Abstract
Mutations of the LAMB3 gene cause a lethal form of junctional epidermolysis bullosa (JEB). We hypothesized that early intra-amniotic gene transfer in a severe murine model of JEB would improve or correct the skin phenotype. Time-dated fetuses from heterozygous LAMB3IAP breeding pairs underwent ultrasound guided intra-amniotic injection of lentiviral vector encoding the murine LAMB3 gene at embryonic day 8 (E8). Gene expression was monitored by immunohistochemistry. The transgenic laminin-β3 chain was shown to assemble with its endogenous partner chains, resulting in detectable amounts of laminin-332 in the basement membrane zone of skin and mucosa. Ultrastructually, the restoration of ~60% of hemidesmosomal structures was also noted. Although we could correct the skin phenotype in 11.9% of homozygous LAMB3IAP mice, none survived beyond 48 h. However, skin transplants from treated E18 homozygous LAMB3IAP fetuses maintained normal appearance for 6 months with persistence of normal assembly of laminin-332. These results demonstrate for the first time long-term phenotypic correction of the skin pathology in a severe model of JEB by in vivo prenatal gene transfer. Although survival remained limited due to the limitations of this mouse model, this study supports the potential for treatment of JEB by prenatal gene transfer.
Keywords: prenatal gene therapy, lentiviral vector, epidermolysis bullosa
INTRODUCTION
Epidermolysis bullosa (EB) is a group of heritable blistering disorders caused by mutations in genes expressed in the cutaneous basement membrane zone (BMZ) that cause considerable morbidity and mortality. Recently, several molecular therapies are being investigated for the treatment of patients with different subtypes of EB, including ex vivo gene therapy,1 protein replacement2 and cellular therapy.3–7 Herlitz junctional epidermolysis bullosa (HJEB) is one of the most severe variants of EB characterized by extensive mucocutaneous blistering and erosions at birth leading to early lethality.8,9 HJEB, an autosomal recessive disease, is caused by mutations in any of the genes encoding the subunit polypeptides laminin-α3, laminin-β3 or laminin-χ2 of the heterotrimeric laminin-332 protein. Currently, there is no specific cure or effective treatment for HJEB. Based on the early onset of severe blister formation, HJEB is a compelling candidate for in vivo prenatal gene therapy.
Potential advantages of prenatal gene therapy include the small size of the fetus, high frequency and accessibility of stem cell populations, and fetal immunologic immaturity that may facilitate tolerance for transgene encoded novel proteins.10,11 The skin is potentially an ideal target for gene correction in utero. Because the fetus is surrounded with amniotic fluid, the entire fetal skin surface can theoretically be exposed to a vector with a single injection into the amniotic cavity. Although the majority of efforts to transduce skin by the intra-amniotic route have achieved efficient gene expression, the duration of expression has been limited to 3–4 weeks, corresponding to the time required for a complete epidermal turnover cycle consistent with failure to transduce the skin stem cell population.12–17
Effective gene therapy for skin disorders will require gene transfer to the skin stem cell populations to achieve long-term expression due to rapid cellular turnover inherent to the epidermis. There have been two types of stem cells identified in the epidermis: the basal cell and the bulge stem cell.18–22 Unfortunately, once the skin becomes stratified through epidermal differentiation, these stem cell populations are no longer accessible via the amniotic fluid. However, there is a stage during early embryonic development when the skin is comprised of only a single layer and all nascent skin stem cell populations are exposed to the amniotic fluid.23 Based on this opportunity, in our previous study we injected lentiviral vector encoding the murine LAMB3 gene under control of the human keratin 5 promoter into the amniotic cavity at embryonic day 8 or 9 (E8/9) of gestation in mouse embryos. We demonstrated efficient transfer of reporter gene to the skin stem cell populations with subsequent life-long expression of the transgene.24
In this study, we have applied the early gestational intra-amniotic gene transfer (IAGT) method to the murine model of HJEB, the homozygous LAMB3IAP mouse, which completely lacks laminin-332 manifest by severe perinatal blistering and demise in the perinatal period.25 Our results demonstrate for the first time long-term phenotypic correction of the skin pathology in a model of severe JEB by in vivo prenatal gene transfer. Although survival remained limited due to the limitations of this mouse model, this study supports the potential for clinical treatment of JEB by prenatal gene transfer.
RESULTS
Viability of fetuses following IAGT
A total of 566 fetal mice from 68 pregnant females derived from heterozygous LAMB3IAP timed matings were injected with lentiviral vector encoding laminin-β3 on embryonic day 8 (E8). We used an ultrasound guided injection system to visualize and inject into the amniotic space as previously described.26,27
To detect any improvement in survival of homozygous EB mice after IAGT, we analyzed a subset of 162 injected mice from 20 of the 68 pregnant females 48 h after birth (P2). Their survival rate was 24.7% (40/162) and genotyping of these mice revealed that 65.0% of these (26/40) were heterozygous and 35.0% (14/40) were wild type (WT). There were no surviving homozygous LAMB3IAP mice in this group, demonstrating that we had not improved survival in this model by IAGT beyond 48 h. To further assess the results of IAGT, and avoid the variables involved in neonatal survival in this mouse model, we decided to harvest the injected mice before birth. The subsequent 408 fetuses from 48 of the 68 pregnant females were analyzed before birth (on E18, 19 or 20). The total survival rate until harvest was 55.2% (223/408) and genotyping of these mice revealed that 50.2% (112/223) were heterozygous, 30.9% (69/223) were WTand 18.8% (42/223) were homozygous (Table 1).
Table 1.
Genotype and phenotype of mice undergoing E8 IAGT
| Total E8 IAGT dam (N=68) | Analyzed 48 h after birth (N=20) | Analyzed before birth (N=48) |
|---|---|---|
| E8 intra-amniotic injection | ||
| Pregnant pups | 162 | 408 |
| Injected pups | 162 (100) | 404 (99.0) |
| Survival pups | 40 (24.7) | 223 (55.2) |
| Genotype of survival pups | Total=40 | Total=223 |
| WT | 14 (35) | 69 (30.9) |
| Hetero | 26 (65) | 112 (50.2) |
| Null | 0 (0) | 42 (18.8) |
| Phenotype of null pups | Total=42 | |
| Null phenotype | 37 (88.1) | |
| WT phenotype | 5 (11.9) Po0.005a |
Abbreviations: IAGT, intra-amniotic gene transfer; WT, wild type.
P-value for skin phenotype comparison between E8 IAGT treated null pups (n=42) and non-treated null pups (n=89) by using the Fisher’s exact test.
