Abstract
Aims:
With increasing attention to renovascular causes and targets for hypertension arises a critical need for more detailed knowledge of renal arterial anatomy. However, a standardized nomenclature is lacking.
Methods and results:
1000 hypertensive patients underwent invasive selective renal artery angiography in 9 centers. Further, renovasography was performed in 249 healthy swine as a surrogate for normotensive anatomy. Anatomical parameters were assessed by quantitative vascular analysis. Mean blood pressure was 168/90 ± 26/17 mmHg. The right main renal artery was longer than the left (41±15 mm vs. 35±13 mm, p<0.001), but left had a greater diameter (5.4±1.2 vs. 5.2±1.2 mm, p<0.001). Accessory renal arteries and renal artery disease were documented in 22% and 9% of the patients, respectively. Other than exhibiting a longer left main renal artery in uncontrolled hypertensives (+2.7 mm, p=0.034) there was no anatomical difference between patients with controlled and uncontrolled hypertension. Main renal artery mean diameter was smaller in patients with impaired kidney function (GFR <90 ml/min, left −0.5 mm, right −0.4 mm, both p<0.001).
Conclusion:
Renal arterial anatomy differs between sides but shows no difference between patients with and without blood pressure control. Impaired GFR was associated with small main renal artery diameter.
Keywords: Hypertension, Clinical research, other imaging modalities
Condensed abstract
Hypertension is highly prevalent and associated with cardiovascular morbidity and mortality. With increasing attention to renovascular causes and targets for hypertension arises a critical need for more detailed knowledge of renal arterial anatomy. 1000 hypertensive patients underwent invasive selective renal artery angiography. Further, renovasography was performed in 249 healthy swine to implement an animal model as a surrogate for normotensive anatomy. Renal arterial anatomy differs in human hypertensive patients and in normotensive swine between sides but shows no difference between patients with and without blood pressure control. Impaired GFR was associated with small main renal artery diameter.
Introduction
Hypertension remains a major risk factor for the most significant cardiovascular events such as stroke, myocardial infarction and heart failure and one of the most prevalent chronic conditions [1]. Moreover, despite its prevalence and the availability of safe and effective antihypertensive drugs, blood pressure control remains poor [2-4]. These issues, long appreciated, have taken on a renewed urgency given the emergence of novel means of blood pressure control. Frequent causes of secondary hypertension are fibromuscular dysplasia (FMD) and atherosclerotic renal artery disease (RAD), the latter accounting for 90% of all cases of renal artery stenosis (RAS) [5] and is closely associated with advanced atherosclerotic diseases and poor outcome [6-10]. Frustrations in the rampant nature of hypertension and the tribulations of new approaches have been linked to an underappreciation for precise anatomic understanding of the vessels and nerves that perfuse and innervate the kidneys. Anatomic variations may affect blood pressure control as a recent study suggested an association between the presence of accessory renal arteries and resistant hypertension [11]. Knowledge of the renal arterial anatomy appears crucial for a profound pathophysiological understanding of hypertension but also for the development of endovascular treatment options [12,13]. Morphometric data of the renal vascular tree in patients with hypertension [14,15] and a consistent and standardized nomenclature are lacking [16-18]. The present study sought to develop a standardized nomenclature for renal anatomy considering the complexity and variation of the renal arterial tree and assess the applicability of the nomenclature in 1000 patients with hypertension undergoing renal arteriography.
Methods
A total of 1000 hypertensive patients underwent selective invasive renal angiography in 8 European (Bad Krozingen, Galway, Gßn, Hamburg, Heidelberg, Homburg/Saar, Utrecht, Zürich) and one Australian center (Melbourne) in preparation of an invasive antihypertensive treatment. Therefore, patients with hemodynamically significant renal artery stenosis diagnosed by non-invasive means were excluded in advance. All participating patients provided written informed consent. Patients were at least 18 years old, had a systolic office blood pressure (SBP) of ≥140 mmHg or were treated with antihypertensive therapy. All patients underwent a complete medical history, physical examination and routine blood chemistry. Glomerular filtration rate (GFR) was assessed using cystatin C measurements. Attended office blood pressures (OBP) were obtained with an automated oscillometric device (e.g. Omron HEM-705 monitor, Omron Healthcare, Vernon Hills, Illinois, USA). All blood pressure measurements were performed in concordance with the Joint National Committee VII Guidelines [19]. The current antihypertensive medication was confirmed by direct questioning.
Renal angiography and quantitative vascular analysis (QVA)
All procedures were performed by experienced interventionalists referring to an experience of at least 10 renal interventions per year. Procedural data were recorded, and two experienced investigators blinded to patient’s characteristics assessed QVA using the CAAS II Research System (Pie Medical, Netherlands).
Anatomical parameters
Morphometric parameters such as minimum, mean and maximum diameter as well as length were documented for the main renal artery and in particular for the proximal (p), middle (m) and distal (d) segments as previously described [13]. The division point in two or more consecutive branches of at least 3 mm in diameter defined the end of the main renal artery.
