Abstract
Background
Patients with HPV-positive oropharyngeal squamous cell carcinoma (OPC) have high survival rates when treated with radiotherapy plus cisplatin. Whether replacement of cisplatin with cetuximab, an antibody against the epidermal growth factor receptor, can preserve high survival rates and reduce treatment toxicity is unknown.
Methods
In a randomized, non-inferiority, multicenter trial, patients with locoregionally-advanced p16-positive OPC were stratified by American Joint Committee on Cancer T (T1-T2 vs. T3-T4) and N (N0-N2a vs. N2b-N3), Zubrod Performance Status (0 vs. 1), and tobacco smoking history (≤ vs. >10 pack-years) and randomized 1:1 to radiotherapy plus cetuximab 400 milligrams per square meter of body surface area (mg/m2), followed by 250 mgs/m2 for seven weekly doses or cisplatin 100 mgs/m2 for two doses, 21 days apart. The sample size was 800 eligible patients. The primary endpoint was overall survival (OS) with non-inferiority margin 1.45 (hazard ratio).
Findings
From June 2011 through July 2014, 849 patients (805 eligible; 399 cetuximab; 406 cisplatin) were randomized at 182 centers in the United States and Canada. With median follow-up 4·5 years, radiotherapy plus cetuximab did not meet the non-inferiority criterion for OS. Estimated 5-year OS was 77·9% (95% confidence interval [CI] 73·4-82·5) in cetuximab group versus 84·6% (95%CI=80·6-88·6) in cisplatin group (hazard ratio [HR], 1·45, 1-sided 95% upper CI, 1·94; non-inferiority p=0·5056; 1-sided log-rank p=0.0163). PFS was significantly lower in cetuximab group than in cisplatin group (HR 1·72, 95%CI=1·29-2·29; 5-year rates, 67·3% vs. 78·4%), and LRF was significantly higher (HR 2·05, 95%CI=1·35-3·10; 5-year rates, 17·3% vs. 9·9%). The rate of moderate-to-severe toxicity that was acute (77·4% vs. 81·7%, p=0·1586) and late (16·5 vs. 20·4%, p=0·1904) was similar in the cetuximab and cisplatin groups, respectively.
Interpretation
For patients with HPV-positive OPC, radiotherapy plus cetuximab demonstrated inferior OS and PFS compared to radiotherapy plus cisplatin; toxicity rates were similar (NCT01302834).
Funding
National Cancer Institute USA, Eli Lilly and The Oral Cancer Foundation
Introduction
Human papillomavirus (HPV) is the cause of a subgroup of oropharyngeal squamous cell carcinomas (OPC) that is rising in incidence in many countries including the United States. Survival rates are higher for HPV-positive than HPV-negative OPC when treated with radiotherapy plus high-dose cisplatin (3-year survival, 82·4% versus 57·1%).1 The high survival rate together with young age at diagnosis has promoted increased concern regarding late treatment-related toxicity for patients with HPV-positive OPC.
The addition of platinum-based chemotherapy to radiotherapy is estimated to result in an absolute 5-year survival benefit for head and neck squamous cell carcinoma (HNSCC) of 8%.2 Benefit is similar for OPC.3 However, moderate-to-severe acute toxicity is greater with the addition of cisplatin.4 Moreover, the combined rate of severe dysphagia, feeding tube-dependence or death without cancer progression after radiotherapy plus cisplatin is as high as 43% at 3 years.5 In a landmark trial (IMC9815), the addition of cetuximab, an antibody against the epidermal growth factor receptor (EGFR), to radiotherapy improved survival for HNSCC without increased toxicity6,7. Absolute 5-year survival benefit was 9·2%, and subgroup analysis suggested similar benefit for OPC.8 The relative risks and benefits of cetuximab versus cisplatin when added to radiotherapy for patients with locoregionally-advanced HNSCC are unknown.
We conducted a randomized clinical trial with a classical non-inferiority study design to compare OS for patients with HPV-positive OPC when treated with radiotherapy plus cetuximab versus cisplatin. NRG Oncology RTOG 1016 investigated the hypothesis that cetuximab would maintain high cure rates and reduce rates of acute and late toxicity.
METHODS
Patients
Eligibility criteria included histologically confirmed HPV-positive OPC; American Joint Committee on Cancer (AJCC) 7th edition clinical categories T1-T2, N2a-N3 or T3-T4, N0-N3 M0; Zubrod Performance Status (PS) 0-1; age ≥ 18 years; and adequate bone marrow, hepatic and renal function. HPV status was determined by the established and validated surrogate of immunohistochemistry for p16-expression in a centralized laboratory,9 and tumors were classified as p16-positive if strong and diffuse nuclear and cytoplasmic staining was present in ≥70% of tumor cells.9 Patients provided their lifetime cigarette exposure history at enrollment via a standardized computer-assisted self-interview.
Trial Design and Treatment
Patients were stratified by T category (T1-T2 vs. T3-T4), N category (N0-N2a vs. N2b-N3), Zubrod PS (0 vs. 1), and tobacco smoking history (≤ vs. >10 pack-years) and randomized in a 1:1 ratio by the permuted block method to receive either intravenous cetuximab at a loading dose of 400 milligrams per square meter of body surface area (mg/m2) 5-7 days prior to radiotherapy initiation followed by 250 mg/m2 weekly for 7 doses (total 2150 mg/m2) or cisplatin 100 mg/m2 on days 1 and 22 of radiotherapy (total 200 mg/m2). All patients received accelerated intensity modulated radiotherapy (IMRT) delivered to 70 Gy in 35 fractions over 6 weeks, 6 fractions per week (with 2 fractions 1 day per week, at least 6 hours apart). This regimen of accelerated radiotherapy plus cisplatin was chosen as the control arm to align with the investigational and control arms of RTOG 01291 and RTOG 0522, respectively, as these trials provided comprehensive data on the survival outcomes for HPV-positive OPC. In addition, RTOG 0129 showed that accelerated fractionated radiotherapy over 6 weeks with 2 cycles of cisplatin yielded similar outcomes as conventionally fractionated radiotherapy over 7 weeks with 3 cycles of cisplatin with better chemotherapy compliance.
Assessments
Adverse events (AE) were evaluated by NCI Common Terminology Criteria for Adverse Events Version 4 (CTCAE V4) and were assessed at baseline, weekly during radiotherapy, end of treatment, and 1 and 3 months after treatment completion. Criteria for dose reduction or delay were pre-specified. Per protocol disease assessment (physical exam, including laryngopharyngoscopy, and if indicated, computed tomography [CT] or magnetic resonance imaging [MRI] of the head and neck) and late AE were required every 3 months for 2 years, every 6 months through year 5, and then annually. Chest X-ray or CT chest were performed annually. Dental health was assessed according to a 5-point scale developed for this trial: normal; mild changes/good dental health; moderate/fair dental health; severe changes in dental health; and life-threatening dental condition. Quality of life outcomes (detailed in the protocol) were assessed at baseline, end of treatment, and at 3, 6, and 12 months after treatment completion. Quality assurance review of chemotherapy and radiotherapy was performed per standard NRG Oncology protocol (Table S1).
RTOG 1016 was registered with the National Cancer Institute (NCT01302834) and approved by institutional review boards of participating institutions. Patients provided written informed consent.