Restoration of laminin-β3 and laminin-332 expression
To determine whether the LAMB3 gene was expressed in the BMZ of the treated homozygous LAMB3IAP mice, we performed immunofluorescent staining with monoclonal anti-laminin-β3 antibody on skin harvested at E18/19/20. As expected, untreated homozygous LAMB3IAP mice showed no immunoreactivity to anti-laminin-β3 antibody (Figure 1a). However, homozygous LAMB3IAP mice after treatment by E8 IAGT expressed laminin-β3 at the basement membrane in a linear pattern (Figure 1e).
Figure 1.
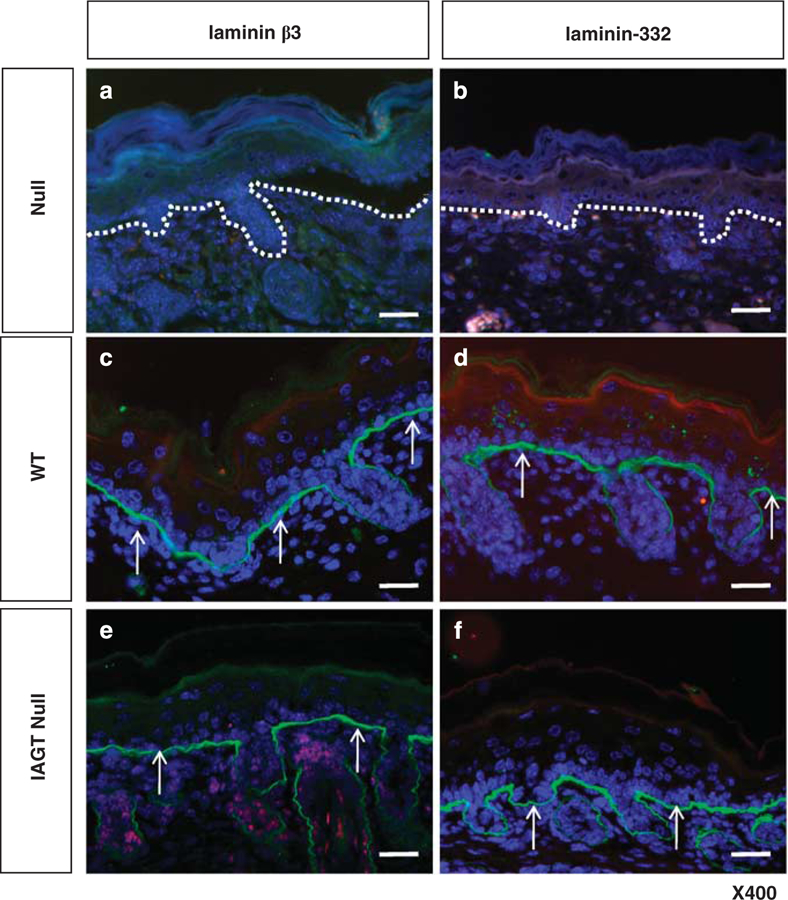
Immunofluorescence analysis of skin samples from untreated homozygous LAMB3IAP mice (defined as ‘null’) (a, b), WT mice (defined as ‘WT’) (c, d) and homozygous LAMB3IAP mice treated by E8 IAGT with lentiviral-LAMB3 (defined as ‘IAGT null’) (e, f). No expression of either laminin-β3 or laminin-332 is seen in the BMZ in untreated null mice. The white dotted lines indicate the position of the basal lamina. (a, b) WT mice are positive for laminin-β3 and laminin-332 staining in a linear pattern along the basement membrane. (c, d) Treated null mice stain positive for laminin-β3 and laminin-332 at the basement membrane in a similar pattern to WT mice arrows (arrows, e, f). The sections were counterstained with 4’,6-diamidino-2-phenylindole to visualize the cells (blue) (scale bar=100 µm).
Since the LAMB3 gene encodes one subunit of the heterotrimeric laminin-332 molecule, restoration of synthesis of the b3 subunit should restore assembly of functional laminin-332 molecules. To assess the restoration of the laminin-332 complex, we performed immunofluorescent staining with polyclonal anti-laminin-332 antibody on skin sections of E8 IAGT treated homozygous LAMB3IAP mice harvested at E18/19/20. Treated homozygous LAMB3IAP mice demonstrated expression of laminin-332 at the basement membrane in a linear pattern (Figure 1f).
Assessments of restoration of skin phenotype
To determine whether expression of the LAMB3 transgene and concomitant restoration of laminin-332 could rescue the skin phenotype, we performed phenotypic assessment by the mechanical skin disruption test (pinch test). In untreated homozygous LAMB3IAP mice, when we performed the pinch test, all null mice failed the test at the first attempt (n=89). We harvested a total of 223 E8 IAGT mice by cesarean section at E18/19/20. No fetal mice demonstrated evidence of skin blistering at delivery. We performed the pinch test and found that in 37 out of 223 fetal mice the skin was disrupted. On subsequent genotyping, 42 homozygous mice were identified. Of the 42 genotypically homozygous mice treated by E8 IAGT, five (11.9%) mice demonstrated phenotypic correction by this relatively rigorous test (Table 1). None of the genotypically WT or heterozygous mice had a positive pinch test. Statistical analyses (Fisher’s exact test) confirmed that E8 IAGT homozygous LAMB3IAP mice significantly restored the skin phenotype compared with untreated LAMB3IAP homozygous mice (Table 1). To assess why the majority of treated null mice failed the pinch test, we performed immunofluorescent staining for laminin-β3 and laminin-332 at the site of mechanical disruption. Despite the presence of mechanical disruption, we were able to detect laminin-β3 and laminin-332 expression at the BMZ within the lesions (Figure 2). We also assessed viral copy number by qPCR analysis in skin between two null mice that failed and two null mice that passed the pinch test but found no correlation between copy number and phenotypic restoration (data not shown).
Figure 2.
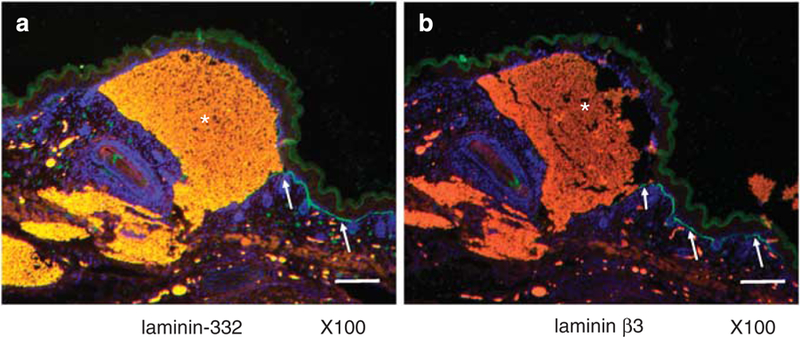
Immunofluorescence analysis of blistered skin samples from E8 IAGT null mice. Both laminin-β3 (a) and laminin-332 (b) were expressed as a linear pattern (arrows) in the BMZ surrounding the blistered area with contained hemorrhage (*) (scale bar=25 µm).