Renal arteries other than the main renal arteries were defined as accessory renal arteries. These could be of similar size and penetrating the hilus or smaller and supplying a minor part of the kidney. Every accessory renal artery was evaluated regarding mean diameter and length. For further comparisons and analysis, the largest caliber vessel of each side was determined. Moreover, the branches of 800 patients were analyzed. The largest branches of each side were ascertained regarding length and diameter. Furthermore, the mean diameter and length were calculated. Renal artery disease included patients with non-significant renal artery stenosis (<50%), prior renal angioplasty or stenting.
Nomenclature of renal arteries
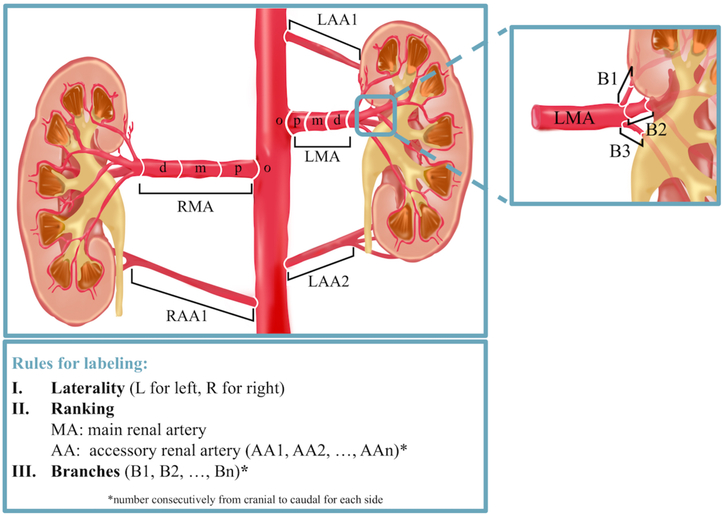
The nomenclature used for QVA was based on a basic three-letter code (Figure 1). All vessels proximal to the kidney’s parenchyma shadow with a mean diameter of at least 3 mm were considered. The first letter indicated the laterality of the kidney (L for left and R for right kidney). The following two letters differentiated between the main renal artery (MA, the largest in diameter) and accessory renal arteries (AA). Subsequent branches were labeled with an additional B and as with accessory renal arteries numbered from cranial to caudal. Figure 2 gives an example of the application of the nomenclature for right renal arteries.
Figure 1: Nomenclature of renal arteries.
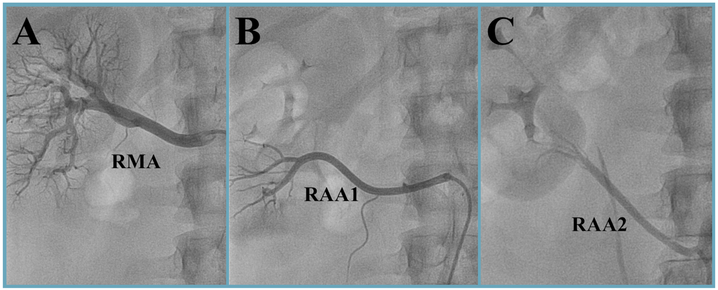
Figure 2: Invasive selective renal artery angiography.
Renal artery anatomy of a 25-year-old man. The right main renal artery (RMA) and the upper accessory renal artery (RAA1) both arise from the aorta. The lower accessory renal artery (RAA2) originates from the right common iliac artery.
Animal model
Renal angiography, subsequent QVA and statistical analysis of the main renal arteries were performed in 249 healthy juvenile Yorkshire domestic farm swine at CBSET, Inc. (Lexington, Massachusetts, USA) in accordance with the Guide for the Care and Use of Laboratory Animals under an approved institutional animal care and use committee-approved protocol. The animal model was introduced as a surrogate for renal artery anatomy in normotensive humans. The QVA was obtained using the Centricity® Cardiology CA1000 Cardiac Review 2.0 software (GE Healthcare, Wauwatosa, Wisconsin, USA).
Statistical analysis
Data management and all statistical analysis were done with IBM SPSS Statistics (version 23.0; SPSS Inc., Chicago, Illinois, USA). Data are presented as the mean ± standard deviation (SD) for continuous variables and as numbers (%) for categorical variables unless otherwise specified. Comparisons between groups were performed using Pearson's χ2-test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test or the Kruskal-Wallis test for continuous variables where appropriate. A two-tailed p-value <0.05 was defined to be statistically significant.
Results
Patient population
Patients average age was 63.7±10.7 years, 57% were male with a body mass index (BMI) of 30.4±5.4 kg/m2. Coronary artery disease (CAD) and type 2 diabetes were prevalent in 270 (27%) and 375 (38%) patients, respectively. Despite an average of 4.8±1.7 prescribed antihypertensive drugs, SBP and DBP was 168±26 mmHg and 90±17 mmHg, respectively. The mean heart rate (HR) was 66.9±11.6 beats per minute (bpm). According to the 2013 ESH/ESC guidelines [20] only 123 patients (12%) achieved blood pressure control while 862 patients (88%) had uncontrolled hypertension (Table 1).