End Points
RTOG 1016 was initially designed to investigate whether radiotherapy plus cetuximab results in 5-year overall survival (OS) not lower than radiotherapy plus cisplatin by more than 9% (hazard ratio [HR] <1·4), based on survival estimates generated from patients with pl6-positive OPC in RTOG 01292. Using a group sequential design based on Haybittle’s boundary with 3 interim analyses, 1-sided alpha 0·05, and 80% power, 600 randomized and eligible patients were required. The expected study duration was 8·5 years. In December 2013, the study was amended to reflect higher survival rates noted for patients with pl6-positive OPC in a later trial, RTOG 0522.10 Under the original design sample size and RTOG 0522 survival estimates, the expected study duration would have been increased by 5 years. The redesign (undertaken before any interim analysis had been conducted) called for a non-inferiority margin of 1·45 for the HR, larger than the initial margin, but with a smaller absolute difference, 7·6%, at 5 years. Using a group sequential design based on Haybittle’s boundary with 3 interim analyses (after 45, 90, and 135 of 180 deaths), 1-sided alpha 0·05, and 80% power, 800 randomized and eligible patients were required; to allow for 20% non-randomization and ineligibility, planned enrollment was up to 1000 patients. The revised expected study duration was 8·15 years. The primary endpoint was OS, defined as time from randomization to death due to any cause. Secondary endpoints included: progression-free survival (PFS: time from randomization to cancer progression or death); locoregional failure and distant metastasis (LRF; DM; Table S2); second primary tumors (SPT); overall and type-specific treatment-related (definitely, probably, or possibly related) adverse events (AEs) that were acute (≤180 days) or late (>180 days) relative to treatment completion; early death (death due to AE or within 30 days of treatment completion); feeding tube placement; dental health; and quality of life. Clinical or radiographic evidence of progression was investigator-assessed by clinical exam, imaging and/or biopsy. Quality of life assessments were optional and limited to the first 400 patients who consented. Only the EORTC QLQ-H&N35 swallowing domain is included here.11
Statistical Analysis
Primary analysis was based on the modified intent-to-treat approach, whereby all patients meeting eligibility criteria are included. Sensitivity analyses were performed for the primary endpoint in the per protocol subset, defined as 70 Gy of radiation and 200 mg/m2 of cisplatin or 8 doses of cetuximab, and in all randomized patients. For the primary endpoint, if the upper limit of the 1-sided 95% confidence interval (CI) for the HR estimated from the Cox proportional hazards model (unadjusted) was <1·45, non-inferiority of cetuximab would be concluded. In addition, the arms were compared by log-rank test, with reference to the 1-sided alternative hypothesis of cetuximab failure rate > cisplatin failure rate (non-pre-specified). All other tests and 95%CI were 2-sided. OS and PFS were estimated by Kaplan-Meier method with groups compared by log-rank test. LRF, DM, and SPT were estimated using cumulative incidence functions with groups compared by cause-specific log-rank tests and HRs.12 The proportional hazards assumption for the Cox model was verified by supremum test with 1000 simulations. Safety analysis was limited to eligible patients who started protocol treatment. Mean raw T-scores and A-scores were calculated per the TAME method.13 AE and feeding tube rates were compared by Fisher’s exact test. Mean T-scores, A-scores, and EORTC QLQ-H&N35 subscale scores on the swallowing domain change from pretreatment to 1-year11 were compared by t test with unequal variances. The Benjamini-Hochberg procedure with 5% false discovery rate was used to adjust for multiple comparisons of AE rates and for unplanned analysis of OS and PFS treatment effect in subgroups. A copy of the full protocol is available at the NRG Oncology website (www.nrgoncology.org).
At the third interim analysis, although neither efficacy nor futility boundaries were crossed, the point estimate for the HR exceeded the non-inferiority margin. A recent methodology article established that non-inferiority trials can be reliably stopped for futility if the observed HR equals or exceeds the pre-specified non-inferiority margin after at least 50% of events.14 In addition, the protocol futility boundary is relatively conservative (unlikely to lead to stopping except for a large deviation from noninferiority). The observed hazard ratio at the current information would need to exceed 1·56 to satisfy the futility boundary. While such a boundary protects against erroneous early stopping for futility, the property that the rule requires an estimate above the upper boundary late in follow-up can be seen as permitting undue risk. Based on these considerations, the NRG Oncology Data Monitoring Committee recommended results be disclosed.
Role of the Funding Source
The trial was designed by the first authors. The first and last authors attest to the accuracy and completeness of the data and analyses and vouch for adherence of the trial to the protocol. Industry sponsors had no role in the study design; in the collection, analysis and interpretation of data, in the writing of the report; and in the decision to submit the paper for publication. A copy of the manuscript was provided to the industry sponsor at the time of submission.
RESULTS
Patients and Treatment
From June 9, 2011 through July 31, 2014, 987 patients were enrolled, of whom 849 underwent randomization at 182 centers in the United States and Canada (CONSORT Flow Diagram). The data cutoff was May 14, 2018. A total of 805 eligible patients were assigned to receive cetuximab (n=399) or cisplatin (n=406). The characteristics of the eligible study population are shown in Table 1. Patients were predominantly male, white and had a median age of 58 years (interquartile range [IQR] 52-63). A history of more than 10 pack-years of cigarette smoking was reported by 38% (n=303), and 71% (n=573) were determined to be in the low-risk group as previously defined in a recursive partitioning analysis conducted in RTOG 01291.
CONSORT Flow Diagram.
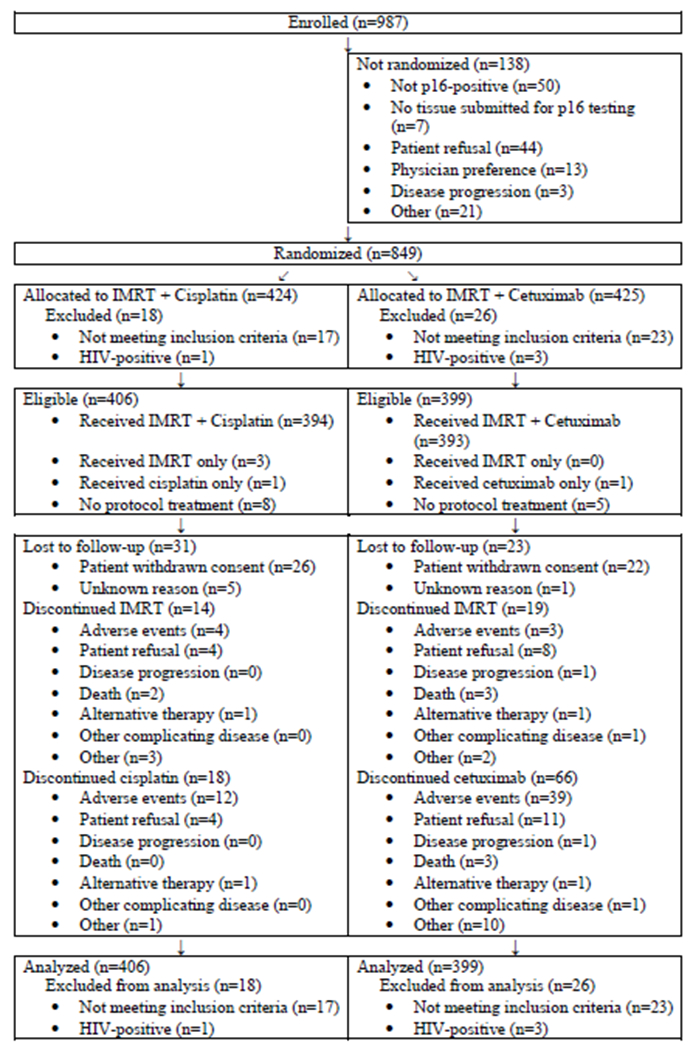
Table 1.