Restoration of hemidesmosomal structures by E8 IAGT
Based on the original description, the homozygous LAMB3IAP mouse is characterized by the formation of abnormal hemidesmosomal structures.25 The hemidesmosomes appear less distinct and inner and outer plaques more diffuse than in WT mice, and the sub-basal dense plate (SBDP) is completely absent. To determine whether the restoration of laminin-b3 could correct the defect in hemidesmosome formation at the BMZ, electron microscopy was performed at E19 on skin sections of E8 IAGT treated homozygous LAMB3IAP mice, untreated homozygous LAMB3IAP mice (negative control) and WT mice (positive control). As shown in Figure 3a, E8 IAGT of LAMB3 transgene could restore normal hemidesmosomal structures, thus demonstrating correction of the major ultrastructual abnormality seen in HJEB skin.
Figure 3.
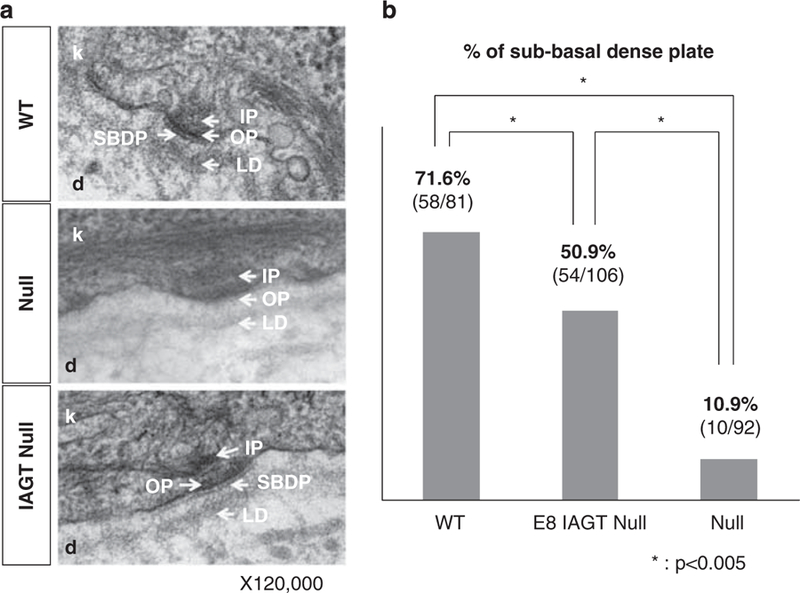
Transmission electron microscopy of dermo-epidermal junction. (a) Electron micrographs of skin sections demonstrate the junction between the basal keratinocytes (k) of the epidermis and the upper reticular dermis (d). In the WT mice, the hemidesmosomes are visible at the basal surface of the keratinocytes and are comprised of an inner plaque (IP), outer plaque (OP) and SBDP. The BMZ, consisting of the electron-opaque lamina lucida and the electron-dense lamina densa, runs parallel to the keratinocyte basal membrane. In homozygous LAMB3IAP mice (null), the hemidesmosomes are present but appear less distinct and the inner and OPs are more diffuse than in the WT mice, and the SBDP is absent. In homozygous LAMB3IAP mice treated by E8 IAGT with lentiviral-LAMB3 (IAGT null), the normal structures of the desmosomes are restored. (b) Bar graph demonstrating the percentage of hemidesmosomes (HD) with a demonstrable SBDP. A blinded examiner with expertise in skin electron microscopy counted the number of the HD and determined the presence or absence of the SBDP. *P-values o0.005 for positive SBDP comparison between WT, E8 IAGT null and non-treated null pups by using the Fisher’s exact test.
To quantitatively assess the efficiency of restoration of hemidesmosomal structures, we compared the percentages of normal hemidesmosomes in an E8 IAGT homozygous LAMB3IAP mouse with an untreated homozygous LAMB3IAP mouse, and a WT mouse. For this purpose, we counted the presence of the SBDP as the critical feature of normal hemidesmosome structure. There are two reasons to choose the SBDP as a landmark: (1) the SBDP is theoretically absent in null mice and (2) the SBDP is comparatively easy to detect regardless of various sectioning plane. In WT mice, 58 out of 81 (71.6%) hemidesmosomes had a detectable SBDP. In contrast, only 10 out of 92 (10.9%) hemidesmosomes in the untreated homozygous LAMB3IAP mouse contained a SBDP. In the treated homozygous LAMB3IAP mouse, 54 out of 106 (50.9%) hemidesmosomes contained a SBDP. If we normalize the data to a 100% frequency of normal hemidesmosomes in WT and 0% in LAMB3IAP homozygous mice, the hemidesmosome restoration rate in E8 IAGT homozygous LAMB3IAP mice was 65.9% (restoration rate=(50.9–10.9)×100/(71.6–10.9)%). Statistical analyses (Fisher’s exact test) confirmed that E8 IAGT homozygous LAMB3IAP mice significantly restored the hemidesmosomal structures compared with untreated LAMB3IAP homozygous mice (Figure 3b).
Skin graft assessment
As we were unable to improve survival in this murine model by IAGT, we elected to assess long-term correction of the skin phenotype in a skin transplant model. The donor skins were harvested from E8 IAGT homozygous LAMB3IAP fetuses (the LAMB3IAP mice are on a B6 background) at E18 and they were grafted onto 6- to 8-week-old, female B6-GFP transgenic mice. We used GFP transgenic mice to allow easy recognition of the grafted skin, and allow identification of any recipient-derived cells in the skin grafts. The donor skin was harvested from treated homozygous LAMB3IAP mice that were assessed by the pinch test before harvest and skin transfer. To be sure that only treated homozygous skin was transplanted, all of the grafted donor skin came from mice that failed the mechanical disruption test. A total of five donor skin samples from five mice were transplanted and four of the five successfully engrafted (80%). The skin grafts on the recipients maintained a normal appearance for 6 months at which time the experiment was terminated. We assessed the grafted skin by the pinch test after a skin graft was established with no disruption observed and no visible subsequent blister formation. Skin grafts of age-matched untreated homozygous LAMB3IAP fetal mice (N=14) uniformly failed to engraft within 2 weeks after transplantation (0%). As our technical control, age-matched B6 fetal skin was transplanted onto GFP transgenic mice (N=5) and 5 of 5 grafts were successful (100%) (Figure 4A). We confirmed the persistence of the skin grafts by fluorescence stereomicroscopy, which demonstrated GFP-negative skin grafts, surrounded by GFP-positive recipient skin (Figure 4B). Immunohistology of the skin grafts demonstrated LAMB3 transgene expression with normal laminin-β3 chain assembly as evidenced by laminin-332, in the BMZ. As expected, there were many GFP-positive cells in the dermis, but we could not detect any GFP-positive keratinocytes in the skin grafts (Figure 5).
