Patients with sickle cell disease (SCD) manage most pain symptoms or events they experience at home without seeking medical help (Smith et al., 2008). Thus, to have the largest impact on patients’ functioning and quality of life, there should be more focus on assessment and treatment of daily pain rather than episodic acute pain that requires emergent care (Amr, Amin, & Al-Omair, 2011). To accomplish this, we need a better understanding of daily pain patterns and factors influencing changes in pain level day-to-day, which currently pose a challenge for medical management (Smith et al., 2008). There is limited information, however, on the daily variability of pain and what factors are associated with increases in SCD pain frequency or intensity. Innovative techniques are critically needed to better understand, describe, and allow better pain treatment. In the current study, we aim to use a mobile e-diary app to describe day-to-day patterns in SCD-related pain symptoms and identify the clinical and demographic factors associated with differences in daily pain level among adult patients with SCD.
METHODS
Three sickle cell centers located in large urban medical centers utilized the Sickle cell Mobile Application to Record symptoms via Technology (SMART) for ongoing studies: This study combines data from two independent clinical trials with similar daily pain tracking protocols (ClinicalTrials.gov NCT01833702, NCT02384590). The clinical trial conducted at University of Pittsburgh (Pitt) and Vanderbilt University (Vanderbilt) asked participants to use SMART for at least 6 months, and the clinical study at Duke University (Duke) asked participants to use the app for at least 1 month. Eligible participants were all >18yo with a confirmed diagnosis of SCD.
All participants were given SMART installed on an iOS mobile device (iPad or iPhone), either their own or one that we provided, to track their daily pain and interventions used. Pain was recorded on a visual analog scale (VAS) with location of pain as a drop-down list. The list of pharmacological interventions taken was customized for each patient and generated from the patient’s current medications listed in medical records. Participants received push-notification reminders to record symptoms twice a day. Additional details regarding SMART have been described in previous reports (Shah, Hemoglobin 2014; Jonassaint, Hemoglobin 2014). Participants received no payment for their entries.
Analysis.
We categorized five sickle-cell genotype groups: type HgbSS, HgbSC, Sβ+, Sβ0 and SOarab. T-tests and ANOVAs compared the mean of the within-patient averaged pain levels. To account for within-patient correlation in the VAS pain reports, we fitted several linear mixed models, including both fixed effects for the independent variables and random effects at the patient and institutions level. We modeled a compound symmetric correlation matrix for within-patient pain reports. The multivariate models included as covariates institution, gender, age, hydroxyurea use, folic acid use, long-acting and short-acting opioid use, and non-opioid pain medications. Time of day was assessed as a categorical variable.
RESULTS
The sample includes 47 patients (mean age 33 years old ± 11.6) from the three institutions: Duke (n = 19), Vanderbilt (n = 9), and Pitt (n = 20). Eight participants were excluded from analysis due to having ≤ 5 entries (n=6) or VAS pain reports never exceeded 0 (n=2). The final analyzed sample included 39 participants with varying types of SCD including type SS/SO-Ara (n=23, 60%), SC (n=8, 21%), Sβ+ (n=5, 13%), and Sβ0 (n=3, 7%). Most participants were prescribed HU (69%) and folic acid (66%). In addition, the majority were treated with pain medications including long-acting narcotics (74%), short-acting narcotics (88%), and non-opioids (74%). The 8 excluded participants were younger, and less likely to be taking FA vitamin or short-acting opioids.
Participants used the SMART app for 164.6 ± 109.6 days, with a mean of 67.2 ± 60.4 pain reports per participant. The most frequent reporting occurred between 18–24 hrs (n=911) and the least frequent between 0–7 hrs (n=221). Mean pain scores over the total study period was 4.7 ± 2.1, as measured by the electronic VAS. The median use of SMART was similar at each institution and there were no use differences by demographic or clinical factors. A rapid decline in reporting occurred from week 1 with 7.38 mean reports to week 6 with 3.72 mean reports. Reporting stayed relatively stable thereafter not falling below 3.0 reports on average until week 19. Although most participants decreased their frequency of reporting over the course of the study, n=7 did not show such decreasing pattern until their last report.
Medications, clinical factors and pain level.
The linear mixed models showed sickle cell genotype SC was associated with significantly lower mean VAS pain levels than either of the most severe genotypes SS or Sβ0 Thal (b=2.15; p=.0.04). Using folic acid (regression coefficient (b)=−.39; p=.04) and non-opioid pain medications (b=−2.06; p=.006) was also significantly associated with lower VAS pain level as compared to those who reported not using those medications. There was a statistical trend toward the use of short-acting opiates being associated with higher VAS pain level (b=1.33; p=.09). There was no effect on VAS pain for age, gender, HU, long-acting opioids, or institution differences. After controlling for the effect of other covariates (SCD diagnosis, age, gender, HU, folic acid, long-acting narcotics, short-acting narcotics, and non-opioids), using folic acid (b=−0.41, p=0.03) and non-opioids (b=−2.25, p=0.004) was still significantly associated with lower VAS pain levels.