Patient and Tumor Characteristics According to Assigned Treatment.
| IMRT + Cisplatin (n=406) |
IMRT + Cetuximab (n=399) |
Total (n=805) |
|
|---|---|---|---|
| Age (years) | |||
| ≤ 65 | 344 (85%) | 345 (86%) | 689 (86%) |
| > 65 | 62 (15%) | 54 (14%) | 116 (14%) |
| Mean (standard deviation) | 57·7 (8·1) | 57·4 (7·8) | 57·6 (8·0) |
| Median (1st - 3rd quartile) | 58 (52-63) | 58 (52-63) | 58 (52-63) |
| Min - max | 33 - 83 | 33 - 80 | 33 - 83 |
| Gender | |||
| Male | 373 (92%) | 355 (89%) | 728 (90%) |
| Female | 33 (8%) | 44 (11%) | 77 (10%) |
| Race | |||
| White | 380 (94%) | 367 (92%) | 747 (93%) |
| Black or African American | 17 (4%) | 19 (5%) | 36 (4%) |
| Other | 2 (<1%) | 8 (2%) | 10 (1%) |
| Unknown | 7 (2%) | 5 (1%) | 12 (1%) |
| Ethnicity | |||
| Hispanic or Latino | 11 (3%) | 15 (4%) | 26 (3%) |
| Not Hispanic or Latino | 383 (94%) | 369 (92%) | 752 (93%) |
| Unknown | 12 (3%) | 15 (4%) | 27 (3%) |
| Zubrod performance status | |||
| 0 | 295 (73%) | 300 (75%) | 595 (74%) |
| 1 | 111 (27%) | 99 (25%) | 210 (26%) |
| Smoking history | |||
| 0 pack-years | 194 (488%) | 181 (45%) | 375 (47%) |
| > 0 - ≤ 10 pack-years | 59 (15%) | 68 (17%) | 127 (16%) |
| >10 pack-years | 153 (38%) | 150 (38%) | 303 (38%) |
| Mean (standard deviation) | 15·0 (23·5) | 14·8 (23·9) | 14·9 (23·7) |
| Median (1st - 3rd quartile) | 2 (0-22) | 3 (0-24) | 2 (0-23) |
| Min - max | 0 - 147 | 0 - 202 | 0 - 202 |
| Primary site | |||
| Tonsillar fossa, tonsil | 202 (50%) | 199 (50%) | 401 (50%) |
| Base of tongue | 174 (43%) | 179 (45%) | 353 (44%) |
| Oropharynx, NOS | 16 (4%) | 15 (4%) | 31 (4%) |
| Pharyngeal oropharynx | 8 (2%) | 5 (1%) | 13 (2%) |
| Soft palate | 4 (1%) | 0 (0%) | 4 (<1%) |
| Vallecula | 2 (<1%) | 1 (<1%) | 3 (<1%) |
| T stage (AJCC 7th Edition) | |||
| T1 | 89 (22%) | 86 (22%) | 175 (22%) |
| T2 | 162 (40%) | 163 (41%) | 325 (40%) |
| T3 | 108 (27%) | 100 (25%) | 208 (26%) |
| T4 | 47 (12%) | 50 (13%) | 97 (12%) |
| N stage (AJCC 7th Edition) | |||
| N0 | 20 (5%) | 14 (4%) | 34 (4%) |
| N1 | 20 (5%) | 25 (6%) | 45 (6%) |
| N2a | 59 (15%) | 56 (14%) | 115 (14%) |
| N2b | 209 (51%) | 208 (52%) | 417 (52%) |
| N2c | 82 (20%) | 83 (21%) | 165 (20%) |
| N3 | 16 (4%) | 13 (3%) | 29 (4%) |
| Overall stage (AJCC 7th Edition) | |||
| III | 29 (7·1%) | 31 (7·8%) | 60 (7·5%) |
| IV | 377 (92·9%) | 368 (92·2%) | 745 (92·5%) |
| Risk group per RTOG 0129 | |||
| Low-risk | 289 (71%) | 284 (71%) | 573 (71%) |
| Intermediate-risk | 117 (29%) | 115 (29%) | 232 (29%) |
| Consented to PRO/QOL Collection | (n=213) | (n=206) | (n=419) |
| No | 17 (8%) | 21 (10%) | 38 (9%) |
| Yes | 196 (92%) | 185 (90%) | 381 (91%) |
Smoking history measured by use of standardized computer-assisted self-interview. PRO, patient-reported outcome; QOL, quality of life.
Cetuximab was administered per protocol in 86% (n=344) of patients (Table S3). A large majority (85%; n=339) in the cetuximab group received at least 7 doses. Mean dose of cetuximab received was 1940·9 mg/m2 (standard deviation [SD] 520.1). In the cisplatin group, chemotherapy was given per protocol in 88% (n=356). Both cycles of cisplatin were delivered in a large majority (93%; n=377). Mean dose received was 184·7 mg/m2 (SD 40.0).
Radiotherapy was delivered per protocol or with acceptable variation in 86% (n=291) and 83% (n=294) in the cetuximab and cisplatin groups, respectively. Distributions of radiotherapy dose, fraction number and total duration in days were equivalent in both groups. At least 95% of the planned 70 Gy dose was delivered to 95% of patients in the cetuximab and cisplatin groups.
Overall Survival
After a median follow-up of 4·5 years, there were 133 deaths: 78 in the cetuximab group and 55 in the cisplatin group. Radiotherapy plus cetuximab did not meet criterion for non-inferiority to radiotherapy plus cisplatin: HR 1·45; 1-sided 95% upper CI 1·94. OS was significantly worse with cetuximab (2-sided 95%CI 1·03-2·05; log-rank p=0·0163). The estimate for 5-year OS was 77·9% (95%CI 73·4-82·5%) in the cetuximab group and 84·6% (95%CI 80·6-88·6) in the cisplatin group (Figure 1A). In the per protocol subset and in all randomized patients, the HR and 1-sided 95% upper CI were 1·40 (2·05) and 1·45 (1·91), respectively.
Figure 1A. Overall Survival According to Assigned Treatment.
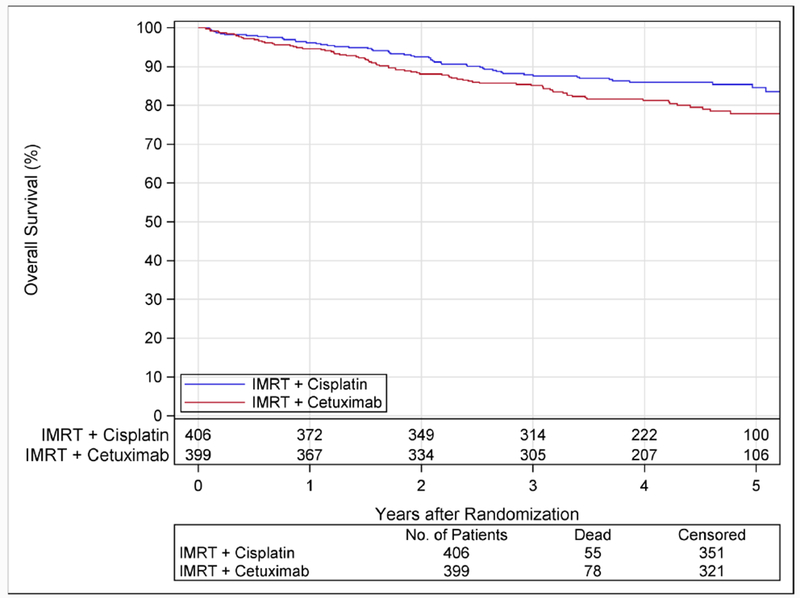
Kaplan-Meier estimates of overall survival are shown according to assigned treatment. After a median follow-up of 4·5 years (range, 0·01-6·5), there were 133 deaths; 78 in the cetuximab group and 55 in the cisplatin group. Radiotherapy plus cetuximab did not meet the criterion for non-inferiority in comparison to radiotherapy plus cisplatin: the hazard ratio was 1·45 with 1-sided 95% upper confidence bound 1·94 (p=0·5056 for non-inferiority). In addition, survival was significantly worse with cetuximab (p=0·0163; 2-sided 95%CI 1·03-2·05). The 5-year estimates were 77·9% (95%CI 73·4-82·5) with cetuximab and 84·6% (95%CI 80·6-88·6) with cisplatin.