Figure 4.
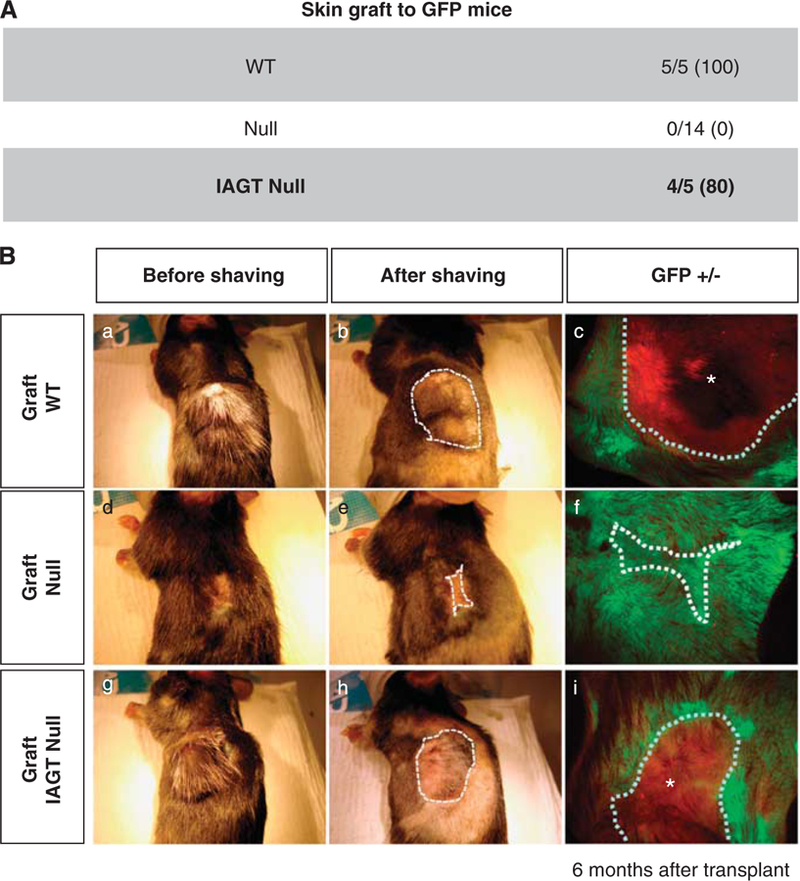
Prolonged skin graft survival after IAGT. The skin from homozygous LAMB3IAP fetal mice treated by E8 IAGT with lentiviral-LAMB3 (IAGT null) at E18 were grafted to the GFP Tg mice. Homozygous untreated LAMB3IAP fetal mice were used as negative controls (null), and C57BL6 fetal mice as positive control (WT). (A) Table demonstrating successful engraftment rates in IAGT null, WT and null mice 6 months after skin grafting. (B) Macroscopic appearance of the grafted skin on GFP Tg mice with optical light (a, b, d, e, g, h) or fluorescent light (c, f, i) 6 months after graft surgery. Null skin graft (middle row) was completely lost and the skin defect was healed with host-derived skin. WT (top row) and E8 IAGT null skin grafts persisted without any obvious blistering or erosions.
Figure 5.
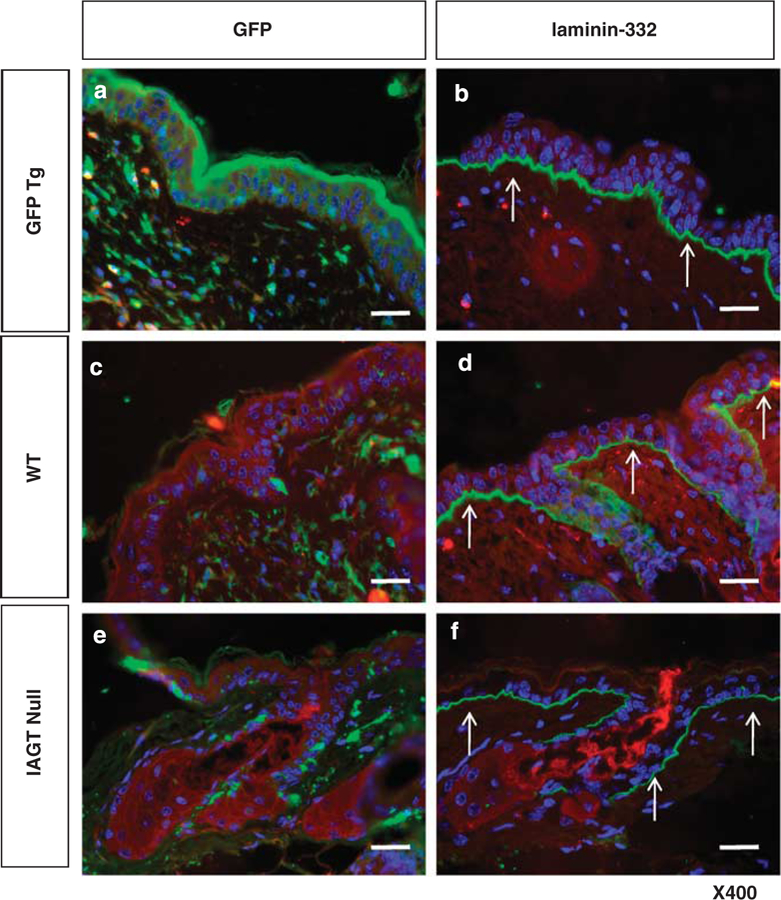
Immunofluorescent histologic appearance of skin grafts at 6 months after placement. (a, b) In the recipient skin, the epidermis is GFP positive and laminin-332 is seen in the BMZ in a linear pattern. (c, d) The WT grafted skin showed GFP-negative epidermis with expression of laminin-332. (e, f, arrows) In the IAGT null skin grafts, skin keratinocytes were GFP negative and expressed laminin-332 in the appropriate linear pattern at the BMZ (arrows). Some GFP-positive cells are seen in the dermis, but these are consistent with the infiltrate of cells derived from the host circulation with the same infiltration seen in WT donor grafts (scale bar=100 µm).