Time of day differences.
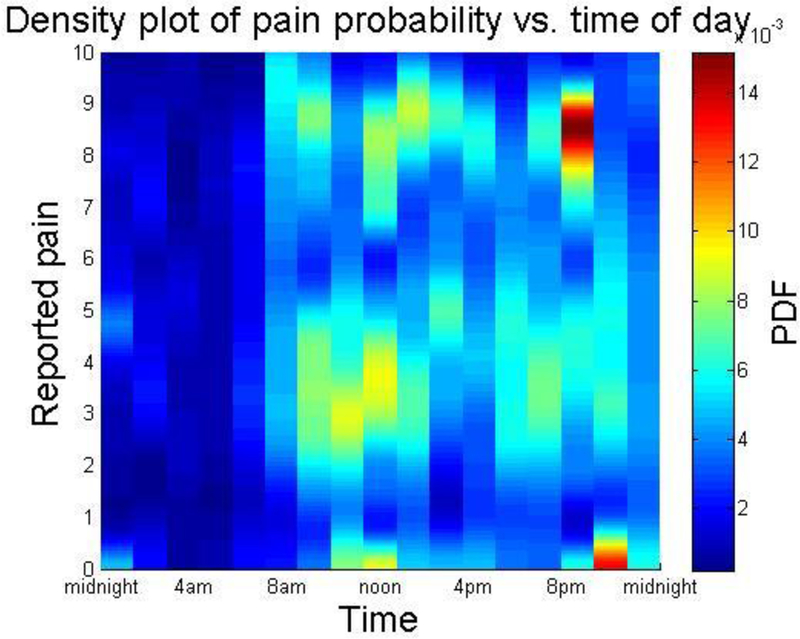
Time of day was associated with VAS pain levels. Density plot of pain probability vs. time of day found a cluster of highest pain reports (Figure 1) between 8 and 9pm. Highest probability of recording was found between 11am and noon, with both higher and lower pain scores recorded frequently during this time.
Figure 1.
PDF = Probability density function
Discussion
On average, patients reported pain using an electronic pain diary app for about 6 months and entered data every 2 to 3 days. There were several trends or significant association in the expected direction, which helps support the validity of this data capture method. For instance, patients taking non-opioid pain medications had 2.3/10 points lower average pain on the VAS, while those prescribed a short-acting opioid for pain had 1.3/10 points higher average pain. Thus, patients were likely fairly accurate in their report of pain using the electronic VAS.
Like other remote pain reporting tools, app use was more frequent at the beginning of the reporting period and then decreased over time (Becker et al., 2015; Runyan et al., 2013; Whitehead & Seaton, 2016). Further, there were significant individual differences in the frequency of reporting, with several individuals reporting fewer than five times and others reporting nearly every day. As attrition and non-adherence are typical for electronic health interventions and remote monitoring tools, future studies of SMART would benefit from implementation of strategies to increase engagement. We also note that participants received no compensation for their reporting in any of these studies, thus there were no external incentives for engagement.
Despite a limited sample size, this study provides strong evidence supporting the use of mobile technology for measuring daily pain and symptoms in SCD. These data suggest that ecological momentary assessment may be an effective and accurate means for evaluating treatment outcomes and pain trajectories in this population.
Table 1.
Demographic and clinic characteristics of the study sample
| Variable | value | N | Percent |
|---|---|---|---|
| Female | 23 | 59.0 | |
| Age | 18–34 yrs >=35 yrs |
24 15 |
61.5 38.5 |
| Use Hydroxyurea (HU) | 27 | 69.2 | |
| Use Folic acid vitamin | 26 | 66.7 | |
| Ever Long-acting medication user Ever Short-acting medication user Ever Non-opioid medication user |
|
29 35 29 |
74.4 89.7 74.4 |
| SCD disease type | HgbSC HgbSS/SO-Ara Sβ+Thal Sβ0Thal |
8 23 5 3 39 |
20.5 59.0 12.8 7.7 |
| Total number of study sample (patients) | |||
Acknowledgements:
The authors would like to acknowledge Andrea Ball from the University of Pittsburgh for her substantive contributions to this manuscript.
Research Funding: This project was supported by grant number 1R21 HS023989 (PI: Shah) and K23 HL135396 (PI: Jonassaint) from the National Heart Lung and Blood Institute.
Appendix Figure 1.