Figure 1B shows the effect of assigned treatment on OS across demographic and clinical subgroups (non-pre-specified). The 1-sided 95% upper CI for the HR was >1·45 for all subgroups. Relative to treatment with cisplatin, patients with a Zubrod PS 1 did significantly worse when treated with cetuximab (HR 2·66, 1-sided 95% upper CI, 4·32), while patients with a Zubrod 0 (HR 1·08, 1-sided 95% upper CI 1·55) did not. However, after adjustment for multiple comparisons, the test for interaction was not significant. Radiotherapy delivery indices were similar across patients stratified by treatment and Zubrod PS (data not shown). Patients with Zubrod 1 versus 0 received a lower mean dose of cetuximab (1879 versus 1961 mg/m2), but not of cisplatin (192 versus 182 mg/m2).
Figure 1B. Overall Survival Treatment Effect in Subgroups.
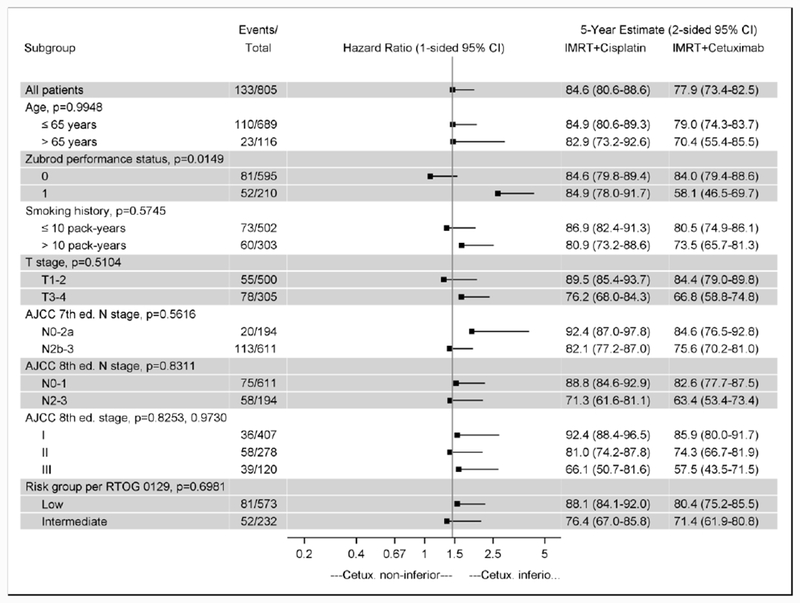
Hazard ratios and 5-year overall survival estimates are shown for subgroups. Risk groups are as defined in RTOG 0129. Low-risk is ≤ 10 pack-years (any N stage) or > 10 pack-years and N0-N2a. Intermediate-risk is > 10 pack-years and N2b-N3. The reference line is at 1·45, the upper bound required for non-inferiority. P-values are for the test for interaction between treatment and subgroup. There is a large difference in the treatment effect for Zubrod 0 and 1: The hazard ratio is 1·08 (1-sided 95% upper confidence bound 1·55) for Zubrod 0 and 2·66 (4·32) for Zubrod 1. The test for interaction is not significant after adjustment for multiple comparisons. Abbreviations: CI, confidence interval.
Secondary disease control endpoints
A total of 198 events of cancer progression or death were reported, including 122 in the cetuximab group and 76 in the cisplatin group. PFS was significantly lower in the cetuximab group than the cisplatin group (HR 1·72, 95%CI 1·29-2·29; p=0·0002; 5-year rates, 67·3 vs. 78·4%; Figure 2A). An analysis of the treatment effect of cetuximab versus cisplatin on PFS in subgroups (non-pre-specified) identified a larger difference for Zubrod 1 (HR 2·68, 95%CI 1·62-4·42) than for Zubrod 0 (HR 1·43, 95%CI 1·01-2·04), but after adjustment for multiple comparisons the difference was not significant.
Figure 2A. Progression-Free Survival According to Assigned Treatment.

Kaplan-Meier estimates of progression-free survival (PFS) are shown according to assigned treatment. Patients assigned to cetuximab had significantly worse PFS (p=0·0002) than patients assigned to cisplatin. The estimated 5-year PFS rates were 67·3% (95%CI 62·4-72·2) with cetuximab and 78·4% (95%CI 73·8-83·0) with cisplatin.
Hazard of LRF in the cetuximab group was more than twice that of the cisplatin group (HR 2·05, 95%CI 1·35-3·10; p=0·0005; 5-year rates, 17·3 vs 9·9%; Figure 2B). Salvage surgery was performed at the primary site or regional lymph nodes in 4% (n=16) and 8% (n=31), respectively, of patients in the cetuximab group, and 3% (n=14) and 6% (n=26) in the cisplatin group.
Figure 2B. Time to Locoregional Failure According to Assigned Treatment.
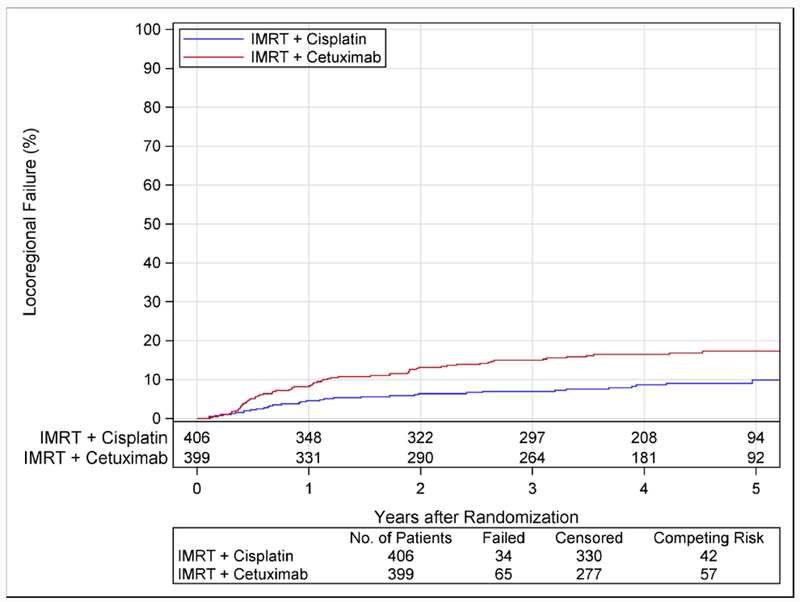
Cumulative incidence estimates of locoregional failure are shown according to assigned treatment. Patients assigned to cetuximab had significantly more locoregional failure (p=0·0005) than patients assigned to cisplatin. The estimated 5-year rates of locoregional failure were 17·3% (95%CI 13·7-21·4) with cetuximab and 9·9% (95%CI 6·9-13·6) with cisplatin.