DISCUSSION
In this study, we used an HJEB mouse model (homozygous LAMB3IAP mouse) to evaluate the feasibility of in vivo prenatal gene therapy for phenotypic correction of the skin pathology of a lethal junctional EB (HJEB). Our study demonstrated that E8 IAGT using lentiviral vector results in expression of laminin-β3 in the dermo-epidermal basement membrane and that the transgene encoded laminin-β3 can be incorporated in the assembly of the heterotrimeric laminin-332 protein. In addition, the laminin-332 can restore structurally normal hemidesmosomes in the BMZ of the HJEB mouse skin. The expression was detected in the skin as well as in the oral mucosa. In addition, we could demonstrate restoration of the skin phenotype by the mechanical disruption test in only 11% of treated homozygous animals. However, as the pinch test is a relatively rigorous, all or nothing assay that does not allow assessment of incremental improvement in phenotype, we decided to assess skin integrity in a model where long-term phenotypic correction could be monitored. In the skin transplant model, we were able to clearly demonstrate an improvement in phenotype with essentially equal skin graft survival and integrity in comparison to normal skin graft controls. In addition to the partial restoration of hemidesmosomes, there are at least two possible explanations for the neonatal failure of the pinch test and subsequent passage of the same test 6 months after transplantation. In adult skin, the additional mechanical support provided by the hair shafts to anchor the epidermis to the dermis, as well as simply the thickness of the skin in the adult versus the neonate may have provided adequate support to prevent shearing with the pinch test. Finally, despite the failure of the pinch test in neonatal skin, the ability of the corrected neonatal skin to engraft compared with complete loss of the non-treated skin supports a significant improvement skin integrity from the in utero treatment.
Despite improvement of the skin phenotype, we demonstrated no effect on survival of the newborn pups in this study. While this may be related to inadequate correction of the skin abnormality, other causes of death in this model may not have been addressed by IAGT and this may also be an issue with clinical application. Newborns with HJEB have major involvement of the airways, gastrointestinal and vesicourinary tracts and possibly abnormalities of T-cell development resulting in immune deficiency.28 In the murine setting, this may have resulted in poor feeding, maternal neglect, hypoproteinemia, electrolyte abnormalities, sepsis and early demise. In the context of potential clinical application, it is important to emphasize that even full correction of the skin phenotype alone may not result in complete correction of manifestations of the disease.
Laminin-332 is one of the key molecules in the BMZ maintaining adhesion between the epidermis and dermis.29 Although other BMZ molecules, such as collagen VII, can be produced not only by the keratinocytes but also by the fibroblasts,30,31 laminin-332 is produced only by the keratinocytes at the basal layer in the epidermis,32 and consequently, correction of the EB skin phenotype in HJEB requires transfer of the LAMB3 gene to the basal layer of the epidermis. There have been several previous experimental studies of prenatal gene transfer to skin.12–15,17 Because the embryonic skin is surrounded by amniotic fluid, the most common mode of gene transfer to the skin has been via IAGT. However, most previous studies have performed IAGT too late in development to achieve skin stem cell transduction, thus resulting in only transient gene expression. We have previously demonstrated that the window of opportunity for direct access to skin stem cell populations in the mouse is limited to the early gestational period from E8 to E9.24 During that period, the skin exists as a single layer of cells, containing all of the nascent stem cells of the skin and skin appendages. Based on our previous marker gene study, we utilized the same approach to perform the IAGT at E8. We used a lentiviral vector construct expressing the LAMB3 gene under control of the human keratin 5 promotor/enhancer (K5E), which was analogous to the GFP-encoding vector that demonstrated high efficiency transduction (~50%) of basal layer stem cells in our previous study.
Similar to our previous study, in this study we appear to have achieved efficient transduction of basal stem cells with long-term expression of the transgene encoded protein. In treated animals, we could demonstrate a linear pattern of expression of laminin-β3 and the assembly of laminin-332 heterotrimer in the cutaneous BMZ. As mentioned above, failure to improve survival in this murine model could be multifactorial and may or may not be reflective of failure to correct the skin phenotype. In addition to skin, HJEB affects the mucous membranes of the oropharynx, esophagus and the genitourinary tract.33,34 While some expression of transgene is achieved in the oropharynx and tongue by E8 IAGT, the buccopharyngeal membrane is still intact and prevents access to stem cells in the posterior pharynx and esophagus.35 Furthermore, the gene expression by E8 IAGT is mostly limited in the epidermal-derived organs.35 This likely results in poor sucking and feeding by the pups with subsequent death from maternal neglect, cannibalism and/or dehydration. However, the relatively normal immunohistochemistry findings in combination with failure of the mechanical disruption test suggested that either the expression was quantitatively inadequate to fully correct the skin phenotype, or that the protein was being expressed but was functionally defective. The observation of associated restoration of laminin-332, confirmed expression of laminin-β3 in a form that can be incorporated into the trimer molecule, consistent with a functionally intact and appropriately expressed protein. We therefore tried to quantitatively assess the relative amount of laminin-332 in treated mice versus untreated and normal mice by analysis of restoration of hemidesmosomes by electron microscopy. As hemidesmosomes are structural anchoring units in the BMZ, the degree of restoration of hemidesmosomes should roughly correspond to the degree of correction of skin integrity. Our analysis documented ~60% restoration of normal hemidesmosome structures in treated homozygous mice. As the mechanical disruption test is an all or nothing, extremely rigorous test of skin integrity, we wondered if we had accomplished a clinically significant degree of skin correction that would prevent skin blistering under normal circumstances of skin exposure. To directly test our treated skin under clinically relevant conditions of normal skin exposure, we performed skin transfer experiments to congenic GFP mice. The reason to use GFP transgenic mice is to allow easy recognition of the grafted skin, and allow identification of any recipient-derived cells in the skin grafts, which can potentially replace the native keratinocytes. On transfer of the treated skin grafts, despite harvest from mice that failed the mechanical disruption test, engrafted at a rate similar to control WT skin grafts and maintained their integrity without blister formation and with normal hair growth for 6 months. This was in marked contrast to grafts from untreated homozygous LAMB3IAP mice, which were uniformly lost within 2 weeks.36 The treated skin grafts showed no replacement by host keratinocytes, by either ingrowth or bone marrow derivation, supporting our conclusion that the expression of laminin-β3 in these grafts by E8 IAGT was quantitatively adequate to support normal skin function but inadequate to withstand the rigorous mechanical disruption test during the neonatal period in most circumstances. These results are supportive of the potential for prenatal gene correction in severe JEB, and perhaps in other forms of EB, and suggest that further improvements in the efficiency of IAGT could completely correct the skin phenotype. In addition, we have previously demonstrated that posterior pharyngeal and esophageal transduction can be achieved after resorption of the buccopharyngeal membrane (E9 in the mouse).35 Therefore, from a clinical perspective, at least some of the extracutaneous manifestations of EB might also be correctable by this approach.