Table 1 –
Baseline characteristics
| Parameter | All patients | Controlled Hypertensi |
Uncontro Hypertension |
p-value* | |||
|---|---|---|---|---|---|---|---|
| Value | N | Value | N | Value | N | ||
| Male (%) | 573 (57%) | 985 | 74 (60%) | 123 | 489 (57%) | 862 | 0.472 |
| Age, years | 63.8±10.8 | 985 | 62.4±10.5 | 123 | 63.9±10.8 | 862 | 0.137 |
| BMI, kg/m2 | 30.4±5.4 | 985 | 30.8±5.1 | 123 | 30.3±5.4 | 862 | 0.353 |
| Type 2 diabetes (%) | 372 (38%) | 985 | 42 (34%) | 123 | 330 (38%) | 862 | 0.353 |
| CAD (%) | 269 (27%) | 985 | 30 (24%) | 123 | 239 (28%) | 862 | 0.374 |
| GFR, ml/(min*1.73m2) | 75.9±29.1 | 875 | 76.8±34.6 | 118 | 75.7±28.1 | 757 | 0.863 |
| Number of antihypertensive dugs | 4.8±1.6 | 985 | 5.0±1.6 | 123 | 4.8±1.7 | 862 | 0.452 |
| SBP, mmHg | 167.9±25.9 | 985 | 130.6±8.5 | 123 | 173.2±23.1 | 862 | <0.001 |
| DBP, mmHg | 89.9±17.1 | 985 | 75.2±10.7 | 123 | 91.9±16.8 | 862 | <0.001 |
| PP, mmHg | 78.0±20.7 | 985 | 55.4±10.2 | 123 | 81.3±19.7 | 862 | <0.001 |
| Heart rate, bpm | 66.9±11.6 | 985 | 66.5±11.8 | 123 | 66.9±11.6 | 862 | 0.499 |
Values are means ± SD or numbers (%).
p-values for comparison of controlled and uncontrolled hypertension (based upon ESH/ESC guidelines, 2013).
BMI = body mass index (kg/m2); CAD = coronary artery disease; DBP = diastolic office blood pressure; GFR = glomerular filtration rate; HR = heart rate; PP = pulse pressure; SBP = systolic office blood pressure.
Renal vascular anatomy
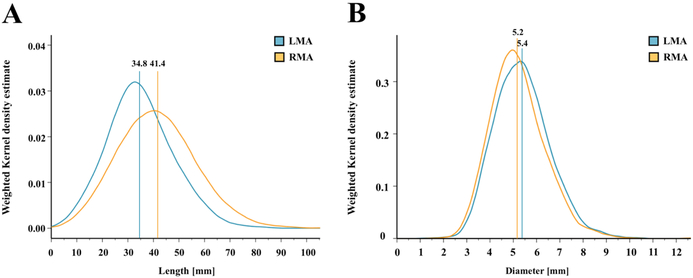
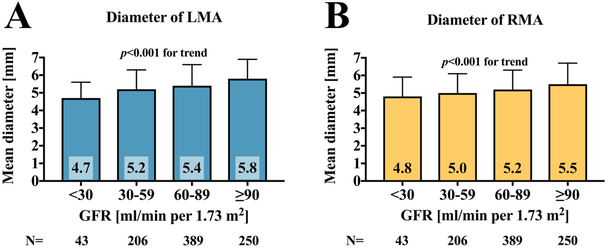
On average, the right main renal artery was longer than the left main renal artery (+6 mm, p<0.001) (Figure 3A), whereas the left main renal artery was of slightly greater diameter (+0.2 mm, p<0.001) (Tables 2 and 3, Figure 3B). The diameters of the main renal arteries were similar in patients with uncontrolled and controlled (SBP <140 mmHg) hypertension (left p=0.641, right p=0.615). Patients with uncontrolled hypertension had longer left main renal arteries (+2.7 mm, p=0.034), whereas the right main renal artery length was not different. When patients were grouped by baseline GFR values, lower GFR was associated with smaller main renal artery diameters (Figure 4). In patients with GFR <30 ml/min/1.73 m2, both right and left main renal artery diameter were smallest when compared to patients with higher GFR values.
Figure 3: Main renal arteries.
Length (A) and diameter (B) of the main renal arteries. Verticals indicate the mean value. LMA: Left main renal artery; RMA: Right main renal artery.