There was no significant difference in rates of DM with cetuximab versus cisplatin (HR 1·49, 95%CI 0·94-2·36; p=0·0885; 5-year rates 11·7% vs. 8·6%). Among those with PFS failure, locoregional failure alone was experienced by 39% (n=47) in the cetuximab group and 30% (n=23) in the cisplatin group. Corresponding numbers for distant metastases alone were 35% (n=43) and 41% (n=31). Nearly all first sites of distant metastases were lung, liver or bone (or a combination thereof) in both groups. Second primary tumor rates were also not significantly different between the treatment groups (HR 0.99, 95%CI 0·61-1.58; p=0·9525; 5-year cumulative incidence rate, cetuximab vs. cisplatin, 10·3% versus 9·9%).
Adverse Events
The early death rate was the same in the cetuximab and cisplatin groups (1·5% with 95%CI 0.6·3.3 for each; p=1·0000). Table 2 shows rates of moderate-to-severe (CTCAE V4 grade 3-4) treatment-related acute and late AE (see also Tables S4 and S5). The rate of one or more grade 3-4 acute AE was similar in the cetuximab and cisplatin groups (77·4 [95%CI 73.0-81.5] vs. 81·7% [95%CI 77.5-85.3]; p=0·1586). Acneiform rash was significantly more frequent in the cetuximab group, whereas myelosuppression, anemia, nausea, vomiting, anorexia, dehydration, hyponatremia, kidney injury, and hearing impairment were significantly more frequent in the cisplatin group.
Table 2.
Prespecified Treatment-Related Adverse Events of Interest or Occurring in at least 5% of Patients According to Assigned Treatment.
| IMRT + Cisplatin | IMRT + Cetuximab | p-value | |
|---|---|---|---|
| Acute period, n | 398 | 394 | |
| Early death | 1·5% | 1·5% | 1.0000 |
| Grade 3-4 overall | 81·7% | 77·4% | 0.1586 |
| Grade 3-4 anemia | 2·8% | 0·0% | 0.0009* |
| Grade 3-4 hearing impaired | 3·0% | 0·3% | 0.0032* |
| Grade 2-3 dry mouth | 49·7% | 53·6% | 0.2872 |
| Grade 3-4 dysphagia | 37·4% | 32·0% | 0.1171 |
| Grade 3-4 mucositis oral | 41·5% | 46·2% | 0.1974 |
| Grade 3 nausea | 19·1% | 8·1% | <0.0001* |
| Grade 3-4 vomiting | 12·1% | 4·1% | <0.0001* |
| Grade 3 fatigue | 5·8% | 4·3% | 0.4178 |
| Grade 3-4 dermatitis radiation | 8·0% | 12·4% | 0.0462 |
| Grade 3-4 lymphocyte count decreased | 17·1% | 17·5% | 0.9252 |
| Grade 3-4 neutrophil count decreased | 15·3% | 0·5% | <0.0001* |
| Grade 3 weight loss | 7·8% | 5·8% | 0.3241 |
| Grade 3-4 white blood cell decreased | 12·1% | 0·0% | <0.0001* |
| Grade 3-4 anorexia | 22·4% | 15·5% | 0.0144* |
| Grade 3-4 dehydration | 15·3% | 6·1% | <0.0001* |
| Grade 3-4 hyponatremia | 5·3% | 1·0% | 0.0008* |
| Grade 3-4 acute kidney injury | 3·3% | 0·3% | 0.0017* |
| Grade 3-4 pharyngeal mucositis | 13·6% | 10·2% | 0.1535 |
| Grade 3-4 rash acneiform | 0·3% | 9·4% | <0.0001* |
| Grade 3-4 pain (all terms) | 14·6% | 12·7% | 0.4694 |
| Mean raw T-score | 3·19 | 2·35 | <0.0001* |
| Late period, n | 383 | 375 | |
| Grade 3-4 overall | 20·4% | 16·5% | 0.1904 |
| Grade 3-4 hearing impaired | 6·3% | 2·1% | 0.0060* |
| Grade 2-3 dry mouth | 32·1% | 33·6% | 0.6991 |
| Grade 3-4 dysphagia | 4·4% | 6·1% | 0.3318 |
| Grade 3 weight loss | 4·4% | 2·9% | 0.3366 |
| Grade 3-4 osteonecrosis of jaw | 2·1% | 0·8% | 0.2234 |
| Grade 3-4 pain (all terms) | 1·3% | 2·1% | 0.4154 |
| Mean raw A-score | 0·38 | 0·27 | 0.1189 |
Tx, treatment; NOS, not otherwise specified.
Adverse event rates were compared by Fisher’s exact test.
T-scores and A-scores were compared by t test with unequal variances.
Significant after adjustment for multiple comparisons.
On the IMRT + Cisplatin arm, there were 6 early deaths:
(1) grade 5 cardiac arrest reported as possibly related to Tx at 1 day after end of Tx;
(2) grade 5 sepsis reported as possibly related to Tx at 4 days after end of Tx;
(3) grade 5 sudden death NOS reported as possibly related to Tx at 18 days after end of Tx;
(4) grade 5 sudden death NOS reported as unrelated to Tx at 2 days after end of Tx;
(5) grade 5 sudden death NOS reported as unrelated to Tx at 2 days after end of Tx;
(6) grade 5 sudden death NOS reported as unrelated to Tx at 7 days after end of Tx.
On the IMRT + Cetuximab arm, there were 6 early deaths:
(1) grade 5 respiratory failure reported as probably related to Tx at 37 days after end of Tx;
(2) grade 5 cardiac arrest reported as possibly related to Tx at 1 day after end of Tx;
(3) grade 5 sudden death NOS reported as possibly related to Tx at 1 day after end of Tx;
(4) grade 5 myocardial infarction reported as possibly related to Tx at 4 days after end of Tx;
(5) grade 5 death NOS reported as possibly related to Tx at 17 days after end of Tx;
(6) grade 5 sudden death NOS reported as unrelated to Tx at 12 days after end of Tx.
An alternative measure of the overall acute toxicity burden for patients is provided by the T-score: the mean number of grade 3-4 acute AE per patient13. Patients in the cetuximab group had a significantly lower T-score than those in the cisplatin group (raw T-score 2·35 versus 3·19; p<0·0001), corresponding to a 40% lower acute toxicity burden.
With regard to late toxicity in the cetuximab versus cisplatin groups, neither overall rates of one or more grade 3-4 AE (16·5 [95%CI 12.9-20.7] vs. 20·4 [95%CI 16.4-24.8], p=0·1904; Table 2) nor mean number of grade 3-4 AE (raw A-score, 0·27 versus 0·38; p=0·1189) were significantly different. Hearing impairment was significantly more common after treatment with cisplatin.
There were no notable differences between groups in treatment-related grade 3-4 AE rates over time (Figure 3A). At 1 year after treatment, 8·5% (95%CI 5.8-12.0) of patients in the cetuximab group and 10·0% (95%CI 7.1-13.6) in the cisplatin group had grade 3-4 AE.
Figure 3A. Treatment-Related Grade 3-4 Adverse Event Rates Over Time According to Assigned Treatment.