From a therapeutic perspective, this approach supports the promise of prenatal gene transfer for the treatment of genetic skin disorders such as EB in which defective protein synthesis occurs at the dermoepidermal interface. However, there are a number of hurdles that would need to be overcome before any clinical application of this approach. First, although we have chosen to focus on skin gene transfer in this study, IAGT is not specific for skin.26,35 While some selectivity occurs due to the limited interface between amniotic fluid and other fetal tissues, we have documented other tissues, including neuroectoderm,35,37 that can be transduced by IAGT. This raises obvious concern about the potential for insertional mutagenesis, developmental effects and the potential for germ line alteration that exists for lentiviral vector-based approaches. These potential risks are, if anything, heightened by early gestational transduction. While greater tissue specificity and safety can probably be accomplished by the use of tissue-specific promoters or by regulated transgene expression, safer gene transfer techniques will need to be developed to alleviate these concerns. The second major impediment is that stage for stage, the timing of our early gestational injections between E8 and E11 in the mouse corresponds to the 21st to 55th day of gestation in human fetal development, a time in pregnancy that precedes current capabilities for prenatal diagnosis. Nevertheless, there has been rapid progress in prenatal diagnosis and in the foreseeable future, new approaches, such as genotyping of fetal cells or free fetal DNA in the maternal circulation, which can be found as early as the 5th week of gestation,38 may allow for even earlier diagnosis of genetic disorders. Dilution of vector in the much larger volume of amniotic fluid in the human is a potential hurdle. However, the volume of human amniotic fluid at 7 weeks gestation is only 1.5 ml39 (murine amniotic fluid volume at E8 is 350 nl) making the delivery of an equivalent concentration of lentiviral vector practically feasible. Finally, correction of skin phenotype alone will not cure the disease, and the prospect of partial treatment may not provide an acceptable quality of life for severely affected patients. However, we believe that with further optimization of this approach, alone or in combination with other therapies, prenatal gene transfer may ultimately have a role in the treatment of this devastating disease.
MATERIALS AND METHODS
Mice
Heterozygous LAMB3IAP mice (obtained from Dr Pamela Swiatek, New York State Department of Health, Buffalo, NY, USA) and C57BL/6TgN actinenhanced green fluorescent protein OsbY01 mice (obtained from Dr Okabe, Osaka University, Genome Information Research Center, Japan) were mated in our breeding colony. Heterozygous LAMB3IAP matings were performed overnight and females were checked for vaginal plugs the next morning. The day of appearance of the vaginal plug was taken as E0. Pregnant females at E8 were utilized for IAGT. Animals were housed in the Laboratory Animal Facility of the Abramson Pediatric Research Center at The Children’s Hospital of Philadelphia and were maintained in sterilized plastic microisolator cages and given sterilized standard laboratory chow and tap water ad libitum. Newborn mice were left with their mother and observed closely. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia, and followed guidelines set forth in the National Institutes of Health ‘Guide for Care and Use of Laboratory Animals’. The genotype of the heterozygous/homozygous LAMB3IAP animals was verified by PCR of the LAMB3 gene and the IAP insertion with a template of genomic DNA from tail samples as previously described.16
Intra-amniotic cavity vector injection at E8
We used an ultrasound guided injection system (Vevo 660; VisualSonics, Toronto, Canada) for intra-amniotic vector injection of E8 pregnant mice. The anesthetic and surgical methods are as we have previously described.26,27 A set volume of 350 nl of vector containing media was injected with an automated syringe.
Immunohistochemistry of laminin-β3/laminin-322
Tissue specimens collected for histology and immunohistochemistry were fixed in 10% buffered formalin solution (Sigma, St Louis, MO, USA) and embedded in paraffin. To evaluate and localize laminin-β3/laminin-322 protein in the harvested tissues, 4 mm sections were obtained using a paraffin microtome (Leica RM2035, Leica Instruments GmbH, Nussloch, Germany). Paraffin sections were incubated overnight at 53 °C and then deparaffinized in serial xylene washes, followed by rehydration through a graded alcohol series to deionized water. Enzymatic pre-treatment using proteinase K (Dako, Carpinteria, CA, USA) was applied to the sections for 10 min at RT (room temperature) followed by rinsing in deionized water and transferred to 0.1 M Tris-buffered saline pH 7.8. The tissue sections were incubated with the blocking solution (protein block serum free; Dako) for 10 min at RT. The blocking solutions were tapped off and the primary antibodies were applied overnight at 4 °C. Antibodies included polyclonal rabbit anti-laminin-332 (a gift from Dr Marinkovich, Stanford University, CA, USA) concentration of 1:400; Anti-laminin-β340 (a gift from Dr Matsui, Toyama University, Toyama, Japan) concentration of 1:50. On the following day, sections were washed for 10 min in Tris-buffered saline buffer at RT and Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) was applied at 1:200 for 30 min at RT. Sections were then rinsed in Tris-buffered saline buffer and mounted with 4′,6-diamidino-2-phenylindole (Invitrogen) and analyzed using a Leica DMR microscope (Leica, Heerburg, Switzerland).
Transmission electron microscopic analysis
After euthanasia, skin samples were obtained from the arms of IAGT treated and untreated homozygous E18 LAMB3IAP mice and of E19 C57BL6 mice, fixed in half strength Karnovsky’s fixative, and embedded in epoxy resin. Ultrathin sections (80 nm) were stained sequentially with saturated uranyl acetate and Reynold’s lead citrate.41 Sections were imaged with a Jeol-1010 Transmission electron microscope (JEOL USA, Peabody, MA, USA).