Table 2 –
Anatomical criteria of main renal arteries
| Parameter | All patients | |
|---|---|---|
| Value | N | |
| Left main renal artery (LMA) | ||
| Length, mm | 34.8±12.5 | 1000 |
| Minimum diameter, mm | 4.4±1.1 | 1000 |
| Mean diameter, mm | 5.4±1.2 | 1000 |
| Maximum diameter, mm | 7.1±1.6 | 1000 |
| Proximal minimum diameter, mm | 4.8±1.2 | 1000 |
| Proximal mean diameter, mm | 5.6±1.3 | 1000 |
| Proximal maximum diameter, mm | 6.9±1.7 | 1000 |
| Middle minimum diameter, mm | 4.8±1.1 | 1000 |
| Middle mean diameter, mm | 5.3±1.2 | 1000 |
| Middle maximum diameter, mm | 5.7±1.3 | 1000 |
| Distal minimum diameter, mm | 4.7±1.2 | 1000 |
| Distal mean diameter, mm | 5.2±1.2 | 1000 |
| Distal maximum diameter, mm | 5.9±1.4 | 1000 |
| Number of branches | 2.2±0.5 | 1000 |
| Mean length of branches, mm | 17.3±7.6 | 1000 |
| Maximum length of branches, mm | 22.4±10.0 | 1000 |
| Mean diameter of branches, mm | 4.0±0.9 | 1000 |
| Maximum diameter of branches, mm | 4.6±1.1 | 1000 |
| Right main renal artery (RMA) | ||
| Length, mm | 41.4±15.0 | 1000 |
| Minimum diameter, mm | 4.2±1.1 | 1000 |
| Mean diameter, mm | 5.2±1.2 | 1000 |
| Maximum diameter, mm | 6.8±1.7 | 1000 |
| Proximal minimum diameter, mm | 4.6±1.1 | 1000 |
| Proximal mean diameter, mm | 5.5±1.3 | 1000 |
| Proximal maximum diameter, mm | 6.5±1.6 | 1000 |
| Middle minimum diameter, mm | 4.6±1.1 | 1000 |
| Middle mean diameter, mm | 5.1±1.1 | 1000 |
| Middle maximum diameter, mm | 5.6±1.3 | 1000 |
| Distal minimum diameter, mm | 4.5±1.2 | 1000 |
| Distal mean diameter, mm | 5.0±1.2 | 1000 |
| Distal maximum diameter, mm | 5.7±1.5 | 1000 |
| Number of branches | 2.2±0.4 | 1000 |
| Mean length of branches, mm | 19.6±9.1 | 1000 |
| Maximum length of branches, mm | 25.6±12.0 | 1000 |
| Mean diameter of branches, mm | 3.9±0.9 | 1000 |
| Maximum diameter of branches, mm | 4.5±1.1 | 1000 |
Values are means ± SD.
LMA = Left main renal artery; RMA = Right main renal artery.
Table 3 –
Comparison between controlled and uncontrolled hypertension
| Parameter | All patients | Controlled Hypertension |
Uncontrolled Hypertension |
p-value* | |||
|---|---|---|---|---|---|---|---|
| Value | N | Value | N | Value | N | ||
| Left main renal artery (LMA) | |||||||
| Length, mm | 34.8±12.5 | 985 | 32.5±11.9 | 123 | 35.2±12.6 | 862 | 0.034 |
| Minimum diameter, mm | 4.4±1.1 | 985 | 4.5±1.0 | 123 | 4.3±1.1 | 862 | 0.152 |
| Mean diameter, mm | 5.4±1.2 | 985 | 5.4±1.0 | 123 | 5.4±1.2 | 862 | 0.641 |
| Maximum diameter, mm | 7.1±1.7 | 985 | 7.1±1.6 | 123 | 7.1±1.7 | 862 | 0.890 |
| Right main renal artery (RMA) | |||||||
| Length, mm | 41.5±15.0 | 985 | 39.1±14.9 | 123 | 41.8±15.0 | 862 | 0.062 |
| Minimum diameter, mm | 4.2±1.1 | 985 | 4.2±1.0 | 123 | 4.2±1.1 | 862 | 0.404 |
| Mean diameter, mm | 5.2±1.2 | 985 | 5.2±1.0 | 123 | 5.2±1.2 | 862 | 0.615 |
| Maximum diameter, mm | 6.8±1.7 | 985 | 6.8±1.7 | 123 | 6.8±1.7 | 862 | 0.941 |
| Accessory renal artery | |||||||
| Left kidney (%) | 122 (12%) | 985 | 22 (18%) | 123 | 100 (12%) | 862 | 0.057 |
| Right kidney (%) | 118 (12%) | 985 | 14 (11%) | 123 | 104 (12%) | 862 | 0.827 |
| Unilateral (%) | 195 (20%) | 985 | 26 (21%) | 123 | 169 (20%) | 862 | 0.690 |
| Bilateral (%) | 24 (2%) | 985 | 5 (4%) | 123 | 19 (2%) | 862 | 0.209 |
| Accessory renal artery left kidney | |||||||
| Length, mm | 46.5±17.8 | 116 | 41.1±15.4 | 20 | 47.6±18.2 | 96 | 0.226 |
| Mean diameter, mm | 2.8±0.8 | 121 | 3.0±0.8 | 22 | 2.7±0.8 | 99 | 0.195 |
| % Diameter LMA | 54.7±19.2 | 121 | 57.8±17.8 | 22 | 54.0±19.5 | 99 | 0.204 |
| Accessory renal artery right kidney | |||||||
| Length | 47.8±18.9 | 103 | 41.5±17.8 | 12 | 48.6±18.9 | 91 | 0.377 |
| Mean diameter, mm | 2.6±0.8 | 117 | 2.4±0.5 | 14 | 2.6±0.8 | 103 | 0.977 |
| % Diameter RMA | 51.7±15.3 | 117 | 48.5±10.1 | 14 | 52.1±15.9 | 103 | 0.662 |
| Renal artery disease† | |||||||
| Left kidney (%) | 55 (6%) | 985 | 11 (9%) | 123 | 44 (5%) | 862 | 0.083 |
| Right kidney (%) | 55 (6%) | 985 | 3 (2%) | 123 | 52 (6%) | 862 | 0.104 |
| Unilateral (%) | 72 (7%) | 985 | 10 (8%) | 123 | 62 (7%) | 862 | 0.709 |
| Bilateral (%) | 19 (2%) | 985 | 2 (2%) | 123 | 17 (2%) | 862 | 1.000 |
Values are means ± SD or numbers (%).