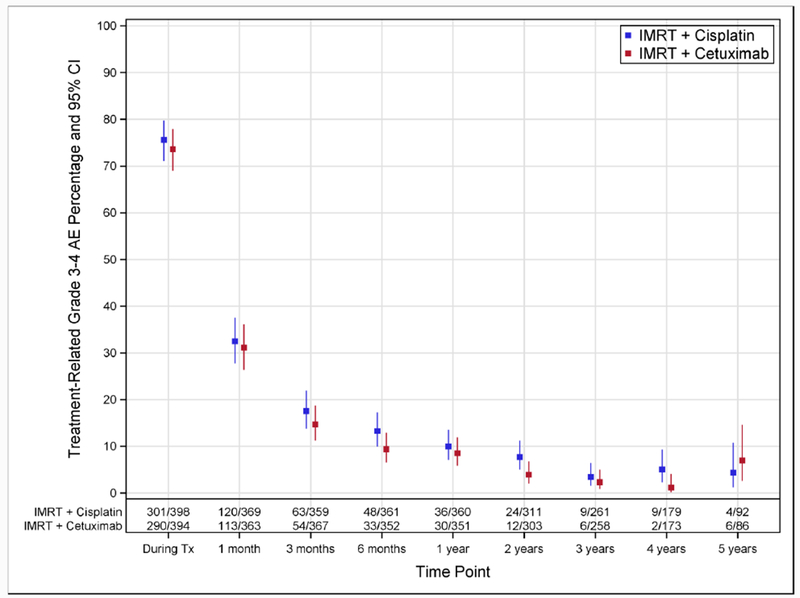
Percentages of patients with treatment-related grade 3-4 adverse events, along with 95% exact confidence intervals, are shown according to assigned treatment. Time points 1 month and later are relative to the end of treatment. The following windows around each time point were used: 1 month, −2 to +4 weeks; 3 months, −4 to +6 weeks; 6 months, −6 to +8 weeks; 1 year and later, ± 3 months. Abbreviations: AE, adverse event; CI, confidence interval; Tx, treatment.
At treatment completion, 57·3% (95%CI 52.2-62.2) of patients in the cetuximab group and 61·5% (95%CI 56.5-66.3) in the cisplatin group had a feeding tube (Figure 3B). These rates dropped to 8·4% (95%CI 5.8-11.8) and 9·2% (95%CI 6.5-12.7) at 1 year after treatment (p=0·7946), respectively.
Figure 3B. Feeding Tube Rates Over Time According to Assigned Treatment.
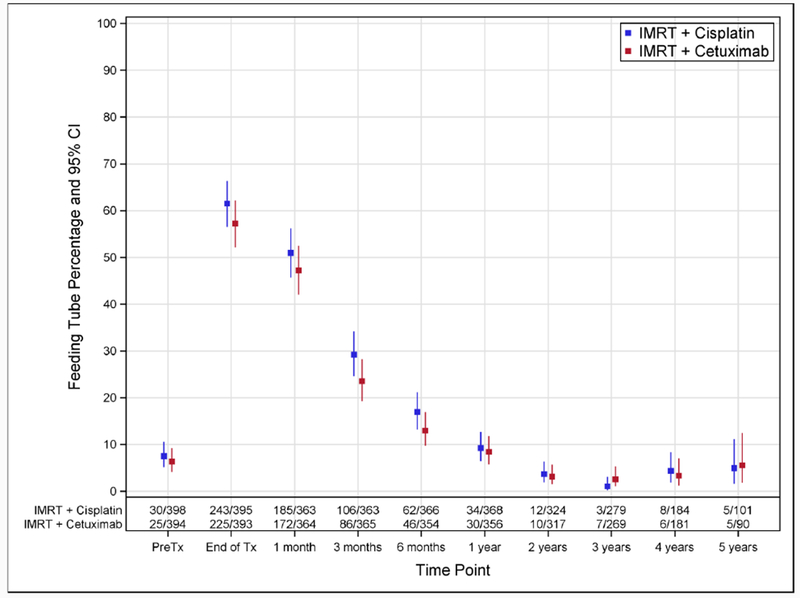
Percentages of patients with a feeding tube, along with 95% exact confidence intervals, are shown according to assigned treatment. Time points 1 month and later are relative to the end of treatment. The following windows around each time point were used: 1 month, −2 to +4 weeks; 3 months, −4 to +6 weeks; 6 months, −6 to +8 weeks; 1 year and later, ± 3 months. Abbreviations: CI, confidence interval; Tx, treatment.
EORTC QLQ-H&N35 completion patterns, completion rates, and reasons missing were similar between the groups. Patient-reported severity of swallowing problems increased in both the cetuximab and cisplatin groups from pretreatment to end of treatment, but no difference was observed between groups in change scores from baseline (means 47·43 vs. 47·99; p=0·8643; Table S6). At 1 year, the cetuximab group had a statistically significant increase in symptoms from pretreatment than did the cisplatin group (7·61 vs. 2·52; p=0·0382), but the difference is below the estimated clinically important difference.15
Before treatment, 75% (n=294) of patients in the cetuximab group had normal or mild changes/good dental health and the mean number of native teeth in place was 21·4 compared to 71% (n=283) and 20·9 in the cisplatin group (Table S7). At 1 year after treatment, these rates were 84% (n=223) and 87% (n=233) with mean number of teeth lost 1·64 and 1·05 for the cetuximab and cisplatin groups, respectively.
Discussion
Radiotherapy plus cetuximab led to inferior OS when compared to radiotherapy plus cisplatin for patients with locoregionally-advanced HPV-positive OPC. Risks of cancer progression or death and locoregional failure were also greater with cetuximab. Profiles of moderate-to-severe acute and late toxicities were different for patients treated with cetuximab versus cisplatin, while rates of one or more such events were similar. Nonetheless, the overall burden of acute toxicity was greater for patients treated with cisplatin than cetuximab as reflected by the T-scores.
RTOG 1016 is the first randomized trial to investigate toxicity amelioration or treatment “de-intensification” for patients with HPV-positive OPC. The design was based on data from the IMC 9815 trial which reported that the addition of cetuximab to radiotherapy improved survival without increased detriment to quality of life.16 Moreover, a subgroup analysis suggested a greater survival benefit from cetuximab was experienced by subgroups with characteristics common to patients with HPV-positive tumors (e.g., oropharyngeal subsite, age < 65 years, good PS).7 A subsequent retrospective biomarker analysis of the IMC 9815 trial suggested that survival benefit was greater from cetuximab for HPV-positive than HPV-negative OPC, although the interaction was not statistically significant.8 Despite these promising data, RTOG 1016 demonstrated that cetuximab is less effective than cisplatin and should not be used alone as a de-intensification strategy for patients with HPV-positive OPC.
Our data are consistent with retrospective studies that reported reduced cancer control with cetuximab versus cisplatin in patients with HPV-positive and HPV-negative HNSCC.17–19 While non-randomized studies are subject to selection bias and confounders (such as PS and comorbidity), two randomized phase 2 trials also observed lower locoregional control rates with radiotherapy plus anti-EGFR antibodies (either cetuximab or panitumumab) versus radiotherapy plus cisplatin.20,21 However, these trials were not adequately powered to evaluate OS or non-inferiority in either HPV-positive or HPV-negative groups. The conclusions from this prospective, non-inferiority trial contradict those of a recent, retrospective meta-analysis of subgroups in clinical trials that concluded cetuximab was not inferior to cisplatin for HPV-positive OPC, cautioning against use of such analyses for clinical decision making22. A randomized trial that showed similar PFS, toxicity and quality of life outcomes for addition of either panitumumab or cisplatin to radiation was not powered for non-inferiority.23 A majority of these studies reported reduced locoregional control with cetuximab, supporting our finding that cisplatin is a more potent radiation sensitizer.