Patient-specific line plots (Spaghetti plot) of the total number of reports per patient by week and a mean curve across all patients (red)
Footnotes
Disclosures:
Conflict of Interest: Jude Jonassaint is owner of SickleSoft, Inc., the IT company responsible for early development of SMART. SickleSoft does not own rights or patents associated with SMART.
Reference
- Albright CL, Pruitt L, Castro C, Gonzalez A, Woo S, & King AC (2005). Modifying physical activity in a multiethnic sample of low-income women: one-year results from the IMPACT (Increasing Motivation for Physical ACTivity) project. Ann Behav Med, 30(3), 191–200. doi: 10.1207/s15324796abm3003_3 [DOI] [PubMed] [Google Scholar]
- Amr MA, Amin TT, & Al-Omair OA (2011). Health related quality of life among adolescents with sickle cell disease in Saudi Arabia. Pan Afr Med J, 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK, Bauserman RL, McCarthy WF, Castro OL, Smith WR, Waclawiw MA, & Anemia I o. t. M. S. o. H. i. S. C. (2010). Hydroxyurea and acute painful crises in sickle cell anemia: effects on hospital length of stay and opioid utilization during hospitalization, outpatient acute care contacts, and at home. J. PAIN SYMPTOM MANAGE., 40(6), 870–882. doi: 10.1016/j.jpainsymman.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Brandl C, Meister S, Nagel E, Miron-Shatz T, Mitchell A, . . . Mertens A (2015). Demographic and health related data of users of a mobile application to support drug adherence is associated with usage duration and intensity. PLoS One, 10(1), e0116980. doi: 10.1371/journal.pone.0116980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake H (2008). Innovation in practice: mobile phone technology in patient care. Br J Community Nurs, 13(4), 160, 162–165. doi: 10.12968/bjcn.2008.13.4.29024 [DOI] [PubMed] [Google Scholar]
- Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, . . . Schori M (2008). National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med, 148(12), 932–938. [DOI] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, & Steiner CA (2010). Acute care utilization and rehospitalizations for sickle cell disease. JAMA, 303(13), 1288–1294. doi: 303/13/1288[pii] [DOI] [PubMed] [Google Scholar]
- Brousseau DC, Panepinto JA, Nimmer M, & Hoffmann RG (2010). The number of people with sickle-cell disease in the United States: National and state estimates. American Journal of Hematology, 85(1), 77–78. doi: Doi 10.1002/Ajh.21570 [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, . . . Bonds DR (1995). Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. New England Journal of Medicine, 332(20), 1317–1322. [DOI] [PubMed] [Google Scholar]
- Dyer O (2003). Patients will be reminded of appointments by text messages. BMJ, 326(7402), 1281. doi: 10.1136/bmj.326.7402.1281-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckard C, Asbury C, Bolduc B, Camerlengo C, Gotthardt J, Healy L, . . . Horzempa J (2016). The Integration of Technology into Treatment Programs to Aid in the Reduction of Chronic Pain. J Pain Manag Med, 2(3). [PMC free article] [PubMed] [Google Scholar]
- Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, . . . Telen MJ (2014). Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol, 89(5), 530–535. doi: 10.1002/ajh.23683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil KM, Anthony KK, Carson JW, Redding-Lallinger R, Daeschner CW, & Ware RE (2001). Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol, 26(3), 163–173. [DOI] [PubMed] [Google Scholar]
- Hochstenbach LM, Zwakhalen SM, Courtens AM, van Kleef M, & de Witte LP (2016). Feasibility of a mobile and web-based intervention to support self-management in outpatients with cancer pain. Eur J Oncol Nurs, 23, 97–105. doi: 10.1016/j.ejon.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Jacob E, Duran J, Stinson J, Lewis MA, & Zeltzer L (2013). Remote monitoring of pain and symptoms using wireless technology in children and adolescents with sickle cell disease. J Am Assoc Nurse Pract, 25(1), 42–54. doi: 10.1111/j.1745-7599.2012.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Stinson J, Duran J, Gupta A, Gerla M, Ann Lewis M, & Zeltzer L (2012). Usability testing of a Smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. J Pediatr Hematol Oncol, 34(5), 326–335. doi: 10.1097/MPH.0b013e318257a13c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassaint CR, Shah N, Jonassaint J, & De Castro L (2015). Usability and Feasibility of an mHealth Intervention for Monitoring and Managing Pain Symptoms in Sickle Cell Disease: The Sickle Cell Disease Mobile Application to Record Symptoms via Technology (SMART). Hemoglobin, 39(3), 162–168. doi: 10.3109/03630269.2015.1025141 [DOI] [PubMed] [Google Scholar]