Lentivector production containing the mouse LAMB3 or GFP under the human keratin 5 promoter/enhancer and cell culture
The human keratin 5 (K5) promoter, previously described,24 was inserted into the HIV-1-based transfer vector, a modified F12 plasmid.42 Inserted directly linked upstream of the promoter was the keratin 5 DNas I-hypersensitive site 4,43 a strong keratinocyte-specific enhancer. The mouse LAMB3 cDNA molecule, derived from total RNA extracted from keratinocytes using standard molecular protocols44 with a coding sequence confirmed to be identical to that of GenBank Accession #NM_008484, was inserted into the lentivector transfer plasmid under the transcriptional control of the human keratin 5 promoter/enhancer (K5E). Alternatively, the GFP reporter was similarly inserted in the transfer plasmid replacing the therapeutic gene. Viral vectors, pseudotyped with the vesicular stomatitis virus-G protein envelope, were generated as previously reported using a three-plasmid transient cotransfection in HEK293-T cells. Viral supernatants were concentrated and, for the GFP vector, titered using serial dilutions as previously described and as determined on the mouse PAM212 and human HaCat T-cell lines (kind gifts from Dr George Cotsarelis, University of Pennsylvania). Titers of injected virus ranged from 1×109 and 3×1010 TUml−1. Virion production of the LAMB3 and also the GFP vectors were determined by measuring p24 by ELISA (ABL Inc., Kensington, MA, USA) according to the manufacturer’s recommendations.
The PAM212 cells were grown in 1:3 Ham’s F-12 medium (Gibco brand, Invitrogen Life Sciences, Carlsbad, CA, USA) and Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin. The HaCat T cells were cultured in Dulbecco’s modified Eagle’s medium with similar supplements.
Fluorescence microscopic analysis
We used fluorescent stereomicroscopy (MZ16FA; Leica) to distinguish between the donor and recipient skin in the skin transfer to GFP mouse experiments. For these studies, mice were euthanized 6 months after placement of the skin grafts and the skin was immediately dissected to allow direct visualization.
Mechanical skin disruption test (pinch test)
To assess the correction of skin phenotype, we performed the mechanical skin disruption or pinch test. The test is performed by pinching the murine skin between two fingers and performing a shearing motion to detach the epidermis. If the epidermis detached from the dermis, the test was recorded as a ‘fail’. If the epidermis maintained its attachment, then it was recorded as a ‘pass’. In animals that ‘passed’ the pinch test, we confirmed the result by using forceps to try to peel off the skin. If the epidermis detached from the dermis with the forceps, the mouse was recorded as a ‘fail’. If the epidermis did not detach from the dermis, even when using the forceps, we classified the mouse as a ‘WT phenotype’.
Skin grafting
Skin grafting was performed on 6- to 8-week-old, female GFP transgenic mice by a modification of the technique described by Billingham et al.44 Briefly, full thickness donor skin grafts (1.5×1.5 cm2) were prepared from the skin of IAGT treated and untreated homozygous E18 LAMB3IAP mice or from E18 C57BL6 mice (as a technical control) and transferred to recipient sites on GFP transgenic mice. Recipient sites were created on the lateral thorax of the mice while carefully preserving the panniculus carnosus. The grafts were covered with petroleum gauze and held in place with a band-aid to create a pressure dressing. Dressings were removed after 5 days. Non-adherent grafts were considered technical failures and were excluded. Adherent grafts were monitored for signs of graft loss (hardening of the graft, and necrosis), and photographs were taken weekly. Grafts were considered lost when >90% of the surface area was necrotic and the graft hardened. The grafted skins were harvested 6 months after skin graft and analyzed by fluorescent stereomicroscopy and immunohistochemistry for laminin-322 and GFP.
Statistical analysis
The data were analyzed by using the Fisher’s exact test.
ACKNOWLEDGEMENTS
We thank Keith Alcorn (The Children’s Hospital of Philadelphia) for animal support, Ray Meade (The Biomedical Imaging Core/Electron Microscopy Resource Laboratory of the University of Pennsylvania) for electron microscopy support and Xiaohua Chen (The Children’s Hospital of Philadelphia) for qPCR support. These studies were supported by NIH/NIAMS Grant R01 AR54876 (JU) and by funds from the Department of Surgery and the Ruth and Tristram C Colket Chair in Pediatric Surgery, Children’s Hospital of Philadelphia (AWF).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 2006; 12: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 2.Remington J, Wang X, Hou Y, Zhou H, Burnett J, Muirhead T et al. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther 2009; 17: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med 2010; 363: 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong T, Gammon L, Liu L, Mellerio JE, Dopping-Hepenstal PJ, Pacy J et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2008; 128: 2179–2189. [DOI] [PubMed] [Google Scholar]
- 5.Uitto J, McGrath JA, Rodeck U, Bruckner-Tuderman L, Robinson EC. Progress in epidermolysis bullosa research: toward treatment and cure. J Invest Dermatol 2010; 130: 1778–1784. [DOI] [PubMed] [Google Scholar]
- 6.Chino T, Tamai K, Yamazaki T, Otsuru S, Kikuchi Y, Nimura K et al. Bone marrow cell transfer into fetal circulation can ameliorate genetic skin diseases by providing fibroblasts to the skin and inducing immune tolerance. Am J Pathol 2008; 173: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolar J, Ishida-Yamamoto A, Riddle M, McElmurry RT, Osborn M, Xia L et al. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood 2009; 113: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhle C, Jiang QJ, Charlesworth A, Bruckner-Tuderman L, Meneguzzi G, Schneider H. Novel and recurrent mutations in the laminin-5 genes causing lethal junctional epidermolysis bullosa: molecular basis and clinical course of Herlitz disease. Hum Genet 2005; 116: 33–42. [DOI] [PubMed] [Google Scholar]
- 9.Laimer M, Lanschuetzer CM, Diem A, Bauer JW. Herlitz junctional epidermolysis bullosa. Dermatol Clin 2010; 28: 55–60. [DOI] [PubMed] [Google Scholar]
- 10.Zanjani ED, Anderson WF. Prospects for in utero human gene therapy. Science 1999; 285: 2084–2088. [DOI] [PubMed] [Google Scholar]
- 11.Schneider H, Coutelle C. In utero gene therapy: the case for. Nat Med 1999; 5: 256–257. [DOI] [PubMed] [Google Scholar]