HPV-negative HNSCC are genetically distinct from HPV-positive OPC. More frequent in HPV-negative HNSCC are EGFR amplification, overexpression and down-stream signaling, whereas mutations downstream of EGFR (i.e. activating in PIK3CA, inactivating in PTEN) that may mediate resistance to EGFR-target therapies are more frequent in HPV-positive OPC.24 Retrospective analyses of clinical trials investigating the addition of anti-EGFR antibodies to chemotherapy for recurrent metastatic HNSCC have observed greater benefit in patients with HPV-negative cancer,25 albeit inconsistently.26 Given the effect of cetuximab on these two cancers may differ, it may not be appropriate to extrapolate the results of RTOG 1016 to HPV-negative HNSCC.
This trial used tumor p16 immunohistochemical expression, an established surrogate for the gold standard of tumor E6/E7 mRNA expression, to determine eligibility.9 P16 analysis was performed and interpreted in a centralized laboratory. P16 expression was considered sufficient for this trial because two standard-of-care regimens were being compared and neither regimen represented true treatment “de-intensification.” We estimate that at most 7% of patients enrolled in the trial may have had HPV-negative cancer11. However, randomization would be expected to balance the distribution in the two groups. A very strong interaction between tumor HPV status and treatment assignment would be necessary to affect inferences drawn from this trial.
In an analysis of RTOG 0129, tumor HPV status, tobacco exposure and tumor and nodal categories were used to assign patients with OPC treated with radiotherapy plus cisplatin into subgroups at low, intermediate and high-risk of death (3-year OS, 93·0% vs. 70·8% vs. 46·2%)1. HPV-positive patients are low-risk unless tobacco pack-years exceed 10 and there are multiple nodes or a node > 6cm, in which case they are intermediate-risk. These data, together with results of the IMC 9815 trial, led to a common clinical practice of substitution of cetuximab for cisplatin in patients from the low-risk group, with worse PS, or older age in the US. Although not powered for subgroup analysis, RTOG 1016 suggests this practice may compromise patient outcomes for those who can receive cisplatin. For platinum ineligible cases, radiotherapy plus carboplatin and 5-fluorouracil with27 or without28 cetuximab or cetuximab alone may be considered, based upon improvements in survival versus radiotherapy alone in clinical trials not exclusive to either HPV-positive OPC nor platinum-ineligible populations. Enrollment in ongoing trials of radiotherapy plus immunotherapy in this patient population should be strongly encouraged where possible. Patients with Zubrod PS 1 had the poorest outcomes with cetuximab in RTOG 1016, a finding which could not be explained by non-compliance to protocol therapy.
RTOG 1016 included all patients with locoregionally-advanced HPV-positive OPC, whereas a majority of de-intensification trials are generally limited to the low-risk group. Phase 2 de-intensification strategies show promising preliminary results for OS and PFS with induction chemotherapy followed by reduced radiotherapy dose or volume in responders28, 29 or cisplatin and radiotherapy dose reduction.29 Cetuximab led to worse outcomes in both low-risk and intermediate-risk groups in RTOG 1016, underscoring the importance of testing de-intensification strategies in non-inferiority trials with a control arm of 70 Gy radiotherapy plus high-dose cisplatin. Five-year survival in RTOG 1016 was higher than the radiotherapy plus cisplatin control arms of RTOG 01291 and 052210, demonstrating the importance of a contemporaneous control arm.
The analysis population was modified intent-to-treat. Five percent of randomized patients were retrospectively declared ineligible and excluded from analysis. However, this is not uncommon in cooperative group trials and has been accounted for by over enrollment to ensure achievement of the required sample size. Moreover, sensitivity analyses that were performed for the primary endpoint in the per protocol subset and in all randomized patients showed similar hazard ratios as the modified intent-to-treat population, confirming the robustness of the survival outcomes.
In summary, radiotherapy plus cetuximab did not meet the criterion for non-inferiority for OS relative to radiotherapy plus cisplatin. In the first randomized trial exclusive to patients with HPV-positive OPC with a primary endpoint of overall survival, radiotherapy plus cisplatin is established as the standard-of-care. Strategies to improve the 5-year PFS rate of 80% achieved with radiotherapy plus cisplatin while reducing toxicity are still needed for HPV-positive OPC.
Supplementary Material
Research in context.
Evidence before this study
Over the past decade, systematic reviews have estimated that patients diagnosed with p16-positive oropharyngeal cancers (OPC) have less than half the risk of death when compared to patients diagnosed with HPV-negative OPC. The high survival rates for patients with HPV-positive OPC have prompted increased concern regarding late toxicity of therapy. On September 28, 2018 we searched the terms “survival” AND “head and neck cancer” AND “meta-analysis” in PubMed and identified several metaanalyses on the effect of adding chemotherapy to radiotherapy for the treatment of locoregionally advanced head and neck cancer. The addition of platinum-based chemotherapy to radiotherapy is estimated to reduce the mortality of head and neck squamous cell carcinoma (HNSCC) by 26%, leading to an absolute 5-year benefit of 8%. The benefit was similar when restricted to the subgroup of patients with OPC. The addition of cisplatin to radiotherapy was shown to significantly increase both the acute and late toxicity of therapy. Only a single randomized trial evaluated the addition of Cetuximab, an antibody to the epidermal growth factor receptor (EGFR), to radiotherapy in locoregionally advanced HNSCC with the primary endpoint of locoregional control and a secondary endpoint of overall survival. Cetuximab was estimated to reduce mortality by 27%, leading to an absolute 5-year survival benefit of 9·2%. Overall acute toxicity, late toxicity and patient reported quality of life were not worse with the addition of cetuximab to radiotherapy. After the regulatory approval of cetuximab in the United States in 2006, cetuximab use significantly increased, and it became a common clinical practice to substitute cetuximab for cisplatin. However, no randomized prospective clinical trials have directly compared overall survival for radiotherapy plus cetuximab to radiotherapy plus cisplatin.
Added value of this study
To the best of our knowledge, NRG Oncology RTOG 1016 is the first randomized, prospective clinical trial exclusive to patients diagnosed with locoregionally advanced HPV-positive OPC. It was designed as a classical non-inferiority study to investigate a hypothesis that the substitution of cetuximab for cisplatin would maintain high cure rates while at the same time reduce acute and late toxicity of therapy. Radiotherapy plus cetuximab did not meet the criterion for non-inferiority for overall survival when compared to radiotherapy plus cisplatin. Cetuximab was estimated to increase the risk of death by 45% (HR 1·45, 95% confidence interval [CI] 1·03-2·05), the risk of cancer progression or death by 72% (HR 1·72, 95% CI 1·29-2·29) and locoregional failure by 105% (95% CI HR 1·35-3·10). Rates of overall moderate to severe acute and late toxicity were similar, although rates of specific toxicities differed significantly.
Implications of all the available evidence
As the first clinical trial exclusive to patients with HPV-positive OPC, this clinical trial establishes radiotherapy plus cisplatin is the standard of care. Cetuximab should not be substituted for cisplatin for patients with HPV-positive OPC who are platinum eligible.
Acknowledgements
Funding: This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), UG1CA189867 (NCORP), U24CA196067 (NRG Specimen Bank), U24CA180803 (IROC) from the National Cancer Institute (NCI), Eli Lilly and The Oral Cancer Foundation (to M.G.)
