increase in the newly synthesized collagen formation, as reflected by the presence of yellow-orange birefringence, which was significantly increased from 15.3 ± 4.3% at baseline to 21.7 ± 3.1% and 26.9 ± 3.7% (P = .014 and .001) at the end of treatment and 3 months posttreatment, respectively (Table III and Fig 5).
Fig 5.
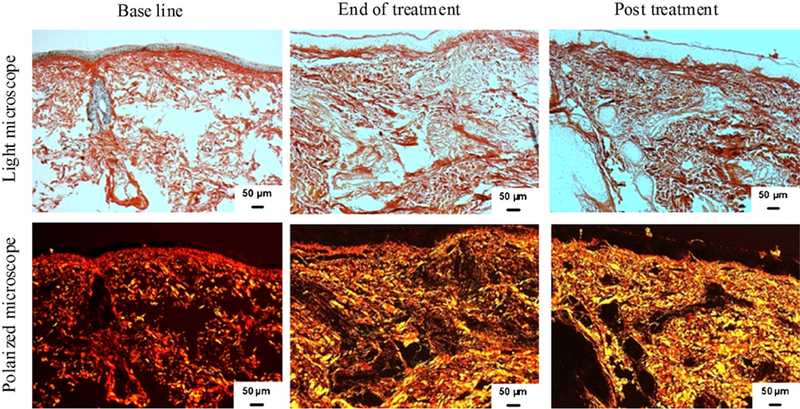
Increase in newly synthesized collagen content in response to radiofrequency treatment. Representative examples of skin tissues stained with picrosirius red viewed under bright field (top) and polarized field (bottom). Bright field captures total collagen content. Polarized light showed yellow to orange birefringence reflecting newly synthesized collagen in yellow and total collagen in red. Note increase in newly synthesized collagen as reflected by yellow after RF treatment.
DISCUSSION
Facial rejuvenation is a developing art, and a science. For a long time, the treatment of photoaged skin and the reversal of the signs of aging were focused on ablative laser resurfacing techniques, as they yield impressive results.18 Recently, the possibility of complications, prolonged recovery time, and avoidance of sun exposure essential to sustain optimal results were reasons to decrease the attractiveness of ablative resurfacing.19 There is now an increased interest in a wide range of nonablative treatments of skin aging, which are used to rejuvenate skin with minimal downtime and complications.7 The basic issue with all studies on nonablative rejuvenation relates to the methodology, as there are few standard and objective approaches to the depth of wrinkles, and the elasticity of skin studies. The clinical results are eventually dependent on the subjective observations of physicians, volunteers, or both. Photodocumentation has also been shown to be an insufficient way of representing the quality and efficacy of treatment.20–22 In this study, we aimed to improve the subjective evaluation in the context of objective means of evaluating the effects of monopolar RF on skin tightening and appearance. This was accomplished with histochemical and immunostaining techniques, and histometric evaluation of skin at the baseline, end of treatment, and 3 months posttreatment. Monopolar RF was approved by the Food and Drug Administration in 2002 for the nonablative treatment of wrinkles and skin tightening, and for full-face treatment in 2004.23,24 Many studies reported that RF is best suited for patients with early signs of aging, with mild to moderate wrinkles.14,25–27 So, the focus of this study was on subjects with relatively mild to moderate degree of photoaging (Glogau I-II). In our study, evaluation of subjects’ clinical results showed noticeable improvement at the end of treatment; with continued improvement 3 months posttreatment. Improvements in skin tightening increased from 35% to 40% at the end of treatment to 70% to 75% at 3 months posttreatment. Appearance of facial rhytides was improved from 40% to 45% at the end of treatment to 90% to 95% at 3 months posttreatment. These mechanical properties of the skin can also be objectively measured using different clinical methods based on two main principles: (1) force is applied, and the decrease in the force generated by this distortion is calculated based on time; and (2) in contrast, a twist is applied to the skin, and the recoil time is measured.28 Furthermore, additional methods to assess skin elasticity and recoil include suction chamber method, twistometry, levarometry, indentometry, gas-bearing electrodynamo-meter, video-microscopy, skin chip technology, and ballistometry.28–30 In previous studies of the RF device, authors gave subjects a single RF treatment, and evaluated the results. Ruiz-Esparza and Gomez29 found that 14 of 15 volunteers had up to 50% improvement in skin tightening, and in nasolabial fold and periorbital wrinkles, 3 months after the treatment session with single treatment and multiple passes. However, two previous studies have shown that multiple treatments with multiple passes could give improved results.30,31 Jacobson et al30 treated 24 adult patients with the ThermaCool system (Solta Medical Inc). The subjects received treatments every 1 to 3 months. The investigators did not specify how many total treatments were applied in their study, yet they stated that, “for patients who received more than a single treatment, it appeared that subsequent treatment sessions further improved their laxity.” In a more recent study, Sukal and Geronemus31 treated patients with two passes on the forehead, 3 on the cheeks, and one on the neck; each patient received 1 to 3 treatments spaced 4 weeks apart. The authors reported that patients had visible improvement at 1-month follow-up and even greater improvement at the 3-month follow-up evaluation.