p-values for comparison of controlled and uncontrolled hypertension (based upon ESH/ESC guidelines, 2013).
Renal artery disease included patients with non-significant renal artery stenosis (<50%) or prior renal angioplasty or stenting.
LMA = Left main renal artery; RMA = Right main renal artery.
Figure 4: Mean diameter grouped by baseline GFR.
Comparison of left (A) and right main renal artery (B) mean diameter when grouping patients by baseline GFR. p-values are comparison between groups. GFR: glomerular filtration rate; LMA: left main renal artery; RMA: right main renal artery.
Accessory renal arteries were present unilaterally in 197 (20%) and bilaterally in 24 (2%) patients. In male patients, the presence of unilateral and bilateral accessory renal arteries was higher compared to female patients (unilateral p<0.001; bilateral p=0.009). Accessory renal arteries were also more common in men when comparing the prevalence for each side separately (left 15% vs. 6%, p<0.001; right 14% vs. 9%, p=0.04). The presence of accessory renal arteries did neither differ between sides (p=0.681) nor between patients with uncontrolled and controlled hypertension (p=0.397), respectively. The mean diameter of the left accessory renal artery was greater than the diameter of the right accessory renal artery (+0.2 mm, p=0.019) whereas the lengths were similar (p=0.595). The length of main renal arteries was related to the presence of accessory renal arteries. Patients with multiple renal arteries had longer left and right main renal arteries when compared to patients with solitary renal arteries (left +5.2 mm, right +6.9 mm, both p<0.001).
The branches of the left and right main renal arteries did not differ in terms of mean and maximum diameter (p=0.215 and p=0.204). The branches of the right main renal artery were longer, both for mean and maximum length (mean +2.3 mm, p<0.001; maximum +3.2 mm, p<0.001).
Taken together, renal artery disease was diagnosed unilaterally in 93 (9%) patients and bilaterally in 19 (2%) patients. In comparison to patients without renal artery disease, patients with renal artery disease were older (66.6±10.7 vs. 64.4±10.7 years, p=0.003), had a higher prevalence of diabetes (51% vs. 37%, p=0.010) and CAD (37% vs. 27%, p=0.044), a lower DBP (87±16 mmHg vs. 90±17 mmHg, p=0.041) and a lower heart rate (64±9 bpm vs. 67±12 bpm, p=0.022) whereas SBP (167±22 mmHg vs. 168±26 mmHg, p=0.668) and PP (79±18 mmHg vs. 78±21 mmHg, p=0.309) were similar in both groups.
Renal vascular anatomy in porcine model
In 249 normotensive juvenile swine, the right main renal artery was longer than the left (+6.7 mm, p<0.001) whereas the left main renal artery was of greater diameter (+0.13 mm, p<0.001).
Discussion
The renal vascular anatomy typically shows a broad interindividual variety in the general population [17] necessitating a standardized nomenclature. To the best of our knowledge, no accepted nomenclature for renal angiograms has been validated practically thus far [17,21]. We introduced a nomenclature which grasps the complexity of renal arterial anatomy and can be applied for clinical and research purposes. In addition, we implemented the nomenclature by analyzing the renal vascular tree of 1000 people with hypertension and subsequently introduced the animal model as a surrogate for renal artery anatomy in normotensive humans. The major findings were i) accessory renal arteries are more common in men than in women, ii) blood pressure control neither correlates with morphological parameters nor with the presence of accessory renal arteries, and iii) low GFR is associated with small diameters of main renal arteries.