Role of the Funding Source: The funding source had no role in study design; in collection, analysis, or interpretation of data; writing of the report; or in the decision to submit for publication.
Declaration of Interests: Drs. Adelstein, Blakaj, Burtness, Dunlap, Eisbruch, Galloway, Galvin, Harari, Harris, Jones, Le, Razaq, Ridge, Ringash, Seaward, Spencer, Sturgis, Trotti and Yao has nothing to disclose. Dr. Beitler reports grants from NRG, during the conduct of the study. Dr. Colevas reports other from COTA, INC, from Keyquest Health, from LOXO Oncology, from Atara Biotherapeutics, from Audro Biotech, Inc, from Pfizer, other from Threshold Pharmaceuticals, other from AstraZeneca, other from Innate Pharma, other from Bristol-Squibb Pharmaceuticals, other from Cellsight Technologies, Inc, other from Tessa Therapeutics. Dr. Dignam reports other from Merck Pharmaceuticals. Dr. Gillison reports grants from The Oral Cancer Foundation , other from National Cancer Institute USA, during the conduct of the study; personal fees and other from Bristol-Myers Squibb, personal fees from TRM Oncology, personal fees from Genocea Biosciences Inc, personal fees from EMD Serono, personal fees from Merck & Co, personal fees from Lilly, personal fees from AstraZeneca, personal fees from NewLink Genetics Corporation, personal fees from Aspyrian Therapeutics, personal fees from Celgene Corp, personal fees from Amgen, outside the submitted work; and Dr. Gillison received a Damon-Runyon Clinical Investigator Award from 2000-2005, which was supported by a grant from Lilly Inc. Dr. Koyfman reports grants from Merck. Dr. Phan reports other from Accuray Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maura L Gillison, Department of Thoracic Head and Neck Medical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Andy M Trotti, Department of Radiation Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Jonathan Harris, NRG Oncology Statistics and Data Management Center, American College of Radiology, Philadelphia, PA, USA.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA.
Paul M Harari, Department of Human Oncology, University of Wisconsin Hospital and Clinics, Madison, WI, USA.
David J Adelstein, Department of Hematology and Medical Oncology, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA.
Erich M Sturgis, Department of Head and Neck Surgery, MD Anderson Cancer Center, Houston, TX, USA.
Barbara Burtness, Department of Medicine, Yale School of Medicine and Yale Cancer Center, New Haven, CT, USA.
John A Ridge, Department of Surgical Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Jolie Ringash, Department of Radiation Oncology, Princess Margaret Cancer Centre and the University of Toronto, Toronto, Ontario, Canada.
James Galvin, Imaging and Radiation Oncology Core Group, Philadelphia, PA, USA.
Min Yao, Department of Radiation Oncology, Case Western Reserve/University Hospitals Seidman Cancer Center, Cleveland, OH, USA.
Shlomo A Koyfman, Department of Radiation Oncology, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA.
Dukagjin M Blakaj, Department of Radiation Oncology, The Ohio State University, Columbus, OH, USA.
Mohammed A Razaq, Department of Hematology/Oncology, University of Oklahoma Health Sciences Center, Norman, OK, USA.
A Dimitrios Colevas, Department of Medicine (Oncology), Stanford University, Stanford, CA, USA.
Jonathan J Beitler, Department of Radiation Oncology, Emory University, Atlanta, GA, USA.
Christopher U Jones, Department of Radiation Oncology, Sutter Cancer Research Consortium, Novato, CA, USA.
Neal E Dunlap, Department of Radiation Oncology, James Graham Brown Cancer Center, University of Louisville, Louisville, KY, USA.
Samantha A Seaward, Department of Radiation Oncology, Kaiser Permanente, Oakland, CA, USA.
Sharon Spencer, Department of Radiation Oncology, University of Alabama at Birmingham Medical Center, Birmingham, AL, USA.
Thomas J Galloway, Department of Radiation Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Jack Phan, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
James J Dignam, Department of Public Health Sciences, University of Chicago, Chicago, IL, USA and NRG Oncology Statistics and Data Management Center, American College of Radiology, Philadelphia, PA, USA.
Quynh Thu Le, Department of Radiation Oncology, Stanford University, Stanford, CA, USA.
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–8. [DOI] [PubMed] [Google Scholar]
- 5.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78. [DOI] [PubMed] [Google Scholar]
- 7.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal DI, Harari PM, Giralt J, et al. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol 2016;34:1300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol 2012;36:945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014;32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 1999;17:1008–19. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978;34:541–54. [PubMed] [Google Scholar]
- 13.Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol 2007;8:613–24. [DOI] [PubMed] [Google Scholar]
- 14.Korn EL, Freidlin B. Interim monitoring for non-inferiority trials: minimizing patient exposure to inferior therapies. Ann Oncol 2018;29:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osoba D Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. Int J Cancer Suppl 1999;12:132–7. [DOI] [PubMed] [Google Scholar]
- 16.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 2007;25:2191–7. [DOI] [PubMed] [Google Scholar]
- 17.Riaz N, Sherman E, Koutcher L, et al. Concurrent Chemoradiotherapy With Cisplatin Versus Cetuximab for Squamous Cell Carcinoma of the Head and Neck. Am J Clin Oncol 2016;39:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang C, Chan C, Jiang W, et al. Concurrent cetuximab versus platinum-based chemoradiation for the definitive treatment of locoregionally advanced head and neck cancer. Head Neck 2015;37:386–92. [DOI] [PubMed] [Google Scholar]
- 19.Barney CL, Walston S, Zamora P, et al. Clinical outcomes and prognostic factors in cisplatin versus cetuximab chemoradiation for locally advanced p16 positive oropharyngeal carcinoma. Oral Oncol 2018;79:9–14. [DOI] [PubMed] [Google Scholar]
- 20.Giralt J, Trigo J, Nuyts S, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol 2015;16:221–32. [DOI] [PubMed] [Google Scholar]
- 21.Magrini SM, Buglione M, Corvo R, et al. Cetuximab and Radiotherapy Versus Cisplatin and Radiotherapy for Locally Advanced Head and Neck Cancer: A Randomized Phase II Trial. J Clin Oncol 2016;34:427–35. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Zhang J, Shi C, Liu L, Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC Cancer 2016;16:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siu LL, Waldron JN, Chen BE, et al. Effect of Standard Radiotherapy With Cisplatin vs Accelerated Radiotherapy With Panitumumab in Locoregionally Advanced Squamous Cell Head and Neck Carcinoma: A Randomized Clinical Trial. JAMA Oncol 2016. [DOI] [PubMed] [Google Scholar]
- 24.Hayes DN, Van Waes C, Seiwert TY. Genetic Landscape of Human Papillomavirus-Associated Head and Neck Cancer and Comparison to Tobacco-Related Tumors. J Clin Oncol 2015;33:3227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 2013;14:697–710. [DOI] [PubMed] [Google Scholar]
- 26.Vermorken JB, Psyrri A, Mesia R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol 2014;25:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Y, Auperin A, Sire C, et al. Improved Outcome by Adding Concurrent Chemotherapy to Cetuximab and Radiotherapy for Locally Advanced Head and Neck Carcinomas: Results of the GORTEC 2007–01 Phase III Randomized Trial. J Clin Oncol 2018:JCO2017762518. [DOI] [PubMed] [Google Scholar]
- 28.Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004;22:69–76. [DOI] [PubMed] [Google Scholar]
- 29.Chera BS, Amdur RJ, Tepper JE, et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2018;124:2347–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