Initial studies revealed that after a single treatment, patients showed better results with multiple passes as compared with a single pass.30 Furthermore, the authors demonstratedthatmultiplesessionsimproved results over a single session. Further review of the literature indicates similar findings.14,26,30,31
The in vivo response to thermal wound healing consists of 3 consecutive stages: inflammatory, proliferative, and remodeling.3 This might explain why clinically visible results were only achieved between 3 and 6 months after the start of treatment. Photoaged skin is associated with a decrease in epidermal thickness with flattening of the rete ridges.32 Although observed RF energy targets the dermal layer, in this study we observed striking changes in histologic features of the epidermis that need further studies to be explained; we showed a noticeable increase in epidermal thickness at the end of treatment, and at 3 months posttreatment, especially in the granular cell layer (Fig 2). These findings suggest that proliferation of cells in the epidermis is increased, and perhaps may contribute to the improvement of skin appearance.
Unlike most lasers that target specific chromophores, the output energy of the monopolar RF is chromophore independent; it is transformed into heat mainly by water within the tissues. As a result, the energy is delivered to 3-dimensional levels of the dermis.10 The depth of thermal injury is limited to 100 to 400 µm below the epidermis, the area where most elastotic material is histologically seen.33 Microscopic changes associated with wrinkles occur primarily in the dermis. In sun-damaged skin, the main dermal alteration is the deposition of large clumps of abnormal elastotic material, replacing the normally collagen-rich dermis.34 In this study, we evaluated the changes induced by RF on total elastin, as it is one of the major changes occurring in aged skin. Our results showed an insignificant change in total elastin content at the end of treatment, followed by significantly decreased elastic tissue 3 months posttreatment. This decrease was accompanied by downward placement and subsidence of the elastotic materials with reorientation of the elastic fibers. Improvement of the quality of elastic fibers and solar elastosis can be explained by the effect of RF on collagen formation and newly synthesized collagen, which replaces the elastotic materials with the redirection of dermal matrix fibers. The reorientation of elastic fibers may reflect the synthesis of new elastic fibers with proper assembly.
It is speculated that heat generated by RF affects the molecular structure of the triple helix of the collagen molecule, with subsequent breakage of intramolecular hydrogen bonds, resulting in collagen fibril denaturation with immediate contraction.8 Over time, as a thermally mediated healing response, fibroblasts are stimulated to enhance new collagen deposition and remodeling, resulting in further collagen tightening, and an overall increase in collagen content.11 Bassichis et al35 have revealed an additional potential mechanism of action for monopolar RF; the subcutaneous fat lobules are separated by an interlacing network of collagen-based fibrous septa. As RF energy usually follows the path of least resistance, fibrous septa are preferentially heated, resulting in the contraction of collagen fibers,35 which is thought to be the key in subsequent remodeling of subcutaneous tissue and tightening of the skin, which becomes attached to the underlying structures.36
In our study, we assessed the effect of RF on collagen content and formation, starting with the evaluation of collagen presentation under the epidermis. We found a slight increase in the narrow collagen band grenz zone thickness present at the dermoepidermal junction, followed by a significant increase in thickness 3 months posttreatment. Normal dermal collagen fibers account for approximately 80% of its dry weight, and are responsible for its tensile properties. Dermal collagen is primarily composed of type I (80%–85%) and type III (10%–15%) collagen.5,34 Wrinkle reduction, by means of thermal heat delivered to the dermis, is based on the stimulation of new collagen formation, and in this study, quantitative evaluation of dermal collagen revealed a significant increase in both type I and III collagens at the end of treatment. These findings are in agreement with previous studies demonstrating new formation of type I and III collagens after RF treatment.37–39 However, our study showed a continued significant increase in type I and III collagens 3 months posttreatment. We further assessed the effect of RF on new collagen formation, and whether the increase in collagen level as observed by immunohistochemistry was a result of the enhancement of newly synthesized collagen formation. Detection of newly synthesized collagen with picrosirius red under polarized microscopic examination showed significantly increased newly synthesized collagen at the end of treatment, and at 3 months posttreatment, compared with baseline, reflecting the positive response obtained by RF on both total collagen and new collagen formation.