The liver forces the right kidney to be lower, more medially displaced and smaller than the left. The renal arteries arise from the lateral aspect of the aorta below the level of the superior mesenteric artery and because the right artery passes posterior to the inferior vena cava it is longer than the left, and indeed our findings confirm these observations [14,22]. We add however also what these studies did not show [22], that the left main renal artery is of larger diameter. Our data also suggest that accessory renal arteries are associated with longer main renal arteries, in contrast to previous studies that described solitary renal arteries as longer [14]. The main renal artery mean diameter was neither affected by the presence of uncontrolled hypertension nor by the presence of accessory renal arteries. In the former respect, previous studies stated inconclusive findings. Whereas Palmieri et al. also found no difference between the diameter of main renal arteries with and without accessory renal arteries [14], a small study using computer tomography documented main renal arteries to be smaller in the presence of accessory renal arteries [23]. Because our patient group consists almost exclusively of patients with hypertension, many of which lack blood pressure control, we introduced an animal model as a surrogate for normotensive patients. Even though the porcine renovascular anatomy is very similar in size to humans [24], the length and diameter may not be exactly translated to human population. Yet, the data of the human and the porcine renal angiography both show the same proportions in size between right and left main renal arteries and may therefore allow to draw conclusions on general population.
Our data also showed a positive relationship between main renal artery mean diameter and renal function as measured by GFR. Several pathophysiological mechanisms should be considered. Small renal diameters, especially in relation to renal mass, can cause an increase in renal sympathetic nerve activity (RSNA) [25]. Elevated RSNA increases renin secretion rate resulting in vasoconstriction and a subsequent decrease in renal blood flow (RBF) and GFR [25,26]. Therefore, small renal diameters may both be consequence of reduced renal blood flow and reflect higher sympathetic tone which can potentially be affected by the means of renal denervation [27]. In the long term, flow-mediated decreases in shear stress may trigger endothelium-dependent inward arterial remodeling, leading to a narrowing of the renal arteries [28]. The association between GFR and main renal artery diameter is in line with findings in patients with renal artery stenosis, where small and minimal reference diameters were associated with low GFR and resistant hypertension [29]. The reference diameter was determined by averaging the diameters of both main renal arteries [29].
Accessory renal arteries were identified in 22% (unilaterally in 20%, bilaterally in 2%) of the patients. Two extensive meta-analyses calculated a slightly higher prevalence of 23.3% and 28.2% for accessory renal arteries ranging from 4% to 61.5% and 8.7% to 75.7%, respectively [17,21]. We documented accessory renal arteries to be more common in males than in females whereas previous studies provide inconclusive evidence of gender-specific differences concerning prevalence [14,17]. However, the general role of accessory renal arteries in the development of hypertension remains elusive. A recently published study on the importance of accessory renal arteries for nonresponse to renal denervation showed accessory renal arteries to be overrepresented in resistant hypertensives and nonresponders [11]. The authors argue, that an insufficient focal perfusion due to a mismatch between arterial perfusion and renal mass may result in an increased renin secretion [30,31]. A review of magnetic resonance angiography data suggests accessory renal arteries to be a vascular anomaly rather than an anatomical cause of hypertension [32].
Renal artery disease was diagnosed unilaterally in 7% and bilaterally in 2%. Patients with significant renal artery stenosis were excluded in advance to renal angiography to minimize selection bias. The overall prevalence of 9% for renal artery disease, however, was high compared to previous studies analyzing patients undergoing diagnostic cardiac catheterization [33,34]. This may be related to the underlying hypertension in the present cohort. Several studies have shown that the prevalence of renal artery stenosis is higher among patients with uncontrolled hypertension (i.e. 15-40%) compared to people with hypertension in general [35].
As with all studies the limitations of our work should be acknowledged. Even though renal angiography of healthy swine showed similar results as our human patient group which consists almost exclusively of patients with hypertension, many of which lack blood pressure control, the results may not be translated to the general population [24]. Selective invasive renal angiography primarily provides two-dimensional images which may have reduced the accuracy of our measurements. The use of local vasodilators prior to imaging was at the interventionalist’s discretion. Thus, the extent of vascular tone may have also affected the measurements. To the best of our knowledge, there has been no consistent, standardized and generally accepted nomenclature of the renal vascular tree which could facilitate comparisons between different studies. For further investigations, we proposed a new intuitive nomenclature which meets the requirements of the complex renal arterial anatomy. Future studies using less invasive diagnostic modalities such as magnetic resonance angiography [36] and computer tomography (CT) [37] are needed to compare healthy individuals without an indication for renal angiography with those affected by hypertension.
Conclusion
Renal arterial anatomy differs significantly when comparing renal arteries by sides or gender but not when comparing patients with hypertension with and without blood pressure control. Further, accessory renal arteries are more common among men than women. Impaired renal function measured by GFR is associated with small main renal artery mean diameter.
Impact on daily practice
With increasing attention to renovascular causes and targets for hypertension arises a critical need for more detailed knowledge of renal arterial anatomy. We proposed a new intuitive nomenclature which meets the requirements of the complex renal arterial anatomy and implemented it by analyzing the renal vascular tree of 1000 people with hypertension. Renal arterial anatomy differs significantly between sides and gender, but not when comparing patients with hypertension with and without blood pressure control.