- Krishna S, Boren SA, & Balas EA (2009). Healthcare via cell phones: a systematic review. Telemed J E Health, 15(3), 231–240. doi: 10.1089/tmj.2008.0099 [DOI] [PubMed] [Google Scholar]
- Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, . . . Segal JB (2008). Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Annals of Internal Medicine, 148(12), 939–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew SM, Tong SF, Lee VK, Ng CJ, Leong KC, & Teng CL (2009). Text messaging reminders to reduce non-attendance in chronic disease follow-up: a clinical trial. Br J Gen Pract, 59(569), 916–920. doi: 10.3399/bjgp09X472250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorey FW, Arnopp J, & Cunningham GC (1996). Distribution of hemoglobinopathy variants by ethnicity in a multiethnic state. Genet Epidemiol, 13(5), 501–512. doi: 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4 [pii] 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-410.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4[pii] 10.1002/(SICI)1098-2272(1996)13:5<501::AID-GEPI6>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- McClellan CB, Schatz JC, Puffer E, Sanchez CE, Stancil MT, & Roberts CW (2009). Use of handheld wireless technology for a home-based sickle cell pain management protocol. J Pediatr Psychol, 34(5), 564–573. doi: 10.1093/jpepsy/jsn121 [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. Division of Blood Diseases and Resources. (2002). The management of sickle cell disease NIH publication no 02–0117 Retrieved from http://purl.access.gpo.gov/GPO/LPS22097
- Palermo TM, Valenzuela D, & Stork PP (2004). A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain, 107(3), 213–219. [DOI] [PubMed] [Google Scholar]
- Rabb LM, Grandison Y, Mason K, Hayes RJ, Serjeant B, & Serjeant GR (1983). A trial of folate supplementation in children with homozygous sickle cell disease. Br J Haematol, 54(4), 589–594. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Steenbergh TA, Bainbridge C, Daugherty DA, Oke L, & Fry BN (2013). A smartphone ecological momentary assessment/intervention “app” for collecting real-time data and promoting self-awareness. PLoS One, 8(8), e71325. doi: 10.1371/journal.pone.0071325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnog JB, Duits AJ, Muskiet FA, ten Cate H, Rojer RA, & Brandjes DP (2004). Sickle cell disease; a general overview. Neth J Med, 62(10), 364–374. [PubMed] [Google Scholar]
- Shah N, Jonassaint J, & De Castro L (2014). Patients welcome the Sickle Cell Disease Mobile Application to Record Symptoms via Technology (SMART). Hemoglobin, 38(2), 99–103. doi: 10.3109/03630269.2014.880716 [DOI] [PubMed] [Google Scholar]
- Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, . . . Roseff SD (2008). Daily assessment of pain in adults with sickle cell disease. Ann Intern Med, 148(2), 94–101. [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Muse ED, & Topol EJ (2013). Can mobile health technologies transform health care? JAMA, 310(22), 2395–2396. doi: 10.1001/jama.2013.281078 [DOI] [PubMed] [Google Scholar]
- Stinson JN, Petroz GC, Stevens BJ, Feldman BM, Streiner D, McGrath PJ, & Gill N (2008). Working out the kinks: testing the feasibility of an electronic pain diary for adolescents with arthritis. Pain Res Manag, 13(5), 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, & Broderick JE (2007). Real-time data collection for pain: appraisal and current status. Pain Med, 8 Suppl 3, S85–93. doi: 10.1111/j.1526-4637.2007.00372.x [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, & Hufford MR (2002). Patient non-compliance with paper diaries. BMJ, 324(7347), 1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, & Nagel RL (2004). Sickle-cell disease. Lancet, 364(9442), 1343–1360. doi: S0140673604171924[pii] [DOI] [PubMed] [Google Scholar]
- Sutton D, Stanley P, Babl FE, & Phillips F (2008). Preventing or accelerating emergency care for children with complex healthcare needs. Arch Dis Child, 93(1), 17–22. doi: 10.1136/adc.2007.117960 [DOI] [PubMed] [Google Scholar]
- Whitehead L, & Seaton P (2016). The Effectiveness of Self-Management Mobile Phone and Tablet Apps in Long-term Condition Management: A Systematic Review. J Med Internet Res, 18(5), e97. doi: 10.2196/jmir.4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Shah AK, Watson M, & Mankad VN (1995). Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Health Rep, 110(1), 80–86. [PMC free article] [PubMed] [Google Scholar]
- Zennadi R, Moeller BJ, Whalen EJ, Batchvarova M, Xu K, Shan S, . . . Telen MJ (2007). Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood, 110(7), 2708–2717. doi: blood-2006-11-056101[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, O’Branski EE, Rosse WF, & Ware RE (1999). Hemoglobin S/O(Arab): thirteen new cases and review of the literature. Am J Hematol, 60(4), 279–284. [DOI] [PubMed] [Google Scholar]