In spite of the protective mechanisms provided with the RF tip, one volunteer developed slight erythema and mild transient hyperpigmentation 2 days after the fourth session. This complication might have occurred as a result of uneven contact of the treatment tip with skin surface, resulting in an accumulation of RF energy in a single treatment area. This complication subsided without relapse. One obvious limitation to our study is the relatively small number of volunteers. Nevertheless, the results showed evidence of clinical and histologic improvement after RF treatment. Although previous publications have suggested improvement after RF with skin changes, including face tightening, few have histologically analyzed the skin of the volunteers treated.8,34,40,41
In conclusion, monopolar RF is an effective and valuable procedure that can be used to tighten and rejuvenate photoaged skin, and contour facial skin laxity. This modality stimulates the repair process, and reverses the clinical, and the histopathological, signs of aging, with the advantage of relatively risk-free procedure and with little downtime.
CAPSULE SUMMARY.
Monopolar radiofrequency is a valuable procedure that can be used to effectively tighten and rejuvenate photoaged skin with little downtime.
Tightening appears to continue for 3 months after the end of radiofrequency treatment.
Radiofrequency showed long-term effects by enhancing collagen synthesis and content.
Acknowledgments
Supported by the Cultural and Educational Bureau of the Republic of Egypt and Egyptian Scholar Program (Dr Medhat); and National Institutes of Health R01 AR28450 (Dr Uitto).
The authors thank Carol Kelly and Alicia Dowling for their help in the preparation of this article.
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol 2002;11:398–405. [DOI] [PubMed] [Google Scholar]
- 2.Kligman LH, Kligman AM. The nature of photoaging: its prevention and repair. Photodermatol 1986;3:215–27. [PubMed] [Google Scholar]
- 3.Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs 2008;20:177–83. [PubMed] [Google Scholar]
- 4.Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging: age-associated connective tissue alterations in the dermis. J Am Acad Dermatol 1989;21:614–22. [PubMed] [Google Scholar]
- 5.Uitto J The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol 2008; 7:s12–6. [PubMed] [Google Scholar]
- 6.El-Domyati M, Attia S, Saleh F, Ahmad H, Uitto J. Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J Cosmet Dermatol 2004;3:191–201. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Geronemus RG. Nonablative laser and light therapies for skin rejuvenation. Arch Facial Plast Surg 2004;6: 398–409. [DOI] [PubMed] [Google Scholar]
- 8.Zelickson BD, Kist D, Bernstein E, Brown DB, Ksenzenko S, Burns J, et al. Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: a pilot study. Arch Dermatol 2004;140: 204–9. [DOI] [PubMed] [Google Scholar]
- 9.Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol 2008;58: 719–37. [DOI] [PubMed] [Google Scholar]
- 10.Atiyeh BS, Dibo SA. Nonsurgical nonablative treatment of aging skin: radiofrequency technologies between aggressive marketing and evidence-based efficacy. Aesthetic Plast Surg 2009;33:283–94. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Esparza J Nonablative radiofrequency for facial and neck rejuvenation. A faster, safer, and less painful procedure based on concentrating the heat in key areas: the ThermaLift concept. J Cosmet Dermatol 2006;5:68–75. [DOI] [PubMed] [Google Scholar]
- 12.Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol 2007;25:487–91. [DOI] [PubMed] [Google Scholar]
- 13.Kist D, Burns AJ, Sanner R, Counters J, Zelickson B. Ultrastructural evaluation of multiple pass low energy versus single pass high energy radio-frequency treatment. Lasers Surg Med 2006; 38:150–4. [DOI] [PubMed] [Google Scholar]
- 14.Bogle MA, Ubelhoer N, Weiss RA, Mayoral F, Kaminer MS. Evaluation of the multiple pass, low fluence algorithm for radiofrequency tightening of the lower face. Lasers Surg Med 2007;39:210–7. [DOI] [PubMed] [Google Scholar]
- 15.Glogau RG, Matarasso SL. Chemical peels: trichloroacetic acid and phenol. Dermatol Clin 1995;13:263–76. [PubMed] [Google Scholar]
- 16.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 2005;22:97–104. [Google Scholar]
- 17.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 1994; 89:397–410. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch RJ, Dayan SH. Nonablative resurfacing. Facial Plast Surg 2004;20:57–61. [DOI] [PubMed] [Google Scholar]