Acknowledgments
We would like to thank Jeannette Zimolong, and Kathrin Gaspard for excellent technical assistance.
Disclosures
FM, CU, and MB are supported by the Ministry of Science and Economy of the Saarland. FM is supported by the Deutsche Hochdruckliga. CU and MB are supported by the Deutsche Forschungsgemeinschaft (KFO 196). FM and MB are supported by Deutsche Gesellschaft für Kardiologie. MS is supported by an NHMRC Senior Research Fellowship. ERE is funded in part by a grant from the US National Institutes of Health (R01 GM 49039). All authors except LL, ART, ERE, SK and BS received scientific support and speaker honorarium from Medtronic Ardian Inc.
Abbreviations
- CAD
coronary artery disease
- DBP
diastolic blood pressure
- GFR
glomerular filtration rate
- QVA
quantitative vascular analysis
- RAD
renal artery disease
- RSNA
renal sympathetic nerve activity
- SBP
systolic blood pressure
References
- 1.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–57. [DOI] [PubMed] [Google Scholar]
- 2.Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, McAlister FA, Johansen H, Baclic O, Campbell N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulenthiran S, Ewen S, Böhm M, Mahfoud F. Hypertension up to date: SPRINT to SPYRAL. Clin Res Cardiol. 2017;0:1–10. [DOI] [PubMed] [Google Scholar]
- 4.Ewen S, Cremers B, Meyer MR, Donazzan L, Kindermann I, Ukena C, Helfer AG, Maurer HH, Laufs U, Grassi G, Böhm M, Mahfoud F. Blood pressure changes after catheter-based renal denervation are related to reductions in total peripheral resistance. J Hypertens. 2015;33:2519–25. [DOI] [PubMed] [Google Scholar]
- 5.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–42. [DOI] [PubMed] [Google Scholar]
- 6.Chrysochou C, Kalra PA. Epidemiology and natural history of atherosclerotic renovascular disease. Prog Cardiovasc Dis. 2009;52:184–95. [DOI] [PubMed] [Google Scholar]
- 7.Aboyans V, Desormais I, Magne J, Morange G, Mohty D, Lacroix P. Renal artery stenosis in patients with peripheral artery disease: prevalence, risk factors and long term prognosis. Eur J Vasc Endovasc Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Zheng B, Ma Q, Zheng L-H, Yong Q, He Y-H, Liu J-H. Analysis of renal artery stenosis in patients with heart failure: A RASHEF study. Chin Med J (Engl). 2015;128:2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amighi J, Schlager O, Haumer M, Dick P, Mlekusch W, Loewe C, Böhmig G, Koppensteiner R, Minar E, Schillinger M. Renal artery stenosis predicts adverse cardiovascular and renal outcome in patients with peripheral artery disease. Eur J Clin Invest 2009;39:784–92. [DOI] [PubMed] [Google Scholar]
- 10.Mui K-W, Zeebregts CJ, van den Hout H, van Baal JG, Navis G, Jan-Woittiez A. Impact of incidental renal artery stenosis on long-term mortality in patients with peripheral arterial disease undergoing vascular procedure. J Vasc Surg Off Publ Soc Vasc Surg [and] Int Soc Cardiovasc Surgery, North Am Chapter. 2011;54:785–90. [DOI] [PubMed] [Google Scholar]
- 11.VonAchen P, Hamann J, Houghland T, Lesser JR, Wang Y, Caye D, Rosenthal K, Garberich RF, Daniels M, Schwartz RS. Accessory renal arteries: prevalence in resistant hypertension and an important role in nonresponse to radiofrequency renal denervation. Cardiovasc Revascularization Med. 2016;17:470–3. [DOI] [PubMed] [Google Scholar]
- 12.Mahfoud F, Edelman ER, Böhm M. Catheter-based renal denervation is no simple matter. J Am Coll Cardiol. 2014;64:644–6. [DOI] [PubMed] [Google Scholar]
- 13.Ewen S, Ukena C, Lüscher TF, Bergmann M, Blankestijn PJ, Blessing E, Cremers B, Dörr O, Hering D, Kaiser L, Nef H, Noory E, Schlaich MP, Sharif F, Sudano I, Vogel B, Voskuil M, Zeller T, Tzafriri AR, Edelman ER, Lauder L, Scheller B, Böhm M, Mahfoud F. Anatomical and procedural determinants of catheter-based renal denervation. Cardiovasc Revascularization Med. 2016;17:474–9. [DOI] [PubMed] [Google Scholar]
- 14.Palmieri BJ, Petroianu A, Silva LC, Andrade LM, Alberti LR. Study of arterial pattern of 200 renal pedicle through angiotomography. Rev Col Bras Cir. 2011;38:116–21. [DOI] [PubMed] [Google Scholar]
- 15.Talenfeld AD, Schwope RB, Alper HJ, Cohen EI, Lookstein RA. MDCT angiography of the renal arteries in patients with atherosclerotic renal artery stenosis: implications for renal artery stenting with distal protection. Am J Roentgenol. 2007;188:1652–8. [DOI] [PubMed] [Google Scholar]
- 16.Stephens FD. Ureterovascular hydronephrosis and the ‘aberrant’ renal vessels. J Urol. 1982;128:984–7. [DOI] [PubMed] [Google Scholar]