- 12.Douar AM, Adebakin S, Themis M, Pavirani A, Cook T, Coutelle C. Foetal gene delivery in mice by intra-amniotic administration of retroviral producer cells and adenovirus. Gene Therapy 1997; 4: 883–890. [DOI] [PubMed] [Google Scholar]
- 13.Waddington SN, Buckley SM, Bernloehr C, Bossow S, Ungerechts G, Cook T et al. Reduced toxicity of F-deficient Sendai virus vector in the mouse fetus. Gene Therapy 2004; 11: 599–608. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, Tanigawa M, Kikuchi N. Nonviral gene transfer to surface skin of mid-gestational murine embryos by intraamniotic injection and subsequent electroporation. Mol Reprod Dev 2004; 69: 268–277. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizawa J, Li XK, Fujino M, Kimura H, Mizuno R, Hara A et al. Successful in utero gene transfer using a gene gun in midgestational mouse fetuses. J Pediatr Surg 2004; 39: 81–84. [DOI] [PubMed] [Google Scholar]
- 16.Muhle C, Neuner A, Park J, Pacho F, Jiang Q, Waddington SN et al. Evaluation of prenatal intra-amniotic LAMB3 gene delivery in a mouse model of Herlitz disease. Gene Therapy 2006; 13: 1665–1676. [DOI] [PubMed] [Google Scholar]
- 17.Endoh M, Koibuchi N, Sato M, Morishita R, Kanzaki T, Murata Y et al. Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound. Mol Ther 2002; 5: 501–508. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005; 11: 1351–1354. [DOI] [PubMed] [Google Scholar]
- 19.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 2005; 9: 855–861. [DOI] [PubMed] [Google Scholar]
- 20.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990; 61: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004; 116: 769–778. [DOI] [PubMed] [Google Scholar]
- 22.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA 2000; 97: 13473–13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne C, Hardman M, Nield K. Covering the limb–formation of the integument. J Anat 2003; 202: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo M, Zoltick PW, Peranteau WH, Radu A, Muvarak N, Ito M et al. Efficient in vivo targeting of epidermal stem cells by early gestational intraamniotic injection of lentiviral vector driven by the keratin 5 promoter. Mol Ther 2008; 16: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuster JE, Guarnieri MH, Ault JG, Flaherty L, Swiatek PJ. IAP insertion in the murine LamB3 gene results in junctional epidermolysis bullosa. Mamm Genome 1997; 8: 673–681. [DOI] [PubMed] [Google Scholar]
- 26.Endo M, Zoltick PW, Chung DC, Bennett J, Radu A, Muvarak N et al. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Ther 2007; 15: 579–587. [DOI] [PubMed] [Google Scholar]
- 27.Henriques-Coelho T, Gonzaga S, Endo M, Zoltick PW, Davey M, Leite-Moreira AF et al. Targeted gene transfer to fetal rat lung interstitium by ultrasound-guided intrapulmonary injection. Mol Ther 2007; 15: 340–347. [DOI] [PubMed] [Google Scholar]
- 28.Kim MG, Lee G, Lee SK, Lolkema M, Yim J, Hong SH et al. Epithelial cell-specific laminin 5 is required for survival of early thymocytes. J Immunol 2000; 165: 192–201. [DOI] [PubMed] [Google Scholar]
- 29.Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat 1998; 193 (Pt 1): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley JR, Rubinstein N, Klaus-Kovtun V. Epidermolysis bullosa acquisita antigen is synthesized by both human keratinocytes and human dermal fibroblasts. J Invest Dermatol 1985; 85: 542–545. [DOI] [PubMed] [Google Scholar]
- 31.Woodley DT, Krueger GG, Jorgensen CM, Fairley JA, Atha T, Huang Y et al. Normal and gene-corrected dystrophic epidermolysis bullosa fibroblasts alone can produce type VII collagen at the basement membrane zone. J Invest Dermatol 2003; 121: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 32.Amano S, Nishiyama T, Burgeson RE. A specific and sensitive ELISA for laminin 5. J Immunol Methods 1999; 224: 161–169. [DOI] [PubMed] [Google Scholar]
- 33.Hata D, Miyazaki M, Seto S, Kadota E, Muso E, Takasu K et al. Nephrotic syndrome and aberrant expression of laminin isoforms in glomerular basement membranes for an infant with Herlitz junctional epidermolysis bullosa. Pediatrics 2005; 116: e601–e607. [DOI] [PubMed] [Google Scholar]
- 34.Laimer M, Lanschützer CM, Nischler E, Klausegger A, Diem A, Pohla-Gubo G et al. [Hereditary blistering diseases. Symptoms, diagnosis and treatment of epidermolysis bullosa]. Hautarzt 2009; 60: 378–388. [DOI] [PubMed] [Google Scholar]
- 35.Endo M, Henriques-Coelho T, Zoltick PW, Stitelman DH, Peranteau WH, Radu A et al. The developmental stage determines the distribution and duration of gene expression after early intra-amniotic gene transfer using lentiviral vectors. Gene Therapy 2010; 17: 61–71. [DOI] [PubMed] [Google Scholar]
- 36.Sakai N, Waterman EA, Nguyen NT, Keene DR, Marinkovich MP. Observations of skin grafts derived from keratinocytes expressing selectively engineered mutant laminin-332 molecules. J Invest Dermatol 2010; 130: 2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stitelman DH, Endo M, Bora A, Muvarak N, Zoltick PW, Flake AW et al. Robust in vivo transduction of nervous system and neural stem cells by early gestational intra amniotic gene transfer using lentiviral vector. Mol Ther 2010; 18: 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uitto J, Pfendner E, Jackson LG. Probing the fetal genome: progress in non-invasive prenatal diagnosis. Trends Mol Med 2003; 9: 339–343. [DOI] [PubMed] [Google Scholar]
- 39.Weissman A, Itskovitz-Eldor J, Jakobi P. Sonographic measurement of amniotic fluid volume in the first trimester of pregnancy. J Ultrasound Med 1996; 15: 771–774. [DOI] [PubMed] [Google Scholar]
- 40.Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin-5 subunits. J Biol Chem 1995; 270: 23496–23503. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 1963; 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 2003; 100: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman CK, Sinha S, Bolotin D, Fan J, Fuchs E. Dissection of a complex enhancer element: maintenance of keratinocyte specificity but loss of differentiation specificity. Mol Cell Biol 2002; 22: 4293–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakano A, Chao SC, Pulkkinen L, Murrell D, Bruckner-Tuderman L, Pfendner E et al. Laminin 5 mutations in junctional epidermolysis bullosa: molecular basis of Herlitz vs non-Herlitz phenotypes. Hum Genet 2002; 110: 41–51. [DOI] [PubMed] [Google Scholar]
- 45.Billingham RE, Krohn PL, Medawar PB. Effect of cortisone on survival of skin homografts in rabbits. Br Med J 1951; 1: 1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]