- 19.Carruthers J, Carruthers A. Shrinking upper and lower eyelid skin with a novel radiofrequency tip. Dermatol Surg 2007;33: 802–9. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg DJ, Rogachefsky AS, Silapunt S. Non-ablative laser treatment of facial rhytides: a comparison of 1450 diode laser treatment with dynamic cooling device as opposed to treatment with dynamic cooling alone. Lasers Surg Med 2002;30:79–81. [DOI] [PubMed] [Google Scholar]
- 21.Fournier N, Dahan S, Barneon G, Diridollou S, Lagarde JM, Gall Y, et al. Nonablative remodeling: clinical, histologic, ultrasound imaging, and profilometric evaluation of a 1540 nm Er:glass laser. Dermatol Surg 2001;27:799–806. [DOI] [PubMed] [Google Scholar]
- 22.Grema H, Greve B, Raulin C. Facial rhytidesesubsurfacing or resurfacing? A review. Lasers Surg Med 2003;32:405–12. [DOI] [PubMed] [Google Scholar]
- 23.De Felipe I, Del Cueto SR, Perez E, Redondo P. Adverse reactions after nonablative radiofrequency: follow-up of 290 patients. J Cosmet Dermatol 2007;6:163–6. [DOI] [PubMed] [Google Scholar]
- 24.Dover JS, Zelickson B, Burns J, Hughes C, Hugo B, Chan H, et al. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low-energy multiple-pass technique leading to a clinical end point algorithm. Dermatol Surg 2007;33:900–7. [DOI] [PubMed] [Google Scholar]
- 25.Alster TS, Tanzi E. Improvement of neck and cheek laxity with a nonablative radiofrequency device: a lifting experience. Dermatol Surg 2004;30:503–7. [DOI] [PubMed] [Google Scholar]
- 26.Abraham MT, Vic Ross E. Current concepts in nonablative radiofrequency rejuvenation of the lower face and neck. Facial Plast Surg 2005;21:65–73. [DOI] [PubMed] [Google Scholar]
- 27.Fisher GH, Jacobson LG, Bernstein LJ, Kim KH, Geronemus RG. Nonablative radiofrequency treatment of facial laxity. Dermatol Surg 2005;31:1237–41. [DOI] [PubMed] [Google Scholar]
- 28.Léveˆque JL. Quantitative assessment of skin aging. Clin Geriatr Med 2001;17:673–89. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Esparza J, Gomez JB. The medical face lift: a noninvasive, nonsurgical approach to tissue tightening in facial skin using nonablative radiofrequency. Dermatol Surg 2003;29:325–32. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson LG, Alexiades-Armenakas M, Bernstein L, Geronemus RG. Treatment of nasolabial folds and jowls with a noninvasive radiofrequency device. Arch Dermatol 2003;139:1371–2. [DOI] [PubMed] [Google Scholar]
- 31.Sukal SA, Geronemus RG. Thermage: the nonablative radio-frequency for rejuvenation. Clin Dermatol 2008;26:602–7. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Esparza J, Gomez JB. Nonablative radiofrequency for active acne vulgaris: the use of deep dermal heat in the treatment of moderate to severe active acne vulgaris (thermo-therapy); a report of 22 patients. Dermatol Surg 2003;29:333–9. [DOI] [PubMed] [Google Scholar]
- 33.Dierickx CC. The role of deep heating for noninvasive skin rejuvenation. Lasers Surg Med 2006;38:799–807. [DOI] [PubMed] [Google Scholar]
- 34.El-Domyati M, Attia S, Saleh F, Ahmad H, Uitto J. Trichloroacetic acid peeling versus dermabrasion: a histometric, immunohistochemical, and ultrastructural comparison. Dermatol Surg 2004;30:179–88. [DOI] [PubMed] [Google Scholar]
- 35.Bassichis BA, Dayan S, Thomas JR. Use of a nonablative radiofrequency device to rejuvenate the upper one-third of the face. Otolaryngol Head Neck Surg 2004;130:397–406. [DOI] [PubMed] [Google Scholar]
- 36.Taylor MB, Prokopenko I. Split-face comparison of radiofrequency versus long-pulse Nd-YAG treatment of facial laxity. J Cosmet Laser Ther 2006;8:17–22. [DOI] [PubMed] [Google Scholar]
- 37.Sarradet MD, Hussain M, Goldberg DJ. Electrosurgical resurfacing: a clinical, histologic, and electron microscopic evaluation. Lasers Surg Med 2003;32:111–4. [DOI] [PubMed] [Google Scholar]
- 38.England LJ, Tan MH, Shumaker PR, Egbert BM, Pittelko K, Orentreich D, et al. Effects of monopolar radiofrequency treatment over soft-tissue fillers in an animal model. Lasers Surg Med 2005;37:356–65. [DOI] [PubMed] [Google Scholar]
- 39.Fenelon G, Franco M, Mora O, Katchburian E, De Paola AA. Combined therapy with steroids and antioxidants prevents ultrastructural damage surrounding chronic radiofrequency lesions. Pacing Clin Electrophysiol 2004;27:65–72. [DOI] [PubMed] [Google Scholar]
- 40.Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J 2005;25:638–42. [DOI] [PubMed] [Google Scholar]
- 41.De Felipe I, Redondo P. Animal model to explain fat atrophy using nonablative radiofrequency. Dermatol Surg 2007;33: 141–5. [DOI] [PubMed] [Google Scholar]