- 17.Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat. 2001;23:33–8. [DOI] [PubMed] [Google Scholar]
- 18.Holden A, Smith AJP, Dukes P, Pilmore H, Yasutomi M. Assessment of 100 live potential renal donors for laparoscopic nephrectomy with multi-detector row helical CT. Radiology. 2005;237:973–80. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard RH, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak AF, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Burnier M, Ambrosioni E, Caulfield MJ, Coca A, Olsen MH, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Ferrari R, Hasdai D, Hoes AW, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Gillebert TC, Rosei EA, Anker SD, Bauersachs J, Hitij JB, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Ž, Rydén L, Sirenko Y, Stanton AV, Struijker-Boudier H, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC Guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2159 LP–2219. [DOI] [PubMed] [Google Scholar]
- 21.Natsis K, Paraskevas G, Panagouli E, Tsaraklis A, Lolis E, Piagkou M, Venieratos D. A morphometric study of multiple renal arteries in Greek population and a systematic review. Rom J Morphol Embryol. 2014;55:1111–22. [PubMed] [Google Scholar]
- 22.Schönherr E, Rehwald R, Nasseri P, Luger AK, Grams AE, Kerschbaum J, Rehder P, Petersen J, Glodny B. Retrospective morphometric study of the suitability of renal arteries for renal denervation according to the Symplicity HTN-2 trial criteria. BMJ Open. 2016;6:e009351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uysal Ramadan S, Yigit H, Gokharman D, Tuncbilek I, Dolgun NA, Kosar P, Kosar U. Can renal dimensions and the main renal artery diameter point to the presence of an accessory renal artery? A 64-slice CT study. Diagnostic Interv Radiol. 2010;17:266–71. [DOI] [PubMed] [Google Scholar]
- 24.Sakakura K, Ladich E, Edelman ER, Markham P, Stanley JRL, Keating J, Kolodgie FD, Virmani R, Joner M. Methodological standardization for the pre-clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv. 2014;7:1184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. AJP Regul Integr Comp Physiol. 2010;298:R245–53. [DOI] [PubMed] [Google Scholar]
- 26.Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–8. [DOI] [PubMed] [Google Scholar]
- 27.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–64. [DOI] [PubMed] [Google Scholar]
- 28.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science (80-). 1986;231:405–7. [DOI] [PubMed] [Google Scholar]
- 29.Zanoli L, Rastelli S, Marcantoni C, Tamburino C, Laurent S, Boutouyrie P, Castellino P. Renal artery diameter, renal function and resistant hypertension in patients with low-to-moderate renal artery stenosis. J Hypertens. 2012;30:600–7. [DOI] [PubMed] [Google Scholar]
- 30.Houghland T, Hamann JJ, Nemoto NT, Garberich RF, Rosenthal K, Lesser JR, Schwartz RS. Abstract 13836: Accessory renal arteries perfuse a substantial fraction of renal mass: studies in renal denervation patients. Circulation. 2014;130:A13836 LP–A13836. [Google Scholar]
- 31.Nemoto N, Hamann J, Lesser J, Schwartz R. Renal denervation in patients with accessory renal arteries: renal mass is directly proportional to total renal artery cross sectional area and implications for therapy. J Am Coll Cardiol. 2014;63:A2084. [Google Scholar]
- 32.Gupta A, Tello R. Accessory renal arteries are not related to hypertension risk: a review of MR angiography data. Am J Roentgenol. 2004;182:1521–4. [DOI] [PubMed] [Google Scholar]
- 33.Shukla AN, Madan TH, Jayaram AA, Kute VB, Rawal JR, Manjunath AP, Udhreja S. Prevalence and predictors of renal artery stenosis in patients undergoing peripheral and coronary angiography. Int Urol Nephrol. 2013;45:1629–35. [DOI] [PubMed] [Google Scholar]
- 34.Crowley JJ, Santos RM, Peter RH, Puma JA, Schwab SJ, Phillips HR, Stack RS, Conlon PJ. Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J. 1998;136:913–8. [DOI] [PubMed] [Google Scholar]
- 35.Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–54. [DOI] [PubMed] [Google Scholar]
- 36.King BF. MR angiography of the renal arteries. Semin Ultrasound CT MRI. 1996;17:398–403. [DOI] [PubMed] [Google Scholar]
- 37.El Fettouh HA, Herts BR, Nimeh T, Wirth SL, Caplin A, Sands M, Ramani AP, Kaouk J, Goldfarb DA, Gill IS. Prospective comparison of 3-dimensional volume rendered computerized tomography and conventional renal arteriography for surgical planning in patients undergoing laparoscopic donor nephrectomy. J Urol. 2003;170:57–60. [DOI] [PubMed] [Google Scholar]